Abstract
The selective killing of cancer cells without toxicity to normal nontransformed cells is an idealized goal of cancer therapy. Thus, there has been much interest in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), a protein that appears to selectively kill cancer cells. TRAIL has been reported to trigger apoptosis and under some circumstances, an alternate death signaling pathway termed necroptosis. The relative importance of necroptosis for cell death induction in vivo is under intensive investigation. Nonetheless, many cancer cells (particularly those freshly isolated from cancer patients) are highly resistant to TRAIL-mediated cell death. Therefore, there is an underlying interest in identifying agents that can be combined with TRAIL to improve its efficacy. There are numerous reports in which combination of TRAIL with standard antineoplastic drugs has resulted in enhanced cancer cell death in vitro. However, many of these chemotherapeutic drugs are nonspecific and associated with adverse effects, which raise serious concerns for cancer therapy in patients. By contrast, natural products have been shown to be safer and efficacious alternatives. Recently, a number of studies have suggested that certain natural products when combined with TRAIL can enhance cancer cell death. In this review, we highlight molecular pathways that might be targeted by various natural products to promote cell death, and focus on our recent work with withanolides as TRAIL sensitizers. Finally, we will suggest synergistic approaches for combining active withanolides with various forms of immunotherapy to promote cancer cell death and an effective antitumor immune response.
Keywords: TRAIL, Apoptosis, Necroptosis, Poly (I:C), PIVAC15
Introduction
Programmed cell death (PCD) plays a pivotal role in organogenesis and sculpting of complex tissues during early stages of development and maintaining tissue homeostasis in adult life by eliminating cellular debris. Therefore, PCD is considered as an evolutionary adaptation which is an absolute necessity to ensure proper balance of cell division and act as a surveillance system to suppress viral infections and cancer development [1]. Apoptosis is a form of PCD that has been extensively studied. It usually occurs in the absence of inflammation since apoptotic cells are rapidly cleared by phagocytic cells. Insufficient apoptosis can lead to uncontrolled cell division culminating in various malignancies [2]. Necrosis on the other hand is considered to be a form of cell death characterized by early plasma membrane permeabilization and organelle swelling and is accompanied with a significant inflammatory response [3]. However, recent studies have led to a revelation that a subset of necrosis, known as necroptosis, is a regulated form of cell death [4, 5]. In this review, we will outline current information on molecular signaling pathways that control apoptosis and necroptosis. We will particularly focus on cancer cell death that could occur in the context of an immune response, such as TNF family death receptor signaling or signaling through toll-like receptors (TLRs). We will further describe how this cell death signaling can be amplified by certain pharmacological interventions and, finally, suggest rational ways whereby agents that enhance cell death signaling might be optimally combined with cancer immunotherapy.
Competing programs of cancer cell death signaling: apoptosis versus necroptosis
Apoptosis
The most studied and coordinated form of PCD is apoptosis. The mechanisms controlling apoptosis involve a cascade of energy-requiring molecular events and occur through two distinct pathways called intrinsic (mitochondrial) pathway relying on intracellular events or the extrinsic pathway which signals after binding of death ligands belonging to the tumor necrosis factor (TNF) superfamily to specialized death receptors (DRs) present on the cell surface. Members of this superfamily include Fas ligand (Fas L), TNF and TRAIL. Both intrinsic and extrinsic apoptosis signaling pathways are critically controlled by a family of serine proteases called caspases [6, 7]. Since many cell stresses (including much standard chemotherapy) engage the intrinsic apoptosis signaling pathway, mutations found in many cancer cells allow them to evade this signaling pathway. Therefore, in this review, we will focus on extrinsic apoptosis signaling involving members of the TNF superfamily. Of these, TRAIL has been anticipated to be a promising candidate for cancer immunotherapy due to its selective tumor killing and low toxicity. However, resistance to TRAIL therapy remains a challenge facing the development of anticancer strategies.
Apoptosis signaling by TRAIL
Apoptosis signaling in response to TRAIL (also known as Apo2/L) has been extensively reviewed in detail by others [8, 9]. Briefly, TRAIL is a member of the TNF receptor ligand superfamily, which induces apoptosis in tumor cells but not in normal cells, thus delivering therapeutic benefit in various malignancies. In humans, four transmembranes and one soluble receptor for TRAIL have been identified. Of these, two (TRAIL-R1 or DR4 and TRAIL-R2 or DR5) contain cytoplasmic death domains (DDs) and have the capacity to induce apoptotic cell death and are the targets of developing cancer therapies. These DDs are also crucial for engaging downstream signaling components that, depending on the cellular context, promote either apoptotic or pro-survival pathways. The other three receptors DcR1 (TRAIL-R3), DcR2 (TRAIL-R4) and the soluble Osteoprotegerin (OPG) lack functional death domains and function as decoy receptors. They can sequester TRAIL and lead to inhibition or suppression of apoptosis [10]. The enhanced expression of the decoy receptors for TRAIL in normal tissues versus tumor cell lines may account for the selective killing of tumor cell lines to TRAIL-mediated apoptosis [11]. Alternatively stressed cells, such as cancer cells, often express higher levels of DR5 on their cell surface as described later in this review.
TRAIL is known to activate both intrinsic and the extrinsic apoptotic pathways based on the cell type. As illustrated in Fig. 1, the proximal apoptotic signaling pathway of TRAIL is triggered by binding of trimerized TRAIL to DR4 and/or DR5, followed by receptor clustering within the lipid rafts leading to the recruitment of Fas-associated protein with death domain (FADD). The receptor–ligand and FADD complex in turn recruit procaspase-8 to the activated receptor, resulting in the formation of death-inducing signaling complex (DISC) and subsequent activation of caspase-8 through oligomerization and self-cleavage. Activated caspase-8 then activates effector caspase-3. Caspase-3 is then able to cleave many downstream substrates to initiate apoptosis [12].
