Abstract
Toll-like receptor 4 (TLR4) is an important trigger of the immune response against hepatitis B virus (HBV) infection and liver injuries. The roles of HBV reactivation versus TLR4-dependant immune response may be critical factors in preventing radiation-induced liver diseases (RILDs) after liver cancer radiotherapy. This study consists of three phases. In the primary phase, livers of mutant TLR4 (TLR4−) mice were irradiated with 30 Gy in either the absence or presence of HBV infection. The latter was done by introduction of plasmid pAAV/HBV 1.2. In the advanced phase, RILDs were compared in normal TLR4 (TLR4+) versus TLR4− mice. In the validation phase, 28 liver cancer patients who had undergone radiotherapy before hepatectomy were enrolled. Liver biopsies near tumors, irradiated with 35–48 Gy, were used to construct tissue microarrays. HBV reactivation, TLR4 expression, and severity of RILDs were studied in both mouse and human. More HBV reactivation, without significant RILD, was observed in irradiated versus unirradiated TLR4− mice. RILD scores of TLR4+ mice were higher than TLR4− mice. In humans, serious RILDs tended to develop in patients with high TLR4 expression, but not in patients with low TLR4 or high HBV surface antigen expression. High TLR4 expression was seen in only 2 of 12 HBV-reactive patients, but in HBV-nonreactive patients, it was seen in 6 of 9 (P < 0.03). In summary, RILDs correlated with high TLR4 expression, but not with HBV reactivation, which is inhibited in liver with high TLR4 expression after liver cancer radiotherapy.
Keywords: Hepatitis B virus, Liver cancer, Toll-like receptor, TLR4 mutation, Radiotherapy
Introduction
Hepatitis B virus (HBV) is recognized as a critically important risk factor for hepatocellular carcinoma (HCC) [1]. For most patients with inoperable and/or locally advanced HCC, radiotherapy (RT) can be carried out as an effective treatment. Although viruses, theoretically, should be killed by irradiation, RT in fact can activate HBV either indirectly, by inducing cytokines released from neighboring nonhepatocytes, or directly, via immunosuppression [2]. Immune responses are crucial for viral clearance during HBV infection [3]. The host defense may be suppressed by HBV via disabling activities of the toll-like receptors (TLRs), including TLR4, which are critical in provoking innate immunity as part of antiviral responses [4, 5]. For example, due to a normal TLR4-dependant immune response, HBV-affected mouse models have not been set up successfully in TLR4 normal (C3H/HeN) mice. However, in a background, containing a mutation in the TLR4 gene, an HBV-affected mouse model has been developed in C3H/HeJ mice [3].
Besides radiation-induced HBV reactivation, severe radiation-induced liver diseases (RILDs) with chronic hepatitis B have also been observed in many previous studies [2]. Therefore, can we deduce that HBV reactivation, in and of itself, is sufficient to cause serious RILDs, without taking into consideration of the TLR4-dependant immune mechanism? Or is the question rather: Which process plays a more vital role in the pathogenesis of RILDs–HBV reactivation or TLR4-dependant immune response?
The present study consists of three phases. Breeder mice of C3H/HeN and C3H/HeJ strains were included in the primary and advanced phases. In C3H/HeJ mice, there is a single mutation (A instead of C) at position 2342 of the TLR4 cDNA sequence. This mutation is not present in C3H/HeN (normal TLR4, TLR4+) mice [6]. In the primary phase, with an innate TLR4 immunodeficiency resulting from the single mutation, treatment of C3H/HeJ (mutant TLR4, TLR4−) mice by hydrodynamic injection of plasmid pAAV/HBV 1.2 resulted in HBV infection of the injected mice within 3–5 weeks [7]. The livers of TLR4− mice were irradiated with 30 Gy in either the absence or presence of HBV infection. Although more HBV reactivation was seen after irradiation, there were no significant differences in RILDs of HBV-infected versus HBV-uninfected TLR4− mice after RT. We hypothesize that this effect is associated with TLR4 immunodeficiency. Therefore, the primary phase was followed by an advanced study phase, in which RILDs were compared in TLR4+ versus TLR4− mice to evaluate the role of TLR4-dependant immune response in RILDs. In the validation phase, 28 liver cancer patients who had undergone RT before hepatectomy in our hospital were enrolled to evaluate the relative roles of HBV reactivation versus TLR4-dependant immune response in existing clinical cases of RILDs.
Materials and methods
Animals
Male C3H/HeJ (TLR4−) and C3H/HeN (TLR4+) mice, 5–6 weeks of age, were kept under standard pathogen-free conditions. All mice were given a normal laboratory diet and water ad libitum and handled according to protocols approved by the Animal Care Ethics Committee of Zhongshan Hospital, Fudan University.
Primary study phase: HBV reactivation and RILD in TLR4− mice
Groups of experimental mice
There were four groups of animals in the experiments, each containing eight male mice, into which TLR4− males were randomized. The first group contained TLR4− mice infected with HBV, and it was termed the TLR4−/HBV/control. The second group contained TLR4−/HBV mice that were treated by RT (TLR4−/HBV/RT). The third group contained uninfected TLR4− mice (TLR4−/control), and the fourth group contained uninfected TLR4− mice that were treated by RT (TLR4−/RT).
