Abstract
Classical MHC molecules present processed peptides from endogenous protein antigens on the cell surface, which allows CD8+ cytotoxic T lymphocytes (CTLs) to recognize and respond to the abnormal antigen repertoire of hazardous cells, including tumor cells. The light chain, β2-microglobulin (β2m), is an essential constant component of all trimeric MHC class I molecules. There is convincing evidence that β2m deficiency generates immune escape phenotypes in different tumor entities, with an exceptionally high frequency in colorectal carcinoma (CRC) and melanoma. Damage of a single β2m gene by LOH on chromosome 15 may be sufficient to generate a tumor cell precommitted to escape. In addition, this genetic lesion is followed in some tumors by a mutation of the second gene (point mutation or insertion/deletion), which produces a tumor cell unable to express any HLA class I molecule. The pattern of mutations found in microsatellite unstable colorectal carcinoma (MSI-H CRC) and melanoma showed a striking similarity, namely the predominance of frameshift mutations in repetitive CT elements. This review emphasizes common but also distinct molecular mechanisms of β2m loss in both tumor types. It also summarizes recent studies that point to an acquired β2m deficiency in response to cancer immunotherapy, a barrier to successful vaccination or adoptive cellular therapy.
Keywords: HLA class I, β2 microglobulin, Microsatellite instability, Human leukocyte antigen, Tumor immune escape, Loss of heterozygosity
Introduction
The classical MHC class I molecule is a trimeric complex consisting of a variable heavy chain, encoded by human HLA-A, HLA-B, and HLA-C genes, the processed antigen peptide bound to the heavy chain groove, and the constant β2m light chain. Specific recognition of this complex by the T-cell receptor triggers cytotoxic T lymphocyte (CTL) activity, which is currently exploited in active (vaccination) and passive (adoptive cellular therapy [ACT], antibody therapy) cancer immunotherapies. Although ACT and antibody therapy achieved remarkable clinical responses in recent clinical trials, their efficacy is dependent on the MHC class I expression level of the tumor. Various studies have indicated that CD8-positive CTLs play a major role in destroying virus-infected cells and tumor cells [1]. CTL-mediated tumor rejection is not only observed in cancer but also in allograft rejection, graft-versus-host disease, and autoimmune diseases, in which identical molecular pathways lead to T-cell activation, suggesting the existence of an immunological constant of rejection [2].
Evidence is accumulating that loss of MHC class I expression is an obstacle to successful cancer immunotherapy [3, 4]. The implication of β2m gene in the generation of HLA class I-loss tumor variants is well established [5], and different types of mutation can knock down the capacity to synthesize the β2m protein required to produce a functionally active HLA class I molecule [6–8]. A number of these mutations have been detected in analyses of cell lines and tumor tissue, ranging from insertions and deletions of nucleotides in repetitive sequence motifs to single base substitutions in one β2m allele in combination with the loss of large segments of chromosome 15q21 encompassing the second β2m allele [9, 10]. These mutations were found to modify β2m expression, inhibiting transcription of the gene or, more frequently, by abrogating translation of the mRNA in some cases or by the synthesis of a nonfunctional protein.
Recent data obtained in our laboratory indicate that one β2m gene copy is inactivated by loss of heterozygosity (LOH) on chromosome 15 in various human tumors [11]. We also reported evidence in some tumors of LOH on chromosome 15q21, which contains the β2m gene, implying that these tumor cells, with an apparently normal HLA class I expression, can already harbor one hit in one β2m gene [11]. These tumor cells are therefore precommitted to an HLA class I total loss phenotype. These findings also suggest that the complete loss of HLA class I antigen expression by this particular molecular mechanism results from successive mutational events [8]. However, other molecular mechanisms can be used by some tumor cells to produce HLA class I total loss, for example, the coordinated downregulation of the transcription of HLA class I heavy chain β2m and antigen presentation machinery genes in bladder carcinomas [12]. There are also human tumors with a high frequency of HLA class I total loss phenotype in which the β2m mutation is not involved, for example, 40 % of prostate cancers [13] and 52 % of breast cancers. (I. Maleno unpublished results), although the precise molecular mechanism is not known.
The present review describes the different β2m mutations reported in tumor tissues and cell lines and examines whether they follow a particular distribution pattern. We focus on data obtained from colorectal cancer (CRC) with mismatch repair deficiency that exhibit the high microsatellite instability phenotype (MSI-H) and melanoma, in which β2m alterations are a common mechanism for generating the HLA class I total loss phenotype. Our analysis reveals specific β2m gene mutation patterns in MSI-H CRCs and melanomas, suggesting a possible mechanistic origin of these mutations in the context of molecular cancer pathways.
Somatic β2-microglobulin mutations in human cancer
The β2m gene consists of 4 exons that encode a protein of 119 amino acids in length. Exons 1 and 2 contain a total of 4 repetitive nucleotide sequences: exon 1 harbors a [CT]4 motif, encompassing codon 13–15, and exon 2 harbors two A5 repeats, ranging from codon 67–68 and 94–95, and one C5 sequence encompassing codon 91–92. Tables 1 and 2 summarize the different deletions, insertions, and single base substitutions that have been identified in the β2m gene of tumor cells and associated with a total loss of HLA class I expression.
Table 1.
Summary of deletion and insertion mutations in β2m gene in human tumors
| Mutation1 | Site2 | β2m expression3 | HLA expression3 | LOH4 | MSI5 | References | |
|---|---|---|---|---|---|---|---|
|
Co1 (LoVo) |
del CT, Homozygous | 13–15 codons, Ex1 | − | − | + | + | [36] |
|
Co2 (SW48) |
del CTCT, Heterozygous del A, Heterozygous |
13–15 codons, Ex1 a47 codon, Ex2 |
− | − | − | − | [36] |
| Co3 | del CT | 13–15 codons, Ex1 | ± | ± | − | − | [36] |
|
Co4 (HRA19) |
del TCTT, Heterozygous | 14–15 codons, Ex1 | ± | ± | − | − | [36] |
|
Co5 (C14) |
del CT, Heterozygous | 13–15 codons, Ex1 | ± | ± | − | [14] | |
|
Co6 (C108) |
del CT, Heterozygous | 13–15 codons, Ex1 | ± | ± | + | [14] | |
|
Co7 (13971/92) |
del CT, Homozygous | 13–15 codons, Ex1 | − | − | + | [14] | |
|
Co8 (H630) |
del CT, Hemi- or Homozygous | 15 codon, Ex1 | − | − | + | [69] | |
|
Co9 (CO-132) |
del CT | 13–14 codons, Ex1 | − | − | + | [25] | |
|
Co10 (CO-135) |
del CT | 13–14 codons, Ex1 | − | − | + | [25] | |
| Co11 | del TTCT | 15–16 codons, Ex1 | − | − | + | [48] | |
| Co12 | del CT | 16 codon, Ex1 | − | − | + | [48] | |
| Co13 | ins CC | 13 codon, Ex1 | − | − | [70] | ||
|
Co14 (CRC-6) |
ins TT | 14–15 codons, Ex1 | − | − | + | [71] | |
|
Co15 (StM185) |
del A | 67–68 codons Ex2 | + | ||||
|
Co16 (CO-86) |
del CA, Heterozygous del A, Heterozygous |
25 codon, Ex2 67 codon, Ex2 |
− | − | + | [25] | |
| Co17 | del A | 68 codon, Ex2 | − | − | + | [48] | |
| Co18 | del A | 95 codon, Ex2 | − | − | + | [24] | |
