Abstract
The involvement of a smouldering microenvironment is currently considered a cancer hallmark and a required step for tumour cells to disable specific immunity while promoting angiogenesis and stroma remodelling. Nevertheless, the molecular pathways driving such aberrant interactions in human cancer and their actual implication in disease progression are still poorly defined. Here, we will report about the remarkable efforts devoted by our group as well as many other scientists to dissect this process focusing on tumour-mediated activation of myeloid dysfunctional pathways occurring in cancer patients. Indeed, myeloid-derived suppressor cells (MDSC), playing a crucial role as cellular regulators of immune responses, have been extensively shown to restrain tumour immunity through a vast array of molecular mechanisms and to promote tumour progression in different murine models. Although in mice the phenotypic features of these cells were defined initially rather generally by Gr1+ and CD11b+ co-expression, more recent studies have unravelled the actual complexity of this population and the existence of different cell subsets. This complexity is even more remarked in the human setting, where heterogeneous populations of myeloid cells with variable phenotype and immunosuppressive features have been described in patients affected by different types of tumours. The lack of homogeneous properties of human MDSC has made these cells a controversial and still unacknowledged player in cancer-related immune suppression and disease progression. Nevertheless, with the efforts of the scientific community, MDSC will soon reveal their key role thereby becoming novel targets for innovative therapeutic strategies.
Keywords: Tumour exosomes, Myeloid dysfunctions, CD14+HLA-DRneg/low, T cells, Cancer vaccines, CITIM 2011
Sculpting of myeloid cells by human cancer
Tumour cells survive and metastasize thanks to their ability of recruiting different components of the host in a sort of “harmful alliance” [1]. In fact, through the release of soluble mediators or by activating specific cell subsets, tumour cells engage different microenvironment components to sustain their own growth and expansion. Among these mechanisms, myeloid-derived suppressor cells (MDSC) are supposed to play a central role in local and systemic tumour progression, providing a favourable microenvironment in which transformed cells can proliferate, acquire new mutations, expand and evade host immunosurveillance [2].
MDSC represent a phenotypically heterogeneous population of myeloid cells at different stages of maturation, found in tumour-bearing mice and in cancer patients and able to suppress multiple phases of the immune response. There is also evidence that this cell subset is involved in a whole array of non-immunological functions, such as promotion of angiogenesis, tumour local invasion and metastases [3]. Several studies indicated that MDSC accumulate in peripheral blood, lymphoid tissues as well as draining tumour sites of cancer-bearing hosts [4], where they exert a highly pleiotropic immune suppressive activity depending on the microenvironment context. Although these cells have been shown to differentiate into normal dendritic cells and macrophages once removed from tumour milieu, more recent data in murine models are suggesting that MDSC may retain their suppressive activity in vivo as a sort of constitutive feature [5]. In contrast, they have been reported to convert to endothelial cells at tumour site, illustrating their high plasticity profile and the multi-faced pro-tumour effect [6].
Here, we are reporting about our experience in characterizing phenotypic and functional features of MDSC from melanoma as well as prostate carcinoma patients, in the scenario of the recent finding available on MDSC in human cancer and the potential role of this pathway as target for novel therapeutic interventions.
MDSC in melanoma patients
By priming a vast repertoire of T-cell responses extensively exploited to throw the fundaments of human tumour immunology, melanoma can be considered the emblem of immunogenic neoplasia [7]. However, this cancer is also the paradigm of immune escape, exploiting diversified strategies to blunt immune responses by affecting different components of innate and adaptive immunity [8]. Nevertheless, despite the important findings achieved in the field, no univocal view of the molecular pathways underlying melanoma immunosuppressive properties, especially in the early steps of disease, has been currently reached.
If the nature of leucocyte infiltrate in primary melanoma could represent a sensor of the cross-talk pathways regulating the initial phases of disease development, it should be mentioned that, together with T lymphocytes, cells expressing monocyte/macrophage markers seem to be quantitatively predominant, while granulocytes are rarely detected and other immunoregulatory cells, such as Treg, are present in small number [9]. The recent report that CD68+ macrophage infiltration at the tumour invasive front and the presence of CD163+ myeloid cells in tumour stroma are independent predictors of poor survival in AJCC stage I/II melanoma [10] suggests that cells of myeloid origin, early recruited by melanoma microenvironment, may confer a more aggressive phenotype to the disease.
