Abstract
Aim of study
To evaluate the feasibility of ipilimumab treatment for metastatic melanoma outside the boundaries of clinical trials, in a setting similar to that of daily practice.
Methods
Ipilimumab was available upon physician request in the Expanded Access Programme for patients with life-threatening, unresectable stage III/IV melanoma who failed or did not tolerate previous treatments and for whom no therapeutic option was available. Induction treatment with ipilimumab 10 mg/kg was administered intravenously every 3 weeks, for a total of 4 doses, with maintenance doses every 12 weeks based on physicians’ discretion and clinical judgment. Tumors were assessed at baseline, Week 12, and every 12 weeks thereafter per mWHO response criteria, and clinical response was scored as complete response (CR), partial response (PR), stable disease (SD), or progressive disease. Durable disease control (DC) was defined as SD at least 24 weeks from the first dose, CR, or PR.
Results
Disease control rate at 24 and 60 weeks was 29.6% and 15%, respectively. Median overall survival at a median follow-up of 8.5 months was 9 months. The 1- and 2-year survival rates were 34.8% and 23.5%, respectively. Changes in lymphocyte count slope and absolute number during ipilimumab treatment appear to correlate with clinical response and survival, respectively. Adverse events were predominantly immune related, manageable, and generally reversible. One patient died from pancytopenia, considered possibly treatment related.
Conclusion
Ipilimumab was a feasible treatment for malignant melanoma in heavily pretreated, progressing patients. A sizeable proportion of patients experienced durable DC, including benefits to long-term survival.
Keywords: Ipilimumab, Cytotoxic T-lymphocyte antigen 4, Advanced melanoma, Immunotherapy, Compassionate use, Monoclonal antibody
Introduction
The World Health Organization estimates that worldwide, 48,000 deaths occur yearly due to the malignant melanomas associated with excess exposure to ultraviolet radiation alone [1]. The median survival time for patients with stage IV melanoma is 6–9 months and the 1-year survival rate is about 25% [2, 3]. The poor prognosis is largely due to a current lack of effective systemic therapies for advanced melanoma that challenges its management. Standard chemotherapeutic agents such as dacarbazine and temozolomide demonstrate very low response rates [4]. Combining these with biological agents may increase response, but no survival benefit has been demonstrated [5]. Response to dacarbazine monotherapy, the current standard of care for first-line therapy of advanced melanoma, is short-lived with an objective response rate of only 3.6% [6]. High-dose interleukin-2 induces durable responses in 16% of patients, but has limited utility due to substantial toxicity and the need for administration in a hospital equipped with an intensive care facility [7]. Furthermore, it is not approved in Europe. The currently poor prognosis of patients with advanced melanoma and the limitations of current therapies highlight the unmet need for more effective and better tolerated treatments.
Melanoma is highly immunogenic [8], and various immunotherapies are being investigated that take advantage of the antitumor immune response. Ipilimumab (Bristol-Myers Squibb, New York City, NY, USA) is a fully human monoclonal antibody that neutralizes cytotoxic T-lymphocyte antigen-4 (CTLA-4) [9, 10]. By blocking this key negative regulator of T-cell activation, ipilimumab acts to potentiate the antitumor immune response [11]. Durable responses and disease stabilization have been demonstrated for ipilimumab in phase II clinical trials in advanced melanoma [9, 12–14], with 3-year survival rates ranging from 23% to 34% [15]. In the phase III, randomized, controlled trial MDX010-20, ipilimumab demonstrated a statistically significant improvement in overall survival in patients with previously treated, advanced melanoma [16]. Ipilimumab clinical activity appears to be independent of a number of negative prognostic factors [17], and response to treatment may follow a period of apparent disease progression [18, 19].
A number of inflammatory, immune-related adverse events (irAEs) have been associated with ipilimumab that are a direct consequence of its mechanism of action. The most common irAEs seen in clinical trials are rash, diarrhea/colitis, and hepatitis [12, 13, 20–22]. Patients treated with ipilimumab experience irAEs that are generally low grade, manageable, and reversible; however, severe and potentially life-threatening irAEs (e.g., colitis and hepatitis) can occur [13, 21]. The majority of these grade 3/4 irAEs are manageable by implementing irAE management algorithms [21–24]. Evidence suggests that the occurrence of irAEs in patients may be associated with tumor regression and prolonged time to relapse [13], however, patients who have not experienced an irAE have also demonstrated DC upon ipilimumab therapy [14, 25].
The Ipilimumab Expanded Access Programme (formerly known as the Ipilimumab Compassionate Use Programme) was initiated to provide ipilimumab to patients who could not receive it within clinical trials. The program allowed physicians to request ipilimumab on a compassionate use basis for patients with life-threatening, unresectable stage III or IV melanoma who failed or could not tolerate previous treatments and for whom no alternative drug, therapy, or trial was available. The physicians concluded, based upon available data on benefit and risk, that administration of ipilimumab at a dose of 10 mg/kg induction/maintenance was an appropriate treatment for their patient.
Experiences from the Expanded Access Programme offer an opportunity to examine the utility of ipilimumab in a setting that more closely mirrors daily clinical practices utilized outside the strict limits of clinical trials. The objective of this manuscript is to describe clinical experiences with ipilimumab in patients with advanced melanoma participating in an open-label, Expanded Access Programme at a single Italian medical institution. Patients included individuals with less common disease subtypes and metastasis to various organs, including the brain.