| Co19 | ins A | 68 codon, Ex2 | − | − | + | [48] | |
| Co20 | ins A | 95–96 codons, Ex2 | − | − | + | [24] | |
| Co21 | del C | 92 codon, Ex2 | − | − | + | [24] | |
| Co22 | ins C | 92–93 codons, Ex2 | − | − | + | [24] | |
|
Co23 (CO-117) |
del C, Heterozygous del CCGTG, Heterozygous |
91 codon, Ex2 101–102 codons, Ex2 |
− | − | + | [25] | |
|
Co24 (HCT) |
del 11pb, Heterozygous | 23–27 codons, Ex2 | − | − | − | [72] | |
|
Co25 (StM78) |
del TG | 8–9 codons, Ex1 | − | [14] | |||
| Co26 | del C | 34 codon, Ex2 | − | − | + | [14] | |
| Co27 | del C | 110 codon, Ex2 | − | − | [70] | ||
|
Co28 (CRC-16) |
del ACTACACT | 86–88 codons, Ex2 | − | − | − | − | [71] |
|
Me1 (Me1386) |
del CT, Hemi- or Homozygous | 13–15 codons, Ex1 | − | − | + | [26] | |
|
Me2 (GR-34) |
del TTCT | 15–16 codons, Ex1 | − | − | + | − | [7] |
|
Me3 (1106Mel) |
del CT, Hemi- or Homozygous | 13–15 codons, Ex1 | − | − | + | [27] | |
|
Me4 (1180Mel) |
del CT, Hemi- or Homozygous | 13–15 codons, Ex1 | − | − | + | [27] | |
|
Me5 (1259Mel) |
del CT, Hemi- or Homozygous | 13–15 codons, Ex1 | − | − | + | [27] | |
|
Me6 (SK-MEL-33) |
del G, Hemi- or Homozygous | 96 codon, Ex2 | − | − | + | [73] | |
|
Me7 (Mel249) |
del AT, Hemizygous | 62 codon, Ex2 | − | − | + | [37] | |
|
Me8 (FO-1) |
del 3 kb, Hemizygous | First exon and a segment of first intron | − | − | + | [9] | |
|
Me9 (Me9923) |
del 14 bp, Homozygous | 79–83 codons, Ex2 | − | − | + | [26] | |
|
Me10 (UKRV-Mel-2b) |
del 498 bp, Hemi- or Homozygous | −426 to +72 nt, including the whole exon 1 | − | − | + | − | [8] |
|
Re1 (fibroblastoid RCC52) |
del G, Heterozygous del CT, Heterozygous |
6 codon, Ex1 13–15 codons, Ex1 |
− | − | − | [28] | |
|
Re2 (epitheloid RCC52) |
del CT, Hemizygous | 13–15 codons, Ex1 | − | − | + | [28] | |
|
LyT1 (T19) |
del CT, Hemizygous | 14–15 codons, Ex1 | − | − | + | − | [29] |
|
LyT2 (T18) |
del TG, Hemizygous | 46–47 codons, Ex2 | − | − | + | − | [29] |
| Cer | del TC or del CT | 14–15 codons, Ex1 | − | [30] |
1Frameshift mutations affecting repeat sequences of the β2m gene were described by Yamamoto et al. [58] in gastric, colorectal, and hereditary nonpolyposis colorectal cancers (HNPCC) (22, 26, and 19 frameshift mutations, respectively), but they have not been included in the table because of the lack of immunohistochemical studies to characterize the HLA Class I expression
2Site indicates codon number from ATG site, and exon in which it is located
3β2m and HLA expression: ± (weak); −(negative)
4LOH at chromosome 15q: + (presence of LOH); −(absence of LOH)
5MSI phenotype: + (presence of MSI); −(absence of MSI)
aSite indicated by authors (Ref. [36]) did not correspond with our scheme of gene codons of β2m gene
Table 2.
Summary of single base substitution mutations in β2m gene in human tumors
| Mutation1 | Site2 | Amino acid substitution | β2m expression3 | HLA expression3 | LOH4 | MSI5 | References | |
|---|---|---|---|---|---|---|---|---|
|
Co29 (C84) |
G → A, Heterozygous | 54 codon, Ex2 | Asp54Asn | ± | ± | − | [36] | |
|
Co30 (C84T) |
G → A, Heterozygous | Nonrepetitive sequence, Ex2 | ± | ± | + | [14] | ||
|
Co31 (C43) |
G → A, Heterozygous | Upstream of ATG | ± | ± | − | [69] | ||
| Co32 | G → A | 54 codon, Ex2 | Asp54Asn | + | [24] | |||
| Co33 | G → A | 67 codon, Ex2 | Glu67Lys | + | H | [70] | ||
| Co34 | A → G | a68 nucleotide, Ex2 | − | − | [70] | |||
| Co35 | A → G | ATG | Met1Val | − | − | [70] | ||
| Co36 | C → T | 6 codon, Ex1 | Ala6Val | + | + | [70] | ||
|
Co24 (HCT) |
C → A, Heterozygous | 30 codon, Ex2 | Tyr10Stop | − | − | − | [72] | |
|
Co37 (HCT15/DLD1) |
C → A, Heterozygous | 30 codon, Ex2 | Tyr10Stop | − | − | + | [36] | |
|
Co38 (3624/91) |
C → G, Homozygous | 11 codon, Ex1 | Ala11Gly | − | − | − | [14] | |
|
Co39 (3822/93) |
CT → GG, Homozygous | Nonrepetitive sequence, Ex2 | − | − | + | [14] | ||
| Co40 | G → T | 63 codon, Ex2 | Gly63Stop | − | − | + | [24] | |
| Co41 | T → A | 50 codon, Ex2 | Phe50Ile | + | − | [70] | ||
| Co42 |
T → A T → G |
5 codon, Ex1 2 codon, Ex1 |
Val5Glu Ser2Ala |
H | H | [70] | ||
| Co43 | C → A | 108 codon, Ex2 | Ser108Stop | + | H | [70] | ||
|
Co37 (HCT15/DLD1) |
G → T, Heterozygous | Last base of IVS1b | − | − | [36] | |||
|
Me11 (1074Mel) |
G → A, Hemizygous | ATG | Met1Ile | − | − | + | [27] | |
|
Me12 (LB1622-Mel) |
T → A, Hemizygous | ATG | Met1Lys | − | − | + | − | [6] |
|
Me13 (BB74-Mel) |
C → G, Hemizygous | 31 codon, Ex2 | Ser31Stop | − | − | + | − | [6] |
|
Me9 (Me9923) |
C → G, Homozygous | 86 codon, Ex2 | Tyr86Stop | − | − | + | [26] | |
|
Me14 (1174Mel) |
C → G, Hemizygous | 31 codon, Ex2 | Ser31Stop | − | − | + | [27] | |
|
Me15 (VMM5b) |
C → G, Hemizygous | 45 codon, Ex2 | Cys45Top | + | − | + | [74] | |
|
Me16 (DNR-DC-M010) |
G → T, Hemizygous | 67 codon, Ex2 | Glu67Stop | − | − | + | [42] | |
|
Me17 (Me18105) |
A → G, Homozygous | IVS1b | − | − | + | [26] | ||
|
Me18 (Mel499) |
T → A | IVS1b | − | − | [37] | |||
|
Lu1 (H2009) |
A → G, Hemi- or Homozygous | ATG | Met1Val | − | − | + | [69] | |
|
Lu2 (C831L) |
C → T | 22 codon, Ex1 | Gln22Stop | − | − | − | [75] | |
|
Ly (Daudi) |
G → C, Homozygous | ATG | Met1Ile | − | − | [5] |
1Point mutations affecting β2m gene were described by Yamamoto et al. [58] in gastric, colorectal, and hereditary nonpolyposis colorectal cancers (HNPCC), but they have not been included in the table because of the lack of immunohistochemical studies to characterize the HLA Class I expression
2Site indicates codon number from ATG start codon (= 1), and exon in which it is located
3β2m and HLA expression: + (positive); ± (weak); H (heterogeneous); − (negative)
4LOH at chromosome 15: + (presence of LOH); −(absence of LOH)
5MSI phenotype: + (presence of MSI); − (absence of MSI)
aSites indicated by authors (Ref. [70]) did not correspond with our scheme of gene codons of β2m gene
bSingle base substitutions in first intron affect splice sites resulting in microdeletions or insertions: Co4/5 (HCT-15/DLD-1 cell line) and Me7 (Me18105 cell line): deletion of the first 11 pb of exon 2; Me17 (Mel499): insertion of 27 and 407 pb in IVS1)
Microdeletions/insertions in repetitive nucleotide motifs of the β2-microglobulin gene
As shown in Table 1, frameshift mutations at coding mononucleotide repeats have been most frequently detected in MSI-H CRC samples (Co), both in cell lines and colon cancer tissues. There is a high frequency of nucleotide deletions (del) affecting repeat regions of exon 1 and exon 2 (Fig. 1). The [CT]4 region of exon 1 has been defined as a mutation hotspot in MSI-H CRC [7, 14]. Fourteen out of a series of 28 (including CRCs tumor specimens and tumor cell lines) were found to harbor deletions/insertions in this repetitive sequence [7] (Table 1). Mutations in the CT repeat of exon 1 have been associated with a mutator phenotype in CRC [7, 15], reflecting an increase in genetic instability during tumor development due to defects in the DNA mismatch repair (MMR) system [16, 17]. These defects are generally caused by a loss of mismatch repair (MMR) function in cancer cells, secondary to inactivation of MMR genes such as MLH1 or MSH2 [18, 19]. In sporadic CRCs, the MLH1 gene could be silenced by promoter hypermethylation [20]. In this context, it has been reported that most Lynch syndrome cases have germline mutations in MSH2 or MLH1 genes [21], favoring the development of CRC and other tumors with microsatellite instability (MSI) [17, 22]. MSI-H phenotype was detected in 18 out of the 28 CRCs listed in Table 1.