In the attempt to unravel immunosuppressive mechanisms limiting T-cell response and favouring melanoma progression, we focused on studying whether a cell subset echoing the MDSC population extensively characterized in tumour-bearing mice [2], could be detected in melanoma patients. At difference from other cancers, we could not detect in stage IV melanoma patients any peripheral accumulation of bona fide MDSC defined as CD34+, lineagenegHLA-DRneg or CD11b+CD15+ cells. In contrast, we observed the expansion of a circulating CD14+ monocyte subset expressing low levels of HLA-DR and highly suppressing T-cell responses through the release of TGFβ [11]. The spontaneous ex vivo production of this cytokine by CD14+HLA-DRneg/low monocytes significantly impairs proliferation, TCR-ζchain expression and effector functions of OKT3-triggered T lymphocytes, whereas no involvement of the arginase I (ARG-1) and inducible nitric oxide synthase (iNOS) pathways, analysed to parallel murine studies, is detected [11]. CD14+CD11b+HLA-DRneg/low cells are significantly expanded in all advanced melanoma patients analysed so far (n ≈ 70), while they result barely detectable (<0.5%) in healthy donors. The frequency of these cells in PBMC inversely correlates with immune responses to a cancer vaccine, confirming the detrimental effect of MDSC on tumour immunity. In line with data in murine models [12], we observed that CD14+CD11b+HLA-DRneg/low cells significantly increased upon the administration of GM-CSF [11], thus emphasizing the detrimental role this growth factor potentially plays in melanoma patients [13].
Interestingly, the accumulation of CD14+CD11b+HLA-DRneg/low cells above healthy subjects’ values is an early event in melanoma, as it can be observed in stage II–III patients with a further but minor enhancement in stage IV disease. In our hands, this is in contrast with the accumulation of T-regulatory cells (Treg) that instead appears to significantly occur only in stage IV melanoma patients (Filipazzi et al., unpublished observation). In addition, cells expressing monocyte markers such as CD68 and producing TGFβ can be abundantly found in primary melanoma lesions with >2 mm thickness, suggesting a crucial role of this pathway in initial tumour development and progression (Filipazzi et al., unpublished observation). CD14+CD11b+HLA-DRneg/lowTGFβ+ cells may thus represent a relevant component of the MDSC population, whose pathological in vivo accumulation is likely driven by melanoma intrinsic pathways involving the expression and/or release of specific immunomodulating factors.
The heterogeneous features of MDSC in cancer patients
Given the high plasticity of myeloid subpopulations, heterogeneous features in terms of phenotype and function in immunoregulatory cells stemming from this compartment are in a way expected. Indeed, even in murine models, where MDSC were originally defined as Gr-1/CD11b co-expressing cells, two subsets, the CD11b+Gr-1high granulocyte-like (CD11b+Ly-6G+Ly6Clow) and CD11b+Gr-1low monocyte-like (CD11b+Ly-6G−Ly6Chigh) MDSC, have been recently reported, and further subgroups are continuously emerging [4]. In contrast, human MDSC have been elusive to identify since the initial studies as they soon appeared to express varied phenotypes and suppressive patterns, likely depending on the cytokine/growth factor profile of different tumour histologies. The lack of defined and homogeneous markers to spot these cells has been profoundly penalizing the assessment of the biological/clinical impact MDSC might play in cancer patients.
To echo the data obtained in murine models, human MDSC are presently divided into two main subsets: a monocytic subpopulation (Mo-MDSC), characterized by the expression of CD14, and a granulocytic subpopulation (G-MDSC), identified by positivity for CD15; both subtypes are reported to express the common myeloid markers CD11b and CD33, with minimal or no expression of maturation myeloid markers such as HLA-DR [14]. Recently additional and more specific molecules have been proposed for human MDSC identification, such as CD115 and CD124 [15, 16], VEGF-R1 [17], MMP9/MMP8 [18], the activated form of Stat3, as well as the overexpression of CD80, CD83 and DC-Sign for Mo-MDSC [19]. In Table 1, we report the attempt to summarize the MDSC phenotypes so far described in patients with different tumour histologies.
Table 1.