Patients and methods
Patient population
Patients eligible for the program had serious or immediately life-threatening, histologically confirmed, unresectable stage III or IV skin, ocular, or mucosal melanoma with no alternative treatment options, no treatment with ipilimumab through a clinical study, and whose physicians requested compassionate use of ipilimumab. Patients with asymptomatic brain metastases were also allowed. An Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2 was required, and an interval of at least 28 days since treatment with chemotherapy, biochemotherapy, surgery, radiation, or immunotherapy was recommended. Main exclusion criteria were any other systemic therapy for melanoma; concomitant autoimmune diseases or other malignancies; and known HIV, hepatitis B, or hepatitis C infection.
Treatment design
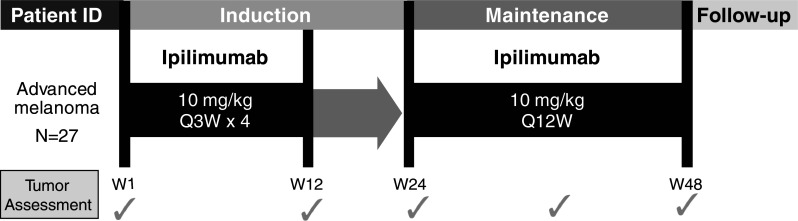
The treatment design is shown in Fig. 1. Ipilimumab 10 mg/kg was administered intravenously over 90 min every 3 weeks, for a total of 4 induction doses. Eligible patients could receive maintenance therapy with ipilimumab 10 mg/kg every 12 weeks, as tolerated, for as long as the physician believed that benefit would be derived from treatment. Treatment was discontinued upon a patient’s withdrawal of consent; pregnancy; or any clinical adverse event (AE), laboratory abnormality, or development of illness that, in the opinion of the physician, indicated that a patient’s continued participation in the program was not advisable. Due to the unique mechanism of action of ipilimumab, kinetics and patterns of response may differ from that of other anticancer therapies; therefore, patients with progressive disease undergo confirmatory scans no less than 4 weeks since the prior scan to verify the reliability of the radiologic finding before discontinuation of ipilimumab. If disease progression prior to Week 12 was followed by objective response, discontinuation was not required if ipilimumab was tolerated. Also, an increase in tumor size due to tumor infiltrating lymphocytes may be mistaken for disease progression; therefore, treatment was to be continued until evidence of tumor burden increase (25% increase from baseline in the sum of the product of the diameters of defined index lesions). Small new lesions, by themselves, were not criteria for discontinuation as long as index lesions were stable or decreasing. Progressive disease was to be confirmed 4 weeks later.
Fig. 1.
Treatment design. ID identification, W week
Patients were considered for ipilimumab reinduction in the Expanded Access Programme based on interim results from the CA184-025 study that evaluated the clinical response to and safety of ipilimumab reinduction therapy in patients from three completed parent phase II clinical trials (CA184-004, CA184-007, CA184-008, and CA184-022). The patients who received reinduction demonstrated disease progression following initial disease control in response to induction dosing with ipilimumab. An interim analysis of data from CA184-025 suggested that 50% of these patients responded to reinduction with 10 mg/kg ipilimumab [26].
Program ethics
This Expanded Access Programme was approved by a local Independent Ethics Committee, and all patients provided a signed informed consent form.
Analysis of safety
All patients who received ipilimumab in the Expanded Access Programme were monitored and assessed continuously for safety and AEs, including irAEs. AEs and other symptoms were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. AEs, serious AEs (SAEs), and irAEs were recorded and analyzed.
Analysis of clinical response
Tumor assessments (TA) utilizing helical (spiral) CT scans of brain, chest, abdomen, and pelvis were performed per previously reported modified World Health Organization (mWHO) criteria [27] at baseline, Week 12 (±2 weeks), Week 24, and every 12 weeks thereafter. Clinical response was scored as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). Disease control (DC) was defined as SD continuing for at least 24 weeks from the first ipilimumab dose, CR, or PR; disease control rate (DCR) was the percentage of patients in these three categories. Overall response (OR) was evaluated per time point of tumor assessment.
Statistical methodology
Data were analyzed using descriptive statistics (e.g., mean, median, standard deviation, and range). The Cox proportional hazard model was used to investigate the correlation between clinical response and baseline biohumoral and peripheral blood count parameters: P-value < 0.05 was considered statistically significant. A multivariate model was used to identify independent factors using the biohumoral parameters collected in the Expanded Access Programme as independent variables. To investigate the role of biohumoral parameters and peripheral blood counts, the slope for the change between baseline and Week 4 values relative to baseline was calculated. A slope <0 indicates a decrease in the parameter from baseline to Week 4 [28, 29]. Overall survival was estimated by the Kaplan–Meier method, median survival was reported with 95% confidence intervals (CI), and standard error (SE) was calculated for rates.
Results
Patients
Between February and September 2008, 27 patients (14 men, 13 women) with unresectable stage III or IV malignant melanoma were treated with ipilimumab at the University Hospital of Siena as part of an ipilimumab Expanded Access Programme. Patients ranged in age from 23 to 77 years (median = 55 years). Characteristics of patients at baseline are presented in Table 1: 2 (7.4%) and 25 (92.6%) patients had stage III and stage IV disease, respectively. Twenty-one patients (77.8%) had stage M1c disease, including 8 (29.6%) with surgically removed (2) or radiotherapy-stabilized (6) brain metastases that did not require steroid treatment. All patients had an ECOG PS of 0 (55.6%) or 1 (44.4%) and 11 patients (41%) had lactate dehydrogenase (LDH) levels >1× upper limit of normal (ULN). Patients received a median of 3 prior therapies for metastatic disease (range: 1–5). All patients received at least one dose of ipilimumab, 78% of patients completed the induction phase, and 41% continued with maintenance therapy. During induction, three patients discontinued treatment due to serious AEs, and three due to disease progression.
