Abstract
Background
Based on their tumor-associated expression pattern, cancer/testis antigens (CTAs) are considered potential targets for cancer immunotherapy. We aim to evaluate the expression of CTAs in non-Hodgkin’s lymphoma (NHL) samples and the ability of these patients to elicit spontaneous humoral immune response against CTAs.
Methods
Expression of MAGE-A family, CT7/MAGE-C1, CT10/MAGE-C2, GAGE and NY-ESO-1 was analyzed by immunohistochemistry in a tissue microarray generated from 106 NHL archival cases. The humoral response against 19 CTAs was tested in 97 untreated NHL serum samples using ELISA technique.
Results
11.3 % of NHL tumor samples expressed at least 1 CTA. MAGE-A family (6.6 %), GAGE (5.7 %) and NY-ESO-1(4.7 %) were the most frequently expressed antigens. We found no statistically significant correlation between CTA positivity and clinical parameters such as NHL histological subtype, Ann Arbor stage, international prognostic index score, response to treatment and overall survival. Humoral response against at least 1 CTA was observed in 16.5 % of NHL serum samples. However, overall seroreactivity was low, and strong titers (>1:1000) were observed in only two diffuse large B-cell lymphomas patients against CT45.
Conclusion
Our findings are in agreement with most of published studies in this field to date and suggest an overall low expression of CTAs in NHL patients. However, as many new CTAs have been described recently and some of them are found to be highly expressed in NHL cell lines and tumor samples, further studies exploring the expression of different panels of CTAs are needed to evaluate their role as candidates for immunotherapy in NHL patients.
Keywords: Non-Hodgkin’s lymphoma, Cancer/testis antigens, Humoral response
Background
The search for tumor-specific antigens as potential targets for immunotherapeutic approach has been challenging for several decades. Cancer/testis antigens (CTAs) have emerged as attractive candidates for cancer-specific immunotherapy due to their particular characteristics of high immunogenicity with no or highly restricted expression in normal tissues [1]. Several studies have demonstrated that these antigens are able to elicit specific humoral and T-cell-mediated cytotoxic immune responses in cancer patients, pointing them as possible cancer vaccine targets. One common feature of CTA expression is its induction by the DNA methyl-transferase 1 inhibitors, 5-aza-2-deoxycytidine and/or by histone deacetylase inhibitors [16–18]. These findings, together with the inclination for global hypomethylation in cancer, suggest that CpG island hypomethylation at the promoter regions of these genes is the possible mechanism for transcriptional activation of CTA genes in cancer [1]. There are more than 100 CTA genes reported in the literature to date [2]. About 30 of these CTA genes are encoded by multigene families on chromosome X (classical CTAs) [3]. These CTAs are of particular interest because almost all CT-X present higher immunogenicity and highly restricted expression pattern in normal tissues compared to those not encoded on chromosome X (non-classical CTAs). Some non-classical CTAs like SCP-1, [4], OY-TES-1 [5], SPO11 [6] and BORIS [7] have an established role in gametogenesis, but in the tumorigenesis field, the biological function of most CTAs remains poorly understood. Recent studies provided some evidence that CTAs may have antiapoptotic properties rather than regulating cell proliferation or adhesion in cancer. [8–15]. The frequency of CTA expression is highly variable among different tumor types: melanoma, ovarian cancer and lung cancer are considered tumors with high frequency of CTA expression, while hematopoietic malignancies, renal, colon and pancreatic cancers, have been described as tumors with low frequency of CTA expression [1]. Some exceptions to this observation among hematopoietic malignancies are the high expression of CT7/MAGE-C1 in multiple myeloma [19, 20], and CT45 in classical Hodgkin lymphoma [21, 22]. Studies correlating CTA expression with clinicopathological features in different tumor types have demonstrated the association of CTA positivity with higher tumor grade, advanced stage or metastatic disease and worse clinical outcome [19, 23–31]. Some studies performed in cancer cell lines have suggested the correlation of CTA expression with resistance to some antineoplastic agents and gamma-irradiation. It could explain, in part, the poor prognosis of these patients [15, 32].
Despite being frequent hematologic malignancy, there are few studies evaluating the expression of CTAs in non-Hodgkin’s lymphoma (NHL) to date. The largest study accessing CTA expression in NHL analyzed the expression of 8 classical and non-classical CTAs (MAGE-A3, MAGE-A4, CT7, SSX-1, SSX-2, SSX-4, SCP-1 and HOM-TES-85) using RT-PCR technique in 93 NHL samples [33]. It was demonstrated that diffuse large B-cell lymphomas (DLBCL), a subtype of B-cell lymphoma, showed the highest frequency of CTA expression. SCP-1 (7/28), SSX-1 (5/28) and CT7 (2/28) were the CTAs more frequently expressed in this subgroup, and among T-cell lymphomas, the majority of samples (9/15) expressed SCP-1 (6/9 peripheral T-cell lymphomas, 2/4 angioimmunoblastic lymphoma and 1/2 precursor T-cell lymphoblastic lymphoma). Another study demonstrated a significant cytotoxic T-cell response in 21/29 HLA-A*0201-positive DLBCL patients with CT63/PASD1 expression using SEREX (Serological Analysis of Recombinant cDNA Expression) technique, identifying CT63/PASD1 as one of the most important candidates for cancer vaccines in DLBCL [34–36]. Recently, Chen et al. [21] also described the expression of CT45 in 42/72 (58 %) of classical Hodgkin’s lymphoma (cHL) and 28/126 (22 %) of DLBCL samples by immunohistochemistry. Interestingly, despite the remarkable high CT45 expression in cHL, only 1 of 67 patients had detectable anti-CT45 antibodies, suggesting low immunogenicity of this CTA or a suppressed immune response in cHL patients. Except for the CTAs described above, the available studies suggest an overall low expression of CTA in NHL.
