Abstract
IL-22-producing CD4+ T cells (IL-22+CD4+ T cells) and Th22 cells (IL-22+IL-17−IFN-γ−CD4+ T cells) represent newly discovered T-cell subsets, but their nature, regulation, and clinical relevance in gastric cancer (GC) are presently unknown. In our study, the frequency of IL-22+CD4+ T cells in tumor tissues from 76 GC patients was significantly higher than that in tumor-draining lymph nodes, non-tumor, and peritumoral tissues. Most intratumoral IL-22+CD4+ T cells co-expressed IL-17 and IFN-γ and showed a memory phenotype. Locally enriched IL-22+CD4+ T cells positively correlated with increased CD14+ monocytes and IL-6 and IL-23 detection ex vivo, and in vitro IL-6 and IL-23 induced the polarization of IL-22+CD4+ T cells in a dose-dependent manner and the polarized IL-22+CD4+ T cells co-expressed of IL-17 and IFN-γ. Moreover, IL-22+CD4+ T-cell subsets (IL-22+IL-17+CD4+, IL-22+IL-17−CD4+, IL-22+IFN-γ+CD4+, IL-22+IFN-γ−CD4+, and IL-22+IL-17+IFN-γ+CD4+ T cells), and Th22 cells were also increased in tumors. Furthermore, higher intratumoral IL-22+CD4+ T-cell percentage and Th22-cell percentage were found in patients with tumor-node-metastasis stage advanced and predicted reduced overall survival. In conclusion, our data indicate that IL-22+CD4+ T cells and Th22 cells are likely important in establishing the tumor microenvironment for GC; increased intratumoral IL-22+CD4+ T cells and Th22 cells are associated with tumor progression and predict poorer patient survival, suggesting that tumor-infiltrating IL-22+CD4+ T cells and Th22 cells may be suitable therapeutic targets in patients with GC.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-012-1241-5) contains supplementary material, which is available to authorized users.
Keywords: Gastric cancer, IL-22+CD4+ T cells, Th22 cells, Tumor progression, Tumor survival
Introduction
Gastric cancer (GC) is the second most frequent cause of oncological death worldwide and carries an especially poor prognosis [1]. Despite efforts to introduce new treatments into traditional therapies, such as surgery, chemotherapy, and radiotherapy, the control of GC at an advanced stage remains difficult. Clinical progression and therapeutic efficacy of GC are influenced by the cross-talks between different immune cells that have influence on tumor progression as well as therapeutic efficacy. It has been shown that tumor-infiltrating lymphocytes (TILs), especially CD3+ T cells, correlated with GC patients’ outcome [2]. Thus, it is important to understand the T-cell immunoregulation in GC. The acquired knowledge may help to develop novel treatment strategies and to improve the efficacy of immunotherapy.
CD4+ T cells constitute a major component of TILs within the tumor microenvironment. Naïve CD4+ T cells can develop into four types of T cells: Th1, Th2, Th17, and regulatory T cells (Tregs), based on the local cytokine milieu. The balance between these four types of T cells has been shown important for the overall immune outcome in several mouse models [3] although information in humans is rather limited. IL-22+CD4+ T cells, based on its secretion of cytokine IL-22, have been reported recently [4]. Although some IL-22+CD4+ T cells also produce other cytokines such as IL-17 [5], some IL-22+CD4+ T cells expressed IL-22 but not IL-17 or IFN-γ [6]. The later is now termed as Th22 cells [7, 8]. Th22 cells could be generated from naïve T cells in the presence IL-6 [8]. Moreover, Th22 cells and/or IL-22+CD4+ T cells have been reported to be increased in many inflammatory diseases such as psoriasis [9] and rheumatoid arthritis [10] and to be involved in epidermal immunity and remodeling [7]. Notably, in humans, virtually nothing is known about the phenotype, regulation, and clinical relevance of Th22 cells as well as IL-22+CD4+ T cells in GC. Here, we show that IL-22+CD4+ T cells and Th22 cells accumulate with GC progression and predict patient survival following surgery.
Materials and methods
Patients and tissue samples
Fresh tumor-draining lymph nodes (TDLN), autologous non-tumor, peritumoral, or tumor gastric tissues (non-tumor tissues, at least 5-cm distant from tumor site) were obtained from patients who underwent surgical resection at the Southwest Hospital of the Third Military Medical University. None of the patients had received radiotherapy or chemotherapy before sampling. Individuals with autoimmune disease, infectious diseases, and multi primary cancer were excluded. The clinical stages of tumors were determined according to the TNM classification system of International Union Against Cancer (Edition 7). The study was approved by the Ethics Committee of the Southwest Hospital of the Third Military Medical University. The written informed consent was obtained from each subject. Helicobacter pylori (H. pylori) infection was determined by serology test for specific anti-H. pylori antibodies (Abs).
Immunofluorescence
Paraformaldehyde-fixed cryostat sections of tumor tissues were washed in PBS and blocked for 30 min with 20 % rabbit serum in PBS. Sections were incubated with goat anti-human IL-22 antibody (Ab) (R&D Systems, Minneapolis, MN, USA) diluted in 5 % rabbit serum. The bound Ab was detected with FITC-conjugated rabbit anti-goat Ab (Zhongshan Biotechnology, China). After washing with PBS, sections were blocked for 30 min with 20 % goat serum in PBS and incubated with mouse anti-human CD4 Ab (eBioscience, San Diego, CA, USA) diluted in 5 % goat serum. The bound Ab was detected with TRITC-conjugated goat anti-mouse Ab (Zhongshan Biotechnology). After washing with PBS, slides were examined with a confocal fluorescence microscope (LSM 510 META, Zeiss, Jena, Germany).
Cell isolation
Fresh tumor and non-tumor tissues were used for the isolation of TIL and non-tumor-infiltrating lymphocytes (NTILs). In brief, fresh tumor and non-tumor tissues were washed three times in RPMI 1640 before cut into small pieces. The specimen were then collected in RPMI 1640 containing 1 mg/ml collagenase IV (Sigma-Aldrich, St. Louis, MO) and 10 mg/ml DNase I (Roche, Basel, Switzerland) and mechanically dissociated by using the gentle MACS Dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany). Dissociated cell suspensions were further incubated 1 h at 37 °C under continuous rotation and filtered through 70-μm cell strainers to obtain cell suspensions. Fresh TDLN were gently minced and passed through 70-μm cell strainers to obtain cell suspensions. The cell suspensions were then used for flow cytometry analysis.