Table 1.
Patient characteristics at baseline
| Characteristic | N = 27 |
|---|---|
| Gender | |
| Male/female—n (%) | 14 (52)/13 (48) |
| Age, years, median (range) | 55 (23–77) |
| Melanoma diagnosis, n (%) | |
| Cutaneous | 23 (85.2) |
| Uveal | 3 (11.1) |
| Mucosal | 1 (3.7) |
| Disease stage, n (%) | |
| III | 2 (7.4) |
| IV | 25 (92.6) |
| M stage, n (%) | |
| M0 (stage III) | 2 (7.4) |
| M1a | 3 (11.1) |
| M1b | 1 (3.7) |
| M1c | 21 (77.8) |
| M1c—brain | 8a (29.6) |
| LDH >1× ULN, n (%) | 11 (40.7) |
| ECOG PS, n (%) | |
| 0 | 15 (55.6) |
| 1 | 12 (44.4) |
| Prior therapies for metastatic disease, median (range) | 3 (1–5) |
ECOG PS Eastern Cooperative Oncology Group performance status, LDH lactate dehydrogenase, ULN upper limit of normal
aEvidence in 6, history in 2
Clinical response
Results for clinical response are summarized in Table 2. At Week 12, 1 of 21 patients had PR and 5 had SD; at Week 24, 3 of 11 patients had PR, and 5 had SD (DCR = 30%; 8/27); at Week 48, 2 of 6 patients had PR and 4 had SD (DCR = 22%; 6/27); at Week 60, 2 of 6 patients had PR, and 2 had SD (DCR = 15%; 4/27); and at Weeks 84 and 96, 2 of 4 patients had PR, and 2 had SD (DCR = 15%; 4/27)—these patients remain on treatment.
Table 2.
Clinical response to ipilimumab
| Response measure | Study week | |||||
|---|---|---|---|---|---|---|
| W12 (n = 27) | W24 (n = 27) | W48 (n = 27) | W60 (n = 27) | W84 (n = 27) | W96 (n = 27) | |
| OR, n (%) | ||||||
| CR | 0 | 0 | 0 | 0 | 0 | 0 |
| PR | 1 (3.7) | 3 | 2 | 2 | 2 | 2 |
| SD | 5 (18.5) | 5 | 4 | 2 | 2 | 2 |
| DC, n (%) | NAa | 8 (29.6) | 6 (22.2) | 4 (15) | 4 (15) | 4 (15) |
CR complete response, DC disease control, OR overall response, PR partial response, SD stable disease, W week
aNot applicable—by definition, SD must be of at least 24 weeks duration to be included in the disease control category
Several patterns of response to ipilimumab were observed that differ from those seen with most anticancer agents; this finding is consistent with observations made in phase II trials [19, 30]. Some treatment-responsive patients demonstrated a slow and steady decline in tumor volume, and new lesions sometimes appeared with a subsequent decline in total tumor burden. Also, in some cases, disease initially progressed after initiation of ipilimumab therapy, with subsequent stabilization or partial improvement seen upon continued treatment. Finally, some patients who continued treatment after PD remain alive and in generally good health (Table 3). These response patterns are illustrated further in the case studies presented later in this article.
Table 3.
Patterns of clinical response after initial PD
| Patient ID | Study week | ||||||
|---|---|---|---|---|---|---|---|
| W12 | W18 | W24 | W36 | W48 | W60 | W72 | |
| Patients with initial progressiona | |||||||
| #7 | PD | SD | SD | SD | SD | PR | – |
| #8 | PD | NA | SD | SD | SD | SD | – |
| #25 | PD | PD | PD | PD | – | – | – |
| #26 | PD | PR | PD | PD | PD | PD | – |
| Reinduced patientsb | |||||||
| #2 | SD | SD | SD | SD | SD | PD | Reinduction |
| #9 | SD | NA | PR | PR | PD | PD | Reinduction |
PD progressive disease, PR partial response, SD stable disease, W week, NA not available
aThese patients showed a SD or PR: continued treatment and observation might be beneficial in patients experiencing SD or even PD without performance status worsening
bThese patients progressed after disease control during the maintenance phase and were reinduced with ipilimumab using the same dosing schedule and remain on treatment
Two patients progressed during the maintenance phase and were reinduced with ipilimumab using the same dosing schedule. Upon reinduction, the W24 TA showed PD in one patient and SD in the other.
In patient populations generally excluded from phase II trials, disease remains stable at W120 in 1 of 3 patients with ocular melanoma; a PR has been demonstrated in the case with mucosal melanoma (see Case 2 below). Among the 6 patients with evidence of brain metastases, brain disease remained stable for 36 weeks in one patient and progressed with no evidence of treatment-related neurological AEs in the remaining five patients.
Survival
As of October 2010, at a median follow-up of 9.6 months, median overall survival (27 patients) was 9.6 months (95% CI: 4.2–15; range: 3.1–29.8). The 1-year survival rate was 34.8% (SE: 9.6%) with a survival rate at 2 years of 23.5% (SE: 8.4%). Median duration of response (8 patients) was 120 weeks (range: 29–128 weeks).
