Abstract
Background
Multiple myeloma (MM) is the malignancy with the most frequent expression of the highly immunogenic cancer–testis antigens (CTA), and we have performed the first analysis of longitudinal expression, immunological properties, and fine specificity of CTA-specific antibody responses in MM.
Methods
Frequency and characteristics of antibody responses against cancer–testis antigens MAGE-A3, NY-ESO-1, PRAME, and SSX-2 were analyzed using peripheral blood (N = 1094) and bone marrow (N = 200) plasma samples from 194 MM patients.
Results
We found that antibody responses against CTA were surprisingly rare, only 2.6 and 3.1 % of patients evidenced NY-ESO-1- and SSX-2-specific antibodies, respectively. NY-ESO-1-specific responses were observed during disease progression, while anti-SSX-2 antibodies appeared after allogeneic stem cell transplantation and persisted during clinical remission. We found that NY-ESO-1- and SSX-2-specific antibodies were both capable of activating complement and increasing CTA uptake by antigen-presenting cells. SSX-2-specific antibodies were restricted to IgG3, NY-ESO-1 responses to IgG1 and IgG3. Remarkably, NY-ESO-1-positive sera recognized various non-contiguous regions, while SSX-2-specific responses were directed against a single 6mer epitope, SSX-285–90.
Conclusions
We conclude that primary autoantibodies against intracellular MM-specific tumor antigens SSX-2 and NY-ESO-1 are rare but functional. While their contribution to disease control still remains unclear, our data demonstrate their theoretic ability to affect cellular anti-tumor immunity by formation and uptake of mono- and polyvalent immune complexes.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1588-x) contains supplementary material, which is available to authorized users.
Keywords: Multiple myeloma, Cancer–testis antigens, Antibody responses, Tumor immunology, Graft-versus-myeloma effect, Stem cell transplantation
Introduction
A subgroup of cancer patients, at one point during the course of their disease, will develop spontaneous antibody responses against antigens expressed by their own tumors [1]. Unfortunately, little is known about the quality of these responses over time, their immunological properties, and especially their fine specificity. This in turn complicates assessing their contribution to an integrated anti-tumor immune response.
Cancer–testis antigens (CTA) have a tumor-restricted expression, being absent from almost every type of healthy tissue, whereas they can be found in a large variety of cancers, and certain members of this gene family are of remarkable immunogenicity. Multiple myeloma (MM) is probably the human malignancy with the broadest and most frequent expression of CTA [2–4]. Moreover, CTA represent a negative prognostic factor in this tumor type, and the presence of certain CTA is central to the survival of myeloma cells [5]. Two CTA expressed in the bone marrow (BM)-residing tumor cells of MM patients [2, 3], namely NY-ESO-1 and SSX-2, are of particular interest for the evaluation of serological responses in MM because their immunogenicity has already been demonstrated in other tumor types and clinical trials targeting these antigens in solid tumors are currently underway [6, 7].
We and others have previously shown that antibody responses against these CTA occur in MM patients but the incidence, persistence and functionality was so far unaddressed and a matter of controversy due to small cohort sizes [2, 8]. In this descriptive analysis, we have asked for the first time how frequently, at which timepoints during the course of the disease, and under which clinical conditions antibody responses against 4 different CTA (MAGE-A3, NY-ESO-1, PRAME, SSX-2) occur in MM patients. Furthermore, we have analyzed the immunological function and the fine specificity of the autologous serological responses directed against both NY-ESO-1 and SSX-2.
Materials and methods
Patients
All patients were admitted for treatment or diagnostic purposes to the University Medical Center Hamburg-Eppendorf. Repeated blood and BM samples were obtained during routine diagnostic procedures, and all participants provided informed consent prior to sample collection. A total of 1,094 peripheral blood (PB) plasma samples and 200 BM plasma samples were collected from 195 consecutive MM patients. In addition, PB sera of 100 anonymous healthy blood donors were collected. Samples were harvested as previously described [3]. In the patients, progression/active disease was defined as pathological serum paraprotein concentrations, progression of osteolysis, or >10 % plasma cells in the BM. This study was performed in accordance with the declaration of Helsinki. The protocol had received approval by the local ethics committee (decision number OB-038/06).
Cell lines
Cell line K562 was obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany), and cell lines SK-MEL-19 and SK-MEL-21 were kindly provided by the New York branch of the Ludwig Institute for Cancer Research (LICR). Lines were maintained in RPMI-1640 or IMDM with 10 % fetal calf serum (FCS) and penicillin/streptomycin.
Proteins and peptides
Full-length NY-ESO-1 and SSX-2 proteins expressed in E. coli were kindly provided by the LICR. Full-length MAGE-A3 protein expressed in baculovirus was provided by GlaxoSmithKline Biologicals (Rixensart, Belgium). Full-length PRAME protein expressed in the wheat germ system was obtained from Abnova (Taipei, Taiwan). Recombinant influenza nucleoprotein (FLU) expressed in E. coli was purchased from Imgenex (San Diego, CA), and control protein glutathione S-transferase (GST) was obtained from Cell Systems (St. Katharinen, Germany). Tetanus toxoid (TT) was provided by Chiron Behring (Marburg, Germany).
We further expressed full-length proteins using codon-optimized ORF clones of NY-ESO-1, SSX-2 and TNFα clones (Qiagen, Hilden, Germany) and SSX-1, SSX-5, and MAGE-C1 (MWG Biotech, Ebersberg, Germany) with an amino-terminal 6x-His-tag in BL21[DE3] cells (New England Biolabs, Frankfurt am Main, Germany). Proteins were purified under denaturing conditions by nickel-affinity chromatography (Supplementary Figure 1A) according to the manufacturer’s instructions (Sigma-Aldrich, Hamburg, Germany). Proteins were stored in PBS/1 % sodium azide at −20 °C. NY-ESO1 and SSX-2 were labeled with fluorescein isothiocyanate isomer I (FITC; Sigma-Aldrich) in bicarbonate buffer according to the manufacturer’s instructions and cleaned up using PD-10 columns (Thermo Scientific).
NY-ESO-1 20mer peptides, as well as SSX-2 20mer and 18mer peptides overlapping by 10 amino acids and spanning the complete NY-ESO-1 and SSX-2 sequences, respectively, were obtained from Multiple Peptide Systems (San Diego, CA). 10mer SSX-2 peptides overlapping by 5 amino acids and spanning amino acid region 71–95 were obtained from Peptides and Elephants (Potsdam, Germany).
Real-time PCR
Extraction of total RNA was performed using the RNeasy Mini Kit (Qiagen). Reverse transcription and conventional and quantitative PCR were performed as previously described [3]. Primers for target genes and probes #23 and #17 used in real-time PCR (NY-ESO-1 and LAGE-1) were obtained commercially from Roche Diagnostics (Mannheim, Germany). The primer sequences were as follows: LAGE F 5ʹ-GTG TCC GGC AAC CTA CTG TT-3ʹ and LAGE R 5ʹ-CAC ATC AAC AGG GAA AGC TG-3ʹ; NY-ESO-1 F: 5ʹ-CTA TGT CAG TTC AGG ACC AGG A-3ʹ and NY-ESO-1 R: 5ʹ-CTG ACC GTG GGG TGT AGG-3ʹ. Samples were analyzed using a LightCycler system (Roche Diagnostics).
Immunohistochemistry
Immunohistochemistry on formalin-fixed, paraffin-embedded archival tissue was performed using an anti-NY-ESO-1 monoclonal antibody (clone E978; Santa Cruz) as previously described [2].