Flow cytometry
The following Abs were used to stain single-cell suspensions from tissues: CD3-APC-H7, IL-17-PE, IFN-γ-PE-Cy7 (BD Pharmingen, San Diego, CA, USA), CD4-FITC or PE, CD45RA-PE-Cy7, IL-22-Alexa Fluor 647, CD14-PerCP-Cy5.5, IL-4-PE, IL-9-PE, PD-1-FITC, CD25-PE-Cy7 (eBioscience), IL-17-PE-Cy7, CD45-PE-Cy7, HLA-DR-PE-Cy7, Foxp3-Alexa Fluor 488 (Biolegend, San Diego, CA, USA). Cells were incubated with appropriate surface Abs. For intracellular molecular measurements, cells were stimulated for 5 h with phorbol 12-myristate 13-acetate (PMA; 50 ng/mL; Sigma-Aldrich) plus ionomycin (1 μg/ml; Sigma-Aldrich) in the presence of Golgistop reagent (BD Pharmingen). Intracellular cytokine staining was performed after fixation and permeabilization, using Perm/Wash solution (BD Pharmingen). Cells were analyzed by flow cytometry with a FACSCanto II (BD Biosciences, San Diego, CA, USA). Data were analyzed with Flowjo software (TreeStar) or FACSDiva software (BD Biosciences).
In vitro monocyte-T-cell co-culture system
Peripheral blood mononuclear cells (PBMC) from GC patients were isolated by Ficoll density gradient centrifugation. Fresh peripheral blood CD14+ monocytes and CD4+ T cells were selected using positive isolation and negative isolation kits, respectively (StemCell Technologies, Vancouver, Canada). In a 5-d incubation, bead-purified peripheral CD4+ T cells were co-cultured (2 × 105 cells/well in 96-well plates) with or without autologous blood monocytes at 2:1 ratio in the presence or absence of different concentrations (1, 2, 5, and 10 ng/ml) of recombinant human (h) IL-6 (Peprotech, Rocky Hill, NJ, USA) or IL-23 (Peprotech) in 200 μl RPMI 1640 medium supplemented with 10 % FCS containing h IL-2 (10 IU/ml), anti-CD3 (2 μg/ml), and anti-CD28 (1 μg/ml) Abs. After 5-d incubation, the supernatants were harvested for ELISA, and the cells for intracellular cytokine staining.
Intracellular cytokine staining for CD4+ T cells
Cultured CD4+ T cells were stimulated with PMA (50 ng/ml) and ionomycin (1 μg/ml) in the presence of Golgistop for 6 h. Then, cells were stained with PE-conjugated anti-CD4 Ab (BD Pharmingen). After washing, cells were fixed and permeabilized with Cytofix/Cytoperm (BD Pharmingen) and stained with Alexa Fluor 647-conjugated anti-IL-22 (eBioscience) and PE-Cy7-conjugated anti-IFN-γ (BD Pharmingen)/PE-Cy7-conjugated anti-IL-17 (Biolegend) Abs. Then, cells were resuspended and analyzed by flow cytometry with a FACSCanto II (BD Biosciences). Data were analyzed with Flowjo software (TreeStar) or FACSDiva software (BD Biosciences). Cellular debris was eliminated from the analysis using a gate on forward and side scatter.
Enzyme-linked immunosorbent assay (ELISA)
GC tissues from specimens were collected, homogenized in 1-ml sterile PBS, and centrifuged. Concentrations of cytokines in the tissue supernatants or T-cell co-culture system were determined using ELISA kits for IL-6, IL-23 (eBioscience), and IL-22 (R&D Systems) according to the manufacturer’s instructions.
Statistical analysis
Results are expressed as mean ±SEM. The statistical significance of differences between two groups was determined by the Student’s t test. For multi-group data analysis, an ANOVA analysis was used. Correlations between parameters were assessed using the Pearson correlation analysis and linear regression analysis, as appropriate. Overall patient survival was defined as the interval between date of surgery and date of death or last follow-up, whichever occurred earlier. The known tumor-unrelated deaths (e.g., intercurrent disease and accidental death) were excluded from the death record for this study. Cumulative survival time was calculated by the Kaplan–Meier method, and survival was measured in months; the log-rank test was applied to compare between two groups. Multivariate analysis of prognostic factors for overall patient survival was performed using the Cox proportional hazards model. SPSS statistical software (version 13.0) was used for all statistical analysis. All data were analyzed using two-tailed tests, and P < 0.05 was considered statistically significant unless otherwise specified.
Results
Patients’ characteristics
A total of 76 never-treated GC patients were collected from March 2010 to August 2011. The baseline clinical and pathological characteristics were presented in Table 1.
Table 1.