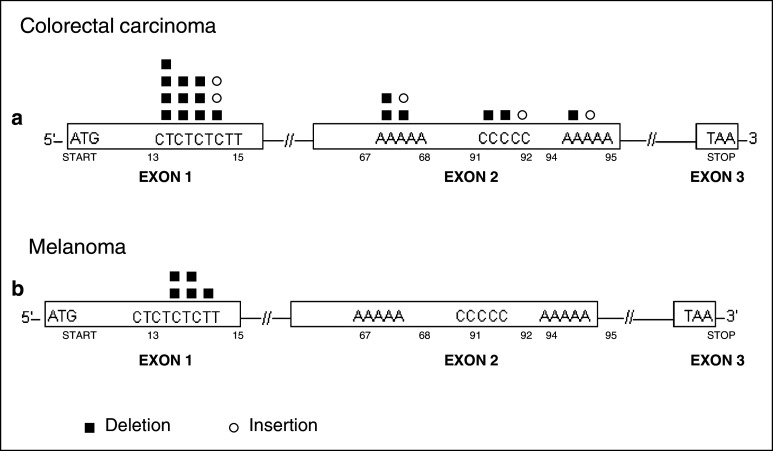
Fig. 1.
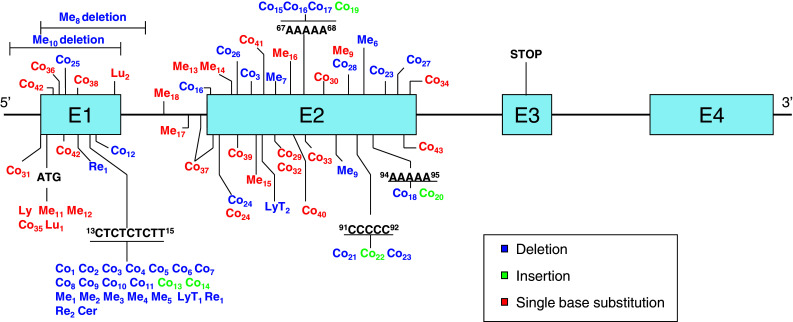
β2m gene alterations in human tumors. Summary of the different β2m gene mutations found in tumor samples and cell lines of melanoma (Me, n = 18), colon cancer (Co, n = 43), Daudi lymphoma (Ly, one case), lung cancer (Lu, 2 cases), sarcomatoid renal carcinoma (Re, 2 cases), cervical cancer (Cer, one case), and testicular diffuse large B cell lymphoma (LyT, 2 cases). The type of mutation is indicated by color according to the legend
Tumors with MSI are characterized by a form of genetic instability that manifests as frameshift mutations, deletions, or insertions in microsatellite DNA and as short repetitive sequences. DNA mononucleotide, dinucleotide, trinucleotide, and tetranucleotide repeats are intrinsically susceptible to slipped-strand mispairing during replication, producing a bulge composed of an unpaired repeat unit. If the bulge is formed on the template strand, the result is a deletion, whereas a bulge on the primer strand yields an insertion [18, 23]. The β2m mutation frequency in MSI-H adenomas is around 15 % and reaches about 30 % in MSI-H CRCs [24].
The mononucleotide repetitive regions (two A5 and one C5) in exon 2 of the β2m gene are also prone to accumulate mutations (Fig. 1). Interestingly, some of the tumors of an MSI phenotype were heterozygous and contained two different deletions, each in a copy of the β2m gene [25]. Both Co16 and Co23 tumors harbored a microdeletion in a mononucleotide repeat sequence of exon 2, while the other mutation was located in a nonrepetitive regions of exon 2 (Fig. 1).
Although MSI-H mainly occurs in CRCs, the [CT]4 repeat is also a mutation hotspot in other types of tumor. Half of the deletions (5 out of 10) detected in melanoma (Me) cell lines were located in this repetitive region, as was also the case for two renal carcinoma cell lines (Re), one testicular diffuse large B lymphoma (LyT), and one cervical cancer cell line (Cer) (see Fig. 1) [7, 26–30]. Melanoma cell lines have been investigated for the presence of MSI [31–34], but only a small proportion of the primary melanomas reported in the literature present a MSI-H pattern, and widespread alterations in the genome have been found [35]. Our group analyzed 30 melanoma cell lines for the presence of an MSI phenotype, including five listed in Tables 1 and 2 (Me2, Me10, Me12, Me13, Me16); we highlight the typical deletion in CT repeat observed in Me2. However, none of the cases showed genomic instability according to the frequency of mutations at conventional mono- and di-nucleotide microsatellite loci [19], indicating that deletions/insertions at CT can occur in melanoma in the absence of the MSI phenotype. Unlike MSI-H CRCs, which contain insertions and deletions in repetitive regions of exon 1 and exon 2, melanomas only show deletions in repetitive regions of exon 1 of the β2m gene (see Fig. 2).
Fig. 2.
Distribution of frameshift mutations in repeat sequences of the β2m gene in colon cancers (a) and melanoma (b). Schematic codon sequence of the β2m gene. Deletions and insertions both affect mono (A, C) and dinucleotide repeats (CT, CC, TT), but tetranucleotide sequences (CTCT, TCTT, TTCT) are only affected by deletions. In colon tumors (a), deletions and insertions were detected throughout the gene repeat sequences whereas in melanoma samples (b), deletions were only observed in the mutation hotspot of exon 1 (CT repeat sequence)
Single nucleotide substitutions in the β2 -microglobulin gene
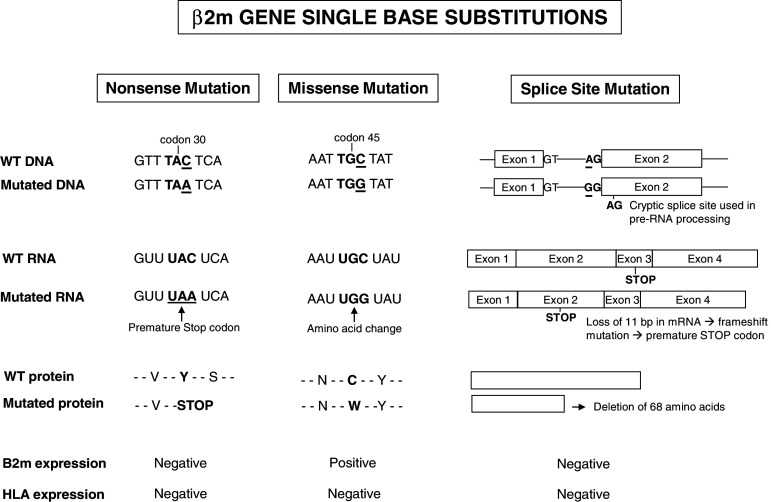
In addition to microdeletion/microinsertions in repetitive sequences, there have been reports of single nucleotide substitutions in the β2m gene that lead to nonsense or missense mutations or modify the splicing of the pre-mRNA (Fig. 3). These three types of point mutation can be generated by transversions (pyrimidine to purine exchange and vice versa) or transitions (purine to purine or pyrimidine to pyrimidine exchange). Transversions and transitions have both been observed in colon carcinoma and melanoma (Table 2).
Fig. 3.
Schematic representation of single base substitutions affecting β2m gene in human tumors. Nonsense mutation: a single nucleotide substitution in tumor DNA results in a premature stop codon. Missense mutation: a single nucleotide substitution alters the codon sequence and replace one amino acid by another in the gene product. Finally, a single nucleotide substitution in the splicing acceptor site (AG) activates a new “cryptic splice site” and introduces a premature stop codon
Nonsense mutations that generate a premature stop codon (Table 2) are highly frequent in CRCs and melanoma. These mutations lead to the production of truncated β2m proteins that are generally undetectable by immunohistochemistry or ELISA (Fig. 3). One exception was sample Co43, in which the change from C to A at codon 108 at the end of exon 2 generated a stop codon but did not affect the HLA class I expression. This may be explained by expression of the second intact parental copy of the β2m gene, or it may be the case that deletion of the last 11 C-terminal amino acids does not severely impact β2m stability and function.