TLR4− mice infected with HBV
The 16 male TLR4− mice, 5 weeks of age, were infected with HBV by exposure to plasmid pAAV/HBV 1.2 DNA (kindly provided by Prof. Pei-Jer Chen), which contained the HBV fragment spanning nucleotides 1400–3182/1–1987 flanked by inverted terminal repeats of AAV [8]. Ten milligrams of plasmid pAAV/HBV 1.2 DNA dissolved in a volume of 0.9 % (w/v) NaCl solution equivalent to 0.1 ml/g of mouse body weight (around 2.0 ml) was injected into the tail veins of mice within 5–7 s, following the hydrodynamics-based transfection protocol [7].
Radiation treatments of mice
Livers of 16 male mice, 6 weeks of age, that had been divided into the TLR4−/HBV/RT (at day 8 after HBV infection) and TLR4−/RT groups were irradiated with 30 Gy in a single fraction by helical tomotherapy (Tomo Therapy, Madison, WI, USA) using 6-MV X-irradiation (dose rate, 8.8 Gy/min). Before RT, animals were anesthetized and immobilized in the supine position for scanning by computed tomography (CT) simulation (Siemens, Munich, Germany). Treatment plans were originally performed using the ADAC Pinnacle3 software, version 7.6 (ADAC Inc.; Milpitas, CA, USA) and subsequently transferred to the HT-treatment planning system (TPS). On each planning CT scan image, nearly the entire mouse livers were outlined as the clinical target volumes; the lungs, gastrointestinal tracts, kidneys, and spinal cords were delineated as critical organs at risk. Image-guided radiation therapy was conducted by megavoltage CT to verify the treatment setup before each RT.
Levels of HBV surface antigen (HBsAg) and HBV-DNA in sera of mice
After mice were infected with HBV, blood samples were obtained before RT and subsequently at 10 and 20 days after RT. Blood was collected by retro-orbital bleeding, and mice were generally anesthetized by intraperitoneal injections of ketamine during the procedure. Mouse serum levels of HBsAg were quantified using enzyme-linked immunosorbent assay (ELISA) kits (Shanghai Kehua Bio-engineering Co., Shanghai, China) following the manufacturer’s protocol. After serum DNA was purified, copy numbers of HBV-DNA were measured by real-time polymerase chain reaction (PCR) analysis, using Quantitative Diagnostic Kits for HBV DNA (Qiagen, Shenzhen, China) (primer sequences not available).
Mouse liver histological evaluation
Mice were killed 20 days after RT, at which time the irradiated liver lobes were harvested, fixed in 4 % (w/v) neutral buffered formalin and then embedded in paraffin. Hepatic sections (2–3 μm thick) stained with hematoxylin and eosin (HE) were examined microscopically at a magnification of 100× to evaluate the severity of RILDs. In a blinded fashion by two independent observers, the severity of RILDs was scored using the Fudan acute RILD histological scoring system, according to the degree of interface hepatitis (piecemeal necrosis), parenchymal injury (apoptosis and spotty necrosis), inflammation, and hepatic veno-occlusive and sinusoidal obstruction syndrome and based on the Ishak score and grading of disease activity in chronic hepatitis developed by Batts and Ludwig [9, 10]. The scoring from 0 to 4 ranges from no effects (0), scattered/mild (1), mild/moderate (2), moderate (3), to widespread severe liver damage (4).
Advanced study phase: TLR4 and RILD in TLR4− and TLR4+ mice
To evaluate the role of TLR4-dependant immune response in RILDs, 16 TLR4+ male mice were randomized into one of two groups: TLR4+/control and TLR4+/RT groups. In addition, 16 TLR4− mice of TLR4−/control and TLR4−/RT groups, from the primary phase, would also be enrolled as controls. In this phase, liver radiation treatments and histological evaluation of TLR4+ mice were also performed following the experimental protocols established in the earlier phase.
Validation phase in 28 liver cancer patients undergoing RT prior to hepatectomy
Patients
In this phase, we retrospectively analyzed clinical evidence of HBV reactivation, TLR4-dependant immune response and RILDs of 28 liver cancer patients (22 HCC and 6 intrahepatic cholangiocarcinoma), who had undergone hepatectomy about 2 months after liver RT in Zhongshan Hospital between January 2005 and May 2013. None of the patients had been treated with chemotherapy around the time of pre-hepatectomy liver RT. Twenty-one of the patients had an HBV infection history (HBV+), and seven of the patients did not (HBV−). Approval for this study was obtained from the Zhongshan Hospital Research Ethics Committee.
Assessment of HBV reactivation post-RT according to serum HBV DNA levels
We retrospectively compared serum HBV DNA levels pre- and post-RT, detected by real-time PCR analysis by the Clinical Laboratory Department of our hospital. Patients were further categorized into two groups: patients post-RT with or without HBV reactivation.
Tissue microarrays
Directed by RT isodose distribution graphs stored in TPS computers, areas of liver adjacent to tumors that had been irradiated with 35–48 Gy (10–25 fractions) were used in further histological studies with tissue microarrays (TMA). Slides with HE-stained sections were screened for optimal peritumoral liver tissues, which were then used to construct TMAs. Cores measuring 2.0 mm along the longest dimension were punched from peritumoral liver areas in formalin-fixed, paraffin-embedded samples. Slides containing sections (4-μm thickness) from the resulting TMA blocks were constructed following standard techniques.
