Abstract
The management of acute lymphoblastic leukemia (ALL) patients has witnessed profound changes in recent years. Nonetheless, most patients tend to relapse, underlining the need for new therapeutic approaches. The anti-leukemic potential of natural killer (NK) cells has over the years raised considerable interest. In this study, we developed an efficient method for the expansion and activation of NK cells isolated from healthy donors and ALL patients for clinical use. NK cell products were derived from peripheral blood mononuclear cells of 35 healthy donors and 4 B-lineage ALL by immunomagnetic CD3 T cell depletion followed by CD56 cell enrichment. Isolated NK cells were expanded and stimulated in serum-free medium supplemented with irradiated autologous feeder cells and autologous plasma in the presence of clinical grade interleukin (IL)-2 and IL-15 for 14 days. Healthy donor NK cells expanded on average 34.9 ± 10.4 fold and were represented, after expansion, by a highly pure population of CD3−CD56+ cells showing a significant upregulation of natural cytotoxicity receptors, activating receptors and maturation markers. These expanded effectors showed cytolytic activity against K562 cells and, most importantly, against primary adult B-lineage ALL blasts. NK cells could be efficiently isolated and expanded—on average 39.5 ± 20.3 fold—also from primary B-lineage ALL samples of patients in complete remission. The expanded NK cells from these patients showed a significantly increased expression of the NKG2D- and DNAM1-activating receptors and were cytotoxic against K562 cells. These data provide the basis for developing new immunotherapeutic strategies for the management of ALL patients.
Keywords: NK, Expansion, ALL, Immunotherapy
Introduction
Natural killer (NK) cells are a subset of peripheral blood lymphocytes immunophenotypically characterized by the expression of the CD56 surface antigen and lack of the CD3 and T cell receptor proteins [1]. They can recognize and kill a variety of virus-infected or tumor-transformed cells, without prior sensitization of their targets, in a human leukocyte antigen (HLA)-unrestricted fashion [2].
NK cells express killer-cell immunoglobulin-like receptors (KIRs), which display either inhibitory (KIR2DL and KIR3DL) or activating (KIR2DS and KIR3DS) functions, through the recognition of allotypic determinants of HLA class I molecules [3]. Beside KIRs, activating receptors responsible for NK cell tumor recognition include the natural cytotoxicity receptors (NCRs: NKp30, NKp44 and NKp46) [4], NKG2D [5, 6] and DNAM1 [7]. It is the selective engagement of activating receptors, in the absence of efficient inhibitory interactions, that renders infected or transformed target cells susceptible to NK cell-mediated killing while sparing normal autologous cells [8, 9].
Progress in our understanding of the mechanisms of receptor-dependent NK cell activity has promoted great interest in the potential value of these cells in the setting of adoptive immunotherapy of cancer [10], including hematologic malignancies [11]. Earlier studies have shown that leukemic blasts may be susceptible to the lytic action of lymphokine-activated killer (LAK) cells [12–14]. More recently, preclinical and clinical data from the haploidentical T cell depleted transplantation field have shown that haploidentical KIR ligand-mismatched NK cells play a primary role as anti-leukemia effector cells in patients with acute myeloid leukemia (AML) [15–17].
The role of NK cells in immunosorveillance of acute lymphoblastic leukemia (ALL) has not been extensively examined and is still controversial. ALL has been previously considered to be less sensitive to NK cell lysis than AML cells [18]. Recent studies addressing the graft-versus-leukemia potential of donor-versus-recipient NK cell alloreactivity report that beneficial effects are more evident for pediatric than adult ALL patients [19]. However, in vitro studies seem to support a promising role for adoptive cellular therapies in the treatment of ALL patients. Jardine et al. [20] recently demonstrated that bortezomib, valproate and troglitazone can upregulate NK-activating ligands on a proportion of adult B-lineage ALL samples, making leukemic cells susceptible to NK-mediated lysis through the NK-activating receptor NKG2D. Furthermore, our group has previously reported the possibility of expanding, in co-culture with an irradiated Epstein–Barr virus (EBV)-positive lymphoblastoid B cell line as feeder, cytotoxic NK cells with killing activity against allogeneic as well as autologous blasts from both adult and pediatric ALL patients in complete remission (CR) [21].
Until recently, clinical protocols have been mainly based on the use of LAK cells or of freshly isolated or short-term activated NK cells [22, 23]. One of the major challenges toward a broader utilization of these cells as an immunotherapy tool in cancer is represented by the need of producing large numbers of functional effectors with anticancer potential. Several protocols of ex vivo NK cell expansion and activation have been investigated, including long-term culture with cytokines (IL-2, IL-4, IL-7 and IL-12) [24, 25] and the use of feeder cells such as peripheral blood mononuclear cells (PBMCs) [26], the K562 [27] or EBV-transformed lymphoblastoid cell lines [28], monocytes [29] or umbilical cord mesenchymal cells [30]. Alici et al. [31] reported the possibility of expanding NK cells from multiple myeloma patients after co-culture of PBMCs with IL-2 in combination with an anti-CD3 antibody; more recently, a 100-fold expansion of NK cells with a purity of nearly 90 % was reported by co-cultivating PBMCs with the Jurkat T cell subline KL-1 [32]. Despite the excellent NK expansion fold, most of these approaches are valuable and cost-effective for the autologous setting, as CD3+ T contaminating lymphocytes could pose a risk of graft-versus-host disease (GVHD) in the allogeneic context [33]. In addition, the use of allogeneic feeder cell sources may have different effects on the NK cell product for clinical application, which need to be further investigated [34].
On these premises, the aims of this study were as follows: (1) to define a new method for an efficient ex vivo expansion and activation of highly pure NK cells from healthy donors and ALL patients using good manufactory practice (GMP)-compliant components and (2) to investigate if these ex vivo expanded and activated NK cells could target primary ALL blasts, in order to suggest a possible new immunotherapeutic strategy for the management of ALL patients.
Materials and methods
Cells and culture media
The K562 (human erythroblastoid cell line; ATCC) cell line was cultured in RPMI-1640 medium supplemented with 10 % heat-inactivated fetal bovine serum (FBS, HyClone, South Logan, UT), 1 % l-glutamine and 1 % Pen-strepto (Euro-Clone, Italy) at 37 °C in 5 % CO2. Human PBMCs were isolated from 35 healthy donors and 4 adult patients with B-lineage ALL in CR, diagnosed at our center. Primary leukemic cells, used as targets in cytotoxicity experiments, were collected from 11 adult patients with B-lineage ALL, managed at our center between December 2011 and February 2013. Informed consent for blood collection and biologic studies was obtained from patients and donors in accordance with the Declaration of Helsinki. The study was approved by the local Ethics Committee.
NK cell enrichment
Peripheral blood mononuclear cells (PBMCs) from healthy donors and B-ALL patients were isolated over Ficoll-Histopaque (Axis-Shield, Oslo, Norway). For NK cell enrichment, a two-step immunomagnetic procedure was used, consisting of a CD3+ T cell depletion followed by a CD56+ cell positive selection on an autoMACS separator (Miltenyi Biotec, Bergisch Gladbach, Germany). The starting material was represented by a mean of 156.3 ± 49.2 × 106 PBMCs for healthy donors and 56.3 ± 25.6 × 106 PBMCs for ALL patients. Isolated cells were analyzed immediately after purification for phenotypic markers and then expanded.
