Abstract
A retrospective cohort study including 112 patients suffering from esophageal squamous cell carcinoma (ESCC) was performed to investigate the expression of B7-H4 in ESCC and determine its association with patient’s clinicopathological parameters and survival. Expression levels of B7-H4 on tumor cells and densities of tumor infiltrating lymphocytes (TILs) in the surgical specimens of ESCC tissues were characterized using immunohistochemical assays. Uni- and multivariate analyses were performed to evaluate the prognostic value of B7-H4 expression levels and densities of TILs in tumor sections. Positive B7-H4 immunostaining was observed in 107 of 112 (95.5%) of ESCC tissue sections. We further divided all patients into two major subgroups, a lower B7-H4 expression group with 46 patients and a higher B7-H4 expression group with 66 patients. We found that expression levels of B7-H4 on tumor cells were significantly correlated with patient’s gender (P = 0.0288), distant metastasis (P = 0.0500), and TNM stage (P = 0.0258). Moreover, tumor cell B7-H4 expression was inversely correlated with densities of CD3+ T cells in tumor nest (P = 0.0424) and CD8+ T cells in tumor stroma (P = 0.0229). The overall survival rate of the patients with higher B7-H4 expression was significantly worse than that of the patients with lower B7-H4 expression (P = 0.0105, Hazard Ratio: 1.854, 95%CI:1.152–2.902). Markers of cell-mediated immune responses such as CD3, CD8, and T-bet were associated with better patient survival. The present study demonstrated that B7-H4 expression in human ESCC is associated with cancer progression, reduced tumor immunosurveillance and worse patient outcomes. B7-H4 can serve as a novel prognostic predictor for human ESCC and a potential target for the immune therapy against this malignancy.
Keywords: B7-H4, Esophageal squamous cell carcinoma, Tumor infiltrating lymphocytes, Prognosis, Tumor immune surveillance
Introduction
Esophageal cancer is one of the most common malignancies and was the 9th leading cause of cancer death in 2009 [1]. Esophageal cancer can be generally divided into two major types, squamous cell carcinoma (SCC) and adenocarcinoma according to the etiological and histopathological classification, and the former is the major type of esophageal cancer in China [2, 3]. Surgical resection of the tumor at the primary site is the standard treatment for esophageal cancer, which shows favorable trends in patient’s postoperative prognosis [4]. As of now, many adjuvant therapies have been available in the treatment of esophageal cancers, including chemotherapy, radiotherapy, and immunotherapy. Nevertheless, due to the aggressive nature of this cancer, approximately 50–60% of esophageal cancer patients die mainly due to metastases after curative operation [5, 6]. The 5-year survival rate of esophageal cancer patients especially those diagnosed at advanced stages (i.e., stage III or IV) remains disappointing [7]. Therefore, it is important to discover novel biomarkers for early detection and new targets for the treatment of esophageal cancer.
The activation of antigen-specific T lymphocytes is modulated by co-stimulatory molecules, such as members of the B7 family of molecules, which deliver either positive or negative signals [8]. Recent studies demonstrate that inhibitory B7 family ligands are highly expressed in human cancer cells, and their protein levels in tumors significantly correlate with patients’ clinicopathological features and postoperative prognosis (reviewed in Ref. [9]). B7-H4 (also known as B7x and B7S1) is a member of the B7 family, and it was shown to inhibit T-cell functions such as proliferation, cytokine production, and development of cytotoxicity [10]. It has been shown that B7-H4 is highly expressed in tumor cells in human cancer tissues, and its protein levels are associated with various clinicopathological parameters and postoperative prognosis [11–18]. However, whether B7-H4 is expressed in esophageal cancer tissues and its relationship with TILs are not clear.
In the present study, we investigated tumor microenvironment by examining B7-H4 protein expression as well as densities of various TIL subsets in human ESCC tissues using the immunohistochemical method. B7-H4 expression in relation to patients’ clinicopathological parameters and densities of TILs was analyzed. Prognostic values of these markers were evaluated in a log-rank survival analysis. We showed that expression levels of the inhibitory costimulatory molecule B7-H4 in human ESCC tissues were significantly correlated with distant metastasis, cancer progression, densities of TILs, and poorer patient outcome. In addition, we demonstrated that markers of cell-mediated immune responses such as CD3, CD8, and T-bet were associated with better patient survival. Thus, our work identifies B7-H4 as an important factor for shaping tumor microenvironment, a novel target for human ESCC immunotherapy, and a new biomarker for postoperative prognosis in human ESCC.
Materials and methods
Development and characterization of the monoclonal antibody 3C8 against human B7-H4
Six-week-old BALB/c mice (Beerkape, Shanghai, China) were immunized by four injections of 1 × 107 mitomycin C-treated 293T-B7-H4 cells at a 21-day interval, respectively. Four days after the final injection, the splenocytes of immunized mice were fused with murine myeloma SP2/0 cells according to the standard procedure. After hypoxanthine/aminopterin/thymidine (HAT) selection, hybridoma cell lines secreting mouse anti-human B7-H4 monoclonal antibody 3C8 were obtained. The antibody was identified by its strong reactivity with 293T-B7-H4 and by its absence of cross-reactivity with 293T-mock cells and other B7 family molecules transgenic 293T cells. The monoclonal antibody 3C8 was purified from mouse ascites on protein G-Sepharose CL4B (Pharmacia, Uppsala, Sweden) affinity columns.