Phenotypic features of MDSC described in cancer patients, according to the tumour histotype
| Disease type | Phenotype | References |
|---|---|---|
| Monocytic MDSC | ||
| Melanoma | CD14+HLA-DRneg/low |
Filipazzi et al. [11] Poscke et al. [19] |
| CD14+IL4Ralpha+ | Mandruzzato et al. [15] | |
| RCC | CD14+HLA-DRneg/low | Van Cruijsen et al. [44] |
| Colon carcinoma | CD14+IL4Ralpha+ | Mandruzzato et al. [15] |
| HNSCC | CD14+ | Serafini et al. [45] |
| Multiple myeloma | CD14+HLA-DRneg/low | Brimnes et al. [21] |
| CD14+ | Serafini et al. [45] | |
| HCC | CD14+HLA-DRneg/low | Hoechst et al. [22] |
| Prostate cancer | CD14+HLA-DRneg/low | Vuk-Pavlovic et al. [23] |
| T Cell NHL | CD14+HLA-DR−B7-H1+ | Wilcox et al. [24] |
| Granulocytic MDSC | ||
| RCC | Lin−HLA-DR−CD33+ |
Mirza et al. [46] Kusmartsev et al. [30] |
| CD33+HLA-DR− | Ko et al. [47] | |
| CD11b+CD14−CD15+ |
Zea et al. [25] Rodriguez et al. [48] |
|
| CD15+CD14− | Ko et al. [47] | |
| NSCLC | CD11b+CD14−CD15+CD33+ | Liu et al. [49] |
| Melanoma | Lin−HLA-DR−CD33+ | Daud et al. [50] |
| CD15+IL4Ralpha+ | Mandruzzato et al. [15] | |
| Colon carcinoma | CD15+IL4Ralpha+ | Mandruzzato et al. [15] |
| CD15+granulocytes | Schmielau and Finn [28] | |
| HNSCC | Lin−HLA-DR− | Almand et al. [51] |
| CD11b+CD14−CD33+ |
Corzo et al. [52] Corzo et al. [53] |
|
| SCChighCD66b+ | Brandau et al. [31] | |
| Breast carcinoma | Lin−/lowHLA-DR−CD33+CD11b+ | Diaz-Montero et al. [20] |
| CD15+granulocytes | Schmielau and Finn [28] | |
| Pancreas carcinoma | CD15+granulocytes | Schmielau and Finn [28] |
| Lung carcinoma | CD11b+CD33+ | Srivastava et al. [54] |
This large amount of novel candidate markers further emphasizes the complexity in defining these cells, likely due to the dynamic and plastic nature of the myeloid compartment and its ability to readily respond to tumour-delivered signals. In this view, not only tumour histology and tumour stage, but also tumour burden, site of metastatic lesions and previous lines of treatments (particularly in case of drugs impacting on bone marrow function) should be taken into account when MDSC are studied and characterized in cancer patients. Nevertheless, recent evidences indicate that peripheral blood accumulation of MDSC (Lin−/low, HLA-DR−, CD33+CD11b+) is generally correlated with tumour stage and burden in patients with solid tumours [20].
It is worth underlining that, since our initial report in advanced melanoma patients treated with GM-CSF, CD14+CD11b+HLA-DRneg/low have been found in patients with several cancer histologies, including melanoma [19], multiple myeloma [21], hepatocarcinoma [22], prostate cancer [23] and T-cell non-Hodgkin lymphoma [24]. We specifically searched for CD14+CD11b+HLA-DRneg/low cells in PBMC of prostate carcinoma patients as well, focussing on early disease (i.e. patients with biochemical recurrence, hormone naive or resistant), which is the clinical setting where cancer vaccines are presently tested in our institution. No accumulation was actually detected, suggesting that, in contrast to melanoma, monocyte dysfunctions might be a late event in prostate cancer, as indeed recently reported [23].
Nevertheless, although the suppressive mechanisms on T-cell activity do not appear to always overlap, this evidence suggests that the ability to affect myeloid differentiation towards immature and defective monocytes might be a rather common feature of human cancer. In this view, it comes easy to hypothesize that the release of monocyte-impacting growth factors and cytokines (such as for instance GM-CSF), reported to occur in several tumour histotypes, might be at the origin of this phenomenon.
Suppressive pathways of human MDSC
An additional feature that could be used for identifying MDSC is the immunosuppressive pathway these cells utilized for exerting their negative regulation on T-cell immunity. Knowing the pathway is thus essential to understand whether the detrimental activity of MDSC could be counteracted by targeting specific suppressive mechanisms (by for instance antagonistic mAb, small molecules or other selective inhibitors). However, according to the data presently available, these patterns appear to be even more heterogeneous and undefined than those referring to MDSC phenotype. At this regard, one of the first issues to be addressed is the potential stability of MDSC function once the cells are removed from the in vivo environment and tested ex vivo. Although experiments in murine models are beginning to investigate this point, reporting both transient and stable immunosuppressive features of ex vivo analysed MDSC [5], little information is available to our knowledge in human setting. Although we did not have the chance to test longer-term stability of CD14+CD11b+HLA-DRneg/low cells from melanoma patients, we observed that the cells spontaneously released significant amount of TGFβ, reaching the maximum level at 6 h [11]. Under the same experimental conditions, we recently observed that, in addition to TGFβ, these MDSC secrete a vast array of immunosuppressive, proangiogenic and proinflammatory soluble factors including IL1beta, IL6, IL8, CCL2 and VEGF (Filipazzi P., unpublished observation), confirming the pleiotropic and plastic features of CD14+CD11b+HLA-DRneg/low cells. Our experience thus indicates that, in the absence of information about the stability of MDSC functional properties, a short-term ex vivo analysis could provide sufficient information and hence be preferred to longer in vitro culture, risking instead altering the original cell features.