Considering that the available information about CTA expression in lymphomas is scarce and heterogeneous regarding methods and samples, we immunohistochemically investigated the protein expression of a panel of CTAs in NHL tissue samples. We also studied the spontaneous humoral immune response in sera of NHL patients to evaluate the potential of CTAs as prognostic markers and candidates for immunotherapeutic approach in NHL patients.
Patients and methods
We retrospectively reviewed all cases of NHL diagnosed between 2003 and 2007 at the Hematology Service of Universidade Federal de Sao Paulo. The histology was reviewed by an experienced hemopathologist (A.C.A.), and NHL cases with sufficient material in paraffin blocks for tissue microarray (TMA) construction were included in this study. All patients included in this study were staged, classified according to the international prognostic index (IPI) and treated according to NHL treatment guidelines available from 2003 to 2007 [37]. Due to unavailability of rituximab in our public hospital at this time, B-cell lymphoma patients were uniformly treated with CHOP-like chemotherapy regimens without anti-CD20 monoclonal antibody.
This research was submitted to the Brazilian Research Council and approved by the Ethical Review Committee of our Institution according to the Declaration of Helsinki (Ethics Committee Approval 0998/07), and all patients provided written informed consent.
Tissue microarray (TMA)
Formalin-fixed paraffin-embedded tissues of 106 previously untreated NHL patients were obtained from the archives of the Department of Pathology, Hospital São Paulo, UNIFESP, Brazil. According to the World Health Organization (WHO) classification [38], the NHL cases consisted of 56 DLBCL, 10 follicular lymphomas, 9 peripheral T-cell lymphomas, 7 small lymphocytic lymphomas, 5 MALT (mucosa-associated lymphoid tissues) lymphomas, 4 marginal zone lymphomas, 3 lymphoplasmacytic lymphomas, 3 T-cell lymphoblastic lymphomas, 2 B-cell lymphoblastic lymphomas, 2 mantle cell lymphomas, 2 anaplastic large cell lymphomas, 2 mycosis fungoides and 1 adult T-cell leukemia/lymphoma. Table 1 summarizes the clinical data of these patients. Slides from all cases were reviewed and representative blocks were chosen for tissue microarray assembly.
Table 1.
Clinical data of non-Hodgkin’s lymphoma patients included in TMA study (n = 106)
| Clinical data | n | % |
|---|---|---|
| Age | ||
| ≤60 | 67 | 63.2 |
| >60 | 39 | 36.8 |
| Median age: 54 (17–91) | ||
| Gender | ||
| Male | 55 | 51.9 |
| Female | 51 | 48.1 |
| HIV status | ||
| Negative | 97 | 91.5 |
| Positive | 9 | 8.5 |
| Tumor cell origin | ||
| B-cell | 89 | 84.0 |
| T-cell | 16 | 15.1 |
| Non-B/non-T | 1 | 0.9 |
| Clinical behavior | ||
| Indolent | 32 | 30.2 |
| Aggressive | 74 | 69.8 |
| DLBCL/Non-DLBCL | ||
| Non-DLBCL | 50 | 47.2 |
| DLBCL | 56 | 52.8 |
| Ann Arbor staging | ||
| Early (I or II) | 41 | 38.7 |
| Advanced (III or IV) | 65 | 61.3 |
| IPI | ||
| Low | 31 | 29.2 |
| Intermediate (low and high intermediate) | 46 | 43.4 |
| High | 19 | 17.9 |
| Not available | 10 | 9.4 |
| Response | ||
| Complete response | 44 | 41.5 |
| Partial response | 22 | 20.8 |
| Stable disease | 0 | 0 |
| Progressive disease | 38 | 35.8 |
| Not available | 2 | 1.9 |
IPI international prognostic index
Core-needle biopsies of paraffin-embedded tissue were obtained and then re-embedded in an array master block using techniques originally developed by Kononen et al. [39] and then modified by Hedvat et al. [40]. A Beecher Instruments (Sun Prairie, WI, USA) arraying device was used to assemble the arrays. Three core-needle biopsies (1.0 mm diameter) from tumor representative areas of each NHL case were included on tissue microarray blocks.
All CTAs chosen for immunohistochemical analysis in this study were classical CTAs due to their higher immunogenicity and highly restricted expression in normal tissues. The following monoclonal antibodies (to the following antigens) were included in our panel: MA454 (MAGE-A1), M3H67 (several MAGE-A antigens), 57B (MAGE-A1, -A3, -A4, -A6 and -A12), MAGE-A10#9 (MAGE-A10), CT7-33 (CT7/MAGE-C1), CT10#5 (CT10/MAGE-C2), clone #26 (GAGE-family, B&D Transduction Labs, Lexington, KY) and E978 (NY-ESO-1). The specificity of each antibody has been tested in previous studies [28, 41–48]. A heat-based antigen-retrieval method was used for all antibodies (vegetable steamer [Oster-Sunbeam, Ft. Lauderdale, FL, USA] 90 °C, 30 min). Except for mAb E978, a biotinylated horse anti-mouse secondary antibody (1:200; Vector, Burlingame, CA, USA) was used to detect primary antibody, followed by an avidin–biotin system (ABC-elite kit, Vector). E978 was detected with Immunovision kit (Leica Microsystems, Buffalo Grove, MN, USA). 3,3′-Diaminobenzidine tetrahydrochloride (Biogenex; San Ramon, CA, USA) served as chromogen. Endogenous peroxidase was suppressed by 1 % H2O2 for 20 min. The extent of tumor staining was graded based on the amount of immunopositive tumor cells as follows: ≤25 %: +, 26–50 %: ++, 51–75 %: +++ and 76–100 %: ++++). Testis with preserved spermatogenesis was used as positive controls, and reactive lymph nodes and tonsils samples were used as negative controls for all antibodies.