Human liver histological evaluation (immunohistochemistry and HE)
TMA sections were stained with HE or subjected to immunohistochemistry (IHC) with antibodies against TLR4 (1:100 dilution) (Abcam, Cambridge, UK) or mouse anti-HBsAg (1:100 dilution) (KeyGEN BioTECH, Nanjing, China) and were visualized with the GTVision™III Detection Sytem/Mo & Rb kit (Gene Tech, Shanghai, China), according to the manufacturer’s instructions. The evaluation of TLR4 and HBsAg expression was performed by three of the authors who were blinded to patient outcome. Five high-power fields (magnification, 100×) were randomly selected. According to the staining percentage and intensity of positive cells counted in each core, immunoreactivity seen by IHC staining was categorized as follows: negative (−), weak or mild (+), moderate (++), or strong (+++). For statistical analysis, staining scores of cells expressing TLR4 and HBsAg were categorized as follows: − or + (low expression), and ++ or +++ (high expression) for TLR4; − (low expression), and +, ++, or +++ (high expression) for HBsAg. Patients were also divided into high- and low- TLR4 or HBsAg expression groups [11]. In TLR4 high- and low-expression groups, the numbers of cases with HBV infection pre-RT, HBV reactivations post-RT, or high HBsAg expression on IHC were further analyzed.
The HE-stained TMA sections were used to compare severity of RILDs in patients, which was scored by the Fudan acute RILD histological system in a blinded fashion by two independent observers. Significant RILD was defined as “RILD score ≥3.” The differences in RILD scores of patients were further analyzed between groups with or without HBV reactivation, with high or low TLR4 expression, and with high or low HBsAg expression.
Statistics
Statistical analyses were done with SPSS 17.0 software (SPSS for Windows, version 17.0). Values were presented as the mean ± standard error of the mean (SEM). Differences in quantitative data obtained for serum HBsAg levels, serum HBV-DNA copy numbers, and RILD scores were evaluated by t test or analysis of variance. Differences in frequency data, such as numbers of cases with HBV infection, occurrence of HBV reactivation, or low HBsAg expression in high-TLR4 expression groups were evaluated by Fisher’s exact probability test. Differences with a P value less than 0.05 were deemed to be statistically significant.
Results
More HBV reactivation in RT-treated versus untreated TLR4− mice
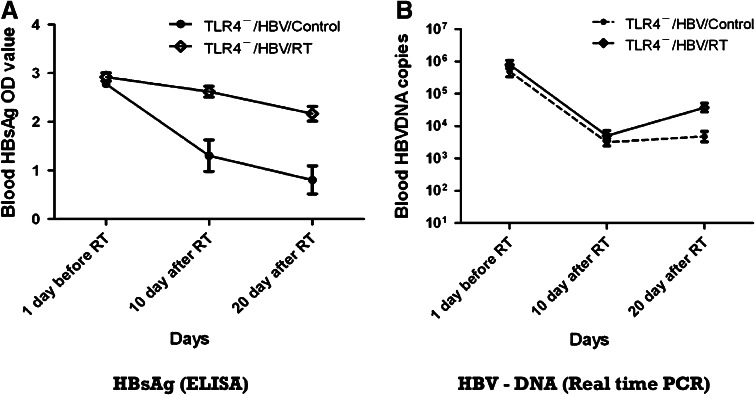
Serum HBsAg levels, assessed by ELISA, were higher in the TLR4−/HBV/RT than TLR4−/HBV/control group after RT (OD values: at 10 days post-RT, 2.62 ± 0.11 vs. 1.31 ± 0.32; P < 0.01; at 20 days post-RT, 2.17 ± 0.15 vs. 0.81 ± 0.29; P = 0.02), although there were no significant difference between the two groups before RT (OD values: at 1 day pre-RT, 2.92 ± 0.083 vs. 2.78 ± 0.073; P = 0.216) (Fig. 1a). Moreover, serum HBV-DNA copy numbers (copies ml−1) measured by real-time PCR, in spite of a general decreasing trend, showed less reduction in the TLR4−/HBV/RT than in the TLR4−/HBV/control group at 20 days post-RT (5.36 ± 1.94 × 104 vs. 7.14 ± 2.16 × 103; P = 0.043) (Fig. 1b). In contrast, the copy numbers were similar in the TLR4−/HBV/RT and TLR4−/HBV/control groups both at 1 day pre-RT (1.00 ± 0.20 × 106 vs. 7.08 ± 1.91 × 105, respectively; P = 0.301) and 10 days post-RT (7.67 ± 2.31 × 103 vs. 3.99 ± 0.99 × 103, respectively; P = 0.176).
Fig. 1.