As for the phenotypic profile, immunosuppressive mechanisms of human MDSC have been mostly searched mirroring murine data. Indeed, l-arginine depletion by ARG-1 has been the first pathway to be reported in MDSC from renal carcinoma patients [25], but more variegated immunosuppressive properties have been subsequently detected, such as iNOS- and NOX2-mediated production of reactive oxygen and nitrogen species [26], VEGF expression [17], cysteine depletion [27], TGFβ secretion [11] as well as induction of Treg [22]. Considering the morphologic and phenotypic division in monocyte and granulocyte-like cells, Mo-MDSC have been described to primarily produce NO for immune suppression, along with a small amount of ROS, whereas G-MDSC tend to inhibit T-cell function primarily via the production of large amounts of reactive oxygen species (ROS), in addition to a small amount of nitric oxide (NO) [4, 26].
Regarding G-MDSC, major effort has been made to understand a potential role of human polymorphonuclear leucocytes (PMN) in cancer-related immunosuppression. The presence of “suppressive” granulocytes has been mostly reported in advanced cancer patients, as associated with CD11b+CD15+/CD66b+ cells co-migrating in the PBMC band during gradient separation, according to the so called low density phenotype, and expressing ARG-1 [25, 28]. Indeed, the expression of this enzyme, ARG1 together with other pathways such as production of reactive oxygen species, has gained human peripheral blood neutrophils a position in the ever-growing family of MDSC. However, the constitutive expression of ARG-1 in human PMN [29], at difference with mouse myeloid cells, and the numerical predominance of these cells among circulating leucocytes, makes the discrimination between MDSC and normal granulocytes rather challenging.
If the pathways of immunosuppression reflect the heterogeneity of the phenotypic features, the negative effects of human MDSC on T-cell immunity appear to be rather consistent. Indeed, these cells have been shown to strongly block T-cell effector function, inhibiting the release of cytokines such as IFNγ and impairing TCR-ζchain expression [30]. In addition to impair T-cell proliferation in response to TCR triggering, MDSC can impair the migratory properties of activated T lymphocytes, as reported in patients with HNC, lung and urinary cancers [31].
A crucial property of murine MDSC recently is the ability to promote tumour angiogenesis, either through the secretion of active factors such as MMP9, as well as the acquisition of endothelial cell properties at tumour microenvironment level or in the presence of proangiogenic culture conditions [32]. Interestingly, recent studies confirmed these properties in human MDSC, as suggested by the ability of G-MDSC from RCC patients to promote angiogenesis [18], or to confer proangiogenic activity to healthy donor neutrophils and monocytes cultured in vitro in tumour-conditioned media from melanoma and RCC lines [33–35].
Tumour factors involved in the generation of human MDSC
The most effective strategy for antagonizing the pro-tumourigenic and immunosuppressive effects of MDSC in cancer patients would be with no doubt to interrupt the circuit leading to their accumulation in tumour-bearing hosts. Although this task may certainly sound quite challenging, particularly in clinical setting, the identification of the molecular pathways expressed by tumour cells and exerting myeloid cell conversion towards MDSC is a crucial goal in this direction. Molecules overexpressed in cancer cells, such as for instance S100A9 protein, have been reported to be critical for MDSC accumulation in murine models and could be responsible for immunological abnormalities in human cancer as well [36]. More recently, systemic serum amyloid A-1 (SAA-1), overexpressed by melanoma cells and detected at high concentration in the serum of advanced melanoma patients, appears to promote the accumulation of IL-10-secreting immunosuppressive neutrophils, representing a bona fide subset of human MDSC [37].