Spontaneous humoral immune response analysis by ELISA
Serum samples of 97 NHL cases, including 59 cases from the TMA cohort, were from patients referred from Hospital Sao Paulo, UNIFESP, Brazil. All samples were obtained at diagnosis, before any therapeutic interventions, to avoid treatment-related influences on humoral immune response analyses. According to the WHO classification, the NHL cases consisted of 50 DLBCL, 10 follicular lymphomas, 8 peripheral T-cell lymphomas, 6 MALT lymphomas, 5 marginal zone lymphomas, 4 mantle cell lymphomas, 4 adult T-cell leukemia/lymphoma, 3 small lymphocytic lymphomas, 2 T-cell lymphoblastic lymphomas, 2 B-cell lymphoblastic lymphomas, 2 anaplastic large cell lymphomas and 1 mycosis fungoides. Clinical data of NHL patients included in spontaneous humoral immune response analysis are summarized in Table 2.
Table 2.
Clinical data of non-Hodgkin’s lymphoma patients included in ELISA study (n = 97)
| Clinical data | n | % |
|---|---|---|
| Age | ||
| ≤60 | 64 | 66.0 |
| >60 | 33 | 34.0 |
| Median age: 54 (17–86) | ||
| Gender | ||
| Male | 53 | 54.6 |
| Female | 44 | 45.4 |
| HIV status | ||
| Negative | 92 | 94.8 |
| Positive | 5 | 5.2 |
| Tumor cell origin | ||
| B-cell | 80 | 82.5 |
| T-cell | 16 | 16.5 |
| Null | 1 | 1.0 |
| Clinical behavior | ||
| Indolent | 28 | 28.9 |
| Aggressive | 69 | 71.1 |
| DLBCL/Non-DLBCL | ||
| Non-DLBCL | 47 | 48.5 |
| DLBCL | 50 | 51.5 |
| Ann Arbor staging | ||
| Early (I or II) | 34 | 35.1 |
| Advanced (III or IV) | 62 | 63.9 |
| Not available | 1 | 1.0 |
| IPI | ||
| Low | 29 | 29.9 |
| Intermediate (low and high intermediate) | 48 | 49.5 |
| High | 15 | 15.5 |
| Not available | 5 | 5.2 |
| Response | ||
| Complete response | 41 | 42.3 |
| Partial response | 17 | 17.5 |
| Stable disease | 0 | 0 |
| Progressive disease | 38 | 39.2 |
| Not available | 1 | 1.0 |
IPI international prognostic index
Serum samples were screened for the presence of IgG-specific responses against MAGE-A1, MAGE-A3, MAGE-A4, MAGE-A10, NY-ESO-1, CT7, CT10, CT24, CT45, CT46, CT63, CT83, SSX-1, SSX-2, SSX-4, LAGE-1, GAGE-2, SAGE-1 and XAGE-1, using ELISA technique with recombinant CTA or CTA fragments described by Gnjatic et al. [49]. In each assay, sera of patients with known presence or absence of specific reactivity were used as controls. A positive result was defined as extrapolated reciprocal titers >100.
Statistical analysis
Associations between the variables were tested by the Pearson χ2 test (X2). Mann–Whitney test was used to perform mean comparisons. Overall survival (OS) analyses were performed according to Kaplan–Meier method, and the log-rank was used for analyzing differences between curves. A p value lower than 0.05 was considered as statistically significant.
Results
Tissue microarray (TMA)
Due to the heterogeneous expression pattern of CTAs in most NHL TMA samples, positivity was not graded, and cases were classified as positive for ≥1 CTA (any degree of positivity) or negative (Fig. 1).
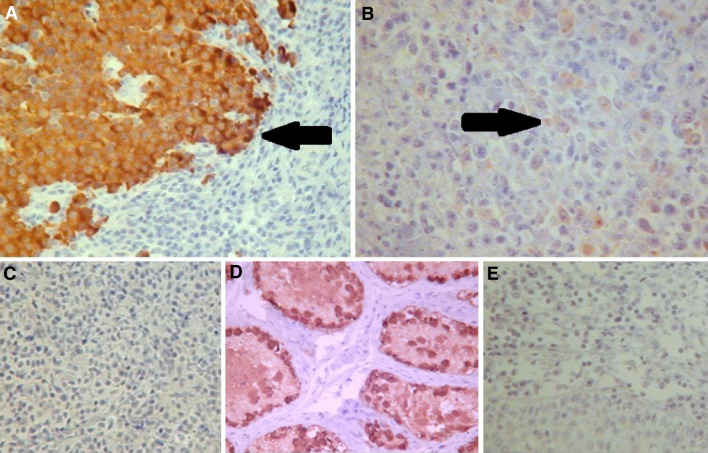
Fig. 1.