Patients and tissue samples
Formalin-fix, paraffin-embedded tumor tissue blocks were collected retrospectively from the Third Affiliated Hospital, Suzhou University (103 cases), and Yixing Tumor Hospital, Jiangsu Wuxi (9 cases), respectively. All of the 112 ESCC patients underwent surgical resection between December 2000 and August 2007 in the two hospitals. None of the patients received chemotherapy or radiotherapy before surgery. The pathologic reports were reviewed, and the tumor–node–metastasis (TNM) stages were assigned according to the American Joint Committee on Cancer staging system [19]. Patients’ clinical parameters are shown in Table 1, and patients’ survival intervals are dated toward the end of March 2010. In addition, 8 normal tissues from the non-malignant portion of esophagus were resected from surgery and used as controls. The protocol for the present study was approved by the ethics committees of the two hospitals.
Table 1.
Correlation between clinical parameters and B7-H4 expression
| Clinical parameters | Cases | B7-H4 Expression | ||||
|---|---|---|---|---|---|---|
| Low | High | P value | ||||
| n | % | n | % | |||
| Gender | ||||||
| Male | 80 | 38 | 47.5 | 42 | 52.5 | 0.0288 |
| Female | 32 | 8 | 25.0 | 24 | 75.0 | |
| Age (years) | ||||||
| <60 | 68 | 27 | 39.7 | 41 | 60.3 | 0.7150 |
| ≥60 | 44 | 19 | 43.2 | 25 | 56.8 | |
| Tumor size (cm) | ||||||
| ≤4 | 43 | 20 | 46.5 | 23 | 53.5 | 0.3556 |
| >4 | 69 | 26 | 37.7 | 43 | 62.3 | |
| Tumor (T) statusa | ||||||
| pT1 | 13 | 9 | 69.2 | 4 | 30.8 | 0.0871b |
| pT2 | 50 | 20 | 40.0 | 30 | 60.0 | |
| pT3 | 38 | 13 | 34.2 | 25 | 65.8 | |
| pT4 | 11 | 4 | 36.4 | 7 | 63.6 | |
| Nodal (N) statusc | ||||||
| N0 | 61 | 30 | 49.2 | 31 | 50.8 | 0.0564 |
| N1 | 51 | 16 | 31.4 | 35 | 68.6 | |
| Distant metastasis (M)d | ||||||
| M0 | 96 | 43 | 44.8 | 53 | 55.2 | 0.0500 |
| M1 | 16 | 3 | 18.8 | 13 | 81.2 | |
| TNM stage | ||||||
| I | 13 | 9 | 69.2 | 4 | 30.8 | 0.0258 b |
| II | 63 | 25 | 39.7 | 38 | 60.3 | |
| III | 20 | 9 | 45.0 | 11 | 55.0 | |
| IV | 16 | 3 | 18.8 | 13 | 81.2 | |
| Total | 112 | |||||
Values in bold signify P < 0.05
aTumor status is classified as follows: pT1 invasion of lamina propria or submucosa, pT2 invasion of muscularis propria, pT3 invasion of adventitia, and pT4 invasion of adjacent structures
bChi-square test for trend
cNodal status is classified as follows: N0 no regional lymph-node metastasis, N1 regional lymph-node metastasis
dDistant metastasis is classified as follows: M0 no distant metastasis, M1 metastasis to cervical nodes, celiac nodes, and other distant metastases
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissues were cut into 3-μm-thick consecutive sections and were dewaxed in xylene, rehydrated and graded ethanol solutions. Antigens were retrieved by heating the tissue sections at 100°C for 30 min in citrate (10 mmol/L, pH6.0) (for B7-H4, CD3, CD8 and T-bet) or EDTA (1 mmol/L, pH9.0) solution (for Foxp3) when needed. Then, the sections were immersed in a 0.3% hydrogen peroxide solution for 30 min to block endogenous peroxidase activity, rinsed in phosphate buffered saline (PBS) for 5 min, and incubated with primary antibodies against B7-H4 (mouse anti-human monoclonal antibody, Clone No. 3C8, final concentration in use, 4 μg/ml), CD3 (mouse anti-human monoclonal antibody, ready to use, Maixin Biotechnology Co., Ltd, Fuzhou, China), CD8 (mouse anti-human monoclonal antibody, ready to use, Maixin Biotechnology Co., Ltd, Fuzhou, China), T-bet (1:120 dilution in use, Clone No. H210, Santa Cruz Biotechnology, Inc., USA), and Foxp3 (1:100 dilution in use, Clone No. PCH101, eBioscience, USA) at 4°C overnight. A negative control was performed by omitting the primary antibodies. The sections were then incubated with horseradish peroxidase-labeled goat against mouse/rabbit secondary antibody (ready to use, Maixin Biotechnology Co., Ltd, Fuzhou, China). Diaminobenzene was used as the chromogen and hematoxylin as the nuclear counterstain. Sections were dehydrated, cleared, and mounted.