Clinical characteristics of 76 patients with gastric cancer
| Variable | No. of patients |
|---|---|
| Gender (male/female) | 47/29 |
| Age (years; median, range) | 56, 31–82 |
| CEA (U/L; < 5/≥5) | 54/22 |
| Tumor size (cm; < 5/≥5) | 47/29 |
| H.pylori antibody (positive/negative) | 41/35 |
| Histological type (differentiated/undifferentiated) | 17/59 |
| Lymphatic invasion (absent/present) | 18/58 |
| Vascular invasion (absent/present) | 66/10 |
| Tumor (T) invasion (T1 + T2/T3 + T4) | 24/52 |
| Lymphoid nodal (N) status (N0 + N1/N2 + N3) | 35/41 |
| Distant metastasis (M) status (M0/M1) | 72/4 |
| TNM stage (I + II/III + IV) | 25/51 |
| CD4+IL-22+ T-cell percentage (median, range)a | 2.4, 0.2–8.2 |
| Th22-cell percentage (median, range)b | 1.1, 0.3–2.3 |
CEA carcinoembryonic antigen
aCD4+IL-22+ T-cell percentage was acquired on CD4+IL-22+cells in CD4+ T cells that gated on CD3+CD4+ cells of tumor tissues
bTh22-cell percentage was acquired on CD4+IL-22+IL-17−IFN-γ−cells in CD4+ T cells that gated on CD3+CD4+ cells of tumor tissues
IL-22+CD4+ T cells are enriched in tumor tissues of GC patients with tumor stage advanced and independently predict survival
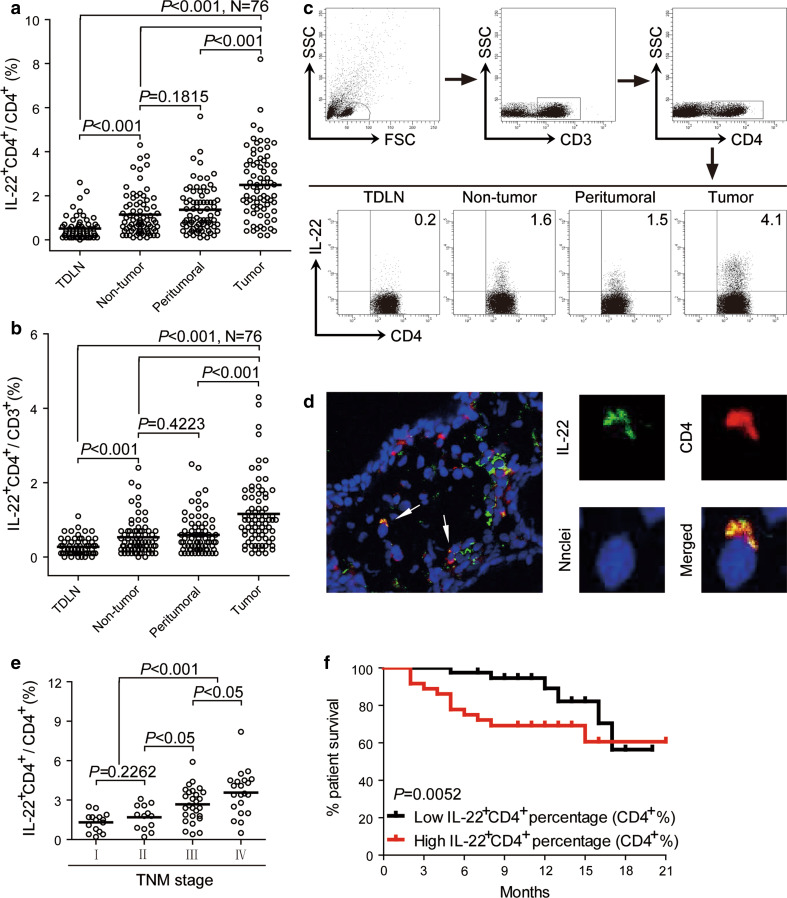
Using flow cytometry, we first evaluated the tissue distribution of IL-22+CD4+ T cells in infiltrating leukocytes freshly isolated from TDLN, autologous non-tumor, peritumoral, or tumor tissues of GC patients. The prevalence of IL-22+CD4+ T cells, not only in CD4+ T cells (Fig. 1a) but also in CD3+ cells (Fig. 1b), was gradually increased in TDLN, non-tumor/peritumoral tissues, and tumor tissues. Notably, the proportion of IL-22+CD4+ T cells was significantly higher in tumors than in other compartments (Fig. 1a, b). In support of this, dual immunofluorescence staining of CD4 and IL-22 showed that IL-22+CD4+ cells were present in GC tumor tissues (Fig. 1d). Moreover, this accumulation of IL-22+CD4+ T cells was most notable from stage II onwards in tumors (Fig. 1e). Taken together, these data indicate that IL-22+CD4+ T cells are increased in tumors of GC patients as tumor progressed.
Fig. 1.
IL-22+CD4+ T cells accumulate in tumor tissues and IL-22+CD4+ T-cell percentage predicts patient survival. a, b Distribution of IL-22+CD4+ T cells in GC patients. Results are expressed as the percentage of IL-22+CD4+ T cells in CD4+ T cells (a) or in CD3+ cells (b) in different tissues. c Dot plots of intracellular cytokine staining for IL-22+CD4+ T cells by gating on lymphocytes, CD3+ cells, and CD4+ T cells. Numbers indicate relative percentages in CD4+ T cells. d Immunofluorescence staining for intratumoral IL-22+CD4+ cells (left 1 panel: original magnification, ×4,000). The arrows indicate representative positive cells in tumor tissues. The green signal represents the staining of IL-22, the red signal represents the staining of CD4, and the blue signal represents the DAPI-stained nuclei (right 4 panels: original magnification, ×100,000). e Percentages of IL-22+CD4+ T cells in CD4+ T cells among TNM stage were compared. f Kaplan–Meier curve for overall survival by median IL-22+CD4+ T-cell percentages. Survival significantly decreased as a function of the percentage of IL-22+CD4+ T cells in CD4+ T cells increased. The horizontal bars in (a, b, e) represent mean values. Each ring in (a, b, e) represents one patient
Next, we evaluated the prognostic value of intratumoral IL-22+CD4+ T-cell percentage on the survival of GC patients. Comparing patients with high (≥2.4 % median level) versus low (<2.4 %) IL-22+CD4+ T-cell percentage level, the 21-month survival rate was significantly higher for those within the high IL-22+CD4+ T-cell percentage group (P = 0.0052) (Fig. 1f). Importantly, this finding that intratumoral IL-22+CD4+ T-cell percentage independently predicted survival was verified by univariate and multivariate analyses using a Cox proportional hazard model (Table 2).
Table 2.