Enzyme-linked immunosorbent assay (ELISA)
ELISA was performed as previously described [9]. During screening, a patient sample was considered positive if its specific OD was higher than the mean OD of 100 samples derived from healthy donors +3 SD. In addition, the OD had to exceed the serum’s background signal on control protein GST by at least 50 %. For the calculation of titers, regression analyses were performed for the linear segment of serum titration curves for positive samples and pooled sera of five representative healthy donors. Titers were defined as the dilution at the intersection of both regression lines.
For antibody subtyping, AP-labeled secondary anti-IgG1, anti-IgG3, and anti-IgG4 antibodies (Southern Biotech) were used at a dilution of 1:3,000. An AP-labeled anti-IgG2 antibody (Beckham Coulter, Krefeld, Germany) was used at a dilution of 1:1,500.
Western blot
Protein lysates were prepared using RIPA lysis buffer containing a protease inhibitor cocktail (Sigma-Aldrich) and denatured for 10 min at 70 °C. Samples containing 50 μg lysate or 3 μg of recombinant protein, respectively, were resolved on 4–12 % Bis–Tris SDS–PAGE gels (Invitrogen, Carlsbad, CA) under reducing conditions. Proteins were blotted on Hybond-ECL nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ) and blocked overnight at 4 °C with Top-Block (Fluka, Buchs, Switzerland). Human sera were applied at a dilution ranging between 1:500 and 1:2,000. An HRP-conjugated antihuman IgG-Fcγ antibody (Sigma-Aldrich) was used at 1:5,000. Beta-actin (Santa Cruz) served as positive control.
Complement activation
The activation of the complement cascade through the classical pathway leads to the deposit of complement factor C3 on the cell membrane. Three micrograms of recombinant protein (NY-ESO-1, SSX-2 or TT) was resolved on 4–12 % Bis–Tris SDS–PAGE gels under reducing conditions. Proteins were blotted and membrane treated as outlined above. As described by Papp et al. [10], this deposit can be detected on nitrocellulose membranes by a C3-specific antibody. Human serum was diluted 1:5 and incubated for 3 h on the nitrocellulose membrane at room temperature prior to stringent washing. Afterward, mouse IgG1 anti-C3 antibody (Thermo scientific) was applied at a dilution of 1:5,000 for 1 h. The membrane was thoroughly washed and exposed to an anti-mouse IgG HRP-labeled antibody (R&D Systems, Minneapolis, MN) for 1 h.
Antigen uptake assay
For the preparation of antigen-specific antibodies, 500 μl whole serum from high-titered patient samples was purified using protein G Sepharose (GE Healthcare) and antigen-coated amine-reactive beads (Thermo Scientific) according to the manufacturer’s instructions. Eluted antibodies were stored in PBS at 4 °C. Specific binding was confirmed by ELISA, as described above (Supplementary Figure 1A). Peripheral blood mononuclear cells (PBMC) were obtained from healthy blood donors at University Medical Center Hamburg and isolated by density gradient centrifugation. Expression of FcγRI, FcγRII, and FcγRIIIB on CD14+ cells was confirmed by flow cytometry (Supplementary Figure 1B) using a FACScalibur flow cytometer (BD Biosciences, Heidelberg, Germany). Antibodies against CD14 (BD Biosciences), CD16b/FcγRIIIB (Beckman Coulter, Krefeld, Germany), CD32/FcγRII (BD Biosciences), and CD64/FcγRI (eBioscience, Frankfurt, Germany) were obtained commercially. Staining and flow cytometry measurements were performed as previously described [11]. For uptake assays, 5 × 106 PBMCs were seeded on poly-l-lysine coated coverslips in a 24-well plate and incubated overnight in RPMI-1640 supplemented with 10 % FCS. Prior to uptake assays, cells were washed twice for 15 min with serum-free RPMI-1640.
Equimolar amounts of purified antigen-specific antibodies equivalent to in vivo concentrations and FITC-labeled CTA were incubated in PBS with agitation for 6 h at room temperature. Immune complexes were added to PBMCs grown on coverslips and subsequently incubated for 30 min at 37 °C. Coverslips were then briefly washed in PBS, fixed using 4 % paraformaldehyde, blocked with 1 % bovine serum albumin (BSA; Sigma-Aldrich) and stained overnight with an anti-CD64 antibody (eBioscience) at 4 °C. The next day coverslips were washed three times with PBS and stained with a Cy3-conjugated anti-mouse IgG antibody (Jackson ImmunoResearch, Suffolk, UK) and 4′,6-diamidino-2-phenylindole (DAPI; Carl Roth, Karlsruhe, Germany) in 1 % BSA for 1 h at room temperature. Coverslips were again washed three times with PBS and mounted on microscope slides using Aqua Polymount (Polysciences, Eppelheim, Germany). Sections were obtained on an Ultraview VOX spinning disk imaging system (PerkinElmer, Waltham, MA) using an oil-immersion 63× objective. For each sample, two 4 × 3 view field regions of comparable cell density were recorded. Instrument settings were adjusted for each donor and antigen. Uptake was defined as numbers of FITC positive vesicles between 0.2 and 20 μm per cell/DAPI-positive nuclei. Values are shown as fold changes over antigen alone. FITC-labeled antigen strongly colocalized with staining of the antigens’ affinity tag using a mouse anti-His antibody (Qiagen) and a secondary Cy3-conjugated anti-mouse IgG antibody (Jackson ImmunoResearch; Supplementary Figure 1C).
Quantification of antigen-specific memory B cells
Antigen-specific B cells were quantified by ELISPOT as previously described [12]. Spots were counted using an ELISPOT reader (AutoimmuneDiagnostika, Strassberg, Germany).
Analysis of SSX-2-specific CD4+ and CD8+ T-cell responses
Read-out-assays were performed following a single cycle of in vitro presensitization, as previously described [13, 14]. T cells were stimulated once with remaining irradiated CD8−CD4− cells either pulsed with individual or with pools of overlapping 20mer SSX-2 peptides.
For the measurement of intracellular cytokines, pulsed T-APC was stained with 0.2 µM 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) for 10 min at 37 °C. T-APC was then washed and was incubated with presensitized effector T cells at a 1:2 ratio in 200 µl X-VIVO-15 at 37 °C for 7 h. Brefeldin-A (Sigma-Aldrich) at 10 μg/ml was added after the first 2 h of culture. Cells were then fixed using FACS Lysing Solution (BD Biosciences) diluted 1:10, permeabilized using Permeabilizing Solution 2 (BD Biosciences), and stained with antibodies against CD4 and interferon (IFN)-γ (BD Biosciences). Co-staining of intracellular FOXP3 was performed using anti-FOXP3 mAb PCH101 (eBioscience, San Diego, CA). Cells were analyzed by flow cytometry with gating on morphologically defined lymphocytes, CD4-positive and CFSE-negative cells.
Statistics
Paired samples t tests were performed to analyze differences between mean values for progression and remission intervals for all antibody-positive patients. Differences between immune complex uptake conditions expressed as logarithmic fold changes over antigen alone were evaluated by Wilcoxon signed-rank test. Results were considered significant if p < 0.05.
Results
Patient characteristics
Analyzing the clinicopathological characteristics of all 195 patients, we found a male predominance, a median age at time of inclusion of 56 years (range 29–78 years), and IgGκ to represent the most common paraprotein subtype (Supplementary Table I). Most patients were diagnosed at advanced stages, and the most common cytogenetic abnormality was deletion 13q14. At the time of inclusion into the study, the majority of patients had been treated with alloSCT, while the second largest group had received autoSCT as maximum therapy. The majority of samples were provided by the Department of Stem Cell Transplantation at University Medical Center Hamburg-Eppendorf.