HBV reactivation after RT in TLR4− mice. a Serum HBsAg levels assessed by ELISA. After HBV infection, OD values of blood HBsAg were higher in TLR4− mice with RT than without RT (P < 0.01, at 10 days post-RT; P = 0.02, at 20 days post-RT). b Serum HBV-DNA copy numbers (copies ml−1) measured by real-time PCR. In spite of a decreasing trend in serum HBV-DNA copy numbers, they were reduced relatively less in TLR4−mice with RT than without RT at 20 days after RT. Abbreviations: HBV hepatitis B virus, RT radiotherapy, TLR4 − toll-like receptor 4 mutation, HBsAg hepatitis B surface antigen, ELISA enzyme-linked immunosorbent assay, OD optical density, PCR polymerase chain reaction
TLR4, but not HBV infection, causes more serious RILD in mice
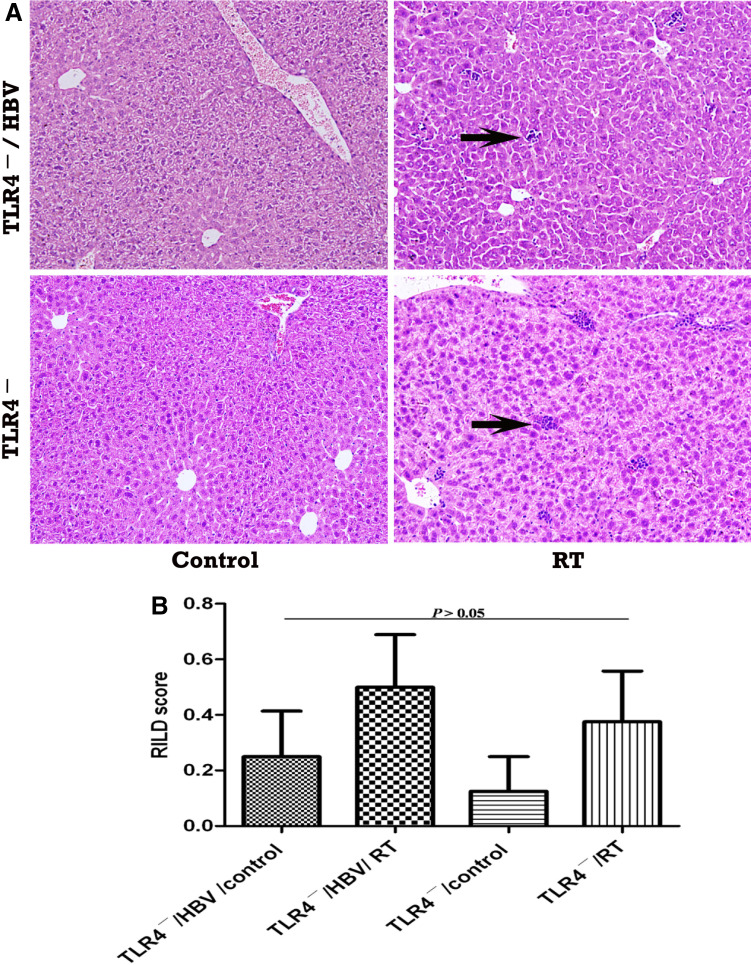
Liver histological changes after RT in each mouse were further evaluated in HE-stained sections and scored. No significant RILDs in mice were observed among TLR4−/HBV/control, TLR4−/HBV/RT, TLR4−/control, or TLR4−/RT groups (RILD scores: 0.13 ± 0.13, 0.38 ± 0.18, 0.25 ± 0.16, and 0.5 ± 0.19, respectively; P > 0.05) (Fig. 2), which indicates that HBV infection would probably not cause a more serious RILD in the absence of a TLR4-dependant response. However, when compared with mice in TLR4−/control, TLR4−/RT, and TLR4+/control groups (RILD scores: 0.25 ± 0.16, 0.5 ± 0.19, and 0.13 ± 0.13, respectively; P > 0.05), the TLR4+/RT mice developed more serious RILDs (RILD score: 2 ± 0.27 vs. the other 3 groups; P < 0.05) (Fig. 3). This further confirms that a TLR4-dependant immune response plays a vital role in mouse RILDs, and it even seems to be the underlying mechanism of RILDs seen here.
Fig. 2.
HBV reactivation after RT does not correlate with severe RILDs in TLR4− mice. a Liver HE staining analysis. Magnification, × 100. Only scattered neutrophil infiltration (arrows) was observed in livers of TLR4− mice with or without HBV infection after RT. b RILD score. Mean RILD score of TLR4− mice with HBV reactivation, after liver RT, was not significantly higher than in mice of the other three groups (P > 0.05). Abbreviations: TLR4 − toll-like receptor 4 mutation, HBV hepatitis B virus, RILD radiation-induced liver disease, HE hematoxylin and eosin, RT radiotherapy
Fig. 3.
RILDs correlate with TLR4 in mice. a Liver HE staining analysis. Magnification ×100. Compared with the scattered neutrophil infiltration (arrows) observed in livers of TLR4− mice, moderate neutrophil infiltration and/or interface hepatitis (arrows) developed in livers of TLR4+ mice after RT. b RILD score. Mean histological score of TLR4+ mice was significantly higher than in mice of the TLR4− groups after liver RT (P < 0.05). Abbreviations: TLR4 − toll-like receptor 4 mutation, TLR4 + normal TLR4, HBV hepatitis B virus, RILD radiation-induced liver disease, HE hematoxylin and eosin, RT radiotherapy
Histological findings in TMAs from 28 liver cancer patients by IHC- and HE-stained sections
Characteristics of the 28 patients in the validation study are shown in Table 1. Expressed TLR4 and HBsAg were observed in the plasmalemma and cytoplasm of hepatocytes (Fig. 4a, arrows). As shown in Table 1, 11 (39.29 %) of the 28 TMA specimens showed high TLR4 expression and 17 (60.71 %) showed low TLR4 expression in peritumoral liver tissues. With regard to HBsAg expressed in peritumoral liver tissues, expression in 9 (32.14 %) specimens was high and in 19 (67.86 %) was low. Assessment and scoring of RILD severity in HE-stained liver TMA slides indicated that 14 (50 %) showed significant RILDs.