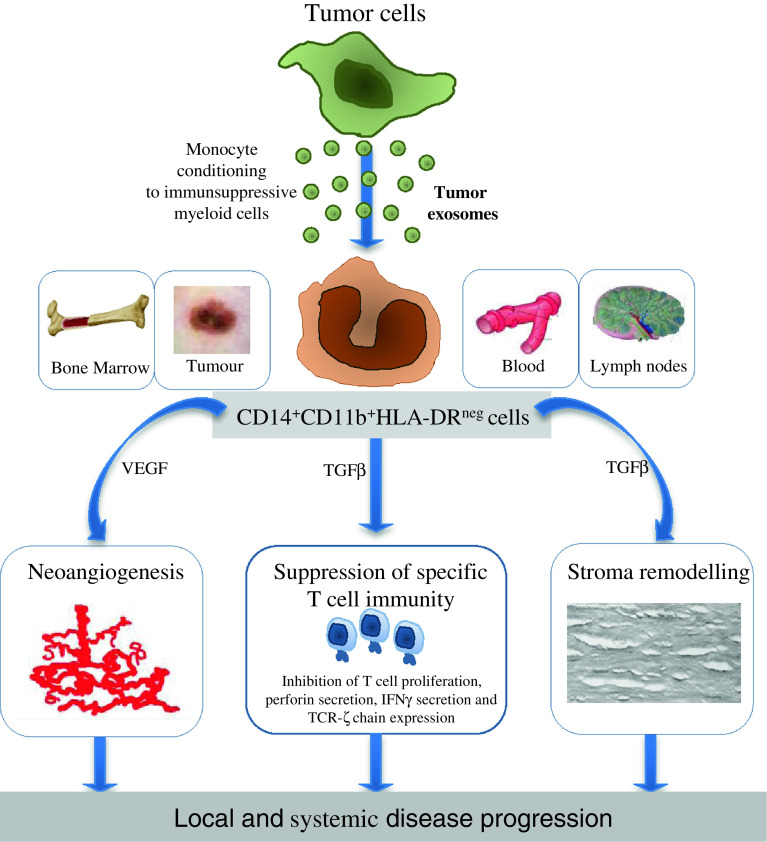
We have addressed this issue years ago by testing whether tumour exosomes, which are nano-sized replicas of the originating cells constitutively and abundantly released in cancer, could be involved in the monocyte conversion to immunosuppressive cells. This approach, besides offering an useful in vitro model to study human MDSC properties, could help identifying the potential pathways responsible for MDSC accumulation in cancer patients. Indeed, tumour exosomes, which are present in significant quantities in biological fluids of cancer patients (including plasma and ascites) [38], are characterized by a remarkable ability to recirculate and could be involved in moulding myeloid differentiation, particularly by reaching the bone marrow and other immune relevant sites [39]. In our in vitro studies, we observed that monocytes from healthy donors can be converted into CD14+CD11b+HLA-DRneg/low cells secreting TGFβ if they are cultured in the presence of exosomes isolated from melanoma or colon carcinoma cell lines, as well as from plasma of melanoma or colon cancer patients [40]. Exosome-converted monocytes induce in T cells an immunosuppressive signature highly resembling that exerted by MSDC isolated from melanoma patients [11] and secrete a panel of proinflammatory and proangiogenic soluble factors largely overlapping with that detected in ex vivo analysed MDSC. A potential involvement of tumour exosomes in MDSC accumulation in cancer patients has been also confirmed by Chalmin and collaborators, who recently reported that exosomes from a human tumour cell line trigger MDSC suppressive function in an Hsp72/TLR2-dependent manner [41]. The definition of the molecular pathways involved in the process of MDSC conversion by tumour exosomes might again be a challenging task, as these organelles contain a broad array of potentially active molecules, ranging from proteins, lipids and nuclear acids including RNA, DNA and miRNA [39]. Studies focused on dissecting this process, with a specific attention to the expression by tumour exosomes of miRNA sequences involved in myeloid compartment differentiation, are presently in progress in our laboratory (Fig. 1).
Fig. 1.
Hypothesis on the role of tumour exosomes in MDSC generation. Exosomes constitutively and abundantly secreted by tumour cells and efficiently recirculating in different body compartments interact with monocytes locally or systemically (in blood, draining lymph nodes and bone marrow). This interaction skews monocyte differentiation towards CD14+CD11b+HLA-DRneg/low cells favouring in turn tumour progression by suppressing T-cell immunity, promoting stroma remodelling and sustaining neoangiogenesis
Tumour cell supernatant has being also utilized to prove a direct involvement of cancer cells in MDSC generation and accumulation, as quite recently reported by several groups, globally showing that normal neutrophils and/or monocytes can be differentiated into MDSC by in vitro culture with conditioned media from melanoma, RCC cells and other solid cancers [33–35]. These studies, although limited to the in vitro setting, underline the crucial role played by cancer cells in MDSC recruitment and activation, pointing to the tumour microenvironment as the major site where most immune dysfunctions involving the myeloid compartment stem from. This key issue, rather underestimated or neglected by studies focused on circulating MDSC, has been elegantly depicted by the recent study of Fridlender et al. [42] who clearly demonstrated in a murine mesothelioma model that neutrophils can be converted into highly immunosuppressive “N2” cells at tumour site through a TGFβ-mediated mechanism.