NY-ESO-1- and SSX-2-specific antibody responses occur spontaneously in the peripheral blood and bone marrow of myeloma patients
We first screened a large number of patient sera using stringent criteria for the definition of seropositivity. Analyzing a median number of 5.4 (range 1–47) serum samples collected per patient during a median follow-up period of 11.4 months (range 1–39 months), we did not observe any antibody responses against CTA commonly expressed in myeloma such as MAGE-A3 and PRAME (data not shown).
However, we found 5 (2.6 %) and 6 (3.1 %) out of 195 patients to evidence anti-NY-ESO-1 and anti-SSX-2 IgG antibodies, respectively, at least at one occasion during the course of their disease. Among all 1,094 sera collected, we found 84 (7.7 %) and 51 (4.7 %) samples to be positive for NY-ESO-1- and SSX-2-specific antibodies, respectively. In contrast, none of the 100 healthy donors evidenced NY-ESO-1- or SSX-2-specific IgG antibodies. Importantly, the relatively infrequent occurrence of anti-NY-ESO-1 or SSX-2 IgG antibody responses in the MM patients was not based on a general B-cell hyporeactivity as we detected strong antibody responses against microbial antigens FLU and TT in the majority of MM patients (data not shown). Furthermore, CTA reactivity did not correlate with absolute whole IgG levels (Supplementary Figure 2). Accordingly, investigating the source of soluble anti-CTA antibodies, we were able to identify for the first time NY-ESO-1-specific antibody secreting B cells in the peripheral blood of myeloma patients using a newly developed ELISPOT technique. NY-ESO-1-specific B cells were only detectable in NY-ESO-1 antibody-positive patients but not in antibody-negative patients (Supplementary Figure 3A).
As the bone marrow represents the immediate tumor microenvironment of MM, we also investigated anti-CTA antibody levels within plasma samples (N = 200) of myeloma patients (N = 75) derived from the BM. We found that in a given patient, anti-CTA antibodies always evidenced similar titers in the PB and in the BM (data not shown). Importantly, antibody responses in the BM environment were completely restricted to those patients who also evidenced CTA-specific humoral responses in their peripheral blood.
NY-ESO-1- and SSX-2-specific antibody responses are observed under opposite clinical conditions
We next analyzed associations between the clinical status of the patients and the presence of anti-CTA antibody responses. We observed that a substantial proportion of samples (55/412) taken at the time of clinical progression of MM evidenced anti-NY-ESO-1 antibodies while only a small minority (3/662) of samples collected during phases of remission were antibody-positive. In contrast, a higher proportion of samples collected at the time of remission (33/662) compared to samples acquired at the time of progression of the disease (12/412) evidenced anti-SSX-2 antibodies. This observation suggested for the first time that development and/or maintenance of humoral responses against NY-ESO-1 might be related to progression of myeloma while anti-SSX-2 antibodies might preferentially be produced in phases of remission.
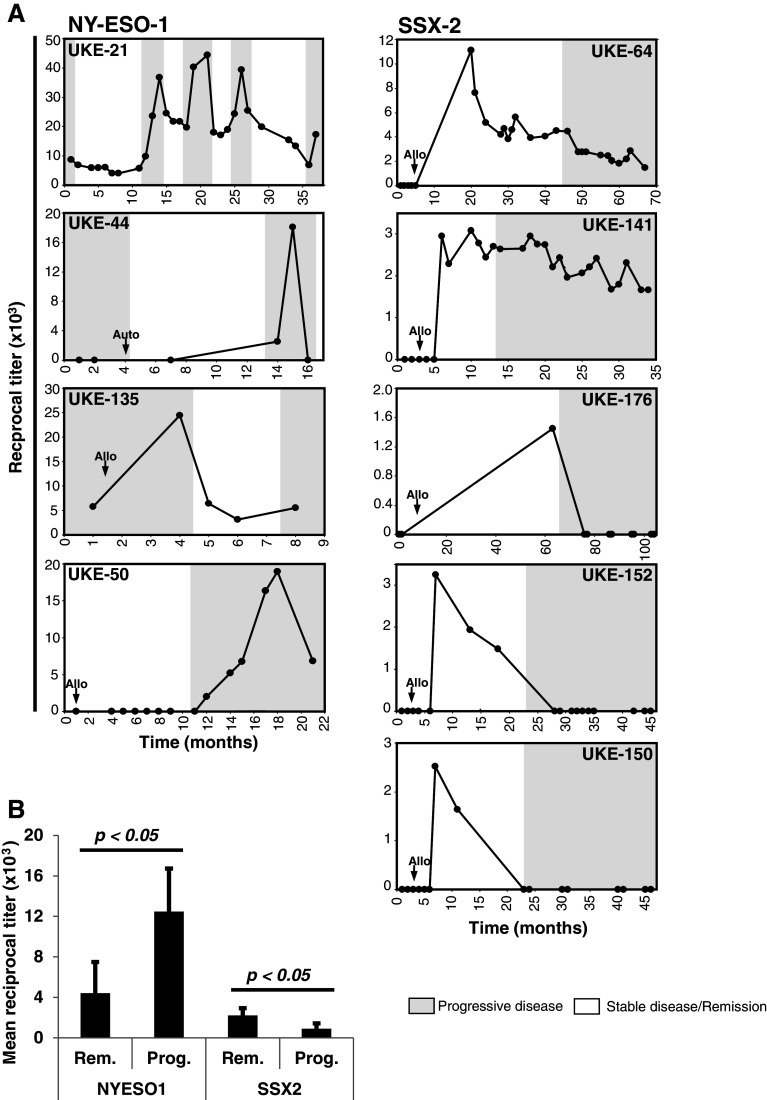
To further investigate the biological significance of the occurrence of NY-ESO-1- and SSX-2-specific antibodies, we next correlated the respective antibody titers with the expression of the CTA in the BM and the clinical course of the disease in individual patients. In all cases of NY-ESO-1 antibody-positive patients, we found the antibody titers to be associated with disease progression and recurrence (Fig. 1a). Titers increased in these five patients significantly upon disease progression and dropped during remission (Fig. 1b), which was independent of total IgG levels (Supplementary Figure 2). Due to the high sequence homology of NY-ESO-1 and LAGE-1, especially in the commonly immunogenic amino-terminal region and the antibodies’ probable cross-reactivity, we next investigated the expression of both antigens in the BM of our patients. We did not detect any expression of LAGE-1 in the cases of UKE-21 and UKE-50 while the number of NY-ESO-1 copies correlated positively with disease burden and increased together with anti-NY-ESO-1 antibody titers (data not shown). For seropositive patients UKE-44 and UKE-135, we were not able to detect any expression of NY-ESO-1 or LAGE-1 mRNA in the BM (data not shown). However, both patients had developed extramedullary manifestations during progression of their disease and we found the respective soft tissue tumors to strongly express of NY-ESO-1 protein as detected by immunohistochemistry (Supplementary Figure 3B).
Fig. 1.