Association of clinical response with biohumoral parameters and peripheral blood count trends
Several variables appear to be associated with survival: baseline levels of albumin (P = 0.05), LDH (P = 0.005), and amylase (P = 0.01); the number of previous lines of therapy (P = 0.01); and tumor response (P = 0.006). The multivariate model suggested that only tumor response, amylase, and LDH appear to independently influence survival. Care must be taken when interpreting the significance of the LDH results due to small sample size—only 40% of these heavily pretreated patients had an LDH elevation, and the distribution of LDH across patients may have influenced this result.
Among peripheral blood count parameters, only change from baseline to Week 4 in absolute lymphocyte count (ALC) appears to be a potential predictor of clinical response to ipilimumab monotherapy. As shown in Table 4a, no patients with negative ALC slope demonstrated DC, suggesting that stable or decreasing values are associated with lack of clinical activity. Furthermore, as shown in Table 4b, patients with an ALC > 1500 cells/μL after 2 ipilimumab treatments (Week 7) had an increased overall survival compared with those having an ALC < 1500 cells/μL at the same time point (41 weeks vs 21 weeks, respectively).
Table 4.
Association of ALC with clinical response and OS
| Clinical response | N | Mean ALC slope | Standard deviation | Fraction with negative slope |
|---|---|---|---|---|
| a ALC slope and response | ||||
| DC | 8 | 0.0879 | 0.08246 | 0/8 |
| PD | 17 | 0.0906 | 0.10034 | 4/17 |
| ALC (cells/μL) | N | Median OS (weeks) |
|---|---|---|
| b ALC and OS | ||
| <1500 | 10 | 21 |
| >1500 | 14 | 41 |
ALC absolute lymphocyte count, DC disease control, PD progressive disease, OS overall survival
Safety and tolerability
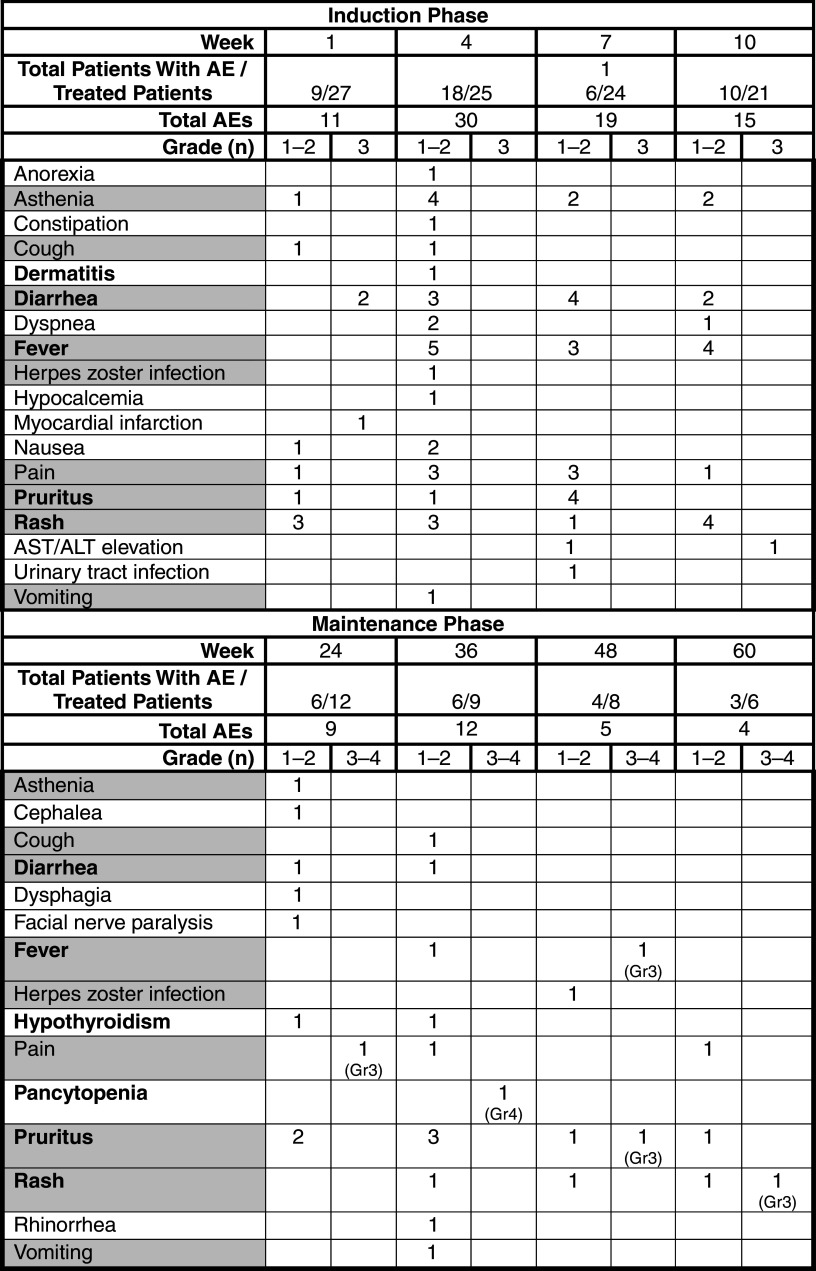
Ipilimumab had an acceptable and manageable safety profile in the Expanded Access Programme that was consistent with that reported in phase II trials [13]. AEs are detailed in Table 5. Most irAEs were of low grade and occurred during the induction phase. One patient experienced a grade 3 myocardial infarction considered unrelated to treatment and was withdrawn from the program. The most common AEs were immune related and included rash, pruritus, fever, and diarrhea. Whereas most were of grades 1 and 2, 2 patients experienced grade 3 diarrhea that resolved within 3 weeks after treatment with antidiarrheals (i.e., loperamide) and intravenous high-dose corticosteroids tapered over 4 weeks following clinical improvement; however, treatment was discontinued due to PD in 1 patient and grade 3 diarrhea upon rechallenge with ipilimumab in another patient. One patient experienced grade 3 elevation in alanine aminotransferase/aspartate aminotransferase (ALT/AST) levels at Week 10 that resolved within 2 weeks after treatment with high-dose steroids tapered over 3 weeks after normalization of ALT/AST values, allowing this patient to continue ipilimumab treatment in the maintenance phase. Grade 4 central pancytopenia was experienced by 1 patient during the maintenance phase and ipilimumab was discontinued. The pancytopenia experienced by this patient was considered to be possibly treatment related and was irreversible despite treatment with supportive medications (i.e., growth factors, transfusions, and antibiotics), immunoglobulins, and immunosuppressive therapy (cyclosporine). This patient also experienced rapid disease progression with the appearance of new brain, lung, and soft tissue metastases that likely contributed to exitus.