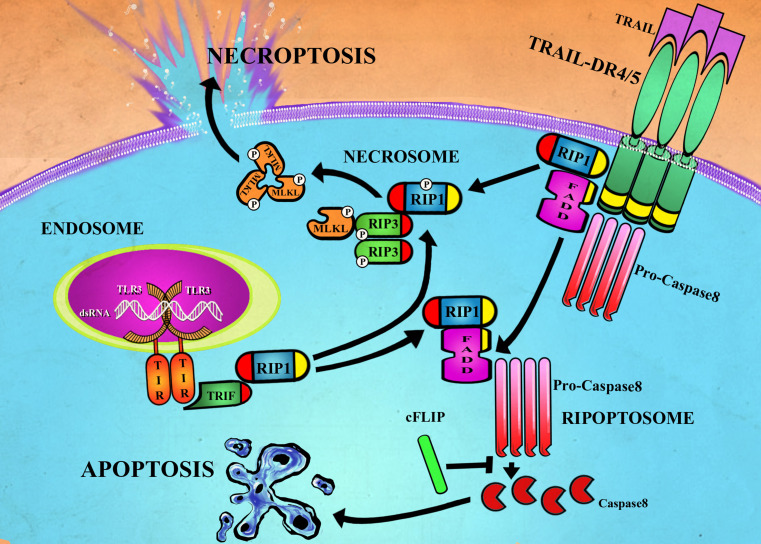
Fig. 1.
Cell death signaling pathways for TRAIL and TLR3. Binding of TRAIL to DR4 or DR5 can result in the formation of the ripoptosome (or complex-IIa) where FADD complexes with RIP via homotypic DD (yellow) to recruit and activate pro-caspase-8 to caspase-8. Binding of double-stranded RNA to TLR3 can also promote ripoptosome formation following interaction between the adapter protein TRIF and RIP1 via homotypic RHIM motifs (red). Under normal circumstances, caspase-8 cleavage of RIP1 and RIP3 limits necroptosis signaling. However, under circumstances where caspase-8 activation is reduced or inhibited, DR4/5 or TLR3 signaling can result in interaction of RIP1 and RIP3 via their RHIM domains subsequently triggering phosphorylation, assembly of the necrosome (or complex IIb) and MLKL-dependent necroptosis
In some cell types, proteolytic caspase-8 further activates BH3-interacting domain death agonist (Bid) by cleavage into a truncated form (tBid). tBid then binds to Bcl-2-associated X protein (Bax) and Bcl-2 homologous antagonist killer (Bak), leading to its translocation to the mitochondrial membrane and change in membrane potential [13, 14]. Thus, TRAIL apoptosis signaling in some cells can be amplified by an additional engagement of the intrinsic apoptosis pathway. However, in cells where a robust activation of caspase-8 occurs, apoptosis proceeds even when the intrinsic signaling pathway is nonfunctional.
Nonetheless, a considerable number of cancer cells, including those freshly isolated from cancer patients, are resistant to TRAIL-mediated apoptosis. One resistance mechanism is attributed to cellular FLICE inhibitory protein (c-FLIP), which shares sequence homology with caspase-8 and acts as a dominant negative, and inhibits caspase-8 activation by competing for FADD binding [15]. Overexpression of c-FLIP has been implicated in conferring resistance to apoptosis induced by death receptor stimulation or by anticancer drugs in a variety of cancers. Another group of molecules involved in TRAIL resistance are the inhibitor of apoptosis (IAP) protein family, which includes X-linked IAP (XIAP), cellular IAP (c-IAP) 1, c-IAP2 and survivin. The IAPs target downstream TRAIL apoptosis signaling by inhibiting the activity of caspases 3, 7 and/or 9. Thus, both blocking the activity of c-FLIP and IAPs may sensitize cancer cells to TRAIL apoptosis [16].
Recent studies have suggested a dichotomy in TRAIL-mediated signaling, indicating various noncanonical (pro-survival) functions of the molecule. TRAIL has been shown to activate nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB), in a TNFR-associated death domain (TRADD) and receptor-interacting protein 1 (RIP1)-dependent pathway, leading to the transcription of antiapoptotic genes such as c-FLIP, c-IAP1, c-IAP2, XIAP and Bcl-XL. Additionally, TRAIL also activates several kinases including phosphoinositide 3-kinase/protein kinase B (PI3K)/AKT) and mitogen-activated protein kinase (MAPK) pathways due to the formation of a secondary complex containing FADD, caspase-8, RIP1, tumor necrosis factor receptor-associated protein (TRAF2) and NEMO [17, 18].
This highlights the critical importance of developing means to counteract TRAIL resistance in cancer cells through combinations of TRAIL receptor agonists with drugs that can inhibit survival signals and/or increase apoptosis signaling.
Therapeutic strategies to amplify apoptotic signaling by TRAIL
A wide range of diverse chemotherapeutic agents has been identified to potentiate cancer cell death in combination with TRAIL. These include DNA-damaging agents such as topoisomerase I and II inhibitors (doxorubicin, epirubicin, etoposide), alkylating agents (cisplatin, carboplatin, oxyplatin) and pyrimidine analogs (5-fluorouracil, gemcitabine) as well as microtubule stabilizing agents such as paclitaxel or docetaxel. Also a number of more recent anticancer drugs such as bortezomib, heat shock protein inhibitors and histone deacetylase inhibitors have been identified as TRAIL-sensitizing agents. The molecular basis of TRAIL sensitization of cancer cells by these standard anticancer agents has been described in more detail in a number of recent reviews [9, 19–22]. Interestingly, a wide range of natural products mostly derived from plants has also displayed some striking effects increasing tumor cell susceptibility to TRAIL receptor agonists.