NY-ESO-1-specific antibodies correlate with tumor load and disease progression while donor-derived anti-SSX-2 antibodies indicate disease remission. a Longitudinal analysis of humoral responses and antigen expression in the BM for individual patients. Black dots indicate reciprocal titers of anti-NY-ESO-1 and anti-SSX-2 antibodies. White dots indicate expression of NY-ESO-1 or SSX-2 RNA, and white triangles demonstrate expression of LAGE and SSX-4 RNA, respectively, in the BM of the patients. Within single graphs, white areas indicate the presence of clinical remission while gray areas indicate recurrence and progression of the disease. The application of alloSCT is indicated by small black arrows. b Comparison of means of reciprocal titer means for each patient during phases of the indicated clinical condition. Paired samples t tests indicate significant differences in antibody titers between clinical conditions for SSX-2 and NY-ESO-1
Compared to anti-NY-ESO-1 antibodies, serological responses against SSX-2 showed a completely different behavior. With the exception of patient UKE-85, who was antibody-positive prior to transplantation and lost reactivity afterward, 5 out of 6 of the anti-SSX-2 seropositive patients developed antibodies after they had received alloSCT (Fig. 1a). In comparison to patients with NY-ESO-1 reactivity, serological responses in SSX-2 antibody-positive patients developed antibodies earlier in these 5 patients, approximately 5–6 months after transplantation. In contrast to anti-NY-ESO-1 immunity, antibody titers specific for SSX-2 after alloSCT correlated negatively with the disease burden in 6 patients (Fig. 1b). Accordingly, in three of the seropositive patients (UKE-150, UKE-152, UKE-176), disease recurrence manifested only after anti-SSX-2 antibodies had become undetectable, and in two patients (UKE-64, UKE-141), the tumor only recurred when SSX-2 antibody titers had already begun to decrease (Fig. 1). BM samples from timepoints preceding alloSCT were available from all patients and none of them evidenced preexisting expression of SSX-2 mRNA. In one seropositive patient (UKE-64), however, SSX-2 was (re-) expressed in the myeloma-infiltrated BM after anti-SSX-2 antibody titers had vanished and relapse of myeloma had taken place (date not shown).
CTA-specific antibodies are capable of promoting complement activation and antigen uptake
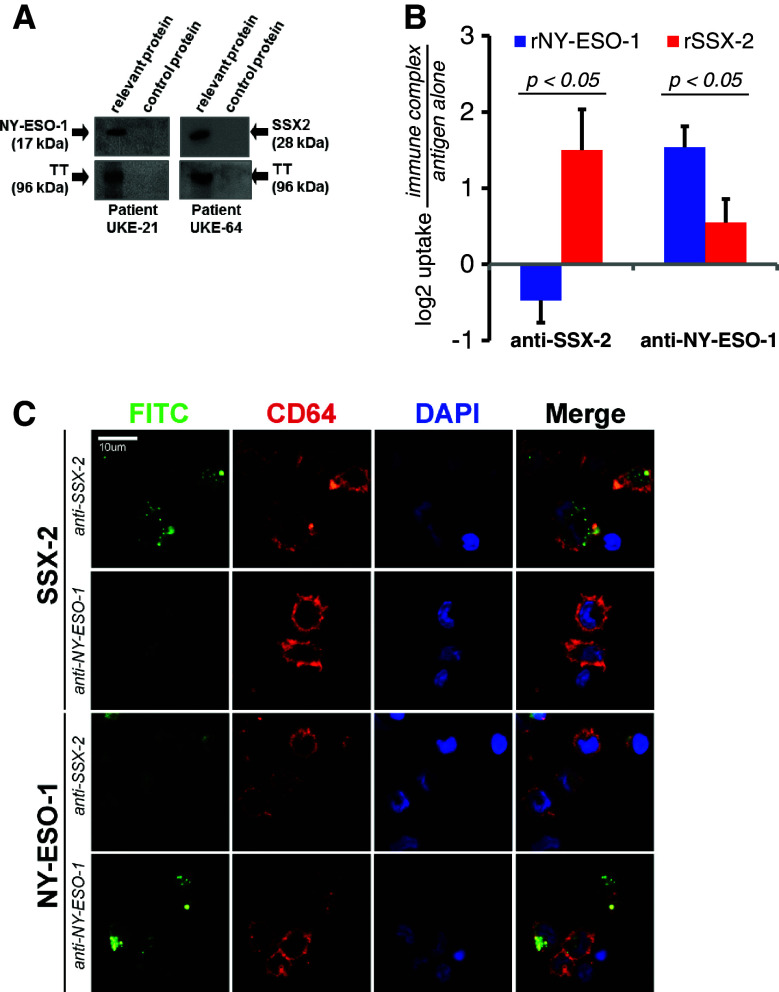
In order to establish whether the observed antibody responses would be at all able to contribute to an anti-tumor immune response, we next analyzed their basic immunological competency. First, we asked whether our patients’ anti-NY-ESO-1 or anti-SSX-2 antibodies, which reacted not only with the respective recombinant proteins but also recognized antigen endogenously expressed by tumor cell lines (Supplementary Figure 3C) were in principle able to directly activate the complement system. Assessing the activation of the classical complement pathway by serum antibodies [10], we found for the first time, both CTA-specific antibodies to be potent inducers of the complement system, as evidenced by C3-deposition on a membrane coated with recombinant protein (Fig. 2a).
Fig. 2.
Functional characteristics of NY-ESO-1- and SSX-2-specific patient antibodies. a Western blot analysis of the complement-activating capacity of NY-ESO-1- and SSX-2-specific antibodies. Tetanus toxoid (TT) served as positive control for complement activation by TT-specific antibodies. Complement activation was detected through a complement factor C3-specific antibody. Sera from NY-ESO-1 antibody-positive patient UKE-21 and from SSX-2 antibody-positive patient UKE-64 were able to activate complement when bound to the respective CTA. The same phenomenon was seen with the patients’ TT-specific antibodies. When exposed to control protein GST, no complement activation occurred. Results of one representative experiment are shown. b Uptake of FITC-labeled CTA protein after immune complex formation with either SSX-2- or NY-ESO1-specific antibodies from high-titered MM patients. Bars indicate mean logarithmic fold changes over antigen alone for the respective CTA for 6 different healthy blood donors. Error bars indicate standard error of means (SEM). c Partial confocal sections of one representative antigen uptake experiment. Formation of specific immune complexes comprised of FITC-labeled antigen, and the corresponding MM patient-derived antibodies increased antigen uptake in CD64+ APCs from one healthy donor. In contrast, the same antibody did not increase uptake in the unspecific context
Formation of antigen–antibody immune complexes can increase the uptake of microbial agents and proteins by antigen-presenting cells in vivo [15, 16]. This process is initiated via engagement of the immune complexes by Fcγ receptors (FcgR) on the APCs’ surface [17]. Uptake via this mechanism has been shown to increase cross-presentation of exogenous antigen by MHC class I molecules and, consequently, activation of cytotoxic T cells [15, 18] providing a mechanism possibly underlying the clinical relevance of antibodies against intracellular but highly immunogenic CTA. We, therefore, analyzed next the ability of patient-derived antibodies to facilitate CTA uptake in vitro. Uptake of FITC-labeled SSX-2 and NY-ESO-1 was then quantified as intracellular numbers of FITC positive vesicles on confocal sections of APCs incubated with immune complexes or free antigen. In six independent experiments using APCs prepared from peripheral blood from different healthy donors, we found that both types of CTA-specific patient antibodies were able to significantly increase uptake of the FITC-labeled cognate antigen by the CD64/FcgRI-positive cells (Figs. 2b, c, p < 0.05).