Table 5.
Summary of adverse events: induction and maintenance phases
AE adverse event, AST aspartate aminotransferase, ALT alanine aminotransferase, Gr grade
Shading indicates AEs common to induction and maintenance phases
Bold text indicates immune-related adverse event
Case studies
Case 1
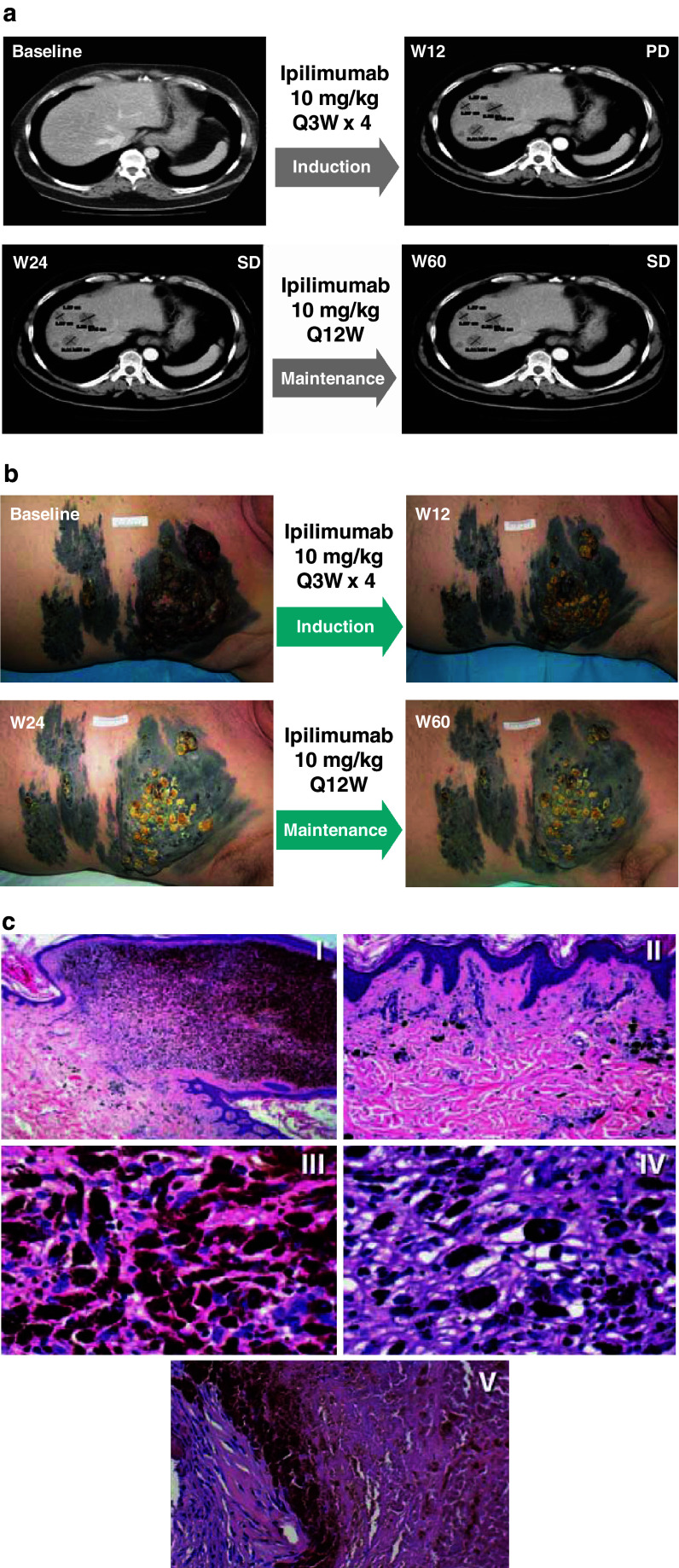
A 61-year-old male patient with stage IV-M1a cutaneous melanoma and elevated baseline LDH levels who had failed 3 courses of chemotherapy received ipilimumab in the Expanded Access Programme. The patient demonstrated disease progression as evidenced by the appearance of multiple liver metastases at the Week 12 TA (Fig. 2a), in spite of initial regression of cutaneous metastases (Fig. 2b). Furthermore, during maintenance therapy, the patient’s initially progressing melanoma stabilized at Week 24 compared with baseline (Fig. 2a, b), with a subsequent stabilization of liver metastases (Fig. 2a) and additional progressive decline in tumor volume of cutaneous metastases (Fig. 2b). Histopathology of cutaneous (Fig. 2c, I–IV) and liver (Fig. 2c, V) biopsies performed at Week 56 and Week 102, respectively, showed largely necrotic tissues in the absence of detectable neoplastic cells. The patient did not experience any irAEs during the induction period, remains alive 128 weeks after initiation of therapy with progression-free survival over 116 weeks and continues on maintenance therapy. This case illustrates that response to ipilimumab therapy can be delayed, and the patient may appear to have initial disease progression. In addition, the case provides an example of a patient who achieved a response to ipilimumab therapy without any irAE; although further confirmation is needed, this suggests that the correlation between the occurrence of irAEs and DC may not be as straightforward as previously thought.