Natural products that sensitize cancer cells to TRAIL
Numerous natural products have shown great potential for enhancing cancer cell death in response to TRAIL [23]. Their usual mechanism of action is thought to involve modulation of diverse nonapoptotic pathways and/or the induction of cell stress pathways that result in an amplification of TRAIL cell death signaling. A number of natural products such as wogonin, sulforaphane and melittin can sensitize TRAIL-resistant cells to TRAIL-mediated cell death through the modulation of NF-kB signaling. Others such as chrysin, 6BIO and bufadienolide inhibit STAT3 phosphorylation. Sanguinarine, artesunate and luteolin are reported to inhibit the PI3 K/AKT pathway, while gossypol, curcumin, apigenin and butein induced DRs through activation of ERK1/2. DRs can also be upregulated by activation of the p38 pathway or through the induction of p53. The targeting of the TRAIL cell death pathway by many diverse natural products has been described in great detail in two recent reviews [23, 24]. The various natural products can target a number of signaling pathways, resulting in decrease in cell survival signaling that can result in decreased expression of various antiapoptotic proteins such as cFLIP or Mcl-1, Bcl-2, Bcl-XL, increases in proapoptotic proteins such as Bax or Bak, and/or increases in the cell surface expression of the DRs. Indeed, a large number of natural products are reported to increase DR expression. Many signaling pathways can increase DR expression such as NF-kB and ERK1/2. However, it is noteworthy that increased DR expression following treatment with many natural products is often associated with increased levels of cell stress. Thus, many natural products increase ROS levels, activate JNK or p38 signaling, induce p53 or increase levels of the transcription factor CHOP which occurs in response to ER stress [25]. This raises a concern that the cells are destined to die at a later time in response to the cellular stress caused by the natural product alone, and TRAIL signaling merely increases the kinetics of this cell death. As such, based on the in vitro studies the therapeutic benefit of some of these combinations may be overestimated and possible toxicities in vivo underestimated. Since most studies on natural products described above have only been conducted in vitro, more animal studies are required to determine which natural products can truly synergize with TRAIL to promote cancer cell death in an in vivo setting.
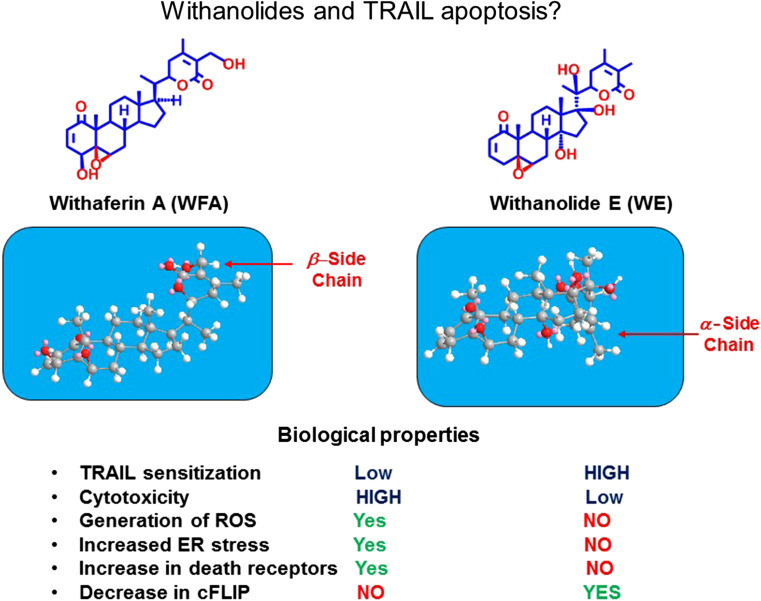
In an attempt to isolate novel TRAIL-sensitizing natural products, we designed a high throughput screen (HTS) encompassing >50,000 pure compounds, natural product extracts and purified natural products [26]. The most promising “hit” was a plant steroidal lactone withanolide E isolated from Physalis peruviana. Interestingly, another member of the withanolide family, withaferin A, has also been reported to sensitize renal carcinoma cells to TRAIL-induced apoptosis [27]. The molecular mechanism of action for withaferin A involved increased cellular production of ROS and the upregulation of DR5 in a CHOP-dependent manner. In addition, slight decreases in the transcription of the antiapoptotic c-FLIP proteins were also reported [27]. Surprisingly, despite the fact that both withanolides were structurally quite similar, major differences were noted in their molecular mechanism of action. Withanolide E was threefold to fourfold more potent than withaferin A as a TRAIL-sensitizing agent. In contrast to withaferin A, withanolide E treatment did not result in either ROS production or increase in levels of ER stress. However, it was much more effective than withaferin A in causing a rapid reduction in levels of the antiapoptotic c-FLIP proteins. Furthermore, when used as a single agent in long-term toxicity assays, withanolide E was much less toxic than withaferin A. The major molecular mechanism of action for the sensitizing effect of withanolde E was due to its ability to produce a rapid drop in the c-FLIP levels due to increased degradation of the proteins. It is quite striking that the biological effects of these withanolides are so different. Preliminary structure–activity relationship (SAR) studies suggest that the 17-beta hydroxy group and the resultant alpha orientation of the lactone side chain significantly alter the shape of withanolide E as compared to withaferin A (Fig. 2), and this may be critical for the TRAIL-sensitizing effects of withanolide E. The molecular mechanism whereby withanolide E rapidly reduces c-FLIP levels is currently under further investigation. Administration of withanolide E in combination with an agonist antibody to DR5 resulted in a much better therapeutic response than either agent alone in a human renal carcinoma xenograft model in athymic mice (Fig. 3). Furthermore, no obvious toxicities were observed in the mice in response to this combination [28]. In ongoing SAR studies, we are now testing a large panel of withanolide analogues for their relative ability to sensitize cancer cells to cell death in response to death ligands. We have identified some analogues that are fourfold to sixfold more potent that withanolide E. All the highly active withanolides so far identified are 17-beta hydroxy withanolides, and the most active are now being used for in vivo combination with agonist anti-DR5 antibodies in various mouse cancer models.