a Illustration of diffuse positivity in tumor representative areas of a lymphoplasmacytic lymphoma sample using MA454 antibody (400×). b Illustration of focal positivity in a diffuse large B-cell lymphoma (DLBCL) sample using MA454 antibody (400×). c Illustration of a negative DLBCL sample using MA454 antibody (400x). d Testis sample used as positive control for MA454 antibody (400×). e Tonsils sample used as negative control for MA454 antibody (400×)
Immunohistochemical analyses found that 12 of 106 (11.3 %) NHL samples were positive for at least 1 of 8 antibodies included in our panel. We grouped together the anti-MAGE-A family antibodies—MA454, M3H67, 57B and MAGE-A10#9 due to the overlapping reactivity against several members of MAGE-A family seen in some of these antibodies [41–48]. The most frequently expressed CTA was MAGE-A family (6.6 %), followed by GAGE and NY-ESO-1, which were positive in 5.7 % and 4.7 % of NHL samples, respectively. The 12 CTA-positive cases were: 9/56 DLBCL, 1/2 anaplastic large cell lymphoma, 1/3 lymphoplasmacytic lymphoma and 1/9 peripheral T-cell lymphoma.
We found no statistically significant correlation between CTA positivity and clinical data (Table 3), except for the unexpected finding of higher CTA expression among early stage (19.5 %) compared to advanced stage NHL patients (6.2 %).
Table 3.
Analysis of CTA expression by immunohistochemistry in different non-Hodgkin’s lymphoma subgroups (n = 106)
| Clinical data | N | 0 CTA ( %) | N | ≥1 CTA (%) | p value |
|---|---|---|---|---|---|
| NHL cell origin | |||||
| B-cell | 79 | 88.8 | 10 | 11.2 | 0.928 |
| T-cell | 14 | 87.5 | 2 | 12.5 | |
| Null | 1 | 100.0 | 0 | 0 | |
| NHL clinical behavior | |||||
| Indolent | 31 | 96.9 | 1 | 3.1 | 0.102 |
| Aggressive | 63 | 85.1 | 11 | 14.9 | |
| DLBCL/non-DLBCL | |||||
| Non-DLBCL | 47 | 94.0 | 3 | 6.0 | 0.130 |
| DLBCL | 47 | 83.9 | 9 | 16.1 | |
| Ann Arbor staging | |||||
| I or II | 33 | 80.5 | 8 | 19.5 | 0.046 |
| III or IV | 61 | 93.8 | 4 | 6.2 | |
| IPI | |||||
| 0 or 1 | 27 | 87.1 | 4 | 12.9 | 0.990 |
| 2 or 3 | 41 | 89.1 | 5 | 10.9 | |
| 4 or 5 | 17 | 89.5 | 2 | 10.5 | |
| Not available | 9 | 90.0 | 1 | 10.0 | |
| Response | |||||
| Complete response | 41 | 93.2 | 3 | 6.8 | 0.230 |
| Non-complete response | 51 | 85.0 | 9 | 15.0 | |
| Not available | 2 | 100 | 0 | 0 | |
IPI international prognostic index
The median OS of NHL patients included in our TMA study was 65 months. Survival analysis demonstrated higher median OS among indolent NHL (70 months) compared to aggressive NHL (12 months) (p = 0.0002) and worse outcome in DLBCL patients (10 months) compared to the non-DLBCL subgroup (70 months) (p = 0.0015). IPI could identify low (median OS not reached), intermediate (14 months) and high-risk (13 months) patients in our cohort (p = 0.002). Despite the difference found in median OS between NHL patients with no CTA expression (65 months) and those who expressed at least one CTA (11 months), it was not statistically significant (p = 0.0947). When Cox’s multivariate analysis was applied, only IPI (p < 0.01) and lymphoma aggressiveness (p < 0.01) remained as significant prognostic factors.
Spontaneous humoral immune response (ELISA) in NHL
ELISA assay demonstrated spontaneous humoral immune response against at least 1 CTA from our broad panel in 16 of 97 (16.5 %) NHL serum samples. However, reactivity was low for almost all patients, and strong reactivity (titers >1:1000) was observed in only two DLBCL patients positive for CT45.
The highest positivity was observed against MAGE-A family (8.2 %), when analyzed together. Analyzing individually, CT45 (5.2 %), NY-ESO-1 (5.2 %) and MAGE-A4 (5.2 %) were the most frequent CTAs. Among DLBCL patients, CT45 and NY-ESO-1 were the CTAs most frequently recognized by serum antibodies, with positivity of 8.0 % (3/35) and 6.0 % (2/35), respectively.
Restricting the panel to the 9 CTAs included in TMA, 13 (13.4 %) of 97 NHL sera samples were positive for at least 1 CTA of the panel. MAGE-A4 (4.1 %), MAGE-A3 (3.1 %) and NY-ESO-1 (3.1 %) were the most frequently expressed CTAs.