Fig. 2.
Case study 1. a CT scans of patient’s liver at baseline and at Week 12, Week 24, and Week 60 after the initiation of ipilimumab therapy. The patient developed liver metastases at Week 12. At Weeks 24 and 60, whereas these lesions persisted, no new metastases developed and the patient achieved stable disease. b Photographs of melanoma lesions on patient’s torso at baseline and at Week 12, Week 24, and Week 60 after the initiation of ipilimumab therapy document the slow progressive decline in melanoma tumor volume. c Histopathology of cutaneous biopsy at Week 56 confirms the observed progressive decline in tumor burden as a result of ipilimumab therapy. (I) Nodular, hyperpigmented component of the lesion surrounded by pigmented flat areas (original magnification 25×). (II) Detail of the flat area, showing regressive changes with mild fibrosis, melanophages, and blood vessels (original magnification 100×). (III) and (IV) Bland nuclei of mostly melanin-laden macrophages and lymphocytes are detectable both in the upper. (III) and lower (IV) more fibrotic areas of the nodular lesion (original magnification 400×). Histologic examination of liver melanoma metastases at Week 102 showed massive necrosis of melanocytes. On left, well-preserved fibroblasts with rare lymphocytes inside of a fibrotic septum, and melanophages are recognizable (original magnification 200×) (V)
Case 2
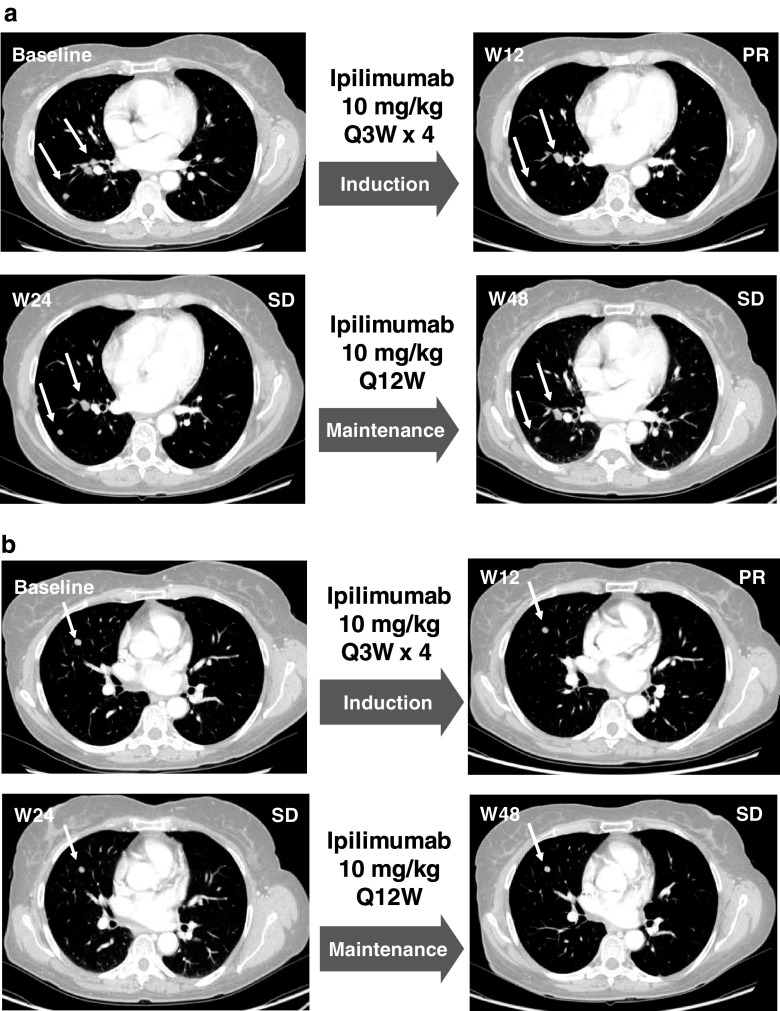
A 50-year-old female patient with stage IV-M1c mucosal melanoma metastasized to the lung, bone, and soft tissue and baseline LDH levels in the normal range, failed 3 courses of chemotherapy and was subsequently treated with ipilimumab in the Expanded Access Programme. At Week 12 of ipilimumab therapy, the patient demonstrated PR as evidenced by lung metastases that remained stable at Week 24 TA and thereafter. CT scans of two distinct foci over time are available in Fig. 3a and b. This patient continued to maintain a PR at subsequent TA and is still alive 105 weeks after initiation of ipilimumab treatment. The patient experienced grade 2 thyroiditis, an irAE, at Week 12 that was successfully treated with the thyroid hormone replacement therapy levothyroxine. This case illustrates that durable disease stabilization, a typical pattern of clinical response to ipilimumab identified in patients with cutaneous melanoma, can also be observed in mucosal melanoma. Patients with stabilized disease can survive for months to years, making this type of response clinically meaningful [31, 32].