Evaluation of immunohistochemical staining
The B7-H4 immunostaining densities were assessed according to the H-score method described by Hammes et al. [20]: H-score = (% tumor cells unstained × 0) + (% tumor cells stained weak × 1) + (% tumor cells stained moderate × 2) + (% tumor cells stained strong × 3). The H-scores ranged from 0 (100% negative tumor cells) to 300 (100% strong staining tumor cells). The method of assessing the density of TILs was as described by our previous study [17]. In brief, TILs in both tumor stroma and tumor nest were determined according to the immunohistochemical staining. First, the TILs in tumor stroma were examined at low magnification (×40) and categorized according to density as follows: Grade 0, scanty; Grade 1, moderate infiltration; Grade 2, abundant infiltration; and Grade 3, massive infiltration. The group containing Grade 0 and Grade 1 was defined as low infiltration group, and another group containing Grade 2 and Grade 3 was defined as high infiltration group. Second, the TILs in tumor nest were counted as follows: five areas in tumor nest with the most intense infiltrating lymphocytes were selected at low magnification (×40), and then the TILs were counted and recorded at high power field (HPF, ×200 magnification). Results from the five areas were averaged and used in the statistical analysis.
Statistical analysis
The correlations between B7-H4 expression and patient’s clinicopathological parameters and the infiltrating densities of TILs in tumor tissues were analyzed by chi-square test. B7-H4 expression and the densities of TILs expression in relation to patient’s postoperative prognosis were examined by log-rank survival analysis, in which the minimum P value seek was conducted according to the method from the literatures and our previous study [21–23] using different H-score values or infiltrating densities as cutoffs, respectively. All statistical analyses were performed using the GraphPad Prism 4.0 software package (GraphPad Software, Inc., San Diego, USA) without any special comments. All statistical tests were two tailed.
Results
B7-H4 expression in human ESCC tissues
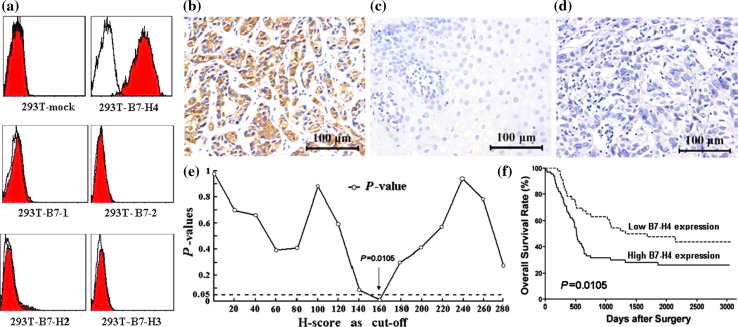
The mouse anti-human B7-H4 monoclonal antibody 3C8 strongly stained 293T-B7-H4, but did not stain 293T-mock cells or 293T cells expressing other B7 family molecules (Fig. 1a). This antibody was then used in the immunohistochemical assay. Positive B7-H4 immunohistochemical staining was predominantly observed on the membrane and in cytoplasm of tumor cells (Fig. 1b), while no staining was found in normal esophageal tissues (Fig. 1c). One hundred and seven out of 112 (95.5%) specimens of ESCC tissues showed positive B7-H4 immunohistochemical staining. In order to further investigate the clinical relevance of B7-H4 protein levels in the ESCC tissues, we categorized 112 patients into two major subgroups according to the intensity of B7-H4 immunohistochemical staining, i.e., the lower B7-H4 expression group, 46 cases (including H-score = 0, 5 cases; 0 < H-score ≤ 160, 41 cases), and the higher B7-H4 expression group (H-score > 160), 66 cases. The cutoff value of H-score = 160 was selected according to the minimum P value seek in the log-rank survival analysis (Fig. 1e and f).
Fig. 1.
The monoclonal mouse anti-human B7-H4 antibody 3C8 was established and used in the immunohistochemical assay. a Flow cytometry analysis showed that the antibody specifically stained 293T-B7-H4 transgenic cells, and the staining was absent in the cross-reactions with 293T-mock cells and other B7 family molecules transgenic 293T cells. b Immunohistochemistry showed that positive B7-H4 immunochemical staining was predominantly observed on the membrane and in cytoplasm of tumor cells. c Negative B7-H4 immunochemical staining in normal esophageal tissue. d Negative control. e The minimum P value seek in the log-rank survival analysis of B7-H4 expression in ESCC tissues was performed, and when the cutoff value of H-score = 160 was selected, the minimal P value = 0.0105 was found. f The log-rank survival analysis was performed when H-score = 160. Scar bar = 100 μm
Correlation between B7-H4 expression and patient’s clinicopathological features
The correlation between tumor cell B7-H4 expression and patients’ clinicopathologic parameters is shown in Table 1. It demonstrates that B7-H4 expression in human ESCC tissues is significantly correlated with patient’s gender (P = 0.0288), distant metastasis (P = 0.0500), and TNM stage (P = 0.0258), whereas it is not correlated with patient’s age, tumor size, tumor status, and nodal status. It is worth mentioning that there was a trend toward a positive correlation between B7-H4 expression and tumor status (P = 0.0871) as well as nodal status (P = 0.0564). Thus, our data demonstrate that the level of B7-H4 protein is positively correlated with advanced clinicopathological parameters and support the idea that B7-H4 is involved in the progression of human ESCC.