Fig. 2.
Chemical structures and properties of withaferin A and withanolide E evaluated for TRAIL-induced apoptosis. Despite similar overall structures, the biological properties of withaferin A and withanolide E are quite different. This may be due to the difference in overall shape of the molecule, where the 17-beta hydroxy group of withanolide E and the alpha orientation of the lactone side chain result in a change in shape that seems crucial for TRAIL apoptosis sensitizing activity
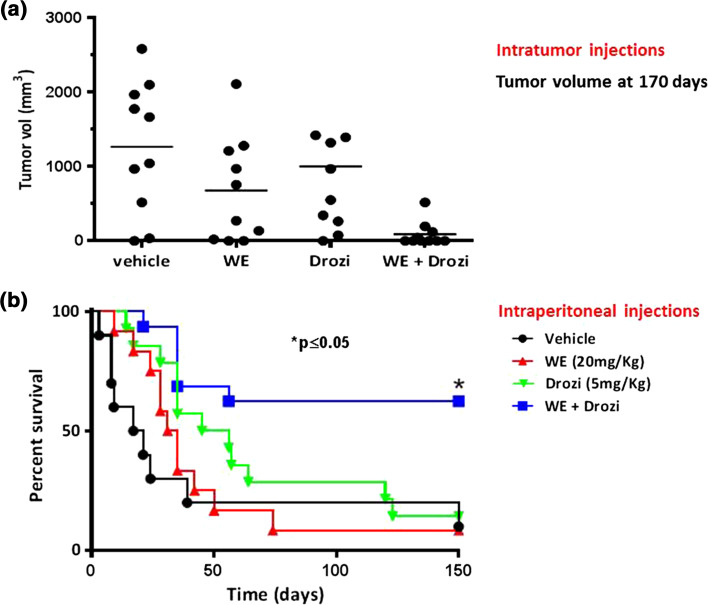
Fig. 3.
Withanolide E enhances death receptor-induced apoptosis in vivo [reproduced from [28]] . Athymic nude mice were injected subcutaneously with 1 × 106 ACHN cells. When tumors reached approximately 100 mm3 in diameter, mice were randomly assigned into four groups for therapy. Mice were treated with either vehicle control, withanolide E (20 mg/kg), DR5 agonist antibody; drozitumab (5 mg/kg) or the combination of withanolide E plus drozitumab twice weekly for 4 weeks as described in (27). a Tumor size at 75 days (intratumor), b survival up to 150 days (intraperitoneal)—pooled from two separate experiments with similar findings. Numbers of mice were as follows: vehicle control (n = 10), withanolide E (n = 10), drozitumab (n = 14), and withanolide E plus drozitumab (n = 16). Using the log-rank Mantel–Cox test, survival was significantly higher in the group receiving the withanolide E plus drozitumab combination (p < 0.05)
Necroptosis
Necroptosis is a form of regulated necrosis that can be activated by a variety of stimuli. However, in contrast to apoptosis, necroptosis does not require caspase activation. Indeed, it often occurs under conditions where caspases are not strongly activated. Among the major inducers of necroptosis are DRs of (Tumor necrosis factor receptor) TNFR family including TNFR1, TNFR, Fas, TRAIL-R1 and TRAIL-R2 [29]. Necroptosis can also be initiated by members of the pattern recognition receptor (PRR) family, which include TLRs, cytosolic NOD-like receptors (NLRs) and retinoic acid-inducible gene I-like receptors (RIG1) [30]. The most extensively studied pathway of necroptotic cell death is the one triggered by TNF family members.