NK cell expansion
For ex vivo cell expansion, isolated NK cells (1 × 105/mL) were suspended in Cellgro SCGM serum-free medium (CellGenix, Freiburg, Germany) supplemented with 5 % autologous plasma, 500 U/mL IL-2 (Aldesleukin, Proleukin, Chiron, Amsterdam, The Netherlands) and 50 ng/mL IL-15 (Cellgro, CellGenix,) in the presence of irradiated (35 Gy) autologous monocytes, T and B cells as feeder (2.5 × 105/mL) and cultured for 14 days at 37 °C. IL-2 and IL-15 were also added to the culture medium during the last 24 h of the expansion period. Only clinical grade materials were used.
Total cell numbers were assessed by staining cells with Trypan blue dye (Sigma, St. Louis, MO) on day 14. The fold expansion was calculated by dividing the absolute number of viable NK cells present at the end of the culture (day 14) by the absolute number of viable NK cells at the beginning of the culture (day 0).
The final product was directly evaluated for purity, viability, phenotype and cytotoxic activity or cryopreserved in human serum type AB supplemented with 5 % DMSO (Sigma) at 20 − 50 × 106 cells/mL per vial. For functional tests on cryopreserved NK cells, expanded cells were thawed at 37 °C and used after overnight culture.
Immunofluorescence and flow cytometry
Freshly isolated or expanded NK cells were analyzed by immunofluorescence using different combinations of the following monoclonal antibodies (mAbs): FITC-conjugated anti-CD16, anti-CD57, anti-CD14, anti-CD25; PE-conjugated anti-CD56; PercP-conjugated anti-CD19, anti-CD69; APC-conjugated anti-CD3, anti-Nkp30, anti-Nkp44, anti-Nkp46, anti-NKG2C; PeCy7-conjugated anti-CD45 and NKG2A (all from BD Biosciences, San Jose, CA) and FITC-conjugated anti-CD158a, anti-CD158e (R&D System, Minneapolis, MN). The expression of NKG2D and DNAM1 activatory receptor on NK cells was evaluated using anti-NKG2D (R&D System) or anti-DNAM1 (AbD Serotec, Oxford, UK) unconjugated mAbs, followed by secondary FITC-conjugated IgG1 (BioLegend, San Diego, CA) staining. NK cells were defined as CD3−CD56+ cells within the lymphocyte gate and the expression of NK cell receptors was referred to this population.
Flow cytometry was carried out with a FACSCantoI flow cytometer using PAINT-A-GATE and the data were analyzed using the FACSDiva software (BD Bioscience, San Jose, CA). Cell surface expression was quantified as the mean fluorescence intensity (MFI) of values obtained with specific mAbs compared with values given by isotype controls.
Cytotoxicity assays
Cytotoxic activity of expanded NK cells against the K562 cell line and against primary adult ALL blasts cryopreserved at diagnosis, was determined in a standard 4-h 51Chromium (51Cr) release assay. Thawed ALL cells were maintained for 1 day in RPMI-1640 medium supplemented with 10 % heat-inactivated FBS (HyClone), 1 % l-glutamine and 1 % Pen-strepto (Euro-Clone) prior to the assay. The NK effector:target (E:T) cell ratio ranged from 50:1 to 0.39:1, using 2 × 103 target cells in triplicate wells. After incubation at 37 °C, plates were centrifuged, 25 μL of culture supernatant were transferred into Luma plates (Perkin Elmer) and counted in a γ-counter (Top Count, Perkin Elmer, Ontario, Canada). The percentage of specific lysis was calculated as follows: 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release).
Statistical analysis
Statistical analyses were performed using Student’s paired t test. Statistical significance was set at p < .05.
Results
Isolation and expansion of highly pure NK cells from healthy donors
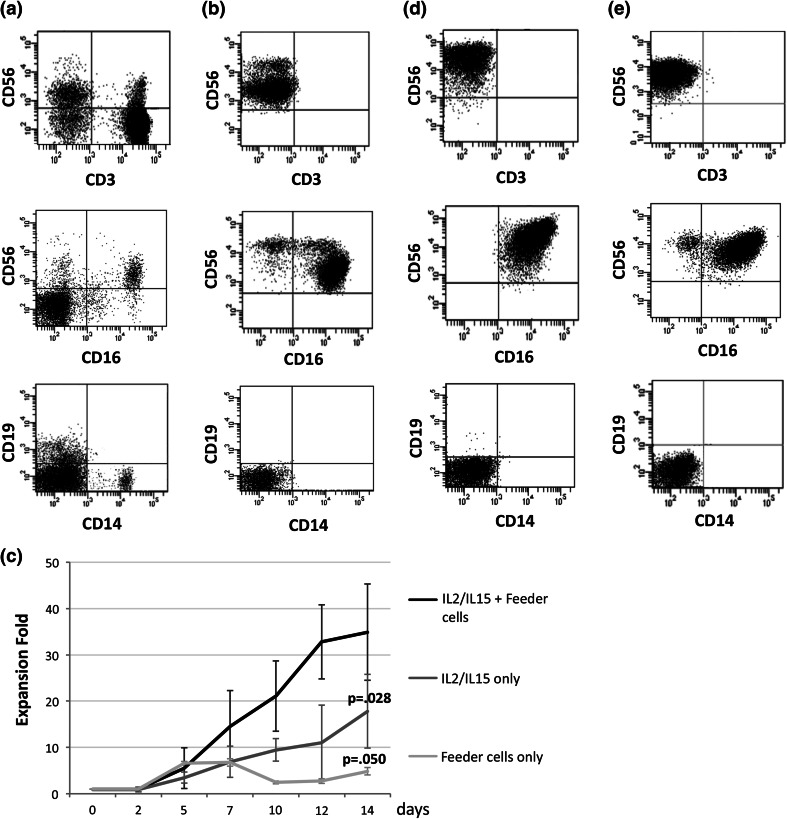
Starting from a mean percentage of NK cells of 9.3 ± 4.4 % (range 2.5–20.2 %) in healthy donor PBMCs, at the end of the isolation process, we obtained an average of 98.0 ± 1.8 % CD56+ cells (range 93.1–99.9 %)—corresponding to a mean of 4.3 × 106 ± 2.5 × 106 NK cells—with a mean yield of 33.2 ± 19.5 %. Upon selection, this cell population was mainly composed of CD56dimCD16+ cells (80.4 ± 12.8 %) with a lower percentage of CD56brightCD16− cells (13.3 ± 10.2 %), thus recapitulating the proportion of the two NK cell subpopulations normally present in human peripheral blood. The mean percentage of CD3+ cells was 0.6 ± 1.4 %; of note, most of these cells were CD56+CD3+ NK-T lymphocytes (0.5 ± 1.3 %; range 0.0 ± 1.4 %), while the CD56−CD3+ contaminating T lymphocytes were 0.1 ± 0.2 % (range 0.0–0.6 %). A minimal contamination by CD19+ B cells, as well as by CD14+ monocytes, was also observed (0.5 ± 0.7 and 0.7 ± 0.2 %, respectively). The phenotype of healthy donor PBMCs and of purified NK cells is summarized in Fig. 1a, b.
Fig. 1.