B7-H4 expression, densities and characteristics of TILs in relation to patient’s prognosis
Univariate analysis demonstrates that the overall survival rate of the subgroup with lower B7-H4 expression is significantly better than that the subgroup with higher B7-H4 expression (P = 0.0105, Hazard Ratio: 1.854, 95%CI, 1.152–2.902, Fig. 1f). As expected, as shown in Table 2, the clinicopathological parameters, such as tumor size, tumor status, nodal status, distant metastasis, and TNM stage, are also of prognostic values. In addition, we also found that densities of certain TIL subsets significantly correlated with patients’ outcomes. TILs such as CD3+ T cells, CD8+ T cells, T-bet+ lymphocytes, and Foxp3+ Tregs infiltrating both in tumor nest and in tumor stroma are included (Fig. 2). Patients with higher CD3+ T-cell infiltration (in either tumor nest, P = 0.0251, or in tumor stroma, P = 0.0320, Fig. 3a) or those with higher T-bet+ lymphocyte infiltration (in tumor nest, P = 0.0363, Fig. 3c) showed better postoperative prognosis than those patients with lower density of these cells. Moreover, the patients with higher numbers of CD3+ T cells in both tumor nest and tumor stroma showed even better survival (P = 0.0124, Fig. 3a). Similarly, the patients with higher numbers of CD8+ or T-bet+ cells in both tumor nest and tumor stroma survived much longer than patients with lower numbers of these cells (Fig. 3b, c). In contrast, although the overall survival rate of patients with lower Foxp3+ Tregs infiltration trends better than those with higher numbers of Tregs, we have not found statistically significant correlation between Foxp3 levels and survival of ESCC patients (Fig. 3d). Our multivariate analysis did not find that B7-H4 expression in ESCC tissues as an independent factor when it was analyzed in independent data sets involving clinicopathological features and densities of TILs, respectively (Table 2).
Table 2.
Univariate and multivariate Cox regression analyses of clinicopathological features, B7-H4 expression, and the densities of TILs in ESCC tissue sections
| Variables | Unfavorable/favorable | HR (95% CI) | P value |
|---|---|---|---|
| Univariate analysisa | |||
| Age (years) | ≥60/<60 | 1.124 (0.702–1.806) | 0.6217 |
| Gender | Male/female | 1.033 (0.619–1.722) | 0.9020 |
| Tumor size (cm) | >4/≤4 | 1.970 (1.196–3.013) | 0.0065 |
| Tumor status | III + IV/I + II | 1.819 (1.173–3.111) | 0.0093 |
| Nodal status | N1/N0 | 1.967 (1.275–3.320) | 0.0031 |
| Distant metastasis | M1/M0 | 4.363 (6.762–47.90) | <0.0001 |
| TNM stage | III + IV/I + II | 2.080 (1.387–4.152) | 0.0017 |
| B7-H4 expression | High/low | 1.854 (1.152–2.902) | 0.0105 |
| CD3-positive T cells in tumor nest | Low/high | 1.888 (1.073–2.862) | 0.0251 |