Missense mutations leading to amino acid exchanges have different effects on HLA class I cell surface expression (Table 2, Fig. 3). For instance, an alteration affecting the start codon (ATG) prevents translation of the mRNA into protein, as first described for a Burkitt lymphoma cell line (Daudi) [5]. Remarkably, the ATG start codon was affected by 5 out of 10 missense mutations found in exon 1 (Table 2). In contrast, other missense mutations can lead to the synthesis of abnormal β2m variants that are merely inefficiently processed, limiting the number of HLA class I cell surface molecules (e.g., low HLA class I expression in Co29, Co30, Co31, Co33, Co42, and Co43). The relevance of the type of amino acid exchange is demonstrated by two further examples: in Co36, the C to T exchange in codon 6 (Ala to Val) did not modify the β2m and HLA class I expression; in melanoma cell line VMM5b (Me15), however, the C to G transversion at codon 45 of exon 2 caused a Cys to Trp change, abolishing the formation of a disulfide bond between residues 45 and 100 of the β2m protein and leading to its degradation by the proteasome (Fig. 3).
Point mutations that disrupt the sites involved in the splicing process of β2m pre-mRNA are another type of mutation observed in colon carcinoma and melanoma cells (Table 2, Fig. 3). These nucleotide exchanges were found to produce the destruction of conserved elements in donor (Me18, Co37) or acceptor (Me17) splice sites at intron I and to the utilization of downstream located cryptic splice sites that result in the deletion or insertion of segments in the mRNA [26, 36, 37]. In the Me18105 cell line (Me17), for example, the A to G transition in the dinucleotide AG splice acceptor site of intron I causes the use of a cryptic splice site in exon 2, which leads to the deletion of 11 bp in the β2m message, producing a frameshift mutation and introducing a premature stop codon in exon 2 (see Fig. 3) [26].
The molecular mechanisms that underlie the generation of single nucleotide substitutions are not known. However, the preference for G to C transversions (21/29) is compatible with deamination induced by activation-induced cytidine deaminase (AID) [38]. Aberrant AID expression has been detected in gastric cancer and human hepatocarcinoma [39, 40], while its expression under physiological conditions is mainly restricted to activated germinal center B cells, inducing somatic hypermutation and class-switch recombination of immunoglobulin genes [41]. The mutagenic action of AID consists of cytosine deamination in the consensus recognition sequence, which results in the formation of uracil for processing by the DNA repair system [38]. In this regard, we analyzed the consensus sequence for AID activity in 19 melanomas and CRCs tumors. Only two melanomas (Me9 and Me16) showed the corresponding consensus sequences close to the C or G changes, suggesting that expression of this enzyme does not play a major role in the generation of single missense and nonsense mutations in the β2m gene. However, we found heterogeneous expression of the AID enzyme in three CRCs but without the consensus sequence for AID.
The concurrence of gene mutations and gene loss generates the β2-microglobulin-deficient cellular phenotype
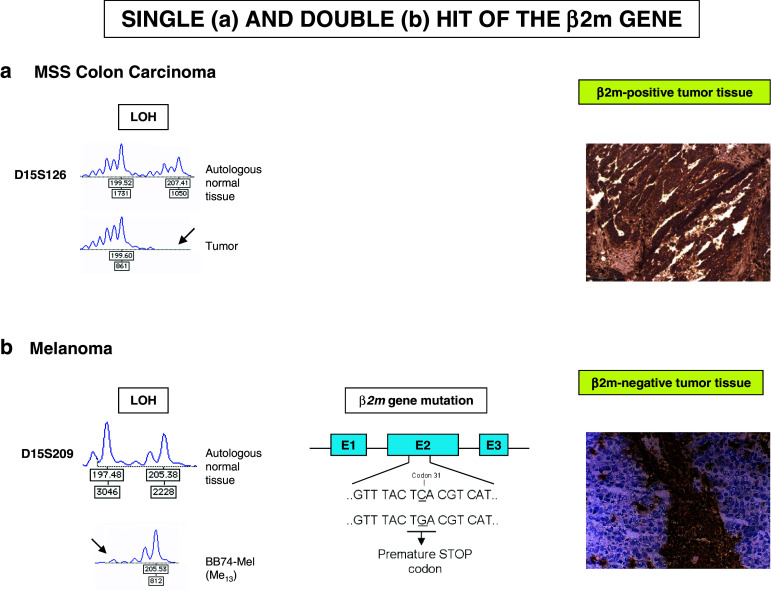
With the exception of three colon carcinoma samples, the great majority of tumors listed in Tables 1 and 2 harbored only a single β2m gene mutation, suggesting loss of the second parental copy of the β2m gene. Loss of chromosomal DNA is detectable at the level of microsatellite markers, which are repetitive sequences of varying length spread over the whole genome. Primers located in the conserved flanking regions of microsatellite markers can be used for their PCR-based amplification, which gives rise to a specific product pattern for each individual. Amplification of microsatellite markers located in chromosome 15q21, to which the β2m gene maps, revealed an altered PCR product pattern for 17 out of 18 melanoma samples in comparison with autologous normal cells, pointing to LOH (Table 1, 2, Fig. 4). In melanoma, therefore, β2m deficiency is generally due to a mutation in one copy of the β2m gene together with complete loss of the second β2m copy [37].
Fig. 4.
Schematic representation of a single and a double hit in the β2m gene. In the first example, an MSS colon carcinoma harbors an LOH in the chromosome 15q21 region but retains HLA class I expression (a). In the second example, a melanoma has alterations affecting both β2m genes (an LOH and a point mutation generating a stop codon) leading to a total loss of HLA class I expression (b)
One example is the cell line UKRV-Mel-2b (Me10) (Table 1), obtained from the metastatic pleural effusion of a melanoma patient [8]. These cells showed a total lack of β2m expression due to a microdeletion of 498 bp in one β2m gene, including the entire exon 1, and a macrodeletion (LOH15q21) that included the entire second copy of the gene. In another melanoma cell line M010-DC-DNR (Me14) obtained from a melanoma patient during metastatic progression (Table 2), β2m deficiency was caused by a mutation in codon 67 of exon 2 in combination with LOH on 15q21 [42].
The molecular mechanisms that lead to LOH on 15q21 appear to be heterogeneous. Comparative genomic hybridization studies on the genome of β2m-deficient cells revealed that the loss of one β2m copy occurred during intra- or inter-chromosomal rearrangements or resulted from the complete loss of one parental chromosome 15 [37]. It has not yet been elucidated whether β2m gene mutation or β2m copy loss is the initiating event [43]. We highlight that LOH on 15q21 can be found in melanoma tumor tissues that still express HLA class I complexes [44]. For instance, analysis of metastases from a melanoma patient revealed low HLA class I expression and LOH on 15q21 in progressing subcutaneous metastases but high HLA class I expression and no LOH on 15q21 in regressing metastases [45]. These data suggest that the loss of one β2m gene copy might be the initial step toward an irreversible HLA class I loss, which is completed by a β2m gene mutation [45].
Besides our findings in melanomas, we detected LOH on 15q21 in 44 % of primary bladder carcinomas, 53 % of breast carcinomas, and 35 % of MSS colon carcinomas [11]. However, this experimental technique is not suitable for CRCs with the MSI-H phenotype, because the microsatellite instability also affects the LOH marker [19]. One possibility to overcome this problem might be the use of heterozygous SNPs within or adjacent to the β2m gene or the application of fluorescent in situ hybridization with specific β2m-labeled probes [8].
In vivo selection of β2-microglobulin-deficient tumors and impact on cancer immunotherapy
Evidences obtained in experimental mouse tumor models indicates that the MHC class I phenotype of a metastatic tumor clone can dramatically change depending on the immune status of the host. Thus, a metastatic colony that was MHC class I negative when the tumor metastasized in a T-cell immunocompetent mouse was MHC class I positive when the tumor metastasized in a T-cell immunodeficient animal [46, 47].