Cell phenotype and expansion of purified NK cells. Representative data of the immunophenotypic analysis of cell-lineage-specific markers on healthy donor NK cells a before and b after the two-step immunomagnetic selection. c Comparison of the mean expansion fold of NK cells from healthy donor PBMCs cultured for 14 days in the presence of IL-2, IL-15 and irradiated autologous feeder cells, alone or in combination. Fold expansion was calculated at the indicated time points by dividing the absolute number of viable NK cells at day 14 by the absolute number of viable NK cells at day 0 and is expressed as mean ± SD. d Immunophenotypic analysis of cell-lineage-specific markers performed on expanded NK cells from one donor (n = 22) showing only CD56brightCD16+ NK cells. e Immunophenotypic analysis of cell-lineage-specific markers performed on expanded NK cells from one donor (n = 24) showing both CD56brightCD16+ and CD56brightCD16dim subpopulations
Following purification, NK cells underwent an in vitro expansion for 14 days. After an initial 5 day long low proliferative phase, the total NK cell population expanded on average 34.9 ± 10.4 fold (range 11.3- to 64.0-fold) by day 14 with a 96.7 ± 4.1 % viability. Collectively, from 0.6 × 106 purified cells, we recovered a mean of 24.4 × 106 ± 9.1 × 106 CD56+ cells per flask. Neither the presence of feeder cells alone nor that of cytokines alone in the culture medium was associated to the same expansion fold (mean expansion fold at day 14: 4.9 ± 0.1, p = .050 and 18.0 ± 4.1, p = .028, respectively) (Fig. 1c).
Immunophenotypic characterization of expanded NK cells from healthy donors
The phenotypic analysis performed on healthy donor expanded cells revealed that CD56+ NK cells were predominant in the culture even at the end of the incubation period, reaching a mean percentage of 99.0 ± 0.9 %. Furthermore, no proliferation of the feeder cell population was observed, as shown by the decrease of both CD56+CD3+ NK-T (0.2 ± 0.3 %) and CD56−CD3+ T (0.01 ± 0.04 %) lymphocytes and the complete disappearance of B lymphocytes and monocytes.
In 10/35 cases (28.6 %), expanded NK cells were characterized by a homogeneous CD56brightCD16+ population (one representative case is shown in Fig. 1d), while in the remaining cases (25/35; 71.4 %), although CD56brightCD16+ NK cells represented the predominant population (80.6 ± 9.4 %), a mean percentage of 14.3 ± 10.6 % CD56brightCD16dim NK cells was present (one representative case is shown in Fig. 1e). Interestingly, when compared to the starting population, expanded NK cells displayed a significantly increased intensity of expression of the CD56 antigen. In particular, during the culture period, CD56 MFI increased from 10,968.7 ± 5,148.3 to 43,496.5 ± 23,122.1 (p < .0001) on CD56brightCD16+ cells and from 2,739.1 ± 1,654.5 to 39,340.4 ± 19,746.8 (p < .0001) on CD56brightCD16dim cells.
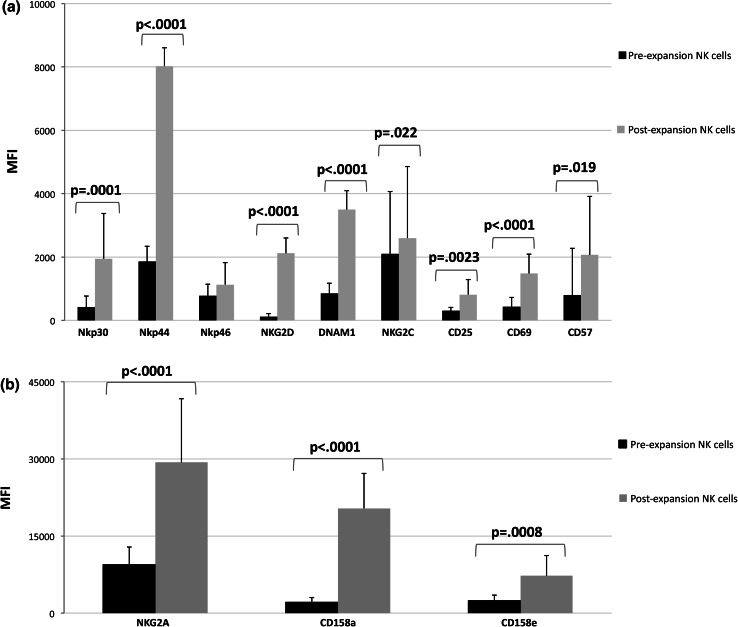
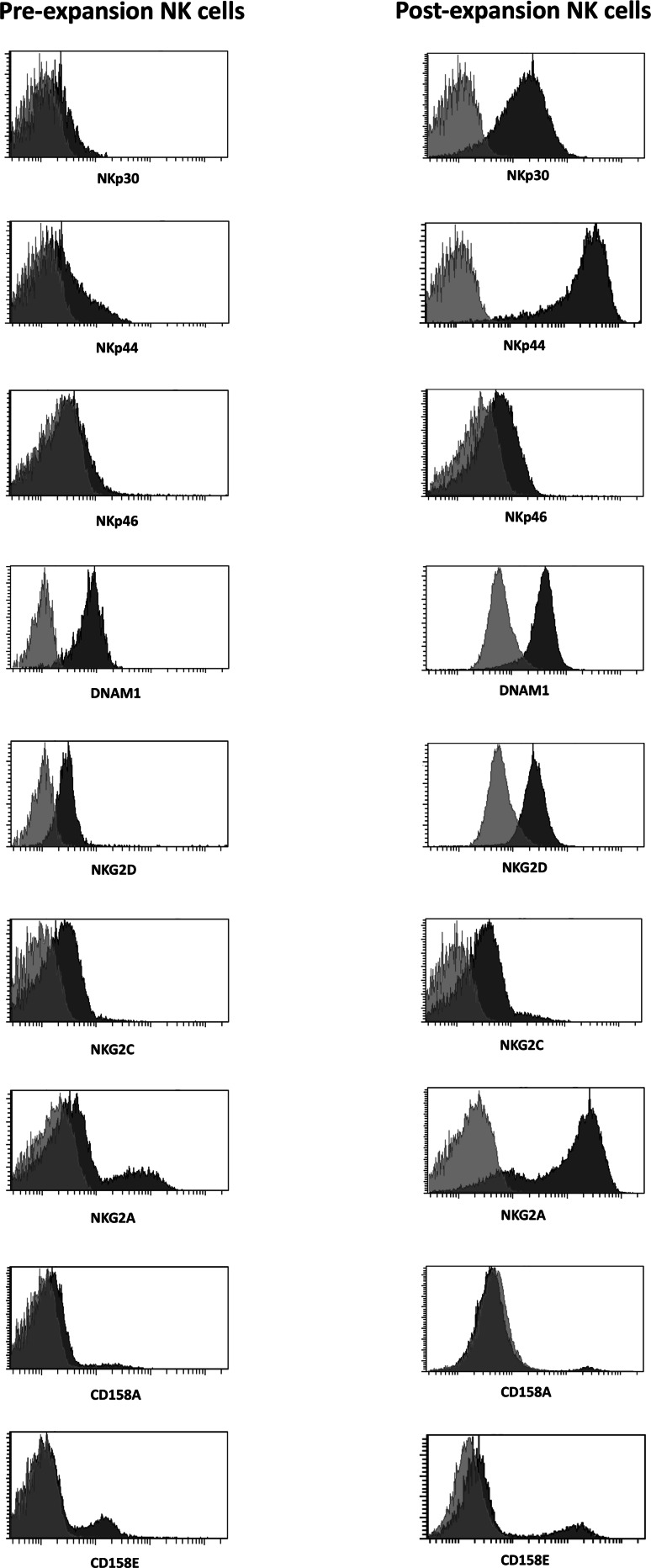
The cell surface phenotype of NK cells was then examined to evaluate the expression of multiple activating or inhibitory receptors as well as maturation markers, before and after expansion. As shown in Fig. 2a, among the activating receptors and maturation markers tested, expanded NK cells showed a significant upregulation of Nkp30 (p = .0001), Nkp44 (p < .0001), NKG2D (p < .0001), DNAM1 (p = <.0001), NKG2C (p = .022), CD25 (p = .0023), CD69 (p < .0001) and CD57 (p = .019), while no substantial changes were observed in the expression levels of Nkp46. The expression of the inhibitory receptor NKG2A and of the inhibitory KIRs CD158a and CD158e was also significantly increased on expanded NK cells (p < .0001; p < .0001 and p = .0008, respectively) (Fig. 2b). Representative histograms of isotypic control versus antibody staining for the receptors tested, showing the comparison of freshly isolated and expanded NK cells, are reported in Fig. 3. Together with an increased expression intensity, a significant increase in the percentage of NK cells expressing Nkp30 (p < .001), NKp44 (p < .001), NKp46 (p = .0006), NKG2C (p = .003), CD25 (p < .001), CD69 (p < .001), CD57 (p < .001) and NKG2A (p < .001) was also observed after expansion (Table 1). Of note, the expression patterns were not affected by cryopreservation and subsequent thawing of the expanded NK cells (data not shown).