Univariate and multivariate analyses of factors associated with survival
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| P value | HR | 95 % CI | P value | |
| Gender (male vs. female) | 0.838 | NA | ||
| Age, years (≥65 vs. <65) | 0.195 | NA | ||
| CEA,U/L (≥5 vs. <5) | 0.751 | NA | ||
| Tumor size, cm (≥5 vs. <5) | 0.550 | NA | ||
| H. pylori antibody (positive vs. negative) | 0.203 | NA | ||
| Histological type (differentiated vs. undifferentiated) | 0.755 | NA | ||
| Lymphatic invasion (positive vs. negative) | 0.555 | NA | ||
| Vascular invasion (positive vs. negative) | 0.152 | NA | ||
| Tumor (T) invasion (T1 + T2 vs. T3 + T4) | 0.941 | NA | ||
| Lymphoid nodal (N) status (N0 + N1 vs. N2 + N3) | 0.451 | NA | ||
| Distant metastasis (M) status (M0 vs. M1) | 0.883 | NA | ||
| TNM stage (I + II vs. III + IV) | 0.943 | NA | ||
| CD4+IL-22+ T-cell percentage (high vs. low)a | <0.001 | 1.654 | 1.242–2.204 | 0.001 |
| Th22-cell percentage (high vs. low)b | 0.045 | 0.524 | 0.245–1.122 | 0.096 |
Cox proportional hazards regression model. Variables used in multivariate analysis were adopted by univariate analysis
CEA carcinoembryonic antigen, HR hazard ratio, CI confidence interval, NA not adopted
aCD4+IL-22+ T-cell percentage was acquired on CD4+IL-22+cells in CD4+ T cells that gated on CD3+CD4+ cells of tumor tissues
bTh22-cell percentage was acquired on CD4+IL-22+IL-17−IFN-γ−cells in CD4+ T cells that gated on CD3+CD4+ cells of tumor tissues
Intratumoral IL-22+CD4+ T cells have an IL-17/IFN-γ co-expression cytokine profile and memory phenotype
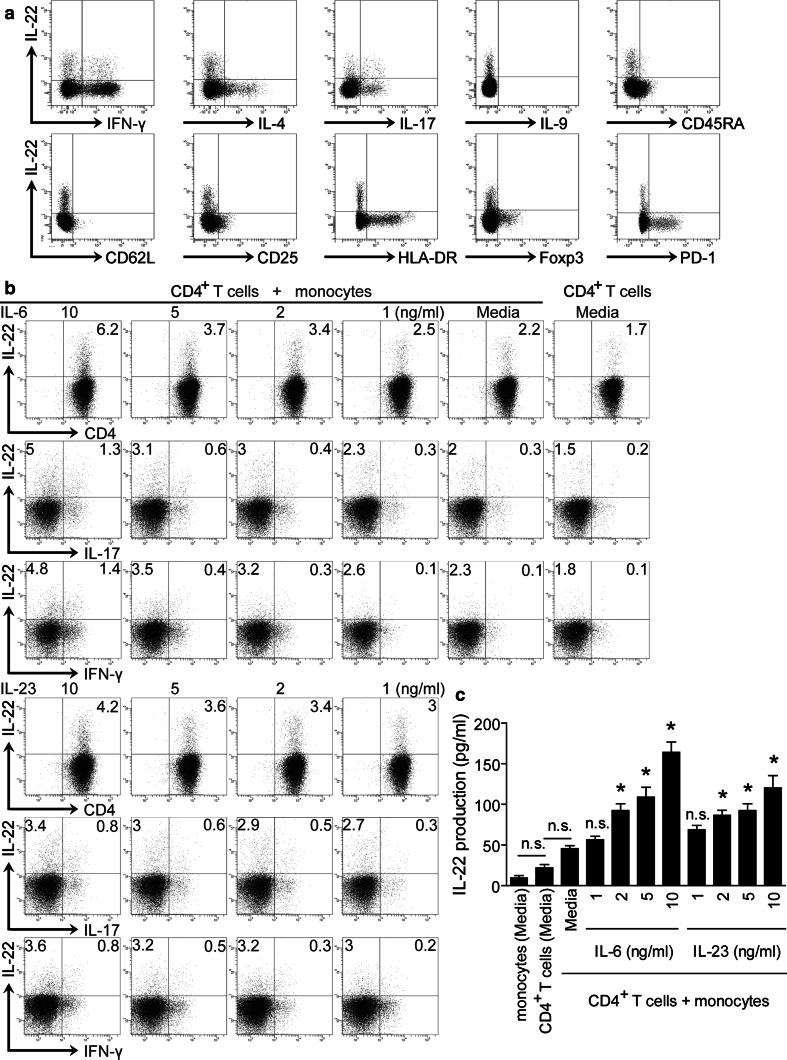
To study phenotypic features of IL-22+CD4+ T cells at tumor site, we first analyzed the cytokine profile of tumor-infiltrating IL-22+CD4+ T cells. Some IL-22+CD4+ T cells co-expressed Th1-cell cytokine IFN-γ and Th17-cell cytokine IL-17 (Fig. 2a), whereas these cells expressed minimal Th2-cell cytokine IL-4 and Th9-cell cytokine IL-9 (Fig. 2a). Furthermore, > 90 % IL-22+CD4+ T cells were CD45RA− and did not express homing molecules CD62L indicating memory T-cell phenotype. We further analyzed the markers for T-cell activation/effector function and immune suppression. IL-22+CD4+ T cells expressed little CD25 and HLA-DR (Fig. 2a), suggesting that IL-22+CD4+ T cells may not be conventional effector T cells. IL-22+CD4+ T cells expressed minimal programmed death 1 receptor (PD-1) and Foxp3 (Fig. 2a), suggesting that IL-22+CD4+ T cells are distinct from Tregs and functionally exhausted PD-1+ T cells. These data above indicate that IL-22+CD4+ T cells exhibit an IL-17/IFN-γ co-expression cytokine profile and memory phenotype in the tumor microenvironment.
Fig. 2.