Gaining comparable data in cancer patients is obviously challenging. Nevertheless, the evidence that myeloid cells with immunosuppressive features can be often detected within the immune infiltrate of human solid cancers (including the well-known M2 macrophages described by Mantovani and collaborators several years ago) [43] and the direct correlation between MDSC frequency in peripheral blood and disease stage [20] emphasize the role of tumour microenvironment as the “epicenter” of MDSC genesis in cancer-bearing hosts. At this regard, it could be hypothesized that not only single defined molecular pathways but also more pleiotropic and complex local alterations, such as those related to cancer-associated metabolic dysfunctions, might be involved [1]. As an example, we are collecting evidence that the acid pH characterizing solid tumour lesions, creates a quite hostile microenvironment for immune responses by inducing anergy in specific T cells (Calcinotto et al., personal communication), promoting exosome release by tumour cells and favouring MDSC differentiation and activity.
Targeting MDSC in cancer patients
Immunosuppression induced by tumours is obviously a major obstacle that must be overcome for successful cancer immunotherapy. Consequently, drugs that can impact on MDSC accumulation and function by (1) promoting MDSC differentiation into myeloid mature cells, thus lacking suppressive activity; (2) inhibiting MDSC expansion from hematopoietic precursors; (3) blocking the signalling pathways that regulate the production of immunosuppressive factors by MDSC; and (4) reducing MDSC accumulation have been evaluated or are currently being explored using murine models or in vitro cultured cells [2], however, only a few reports have demonstrated an effect on human MDSCs in clinical studies. In Table 2, a list of trials testing MDSC modulation by pharmacological intervention in cancer patients is reported.
Table 2.
Therapeutic strategies modulating MDSC frequency and function in cancer patients
| Drug | Cancer patients | Mechanisms | References |
|---|---|---|---|
| ATRA | RCC | Favours differentiation of myeloid progenitors to Dc and macrophages | Mirza et al. [46] |
| Vitamin D3 | Head and neck | Increases HLA-DR and reduces CD34+ cells | Lathers et al. [55] |
| Bevacizumab, VEGF-trap | RCC and several solid tumours | Decrease MDSC VEGFR+ cells |
Kusmartsev et al. [17] Rodriguez et al. [48] Fricke et al. [56] |
| Tk inhibitors (Sunitinib) | RCC | Stat3 and c-kit mediated mechanisms | Ko et al. [47] |
Since it is well established that tumour-derived factors influence myelopoiesis and may induce the accumulation and, eventually, the activation of MDSC, it could be here reasoned that drugs interfering with the ability of tumour cells to produce MDSC-inducing factors such as defined cytokines or exosomes [35, 40] are likely to impact on number and function of these immunosuppressive cells. Indeed, we have evidence that buffering tumour pH can reduce exosome secretion and MDSC activity both in vitro and in melanoma patients (Filipazzi, unpublished).
Conclusions
As beautifully depicted by Youn and Gabrilovich in their recent review, MDSC heterogeneity can indeed be viewed as “a blessing and a curse” [5], at the same time appealing and frustrating researches working in the field. However, this acknowledged level of complexity should not discourage from performing concerted efforts to reach consensus in this arena. As a group mostly involved in clinical studies, we strongly believe that more attention should be paid to the careful and consistent selection of patients to be included in MDSC studies, particularly in terms of disease features and treatment history. Multi-centric clinical trials could offer a great opportunity to assess MDSC phenotypic/functional features in a consistent number of patients with comparable disease conditions (i.e. those dictated by inclusion criteria), possibly relying then on MDSC evaluation by a validated central laboratory. Alternatively, of great values would also be the comparative studies focused on the analysis of different MDSC candidates in the same clinical setting. These studies should also include a potential correlation with disease course and prognosis, as well as with immune responses in case of immune-based therapies, to provide at least a first level of clinical implications. Finally, it should be considered to routinely include the evaluation of MDSC frequency in the standard immunomonitoring of patients enrolled in cancer vaccines, again to unravel any potential influence of this parameter on the immunization strategy. Such kind of efforts would certainly provide unique and definitive information about the real impact MDSC play in cancer patients and would hopefully contribute to dispel the doubts and the confusion about these fascinating cells.