| CD3-positive T cells in tumor stroma | Low/high | 1.690 (1.045–2.640) | 0.0320 |
| CD8-positive T cells in tumor nest | Low/high | 1.451 (0.919–2.367) | 0.1079 |
| CD8-positive T cells in tumor stroma | Low/high | 1.469 (0.924–2.318) | 0.1046 |
| T-bet-positive lymphocytes in tumor nest | Low/high | 1.701 (1.041–3.364) | 0.0363 |
| T-bet-positive lymphocytes in tumor stroma | Low/high | 1.496 (0.891–2.374) | 0.1342 |
| Foxp3-positive Tregs in tumor nest | High/low | 1.319 (0.823–2.164) | 0.2415 |
| Foxp3-positive Tregs in tumor stroma | High/low | 1.359 (0.856–2.145) | 0.1953 |
| Multivariate Cox regression analysisb | |||
| Tumor size (cm) | >4/≤4 | 1.621 (0.954–2.755) | 0.0741 |
| Tumor status | III + IV/I + II | 1.375 (0.699–2.705) | 0.3559 |
| Nodal status | N1/N0 | 1.202 (0.620–2.330) | 0.5857 |
| Distant metastasis | M1/M0 | 3.731 (1.705–8.164) | 0.0010 |
| TNM stage | III + IV/I + II | 0.769 (0.290–2.033) | 0.5958 |
| B7-H4 expression | High/low | 1.544 (0.936–2.547) | 0.0892 |
| Multivariate Cox regression analysisc | |||
| CD3-positive T cells in tumor nest | Low/high | 0.691 (0.338–1.413) | 0.3117 |
| CD3-positive T cells in tumor stroma | Low/high | 0.776 (0.421–1.429) | 0.4154 |
| T-bet-positive lymphocytes in tumor nest | Low/high | 0.724 (0.417–1.258) | 0.2522 |
| B7-H4 expression | High/low | 1.555 (0.914–2.647) | 0.1034 |
Values in bold signify P < 0.05
aThe minimum P value seek was conducted according to the method in statistical analysis
bMultivariate Cox regression analysis in SPSS13.0 including clinicopathological features and B7-H4 expression in an independent data set
cMultivariate Cox regression analysis in SPSS13.0 including densities of TILs and B7-H4 expression in an independent data set
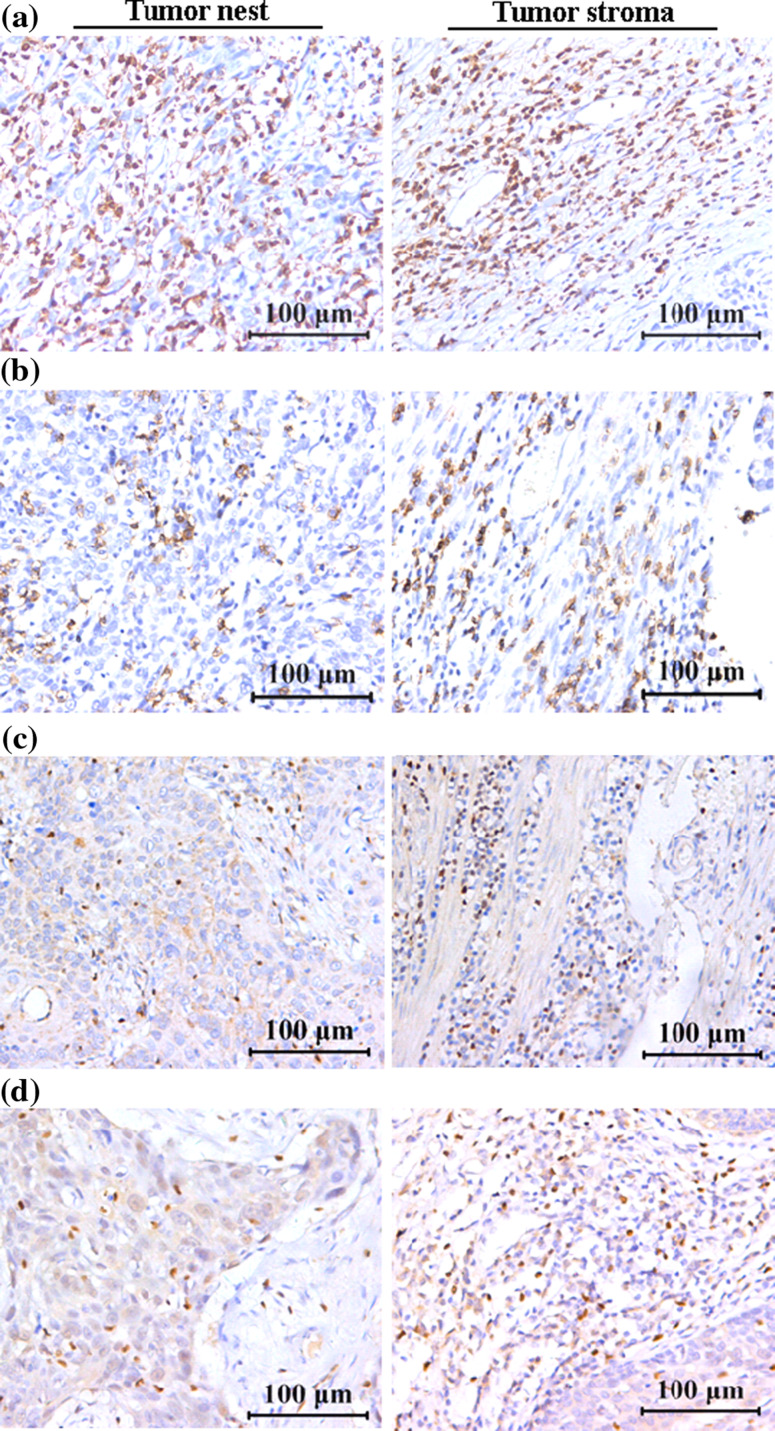
Fig. 2.
Infiltrations of TILs in ESCC tissues (left panel, in tumor nest and right panel, in tumor stroma). a CD3-positive T cells. b CD8-positive T cells. c T-bet-positive lymphocytes. d Foxp3-positive Tregs. Scar bar = 100 μm
Fig. 3.
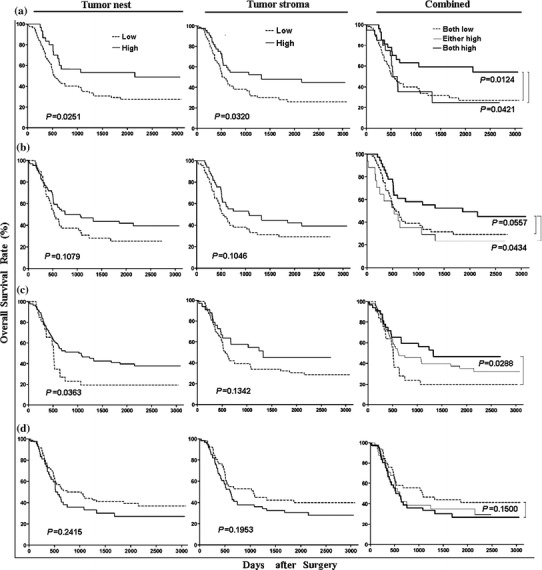
Survival analyses of infiltrating densities of TILs in ESCC tissues (left panel, in tumor nest, middle panel, in tumor stroma and right panel, combined). a CD3-positive T cells. b CD8-positive T cells. c T-bet-positive lymphocytes. d Foxp3-positive Tregs
Correlation between tumor cell B7-H4 expression and densities of TILs
As shown in Table 3, levels of B7-H4 expression on tumor cells were inversely correlated with the density of CD3+ T cells in tumor nest (P = 0.0424) and CD8+ T cells in tumor stroma (P = 0.0229), while they were not correlated with densities of Foxp3-positive cells in tumor nest or in tumor stroma (data not shown). Thus, our data further support a possible role of B7-H4 in suppressing cellular immune surveillance of human ESCC.
Table 3.