Fig. 3.
Case study 2. CT scans of 2 distinct foci of lung metastasis (a and b) at baseline and Weeks 12, 24, and 48 after initiation of ipilimumab therapy. The patient achieved PR at Week 24 followed by SD beginning at Week 24 and lasting through Week 48
Discussion
The clinical response and patterns of response to ipilimumab demonstrated in 27 heavily pretreated patients with advanced melanoma in the Expanded Access Programme were similar to those observed in phase II trials [33]. Ipilimumab induced durable objective responses with disease control achieved in 22% of patients at Week 48, a median overall survival of 9.6 months, and 1- and 2-year survival rates of 34.8% and 23.5%, respectively.
Results of a separate study of ipilimumab in the Expanded Access Programme suggest that changes in ALC that occur after 2 doses of ipilimumab appear to correlate with clinical response [34]. This is consistent with results obtained in our experience (Table 4a, b) since patients with an ALC > 1500 cells/μL after 2 ipilimumab treatments (Week 7) had an increased overall survival. In addition to these results for absolute values, no patients with a negative ALC slope demonstrated DC. The small number of patients precluded the performance of a multivariate analysis. Nevertheless, ALC appears to be a potentially informative biomarker in heavily pretreated metastatic melanoma patients who receive ipilimumab therapy in daily practice.
The safety profile of ipilimumab that we observed was substantially similar to that seen in phase II and III clinical trials [13, 16]. Treatment was associated with mechanism-based irAEs that were generally mild, symptomatically or medically manageable and, in most cases, reversible using product-specific treatment guidelines that were developed during the course of clinical trials [35, 36]. These findings are of particular relevance to the routine use of ipilimumab by community physicians; in fact, strict adherence to these treatment algorithms will contribute greatly to allowing the safe use of this agent in daily practice.
Evidence from previous ipilimumab clinical trials suggests that the occurrence of irAEs may be positively associated with DC [25]. In our experience, this association was not absolute—some patients without irAEs demonstrated DC with ipilimumab.
A number of key findings from phase II and III trials of ipilimumab were confirmed in our experiences in the Expanded Access Programme with 27 heavily pretreated patients with advanced melanoma. The observations that follow have potential clinical implications and should help to guide the comprehensive use of ipilimumab outside clinical trials. DC associated with ipilimumab therapy appears to be more durable and can follow different patterns than those of traditional chemotherapy [14, 30]. DC can be delayed or occur after initial disease progression; therefore, continued treatment and observation may benefit patients experiencing SD or even those with progressive disease not experiencing worsening of performance status. On the basis of this evidence, a novel set of immune-related response criteria (irRC) that more accurately describe clinical response to ipilimumab may be needed to supplement standard mWHO or Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Proposed criteria have been developed [30] and validation is ongoing [37].
Recent evidence from the interim analysis of data from CA184-025 suggests that 50% of patients who progress following initial response to ipilimumab treatment can respond to reinduction therapy [26]; these data, along with our present experience with reinduction of two patients, could have implications for the long-term management of patients with advanced melanoma outside of clinical trials. Nevertheless, it remains unknown which patients are most likely to benefit from ipilimumab reinduction. Biomarkers that predict response to reinduction therapy are needed to help guide daily practice, and efforts to identify these are proceeding in ongoing clinical trials of ipilimumab.
The observations that we made during the Expanded Access Programme demonstrate that ipilimumab is a feasible treatment in daily practice for metastatic melanoma and that clinical responses can be obtained in heavily pretreated, progressing patients. irAEs identified in phase II and III trials of ipilimumab also occurred in this unselected patient population and were generally reversible. Furthermore, a sizeable proportion of these patients experienced durable clinical response, including benefits to long-term survival. Patients’ and treating physicians’ awareness of the patterns of clinical response and side effects related to ipilimumab will contribute greatly to the effective and safe daily use of this agent.
Acknowledgments
The authors wish to acknowledge the excellent nursing support of Angela Iacovelli, Marilena Piccinelli, Massimo Resti, and Sergio Speranza at the University Hospital of Siena. Editorial and writing assistance was provided by StemScientific, funded by Bristol-Myers Squibb Company. This research was supported in part by grants awarded to Michele Maio from the Associazione Italiana per la Ricerca sul Cancro (IG 603) and the Istituto Toscano Tumori. Sergio Speranza was supported in part by a Research Nurse grant from the Istituto Toscano Tumori.