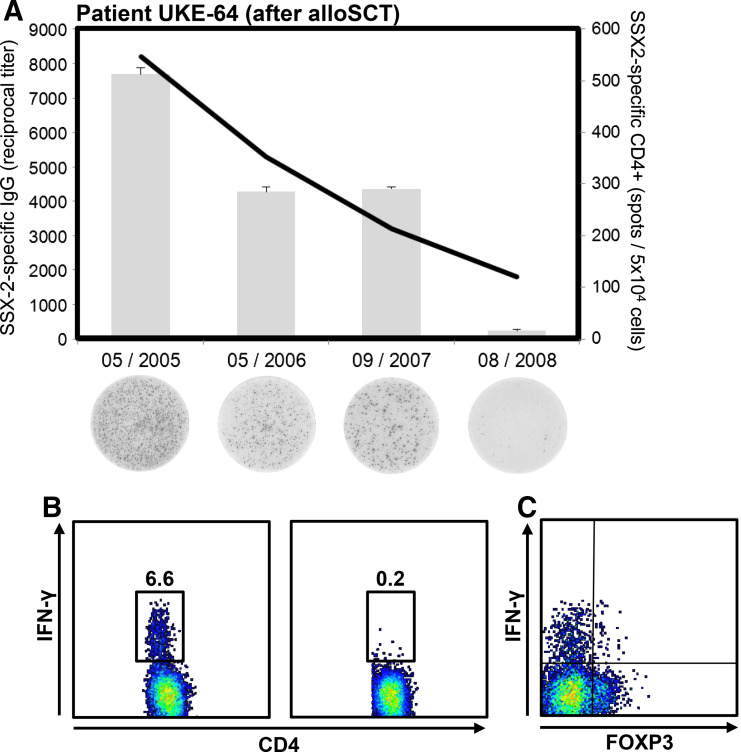
Following up on a potential contribution of these antibodies to CTA-specific cellular immunity, we analyzed the T-cell responses in one patient with anti-SSX-2 antibodies. We found that numbers of SSX-2-specific peripheral T cells were associated with the antibody titer (Fig. 3a) and secreted effector cytokine IFNγ (Fig. 3b). Importantly, none of the SSX-2-specific CD4+ T cells expressed the regulatory T-cell marker FOXP3 (Fig. 3c).
Fig. 3.
Levels of anti-SSX-2 antibodies correlate with the number of SSX-2-specific T cells. a In patient UKE-64, anti-SSX-2 antibody titers were followed after induction by alloSCT and numbers of T cells specific for SSX-2 were determined by IFN-γ ELISPOT. The black line indicates the reciprocal titer of anti-SSX-2 IgG antibodies, and gray bars indicate mean numbers (+SEM) of CD4+ T cells specific for the SSX-231–50 epitope. Below each bars examples of ELISPOT results for the given timepoints are shown. b Dot plots represent examples of intracellular cytokine staining followed by flow cytometry. CD4+ T cells from patient UKE-64 were cultured for 2–3 weeks with a pool of three 20-mer SSX-2 peptides covering amino acid region 31–70. After being re-exposed to the respective SSX-2 peptides, the patient’s CD4+ T cells secreted IFN-γ (left box) while exposure toward T-APC pulsed with an NY-ESO-1 control peptide (right box) did not provoke IFN-γ production. c As indicated by this exemplary dot plot, the patient’s CD4+ T cells specific for SSX-2 were negative for FOXP3 suggesting that these cells did not represent T regulatory cells
IgG1/IgG3-type anti-NY-ESO-1 antibodies recognize multiple non-contiguous epitopes while IgG3-type SSX-2-specific antibodies are restricted to a single 6 amino acid peptide
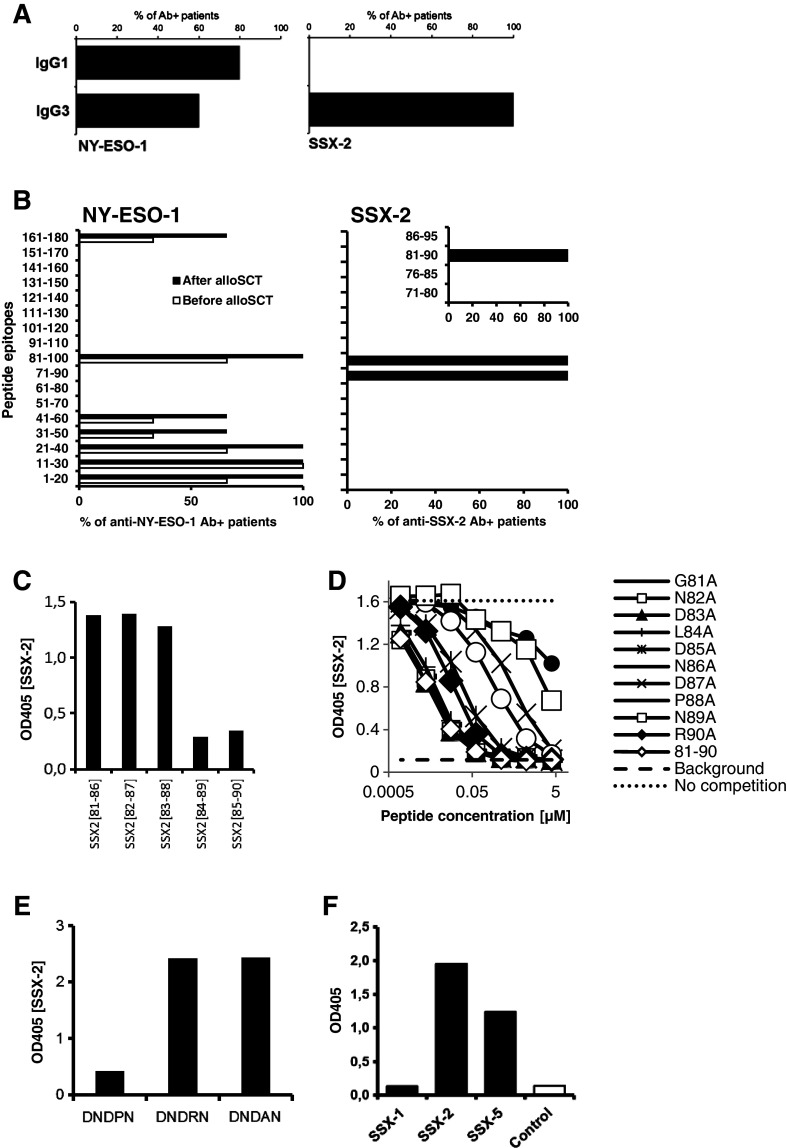
Finally, we set out to identify differences in the biological characteristics of anti-NY-ESO-1 and anti-SSX-2 antibodies. First, when we analyzed the dominant subtypes of our patients’ CTA-specific antibody responses, we found that NY-ESO-1-specific antibodies were mainly of the IgG1 subtype and to a lesser extent of the IgG3 subtype. In contrast, SSX-2 specific antibodies belonged exclusively to the IgG3 subtype (Fig. 4a).
Fig. 4.