Correlation between B7-H4 expression and the densities of TILs in ESCC tissue sections
| B7-H4 expression | Cases | CD3-positive T cells | CD8-positive T cells | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In tumor nest | In tumor stroma | In tumor nest | In tumor stroma | ||||||||||
| Low | High | P value | Low | High | P value | Low | High | P value | Low | High | P value | ||
| Low | 46 | 29 | 17 | 0.0424 | 24 | 22 | 0.1223 | 27 | 19 | 0.3027 | 20 | 26 | 0.0229 |
| High | 66 | 53 | 13 | 44 | 22 | 45 | 21 | 43 | 23 | ||||
Values in bold signify P < 0.05
Discussion
B7-H4 is a member of the B7 gene family, and it has been implicated in negatively regulating the T-cell-mediated immunity. Here, we demonstrate that B7-H4 is overexpressed in human ESCC tissues but not expressed in normal esophageal tissues. Higher B7-H4 expression is found in 58.9% (66/112) of all 112 ESCC patients, and it is positively correlated with distant metastasis, advanced TNM stage, and poor patient outcome, implicating its role in ESCC progression. Furthermore, we demonstrate that protein levels of B7-H4 in tumor cells inversely correlate with densities of CD3+ and CD8+ TILs, supporting the idea that it is involved in avoidance of antitumor immunity by human esophageal cancer cells. Besides B7-H4, we also found that densities of certain subsets of TILs, such as CD3, CD8, and T-bet-positive lymphocytes, are of prognostic values for ESCC, supporting the clinical utility for these markers.
It has been found that increased B7-H4 expression is involved in shaping the tumor microenvironment, and aberrant B7-H4 expression is associated with various clinicopathological features in many human malignancies [9]. B7-H4 expression was significantly higher in invasive breast cancer cells than in the normal breast epithelium, and increased B7-H4 expression was associated with negative progesterone receptor status, HER-2/neu status, and history of neoadjuvant chemotherapy of breast cancer patients [18]. B7-H4 expression is much higher in ovarian papillary serous adenocarcinoma than other less malignant ovarian cancers or normal tissues [16]. Interestingly, B7-H4+ tumor-associated macrophages in ovarian cancer inhibit T-cell activation, and its levels predict poor survival of the patient [24]. Krambeck et al. [15] reported that 59.1% (153/259) renal cell carcinoma (RCC) specimens exhibited positive B7-H4 immunostaining, and B7-H4 expression level was associated with adverse clinical and pathologic features, including constitutional symptoms, tumor necrosis, tumor size, grade, cancer stage, and patient’s survival. Aberrant B7-H4 expression was also found in prostate cancer, and strong expression of B7-H4 is positively correlated with extra capsular extension, seminal vesicle invasion, and distant metastasis [25]. We recently demonstrated that higher B7-H4 expression in cancer cells was significantly associated with poor prognosis of the patients suffering from gastric cancer [26]. As of now, however, the expression of B7-H4 and its clinical significance in human esophageal cancer are not known. Our study, for the first time, revealed that B7-H4 is overexpressed in ESCC, and its expression level is significantly correlated with distant metastasis, advanced TNM stage, and poor patient’s outcome, and shows a trend of positive correlation with the invasion depth and nodal metastasis. Thus, our findings in the present study support that B7-H4 expression might play an important role in ESCC progression and can serve as a good prognostic indicator for this cancer.
The regulatory mechanism of B7-H4 expression in tumor cells still remains to be determined. It has been demonstrated that IL-6 and IL-10 strongly stimulate B7-H4 expression on monocytes, macrophages, myeloid-derived DCs, whereas IL-4 and GM-CSF suppress its expression [27, 28]. IFN-γ is known for the induction of B7-H1 expression; however, its effect on B7-H4 expression is minimal. Tumor-associated T cells stimulated macrophages to produce IL-6 and IL-10, which subsequently promote B7-H4 expression on macrophages themselves [24, 27, 29]. In contrast, none of the cytokines such as IL-4, GM-CSF, IL-6, IL-10, IFN-γ, and TNF-α has any significant effect on tumor B7-H4 expression, suggesting that B7-H4 is differentially regulated in tumor cells and macrophages [27], i.e. B7-H4 is constitutively expressed on tumor cells and its expression is inducible in macrophages.
It has been suggested that the B7-H4 molecule promotes cancer progression via two possible mechanisms, one is to serve as a negative regulator of T-cell-mediated antitumor immunity, and the other one is to render tumor cells refractory to apoptosis [9, 15]. Previous studies from us and other groups showed that the higher expression of B7-family inhibitory molecules by tumor cells is significantly correlated with densities of TILs in some human malignancies [9, 17]. Kryczek et al. [24] reported that B7-H4-positive tumor-associated macrophages positively correlated with the number of Foxp3-positive Tregs in ovarian cancer. Our current study shows that the expression of B7-H4 on esophageal cancer cells is inversely correlated with densities of CD3+ T cells in tumor nest and CD8+ T cells in tumor stroma, suggesting that B7-H4 inhibits the recruitment or survival of tumor infiltrating T lymphocytes in the tumor microenvironment. Collectively, results from all of these studies support an important role of B7-H4 in shaping tumor immune suppressive microenvironment.