Various studies in humans have demonstrated that tumor cells acquire resistance to T-cell recognition by defective HLA class I expression [48–50]. We have proposed to distinguish between reversible (“soft”) and irreversible (“hard”) alterations. Reversible HLA class I alterations are caused by the transcriptional silencing of genes encoding HLA class I heavy chains and antigen processing machinery components. These phenotypes can be reversed by cytokines [50, 51] or by agents that modify histone acetylation or methylation, restoring the tumor’s susceptibility to CTLs [52–54]. In contrast, irreversible alterations usually result from the loss of one HLA haplotype affecting chromosome 6p21, which generates tumor cells that express only one HLA-A, HLA-B, and HLA-C set of genes or from mutations in the β2m gene (hard lesions) [50]. Hence, the “soft” or “hard” nature of an alteration might determine the success or failure of immunotherapy. For instance, tumor escape variants with low HLA class I expression but soft lesions will recover HLA expression after immunotherapy through effect of cytokines released locally in the tumor microenvironment. In contrast, HLA class I-deficient tumor cells with hard lesions will not recover HLA, regardless of the type of immunotherapy.
We propose that T-cell-based therapy may fail due to the loss of HLA class I expression produced by irreversible mechanisms. In particular, the lack of immunotherapeutic efficacy in melanomas may in part be explained by the pre-existence of metastatic tumor lesions that harbor β2m gene mutations generated during tumor progression [6, 8]. In fact, additional selective pressure may be exerted during T-cell-based immunotherapy, favoring the outgrowth of HLA class I-deficient tumor cells with “hard lesions” [55].
Our group previously reported that the poor clinical response of two melanoma patients to vaccination with HLA-A1-restricted MAGE-derived peptides (BB74-Mel [Me12]) and LB1622-Mel [Me13]) correlated with the loss of HLA Class I surface expression in tumor tissues and cell lines due to the presence of LOH on chromosome 15q21 in combination with β2m gene mutations [6]. Likewise, another melanoma patient who did not respond to immunotherapy with IFN-α showed total loss of HLA class I surface expression caused by the concurrence of a β2m gene mutation and LOH on chromosome 15q21 (UKRV-Mel-2b [Me10]) [41]. In this context, we recently reported a higher incidence of 15q21 chromosomal region loss in high-risk BCG-treated bladder carcinomas that relapsed than in those that did not, suggesting an association between hard β2m lesions and tumor escape [56].
There is further evidence of the functional significance of mutations in the β2m gene. First, the frequency of mutations at mononucleotide repeats in the coding region of the β2m gene is much higher than would be expected by chance. The predicted frequency of mutations at microsatellites with a length of 5 nucleotides in MSI-H CRC is lower than 1 % [24, 57], but the observed frequency of β2m mutations reaches 30 % in MSI-H CRCs [24]. Second, no mutations are found at significantly longer repeats in either coding or noncoding regions of other genes, suggesting that β2m mutations may be under positive selective pressure in MSI cancers [58]. Third, mutations that inactivate β2m appear to favor local tumor growth, given the demonstration by immunohistochemistry studies that metastatic lesions are homogenously composed of β2m-negative tumor cells (see Fig. 4).
The data reviewed here suggest the need for strategies to overcome tumor escape mechanisms. This requires a clear definition of the precise molecular mechanisms responsible for HLA alterations. In particular, it is necessary to differentiate between reversible (soft) mechanisms, in which the administration of cytokines (e.g., IFN) can be useful, and irreversible (hard) mechanisms, in which HLA expression can only be restored by transfer of the appropriate wild-type functional gene.
The β2m gene is widely implicated in the generation of HLA class I-loss tumor phenotypes, underlining the importance of developing therapies to correct defects in this gene and thereby restore HLA class I expression [11]. Our group successfully restored HLA class I expression in β2m-negative tumor cells by transduction with a nonreplicating adenovirus vector encoding the wild-type human β2m gene, obtaining recognition of the β2m-transduced tumor cells by cytotoxic T cells. In the same study, intratumoral injection of the β2m recombinant adenoviral vector into a human tumor xenograft of a nude/nude mouse resulted in the re-expression of HLA class I molecules [59].
It should also be taken into account that the level of MHC/HLA class I expression in tumors can affect T- and NK-cell effector mechanisms in an opposite manner according to whether the tumor is growing locally or is in metastatic dissemination. Thus, our group reported that GR9 mouse fibrosarcoma clones expressing elevated levels of H-2 class I molecules are highly immunogenic and induce a T-cell-mediated rejection when growing locally as a primary tumor mass, whereas the same H-2 positive clones produce a large amount of spontaneous metastases in different organs [60]. Conversely, H-2 negative clones of the same mouse fibrosarcoma revealed a low local immunogenicity and grew rapidly but with very few or no spontaneous metastases [61]. Likewise, it has been reported that the loss of HLA class I expression in human uveal melanoma is associated with better patient survival, suggesting that NK cells might play a major role in destroying MHC class I-deficient tumor cells when “blood-borne” to colonize distant tissues [62, 63]. In this context, it was recently reported that the potential metastatic spread of colon cancer cells to the liver is reduced when they carry an HLA class I negative phenotype produced by β2m mutations [64]. In a similar manner, a poor survival of colorectal cancer patients has been associated with tumors expressing intermediate HLA expression in comparison with those with total loss or positive expression, indicating that tumor tissues with ±HLA class I expression can escape from T- and NK-cell cytotoxicity [65]. We favor the proposition that the transfer of the β2m gene and the consequent re-expression and/or enhancement of HLA class I expression promotes T-cell-mediated tumor rejection in solid primary or metastatic lesions. NK cells play a major role when tumor cells are migrating as single cells to induce metastasis. Finally, it is important to note that β2m has also been related to other nonimmunological functions, including enhancement of epithelia/mesenchymal transition [66] and activity as a growth factor and signaling molecule in cancer cells [67, 68].
Conclusions and future directions
Over the past two decades, several studies identified mutations in the β2m gene to be causative for the HLA class I total loss phenotype of tumors. In general, one type of β2m mutation was reported to be present in a cell ranging from single base substitutions to microinsertions/microdeletions. Such mutations were predominantly detected in colorectal carcinoma and melanoma. However, recent data suggest that these β2m mutations coincide with extensive loss of genetic material in chromosome region 15q21, to which the β2m gene maps. LOH in 15q21 can be detected in tumor tissues with apparently “normal” HLA expression. Thus, we propose that in general, the LOH is the primary mutational event that is then followed to the β2m gene mutation as the second hit that produces the MHC class I negative phenotype. Indeed, HLA class I negative tumor tissue obtained from melanoma patients undergoing different types of immunotherapy has been characterized for these two successive mutational events affecting the β2m gene. These HLA class I negative melanoma cells with irreversible “hard” lesions are immunoselected after immunotherapy since are resistant to T-cell recognition and destruction. We have also obtained evidences that β2m LOH also occur with high incidence in breast and bladder carcinoma suggesting an important role of this gene in the generation of tumor escape variants in different tumors. There are tumor entities that acquire total HLA class I loss by other molecular mechanisms. For instance, a coordinated downregulation of the transcription of genes encoding HLA class I heavy chains, β2m and components of the antigen presentation machinery has been found in bladder carcinomas [12] or tumors in which the precise molecular mechanism responsible HLA class I total loss is not known, for example, 40 % of prostate cancers [13] and 52 % of breast cancers. (I. Maleno unpublished data). So, it will be a challenge for the future to define all these mechanisms and to develop strategies to circumvent HLA class I downregulation in order to ensure maximal efficacy of T-cell-based immunotherapy. We favor the idea that the β2m gene can be a target for gene therapy by replacing the damaged gene by a wild-type one, inducing HLA class I expression in HLA-deficient tumor cells or event increasing HLA expression in cells with a weak expression due to LOH in one β2m gene to promote tumor rejection.
Acknowledgments
The authors are grateful to Dr Natalia Aptsiauri for helpful discussions and suggestions, to Dr Isabel Maleno for helping in the preparation of the manuscript, and to Eva García and Ana Isabel Rodríguez for technical assistance. They also thank the Tumor-Tissue Biobank of Virgen de las Nieves University Hospital for providing samples. The study was partially supported by grants from the Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III, Red Genómica del Cáncer (RETICRD 06/020), Consejería de Salud de la Junta de Andalucía, Consejería de Innovación, Ciencia y Empresa Junta de Andalucía, (P08-TIC-4299), Dirección General de Investigación y Gestión del Plan Nacional I + D + i (TIN2009-13489), Proyecto de Investigación de Excelencia (CTS-3952, CVI-4740 and P06/-CTS-02200), and Plan Andaluz de Investigación (PAI, Group CTS) in Spain; and from the European Searchable Tumour Cell Line Database (ESTDAB) project (contract No. QLRI-CT-2001-01325) at http://www.ebi.ac.uk/estdab, the European Network for the identification and validation of antigens and biomarkers in cancer and their application in clinical tumor immunology (ENACT) project (European community LSHC-CT-2004-503306), and the Cancer Immunotherapy project (European community OJ 2004/c158,18234) and the Helmholtz-Gemeinschaft Deutscher Forschungszentren (HGF) “Alliance on Immunotherapy of Cancer”.