IgG subtype analysis and epitope mapping of anti-NY-ESO-1 and anti-SSX-2 antibody responses. a Analysis of the distribution of patient-derived NY-ESO-1- and SSX-2-specific antibodies regarding IgG subtypes. Bars indicate the percentage of seropositive patients evidencing the given subtype of CTA-specific IgG. b Target epitopes of the NY-ESO-1 (left) and SSX-2-specific (right) antibody responses of MM patients were identified using overlapping 20-mer peptides spanning the complete sequence of the respective CTA. Bars indicate percentages of seropositive patients recognizing the given epitope. In the case of SSX-2-specific antibodies, an additional series of overlapping 10-mer peptides was used to further define the exact target epitope of the patients’ humoral responses. Results were validated by three independent experiments. c Competition with 6mer peptides covering SSX-281–90 at 250 μM for binding of full-length SSX-2. d Dilution series of single-alanine substitution variants of initial 10mer SSX-281–90 competing for binding of full-length SSX-2 protein. e Competition with 5mer peptides containing the minimal SSX-2 epitope with and without substitution of proline in position 88 at 250 μM. Competition assays were performed for all SSX-2 antibody-positive patients. Data are shown for one representative patient. f SSX-2-recognizing sera of 6 patients were tested against SSX-1, SSX-2, and SSX-5 full-length proteins in an ELISA assay. Recombinant full-length MAGE-C2 protein was used as a control. Results are shown for one representative patient
Antigen valency and B-cell epitope diversity influence the quality of the interaction of immune complexes with APCs [19]. Using overlapping 20mer peptides spanning the whole sequences of NY-ESO-1 and SSX-2, respectively, we mapped the dominant epitopes of the CTA-specific antibody responses. We found the NY-ESO-1-specific antibodies to target a number of different epitopes. All seropositive patients (n = 5) evidenced antibodies against amino acid regions 1–40 and 81–100, while 3 patients also reacted against regions 31–60 and 161–180 (Fig. 4b). In contrast, SSX-2-specific antibodies of the seropositive patients (N = 6) exclusively recognized two 20mers covering region 71–100 (Fig. 4b). Using first 10mer (Fig. 4b) and then 6mer peptides (Fig. 4c), each overlapping by 5 amino acids, we were able to further pinpoint the epitope recognized by the patients’ SSX-2-specific antibodies. Surprisingly, we found for the first time that all humoral responses against SSX-2 were restricted to the single 6mer DNDPNR mapping to amino acids 85–90 of the whole protein sequence (Fig. 4c). Using single-alanine substitution variants of SSX-281–90, we were able to demonstrate the predominant role of the two asparagine residues in positions 86 and 89, as well as the contained proline and aspartate in positions 88 and 87 (Fig. 4d).
With the exception of P88, the identified peptide is shared among all members of the SSX protein family (Supplementary Figure 4) but lacks homology with other human or microbial sequences [20]. Importantly, substituting P88 for arginine present in the corresponding minimal epitopes of SSX-4, -6, -7, -8, and -9, prevented relevant binding (Fig. 4e). P88 may be crucial for the correct alignment of the asparagine/aspartate side chains representing potential antibody binding sites. Accordingly, the patients’ sera binding to SSX-2 protein also recognized full-length SSX-5 protein, which possesses the same 6mer epitope but not to SSX-1, which shares an overall homology of 78 % with SSX-2 but has a histidine in place of P88 (Fig. 4f). Together, these findings indicate for the first time a very narrow specificity and high selectivity of the myeloma patients’ donor-derived antibody responses for cancer–testis antigen SSX-2.
Discussion
The introduction of new therapeutic agents such as bortezomib or lenalidomide has significantly improved remission rates and survival of multiple myeloma (MM) patients, but the majority of them will still relapse and succumb to the disease within 5 years from diagnosis [21]. AlloSCT has been suggested to improve overall survival and to achieve cures in patients through immune mechanisms involving a graft-versus-myeloma (GvM) effect [22]; however, the targets of these immune reactions and their quality remain poorly understood.
It has been shown for multiple tumor types that patients are capable of developing spontaneous antibody responses against intracellular tumor proteins [1, 23]. The present study constitutes the first longitudinal analysis of antibody responses against the predominantly intracellular cancer–testis antigen family in a human malignancy. Analyzing a large number of plasma samples, we found anti-NY-ESO-1 and anti-SSX-2 antibodies to occur at similar frequencies in MM patients (2.6 and 3.1 %). With the exception of our previous report on SSX-2-specific antibody responses in individual patients [2], the frequency of anti-SSX-2 antibody responses in MM has never been analyzed. In contrast, antibody responses against NY-ESO-1 have previously been reported to occur in 22 % of MM patients [8]. Possible explanations for the lower frequency found in this study are our more restrictive definition of antibody-positivity, differences in cohort composition, and lower numbers of patients in the previous study.
In our current study, we observed NY-ESO-1-specific antibody titers to coincide with increased tumor burden and progression of the disease. This finding is in accordance with previous studies suggesting that NY-ESO-1-specific antibodies represent markers of recurrent and/or progressive disease in melanoma and prostate cancer [24, 25]. It appears that the NY-ESO-1-specific B-cell response is unable to eradicate the malignant NY-ESO-1-positive tumor cells as indicated by the positive association between NY-ESO-1 mRNA copies in the patients’ BM and the antibody titers. This could, on the one hand, imply that NY-ESO-1-specific antibodies indeed represent mere epiphenomena of tumor progression, reflecting the current tumor load and not having any independent clinical effect, or that they even skew anti-tumor immunity toward an ineffective Th2-type response [26]. On the other hand, it could be that the NY-ESO-1-specific humoral immune responses, probably in collaboration with cellular immunity, slow down tumor growth to some degree while eventually being incapable of completely preventing progression of MM. The finding that the association of anti-NY-ESO-1 antibodies with reduced overall survival in melanoma can be reversed by blocking the T-cell inhibitor CTLA-4 [27] would argue for the idea that anti-NY-ESO-1-specific antibodies are in general capable of contributing to tumor control if an appropriate immune context is provided.
In contrast, antibodies against SSX-2, which were most likely donor-derived, occurred early after alloSCT in 5 out of 6 patients. In marked contrast to findings with NY-ESO-1, there was also no detectable SSX-2 expression in the BM of the patients at the time when the antibody responses occurred. Finally, donor-derived antibodies against SSX-2 were preferentially present during periods of remission of the disease. While this difference was statistically significant, we caution that the small number of antibody-positive patients in our study may limit the generalizability of this finding. In 5 out of 6 patients who had become seropositive after alloSCT, clinical recurrence only occurred after SSX-2 antibody titers had shown a decrease or had become undetectable.
Whether autoantibody responses against tumor-specific antigens are able to contribute to an integrated anti-tumor immune response or are even able to reach the tumor site in sufficient quantities remains poorly understood [23]. However, we show here that in MM high levels of CTA-specific antibodies are present at the primary site of the disease. We further demonstrate that the CTA-specific antibodies belong to major effector subtypes IgG1 and IgG3 [28–30] as expected for protein antigens. Their ability to activate the complement cascade would allow them to target MM cells [31, 32] aberrantly expressing CTA on the cell surface, a phenomenon observed during early apoptosis [33, 34] and other pathological processes in malignant cells [18]. Alternatively, uptake of mono- or polyvalent immune complexes of different IgG subtypes by APCs [35, 36] can alter the resulting cellular immune response and promote anti-tumor immunity [35, 37].
While we cannot rule out the possibility that the observed high-titered SSX-2-specific antibody responses play a central role in the immunological control of active MM, it seems unlikely that an isolated SSX-2-specific antibody response would be able to contribute much on its own. Instead our data point to a relatively weak antigenicity of SSX-2, requiring the strongly immunostimulatory setting provided by alloSCT to produce detectable antibody levels. This is supported by a single narrow previously undescribed epitope not shared with other commonly expressed members of the SSX family. Considering this, the presence of high titers of anti-SSX-2 antibodies during remission might instead point toward a more active and competent immune system, which has been demonstrated to play a crucial role in the control of MM, especially in the alloSCT setting [22]. The disappearance of SSX-2-specific antibodies during disease progression may be the result of the overexpression of more antigenic tumor associated proteins, such as NY-ESO-1, overwhelming the capacity of the immune system to maintain SSX-2-specific immunity. The relatively low titers of NY-ESO-1 antibodies during remission may in turn be the result of a reduced expression of NY-ESO-1 as a means to evade T-cell-based immunosurveillance. In addition, NY-ESO-1 protein may be downregulated during remission for functional reasons, such as reduced proliferation.