Interestingly, some of the same components of the signaling complexes required to initiate apoptosis are also critical for necroptosis signaling. For death receptor signaling, a secondary cytoplasmic complex, Complex-IIa, is formed by association of FADD, RIP1, caspase-8 and c-FLIP also known as the ripoptosome [31]. Spontaneous formation of ripoptosome has been attributed to genotoxic stress induced by chemotherapies such as etopsides or downregulation of IAPs by Smac (Second mitochondria-derived activator of caspase) mimetics [32, 33]. Once the ripoptosome is formed, it will trigger caspase-8 activation, resulting in apoptotic cell death initiation. However, if caspases are not fully activated or their activity is blocked by c-FLIP, the protein kinase RIP3 is recruited to the complex, forming a necrosome, which leads to necroptotic cell death [31]. Hence, the ripoptosome serves as a “docking station,” for apoptotic and necroptotic death signaling. The key player in mediating this complex is the serine–threonine kinase activity of the RIP family undergoing several autophosphorylation events that regulate the switch between apoptosis and necroptosis. Phosphorylation of RIP3 allows the binding and phosphorylation of mixed lineage kinase domain-like (MLKL) pseudokinase, a key component of necroptotic cell death [34]. Necroptosis can be completely blocked either by the kinase inactivating mutation in any of the two kinases, or chemically by RIP1 kinase inhibitors (necrostatins), or RIP3 kinase inhibitors. The assembly of necrosome is also characterized by amyloid structures formed during RIP1 and RIP3 oligomerization through the RHIM motif (RIP homotypic interaction motif) [35]. Although RIP1 after oligomerization is dispensable for necroptosis, RIP3 RHIM motif is essential for cell death [36]. The physiological significance of the ripoptosome components RIPK1 and c-FLIP in controlling cell death has been demonstrated most convincingly in gene-targeting experiments in mice [37]. Tissue-specific elimination of either RIP1 or c-FLIP in intestinal epithelium, liver or skin of adult mice can result in extensive cell death that can be due to both apoptosis and necroptosis. This illustrates that c-FLIP and RIP1 are critical modifiers of both forms of programmed cell death during normal tissue homeostasis [38]. Therefore, although the targeting of ripoptosome components could prove to be an effective way to kill cancer cells, extreme caution must be exercised to prevent collateral damage to normal tissues. It would be anticipated that cancer cells undergoing cell death by necroptosis would release a variety of tumor antigens as well as danger-associated molecular patterns (DAMPs) that can trigger substantial local inflammation. There is increasing evidence indicating that many commonly used anticancer agents can trigger necroptotic signaling pathways, thus eliciting cell death in malignant cells [39]. A better understanding of the signaling networks regulating necroptosis in cancer cells is expected to speed up the development of anticancer drugs for therapeutic exploitation of necroptosis for cancer therapy.
TLR3-mediated cell death signaling
TLRs are a group of transmembrane receptors first identified in immune cells, which recognize molecular motifs of pathogen origin and activate immune response. Several reports have shown that they can also be expressed in tumor cells [40, 41]. Recent in vitro studies have reported induction of apoptosis in malignant cells treated with the synthetic TLR3-agonist, double-stranded RNA (dsRNA) mimicker and polyinosinic–polycytidylic acid [poly(I:C)]. These studies have also demonstrated that TLR3-induced apoptosis in cancer cells is dependent on caspase-8 activation, indicating that TLR3 activation triggers the “extrinsic pathway” of apoptosis [41]. Intriguingly, the process of TLR3-mediated apoptosis is independent of the classical death receptors of the TNFR superfamily, since blocking of these receptors (TRAIL-R or Fas) by neutralizing antibodies does not inhibit TLR3-mediated caspase-8-dependent apoptosis [42, 43]. Since TLR3 lacks a death domain, it is probable that it directly engages the “extrinsic pathway” by activating caspase-8. TLR3 possesses a TIR (toll/interleukin-1 receptor) domain that binds to TRIF (TIR-domain-containing adapter-inducing interferon-β) through homotypic TIR-domain interaction. On its C terminus, TRIF possesses a RHIM domain, which can interact with the RHIM domain of RIP1 which then recruits FADD followed by recruitment of caspase-8 via the death effector domains (DED) resulting in the assembly of the ripoptosome [43] (Fig. 1). Since TLR3 behaves like a death receptor and its engagement recruits RIP1, it is realistic to assume that TLR3 could also induce necroptotic cell death when caspase-8 is inhibited. Recent work from our laboratory indicated that active withanolides that sensitized cancer cells to TRAIL apoptosis were also able to sensitize cancer cells to poly(I:C) [a TLR3 ligand]. Hence, active withanolides most likely enhance the activity of a common downstream signaling pathway important for both TRAIL- and poly(I:C)-mediated apoptoses (Unpublished data, Tewary et al.).
Apart from its apoptotic and necroptotic effect on tumor cells, TLR3 ligation by its cognate ligands is also known to promote innate and adaptive immune responses leading to activation of myeloid cells including professional antigen-presenting cells (APCs) which can further cross prime CD8 T cells leading to long-lasting immunity and subsequent tumor regression [44]. Hence, targeting TLR3 is of great interest as a means to improve immunotherapeutic approaches to cancer therapy. One of the promising candidates is poly(I:C), which has been thoroughly investigated as potential anticancer adjuvant for immunotherapy in the last several decades due to its capability to induce effective immune responses. Nevertheless, its use was limited due to the cytokine storm produced in vivo, leading to shock, renal failure, coagulopathies and hypersensitivity reactions [45]. However, recent preclinical data with various mouse models suggest that poly(I:C), in pharmacologically safer forms such as Ampligen® or Hiltonol®, can be revived as an excellent candidate for future cancer therapies [46–48]. It is tempting to speculate that in cancers where TLR3 is expressed, administration of poly(I:C) might have multiple beneficial effects. Therefore, it could directly induce death of cancer cells and the release of tumor antigens, in addition to its well-described promotion of anticancer immune responses [48, 49].
Future directions
Promising preclinical data showing the potent tumoricidal activity of a number of TRAIL receptor agonists paved the way for clinical testing. Both recombinant human TRAIL/Apo-2L (Dulanermin) as well agonist antibodies to DR4 and DR5 have been tested in a number of clinical trials in cancer patients [7]. Although these agents were well tolerated with minimal adverse events, cancer patients with objective responses were few in number, so it remains unclear why these reagents that performed so well in preclinical studies failed to achieve marked effects in humans. One possible explanation is that in the cancer cells of the patients the apoptotic signaling threshold required to trigger cell death was rarely reached. Along similar lines, Dulanermin and the agonist antibodies to DR4 and DR5 are relatively weak agonists; thus, although possible toxic adverse events were minimized this came at the expense of significant antitumor effects. Recently, there has been renewed attention on the generation of TRAIL death receptor “superagonists.” Some of these agents are much more potent than recombinant TRAIL or agonist antibodies in preclinical studies [50]. Thus, the combination of these more potent TRAIL reagents, combined with novel sensitizing compounds that directly impact TRAIL death signaling pathways, may offer the hope for more dramatic anticancer response in the future.