Table 1.
Characteristics of the 28 liver cancer patients undergoing RT before hepatectomy
| Pt | Sex | Age | HBV infection | HBV reactivation | IHC-stained TMA | HE-stained TMA | |
|---|---|---|---|---|---|---|---|
| TLR4 | HBsAg | RILD score | |||||
| 1 | M | 46 | Yes | Yes | − | − | 1 |
| 2 | M | 65 | Yes | Yes | + | − | 1 |
| 3 | M | 78 | Yes | Yes | + | − | 2 |
| 4 | F | 45 | Yes | No | − | − | 3 |
| 5 | M | 52 | Yes | No | + | − | 3 |
| 6 | M | 60 | No | NA | + | − | 3 |
| 7 | M | 40 | Yes | Yes | + | + | 1 |
| 8 | M | 55 | No | NA | ++ | − | 2 |
| 9 | M | 48 | Yes | No | ++ | − | 4 |
| 10 | M | 69 | Yes | No | ++ | − | 4 |
| 11 | M | 55 | Yes | No | ++ | − | 3 |
| 12 | M | 59 | Yes | No | ++ | − | 4 |
| 13 | F | 64 | Yes | No | ++ | − | 2 |
| 14 | M | 49 | No | NA | + | − | 2 |
| 15 | M | 56 | No | NA | ++ | − | 3 |
| 16 | F | 68 | No | NA | ++ | − | 4 |
| 17 | M | 62 | Yes | No | + | − | 2 |
| 18 | M | 44 | Yes | Yes | − | + | 1 |
| 19 | M | 53 | Yes | Yes | + | + | 2 |
| 20 | M | 61 | Yes | Yes | + | − | 2 |
| 21 | M | 59 | Yes | Yes | ++ | + | 4 |
| 22 | M | 42 | Yes | Yes | + | + | 2 |
| 23 | M | 47 | Yes | Yes | + | + | 3 |
| 24 | M | 69 | No | NA | − | − | 2 |
| 25 | M | 49 | Yes | No | ++ | + | 4 |
| 26 | M | 45 | Yes | Yes | + | ++ | 2 |
| 27 | M | 76 | No | NA | + | − | 4 |
| 28 | M | 40 | Yes | Yes | ++ | ++ | 4 |
RT radiotherapy, Pt patient number, HBV, hepatitis B virus; IHC, immunohistochemistry, TMA tissue microarray, HE hematoxylin and eosin, TLR4 toll-like receptor 4, HBsAg hepatitis B surface antigen, RILD radiation-induced liver disease, M male, F female, NA not applicable; Yes, stands for cases with HBV infection pre-RT or with HBV reactivation post-RT; No, stands for cases without HBV infection pre-RT or without HBV reactivation post-RT; the symbols −, +, and ++, represent a negative, weak or mild, and moderate immunoreactivity, respectively
Fig. 4.
RILDs correlate with high TLR4 expression, but not with HBV reactivation, in human. a IHC- and HE-stained tissue microarrays of 28 liver cancer patients. Magnification ×100 and ×400. Compared with patients (such as Pt 18) with high HBsAg (arrows) and low TLR4 expression, patients (such as Pt 10) with high TLR4 expression (arrows) may develop more serious RILDs, such as liver piecemeal necrosis, inflammation, or hepatic veno-occlusive, and sinusoidal obstruction syndrome (arrows). b Grouping of RILD scores by TLR4 or HBsAg expression levels. c Grouping of RILD scores by HBV infection or reactivation. Although differences of RILDs were not observed between patients with or without HBV infection, or between patients with low versus high liver HBsAg expression, it is clear that patients with high TLR4 expression or without HBV reactivation post-RT may develop more serious RILDs. Abbreviations: RILD radiation-induced liver disease, TLR4 toll-like receptor 4, IHC immunohistochemistry, HE hematoxylin and eosin, HBV Hepatitis B virus, HBsAg hepatitis B surface antigen; Pt 10 or Pt 18, correspond to patient numbers 10 or 18 among the 28 patients in our study: Yes, with HBV infection or HBV reactivation; No, without HBV infection or HBV reactivation; RT radiotherapy
Reactivation of HBV post-RT is hindered in patients with high TLR4 expression levels
Serum HBV DNA levels pre- and post-RT were retrospectively analyzed. Twelve (57.14 %) of the 21 HBV+ patients showed HBV reactivation after RT and 9 (42.86 %) did not. There were 6 (66.67 %) of 9 HBV-nonreactive patients showing high liver TLR4 expression, compared to only 2 (16.67 %) of 12 HBV-reactive patients (P = 0.03). This indicates that HBV reactivation post-RT might result from low TLR4 expression in liver. Moreover, although no significant differences in TLR4 levels were found between HBV+ versus HBV− patients, or between patients with low versus high HBsAg expression in liver (P > 0.05), high TLR4 expression in liver might be considered as a mechanism to prevent or hinder HBV reactivation after RT (Table 2).