Acknowledgments
This work was supported by grants from the Italian Association for Cancer Research (AIRC), the Italian Health Ministry, the Monzino Foundation and the Harry Lloyd Melanoma Foundation.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13(1):23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181(8):5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40(11):2969–2975. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8(8):618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 7.Parmiani G, Castelli C, Santinami M, Rivoltini L. Melanoma immunology: past, present and future. Curr Opin Oncol. 2007;19(2):121–127. doi: 10.1097/CCO.0b013e32801497d7. [DOI] [PubMed] [Google Scholar]
- 8.Rivoltini L, Canese P, Huber V, Iero M, Pilla L, Valenti R, Fais S, Lozupone F, Casati C, Castelli C, Parmiani G. Escape strategies and reasons for failure in the interaction between tumour cells and the immune system: how can we tilt the balance towards immune-mediated cancer control? Expert Opin Biol Ther. 2005;5(4):463–476. doi: 10.1517/14712598.5.4.463. [DOI] [PubMed] [Google Scholar]
- 9.Hillen F, Baeten CI, van de Winkel A, Creytens D, van der Schaft DW, Winnepenninckx V, Griffioen AW. Leukocyte infiltration and tumor cell plasticity are parameters of aggressiveness in primary cutaneous melanoma. Cancer Immunol Immunother. 2008;57(1):97–106. doi: 10.1007/s00262-007-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen TO, Schmidt H, Møller HJ, Høyer M, Maniecki MB, Sjoegren P, Christensen IJ, Steiniche T. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J Clin Oncol. 2009;27(20):3330–3337. doi: 10.1200/JCO.2008.19.9919. [DOI] [PubMed] [Google Scholar]
- 11.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25(18):2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 12.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64(17):6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 13.Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2007;18(2):226–232. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- 14.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11(7):802–807. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S, Zanon A, Rossi CR, Nitti D, Bronte V, Zanovello P. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J Immunol. 2009;182(10):6562–6568. doi: 10.4049/jimmunol.0803831. [DOI] [PubMed] [Google Scholar]
- 16.Dugast AS, Haudebourg T, Coulon F, Heslan M, Haspot F, Poirier N, Vuillefroy de Silly R, Usal C, Smit H, Martinet B, Thebault P, Renaudin K, Vanhove B. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol. 2008;180(12):7898–7906. doi: 10.4049/jimmunol.180.12.7898. [DOI] [PubMed] [Google Scholar]
- 17.Kusmartsev S, Eruslanov E, Kübler H, Tseng T, Sakai Y, Su Z, Kaliberov S, Heiser A, Rosser C, Dahm P, Siemann D, Vieweg J. Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J Immunol. 2008;181(1):346–353. doi: 10.4049/jimmunol.181.1.346. [DOI] [PubMed] [Google Scholar]
- 18.Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol. 2011;11(7):856–861. doi: 10.1016/j.intimp.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR−/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70(11):4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 20.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58(1):49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brimnes MK, Vangsted AJ, Knudsen LM, Gimsing P, Gang AO, Johnsen HE, Svane IM. Increased level of both CD4+FOXP3+ regulatory T cells and CD14+HLA-DR−/low myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand J Immunol. 2010;72(6):540–547. doi: 10.1111/j.1365-3083.2010.02463.x. [DOI] [PubMed] [Google Scholar]
- 22.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135(1):234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Vuk-Pavlović S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, Dietz AB. Immunosuppressive CD14+HLA-DRlow/− monocytes in prostate cancer. Prostate. 2010;70(4):443–455. doi: 10.1002/pros.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilcox RA, Wada DA, Ziesmer SC, Elsawa SF, Comfere NI, Dietz AB, Novak AJ, Witzig TE, Feldman AL, Pittelkow MR, Ansell SM. Monocytes promote tumor cell survival in T-cell lymphoproliferative disorders and are impaired in their ability to differentiate into mature dendritic cells. Blood. 2009;114(14):2936–2944. doi: 10.1182/blood-2009-05-220111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, Mier J, Ochoa AC. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65(8):3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 26.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172(2):989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 27.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59:1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61(12):4756–4760. [PubMed] [Google Scholar]
- 29.Munder M, Mollinedo F, Calafat J, Canchado J, Gil-Lamaignere C, Fuentes JM, Luckner C, Doschko G, Soler G, Eichmann K, Müller FM, Ho AD, Goerner M, Modolell M. Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood. 2005;105(6):2549–2556. doi: 10.1182/blood-2004-07-2521. [DOI] [PubMed] [Google Scholar]
