Abstract
Objective
The J-SICT DC Vaccine Study Group provides dendritic cell (DC) vaccines for compassionate use under unified cell production and patient treatment regimens. We previously reported beneficial effects of DC vaccines on the overall survival of 62 patients with advanced non-small cell lung cancer (NSCLC) in a single-center analysis. Here, we extended analysis to 260 patients with NSCLC who were treated at six centers.
Methods
Of the 337 patients who met the inclusion criteria, we analyzed 260 patients who received ≥5 peptide-pulsed DC vaccinations once every 2 weeks.
Results
The mean survival time (MST) from diagnosis was 33.0 months (95 % confidence interval [CI]: 27.9–39.2), and that from time of first vaccination was 13.8 months (95 % CI 11.4–16.8). An erythema reaction at the injection site that was ≥30 mm in diameter was correlated most strongly with overall survival from the first vaccine (≥30 vs. < 30 mm: MST 20.4 vs. 8.8 months, P < 0.001). We reported a similar finding in our previous analysis of patients with advanced pancreatic cancer. Interestingly, although such findings were common between patients with adenocarcinoma and those with other subtypes, the former group experienced significantly prolonged overall survival and a higher response rate for erythema (56.3 vs. 37.3 %, respectively, P = 0.014).
Conclusions
This is the first multicenter study that suggests a possible clinical benefit of DC vaccines for patients with advanced NSCLC, especially those with adenocarcinoma. These findings suggest a specific potential responder population for DC vaccines and warrant further investigation in well-controlled prospective randomized trials.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-016-1872-z) contains supplementary material, which is available to authorized users.
Keywords: Non-small cell lung cancers, Adenocarcinoma, Dendritic cell vaccine, Erythema
Introduction
Non-small cell lung cancers (NSCLCs) account for approximately 85 % of all lung cancer cases [1] and the prognoses of patients with advanced or inoperable/recurrent NSCLCs remains unsatisfactory [2–5]. The development of new therapeutics to treat NSCLCs is thus eagerly needed.
Dendritic cells (DCs) are potent antigen-presenting cells in humans [6], and a number of clinical trials have used DC-based vaccines to target advanced malignancies including lung cancers, worldwide [7–10]. These were early clinical studies with limited numbers of patients, and DC vaccines in those studies elicited antitumor immune responses without serious adverse events. However, the clinical outcomes, including the antitumor effect and prolongation of survival, were rather limited in these studies.
The DC Vaccine Study Group of the Japanese society of immunotherapy and cell therapy (J-SICT; formerly the Japan Society of Innovative Cell Therapy, http://www.j-sict.jp/index.html) is associated with multiple medical centers in Japan. This study group has provided DC vaccines for compassionate use under unified regimens for both cell production and patient treatment, and it has published the clinical data of patients treated with DC-based vaccines. One medical institution from this group recently published a report describing the potentially beneficial effect of DC vaccines on the overall survival of 62 patients with advanced NSCLCs [11].
In the present study, we extended the data set to 260 patients with advanced NSCLC who were treated at one of the six centers in the DC Vaccine Study Group. Importantly, these institutions used a unified Standard Operating Procedure (SOP) to generate DC vaccines based on previous clinical studies with minor modifications [11–18]. They also used the same synthetic peptides of Wilms’ tumor gene 1 (WT1) and/or mucin 1 (MUC1) as tumor antigens, and they used a similar treatment regimen for the vaccinations.
Patients and methods
Patients
This study was a retrospective analysis of patients who underwent institutional review board (IRB)-approved compassionate treatments, but not prospectively planned clinical trials, at six medical centers in Japan. We accept patients for compassionate use of DC vaccines who, along with their family seriously hoped to receive additional treatment with standard therapy or who were dropped from standard therapy due to serious side effects. A total of 337 Japanese patients with advanced NSCLCs were treated between January 2007 and December 2013. Patients who met all of the following inclusion criteria were eligible for the present analyses, as described previously [14]: (1) clinical diagnosis of inoperable NSCLC due to locally advanced or metastatic disease; (2) an expected prognosis of over 4 months; (3) a white blood cell (WBC) count ≥ 2500 cells/μL; (4) hemoglobin (Hb) ≥ 7.0 g/dL; (5) a platelet count ≥ 70,000 counts/μL; and (6) no serious dysfunction of vital organs. The patient data set that was used in the previous single-center analysis [11] was also included in this study when patients met the above criteria. Each patient was evaluated at the time of initial treatment based on the latest version of the TNM classification and staging system, which was version 6 for cases in or before 2010 and version 7 for cases after 2010.