Table 2.
Correlations between TLR4, HBV, and severity of human RILD
| Severity of RILD | TLR4 expression by IHC-stained TMA | ||||
|---|---|---|---|---|---|
| RILD score (mean ± SEM) | p value | High TLR4 (cases) | Low TLR4 (cases) | p value | |
| HBV infection (n = 21/28) | 2.57 ± 0.24 | 0.55 | 8 | 13 | 1.00 |
| No HBV infection (n = 7/28) | 2.86 ± 0.34 | 3 | 4 | ||
| Low HBsAg on TMA (n = 19/28) | 2.68 ± 0.23 | 0.84 | 8 | 11 | 1.00 |
| High HBsAg on TMA (n = 9/28) | 2.6 ± 0.37 | 3 | 6 | ||
| HBV reactivation (n = 12/21) | 2.08 ± 0.31 | 0.02 | 2 | 10 | 0.03 |
| No HBV reactivation (n = 9/21) | 3.22 ± 0.28 | 6 | 3 | ||
| High TLR4 on TMA (n = 11/28) | 3.45 ± 0.25 | <0.01 | |||
| Low TLR4 on TMA (n = 17/28) | 2.12 ± 0.21 | ||||
RILD radiation-induced liver disease, TLR4 toll-like receptor 4, IHC immunohistochemistry, TMA tissue microarray, SEM standard error of the mean, HBV hepatitis B virus, HBsAg hepatitis B surface antigen
High TLR4 expression, but not HBV reactivation, causes severe RILDs after liver cancer RT
From IHC and HE histological findings in the 28 liver cancer patients, no significant differences in RILDs were seen between HBV+ versus HBV− patients (P = 0.55), or between patients with low versus high liver HBsAg expression (P = 0.84). However, significant RILDs did correlate with high TLR4 expression (P < 0.01, vs. low TLR4 expression) and absence of HBV reactivation (P = 0.02, vs. HBV reactivation). Moreover, when compared with patients having HBV reactivation post-RT, those without HBV reactivation, due to there being more patients with high than low TLR4 expression, were likely to develop more serious RILDs. In other words, HBV reactivation post-RT did not cause severe RILDs. Our finding is that high TLR4 expression might not only inhibit HBV reactivation post-RT, but also lead to severe RILDs (Table 2; Fig. 4).
Discussion
The TLR4 protein is an important trigger of the innate immune response, and it can recognize molecules derived from pathogens and endogenous danger signals that act as “damage-associated molecular patterns” (DAMPs) [12] [13]. Though RT is considered mostly immunosuppressive, it is noted also for its immunostimulatory effects [14, 15]. Distressed, injured, or damaged tissues induced by RT may generate DAMPs that stimulate TLR4. The latter may associate with myeloid differentiation primary response protein (MyD88) and Toll/interleukin (IL)-1 receptor domain-containing adaptor inducing interferon (IFN) α (TRIF) under sterile conditions, which can lead to activation of pro-inflammatory cytokines, chemokines, and reactive oxygen species (ROS) [16, 17], thus further promoting damage [13, 18]. Liu et al. [19] have demonstrated that TLR4 knockout mice can be protected from radiation-induced thymic lymphoma by downregulation of IL-6.
However, the role of TLR4-mediated inflammation in RILDs is a complex process. In healthy liver, TLR4 might be expressed in hepatocytes and hepatic nonparenchymal cells (NPCs), such as Kupffer cells (KCs), sinusoidal endothelial cells, and hepatic stellate cells [18]. Expression levels of TLR4 were higher in liver stroma, which contain NPCs, than in HCC stroma, comprising fibrous or necrotic tissue [20, 21]. In addition, despite the constant confrontation with gut-derived lipopolysaccharides, normal liver does not show signs of inflammation, and this phenomenon is thought to be part of a process known as “liver tolerance.” Nevertheless, “liver tolerance” of TLR4 can be broken down and induce an inappropriate immune response in the pathogenesis of alcoholic liver disease, chronic hepatitis B, hepatic fibrosis, and HCC [22]. Chen et al. had found that expression of TLR4 is decreased in HBV+ chronic hepatitis and cirrhosis [23, 24]. Excessive activation of TLR signaling may cause liver damage [18, 25]. In the present study, we have found that TLR4-mutant mice have decreased RILDs due to a defective TLR4-dependant response. Moreover, patients with high TLR4 expression, seen by IHC, present more severe RILDs after liver RT. Therefore, proteins involved with TLR4-dependant immune response in liver may be potential therapeutic targets for prevention and treatment for RILDs.