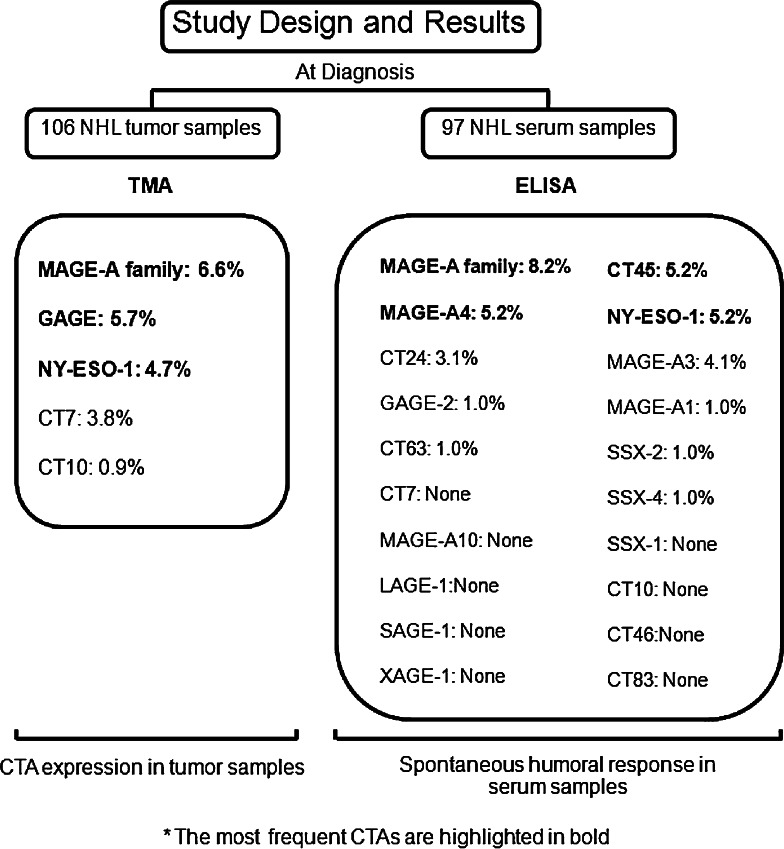
In the 59 NHL cases with both tissue and sera available, we found 3 positive cases by immunohistochemistry and 10 positive cases by ELISA; 2 of 3 positive cases by immunohistochemistry were also positive by ELISA, and 1 of immunohistochemically positive case was negative by ELISA. The summary of study design and results is illustrated in Fig. 2.
Fig. 2.
Summary of study design and results
Discussion
To the best of our knowledge, this is the most extensive study performed in this field, evaluating CTA expression in NHL tumor samples, serum immune response analyses, clinical and prognostic data. Analyzing an extensive panel of CTAs, we found an overall low immunohistochemical expression (11.3 %) and serum reactivity against CTAs in our cohort. MAGE-A family (6.6 %), GAGE (5.7 %) and NY-ESO-1 (4.7 %) were the most frequently expressed CTAs. As NHL corresponds to a very heterogeneous subtype of tumors, we analyzed separately DLBCL, the most frequent subtype of NHL, where high expression of some CTAs was described in previous studies. However, positivity was equally low to the CTAs included in our panel, and we also did not find statistically significant correlation between CTA expression and prognostic factors or patient outcome. Despite the limited available data about CTA expression in lymphomas to date, our findings are in agreement with most studies performed in this field, using RT-PCR and/or immunohistochemical analysis [33].
Most of studies evaluating CTA expression in different tumors have demonstrated a higher CTA expression in higher histological grade and in advanced stage/metastatic diseases [19, 23–31]. In our study, we found this tendency of CTA expression in indolent versus aggressive NHL and in complete response versus non-complete response subgroup, but differences were not statistically significant. An unexpected finding was the higher positivity in early stage compared to advanced stage disease (p = 0.046).
We consider that the poor outcome seen among aggressive NHL patients (most of them DLBCL) in our study probably occurred due to the unavailability of rituximab in Brazilian public hospitals to treat B-cell lymphomas at the time of this study, and the difference in median OS between subgroups according to CTAs positivity likely relates to the known poorer prognosis of aggressive NHL, in whom expression of CTAs was largely confined.
Xie et al. [33] used a CTA panel different from ours. Anti-SCP-1 and commercially available anti-SSX antibodies were not included in our study because SCP-1 is a non-X CTA and both antigens were negative by RT-PCR in a previous study of our collaborating center (LICR—data not published). Among the CTAs studied in both studies (MAGE-A3, MAGE-A4 and CT7), we found lower expression of CT7 in DLBCL (2/56 vs. 2/28) and positive expression of MAGE-A family, which was reported as negative in all DLBCL cases by Xie et al. [33]. The expression of NY-ESO-1 was positive in 4/56 DLBCL of our cohort. Although tested in only 5/28 DLBCL cases, NY-ESO-1 was negative in all samples of Xie’s study. Among T-cell lymphomas, we found CTA expression in only 1/16 samples (a peripheral T-cell lymphoma case with positivity for MAGE-A1 and GAGE). Except for SCP-1, that was positive in 9/15 samples, Xie et al. [33] also demonstrated low positivity of other CTAs in T-cell lymphomas.
Even in a partially different cohort, we considered important to assess the serum response against CTAs in NHL because we had a larger panel available for ELISA assay, allowing us to explore the humoral response of NHL patients against not only the CTAs included in immunohistochemical analysis, but also against other CTAs described as highly expressed in other studies, like CT45 and CT63/PASD1.