Conflict of interest
The authors declare that they have no conflict of interests.
Abbreviations
- β2m
β2-microglobulin
- CRC
Colorectal cancer
- HLA
Human leukocyte antigen
- MHC
Major histocompatibility complex
- LOH
Loss of heterozygosity
- MMR
Mismatch repair
- MSI-H
High microsatellite instability
- MSS
Microsatellite stability
References
- 1.Coulie PG, Karanikas V, Lurquin C, Colau D, Connerotte T, Hanagiri T, Van Pel A, Lucas S, Godelaine D, Lonchay C, Marchand M, Van Baren N, Boon T. Cytolytic T-cell responses of cancer patients vaccinated with a MAGE antigen. Immunol Rev. 2002;188:33–42. doi: 10.1034/j.1600-065X.2002.18804.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang E, Worschech A, Marincola FM. The immunologic constant of rejection. Trends Immunol. 2008;29:256–262. doi: 10.1016/j.it.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Garrido F, Ruiz-Cabello F, Cabrera T, Perez-Villar JJ, Lopez-Botet M, Duggan-Keen M, Stern PL. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today. 1997;18:89–95. doi: 10.1016/S0167-5699(96)10075-X. [DOI] [PubMed] [Google Scholar]
- 4.del Campo Ana B, Carretero J, Aptsiauri N, Garrido F. Targeting tumor HLA class I expression to increase tumor immunogenicity. Tissue Antigens. 2012;79:147–154. doi: 10.1111/j.1399-0039.2011.01831.x. [DOI] [PubMed] [Google Scholar]
- 5.Rosa F, Berissi H, Weissenbach J, Maroteaux L, Fellous M, Revel M. The β2m mRNA in human Daudi cells has a mutated initiation codon but is still inducible by interferon. EMBO J. 1983;2:239–243. doi: 10.1002/j.1460-2075.1983.tb01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benitez R, Godelaine D, Lopez-Nevot MA, Brasseur F, Jiménez P, Marchand M, Oliva MR, van Baren N, Cabrera T, Andry G, Landry C, Ruiz-Cabello F, Boon T, Garrido F. Mutations of the beta2-microglobulin gene result in a lack of HLA class I molecules on melanoma cells of two patients immunized with MAGE peptides. Tissue Antigens. 1998;52:520–529. doi: 10.1111/j.1399-0039.1998.tb03082.x. [DOI] [PubMed] [Google Scholar]
- 7.Pérez B, Benitez R, Fernández MA, Oliva MR, Soto JL, Serrano S, López Nevot MA, Garrido F. A new beta 2 microglobulin mutation found in a melanoma tumor cell line. Tissue Antigens. 1999;53:569–572. doi: 10.1034/j.1399-0039.1999.530607.x. [DOI] [PubMed] [Google Scholar]
- 8.Paschen A, Méndez RM, Jimenez P, Sucker A, Ruiz-Cabello F, Song M, Garrido F, Schadendorf D. Complete loss of HLA class I antigen expression on melanoma cells: a result of successive mutational events. Int J Cancer. 2003;103:759–767. doi: 10.1002/ijc.10906. [DOI] [PubMed] [Google Scholar]
- 9.D’Urso CM, Wang ZG, Cao Y, Tatake R, Zeff RA, Ferrone S. Lack of HLA class I antigen expression by cultured melanoma cells FO-1 due to a defect in Β2m gene expression. J Clin Invest. 1991;87:284–292. doi: 10.1172/JCI114984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Browning M, Petronzelli F, Bicknell D, Krausa P, Rowan A, Tonks S, Murray N, Bodmer J, Bodmer W. Mechanisms of loss of HLA class I expression on colorectal tumor cells. Tissue Antigens. 1996;47:364–371. doi: 10.1111/j.1399-0039.1996.tb02571.x. [DOI] [PubMed] [Google Scholar]
- 11.Maleno I, Aptsiauri N, Cabrera T, Gallego A, Paschen A, López-Nevot MA, Garrido F. Frequent loss of heterozygosity in the β2-microglobulin region of chromosome 15 in primary human tumors. Immunogenetics. 2011;63:65–71. doi: 10.1007/s00251-010-0494-4. [DOI] [PubMed] [Google Scholar]
- 12.Romero JM, Jiménez P, Cabrera T, Cózar JM, Pedrinaci S, Tallada M, Garrido F, Ruiz-Cabello F. Coordinated downregulation of the antigen presentation machinery and HLA class I/β2m complex is responsible for HLA-ABC loss in bladder cancer. Int J Cancer. 2005;113:605–610. doi: 10.1002/ijc.20499. [DOI] [PubMed] [Google Scholar]
- 13.Blades RA, Keating PJ, McWilliam LJ, George NJ, Stern PL. Loss of HLA class I expression in prostate cancer: implications for immunotherapy. Urology. 1995;46:681–686. doi: 10.1016/S0090-4295(99)80301-X. [DOI] [PubMed] [Google Scholar]
- 14.Bicknell DC, Kaklamanis L, Hampson R, Bodmer WF, Karran P. Selection for beta 2-microglobulin mutation in mismatch repair-defective colorectal carcinomas. Curr Biol. 1996;6:1695–1697. doi: 10.1016/S0960-9822(02)70795-1. [DOI] [PubMed] [Google Scholar]
- 15.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 16.Umar A, Kunkel TA. DNA-replication fidelity, mismatch repair and genome instability in cancer cells. Eur J Biochem. 1996;238:297–307. doi: 10.1111/j.1432-1033.1996.0297z.x. [DOI] [PubMed] [Google Scholar]
- 17.Imai K, Yamamoto H. Carcinogenesis and microsatellite instability: the interrelationship between genetics and epigenetics. Carcinogenesis. 2008;29:673–680. doi: 10.1093/carcin/bgm228. [DOI] [PubMed] [Google Scholar]
- 18.Dietmaier W, Wallinger S, Bocker T, Kullmann F, Fishel R, Rüschoff J. Diagnostic microsatellite instability: definition and correlation with mismatch repair protein expression. Cancer Res. 1997;57:4749–4756. [PubMed] [Google Scholar]
- 19.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 20.Herman JG, Umar A, Polyak K, Graff JR, Ahuja N, Issa JP, Markowitz S, Willson JK, Hamilton SR, Kinzler KW, Kane MF, Kolodner RD, Vogelstein B, Kunkel TA, Baylin SB. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A. 1998;95:6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagerstedt Robinson K, Liu T, Vandrovcova J, Halvarsson B, Clendenning M, Frebourg T, Papadopoulos N, Kinzler KW, Vogelstein B, Peltomäki P, Kolodner RD, Nilbert M, Lindblom A. Lynch syndrome (hereditary nonpolyposis colorectal cancer) diagnostics. J Natl Cancer Inst. 2007;99:291–299. doi: 10.1093/jnci/djk051. [DOI] [PubMed] [Google Scholar]
- 22.Speicher MR. Microsatellite instability in human cancer. Oncol Res. 1995;7:267–275. [PubMed] [Google Scholar]
- 23.Bichara M, Pinet I, Schumacher S, Fuchs RP. Mechanisms of dinucleotide repeat instability in Escherichia coli . Genetics. 2000;154:533–542. doi: 10.1093/genetics/154.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kloor M, Michel S, Buckowitz B, Rüschoff J, Büttner R, Holinski-Feder E, Dippold W, Wagner R, Tariverdian M, Benner A, Schwitalle Y, Kuchenbuch B, von Knebel Doeberitz M. Beta2-microglobulin mutations in microsatellite unstable colorectal tumors. Int J Cancer. 2007;121:454–458. doi: 10.1002/ijc.22691. [DOI] [PubMed] [Google Scholar]
- 25.Cabrera CM, Jiménez P, Cabrera T, Esparza C, Ruiz-Cabello F, Garrido F. Total loss of MHC class I in colorectal tumors can be explained by two molecular pathways: β2m inactivation in MSI-positive tumors and LMP7/TAP2 downregulation in MSI-negative tumors. Tissue Antigens. 2003;6:211–219. doi: 10.1034/j.1399-0039.2003.00020.x. [DOI] [PubMed] [Google Scholar]