Overall, our data indicate that NY-ESO-1-specific antibodies, despite their basic functional capabilities, are unlikely to contribute relevantly to an anti-tumor immune response in our patients, but rather represent a marker of disease progression. In contrast, SSX-285–90-restricted IgG3 antibodies are theoretically able to promote anti-tumor immunity, especially when supported by effector T cells targeting the same antigen and seem to correlate with favorable clinical status, however, whether detection of these rare antibodies carries actual predictive value in MM patients will have to be addressed by prospective studies. Future studies will further answer the question if and how these responses actually contribute to an integrated immune response and whether their essential differences in phenotype also lead to different anti-tumor activity. Hopefully, detailed analyses of spontaneously and alloSCT-induced immune responses against CTA will help to further optimize immunotherapeutic approaches for patients with MM.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by grants from the Erich and Gertrud Roggenbuck-Stiftung, Eppendorfer Krebs- und Leukämiehilfe, Deutsche José Carreras Leukämie-Stiftung, Deutsche Krebshilfe, and from the Cancer Research Institute (to Djordje Atanackovic).
Conflict of interest
The authors declare no conflict of interest.
Abbreviations
- ADCC
Antibody-dependent cytotoxicity
- AlloSCT
Allogeneic stem cell transplantation
- APC
Antigen-presenting cell
- AutoSCT
Autologous stem cell transplantation
- BM
Bone marrow
- CFSE
5-(and-6)-Carboxyfluorescein diacetate succinimidyl ester
- CTA
Cancer–testis antigens
- DAPI
4′,6-Diamidino-2-phenylindole
- ELISA
Enzyme-linked immunosorbent assay
- ELISPOT
Enzyme-linked immune spot assay
- FcγR
Fcγ receptor
- FCS
Fetal calf serum
- FLU
Influenza nucleoprotein
- GvM
Graft-versus-myeloma
- IFN
Interferon
- ORF
Open-reading frame
- PB
Peripheral blood
- PBMC
Peripheral blood mononuclear cells
- RT
Reverse transcriptase
- TT
Tetanus toxoid
Footnotes
Tim Luetkens and Sebastian Kobold have contributed equally to this work.
References
- 1.Kobold S, Luetkens T, Cao Y, Bokemeyer C, Atanackovic D. Prognostic and diagnostic value of spontaneous tumor-related antibodies. Clin Dev Immunol. 2010;2010:721531. doi: 10.1155/2010/721531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atanackovic D, Arfsten J, Cao Y, Gnjatic S, Schnieders F, Bartels K, Schilling G, Faltz C, Wolschke C, Dierlamm J, Ritter G, Eiermann T, Hossfeld DK, Zander AR, Jungbluth AA, Old LJ, Bokemeyer C, Kroger N. Cancer–testis antigens are commonly expressed in multiple myeloma and induce systemic immunity following allogeneic stem cell transplantation. Blood. 2007;109(3):1103–1112. doi: 10.1182/blood-2006-04-014480. [DOI] [PubMed] [Google Scholar]
- 3.Atanackovic D, Luetkens T, Hildebrandt Y, Arfsten J, Bartels K, Horn C, Stahl T, Cao Y, Zander AR, Bokemeyer C, Kroger N. Longitudinal analysis and prognostic effect of cancer–testis antigen expression in multiple myeloma. Clin Cancer Res. 2009;15(4):1343–1352. doi: 10.1158/1078-0432.CCR-08-0989. [DOI] [PubMed] [Google Scholar]
- 4.Pabst C, Zustin J, Jacobsen F, Luetkens T, Kroger N, Schilling G, Bokemeyer C, Sauter G, Atanackovic D, Marx A. Expression and prognostic relevance of MAGE-C1/CT7 and MAGE-C2/CT10 in osteolytic lesions of patients with multiple myeloma. Exp Mol Pathol. 2010;89(2):175–181. doi: 10.1016/j.yexmp.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Atanackovic D, Hildebrandt Y, Jadczak A, Cao Y, Luetkens T, Meyer S, Kobold S, Bartels K, Pabst C, Lajmi N, Gordic M, Stahl T, Zander AR, Bokemeyer C, Kroger N. Cancer–testis antigens MAGE-C1/CT7 and MAGE-A3 promote the survival of multiple myeloma cells. Haematologica. 2009;95(5):785–793. doi: 10.3324/haematol.2009.014464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawaguchi S, Wada T, Ida K, Sato Y, Nagoya S, Tsukahara T, Kimura S, Sahara H, Ikeda H, Shimozawa K, Asanuma H, Torigoe T, Hiraga H, Ishii T, Tatezaki SI, Sato N, Yamashita T. Phase I vaccination trial of SYT–SSX junction peptide in patients with disseminated synovial sarcoma. J Transl Med. 2005;3(1):1. doi: 10.1186/1479-5876-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Levy CL, Li YF, El-Gamil M, Schwarz SL, Laurencot C, Rosenberg SA. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29(7):917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Rhee F, Szmania SM, Zhan F, Gupta SK, Pomtree M, Lin P, Batchu RB, Moreno A, Spagnoli G, Shaughnessy J, Tricot G. NY-ESO-1 is highly expressed in poor-prognosis multiple myeloma and induces spontaneous humoral and cellular immune responses. Blood. 2005;105(10):3939–3944. doi: 10.1182/blood-2004-09-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobold S, Tams S, Luetkens T, Cao Y, Sezer O, Bartels BM, Reinhard H, Templin J, Bartels K, Hildebrandt Y, Lajmi N, Marx A, Haag F, Bokemeyer C, Kroger N, Atanackovic D. Patients with multiple myeloma develop SOX2-specific autoantibodies after allogeneic stem cell transplantation. Clin Dev Immunol. 2011;2011:302145. doi: 10.1155/2011/302145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papp K, Vegh P, Miklos K, Nemeth J, Rasky K, Peterfy F, Erdei A, Prechl J. Detection of complement activation on antigen microarrays generates functional antibody profiles and helps characterization of disease-associated changes of the antibody repertoire. J Immunol. 2008;181(11):8162–8169. doi: 10.4049/jimmunol.181.11.8162. [DOI] [PubMed] [Google Scholar]
- 11.Atanackovic D, Cao Y, Luetkens T, Panse J, Faltz C, Arfsten J, Bartels K, Wolschke C, Eiermann T, Zander AR, Fehse B, Bokemeyer C, Kroger N. CD4+ CD25+ FOXP3+ T regulatory cells reconstitute and accumulate in the bone marrow of patients with multiple myeloma following allogeneic stem cell transplantation. Haematologica. 2008;93(3):423–430. doi: 10.3324/haematol.11897. [DOI] [PubMed] [Google Scholar]
- 12.Cao Y, Gordic M, Kobold S, Lajmi N, Meyer S, Bartels K, Hildebrandt Y, Luetkens T, Ihloff AS, Kroger N, Bokemeyer C, Atanackovic D. An optimized assay for the enumeration of antigen-specific memory B cells in different compartments of the human body. J Immunol Methods. 2010;358(1–2):56–65. doi: 10.1016/j.jim.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Atanackovic D, Matsuo M, Ritter E, Mazzara G, Ritter G, Jager E, Knuth A, Old LJ, Gnjatic S. Monitoring CD4+ T cell responses against viral and tumor antigens using T cells as novel target APC. J Immunol Methods. 2003;278(1–2):57–66. doi: 10.1016/S0022-1759(03)00209-6. [DOI] [PubMed] [Google Scholar]