Patients were enrolled at one of six medical centers in Japan (Shinshu University Hospital, Sapporo Hokuyu Hospital, Seren Clinic Tokyo, Seren Clinic Nagoya, Seren Clinic Kobe, or Seren Clinic Fukuoka). Each patient received DC vaccine five times or more as described below. Treatment was performed according to the Declaration of Helsinki, and all participants signed informed consent forms. The treatments were approved by the IRB of each institution (approval numbers: #1199 for Shinshu University Hospital, #15 for Sapporo Hokuyu Hospital, and Medicine 24-4 for Seren Clinic Tokyo, Nagoya, Kobe, and Fukuoka), as described previously [14].
In this study, since extensive loss of data for mutation status could lead to significant bias in statistical analysis, we did not include the information for mutations except for epidermal growth factor receptor (EGFR). The data was collected in this study from 2007 to 2013, ALK mutation diagnostics were approved in 2012 in Japan, and other diagnostics, including K-ras mutations, have not been approved for NSCLCs in Japan.
DC vaccines
Preparation of DCs
DCs were prepared as described previously [11–18]. Briefly, peripheral blood mononuclear cells (PBMCs) were isolated from the leukapheresis products by Ficoll-Hypaque gradient density centrifugation. These PBMCs were placed on tissue-culture plates, and the adherent cells were cultured in medium containing human recombinant granulocyte–macrophage colony-stimulating factor and human recombinant interleukin-4 to generate immature DCs.
Five days later, the DCs were stimulated with OK-432 (10 mg/mL; a lyophilized preparation of Streptococcus pyogenes, Chugai Pharmaceutical Co., Ltd., Tokyo, Japan) and prostaglandin E2 (50 ng/mL) for 24 h. The DCs were then pulsed with peptide antigens according to the HLA-A pattern. The DCs were pulsed with WT1 peptide antigens for 24 h after treatment with OK-432 and prostaglandin E2. MUC1 was added to the DC culture media at the same time as OK-432 and prostaglandin E2. The DCs were cryopreserved and maintained until the day of administration. The phenotype CD14−/low/HLA-DR+/HLA-ABC+/CD80+/CD83+/CD86+/CD40+/CCR7+ was used to define mature DCs.
The cells were prepared by well-trained technical staff in each institutional cell-processing center (CPC) under the SOP provided by tella, Inc. (http://www.tella.jp/en/). Regarding the release criteria, testing for sterility, mycoplasma (polymerase chain reaction [PCR] method), and endotoxins (Endospecy™; Seikagaku Corp., Tokyo) was performed using the supernatant or cell suspension just before tube filling.
Peptide antigens
DCs were pulsed with the WT1 and/or MUC1 peptide antigens, with WT1 being chosen according to patient HLA-A type, as described previously [11, 14] CYTWNQMNL (mutant WT1 peptide, Neo-MPS; San Diego, CA) was chosen for HLA-A*24:02, and RMFPNAPYL (WT1 peptide; Neo-MPS) was chosen for HLA-A*02:01/02:06. The MUC1 long peptide TRPAPGSTAPPAHGVTSAPDTRPAPGSTAP (Greiner Japan, Tokyo) was used for all HLA-A types. We did not include immunohistochemistry when selecting these peptides because previous studies have shown a moderate-to-high frequency of WT1 and MUC1 overexpression in NSCLC (83 and 39 %, respectively) [19, 20].
Patient treatment and clinical assessments
The treatment regimen has been described previously [11, 14]. Briefly, all patients were injected five or more times intradermally with DCs in close proximity to the axial and/or inguinal lymph nodes, once every two weeks. At the time of vaccination, 0.1 mL of intradermal DC vaccine was used in the forearm to assess erythema response. When the patient requested it, OK-432 (to enhance the Th1 response) [21, 22] was administered at 0.5 clinical units (CU) as the initial dose and increased gradually until the patient’s temperature reached 38 °C, which should take place at a dose < 5.0 CU) simultaneously with the DC vaccine as an immunological adjuvant. The clinical parameters studied were sex, age, Eastern Cooperative Oncology Group performance status (ECOG-PS), clinical stage, laboratory data at time of leukapheresis, and combined chemotherapy.
The maximum erythema diameter was measured after 24–48 h, and patients who exhibited erythema ≥ 30 mm in diameter at least once were categorized as having a positive response. All other responses were considered negative. Adverse events were graded and documented according to the Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE v4.0). The Prognostic Nutritional Index (PNI) [14] was used as an index to assess patient nutritional condition. PNI was calculated using the following equation: PNI = 10 × serum albumin (g/dL) + 0.005 × total lymphocyte counts.