Fig. 2.
Changes in the receptor expression patterns of NK cells following expansion. The surface expression levels of activating receptors and maturation markers (a) and of inhibitory receptors (b) on NK cells from healthy donors before and after expansion were assessed by multicolor flow cytometry. For each receptor, analysis was performed on the receptor-positive subsets within the total NK cell population. Results are expressed as mean MFI ± SD
Fig. 3.
Representative histograms of negative isotypic control (light gray) versus antibody (dark gray) staining of freshly isolated versus expanded NK cells from one donor (n = 19) are shown
Table 1.
Percent of NK cells expressing activating and inhibitory receptors, as well as maturation markers, before and after expansion
| Before expansion (mean % SD) | After expansion (mean % SD) | p value | |
|---|---|---|---|
| Nkp30 | 49.2 ± 20.9 | 93.7 ± 4.7 | p < .001 |
| Nkp44 | 13.6 ± 3.4 | 94.9 ± 4.2 | p < .001 |
| Nkp46 | 28.8 ± 4.8 | 50.8 ± 13.0 | p = .0006 |
| NKG2D | 98.7 ± 1.4 | 99.3 ± 1.3 | n.s.* |
| DNAM1 | 99.7 ± 1.3 | 99.4 ± 1.1 | n.s.* |
| NKG2C | 23.5 ± 20.9 | 54.0 ± 32.4 | p = .003 |
| CD25 | 7.9 ± 5.6 | 97.7 ± 2.2 | p < .001 |
| CD69 | 22.3 ± 16.4 | 61.5 ± 20.7 | p < .001 |
| CD57 | 22.1 ± 11.7 | 89.9 ± 18.9 | p < .001 |
| NKG2A | 35.5 ± 23.7 | 78.7 ± 23.1 | p < .001 |
| CD158a | 14.3 ± 13.8 | 15.9 ± 16.3 | n.s.* |
| CD158e | 12.1 ± 9.4 | 10.1 ± 7.8 | n.s.* |
* n.s. not significant
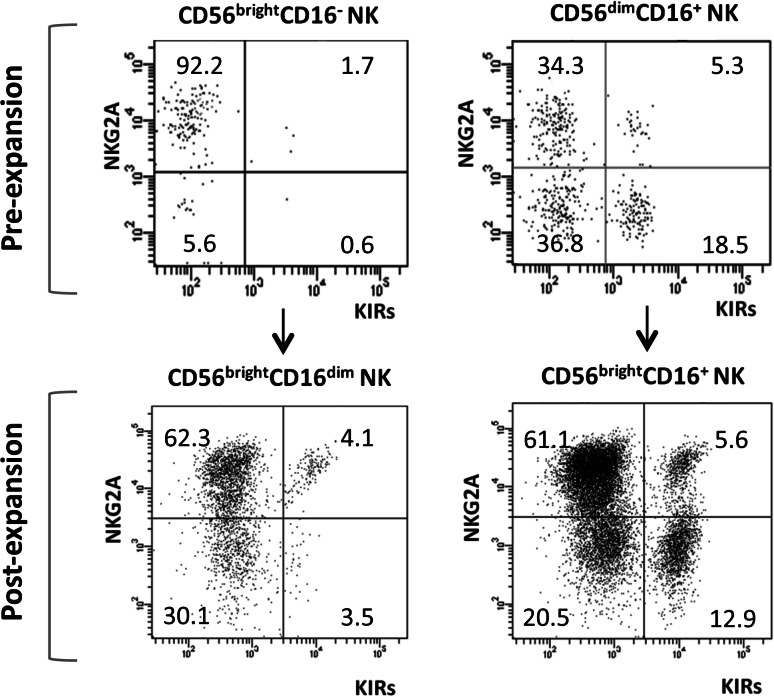
Finally, in order to investigate NK cell differentiation, a multiparametric analysis of NKG2A and inhibitory KIR (CD158a and CD158e) expression was performed on freshly isolated and expanded NK cells from 4 healthy donors. This analysis confirmed the presence of a significantly higher percentage of NKG2A+KIR− cells (92.4 ± 5.1 vs. 36.6 ± 5.6 %, p < .0001) among the CD56brigh compared to the CD56dim freshly isolated NK cell subpopulation, together with a significantly lower percentage of both NKG2A+KIR+ (0.6 ± 0.4 vs. 4.5 ± 3.4 %, p = .017) and NKG2A−KIR+ (0.4 ± 0.4 vs. 15.8 ± 5.6 %, p = .0015) cells in the same subpopulation.
When the same analysis was performed on expanded cells, CD56brightCD16dim NK cells displayed an increase of both NKG2A+KIR+ (from 0.6 ± 0.4 to 3.7 ± 2.2 %) and NKG2A−KIR+ (from 0.4 ± 0.4 to 3.2 ± 3.7 %) cells associated with a significant decrease of NKG2A+KIR− cells (from 92.4 ± 5.1 to 68.5 ± 4.6 %, p = .001), reaching percentages comparable to those observed in CD56brightCD16+ NK cells. Furthermore, a significant increase of NKG2A+KIR− cells (p < .001) followed by a significant decrease of NKG2A−KIR− cells (p = .026) was recorded in the more mature CD56brightCD16+ NK subpopulation. A representative analysis of NKG2A and KIR expression on NK cell subsets before and after expansion is shown in Fig. 4.
Fig. 4.