- 26.Hicklin DJ, Wang Z, Arienti F, Rivoltini L, Parmiani G, Ferrone S. Beta2-Microglobulin mutations, HLA class I antigen loss, and tumor progression in melanoma. J Clin Invest. 1998;101:2720–2729. doi: 10.1172/JCI498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang CC, Campoli M, Restifo NP, Wang X, Ferrone S. Immune selection of hot-spot beta 2-microglobulin gene mutations, HLA-A2 allospecificity loss, and antigen-processing machinery component down-regulation in melanoma cells derived from recurrent metastases following immunotherapy. J Immunol. 2005;174:1462–1471. doi: 10.4049/jimmunol.174.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsieh CH, Hsu YJ, Chang CC, Liu HC, Chuang KL, Chuang CK, Pang ST, Hasumi K, Ferrone S, Liao SK. Total HLA class I loss in a sarcomatoid renal carcinoma cell line caused by the coexistence of distinct mutations in the two encoding β2m genes. Cancer Immunol Immunother. 2009;58:395–408. doi: 10.1007/s00262-008-0565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordanova ES, Riemersma SA, Philippo K, Schuuring E, Kluin PM. Beta2-microglobulin aberrations in diffuse large B-cell lymphoma of the testis and the central nervous system. Int J Cancer. 2003;103:393–398. doi: 10.1002/ijc.10824. [DOI] [PubMed] [Google Scholar]
- 30.Koopman LA, Corver WE, van der Slik AR, Giphart MJ, Fleuren GJ. Multiple genetic alterations cause frequent and heterogeneous human histocompatibility leukocyte antigen class I loss in cervical cancer. J Exp Med. 2000;191:961–976. doi: 10.1084/jem.191.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomlinson IP, Beck NE, Bodmer WF. Allele loss on chromosome 11q and microsatellite instability in malignant melanoma. Eur J Cancer. 1996;32:1797–1802. doi: 10.1016/0959-8049(96)00198-0. [DOI] [PubMed] [Google Scholar]
- 32.Birindelli S, Tragni G, Bartoli C, Ranzani GN, Rilke F, Pierotti MA, Pilotti S. Detection of microsatellite alterations in the spectrum of melanocytic nevi in patients with or without individual or family history of melanoma. Int J Cancer. 2000;86:255–261. doi: 10.1002/(SICI)1097-0215(20000415)86:2<255::AID-IJC16>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 33.Hussein MR, Sun M, Tuthill RJ, Roggero E, Monti JA, Sudilovsky EC, Wood GS, Sudilovsky O. Comprehensive analysis of 112 melanocytic skin lesions demonstrates microsatellite instability in melanomas and dysplastic nevi, but not in benign nevi. J Cutan Pathol. 2001;28:343–350. doi: 10.1034/j.1600-0560.2001.280702.x. [DOI] [PubMed] [Google Scholar]
- 34.Palmieri G, Ascierto PA, Cossu A, Colombino M, Casula M, Botti G, Lissia A, Tanda F, Castello G. Assessment of genetic instability in melanocytic skin lesions through microsatellite analysis of benign naevi, dysplastic naevi, and primary melanomas and their metastases. Melanoma Res. 2003;13:167–170. doi: 10.1097/00008390-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Hussein MR. Genetic pathways to melanoma tumorigenesis. J Clin Pathol. 2004;57:797–801. doi: 10.1136/jcp.2003.015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bicknell DC, Rowant A, Bodmer WF. β2-Microglobulin gene mutations: a study of established colorectal cell lines and fresh tumors. Proc Natl Acad Sci USA. 1994;91:4751–4755. doi: 10.1073/pnas.91.11.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paschen A, Arens N, Sucker A, Greulich-Bode KM, Fonsatti E, Gloghini A, Striegel S, Schwinn N, Carbone A, Hildenbrand R, Cerwenka A, Maio M, Schadendorf D. The coincidence of chromosome 15 aberrations and β2m gene mutations is causative for the total loss of human leukocyte antigen class I expression in melanoma. Clin Cancer Res. 2006;12:3297–3305. doi: 10.1158/1078-0432.CCR-05-2174. [DOI] [PubMed] [Google Scholar]
- 38.Di Noia J, Neuberger M. Molecular mechanisms of antibody somatic hypermutation. Annu Rev Biochem. 2007;76:1–22. doi: 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- 39.Kim CJ, Song JH, Cho YG, Cao Z, Kim SY, Nam SW, Lee JY, Park WS. Activation-induced cytidine deaminase expression in gastric cancer. Tumour Biol. 2007;28:333–339. doi: 10.1159/000124239. [DOI] [PubMed] [Google Scholar]
- 40.Marusawa H, Aberrant AID. Expression and human cancer development. Int J Biochem Cell Biol. 2008;40:1399–1402. doi: 10.1016/j.biocel.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 41.Shivarov V, Shinkura R, Doi T, Begum NA, Nagaoka H, Okazaki IM, Ito S, Nonaka T, Kinoshita K, Honjo T. Molecular mechanism for generation of antibody memory. Philos Trans R Soc Lond B Biol Sci. 2009;364:569–575. doi: 10.1098/rstb.2008.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Del Campo A, Mendez R, Carretero J, Maleno I, Zinchenko S, Ruiz-Cabello F, Kyte JA, Aamdal S, Gaudernak G, Aptsiauri N, Garrido F. Analysis of HLA class I expression in metastatic lesions obtained from a melanoma patient before and after treatment with tumor-mRNA-transfected DCs: immunoselection of cells with beta 2-microglobulin gene alterations. Tissue Antigens. 2010;75:488. [Google Scholar]
- 43.Marusawa H, Aberrant AID. Expression and human cancer development. Int J Biochem Cell Biol. 2008;40:1399–1402. doi: 10.1016/j.biocel.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 44.Carretero R, Romero JM, Ruiz-Cabello F, Maleno I, Rodriguez F, Camacho FM, Real LM, Garrido F, Cabrera T. Analysis of HLA class I expression in progressing and regressing metastatic melanoma lesions after immunotherapy. Immunogenetics. 2008;60:439–447. doi: 10.1007/s00251-008-0303-5. [DOI] [PubMed] [Google Scholar]
- 45.Carretero R, Wang E, Rodriguez AI, Reinboth J, Ascierto ML, Engle AM, Liu H, Camacho FM, Marincola FM, Garrido F, Cabrera T. Regression of melanoma metastases after immunotherapy is associated with activation of antigen presentation and interferon-mediated rejection genes. Int J Cancer. 2012;131:387–395. doi: 10.1002/ijc.26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Lora A, Martinez M, Algarra I, Gaforio JJ, Garrido F. MHC class I-deficient metastatic tumor variants immunoselected by T lymphocytes originate from the coordinated downregulation of APM components. Int J Cancer. 2003;106:521–527. doi: 10.1002/ijc.11241. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Lora A, Algarra I, Gaforio JJ, Ruiz-Cabello F, Garrido F. Immunoselection by T lymphocytes generates repeated MHC class I-deficient metastatic tumor variants. Int J Cancer. 2001;91:109–119. doi: 10.1002/1097-0215(20010101)91:1<109::AID-IJC1017>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 48.Kloor M, Becker C, Benner A, Woerner SM, Gebert J, Ferrone S, von Knebel Doeberitz M. Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res. 2005;65:6418–6424. doi: 10.1158/0008-5472.CAN-05-0044. [DOI] [PubMed] [Google Scholar]
- 49.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 50.Garrido F, Cabrera T, Aptsiauri N. “Hard” and “soft” lesions underlying the HLA class I alterations in cancer cells: implications for immunotherapy. Int J Cancer. 2010;127:249–256. doi: 10.1002/ijc.25270. [DOI] [PubMed] [Google Scholar]