Currently, there is little information concerning whether death by necroptosis occurs in some cancers, and how this might influence therapeutic outcome. This certainly merits further investigation. In addition, there has been much recent interest in how specific forms of cancer cell death may be more immunogenic, and this can affect subsequent anticancer immune responses resulting in improved therapeutic outcomes [51]. The relationship between apoptotic, necroptotic and immunogenic cell death remains to be clarified. One might anticipate that natural products that help promote cancer cell death might be ideal candidates for combination with immunotherapies, assuming their effects on immune responses would be relatively innocuous when compared to standard chemotherapeutic agents. Nonetheless caution is warranted. Active withanolides may also sensitize antigen-presenting cells such as dendritic cells, which abundantly express TLR3, to apoptosis. Studies are currently underway to determine whether a therapeutic window exists in which significant cancer cell death can be induced in the absence of major undesirable side effects.
Acknowledgments
We thank Andrew Sayers for his assistance with the artwork. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and the University of Arizona College of Agriculture and Life Sciences.
Abbreviations
- APCs
Antigen-presenting cells
- Bak
Bcl-2 homologous antagonist killer
- Bax
Bcl-2-associated X protein
- Bid
BH3-interacting domain death agonist
- c-FLIP
Cellular FLICE inhibitory protein
- DAMPs
Danger-associated molecular patterns
- DD
Death domain
- DED
Death effector domains
- DISC
Death-inducing signaling complex
- DRs
Death receptors
- dsRNA
Double-stranded RNA
- FADD
Fas-associated protein with death domain
- Fas L
Fas ligand
- IAP
Inhibitor of apoptosis,
- MAPK
Mitogen-activated protein kinase
- MLKL
Mixed lineage kinase domain-like
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NLRs
NOD-like receptors
- OPG
Osteoprotegerin
- PCD
Programmed cell death
- PI3K/AKT
Phosphoinositide 3-kinase/protein kinase B
- poly(I:C)
Polyinosinic–polycytidylic acid
- PRR
Pattern recognition receptor
- RHIM RIP
Homotypic interaction motif
- RIG1
Retinoic acid-inducible gene I like receptors
- RIP1
Receptor-interacting protein 1
- SAR
Structure–activity relationship
- Smac
Second mitochondria-derived activator of caspase
- TIR
Toll/interleukin-1 receptor
- TLRs
Toll-like receptors
- TNF
Tumor necrosis factor
- TNFR
Tumor necrosis factor receptor
- TRADD
TNFR-associated death domain
- TRAF2
Tumor necrosis factor receptor-associated protein
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
- TRIF
TIR-domain-containing adapter-inducing interferon-β
Compliance with ethical standards
Conflict of interest
Thomas Sayers is an inventor on US patent (No. 9,238,069) Method of Sensitizing Cancer Cells to the Cytotoxic Effects of Death Receptor Ligands for Cancer Treatment assigned to the US Government. Thomas Sayers, A. A. Leslie Gunatilaka and Poonam Tewary have filed a patent application based on ability of active 17-beta hydroxy withanolides to promote death in cancer cells in response to TNF family death ligands and TLR3 ligands such as poly(I:C).
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Fifteenth International Conference on Progress in Vaccination against Cancer (PIVAC 15), held in Tübingen, Germany, 6th–8th October, 2015. It is part of a Cancer Immunology, Immunotherapy series of Focussed Research Reviews and meeting report.
Contributor Information
Poonam Tewary, Phone: 301-846-6551, Email: tewaryp@mail.nih.gov.