Intratumoral IL-22+CD4+ T cells show an IL-17/IFN-γ co-expression and memory phenotype, and IL-22+CD4+ T-cell differentiation is induced by IL-6 and IL-23 in the presence of monocytes. a Multicolor flow cytometry for the co-expression of cytokine profile, CD45RA, CD62L, and the markers associated with T-cell activation/effector function and immune suppression on intratumoral IL-22+CD4+ T cells. b, c Peripheral CD4+ cells were co-cultured for 5 days with or without autologous peripheral CD14+ monocytes at 2:1 ratio supplemented with different concentrations of IL-6 or IL-23. Results are expressed as the percentage of IL-22+CD4+, IL-22+IL-17+CD4+, IL-22+IL-17−CD4+, IL-22+IFN-γ+CD4+, and IL-22+IFN-γ−CD4+ T cells in CD4+ T cells (b). Production of IL-22 in the co-culture systems was detected by ELISA in the culture supernatants (c). * indicates P < 0.05, ** indicates P < 0.01, n.s. indicates P > 0.05 for groups connected by horizontal lines compared in (c), or compared with media controls in (c). Results above are representative of 3 independent experiments
IL-6 and IL-23 can induce the polarization of IL-22+CD4+ T cells
Given the accumulation of IL-22+CD4+ T cells at tumor sites and its correlation with increased local CD14+ monocytes and IL-6 levels (Fig. S1a, b), we co-cultured purified CD4+ T cells and CD14+ monocytes from PBMC of GC patients in the presence of IL-6 to investigate whether they are capable of polarizing CD4+ T cells into IL-22+CD4+ T cells. After 5-d co-culture, IL-6, in the presence of monocytes, significantly induced IL-22+CD4+ T-cell polarization and IL-22 production in a dose-dependent manner (Fig. 2b, c). Moreover, we found that IL-22+CD4+ T cells were positively correlated with IL-23 in tumors (Fig. S1c). So, we repeated above experiments replacing IL-6 with IL-23 with similar observations (Fig. 2b, c). We also found that some polarized IL-22+CD4+ T cells co-expressed IL-17 and/or IFN-γ (Fig. 2b). These data imply that tumor environmental IL-6 and IL-23 may be involved in facilitating the polarization of IL-22+CD4+ T cells.
IL-22+CD4+ T-cell subsets (IL-22+IL-17+CD4+, IL-22+IL-17−CD4+, IL-22+IFN-γ+CD4+, and IL-22+IFN-γ−CD4+ T cells) are increased in tumors
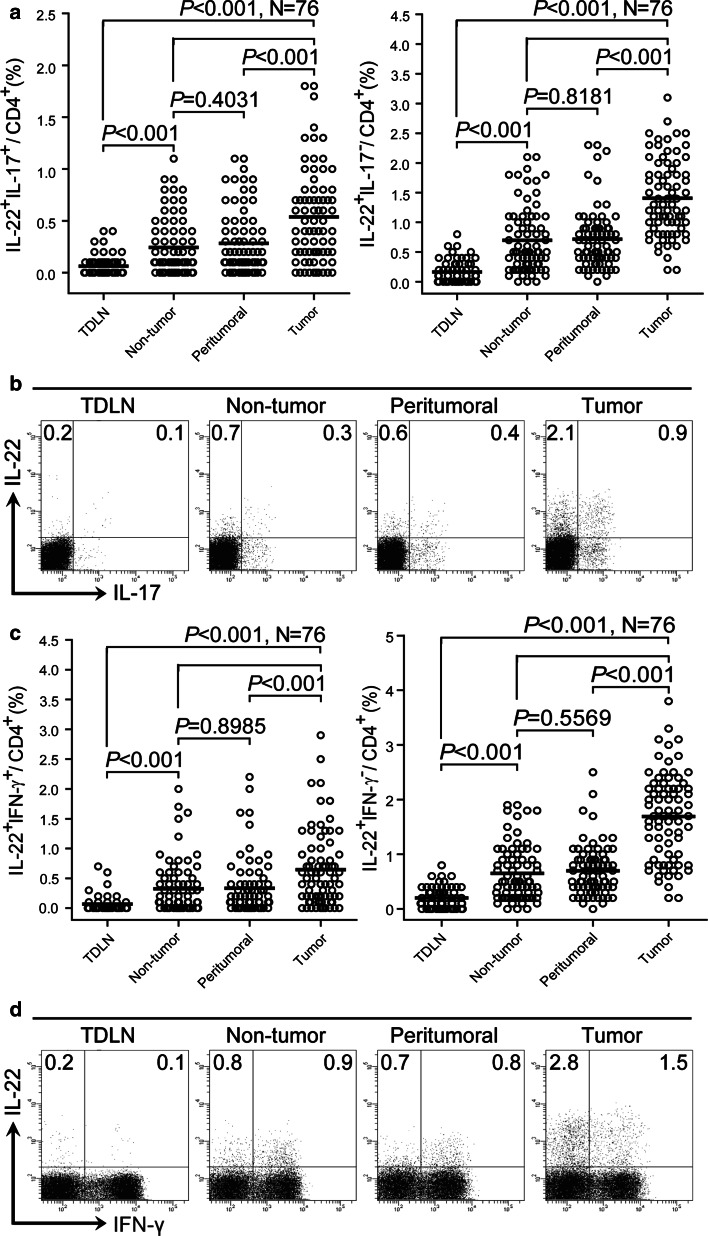
We also divided IL-22+CD4+ T cells into IL-22+IL-17+CD4+ and IL-22+IL-17−CD4+ T cells according to IL-17 production or IL-22+IFN-γ+CD4+ and IL-22+IFN-γ−CD4+ T cells according to IFN-γ production and analyzed their tissue distribution. The prevalence of IL-22+IL-17+CD4+ or IL-22+IL-17−CD4+ T cells in CD4+ T cells was gradually increased in TDLN, non-tumor tissues/peritumoral tissues, and tumor tissues (Fig. 3a, b). Notably, the proportion of IL-22+IL-17+CD4+ or IL-22+IL-17−CD4+ T cells was significantly higher in tumors than in other compartments (Fig. 3a, b). Similar observations were made when analyzing IL-22+IFN-γ+CD4+ or IL-22+IFN-γ−CD4+ T cells (Fig. 3c, d). These data indicate that the in-vitro-induced IL-22+CD4+ T-cell subsets are also reflected by their counterparts in tumors.
Fig. 3.