TIL subsets, particularly Th1, Tc1, and NK cells, perform important roles in host immune responses against cancer cells. T-bet, a transcription factor important for the function of Th1, Tc1, and NK cells, has been shown to be important for adaptive immune response against tumor [30, 31]. On the other hand, regulatory TILs promote tumor progression by inhibiting antitumor immune responses. It has been reported that lower densities of tumor infiltrating lymphocytes are associated with poor outcome in esophageal squamous cell carcinoma [32–35]. However, the characteristics of TILs, particularly the cytolytic and regulatory nature of TILs, were not examined. We then decided to examine the prognostic values of the densities as well as characteristics of TILs in ESCC tissues. As shown in Table 2, we found that patients with higher numbers of tumor infiltrating CD3+ and CD8+ T cells had better postoperative prognosis than patients with lower numbers of these TILs. Despite the fact that many studies showed that the density of Foxp3-positive cells correlated with poor cancer outcome, in the present study in ESCC, we found that the overall survival rate of patients with lower densities of Foxp3+ Tregs seems better than those with higher densities, but the difference was not statistically significant. In contrast, T-bet, a member of the T-box family of transcription factors, which is a critical transcription factor for Th1 cells, Tc1 cells, and NK cells [30], has been shown as a valuable prognostic predictor of ESCC in the present study. T-bet may activate Th1 and Tc1 genetic programs in part by directly controlling IFN-γ gene transcription and inhibit Th2 and Tc2 differentiation [36]. Moreover, our recent study demonstrates that T-bet and Eomes coordinately regulate the migration of antitumor T cells to tumor tissues [31]. Therefore, our data support the idea that the presence of T-bet+ TILs represents an effective antitumor response against ESCC. It remains to be studied how inhibitory factors such as B7-H4 regulates the antitumor Th1/Tc1 response within the tumor microenvironment.
In conclusion, our findings indicate that the inhibitory co-stimulatory molecule B7-H4 is involved in ESCC progression and tumor avoidance of immunosurveillance and could be a useful prognostic indicator for human ESCC. Our data also support efforts to develop immunotherapeutic approaches targeting B7-H4 for the treatment of human ESCC.
Acknowledgments
We thank senior pathologists Chang-qing Lu, Qing Li, Bo Tian, Jun Xie, Cao Wu, Yuan-dong Chen and Prof. Tong-yu Chen (Department of Pathology, the Third Affiliated Hospital of Suzhou University, Jiangsu Changzhou, China) for their expert suggestions and technical assistances. This work was supported by grants from the Major State Basic Research Development Program of China (973 Program: CB51003), the National Natural Science Foundation of China (No. 30930085), and the advanced program of Commission of Technology and Industry for National Defense (A3820060130).
Conflict of interest
The authors declare that they have no competing interests to this paper.
Footnotes
L. Chen and J. Sun contributed equally to this work.
Contributor Information
Bin-feng Lu, Phone: +412-648-9339, FAX: +412-383-8098, Email: binfeng@pitt.edu.
Xue-guang Zhang, Phone: +86-512-65732002, FAX: +86-512-65104908, Email: xueguangzh@yahoo.com.cn.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Stoner GD, Wang LS, Chen T. Chemoprevention of esophageal squamous cell carcinoma. Toxicol Appl Pharmacol. 2007;224(3):337–349. doi: 10.1016/j.taap.2007.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L, Luo G, Tan Y, Wei J, Wu C, Zheng L, Zhang X, Xu N. Immunolocalisation of tissue factor in esophageal cancer is correlated with intratumoral angiogenesis and prognosis of the patient. Acta Histochem. 2010;112(3):233–239. doi: 10.1016/j.acthis.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 4.Hofstetter W, Swisher SG, Correa AM, Hess K, Putnam JB, Jr, Ajani JA, Dolormente M, Francisco R, Komaki RR, Lara A, Martin F, Rice DC, Sarabia AJ, Smythe WR, Vaporciyan AA, Walsh GL, Roth JA. Treatment outcomes of resected esophageal cancer. Ann Surg. 2002;236(3):376–384. doi: 10.1097/00000658-200209000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stathopoulos GP, Tsiaras N. Epidemiology and pathogenesis of esophageal cancer: management and its controversial results (review) Oncol Rep. 2003;10(2):449–454. [PubMed] [Google Scholar]
- 6.Stoner GD, Gupta A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis. 2001;22(11):1737–1746. doi: 10.1093/carcin/22.11.1737. [DOI] [PubMed] [Google Scholar]
- 7.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349(23):2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 8.Greenfield EA, Nguyen KA, Kuchroo VK. CD28/B7 costimulation: a review. Crit Rev Immunol. 1998;18(5):389–418. doi: 10.1615/critrevimmunol.v18.i5.10. [DOI] [PubMed] [Google Scholar]
- 9.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 10.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7–H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18(6):849–861. doi: 10.1016/S1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 11.Miyatake T, Tringler B, Liu W, Liu SH, Papkoff J, Enomoto T, Torkko KC, Dehn DL, Swisher A, Shroyer KR. B7–H4 (DD-O110) is overexpressed in high risk uterine endometrioid adenocarcinomas and inversely correlated with tumor T-cell infiltration. Gynecol Oncol. 2007;106(1):119–127. doi: 10.1016/j.ygyno.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 12.Tringler B, Liu W, Corral L, Torkko KC, Enomoto T, Davidson S, Lucia MS, Heinz DE, Papkoff J, Shroyer KR. B7–H4 overexpression in ovarian tumors. Gynecol Oncol. 2006;100(1):44–52. doi: 10.1016/j.ygyno.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, Wang X. B7–H3 and B7–H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53(2):143–151. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Simon I, Zhuo S, Corral L, Diamandis EP, Sarno MJ, Wolfert RL, Kim NW. B7–h4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res. 2006;66(3):1570–1575. doi: 10.1158/0008-5472.CAN-04-3550. [DOI] [PubMed] [Google Scholar]