Comparison of NKG2A and inhibitory KIR (CD158a and CD158e) expression on NK cell subsets before and after expansion. The percentages of NKG2A+KIRs−, NKG2A+KIRs+, NKG2A−KIRs+ and NKG2A−KIRs− NK cells were analyzed by flow cytometric analyses. Representative FACS dot plots from one donor (n = 35) are presented
Cytolytic activity of expanded NK cells from healthy donors against cancer cell lines and adult ALL blasts
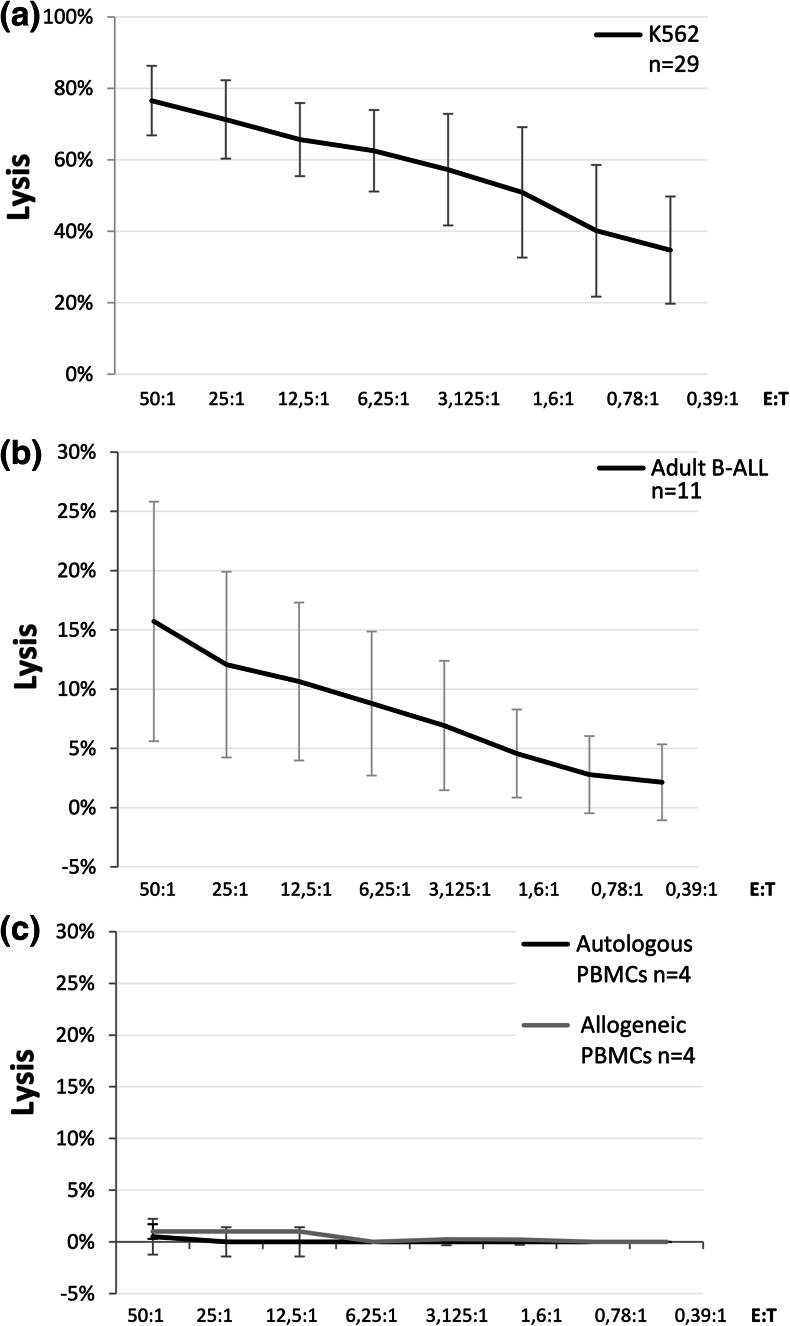
Expanded NK cells from healthy donors mediated an efficient lysis of K562 cell line, as measured by in vitro 51Cr release assay, with a mean percentage of killing at a 50:1 effector to target cell ratio of 75.6 ± 9.7 % (Fig. 5a). Interestingly, these allogeneic effectors exerted a cytotoxic activity also against primary ALL blasts cryopreserved at diagnosis. In fact, among the 11 adult B-ALL samples analyzed, we found a mean cytotoxicity of 16.0 ± 10.1 % at a 50:1 E:T ratio (Fig. 5b). No significant differences in the cytotoxic capacity were observed between donors showing only CD56brightCD16+ NK cells and donors showing both CD56brightCD16+ and CD56brightCD16dim NK cells (mean cytotoxicity at 50:1 E:T ratio against K562 74.6 ± 13.2 versus 69.2 ± 11.0 % and against B-ALL blasts 18.3 ± 11.9 versus 13.1 ± 10.2 %, respectively).
Fig. 5.
Cytolytic activity of expanded NK cells against tumor and normal cells. Cytotoxicity of expanded and activated alloreactive NK cells against the K562 cell line (a), against adult B-ALL primary blasts (b) and against normal autologous and allogeneic PBMCs (c), as measured by the standard 51Cr release assay. Data are expressed as mean percentage of lysis ± SD
Finally, to evaluate the cytolytic potential against normal cells, cytotoxic assays were performed after co-culturing expanded NK cells with autologous as well as allogeneic PBMCs from healthy donors. As illustrated in Fig. 5c, the cytotoxicity against allogeneic PBMCs was irrelevant, being 1.0 ± 1.7 % at a 50:1 E:T ratio, and resulted comparable to the cytotoxicity obtained against autologous PBMCs (mean cytotoxicity at a 50:1 E:T ratio 0.5 ± 0.7 %).
In addition, no significant differences were observed in the cytolytic activity of fresh versus cryopreserved expanded NK cells against the cancer cell line K562 (n = 5; mean cytotoxicity at 50:1 E:T ratio 78.1 ± 4.3 versus 77.4 ± 9.7 %, respectively).
Isolation and expansion of NK cells from ALL patients
To assess the possibility of isolating and expanding NK cells for clinical use in the autologous setting, we collected PBMCs from four patients with B-lineage ALL in CR. NK cells could be efficiently isolated from these patients, reaching a mean yield of 42.1 ± 10.9 % (range 29.6–53 %),—corresponding to a mean of 5.0 × 106 ± 4.6 × 106 NK cells—being this result comparable to that observed with healthy donors. Exposure of selected cells to IL-2, IL-15 and autologous feeder induced a fold expansion of 39.5 ± 20.3 (range 28.1–77.9) with a final population of 98.7 ± 0.8 % CD56+CD3− NK cells, 1.5 ± 0.9 % CD56+CD3+ NK-T and 0.5 ± 0.4 % CD56−CD3+ T cells.