Thomas J. Sayers, Phone: 301-846-5729, Email: sayerst@mail.nih.gov
References
- 1.Kroemer G, Petit P, Zamzami N, Vayssiere JL, Mignotte B. The biochemistry of programmed cell-death. Faseb J. 1995;9(13):1277–1287. doi: 10.1096/fasebj.9.13.7557017. [DOI] [PubMed] [Google Scholar]
- 2.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanduc D, Mittelman A, Serpico R, Sinigaglia E, Sinha AA, Natale C, Santacroce R, Di Corcia MG, Lucchese A, Dini L, Pani P, Santacroce S, Simone S, Bucci R, Farber E. Cell death: apoptosis versus necrosis (review) Int J Oncol. 2002;21(1):165–170. [PubMed] [Google Scholar]
- 4.Martin SJ, Henry CM. Distinguishing between apoptosis, necrosis, necroptosis and other cell death modalities. Methods. 2013;61(2):87–89. doi: 10.1016/j.ymeth.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Feoktistova M, Leverkus M. Programmed necrosis and necroptosis signaling. FEBS J. 2015;282(1):19–31. doi: 10.1111/febs.13120. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003;22(53):8628–8633. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- 7.Amarante-Mendes GP, Griffith TS. Therapeutic applications of TRAIL receptor agonists in cancer and beyond. Pharmacol Ther. 2015;155:117–131. doi: 10.1016/j.pharmthera.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene. 2010;29(34):4752–4765. doi: 10.1038/onc.2010.221. [DOI] [PubMed] [Google Scholar]
- 9.Dimberg LY, Anderson CK, Camidge R, Behbakht K, Thorburn A, Ford HL. On the TRAIL to successful cancer therapy. Predicting and counteracting resistance against TRAIL based therapeutics. Oncogene. 2013;32(11):1341–1350. doi: 10.1038/onc.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahalingam D, Szegezdi E, Keane M, de Jong S, Samali A. TRAIL receptor signalling and modulation: are we on the right TRAIL? Cancer Treat Rev. 2009;35(3):280–288. doi: 10.1016/j.ctrv.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Yagita H, Takeda K, Hayakawa Y, Smyth MJ, Okumura K. TRAIL and its receptors as targets for cancer therapy. Cancer Sci. 2004;95(10):777–783. doi: 10.1111/j.1349-7006.2004.tb02181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pennarun B, Meijer A, de Vries EG, Kleibeuker JH, Kruyt F, de Jong S. Playing the DISC: turning on TRAIL death receptor-mediated apoptosis in cancer. Biochim Biophys Acta. 1805;2:123–140. doi: 10.1016/j.bbcan.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Ozoren N, El-Deiry WS. Defining characteristics of types I and II apoptotic cells in response to TRAIL. Neoplasia. 2002;4(6):551–557. doi: 10.1038/sj.neo.7900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jost PJ, Grabow S, Gray D, McKenzie MD, Nachbur U, Huang DC, Bouillet P, Thomas HE, Borner C, Silke J, Strasser A, Kaufmann T. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature. 2009;460(7258):1035–1039. doi: 10.1038/nature08229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashkenazi A, Herbst RS. To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest. 2008;118(6):1979–1990. doi: 10.1172/JCI34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fulda S. Tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL) Adv Exp Med Biol. 2014;818:167–180. doi: 10.1007/978-1-4471-6458-6_8. [DOI] [PubMed] [Google Scholar]
- 17.Azijli K, Weyhenmeyer B, Peters GJ, de Jong S, Kruyt FA. Non-canonical kinase signaling by the death ligand TRAIL in cancer cells: discord in the death receptor family. Cell Death Differ. 2013;20(7):858–868. doi: 10.1038/cdd.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He WY, Wang Q, Xu J, Xu XL, Padilla MT, Ren GS, Gou X, Lin Y. Attenuation of TNFSF10/TRAIL-induced apoptosis by an autophagic survival pathway involving TRAF2-and RIPK1/RIP1-mediated MAPK8/JNK activation. Autophagy. 2012;8(12):1811–1821. doi: 10.4161/auto.22145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sayers TJ. Targeting the extrinsic apoptosis signaling pathway for cancer therapy. Cancer Immunol Immunother. 2011;60(8):1173–1180. doi: 10.1007/s00262-011-1008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menke C, Bin L, Thorburn J, Behbakht K, Ford HL, Thorburn A. Distinct TRAIL resistance mechanisms can be overcome by proteasome inhibition but not generally by synergizing agents. Cancer Res. 2011;71(5):1883–1892. doi: 10.1158/0008-5472.CAN-10-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amm HM, Oliver PG, Lee CH, Li Y, Buchsbaum DJ. Combined modality therapy with TRAIL or agonistic death receptor antibodies. Cancer Biol Ther. 2011;11(5):431–449. doi: 10.4161/cbt.11.5.14671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Wilt LHAM, Kroon J, Jansen G, de Jong S, Peters GJ, Kruyt FAE. Bortezomib and TRAIL: a perfect match for apoptotic elimination of tumour cells? Crit Rev Oncol Hematol. 2013;85(3):363–372. doi: 10.1016/j.critrevonc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Dai X, Zhang J, Arfuso F, Chinnathambi A, Zayed ME, Alharbi SA, Kumar AP, Ahn KS, Sethi G. Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp Biol Med (Maywood) 2015;240(6):760–773. doi: 10.1177/1535370215579167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasad S, Kim JH, Gupta SC, Aggarwal BB. Targeting death receptors for TRAIL by agents designed by Mother Nature. Trends Pharmacol Sci. 2014;35(10):520–536. doi: 10.1016/j.tips.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Fulda S. Modulation of apoptosis by natural products for cancer therapy. Planta Med. 2010;76(11):1075–1079. doi: 10.1055/s-0030-1249961. [DOI] [PubMed] [Google Scholar]