Unfortunately, CT45 and CT63/PASD1 were not included in our TMA panel because serological reagents for immunohistochemistry were not available. Although CT63/PASD1 has been described as highly expressed in DLBCL patients by SEREX and T-cell cytotoxic response studies [34–36], in our study, CT63/PASD1 was only tested for serum reactivity in NHL patients, and it was negative in all DLBCL samples. The only CT63/PASD1-positive case was a null-cell phenotype anaplastic large cell lymphoma.
We did not find any plausible explanation to the low expression of CTAs in NHL compared to other malignancies. The findings of global low expression of CTAs in hematological malignancies are in agreement with most of studies in this field published to date. Interestingly, there is high expression of CT7 in multiple myeloma, CT45 in HL and CT63/PASD1 in DLBCL. However, as many new CTAs have been identified recently and some of them have found to be highly expressed in hematological malignancies including NHL (Ex. SP17), it is reasonable to think that other CTAs may be highly expressed in NHL. We should consider that NHL is a very heterogeneous group of tumors, and the CTA expression appears to be very heterogeneous within the various NHL subtypes. As our cohort did not include all subtypes of NHL and in many rare subtypes we analyzed only few samples, we are not able to make any general conclusion about CTA expression in NHL. This is an exploratory study, and further studies accessing the expression of different panels of CTAs in a higher cohort are needed to establish the CTA positivity in NHL patients.
Beside the identification of tumor-specific antigens, optimization of antigen delivery strategies is needed to improve the clinical response to anti-tumoral vaccines. Dendritic cells are considered attractive cancer vaccine platform due to their ability to present peptides derived from tumor-associated antigens on MHC class I, activate tumor-specific cytotoxic T-cells and stimulate the growth and differentiation of B-cells [50, 51]. We believe that an ideal situation for a CTA-based vaccine would be the use of a polyvalent vaccine containing the combination of more frequently expressed CTAs, with an optimized delivery system (DCs) and immunomodulatory agents in a patient treated with effective chemotherapy, presenting only minimal residual disease. Considering the potential benefit of immunotherapy, especially in situations with poor outcome like T-cell lymphoma, mantle cell lymphoma and refractory DLBCL, new studies accessing different panels of CTAs in NHL are needed to establish the role of these antigens as candidates for immunotherapy in NHL patients.
Acknowledgments
This work was partially supported by grants from CAPES (RJI) and CNPq (GWBC).
Conflict of interest
The authors do not have any financial or non-financial competing interests for publication of this manuscript.
Footnotes
Riguel J. Inaoka and Achim A. Jungbluth are contributed equally to this manuscript.
References
- 1.Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009;100(11):2014–2021. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida LG, Sakabe NJ, de Oliveira AR, et al. CTdatabase: a knowledge-base of high-throughput and curated data on cancer-testis antigens. Nucleic Acids Res. 2009;37:D816–D819. doi: 10.1093/nar/gkn673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 4.Tureci O, Sahin U, Zwick C, et al. Identification of a meiosis-specific protein as a member of the class of cancer/testis antigens. Proc Natl Acad Sci U S A. 1998;95:5211–5216. doi: 10.1073/pnas.95.9.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ono T, Kurashige T, Harada N, et al. Identification of proacrosin binding protein sp32 precursor as a human cancer/testis antigen. Proc Natl Acad Sci U S A. 2001;98:3282–3287. doi: 10.1073/pnas.041625098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romanienko PJ, Camerini-Otero RD. Cloning, characterization, and localization of mouse and human SPO11. Genomics. 1999;61:156–169. doi: 10.1006/geno.1999.5955. [DOI] [PubMed] [Google Scholar]
- 7.Loukinov DI, Pugacheva E, Vatolin S, et al. BORIS, a novel male germ-line-specific protein associated with epigenetic reprogramming events, shares the same 11-zinc-finger domain with CTCF, the insulator protein involved in reading imprinting marks in the soma. Proc Natl Acad Sci U S A. 2002;99:6806–6811. doi: 10.1073/pnas.092123699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atanackovic D, Hildebrandt Y, Jadczak A, et al. Cancer-testis antigens MAGE-C1/CT7 and MAGE-A3 promote the survival of multiple myeloma cells. Haematologica. 2010;95(5):785–793. doi: 10.3324/haematol.2009.014464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu X, Asa SL, Ezzat S. Fibroblast growth factor 2 and estrogen control the balance of histone 3 modifications targeting MAGE-A3 in pituitary neoplasia. Clin Cancer Res. 2008;14:1984–1996. doi: 10.1158/1078-0432.CCR-07-2003. [DOI] [PubMed] [Google Scholar]