IL-22+IL-17+CD4+, IL-22+IL-17−CD4+, IL-22+IFN-γ+CD4+, and IL-22+ IFN-γ−CD4+ T cells accumulate in tumor tissues. a, c Distribution of IL-22+CD4+ T-cells subsets in GC patients. Results are expressed as the percentage of IL-22+IL-17+CD4+ and IL-22+IL-17−CD4+ T cells in CD4+ T cells (a) or IL-22+IFN-γ+CD4+ and IL-22+IFN-γ−CD4+ T cells in CD4+ T cells (c) in different tissues. b, d Dot plots of intracellular cytokine staining for IL-22+IL-17+CD4+ and IL-22+IL-17−CD4+ T cells (b) or IL-22+IFN-γ+CD4+ and IL-22+IFN-γ−CD4+ T cells (d) by gating on CD4+ T cells. Numbers indicate relative percentages in CD4+ T cells. The horizontal bars in (a, c) represent mean values. Each ring in (a, c) represents one patient
Th22 cells and IL-22+IL-17+IFN-γ+CD4+ T cells are increased in tumors
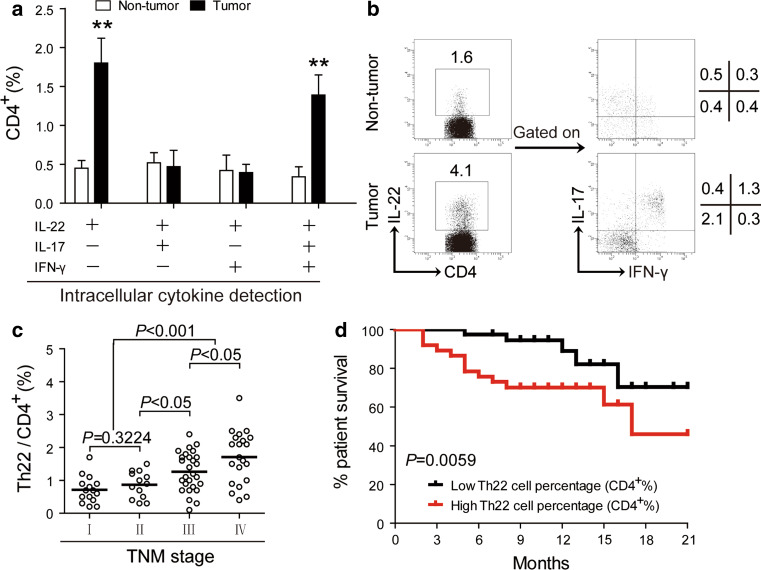
As minimal IL-22+CD4+ T cells were detected in TDLN and similar low number of IL-22+CD4+ T cells detected in non-tumor and peritumoral tissues (Fig. 1), we finally focused on IL-22+CD4+ T-cell subsets in non-tumor and tumor tissues. Th22 cells (IL-22+IL-17−IFN-γ−CD4+ T cells) and IL-22+IL-17+IFN-γ+CD4+ T cells showed clear increase in tumors, whereas IL-22+IL-17+IFN-γ−CD4+ and IL-22+IL-17−IFN-γ+CD4+ T cells showed similar frequency in the two tissues (Fig. 4a, b). Taken together, the IL-22+CD4+ T-cell increase in tumors is therefore further dissected as the consequence of Th22-cell and IL-22+IL-17+IFN-γ+CD4+ T-cell enrichment.
Fig. 4.
Th22 cells and IL-22+IL-17+IFN-γ+CD4+ T cells accumulate in tumors, and Th22 cells correlate with tumor stage and survival in GC patients. a The percentage of IL-22+IL-17−IFN-γ−CD4+ (Th22), IL-22+IL-17+IFN-γ−CD4+, IL-22+IL-17−IFN-γ+CD4+, and IL-22+IL-17+IFN-γ+CD4+ T cells in CD4+ T cells in non-tumor or tumor tissues (n = 76). b Dot plots of intracellular cytokine staining for IL-22+IL-17−IFN-γ−CD4+ (Th22), IL-22+IL-17+IFN-γ−CD4+, IL-22+IL-17−IFN-γ+CD4+, and IL-22+IL-17+IFN-γ+CD4+ T cells by gating on CD4+ T cells. Numbers indicate relative percentages in CD4+ T cells. c Percentages of Th22 cells in CD4+ T cells among TNM stage were compared. d Kaplan–Meier curve for overall survival by median Th22-cell percentages. Survival significantly decreased as a function of the percentage of Th22 cells increased. The horizontal bars in c represent mean values. Each ring in (c) represents one patient
Th22 cells correlate with tumor stage and survival in patients with GC
Next, we studied whether increased Th22 cells were associated with tumor stage. This accumulation of Th22 cells was most notable from stage II onwards (Fig. 4c), suggesting that Th22 cells accumulate at tumor site during tumor progression. We also evaluated the prognostic value of intratumoral Th22-cell percentage on the survival of GC patients. Comparing patients with high (≥1.1 % median level) versus low (<1.1 %) Th22-cell percentage level, the 21-month survival rate was significantly higher for those within the high Th22-cell percentage group (P = 0.0059) (Fig. 1d). However, there was no significant difference of survival rate between high versus low IL-22+IL-17+IFN-γ+CD4+ T-cell percentage level (data not shown). Moreover, using a Cox proportional hazard model, intratumoral Th22-cell percentage significantly predicted survival verified by univariate analyses, but not independently predicted survival verified by multivariate analyses (Table 2).
Th22 cells, IL-22+IL-17+CD4+, IL-22+IFN-γ+CD4+, and IL-22+IL-17+IFN-γ+CD4+ T cells are closely associated with IL-22+CD4+ T cells in tumors
Some IL-22+CD4+ T cells co-expressed either IL-17 or IFN-γ (Fig. 1); IL-22+CD4+ T-cells subsets (IL-22+IL-17+CD4+, IL-22+IL-17−CD4+, IL-22+IFN-γ+CD4+, and IL-22+IFN-γ−CD4+ T cells) (Fig. 3), Th22 cells, and IL-22+IL-17+IFN-γ+CD4+ T cells (Fig. 4) were increased in tumors. We finally investigated the relationships between IL-22+CD4+ T cells and these six subsets in terms of total CD4+ T cells. Interestingly, we found that IL-22+CD4+ T cells were positively correlated with IL-22+IL-17+CD4+ (Fig. S2a) and IL-22+IFN-γ+CD4+ T cells (Fig. S2c), Th22 cells (IL-22+IL-17−IFN-γ−CD4+ T cells) (Fig. S2e), and IL-22+IL-17+IFN-γ+CD4+ T cells (Fig. S2f), but not correlated with IL-22+IL-17−CD4+ (Fig. S2b) and IL-22+IFN-γ−CD4+ T cells (Fig. S2d). However, there was no correlation between Tregs and IL-22+CD4+ T cells (data not shown). These data indicate that IL-22+CD4+ T cells are closely associated with Th22 cells and their IL-17+/IFN-γ+ subsets in tumors.