- 26.Booth NL, Sayers TJ, Brooks AD, Thomas CL, Jacobsen K, Goncharova EI, McMahon JB, Henrich CJ. A cell-based high-throughput screen to identify synergistic TRAIL sensitizers. Cancer Immunol Immunother. 2009;58(8):1229–1244. doi: 10.1007/s00262-008-0637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee TJ, Um HJ, Min DS, Park JW, Choi KS, Kwon TK. Withaferin A sensitizes TRAIL-induced apoptosis through reactive oxygen species-mediated up-regulation of death receptor 5 and down-regulation of c-FLIP. Free Radic Biol Med. 2009;46(12):1639–1649. doi: 10.1016/j.freeradbiomed.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Henrich CJ, Brooks AD, Erickson KL, Thomas CL, Bokesch HR, Tewary P, Thompson CR, Pompei RJ, Gustafson KR, McMahon JB, Sayers TJ. Withanolide E sensitizes renal carcinoma cells to TRAIL-induced apoptosis by increasing cFLIP degradation. Cell Death Dis. 2015 doi: 10.1038/cddis.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou W, Yuan J. Necroptosis in health and diseases. Semin Cell Dev Biol. 2014;35:14–23. doi: 10.1016/j.semcdb.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 30.de Almagro MC, Vucic D. Necroptosis: pathway diversity and characteristics. Semin Cell Dev Biol. 2015;39:56–62. doi: 10.1016/j.semcdb.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Humphries F, Yang S, Wang B, Moynagh PN. RIP kinases: key decision makers in cell death and innate immunity. Cell Death Differ. 2015;22(2):225–236. doi: 10.1038/cdd.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, MacFarlane M, Hacker G, Leverkus M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43(3):449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, MacFarlane M, Cain K, Meier P. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43(3):432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Sun LM, Wang HY, Wang ZG, He SD, Chen S, Liao DH, Wang L, Yan JC, Liu WL, Lei XG, Wang XD. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148(1–2):213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 35.Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, Damko E, Moquin D, Walz T, McDermott A, Chan FK, Wu H. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150(2):339–350. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu XN, Yang ZH, Wang XK, Zhang Y, Wan H, Song Y, Chen X, Shao J, Han J. Distinct roles of RIP1-RIP3 hetero- and RIP3-RIP3 homo-interaction in mediating necroptosis. Cell Death Differ. 2014;21(11):1709–1720. doi: 10.1038/cdd.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silke J, Rickard JA, Gerlic M. The diverse role of RIP kinases in necroptosis and inflammation. Nat Immunol. 2015;16(7):689–697. doi: 10.1038/ni.3206. [DOI] [PubMed] [Google Scholar]
- 38.Tsuchiya Y, Nakabayashi O, Nakano H. FLIP the switch: regulation of apoptosis and necroptosis by cFLIP. Int J Mol Sci. 2015;16(12):30321–30341. doi: 10.3390/ijms161226232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fulda S. Therapeutic exploitation of necroptosis for cancer therapy. Semin Cell Dev Biol. 2014;35:51–56. doi: 10.1016/j.semcdb.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T. TLR3 can directly trigger apoptosis in human cancer cells. J Immunol. 2006;176(8):4894–4901. doi: 10.4049/jimmunol.176.8.4894. [DOI] [PubMed] [Google Scholar]
- 41.Salaun B, Lebecque S, Matikainen S, Rimoldi D, Romero P. Toll-like receptor 3 expressed by melanoma cells as a target for therapy. Clin Cancer Res. 2007;13(15 Pt 1):4565–4574. doi: 10.1158/1078-0432.CCR-07-0274. [DOI] [PubMed] [Google Scholar]
- 42.Weber A, Kirejczyk Z, Besch R, Potthoff S, Leverkus M, Hacker G. Proapoptotic signalling through Toll-like receptor-3 involves TRIF-dependent activation of caspase-8 and is under the control of inhibitor of apoptosis proteins in melanoma cells. Cell Death Differ. 2010;17(6):942–951. doi: 10.1038/cdd.2009.190. [DOI] [PubMed] [Google Scholar]
- 43.Estornes Y, Toscano F, Virard F, Jacquemin G, Pierrot A, Vanbervliet B, Bonnin M, Lalaoui N, Mercier-Gouy P, Pacheco Y, Salaun B, Renno T, Micheau O, Lebecque S. dsRNA induces apoptosis through an atypical death complex associating TLR3 to caspase-8. Cell Death Differ. 2012;19(9):1482–1494. doi: 10.1038/cdd.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S, Yagita H, Sayers TJ, Celis E. Optimized combination therapy using bortezomib, TRAIL and TLR agonists in established breast tumors. Cancer Immunol Immun. 2010;59(7):1073–1081. doi: 10.1007/s00262-010-0834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krown SE, Kerr D, Stewart WE, Field AK, Oettgen HF. Phase-I trials of poly(I, C) complexes in advanced cancer. J Biol Resp Modif. 1985;4(6):640–649. [PubMed] [Google Scholar]
- 46.Jasani B, Navabi H, Adams M. Ampligen: a potential toll-like 3 receptor adjuvant for immunotherapy of cancer. Vaccine. 2009;27(25–26):3401–3404. doi: 10.1016/j.vaccine.2009.01.071. [DOI] [PubMed] [Google Scholar]
- 47.Navabi H, Jasani B, Reece A, Clayton A, Tabi Z, Donninger C, Mason M, Adams M. A clinical grade poly I:C-analogue (Ampligen (R)) promotes optimal DC maturation and Th1-type T cell responses of healthy donors and cancer patients in vitro. Vaccine. 2009;27(1):107–115. doi: 10.1016/j.vaccine.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 48.Nagato T, Lee YR, Harabuchi Y, Celis E. Combinatorial immunotherapy of polyinosinic–polycytidylic acid and blockade of programmed death-ligand 1 induce effective CD8 T-cell responses against established tumors. Clin Cancer Res. 2014;20(5):1223–1234. doi: 10.1158/1078-0432.CCR-13-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho HI, Jung SH, Sohn HJ, Celis E, Kim TG. An optimized peptide vaccine strategy capable of inducing multivalent CD8+ T cell responses with potent anti-tumor effects. Oncoimmunology. 2015 doi: 10.1080/2162402X.2015.1043504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swers JS, Grinberg L, Wang L, Feng H, Lekstrom K, Carrasco R, Xiao Z, Inigo I, Leow CC, Wu H, Tice DA, Baca M. Multivalent scaffold proteins as superagonists of TRAIL receptor 2-induced apoptosis. Mol Cancer Ther. 2013;12(7):1235–1244. doi: 10.1158/1535-7163.MCT-12-1107. [DOI] [PubMed] [Google Scholar]
- 51.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]