- 10.Yang B, O’Herrin SM, Wu J, et al. MAGE-A, MAGE-B, and MAGE-C proteins form complexes with KAP1 and suppress p53-dependent apoptosis in MAGE-positive cell lines. Cancer Res. 2007;67:9954–9962. doi: 10.1158/0008-5472.CAN-07-1478. [DOI] [PubMed] [Google Scholar]
- 11.Peikert T, Specks U, Farver C, et al. Melanoma antigen A4 is expressed in non-small cell lung cancers and promotes apoptosis. Cancer Res. 2006;66:4693–4700. doi: 10.1158/0008-5472.CAN-05-3327. [DOI] [PubMed] [Google Scholar]
- 12.Monte M, Simonatto M, Peche LY, et al. MAGE-A tumor antigens target p53 transactivation function through histone deacetylase recruitment and confer resistance to chemotherapeutic agents. Proc Natl Acad Sci U S A. 2006;103:11160–11165. doi: 10.1073/pnas.0510834103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bai S, He B, Wilson EM. Melanoma antigen gene protein MAGE-11 regulates androgen receptor function by modulating the interdomain interaction. Mol Cell Biol. 2005;25:1238–1257. doi: 10.1128/MCB.25.4.1238-1257.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagao T, Higashitsuji H, Nonoguchi K, et al. MAGE-A4 interacts with the liver oncoprotein gankyrin and suppresses its tumorigenic activity. J Biol Chem. 2003;278:10668–10674. doi: 10.1074/jbc.M206104200. [DOI] [PubMed] [Google Scholar]
- 15.Cilensek ZM, Yehiely F, Kular RK, et al. A member of the GAGE family of tumor antigens is an anti-apoptotic gene that confers resistance to Fas/CD95/APO-1, interferon-gamma, taxol and gamma-irradiation. Cancer Biol Ther. 2002;1:380–387. doi: 10.4161/cbt.1.4.12. [DOI] [PubMed] [Google Scholar]
- 16.Oi S, Natsume A, Ito M, et al. Synergistic induction of NY-ESO-1 antigen expression by a novel histone deacetylase inhibitor, valproic acid, with 5-aza-2′-deoxycytidine in glioma cells. J Neurooncol. 2009;92:15–22. doi: 10.1007/s11060-008-9732-0. [DOI] [PubMed] [Google Scholar]
- 17.Sigalotti L, Fratta E, Coral S, et al. Intratumor heterogeneity of cancer/testis antigens expression in human cutaneous melanoma is methylation-regulated and functionally reverted by 5-aza-2′-deoxycytidine. Cancer Res. 2004;64:9167–9171. doi: 10.1158/0008-5472.CAN-04-1442. [DOI] [PubMed] [Google Scholar]
- 18.De Smet C, Lurquin C, Lethe B, et al. DNA methylation is the primary silencing mechanism for a set of germ line- and tumor-specific genes with a CpG-rich promoter. Mol Cell Biol. 1999;19:7327–7335. doi: 10.1128/mcb.19.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrade VC, Vettore AL, Felix RS, et al. Prognostic impact of cancer/testis antigen expression in advanced stage multiple myeloma patients. Cancer Immun. 2008;8:2. [PMC free article] [PubMed] [Google Scholar]
- 20.Jungbluth AA, Ely S, DiLiberto M, et al. The cancer-testis antigens CT7 (MAGE-C1) and MAGE-A3/6 are commonly expressed in multiple myeloma and correlate with plasma-cell proliferation. Blood. 2005;106:167–174. doi: 10.1182/blood-2004-12-4931. [DOI] [PubMed] [Google Scholar]
- 21.Chen YT, Chadburn A, Lee P, et al. Expression of cancer testis antigen CT45 in classical Hodgkin lymphoma and other B-cell lymphomas. Proc Natl Acad Sci U S A. 2010;107(7):3093–3098. doi: 10.1073/pnas.0915050107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heidebrecht HJ, Claviez A, Kruse ML, et al. Characterization and expression of CT45 in Hodgkin’s lymphoma. Clin Cancer Res. 2006;12:4804–4811. doi: 10.1158/1078-0432.CCR-06-0186. [DOI] [PubMed] [Google Scholar]
- 23.Velazquez EF, Jungbluth AA, Yancovitz M, et al. Expression of the cancer/testis antigen NY-ESO-1 in primary and metastatic malignant melanoma (MM)—correlation with prognostic factors. Cancer Immun. 2007;7:11. [PMC free article] [PubMed] [Google Scholar]
- 24.Goydos JS, Patel M, Shih W. NY-ESO-1 and CTp11 expression may correlate with stage of progression in melanoma. J Surg Res. 2001;98:76–80. doi: 10.1006/jsre.2001.6148. [DOI] [PubMed] [Google Scholar]
- 25.Suyama T, Shiraishi T, Zeng Y, et al. Expression of cancer/testis antigens in prostate cancer is associated with disease progression. Prostate. 2010;70(16):1778–1787. doi: 10.1002/pros.21214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riener MO, Wild PJ, Soll C, et al. Frequent expression of the novel cancer testis antigen MAGE-C2/CT-10 in hepatocellular carcinoma. Int J Cancer. 2009;124:352–357. doi: 10.1002/ijc.23966. [DOI] [PubMed] [Google Scholar]
- 27.Atanackovic D, Luetkens T, Hildebrandt Y, et al. Longitudinal analysis and prognostic effect of cancer-testis antigen expression in multiple myeloma. Clin Cancer Res. 2009;15:1343–1352. doi: 10.1158/1078-0432.CCR-08-0989. [DOI] [PubMed] [Google Scholar]
- 28.Perez D, Herrmann T, Jungbluth AA, et al. Cancer testis antigen expression in gastrointestinal stromal tumors: new markers for early recurrence. Int J Cancer. 2008;123(7):1551–1555. doi: 10.1002/ijc.23698. [DOI] [PubMed] [Google Scholar]