- 30.Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kübler H, Yancey D, Dahm P, Vieweg J. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2008;14(24):8270–8278. doi: 10.1158/1078-0432.CCR-08-0165. [DOI] [PubMed] [Google Scholar]
- 31.Brandau S, Trellakis S, Bruderek K, Schmaltz D, Steller G, Elian M, Suttmann H, Schenck M, Welling J, Zabel P, Lang S. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J Leukoc Biol. 2011;89(2):311–317. doi: 10.1189/jlb.0310162. [DOI] [PubMed] [Google Scholar]
- 32.Yang Li, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Yu, Matrisian LM, Carbone DP, Charles Lin P. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6(4):409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 33.Borden EC, Ko Smith J, Rayman PA, Jacobs B, Ireland J, Lindner D, Finke J (2010) Dual mechanicistic function of MDSC subsets in melanoma resistence. J Clin Oncol Vol 28, no 15 suppl
- 34.Lechner MG, Megiel C, Russell SM, Bingham B, Arger N, Woo T, Epstein AL. Functional characterization of human Cd33+ and Cd11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J Transl Med. 2011;9:90. doi: 10.1186/1479-5876-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185(4):2273–2284. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205(10):2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Santo C, Arscott R, Booth S, Karydis I, Jones M, Asher R, Salio M, Middleton M, Cerundolo V. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat Immunol. 2010;11(11):1039–1046. doi: 10.1038/ni.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, Zaccheddu A, Colone M, Arancia G, Gentile M, Seregni E, Valenti R, Ballabio G, Belli F, Leo E, Parmiani G, Rivoltini L. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128(7):1796–1804. doi: 10.1053/j.gastro.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 39.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 40.Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, Corbelli A, Fais S, Parmiani G, Rivoltini L. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66(18):9290–9298. doi: 10.1158/0008-5472.CAN-06-1819. [DOI] [PubMed] [Google Scholar]
- 41.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, Multhoff G, Hamman A, Martin F, Chauffert B, Solary E, Zitvogel L, Garrido C, Ryffel B, Borg C, Apetoh L, Rébé C, Ghiringhelli F. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120(2):457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 44.van Cruijsen H, van der Veldt AA, Vroling L, Oosterhoff D, Broxterman HJ, Scheper RJ, Giaccone G, Haanen JB, van den Eertwegh AJ, Boven E, Hoekman K, de Gruijl TD. Sunitinib-induced myeloid lineage redistribution in renal cell cancer patients: CD1c+ dendritic cell frequency predicts progression-free survival. Clin Cancer Res. 2008;14(18):5884–5892. doi: 10.1158/1078-0432.CCR-08-0656. [DOI] [PubMed] [Google Scholar]
- 45.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203(12):2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66(18):9299–9307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R, Finke JH. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15(6):2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69(4):1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, Wu YC, Chu Y, Chung FT, Kuo CH, Lee KY, Lin SM, Lin HC, Wang CH, Yu CT, Kuo HP. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14−/CD15+/CD33+ myeloid-derived suppressor cells and CD8 + T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol. 2010;136(1):35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daud AI, Mirza N, Lenox B, Andrews S, Urbas P, Gao GX, Lee JH, Sondak VK, Riker AI, Deconti RC, Gabrilovich D. Phenotypic and functional analysis of dendritic cells and clinical outcome in patients with high-risk melanoma treated with adjuvant granulocyte macrophage colony-stimulating factor. J Clin Oncol. 2008;26(19):3235–3241. doi: 10.1200/JCO.2007.13.9048. [DOI] [PubMed] [Google Scholar]
- 51.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166(1):678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 52.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182(9):5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207(11):2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Srivastava MK, Bosch JJ, Thompson JA, Ksander BR, Edelman MJ, Ostrand-Rosenberg S. Lung cancer patients’ CD4(+) T cells are activated in vitro by MHC II cell-based vaccines despite the presence of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2008;57(10):1493–1504. doi: 10.1007/s00262-008-0490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lathers DM, Clark JI, Achille NJ, Young MR. Phase 1B study to improve immune responses in head and neck cancer patients using escalating doses of 25-hydroxyvitamin D3. Cancer Immunol Immunother. 2004;53(5):422–430. doi: 10.1007/s00262-003-0459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fricke I, Mirza N, Dupont J, Lockhart C, Jackson A, Lee JH, Sosman JA, Gabrilovich DI. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin Cancer Res. 2007;13(16):4840–4848. doi: 10.1158/1078-0432.CCR-07-0409. [DOI] [PubMed] [Google Scholar]