Statistical analyses
Survival curves were plotted using the Kaplan–Meier method, and survival curve comparisons were conducted with the log-rank test. We conducted multivariate analyses using the Cox proportional hazards regression model, and we used laboratory data at the time of leukapheresis in this analysis. The differences between two groups in categorical data were analyzed by Fisher exact probability test or Pearson Chi squared test. P values <0.05 were considered significant. Analyses were conducted using JMP Pro version 11.0 (SAS Institute Japan, Tokyo).
Results
Patient characteristics
Data were collected from 337 patients with advanced NSCLC who met the inclusion criteria and received DC vaccines at one of six medical centers comprising the J-SICT DC Vaccine Study Group. From these cases, the laboratory and clinical data of 260 patients who received five or more administrations of the DC vaccine once every two weeks were analyzed. We used the data from patients who received five or more administrations of the DC vaccine. This is because previous analysis of 62 patients with NSCLC demonstrated that the mean survival times (MSTs) of patients treated with less than 5 and more than 5 DC vaccine administrations were 2 months and 14 months, respectively (P < 0.0001) [11]. Moreover, our preliminary analysis using the overall data set of 337 patients in this study also showed similar results (2.2 months vs. 13.0 months, respectively, P < 0.0001). We confirmed that the group who received fewer than 5 DC vaccine administrations largely contained patients with serious conditions and grade 3–4 ECOG-PS scores, leading such patients to be approved for compassionate usage of the DC vaccines in this analysis. The preliminary analysis concerning patients with poor prognosis demonstrated varied laboratory data leading to their exclusion from further analysis.
The clinical characteristics of all 260 patients, as well as those with adenocarcinoma and other histological subtypes (e.g., squamous cell carcinoma [SCC]), are summarized in Table 1. Among the 260 patients, 207 (79.6 %) had adenocarcinoma, 81 (31.2 %) experienced postoperative relapse, and 179 (68.8 %) were inoperable due to distant metastasis or an intolerable physical condition. Additionally, 185 patients (71.4 %) received DC vaccines combined with simultaneous chemotherapy. Compared with the other histological subtypes (n = 53), the adenocarcinoma group included a significantly higher portion of female patients (37.7 vs. 22.6 %, P = 0.035), showed significantly higher WBC values (mean, 5900 vs. 6768/mL; P = 0.011) and significantly lower serum C-reactive protein (CRP) values (mean, 1.0 vs. 1.9 mg/dL; P = 0.013), and included significantly more patients who received combination chemotherapy (75.2 vs. 56.6 %; P = 0.009).
Table 1.
Patient Characteristics
| Variables | All cases | Adenocarcinoma | Others | P values |
|---|---|---|---|---|
| N = 260* | N = 207 | N = 53 | ||
| Age (years) | ||||
| Median (Range) | 63 (30–91) | 63 (32–89) | 65 (30–91) | 0.146# |
| Sex, no. (%) | ||||
| Male | 170 (65.4) | 129 (62.3) | 41 (77.4) | 0.035+ |