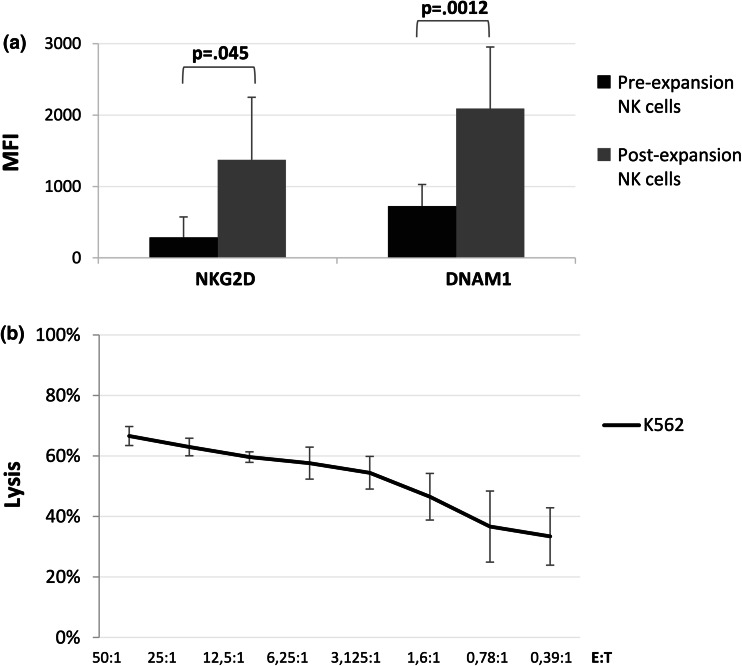
A mean of 18.6 × 106 ± 16.7 × 106 NK cells per flask was obtained after expansion. Expanded NK cells from B-lineage ALL patients were characterized by a significant upregulation of both NKG2D (p = .045)- and DNAM1 (p = .0012)-activating receptors (Fig. 6a), and showed a strong lytic activity against the K562 cell line (n = 4; mean cytotoxicity at a 50:1 E:T ratio 67.6 ± 3.1 %) (Fig. 6b), confirming that functionally active NK cells could be expanded ex vivo from ALL patients.
Fig. 6.
Immunophenotypic and functional characteristics of expanded and activated NK cells from B-ALL patients. a Comparison of NK cell activatory receptor expression pre- and post-expansion. b Cytotoxic activity of expanded NK cells against the K562 cancer cell line. Data are expressed as mean percentage of lysis ± SD of 4 independent experiments
Discussion
In this study, we developed a GMP-compliant method to generate large numbers of highly enriched and activated NK cells for clinical use in ALL patients. These expanded effectors generated both from healthy donors and B-lineage ALL patients in CR demonstrated to be a highly pure NK cell population characterized by a significant upregulation of activating receptors and a strong lytic activity against the K562 tumor cell line. In addition, healthy donor NK cells exerted killing activity also against adult primary B-lineage ALL blasts.
In order to achieve the highest purity of the final product, our isolation strategy consisted of a CD3 depletion followed by a subsequent CD56 positive selection on an autoMACS separator. This selection procedure ensured a minimal contamination by CD3+ T and NK-T lymphocytes and presented the further advantage of using the autologous cells obtained from the positive and negative fractions of the CD3- and CD56-positive selections as a feeder line capable of providing additional stimuli to the cell growth during the expansion culture period.
Due to their small number and their modest cytotoxic potential, isolated NK cells need to be expanded ex vivo in the presence of culture conditions that could generate high numbers of functionally robust NK cells. For this purpose, two doses of IL-2 and IL-15, both clinical grade manufactured, have been used, integrated with irradiated autologous feeder cells. The main advantages of this protocol are (1) the lack of in vivo cytokine infusion which can cause systemic toxicities; (2) the use of IL-15, which plays an essential role in NK cells development, expansion and homeostasis; and (3) the use of autologous feeder cells to prevent the unpredictable effects associated with the use of allogeneic feeder cells. In this model, both cytokines and feeder cells are necessary to obtain an effective NK cell proliferation, since a lower expansion fold was observed in the presence of cytokines or feeder cells alone. Under these conditions, the ratio of NK cell expansion was near to 35 times for healthy donors and to 40 times for B-lineage ALL patients. When comparing these results with previously published protocols, a variability in NK cell expansion fold could be observed. In the protocol used by Koehl et al. [35], the ex vivo expansion of highly purified NK cells after haploidentical stem cells transplantation in children did not involve IL-15 or a feeder cell source. In this case, a median NK cell expansion of only 5 times was obtained. More recent studies reported higher expansion folds, up to 400 times [36]. These differences may be ascribed to critical factors that include the efficiency of the protocol in supporting NK cell activation and growth, the source of feeder cells, additions of animal-derived reagents and grading of culture components, such as culture media, growth factors and serum components. A robust NK cell proliferation has, for example, been reported using a genetically modified K562 cell line as expansion culture feeder [37, 38]. Other promising new developments include the involvement of novel cytokines, such as IL-6 or IL-21, alone or in combination [39], or the expansion of NK cells from umbilical cord blood as an alternative source [40]. However, despite the encouraging results, there are several limitations with regard to GMP-compliant protocols such as the commercial unavailability of the respective clinical-scale cytokines or the lack of marketing authorization for the use of gene-manipulated tumor cell lines.
The expansion conditions used in our study led to a significant growth advantage of CD56+CD16+ CD3− cells, compared to the T- or B-lymphoid populations. The high T cell depletion leading to the presence of a very small proportion of T-lymphoid contaminants (0.01 ± 0.04 %) in the final product is essential to avoid GVHD in the allogeneic setting. Furthermore, also B cell depletion, as observed in our post-expansion NK samples, is an important condition in the context of NK cell-based immunotherapy, being indispensable to prevent EBV reactivation, which may potentially trigger lymphoproliferative diseases in immunocompromised patients.
Although the two main NK cell subsets showed a normal distribution in all donors after enrichment, in line with previous observations [41] some differences were observed at the end of the expansion. In fact, on day 14 all NK cells showed a CD56bright phenotype, with some donors showing only CD56brightCD16+ NK cells and others showing both CD56brightCD16+ and CD56brightCD16dim cells. It has been postulated that an initial pre-activation status of donor NK cells may confine the expansion to the CD56brightCD16+ subpopulation of NK cells [41]. In any case, the presence of the CD16dim subpopulation seemed not to influence the cytotoxic activity of expanded NK cells against both the K562 cell line and B-ALL blasts.
NK cells showed a significant upregulation of Nkp30-, Nkp44-, NKG2C-, NKG2D-, DNAM1-activating receptors and CD25 and CD69 activation markers in response to cell expansion. The net effect of changes in phenotype resulted in expanded NK cells showing high levels of cytotoxicity against the MHC-I− K562 cell line used as control, without any difference between fresh and cryopreserved products. Of note, expanded NK cells did not show cytotoxic activity against autologous as well as allogeneic normal PBMCs, suggesting that although the expression of activating receptors increases on expanded NK cells, these cells are still capable of sparing healthy cells that express self-HLA class I or lack ligands to stimulatory NK cell receptors. Immunophenotypic analyses performed after expansion also showed an increased expression of the inhibitory receptors NKG2A, CD158a and CD158e. We may suppose that its inhibitory activity may be indirectly overridden by the strongly increased expression of the major specific activating receptors.
In line with the study by Béziat et al. [42], in our study, NK cell differentiation seemed to be associated with the decrease of NKG2A and the acquisition of KIRs, as shown by the changes in the percentages of CD56brightCD16dim NK cells expressing these markers after expansion. At the same time, the significant increase of NKG2A+ cells among CD56brightCD16+ cells may be the consequence of the re-education of hyporesponsive NKG2A−KIR− NK cells under in vitro stimulation, as also hypothesized by the authors [42]. However, NK cell maturation steps may be influenced by the culture conditions used in the expansion protocol and this issue needs to be further investigated.