Discussion
In spite of the generalized tumor-mediated immune modulation or immune-mediated tumor progression in cancer patients [11], many malignancies arise at sites of inflammatory immune responses, and TILs are often accumulated and related-cytokines are produced in tumors [12]. In this study, we have shown that in GC IL-22+CD4+ T cells and Th22 cells increased with tumor progression and have a direct correlation with poor prognosis in GC patients. Although IL-22+CD4+ T cells and Th22 cells were discovered recently in human inflammatory disorders [7], to our knowledge, this is the first demonstration of a statistically significant increase of prevalent IL-22+CD4+ T-cell and Th22-cell infiltration of the GC microenvironment.
In this study, we first showed that IL-22+CD4+ T cells with an IL-17/IFN-γ co-expression cytokine profile and memory phenotype were enriched in tumors. Intratumoral IL-22+CD4+ T cells, after loosing the expression of the lymphoid homing molecule CD62L, may reside in tumors for extended time without entering lymphatic or blood circulation [13]. The B7-H1 receptor, PD-1, may be expressed in functionally exhausted T cells [14], and Foxp3 is expressed by Tregs both of which are often found in the tumor microenvironment and contribute to immune suppression [15]. Intratumoral IL-22+CD4+ T cells expressed little PD-1 or Foxp3, and they expressed no CD25 and HLA-DR associated with T-cell activation. Altogether, these expression profiles reveal that IL-22+CD4+ T cells exhibit a phenotype that may be different from other T-cell subsets and their function may be associated with production of cytokines locally within the tumor.
Next, we addressed which immune cells and cytokines contribute to the polarization and regulation of IL-22+CD4+ T cells in GC. Recent studies have shown that IL-6 can convert CD4+ T cells into IL-22+CD4+ T cells [8] and that monocytes respond to tumor-derived signals, such as IL-6 [16], and in turn polarize T-cell responses [17]. Accordingly, our data indicated that intratumoral IL-22+CD4+ T cells positively correlated with tumor-residing monocytes as well as the level of tumor microenvironmental IL-6 and IL-23. Furthermore, IL-6 and IL-23 markedly increased the polarization of IL-22+CD4+ T cells from peripheral CD4+ T cells in a dose-dependent manner in the presence of monocytes; these polarized IL-22+CD4+ T cells in vitro have a similar phenotype of intratumoral IL-22+CD4+ T cells. These data suggest that tumor microenvironment may provide a cell-cytokine milieu that is suitable for the polarization of IL-22+CD4+ T cells.
IL-22 is a recently discovered cytokine belonging to IL-10 cytokine family [18]. IL-10 and IL-24 that also belong to IL-10 cytokine family were also reported to be involved in GC [19, 20]. The expression and regulation of other IL-10 cytokine family members such as IL-19, IL-20, and IL-26 also need further study. Szaflarska et al. showed that preoperative plasma levels of IL-10 were associated with poor prognosis in GC [21]. However, there were no significant differences of IL-22 expression in peripheral blood serum between GC patients and healthy donors (data not shown). Moreover, we did not detect the levels of IL-22 and IL-10 family cytokines in tumor supernatants and the expression of IL-22 receptor in GC tissues in this study, which needs further investigation.
IL-22 was originally thought to be predominantly produced by IFN-γ-producing Th1 cells [22]. Later, it became clear that IL-17-producing T-cell subset is the dominant IL-22 producer [23, 24]. Consistently, our study also showed that many intratumoral IL-22+CD4+ T cells co-expressed Th1 and Th17 cytokines IFN-γ and IL-17. Recently, a human IL-22-producing Th-cell population that is distinct from Th17, Th1, and Th2 cells has been identified and called Th22 cells [8]. Th22 cells are characterized by secretion of IL-22, but not IL-17 or IFN-γ [10]. We further demonstrated that the enriched intratumoral IL-22+CD4+ T cells were closely associated with both IL-22+IL-17+IFN-γ+CD4+ and IL-22+IL-17−IFN-γ−CD4+ (Th22) T-cell subsets, suggesting a polyfunctional cytokine profile and for a potentially synergistical function.
Most importantly, our findings also shed light on the clinical relevance of IL-22+CD4+ T cells and Th22 cells in GC. Specifically, we found that an increased frequency of IL-22+CD4+ T cells and Th22 cells correlated with advanced tumor progression and poorer patient survival. IL-22+CD4+ T cells could also produce other cytokines, such as IL-17 and IFN-γ that play different roles in tumor, respectively [25, 26], and IL-22 is an inflammatory cytokine and produced by activated CD4+ T cells as well as CD8+ T cells, γδ T cells, NKT cells, and so on [27, 28], implying that the increased mortality may not be due to IL-22 alone. Given that the clinical outcome for GC patients remains poor and that few prognostic factors currently exist for this disease following surgery [1], intratumoral IL-22+CD4+ T-cell and Th22-cell frequencies might prove useful clinical markers for GC patients in the future.
In conclusion, our data suggest that increased IL-22+IL-17+IFN-γ+CD4+ T cells and Th22 cells may play important role during GC establishment and progression and may serve as a prognostic marker for poor survival. Targeting IL-22+CD4+ T cells and their function may become a novel therapy for GC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
IL-22+CD4+ T cells correlate with monocytes, IL-6, and IL-23 in tumors. The correlations and correlation coefficients between the percentage of IL-22+CD4+ T cells in total CD4+ T cells and the percentage of CD14+ monocytes in total CD45+ T cells (a) or the concentrations of IL-6 (b) or IL-23 (c) were evaluated and computed in the same tumors. Each dot represents one patient. (TIFF 4118 kb)
IL-22+CD4+ T cells correlate with its IL-17+/IFN-γ+ subsets, Th22 cells, and IL-22+IL-17+IFN-γ+CD4+ T cells in tumors. The correlations and correlation coefficients between the percentages of IL-22+CD4+ T cells and IL-22+IL-17+CD4+ (a), IL-22+IL-17-CD4+ (b), IL-22+IFN-γ+CD4+ (c), IL-22+IFN-γ-CD4+ (d) Th22 (IL-22+IL-17+IFN-γ+CD4+) (e) or IL-22+IL-17+IFN-γ+CD4+ T cells (f) in total CD4+ T cells were evaluated and computed in the same tumors. Each dot represents one patient. (TIFF 10888 kb)
Acknowledgments
This work was supported by grants of the National Natural Science Foundation of China (NSFC, No. 81071412), and National Basic Research Program of China (973 program, No. 2009CB522606).