- 14.Atanackovic D, Altorki NK, Cao Y, Ritter E, Ferrara CA, Ritter G, Hoffman EW, Bokemeyer C, Old LJ, Gnjatic S. Booster vaccination of cancer patients with MAGE-A3 protein reveals long-term immunological memory or tolerance depending on priming. Proc Natl Acad Sci USA. 2008;105(5):1650–1655. doi: 10.1073/pnas.0707140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, Amigorena S. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189(2):371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stuart LM, Ezekowitz RA. Phagocytosis: elegant complexity. Immunity. 2005;22(5):539–550. doi: 10.1016/j.immuni.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Swanson JA, Hoppe AD. The coordination of signaling during Fc receptor-mediated phagocytosis. J Leukoc Biol. 2004;76(6):1093–1103. doi: 10.1189/jlb.0804439. [DOI] [PubMed] [Google Scholar]
- 18.Senovilla L, Vitale I, Martins I, Tailler M, Pailleret C, Michaud M, Galluzzi L, Adjemian S, Kepp O, Niso-Santano M, Shen S, Marino G, Criollo A, Boileve A, Job B, Ladoire S, Ghiringhelli F, Sistigu A, Yamazaki T, Rello-Varona S, Locher C, Poirier-Colame V, Talbot M, Valent A, Berardinelli F, Antoccia A, Ciccosanti F, Fimia GM, Piacentini M, Fueyo A, Messina NL, Li M, Chan CJ, Sigl V, Pourcher G, Ruckenstuhl C, Carmona-Gutierrez D, Lazar V, Penninger JM, Madeo F, Lopez-Otin C, Smyth MJ, Zitvogel L, Castedo M, Kroemer G. An immunosurveillance mechanism controls cancer cell ploidy. Science. 2012;337(6102):1678–1684. doi: 10.1126/science.1224922. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell AJ, Edwards MR, Collins AM. Valency or wahlency: is the epitope diversity of the B-cell response regulated or chemically determined? Immunol Cell Biol. 2001;79(5):507–511. doi: 10.1046/j.1440-1711.2001.01021.x. [DOI] [PubMed] [Google Scholar]
- 20.Cummings L, Riley L, Black L, Souvorov A, Resenchuk S, Dondoshansky I, Tatusova T. Genomic BLAST: custom-defined virtual databases for complete and unfinished genomes. FEMS Microbiol Lett. 2002;216(2):133–138. doi: 10.1111/j.1574-6968.2002.tb11426.x. [DOI] [PubMed] [Google Scholar]
- 21.Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374(9686):324–339. doi: 10.1016/S0140-6736(09)60221-X. [DOI] [PubMed] [Google Scholar]
- 22.Kroger N. Mini-midi-maxi? How to harness the graft-versus-myeloma effect and target molecular remission after allogeneic stem cell transplantation. Leukemia. 2007;21(9):1851–1858. doi: 10.1038/sj.leu.2404775. [DOI] [PubMed] [Google Scholar]
- 23.Kobold S, Lutkens T, Cao Y, Bokemeyer C, Atanackovic D. Autoantibodies against tumor-related antigens: incidence and biologic significance. Hum Immunol. 2010;71(7):643–651. doi: 10.1016/j.humimm.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Jager E, Stockert E, Zidianakis Z, Chen YT, Karbach J, Jager D, Arand M, Ritter G, Old LJ, Knuth A. Humoral immune responses of cancer patients against “cancer–testis” antigen NY-ESO-1: correlation with clinical events. Int J Cancer. 1999;84(5):506–510. doi: 10.1002/(SICI)1097-0215(19991022)84:5<506::AID-IJC10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Fossa A, Berner A, Fossa SD, Hernes E, Gaudernack G, Smeland EB. NY-ESO-1 protein expression and humoral immune responses in prostate cancer. Prostate. 2004;59(4):440–447. doi: 10.1002/pros.20025. [DOI] [PubMed] [Google Scholar]
- 26.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nature Med. 1998;4(5):627–630. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 27.Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, Xu Y, Pogoriler E, Terzulli SL, Kuk D, Panageas KS, Ritter G, Sznol M, Halaban R, Jungbluth AA, Allison JP, Old LJ, Wolchok JD, Gnjatic S. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci USA. 2011;108(40):16723–16728. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedrichs B, Siegel S, Kloess M, Barsoum A, Coggin J, Jr, Rohrer J, Jakob I, Tiemann M, Heidorn K, Schulte C, Kabelitz D, Steinmann J, Schmitz N, Zeis M. Humoral immune responses against the immature laminin receptor protein show prognostic significance in patients with chronic lymphocytic leukemia. J Immunol. 2008;180(9):6374–6384. doi: 10.4049/jimmunol.180.9.6374. [DOI] [PubMed] [Google Scholar]
- 29.Bellucci R, Alyea EP, Chiaretti S, Wu CJ, Zorn E, Weller E, Wu B, Canning C, Schlossman R, Munshi NC, Anderson KC, Ritz J. Graft-versus-tumor response in patients with multiple myeloma is associated with antibody response to BCMA, a plasma-cell membrane receptor. Blood. 2005;105(10):3945–3950. doi: 10.1182/blood-2004-11-4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redpath S, Michaelsen T, Sandlie I, Clark MR. Activation of complement by human IgG1 and human IgG3 antibodies against the human leucocyte antigen CD52. Immunology. 1998;93(4):595–600. doi: 10.1046/j.1365-2567.1998.00472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li P, Jiang N, Nagarajan S, Wohlhueter R, Selvaraj P, Zhu C. Affinity and kinetic analysis of Fcgamma receptor IIIa (CD16a) binding to IgG ligands. J Biol Chem. 2007;282(9):6210–6221. doi: 10.1074/jbc.M609064200. [DOI] [PubMed] [Google Scholar]
- 32.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daeron M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113(16):3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 33.Hansen MH, Nielsen HV, Ditzel HJ. Translocation of an intracellular antigen to the surface of medullary breast cancer cells early in apoptosis allows for an antigen-driven antibody response elicited by tumor-infiltrating B cells. J Immunol. 2002;169(5):2701–2711. doi: 10.4049/jimmunol.169.5.2701. [DOI] [PubMed] [Google Scholar]
- 34.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182(5):1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moeller I, Spagnoli GC, Finke J, Veelken H, Houet L. Uptake routes of tumor-antigen MAGE-A3 by dendritic cells determine priming of naive T-cell subtypes. Cancer Immunol Immunother. 2012;61(11):2079–2090. doi: 10.1007/s00262-012-1272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bevaart L, Jansen MJ, van Vugt MJ, Verbeek JS, van de Winkel JG, Leusen JH. The high-affinity IgG receptor, FcgammaRI, plays a central role in antibody therapy of experimental melanoma. Cancer Res. 2006;66(3):1261–1264. doi: 10.1158/0008-5472.CAN-05-2856. [DOI] [PubMed] [Google Scholar]
- 37.Noguchi T, Kato T, Wang L, Maeda Y, Ikeda H, Sato E, Knuth A, Gnjatic S, Ritter G, Sakaguchi S, Old LJ, Shiku H, Nishikawa H. Intracellular tumor-associated antigens represent effective targets for passive immunotherapy. Cancer Res. 2012;72(7):1672–1682. doi: 10.1158/0008-5472.CAN-11-3072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.