| Female | 90 (34.6) | 78 (37.7) | 12 (22.6) | |
| ECOG performance status score, no. (%) | ||||
| 0 | 78 (30.0) | 64 (30.9) | 14 (26.4) | 0.669+ |
| 1 | 151 (58.1) | 120 (58.0) | 31 (58.5) | |
| 2 | 23 (8.8) | 18 (8.7) | 5 (9.4) | |
| 3 | 8 (3.1) | 5 (2.4) | 3 (5.7) | |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Histological diagnosis, no. (%) | ||||
| Adenocarcinoma | 207 (79.6) | 207 (100) | 0 (0.0) | – |
| Squamous cell carcinoma | 32 (12.3) | 0 (0.0) | 32 (60.4) | |
| Large cell carcinoma | 5 (1.9) | 0 (0.0) | 5 (9.4) | |
| Others (NSCLC)** | 16 (6.2) | 0 (0.0) | 16 (30.2) | |
| Clinical Stage, no. (%) | ||||
| Relapse (preoperative stage) | 81 (31.2) | 67 (32.4) | 14 (26.4) | 0.398+ |
| Stage I | 19 (23.5) | 15 (22.4) | 4 (28.6) | |
| Stage IIA and IIB | 20 (24.7) | 15 (22.4) | 5 (35.7) | |
| Stage IIIA and IIIB | 31 (38.3) | 26 (38.8) | 5 (35.7) | |
| Undetermined | 11 (13.6) | 11 (16.4) | 0 (0.0) | |
| Inoperable | 179 (68.8) | 140 (67.6) | 39 (73.6) | |
| Stage IIB*** | 1 (0.6) | 0 (0.0) | 1 (2.6) | |
| Stage IIIA | 22 (12.3) | 11 (7.9) | 11 (28.2) | |
| Stage IIIB | 34 (19.0) | 23 (16.4) | 11 (28.2) | |
| Stage IV | 113 (63.1) | 100 (71.4) | 13 (33.3) | |
| Undetermined | 9 (5.0) | 6 (4.3) | 3 (7.7) | |
| Initial standard therapy, no. (%) | ||||
| Y | 229 (88.1) | 194 (93.7) | 47 (88.7) | 0.232+ |
| Surgery | 81 (35.4) | 67 (32.4) | 14 (26.4) | |
| Chemotherapy | 185 (80.8) | 179 (86.5) | 41 (77.4) | |
| Radiotherapy | 100 (43.7) | 81 (39.7) | 19 (35.6) | |
| N | 31 (11.9) | 13 (6.3) | 6 (11.3) | |
| Laboratory data at time of leukapheresis (mean ± S.D.) | ||||
| WBC (/L) | 6078 (±2221) | 5900 (±2135) | 6768 (±2430) | 0.011# |
| No. of neutrophil (%) | 64.8 (±11.6) | 64.9 (±11.9) | 64.2 (±10.1) | 0.693# |
| No. of lymphocytes (%) | 23.8 (±9.4) | 23.6 (±9.5) | 24.8 (±9.2) | 0.416# |
| No. of monocytes (%) | 7.6 (±3.8) | 7.6 (±3.9) | 7.6 (±3.2) | 0.971# |
| Hemoglobin (g/dL) | 12.3 (±1.9) | 12.3 (±1.8) | 12.2 (±2.1) | 0.702# |
| Platelet (×104) | 24.5 (±8.1) | 24.3 (±8.0) | 25.2 (±8.7) | 0.460# |
| Albumin (g/dL) | 3.9 (±0.5) | 3.9 (±0.5) | 3.9 (±0.5) | 0.591# |
| LDH (IU/L) | 223 (±110) | 224.2 (±118.0) | 223.2 (±70.8) | 0.955# |
| CRP (mg/dL) | 1.2 (±2.2) | 1.0 (±1.8) | 1.9 (±3.3) | 0.013# |
| Fibrinogen (mg/dL) | 411 (±153) | 406.1 (±156.5) | 435.1 (±133.1) | 0.400# |
| PNI | 45.6 (±5.8) | 45.6 (±5.6) | 46.4 (±5.6) | 0.400# |
| Time to start DC vaccination from diagnosis, months | ||||
| Median (range) | 9.9 (1–105) | 10.9 (1.3–104.9) | 6.6 (1.1–69.6) | 0.036# |
| Number of DC vaccines (/leukapheresis) | ||||
| Median (Range) | 7 (5–34) | 8 (5–34) | 7 (5–21) | 0.002# |
| Standard therapy combined with DC vaccine, no. (%) | ||||
| Chemotherapy | 185 (71.4) | 155 (75.2) | 30 (56.6) | 0.009# |
| Bevacizumab | 22 (8.5) | 19 (9.2) | 3 (5.7) | 0.391# |
| CDDP/CBDCA | 54 (22.6) | 46 (23.6) | 8 (18.2) | 0.429# |
| PTX/DTX | 41 (17.2) | 33 (16.9) | 8 (18.2) | 0.842# |
| PEM | 38 (15.9) | 36 (18.5) | 2 (4.6) | 0.011# |
| 5-FU | 27 (11.3) | 20 (10.3) | 7 (15.9) | 0.304# |
| Others | 29 (12.1) | 25 (12.8) | 4 (9.2) | 0.541# |
| Gefitinib or Erlotinib | 62 (23.8) | 58 (28.0) | 4 (7.6) | <0.001# |