In addition, many studies have revealed that molecules involved with development of radiation-induced inflammation, such as IL-6, can facilitate HBV infection and reactivate HBV after RT [2]. In our studies, HBV reactivation after RT was also confirmed in mice. However, TLR4-dependant response also plays a vital role during the process of HBV reactivation after RT. In an HBV-transgenic mouse model, administration of TLR4 ligands was found to promptly inhibit viral replication mediated by IFNs [26]. From analysis of results obtained in liver cancer patients, we found that patients with high expression of TLR4 also show lower HBV replication, or even no HBV reactivation after RT, than patients with low TLR4 expression. Moreover, due to a normal TLR4-dependant immune response, C3H/HeN (TLR4+) mice were not able to maintain HBV infection status for a lengthy period [3]. This, unfortunately, hindered irradiation of HBV+ livers from TLR4+ mice and precluded further evaluation of the role of HBV versus normal TLR4 in RILDs in the study. These results indicate that TLR4 enhances the immune functions that help clear HBV replication.
Although HBV reactivation was thought to be the underlying mechanism of RILD for carriers in some earlier reports [2], our study reveals that HBV reactivation after RT, without considering TLR4-dependant response, has no significant relationship with severity of RILDs. In fact, immune activity plays a key role in HBV-related liver injuries. As stated earlier, some of the HBV+ patients develop low levels of TLR4 expression in liver, which might lead to less severity of RILDs. The findings substantiate that it is TLR4-dependant immune response, but not HBV reactivation, that plays a vital role in RILDs.
Moreover, the mechanism by which TLR4-dependant immune response promotes RILDs remains to be delineated. Previous studies suggested that radiation sensitivity of hepatocytes might be significantly influenced by changes in levels of certain cytokines, such as IL-6, in the liver microenvironment [27, 28]. The TLR4-dependant immune response might also promote RILDs via enhancing expression levels of some proteins in liver tissue interstitial fluids (a special microenvironment around hepatocytes [29]). This is a possible mechanism involved in severe RILDs after liver RT associated with high expression of TLR4, and it will be further studied by us in future work.
In conclusion, induction of inappropriate TLR4-dependant immune responses could be involved in the pathogenesis of hepatitis B and liver RT. Reactivation of HBV might be triggered by RT, especially when TLR4 expression in liver is low. However, without TLR4-dependant immune response, HBV reactivation per se would not lead to significant RILDs. High TLR4 expression in liver was associated with development of severe RILDs after liver RT. It indicated that use of TLR4 inhibitors along with HBV antiviral drugs might prevent the inflammatory component of RILD and viral reactivation in HBV+ liver cancer patients. Such an effect would favor treatment for HCC and other abdominal cancers with RT in future.
Acknowledgments
We thank Prof. Pei-Jer Chen (National Taiwan University) for kindly providing plasmid pAAV/HBV 1.2. This work was supported by the National Natural Science Foundation of China (Grant 81241012 to Z–C Zeng).
Conflict of interest
None.
Abbreviations
- HBV
Hepatitis B virus
- RILD
Radiation-induced liver disease
- TLR4
Toll-like receptor 4
- RT
Radiotherapy
- HCC
Hepatocellular carcinoma
- HBsAg
Hepatitis B surface antigen
- PCR
Polymerase chain reaction
- TMA
Tissue microarray
- IL
Interleukin
- IFN
Interferon
References:
- 1.Ohishi W, Fujiwara S, Cologne JB, Suzuki G, Akahoshi M, Nishi N, Tsuge M, Chayama K. Impact of radiation and hepatitis virus infection on risk of hepatocellular carcinoma. Hepatology. 2011;53(4):1237–1245. doi: 10.1002/hep.24207. [DOI] [PubMed] [Google Scholar]
- 2.Chou CH, Chen PJ, Lee PH, Cheng AL, Hsu HC, Cheng JC. Radiation-induced hepatitis B virus reactivation in liver mediated by the bystander effect from irradiated endothelial cells. Clin Cancer Res. 2007;13(3):851–857. doi: 10.1158/1078-0432.CCR-06-2459. [DOI] [PubMed] [Google Scholar]
- 3.Chang WW, Su IJ, Lai MD, Chang WT, Huang W, Lei HY. Toll-like receptor 4 plays an anti-HBV role in a murine model of acute hepatitis B virus expression. World J Gastroenterol. 2005;11(42):6631–6637. doi: 10.3748/wjg.v11.i42.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301(5633):640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt M, Raghavan B, Muller V, Vogl T, Fejer G, Tchaptchet S, Keck S, Kalis C, Nielsen PJ, Galanos C, Roth J, Skerra A, Martin SF, Freudenberg MA, Goebeler M. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat Immunol. 2010;11(9):814–819. doi: 10.1038/ni.1919. [DOI] [PubMed] [Google Scholar]
- 6.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in TLR4 gene. Science. 1998;282(5396):2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 7.Chang WW, Su IJ, Lai MD, Chang WT, Huang W, Lei HY. The role of inducible nitric oxide synthase in a murine acute hepatitis B virus (HBV) infection model induced by hydrodynamics-based in vivo transfection of HBV-DNA. J Hepatol. 2003;39(5):834–842. doi: 10.1016/S0168-8278(03)00389-1. [DOI] [PubMed] [Google Scholar]