The main goal of our study was to evaluate whether the expanded NK cell populations were cytolytic against primary blasts from ALL patients. We demonstrated that primary adult B-lineage ALL blasts are recognized and partially killed by expanded NK cells. The different percentages of lytic activity against primary ALL blasts observed among NK cells of donor origin highlight the existence of an expected inter-sample variability and correlated both with an unpredictable intrinsic blast resistance or sensitivity to NK cell activity and—in the case of the allogeneic context—with the degree of HLA/KIR donor-recipient mismatch. In this respect, we have recently analyzed the expression of ligands for NK-activating receptors within molecularly defined subgroups of ALL cases, revealing a higher surface expression of both NKG2D and DNAM-1 ligands on BCR-ABL + blasts. Accordingly, primary blasts from BCR-ABL + B-ALL appeared significantly more susceptible to NK-dependent lysis than B-ALL without molecular aberrations [43], suggesting that an immune-mediated control of leukemia during TKIs maintenance is worthy of being pursued in this subset.
Finally, we documented the possibility of expanding ex vivo cytotoxic effectors with killing capacity against cancer cells from B-lineage ALL patients in CR, pointing to the feasibility of NK cell adoptive transfer in the autologous setting.
In conclusion, our study demonstrates the possibility of expanding functional NK cells under GMP-suitable conditions from healthy donors and from B-lineage ALL patients in CR, providing a basis for the design of new immunotherapeutic strategies for the management of ALL patients based on the infusion of allogeneic or autologous NK cells.
Acknowledgments
Research Grant support: Associazione Italiana per la Ricerca sul Cancro (AIRC), Special Project 5 × 1000, Milan, Italy; Ministero della Salute; Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR); Fondo per gli Investimenti della Ricerca di Base (FIRB).
Conflict of interest
There are no conflict of interests to disclose.
Ethical standard
All patients and donors gave their informed consent for blood collection and biologic studies in accordance with the Declaration of Helsinki. The study was approved by the local Ethics Committee.
Abbreviations
- ALL
Acute lymphoblastic leukemia
- AML
Acute myeloid leukemia
- 51Cr
51Chromium
- CR
Complete remission
- EBV
Epstein–Barr virus
- FBS
Fetal bovine serum
- GMP
Good manufactory practice
- GVHD
Graft-versus-host disease
- HLA
Human leukocyte antigen
- IL-2
Interleukin-2
- KIRs
Killer cell immunoglobulin-like receptors
- LAK
Cells lymphokine-activated killer cells
- mAbs
Monoclonal antibodies
- MFI
Mean fluorescence intensity
- NCRs
Natural cytotoxicity receptors
- NK
Cells natural killer cells
- PBMCs
Peripheral blood mononuclear cells
References
- 1.Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421–2438. [PubMed] [Google Scholar]
- 2.Miller JS. The biology of natural killer cells in cancer, infection, and pregnancy. Exp Hematol. 2001;29:1157–1168. doi: 10.1016/S0301-472X(01)00696-8. [DOI] [PubMed] [Google Scholar]
- 3.Thielens A, Vivier E, Romagné F. NK cell MHC class I specific receptors [KIR): from biology to clinical intervention. Curr Opin Immunol. 2012;24:239–245. doi: 10.1016/j.coi.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 5.Coudert JD, Held W. The role of the NKG2D receptor for tumor immunity. Semin Cancer Biol. 2006;16:333–343. doi: 10.1016/j.semcancer.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Eagle RA, Trowsdale J. Promiscuity and the single receptor: NKG2D. Nat Rev Immunol. 2007;7:737–744. doi: 10.1038/nri2144. [DOI] [PubMed] [Google Scholar]
- 7.Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, McClanahan T, Hannum C, Kitamura T, Nicholl J, Sutherland GR, Lanier LL, Phillips JH. DNAM1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4:573–581. doi: 10.1016/S1074-7613(00)70060-4. [DOI] [PubMed] [Google Scholar]
- 8.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 9.Stewart CA, Laugier-Anfossi F, Vély F, Saulquin X, Riedmuller J, Tisserant A, Gauthier L, Romagné F, Ferracci G, Arosa FA, Moretta A, Sun PD, Ugolini S, Vivier E. Recognition of peptide-MHC class I complexes by activating killer immunoglobulin-like receptors. Proc Natl Acad Sci USA. 2005;102:13224–13229. doi: 10.1073/pnas.0503594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10:230–252. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegler U, Kalberer CP, Nowbakht P, Sendelov S, Meyer-Monard S, Wodnar-Filipowicz A. Activated natural killer cells from patients with acute myeloid leukemia are cytotoxic against autologous leukemic blasts in NOD/SCID mice. Leukemia. 2005;19:2215–2222. doi: 10.1038/sj.leu.2403985. [DOI] [PubMed] [Google Scholar]
- 12.Adler A, Chervenick PA, Whiteside TL, Lotzová E, Herberman RB. Interleukin 2 induction of lymphokine-activated killer (LAK) activity in the peripheral blood and bone marrow of acute leukemia patients. I. Feasibility of LAK generation in adult patients with active disease and in remission. Blood. 1988;71:709–716. [PubMed] [Google Scholar]
- 13.Fierro MT, Liao XS, Lusso P, Bonferroni M, Matera L, Cesano A, Lista P, Arione R, Forni G, Foa R. In vitro and in vivo susceptibility of human leukemic cells to lymphokine activated killer activity. Leukemia. 1988;2:50–54. [PubMed] [Google Scholar]
- 14.Brune M, Castaigne S, Catalano J, Gehlsen K, Ho AD, Hofmann WK, Hogge DE, Nilsson B, Or R, Romero AI, Rowe JM, Simonsson B, Spearing R, Stadtmauer EA, Szer J, Wallhult E, Hellstrand K. Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood. 2006;108:88–96. doi: 10.1182/blood-2005-10-4073. [DOI] [PubMed] [Google Scholar]
- 15.Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, Urbani E, Negrin RS, Martelli MF, Velardi A. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–339. [PubMed] [Google Scholar]
- 16.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 17.Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, Stern M, Pende D, Perruccio K, Burchielli E, Topini F, Bianchi E, Aversa F, Martelli MF, Velardi A. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110:433–440. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pende D, Spaggiari GM, Marcenaro S, Martini S, Rivera P, Capobianco A, Falco M, Lanino E, Pierri I, Zambello R, Bacigalupo A, Mingari MC, Moretta A, Moretta L. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112) Blood. 2005;105:2066–2073. doi: 10.1182/blood-2004-09-3548. [DOI] [PubMed] [Google Scholar]
- 19.Pende D, Marcenaro S, Falco M, Martini S, Bernardo ME, Montagna D, Romeo E, Cognet C, Martinetti M, Maccario R, Mingari MC, Vivier E, Moretta L, Locatelli F, Moretta A. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and re-definition of inhibitory KIR specificity. Blood. 2009;113:3119–3129. doi: 10.1182/blood-2008-06-164103. [DOI] [PubMed] [Google Scholar]