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Pei-wu Yu, Email: yupeiwu01@vip.sina.com.
Quan-ming Zou, Phone: +86-023-68752315, FAX: +86-023-68752315, Email: qmzou@tmmu.edu.cn.
References
- 1.Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477–490. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, Kim WH. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99:1704–1711. doi: 10.1038/sj.bjc.6604738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4 + effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 4.Trifari S, Spits H. IL-22-producing CD4 + T cells: middle-men between the immune system and its environment. Eur J Immunol. 2010;40:2369–2371. doi: 10.1002/eji.201040848. [DOI] [PubMed] [Google Scholar]
- 5.Nograles KE, Zaba LC, Shemer A, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123(e2):1244–1252. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujita H, Nograles KE, Kikuchi T, Gonzalez J, Carucci JA, Krueger JG. Human langerhans cells induce distinct IL-22-producing CD4 + T cells lacking IL-17 production. Proc Natl Acad Sci USA. 2009;106:21795–21800. doi: 10.1073/pnas.0911472106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 9.Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Li JM, Liu XG, et al. Elevated Th22 cells correlated with Th17 cells in patients with rheumatoid arthritis. J Clin Immunol. 2011;31:606–614. doi: 10.1007/s10875-011-9540-8. [DOI] [PubMed] [Google Scholar]
- 11.Schreiber RD, Old LJ, Smyth MJ. Cancer immuno editing: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 12.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7:139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 13.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 14.Curiel TJ, Wei S, Dong H, et al. Blockade of B7–H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 15.Zhou G, Drake CG, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107:628–636. doi: 10.1182/blood-2005-07-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 17.Kuang DM, Peng C, Zhao Q, Wu Y, Zhu LY, Wang J, Yin XY, Li L, Zheng L. Tumor-activated monocytes promote expansion of IL-17-producing CD8 + T cells in hepatocellular carcinoma patients. J Immunol. 2010;185:1544–1549. doi: 10.4049/jimmunol.0904094. [DOI] [PubMed] [Google Scholar]
- 18.Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164:1814–1819. doi: 10.4049/jimmunol.164.4.1814. [DOI] [PubMed] [Google Scholar]
- 19.Sakamoto T, Saito H, Tatebe S, Tsujitani S, Ozaki M, Ito H, Ikeguchi M. Interleukin-10 expression significantly correlates with minor CD8 + T-cell infiltration and high microvessel density in patients with gastric cancer. Int J Cancer. 2006;118:1909–1914. doi: 10.1002/ijc.21598. [DOI] [PubMed] [Google Scholar]
- 20.Yan S, Zhang H, Xie Y, Sheng W, Xiang J, Ye Z, Chen W, Yang J. Recombinant human interleukin-24 suppresses gastric carcinoma cell growth in vitro and in vivo. Cancer Invest. 2010;28:85–93. doi: 10.3109/07357900903095672. [DOI] [PubMed] [Google Scholar]
- 21.Szaflarska A, Szczepanik A, Siedlar M, Czupryna A, Sierzega M, Popiela T, Zembala M. Preoperative plasma level of IL-10 but not of proinflammatory cytokines is an independent prognostic factor in patients with gastric cancer. Anticancer Res. 2009;29:5005–5012. [PubMed] [Google Scholar]
- 22.Gurney AL. IL-22, a Th1 cytokine that targets the pancreas and select other peripheral tissues. Int Immunopharmacol. 2004;4:669–677. doi: 10.1016/j.intimp.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreymborg K, Etzensperger R, Dumoutier L, Haak S, Rebollo A, Buch T, Heppner FL, Renauld JC, Becher B. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J Immunol. 2007;179:8098–8104. doi: 10.4049/jimmunol.179.12.8098. [DOI] [PubMed] [Google Scholar]
- 25.Wakita D, Chamoto K, Ohkuri T, Narita Y, Ashino S, Sumida K, Nishikawa H, Shiku H, Togashi Y, Kitamura H, Nishimura T. IFN-gamma-dependent type 1 immunity is crucial for immunosurveillance against squamous cell carcinoma in a novel mouse carcinogenesis model. Carcinogenesis. 2009;30:1408–1415. doi: 10.1093/carcin/bgp144. [DOI] [PubMed] [Google Scholar]
- 26.Prabhala RH, Pelluru D, Fulciniti M, Prabhala HK, Nanjappa P, Song W, Pai C, Amin S, Tai YT, Richardson PG, Ghobrial IM, Treon SP, Daley JF, Anderson KC, Kutok JL, Munshi NC. Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood. 2010;115:5385–5392. doi: 10.1182/blood-2009-10-246660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 28.Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, Gurney AL. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem. 2000;275:31335–31339. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IL-22+CD4+ T cells correlate with monocytes, IL-6, and IL-23 in tumors. The correlations and correlation coefficients between the percentage of IL-22+CD4+ T cells in total CD4+ T cells and the percentage of CD14+ monocytes in total CD45+ T cells (a) or the concentrations of IL-6 (b) or IL-23 (c) were evaluated and computed in the same tumors. Each dot represents one patient. (TIFF 4118 kb)
IL-22+CD4+ T cells correlate with its IL-17+/IFN-γ+ subsets, Th22 cells, and IL-22+IL-17+IFN-γ+CD4+ T cells in tumors. The correlations and correlation coefficients between the percentages of IL-22+CD4+ T cells and IL-22+IL-17+CD4+ (a), IL-22+IL-17-CD4+ (b), IL-22+IFN-γ+CD4+ (c), IL-22+IFN-γ-CD4+ (d) Th22 (IL-22+IL-17+IFN-γ+CD4+) (e) or IL-22+IL-17+IFN-γ+CD4+ T cells (f) in total CD4+ T cells were evaluated and computed in the same tumors. Each dot represents one patient. (TIFF 10888 kb)