- 15.Krambeck AE, Thompson RH, Dong H, Lohse CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. B7–H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci U S A. 2006;103(27):10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salceda S, Tang T, Kmet M, Munteanu A, Ghosh M, Macina R, Liu W, Pilkington G, Papkoff J. The immunomodulatory protein B7–H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp Cell Res. 2005;306(1):128–141. doi: 10.1016/j.yexcr.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Sun J, Chen L, Zhang G, Jiang J, Zhu M, Tan Y, Wang H, Lu B, Zhang X. Clinical significance and regulation of the costimulatory molecule B7–H3 in human colorectal carcinoma. Cancer Immunol Immunother. 2010;59(8):1163–1171. doi: 10.1007/s00262-010-0841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tringler B, Zhuo S, Pilkington G, Torkko KC, Singh M, Lucia MS, Heinz DE, Papkoff J, Shroyer KR. B7–h4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res. 2005;11(5):1842–1848. doi: 10.1158/1078-0432.CCR-04-1658. [DOI] [PubMed] [Google Scholar]
- 19.Greene FL, Page DL, Fleming ID, et al. AJCC cancer staging manual. 6. New York: Springer; 2002. [Google Scholar]
- 20.Hammes LS, Tekmal RR, Naud P, Edelweiss MI, Kirma N, Valente PT, Syrjanen KJ, Cunha-Filho JS. Up-regulation of VEGF, c-fms and COX-2 expression correlates with severity of cervical cancer precursor (CIN) lesions and invasive disease. Gynecol Oncol. 2008;110(3):445–451. doi: 10.1016/j.ygyno.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 21.Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, Wu WZ, Wang L, Tang ZY, Sun HC. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26(16):2707–2716. doi: 10.1200/JCO.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 22.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 23.Chen L, Di D, Luo G, Zheng L, Tan Y, Zhang X, Xu N. Immunochemical staining of MT2-MMP correlates positively to angiogenesis of human esophageal cancer. Anticancer Res. 2010;30(10):4363–4368. [PubMed] [Google Scholar]
- 24.Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, Chen L, Coukos G, Zou W. Relationship between B7–H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67(18):8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 25.Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, Scardino PT, Sharma P, Allison JP. B7–H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A. 2007;104(49):19458–19463. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang J, Zhu Y, Wu C, Shen Y, Wei W, Chen L, Zheng X, Sun J, Lu B, Zhang X. Tumor expression of B7–H4 predicts poor survival of patients suffering from gastric cancer. Cancer Immunol Immunother. 2010;59(11):1707–1714. doi: 10.1007/s00262-010-0900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, Ochoa A, Chen L, Zou W. B7–H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203(4):871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kryczek I, Wei S, Zou L, Zhu G, Mottram P, Xu H, Chen L, Zou W. Cutting edge: induction of B7–H4 on APCs through IL-10: novel suppressive mode for regulatory T cells. J Immunol. 2006;177(1):40–44. doi: 10.4049/jimmunol.177.1.40. [DOI] [PubMed] [Google Scholar]
- 29.Song H, Park G, Kim YS, Hur I, Kim H, Ryu JW, Lee HK, Cho DH, Choi IH, Lee WJ, Hur DY. B7–H4 reverse signaling induces the apoptosis of EBV-transformed B cells through Fas ligand up-regulation. Cancer Lett. 2008;266(2):227–237. doi: 10.1016/j.canlet.2008.02.067. [DOI] [PubMed] [Google Scholar]
- 30.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–669. doi: 10.1016/S0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Y, Ju S, Chen E, Dai S, Li C, Morel P, Liu L, Zhang X, Lu B. T-bet and Eomesodermin are required for T cell-mediated antitumor immune responses. J Immunol. 2010;185(6):3174–3183. doi: 10.4049/jimmunol.1000749. [DOI] [PubMed] [Google Scholar]
- 32.Yasunaga M, Tabira Y, Nakano K, Iida S, Ichimaru N, Nagamoto N, Sakaguchi T. Accelerated growth signals and low tumor-infiltrating lymphocyte levels predict poor outcome in T4 esophageal squamous cell carcinoma. Ann Thorac Surg. 2000;70(5):1634–1640. doi: 10.1016/S0003-4975(00)01915-9. [DOI] [PubMed] [Google Scholar]
- 33.Chouaib S, Asselin-Paturel C, Mami-Chouaib F, Caignard A, Blay JY. The host-tumor immune conflict: from immunosuppression to resistance and destruction. Immunol Today. 1997;18(10):493–497. doi: 10.1016/S0167-5699(97)01115-8. [DOI] [PubMed] [Google Scholar]
- 34.Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, Kawarada Y, Hida Y, Oshikiri T, Kurokawa T, Suzuoki M, Nakakubo Y, Hiraoka K, Murakami S, Shinohara T, Itoh T, Okushiba S, Kondo S, Katoh H. CD4 + and CD8 + T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63(7):1555–1559. [PubMed] [Google Scholar]
- 35.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61(10):3932–3936. [PubMed] [Google Scholar]
- 36.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295(5553):338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]