- 29.Bellati F, Napoletano C, Tarquini E, et al. Cancer testis antigen expression in primary and recurrent vulvar cancer: association with prognostic factors. Eur J Cancer. 2007;43(17):2621–2627. doi: 10.1016/j.ejca.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Reber HA, Hines OJ, et al. The clinical significance of MAGEA3 expression in pancreatic cancer. Int J Cancer. 2006;118:2269–2275. doi: 10.1002/ijc.21656. [DOI] [PubMed] [Google Scholar]
- 31.Yakirevich E, Sabo E, Lavie O, et al. Expression of the MAGE-A4 and NY-ESO-1 cancer-testis antigens in serous ovarian neoplasms. Clin Cancer Res. 2003;9:6453–6460. [PubMed] [Google Scholar]
- 32.Duan Z, Duan Y, Lamendola DE, et al. Overexpression of MAGE/GAGE genes in paclitaxel/doxorubicin-resistant human cancer cell lines. Clin Cancer Res. 2003;9:2778–2785. [PubMed] [Google Scholar]
- 33.Xie X, Wacker HH, Huang S, et al. Differential expression of cancer testis genes in histological subtypes of non-Hodgkin’s lymphomas. Clin Cancer Res. 2003;9(1):167–173. [PubMed] [Google Scholar]
- 34.Ait-Tahar K, Liggins AP, Collins GP, et al. Cytolytic T-cell response to the PASD1 cancer testis antigen in patients with diffuse large B-cell lymphoma. Br J Haematol. 2009;146(4):396–407. doi: 10.1111/j.1365-2141.2009.07761.x. [DOI] [PubMed] [Google Scholar]
- 35.Cooper CD, Liggins AP, Ait-Tahar K, et al. PASD1, a DLBCL-associated cancer testis antigen and candidate for lymphoma immunotherapy. Leukemia. 2006;20(12):2172–2174. doi: 10.1038/sj.leu.2404424. [DOI] [PubMed] [Google Scholar]
- 36.Liggins AP, Brown PJ, Asker K, et al. A novel diffuse large B-cell lymphoma-associated cancer testis antigen encoding a PAS domain protein. Br J Cancer. 2004;91(1):141–149. doi: 10.1038/sj.bjc.6601875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.NCCN (2007) National Comprehensive Cancer Network. The complete library of practice guidelines in oncology (online). Version 2007. Available from. URL:http://www.nccn.org
- 38.Jaffe E, Harris NL, Stein H, et al. World health organization classification of tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2008. [Google Scholar]
- 39.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 40.Hedvat CV, Hegde A, Chaganti RS, et al. Application of tissue microarray technology to the study of non-Hodgkin’s and Hodgkin’s lymphoma. Hum Pathol. 2002;33:968–974. doi: 10.1053/hupa.2002.127438. [DOI] [PubMed] [Google Scholar]
- 41.Chen YT, Stockert E, Chen Y, et al. Identification of the MAGE-1 gene product by monoclonal and polyclonal antibodies. Proc Natl Acad Sci U S A. 1994;91(3):1004–1008. doi: 10.1073/pnas.91.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jungbluth AA, Stockert E, Chen YT, et al. Monoclonal antibody MA454 reveals a heterogeneous expression pattern of MAGE-1 antigen in formalin-fixed paraffin embedded lung tumours. Br J Cancer. 2000;83(4):493–497. doi: 10.1054/bjoc.2000.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dhodapkar MV, Osman K, Teruya-Feldstein J, et al. Expression of cancer/testis (CT) antigens MAGE-A1, MAGE-A3, MAGE-A4, CT-7, and NY-ESO-1 in malignant gammopathies is heterogeneous and correlates with site, stage and risk status of disease. Cancer Immun. 2003;23(3):9. [PubMed] [Google Scholar]
- 44.Nelson PT, Zhang PJ, Spagnoli GC, et al. Cancer/testis (CT) antigens are expressed in fetal ovary. Cancer Immun. 2007;12(7):1. [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma P, Shen Y, Wen S, et al. Cancer-testis antigens: expression and correlation with survival in human urothelial carcinoma. Clin Cancer Res. 2006;12(18):5442–5447. doi: 10.1158/1078-0432.CCR-06-0527. [DOI] [PubMed] [Google Scholar]
- 46.Oba-Shinjo SM, Caballero OL, Jungbluth AA, et al. Cancer-testis (CT) antigen expression in medulloblastoma. Cancer Immun. 2008;8:7. [PMC free article] [PubMed] [Google Scholar]
- 47.Zhuang R, Zhu Y, Fang L, et al. Generation of monoclonal antibodies to cancer/testis (CT) antigen CT10/MAGE-C2. Cancer Immun. 2006;6:7. [PubMed] [Google Scholar]
- 48.Vaughan HA, Svobodova S, Macgregor D, et al. Immunohistochemical and molecular analysis of human melanomas for expression of the human cancer-testis antigens NY-ESO-1 and LAGE-1. Clin Cancer Res. 2004;10(24):8396–8404. doi: 10.1158/1078-0432.CCR-04-0809. [DOI] [PubMed] [Google Scholar]
- 49.Gnjatic S, Old LJ, Chen YT. Autoantibodies against cancer antigens. Methods Mol Biol. 2009;520:11–19. doi: 10.1007/978-1-60327-811-9_2. [DOI] [PubMed] [Google Scholar]
- 50.Geldmacher A, Freier A, Losch FO, et al. Therapeutic vaccination for cancer immunotherapy: antigen selection and clinical responses. Hum Vaccin. 2011;7:115–119. doi: 10.4161/hv.7.0.14573. [DOI] [PubMed] [Google Scholar]
- 51.Palucka K, Ueno H, Fay J, et al. Dendritic cells and immunity against cancer. Int J Med. 2011;269(1):64–73. doi: 10.1111/j.1365-2796.2010.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]