- 8.Huang LR, Wu HL, Chen PJ, Chen DS. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc Natl Acad Sci USA. 2006;103(47):17862–17867. doi: 10.1073/pnas.0608578103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19(12):1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007;47(4):598–607. doi: 10.1016/j.jhep.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Xu H, Wu Q, Dang S, Jin M, Xu J, Cheng Y, Pan M, Wu Y, Zhang C, Zhang Y. Alteration of CXCR7 expression mediated by TLR4 promotes tumor cell proliferation and migration in human colorectal carcinoma. PLoS ONE. 2011;6(12):e27399. doi: 10.1371/journal.pone.0027399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Astsaturov IA, Bingham CA, McCarthy KM, von Mehren M, Xu W, Alpaugh RK, Tang Y, Littlefield BA, Hawkins LD, Ishizaka ST, Weiner LM. Effective antibody therapy induces host-protective antitumor immunity that is augmented by TLR4 against treatment. Cancer Immunol Immunother. 2012;61(1):49–61. doi: 10.1007/s00262-011-1090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 14.Oblak A, Jerala R. Toll-like receptor 4 activation in cancer progression and therapy. Clin Dev Immunol. 2011;2011:609579. doi: 10.1155/2011/609579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manzano-Alonso ML, Castellano-Tortajada G. Reactivation of hepatitis B virus infection after cytotoxic chemotherapy or immunosuppressive therapy. World J Gastroenterol. 2011;17(12):1531–1537. doi: 10.3748/wjg.v17.i12.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, Andre F, Tursz T, Kroemer G, Zitvogel L. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 17.O’Neill LA. When signaling pathways collide positive and negative regulation of toll-like receptor signal transduction. Immunity. 2008;29(1):12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48(1):322–335. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 19.Liu C, Gao F, Li B, Mitchel RE, Liu X, Lin J, Zhao L, Cai J. TLR4 knockout protects mice from radiation-induced thymic lymphoma by downregulation of IL6 and miR-21. Leukemia. 2011;25(9):1516–1519. doi: 10.1038/leu.2011.113. [DOI] [PubMed] [Google Scholar]
- 20.Eiro N, Altadill A, Juarez LM, Rodriguez M, Gonzalez LO, Atienza S, Bermudez S, Fernandez-Garcia B, Fresno-Forcelledo MF, Rodrigo L, and Vizoso FJ (2013) Toll-like receptors 3, 4 and 9 in hepatocellular carcinoma: Relationship with clinicopathological characteristics and prognosis. Hepatol Res [DOI] [PubMed]
- 21.Wu J, Lu M, Meng Z, Trippler M, Broering R, Szczeponek A, Krux F, Dittmer U, Roggendorf M, Gerken G, Schlaak JF. Toll-like receptor-mediated control of HBV replication by nonparenchymal liver cells in mice. Hepatology. 2007;46(6):1769–1778. doi: 10.1002/hep.21897. [DOI] [PubMed] [Google Scholar]
- 22.Soares JB, Pimentel-Nunes P, Roncon-Albuquerque R, Leite-Moreira A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol Int. 2010;4(4):659–672. doi: 10.1007/s12072-010-9219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z, Cheng Y, Xu Y, Liao J, Zhang X, Hu Y, Zhang Q, Wang J, Zhang Z, Shen F, Yuan Z. Expression profiles and function of Toll-like receptors 2 and 4 in peripheral blood mononuclear cells of chronic hepatitis B patients. Clin Immunol. 2008;128(3):400–408. doi: 10.1016/j.clim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Wu JF, Chen CH, Ni YH, Lin YT, Chen HL, Hsu HY, Chang MH. Toll-like receptor and hepatitis B virus clearance in chronic infected patients: a long-term prospective cohort study in Taiwan. J Infect Dis. 2012;206(5):662–668. doi: 10.1093/infdis/jis420. [DOI] [PubMed] [Google Scholar]
- 25.Gao B, Seki E, Brenner DA, Friedman S, Cohen JI, Nagy L, Szabo G, Zakhari S. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2011;300(4):G516–G525. doi: 10.1152/ajpgi.00537.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Testro AG, Visvanathan K. Toll-like receptors and their role in gastrointestinal disease. J Gastroenterol Hepatol. 2009;24(6):943–954. doi: 10.1111/j.1440-1746.2009.05854.x. [DOI] [PubMed] [Google Scholar]
- 27.Du SS, Qiang M, Zeng ZC, Ke AW, Ji Y, Zhang ZY, Zeng HY, Liu Z. Inactivation of kupffer cells by gadolinium chloride protects murine liver from radiation-induced apoptosis. Int J Radiat Oncol Biol Phys. 2010;76(4):1225–1234. doi: 10.1016/j.ijrobp.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 28.Zhou LY, Wang ZM, Gao YB, Wang LY, Zeng ZC. Stimulation of hepatoma cell invasiveness and metastatic potential by proteins secreted from irradiated nonparenchymal cells. Int J Radiat Oncol Biol Phys. 2012;84(3):822–828. doi: 10.1016/j.ijrobp.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Sun W, Ma J, Wu S, Yang D, Yan Y, Liu K, Wang J, Sun L, Chen N, Wei H, Zhu Y, Xing B, Zhao X, Qian X, Jiang Y, He F. Characterization of the liver tissue interstitial fluid (TIF) proteome indicates potential for application in liver disease biomarker discovery. J Proteome Res. 2010;9(2):1020–1031. doi: 10.1021/pr9009172. [DOI] [PubMed] [Google Scholar]