- 51.Seliger B, Ruiz-Cabello F, Garrido F. IFN inducibility of major histocompatibility antigens in tumors. Adv Cancer Res. 2008;101:249–276. doi: 10.1016/S0065-230X(08)00407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serrano A, Tanzarella S, Lionello I, Mendez R, Traversari C, Ruiz-Cabello F, Garrido F. Reexpression of HLA class I antigens and restoration of antigen-specific CTL response in melanoma cells following 5-aza-2’-deoxycytidine treatment. Int J Cancer. 2001;94:243–251. doi: 10.1002/ijc.1452. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez T, Mendez R, del Campo A, Jimenez P, Aptsiauri N, Garrido F, Ruiz-Cabello F. Distinct mechanisms of loss of IFN-gamma mediated HLA class I inducibility in two melanoma cell lines. BMC Cancer. 2007;7:34. doi: 10.1186/1471-2407-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khan AN, Gregorie CJ. Tomasi TB Histone deacetylase inhibitors induce TAP, LMP, Tapasin genes and MHC class I antigen presentation by melanoma cells. Cancer Immunol Immunother. 2008;57:647–654. doi: 10.1007/s00262-007-0402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Restifo NP, Marincola FM, Kawakami Y, Taubenberger J, Yannelli JR, Rosenberg SA. Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J Natl Cancer Inst. 1996;88:100. doi: 10.1093/jnci/88.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carretero R, Cabrera T, Gil H, Saenz-Lopez P, Maleno I, Aptsiauri N, Cozar JM, Garrido F. Bacillus Calmette-Guerin immunotherapy of bladder cancer induces selection of human leukocyte antigen class I-deficient tumor cells. Int J Cancer. 2011;129:839–846. doi: 10.1002/ijc.25733. [DOI] [PubMed] [Google Scholar]
- 57.Woerner SM, Yuan YP, Benner A, Korff S, von Knebel Doeberitz M, Bork P (2010) SelTarbase, a database of human mononucleotide microsatellite mutations and their potential impact to tumorigenesis and immunology. Nucleic Acids Res 38 (Database issue):D682–D689 [DOI] [PMC free article] [PubMed]
- 58.Yamamoto H, Sawai H, Perucho M. Frameshift somatic mutations in gastrointestinal cancer of the microsatellite mutator phenotype. Cancer Res. 1997;57:4420–4426. [PubMed] [Google Scholar]
- 59.del Campo AB, Aptsiauri N, Mendez R, Zinchenko S, Vales A, Paschen A, Ward S, Ruiz-Cabello F, Gonzalez-Aseguinolaza G, Garrido F. Efficient recovery of HLA class I expression in human tumor cells after β2m gene transfer using adenoviral vector: implications for cancer immunotherapy. Scand J Immunol. 2009;70:125–135. doi: 10.1111/j.1365-3083.2009.02276.x. [DOI] [PubMed] [Google Scholar]
- 60.Perez M, Algarra I, Ljunggren HG, Caballero A, Mialdea MJ, Gaforio JJ, Klein G, Karre K, Garrido F. A weakly tumorigenic phenotype with high MHC class-I expression is associated with high metastatic potential after surgical removal of the primary murine fibrosarcoma. Int J Cancer. 1990;46:258–261. doi: 10.1002/ijc.2910460219. [DOI] [PubMed] [Google Scholar]
- 61.Garrido ML, Perez M, Delgado C, Rojano J, Algarra I, Garrido A, Garrido F. Immunogenicity of H-2 positive and H-2 negative clones of a mouse tumour, GR9. J Immunogenet. 1986;13:159–167. doi: 10.1111/j.1744-313X.1986.tb01097.x. [DOI] [PubMed] [Google Scholar]
- 62.Jager MJ, Hurks HM, Levitskaya J, Kiessling R. HLA expression in uveal melanoma:there is no rule without some exception. Human Immunol. 2002;63:444–451. doi: 10.1016/S0198-8859(02)00389-0. [DOI] [PubMed] [Google Scholar]
- 63.Ma D, Luyten GP, Luider TM, Niederkorn JY. Relationship between natural killer cell susceptibility and metastasis of human uveal melanoma cells in a murine model. Invest Ophthalmol Vis Sci. 1994;36:435–441. [PubMed] [Google Scholar]
- 64.Tikidzhieva A, Benner A, Michel S, Formentini A, Link K-H, Dippold W, von Knebel Doeberitz M, Kornmann M, Kloor M. Microsatellite instability and beta2-microglobulin mutations as prognostic markers in colon cancer: results of the FOGT-4 trial. Br J Cancer. 2012;106:1239–1245. doi: 10.1038/bjc.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watson NF, Madjd Z, Splendlove I, Ellis IO, Scholefield JH, Durant LG. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with poor prognosis. Int J Cancer. 2006;118:6–10. doi: 10.1002/ijc.21303. [DOI] [PubMed] [Google Scholar]
- 66.Josson S, Nomura T, Lin JT, Huang WC, Wu D, Zhau HE, Zayzafoon M, Weizmann MN, Gururajan M, Chung LW. b2-Microglobulin induces epithelial to mesenchymal transition and confers cancer lethality and bone metastasis in human cancer cells. Cancer Res. 2011;71:2600–2610. doi: 10.1158/0008-5472.CAN-10-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang WC, Wu D, Xie Z, Zhau HE, Nomura T, Zayzafoon M, Pohl J, Hsieh CL, Weitzmann MN, Farach-Carson MC, Chung LW. beta2-microglobulin is a signaling and growth-promoting factor for human prostate cancer bone metastasis. Cancer Res. 2006;66:9108–9116. doi: 10.1158/0008-5472.CAN-06-1996. [DOI] [PubMed] [Google Scholar]
- 68.Huang WC, Zhau HE, Chung LW. Androgen receptor survival signalling is blocked by anti-{beta}2-microglobulin monoclonal antibody via a mitogen-activated protein kinase/lipogenic pathway in human prostate cancer cells. J Biol Chem. 2010;285:7947–7956. doi: 10.1074/jbc.M109.092759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen H, Gabrilovich D, Virmanii A, Ratnani I, Girgis K, Nadaf-Rahrovm S, Fernandez-Viña M, Carbone P. Structural and functional analysis of β2 microglobulin abnormalities in human lung and breast cancer. Int J Cancer. 1996;67:756–763. doi: 10.1002/(SICI)1097-0215(19960917)67:6<756::AID-IJC2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 70.De Miranda NF, Nielsen M, Pereira D, van Puijenbroek M, Vasen HF, Hes FJ, van Wezel T, Morreau H. MUTYH-associated polyposis carcinomas frequently lose HLA class I expression—a common event amongst DNA-repair-deficient colorectal cancers. J Pathol. 2009;219:69–76. doi: 10.1002/path.2569. [DOI] [PubMed] [Google Scholar]
- 71.Bernal M, Concha A, Sáenz-López P, Rodríguez AI, Cabrera T, Garrido F, Ruiz-Cabello F. Leukocyte infiltrate in gastrointestinal adenocarcinomas is strongly associated with tumor microsatellite instability but not with tumor immunogenicity. Cancer Immunol Immunother. 2011;60:869–882. doi: 10.1007/s00262-011-0999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gattoni-Celli S, Kirsch K, Timpane R, Isselbacher KJ. Beta 2-microglobulin gene is mutated in a human colon cancer cell line (HCT) deficient in the expression of HLA class I antigens on the cell surface. Cancer Res. 1992;52:1201–1204. [PubMed] [Google Scholar]
- 73.Wang Z, Cao Y, Albino AP, Zeff RA, Houghton A, Ferrone S. Lack of HLA class I antigen expression by melanoma cells SK-MEL-33 caused by a reading frameshift in beta 2-microglobulin messenger RNA. J Clin Invest. 1993;91:684–692. doi: 10.1172/JCI116249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang CC, Ogino T, Mullins DW, Oliver JL, Yamshchikov GV, Bandoh N, Slingluff CL, Jr, Ferrone S. Defective human leukocyte antigen class I-associated antigen presentation caused by a novel β2m loss-of-function in melanoma cells. J Biol Chem. 2006;281:18763–18773. doi: 10.1074/jbc.M511525200. [DOI] [PubMed] [Google Scholar]
- 75.Baba T, Hanagiri T, Ichiki Y, Kuroda K, Shigematsu Y, Mizukami M, Sugaya M, Takenoyama M, Sugio K, Yasumoto K. Lack and restoration of sensitivity of lung cancer cells to cellular attack with special reference to expression of human leukocyte antigen class I and/or major histocompatibility complex class I chain related molecules A/B. Cancer Sci. 2007;98:1795–1802. doi: 10.1111/j.1349-7006.2007.00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]