References
- 1.Chandy BK. WHO—Facts Sheet: health consequences of excessive solar UV radiation. Kuwait Med J. 2006;38:254–258. [Google Scholar]
- 2.Tarhini AA, Agarwala SS. Cutaneous melanoma: available therapy for metastatic disease. Dermatol Ther. 2006;19:19–25. doi: 10.1111/j.1529-8019.2005.00052.x. [DOI] [PubMed] [Google Scholar]
- 3.Korn EL, Liu P-Y, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin OncoI. 2008;26:527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 4.Seetharamu N, Ott PA, Pavlick AC. Novel therapeutics for melanoma. Exp Rev Anticancer Ther. 2009;9:839–849. doi: 10.1586/era.09.40. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: an overview. Oncology (Williston Park) 2009;23:488–496. [PMC free article] [PubMed] [Google Scholar]
- 6.Bedikian AY, Millward M, Pehamberger H, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24:4738–4745. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 7.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin-2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 8.Crowley NJ, Seigler HF. Possibilities of immunotherapy and gene therapy for malignant melanoma. Semin Surg Oncol. 1993;9:273–278. [PubMed] [Google Scholar]
- 9.Langer LF, Clay TM, Morse MA. Update on anti-CTLA-4 antibodies in clinical trials. Expert Opin Biol Ther. 2007;7:1245–1256. doi: 10.1517/14712598.7.8.1245. [DOI] [PubMed] [Google Scholar]
- 10.Morse MA. Technology evaluation: ipilimumab, Medarex/Bristol-Myers Squibb. Curr Opin Mol Ther. 2005;7:588–597. [PubMed] [Google Scholar]
- 11.Fong L, Small EJ. Anticytotoxic T-Lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–5283. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 12.Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12:1005–1016. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber JS. Anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864–872. doi: 10.1634/theoncologist.12-7-864. [DOI] [PubMed] [Google Scholar]
- 14.Di Giacomo AM, Danielli R, Guidoboni M, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58:1297–1306. doi: 10.1007/s00262-008-0642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maio M, Lebbé C, Neyns B et al Three-year survival rates for patients with advanced melanoma who received ipilimumab at 10 mg/kg in phase II trials. Presented at: Perspectives in Melanoma XIV; September 17–18, 2010; Amsterdam, The Netherlands. Poster P-0020
- 16.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolchok JD, dePril V, Linette G et al (2009) Efficacy of ipilimumab 10 mg/kg in advanced melanoma patients (pts) with good and poor prognostic factors. J Clin Oncol 27:[Suppl.; abstr 9036]
- 18.Hamid O, Urba WJ, Yellin M et al (2007) Kinetics of response to ipilimumab (MDX-010) in patients with stage III/IV melanoma. J Clin Oncol 25:[Suppl.; abstr 8525]
- 19.Hodi FS, Hoos A, O’Day S et al (2008) Novel efficacy criteria for anti-tumor activity to immunotherapy using the example of ipilimumab, an anti-CTLA-4 monoclonal antibody. J Clin Oncol 26:[Suppl.; abstr 3008]
- 20.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ledezma B. Ipilimumab for advanced melanoma: a nursing perspective. Oncol Nurs Forum. 2009;36:97–104. doi: 10.1188/09.ONF.97-104. [DOI] [PubMed] [Google Scholar]
- 24.Lin R, Yellin MJ, Lowy I et al (2008) An analysis of the effectiveness of specific guidelines for the management of ipilimumab-mediated diarrhea/colitis: prevention of gastrointestinal perforation and/or colectomy. J Clin Oncol 26:[Suppl.; abstr 9063]
- 25.Lutzky J, Wolchok J, Hamid O et al (2009) Association between immune-related adverse events (irAEs) and disease control or overall survival in patients (pts) with advanced melanoma treated with 10 mg/kg ipilimumab in three phase II clinical trials. J Clin Oncol 27:[Suppl.; abstr 9034]
- 26.Harmankaya K, Minor D, Linette G et al Ipilimumab re-induction after progression in patients with advanced melanoma enrolled in phase II clinical trials. Presented at: Joint ECCO 15–34th ESMO Multidisciplinary Congress; September 20–24, 2009; Berlin, Germany
- 27.O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712–1717. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 28.Aamdal S, Wolchok JD, Weber J, et al. Changes in peripheral blood absolute lymphocyte count (ALC) may guide patient selection for continued treatment with ipilimumab. Eur J Cancer. 2009;7(Suppl):579. [Google Scholar]
- 29.Schmidt H, Hamid O, Nissan A, et al. Identification of tumor biopsy markers as potential predictors of ipilimumab clinical activity in patients with advanced melanoma. Eur J Cancer. 2009;7(Suppl):577. [Google Scholar]
- 30.Wolchok JD, Hoos A, O’Day SJ, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 31.O’Day SJ, Weber J, Lebbe C et al (2009) Effect of ipilimumab treatment on 18-month survival: update of patients (pts) with advanced melanoma treated with 10 mg/kg ipilimumab in three phase II clinical trials. J Clin Oncol 27:(Suppl.; abstr 9033)
- 32.Hersh J, Weber J, Powderly J et al (2009) Long-term survival of patients (pts) with advanced melanoma treated with ipilimumab with or without dacarbazine. J Clin Oncol 27:(Suppl.; abstr 9038)
- 33.O’Day SJ, Weber JS, Hamid O et al Completed phase II clinical trials: experience with 10 mg/kg ipilimumab for the treatment of advanced melanoma. Presented at: World Meeting of Interdisciplinary Melanoma/Skin Cancer Centers; November 19–21, 2009; Berlin, Germany. Poster 41
- 34.Ku GY, Yuan J, Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–1775. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Giacomo AM, Biagioli M, Maio M. The emerging toxicity profiles of anti-CTLA-4 antibodies across clinical indications. Semin Oncol. 2010;37:499–507. doi: 10.1053/j.seminoncol.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Kaehler KC, Piel S, Livingstone E, et al. Update on immunologic therapy with anti-CTLA-4 antibodies in melanoma: identification of clinical and biological response patterns, immune-related adverse events, and their management. Semin Oncol. 2010;37:485–498. doi: 10.1053/j.seminoncol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 37.National Monitoring Center for Clinical Trials (Italy). A single-arm phase II study of a combination of ipilimumab and fotemustine in patients with unresectable stage III or IV melanoma. EudraCT Number 2010-019356-50. http://oss-sper-clin.agenziafarmaco.it/cgi-bin/ricerca_sperim_keyword_pp