- 20.Jardine L, Hambleton S, Bigley V, Pagan S, Wang XN, Collin M. Sensitizing primary acute lymphoblastic leukemia to natural killer cell recognition by induction of NKG2D ligands. Leuk Lymphoma. 2013;54:167–173. doi: 10.3109/10428194.2012.708026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torelli GF, Guarini A, Maggio R, Alfieri C, Vitale A, Foà R. Expansion of natural killer cells with lytic activity against autologous blasts from adult and pediatric acute lymphoid leukemia patients in complete hematologic remission. Haematologica. 2005;90:785–792. [PubMed] [Google Scholar]
- 22.Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, Matory YL, Skibber JM, Shiloni E, Vetto JT, Seipp CA, Simpson C, Reichert CM. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 23.Stern M, Passweg JR, Meyer-Monard S, Esser R, Tonn T, Soerensen J, Paulussen M, Gratwohl A, Klingebiel T, Bader P, Tichelli A, Schwabe D, Koehl U. Pre-emptive immunotherapy with purified natural killer cells after haploidentical SCT: a prospective phase II study in two centers. Bone Marrow Transplant. 2013;48:433–438. doi: 10.1038/bmt.2012.162. [DOI] [PubMed] [Google Scholar]
- 24.Carlens S, Gilljam M, Chambers BJ, Aschan J, Guven H, Ljunggren HG, Christensson B, Dilber MS. A new method for in vitro expansion of cytotoxic human CD3−CD56+ natural killer cells. Hum Immunol. 2001;62:1092–1098. doi: 10.1016/S0198-8859(01)00313-5. [DOI] [PubMed] [Google Scholar]
- 25.McKenna DH, Jr, Sumstad D, Bostrom N, Kadidlo DM, Fautsch S, McNearney S, Dewaard R, McGlave PB, Weisdorf DJ, Wagner JE, McCullough J, Miller JS. Good manufacturing practices production of natural killer cells for immunotherapy: a six-year single-institution experience. Transfusion. 2007;47:520–528. doi: 10.1111/j.1537-2995.2006.01145.x. [DOI] [PubMed] [Google Scholar]
- 26.Luhm J, Brand JM, Koritke P, Hoppner M, Kirchner H, Frohn C. Large-scale generation of natural killer lymphocytes for clinical application. J Hematother Stem Cell Res. 2002;11:651–657. doi: 10.1089/15258160260194794. [DOI] [PubMed] [Google Scholar]
- 27.Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, Eldridge P, Leung WH, Campana D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perussia B, Ramoni C, Anegon I, Cuturi MC, Faust J, Trinchieri G. Preferential proliferation of natural killer cells among peripheral blood mononuclear cells cocultured with B lymphoblastoid cell lines. Nat Immun Cell Growth Regul. 1987;6:171–188. [PubMed] [Google Scholar]
- 29.Miller JS, Oelkers S, Verfaillie C, McGlave P. Role of monocytes in the expansion of human activated natural killer cells. Blood. 1992;80:2221–2229. [PubMed] [Google Scholar]
- 30.Boissel L, Tuncer HH, Betancur M, Wolfberg A, Klingemann H. Umbilical cord mesenchymal stem cells increase expansion of cord blood natural killer cells. Biol Blood Marrow Transplant. 2008;14:1031–1038. doi: 10.1016/j.bbmt.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Alici E, Sutlu T, Björkstrand B, Gilljam M, Stellan B, Nahi H, Quezada HC, Gahrton G, Ljunggren H, Dilber MS. Autologous antitumor activity by NK cells expanded from myeloma patients using GMP-compliant components. Blood. 2008;111:3155–3162. doi: 10.1182/blood-2007-09-110312. [DOI] [PubMed] [Google Scholar]
- 32.Lim SA, Kim TJ, Lee JE, Sonn CH, Kim K, Kim J, Choi JG, Choi IK, Yun CO, Kim JH, Yee C, Kumar V, Lee KM. Ex vivo expansion of highly cytotoxic human NK cells by cocultivation with irradiated tumor cells for adoptive immunotherapy. Cancer Res. 2013;73:2598–2607. doi: 10.1158/0008-5472.CAN-12-2893. [DOI] [PubMed] [Google Scholar]
- 33.Suck G, Koh MB. Emerging natural killer cell immunotherapies: large-scale ex vivo production of highly potent anticancer effectors. Hematol Oncol Stem Cell Ther. 2010;3:135–142. doi: 10.1016/S1658-3876(10)50024-4. [DOI] [PubMed] [Google Scholar]
- 34.Miller JS. Should natural killer cells be expanded in vivo or ex vivo to maximize their therapeutic potential? Cytotherapy. 2009;11:259–260. doi: 10.1080/14653240902888000. [DOI] [PubMed] [Google Scholar]
- 35.Koehl U, Esser R, Zimmermann S, Tonn T, Kotchetkov R, Bartling T, Sörensen J, Grüttner HP, Bader P, Seifried E, Martin H, Lang P, Passweg JR, Klingebiel T, Schwabe D. Ex vivo expansion of highly purified NK cells for immunotherapy after haploidentical stem cell transplantation in children. Klin Padiatr. 2005;217:345–350. doi: 10.1055/s-2005-872520. [DOI] [PubMed] [Google Scholar]
- 36.Harada H, Saijo K, Watanabe S, Tsuboi K, Nose T, Ishiwata I, Ohno T. Selective expansion of human natural killer cells from peripheral blood mononuclear cells by the cell line, HFWT. Jpn J Cancer Res. 2002;93:313–319. doi: 10.1111/j.1349-7006.2002.tb02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shook DR, Campana D. Natural killer cell engineering for cellular therapy of cancer. Tissue Antigens. 2011;78:409–415. doi: 10.1111/j.1399-0039.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lapteva N, Durett AG, Sun J, Rollins LA, Huye LL, Fang J, Dandekar V, Mei Z, Jackson K, Vera J, Ando J, Ngo MC, Coustan-Smith E, Campana D, Szmania S, Garg T, Moreno-Bost A, Vanrhee F, Gee AP, Rooney CM. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy. 2012;14:1131–1143. doi: 10.3109/14653249.2012.700767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin CY, Chuang TF, Liao KW, Huang YJ, Pai CC, Chu RM. Combined immunogene therapy of IL-6 and IL-15 enhances anti-tumor activity through augmented NK cytotoxicity. Cancer Lett. 2008;272:285–295. doi: 10.1016/j.canlet.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 40.Spanholtz J, Tordoir M, Eissens D, Preijers F, van der Meer A, Joosten I, Schaap N, de Witte TM, Dolstra H. High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy. PLoS ONE. 2010;5:e9221. doi: 10.1371/journal.pone.0009221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huenecke S, Zimmermann SY, Kloess S, Esser R, Brinkmann A, Tramsen L, Koenig M, Erben S, Seidl C, Tonn T, Eggert A, Schramm A, Bader P, Klingebiel T, Lehrnbecher T, Passweg JR, Soerensen J, Schwabe D, Koehl U. IL-2-driven regulation of NK cell receptors with regard to the distribution of CD16+ and CD16− subpopulations and in vivo influence after haploidentical NK cell infusion. J Immunother. 2010;33:200–210. doi: 10.1097/CJI.0b013e3181bb46f7. [DOI] [PubMed] [Google Scholar]
- 42.Béziat V, Descours B, Parizot C, Debré P, Vieillard V. NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs. PLoS One. 2010;5:e11966. doi: 10.1371/journal.pone.0011966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torelli GF, Peragine N, Raponi S, Pagliara D, De Propris MS, Vitale A, Bertaina A, Barberi W, Moretta L, Basso G, Santoni A, Guarini A, Locatelli F, Foa’ R. Recognition of adult and pediatric acute lymphoblastic leukemia blasts by natural killer cells. Haematologica. 2014;99:1248–1254. doi: 10.3324/haematol.2013.101931. [DOI] [PMC free article] [PubMed] [Google Scholar]