| Radiotherapy | 38 (15.0) | 32 (15.8) | 6 (11.8) | 0.455# |
| None, including BSC | 26 (10.0) | 48 (23.3) | 20 (37.7) | 0.038# |
| Timing of chemotherapy combined with DC vaccine, no. (%) | ||||
| 1st line | 92 (35.4) | 77 (31.2) | 15 (28.3) | 0.010# |
| 2nd line | 52 (20.0) | 42 (20.3) | 10 (18.9) | |
| 3rd line and more | 71 (27.3) | 61 (29.5) | 10 (18.9) | |
| BSC or without chemotherapy | 43 (16.5) | 26 (12.6) | 17 (32.1) | |
| N.A. | 2 (0.8) | 1 (0.5) | 1 (1.9) |
SCC squamous cell carcinoma, LCC large cell carcinoma, PNI prognostic nutritional index, CDDP cisplatin, CBDCA carboplatin, PTX paclitaxel, DTX docetaxel, PEM pemetrexed, 5-FU 5-fluorouracil, BSC best supportive care, N.A. not available
* Including 3 cases without assessment for erythema
** Histological subtype was not confirmed
*** Standard therapy was not available due to intestinal pneumonitis
# Fisher’s exact probability test
+ Chi squared test
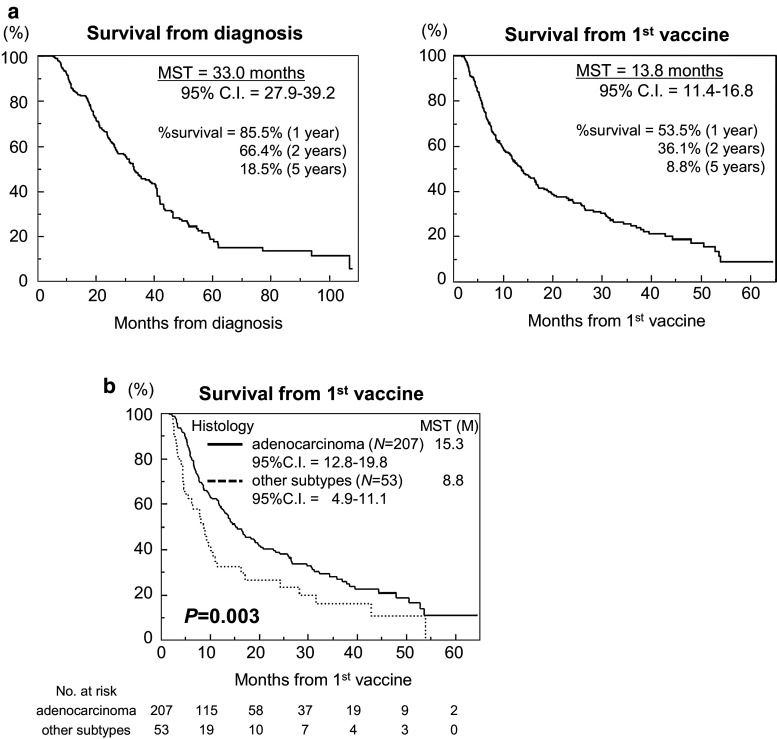
As shown in Fig. 1a, the MST of the 260 NSCLC patients from the time of diagnosis was 33.0 months (95 % confidence interval [CI]: 27.9–39.2 months), and that from the first DC vaccination was 13.8 months (95 % CI 11.4–16.8 months). The survival rates from diagnosis and the first vaccination were 85.5 and 53.5 % after 1 year, 66.4 and 36.1 % after 2 years, and 18.5 and 8.8 % after 5 years, respectively.
Fig. 1.
Overall survival (OS) a Kaplan–Meier plots of the OS times for 260 patients from the time of diagnosis (left graph, MST = 33.0 months) and from the time of first DC vaccination (right graph, MST = 13.8 months). b Kaplan–Meier plots of the OS times of patients with adenocarcinoma (n = 207, MST = 15.3 months) and those with other subtypes (n = 53, MST = 8.8 months) from the time of first DC vaccination (P = 0.003)
Patients with histologically identified adenocarcinoma had better prognoses
Next, we examined whether the histological subtype affected survival, and Fig. 1b illustrates the significantly better prognoses of patients whose tumors were histologically identified as adenocarcinoma (MST 15.3 months, 95 % CI 12.8–19.8 months) compared to the prognoses of patients with tumors of other subtypes (MST 8.8 months, 95 % CI 4.9–11.1 months; P = 0.003). The disease control rate (DCR = % complete response [CR] + partial response [PR] + stable disease [SD]) 3 months after first vaccination, according to the RECIST criteria (Supplementary Table S1), tended to be better in the adenocarcinoma group (43.0 %) than in the group of other subtypes (30.2 %), albeit without statistical significance (P = 0.065).
Prognostic factors related to the survival from the time of first DC vaccination
Univariate analyses with log-rank tests demonstrated that several baseline and laboratory factors previously shown to be prognostic for longer survival in advanced pancreatic cancer, namely body mass index (BMI) [23–28], distant metastasis (pleural dissemination, brain, bone, and liver), ECOG-PS [29, 30], neutrophil to lymphocyte ratio (NLR) [28, 31–33], serum albumin (Alb) [31], CRP [34, 35], and PNI [33], were associated with survival from the time of first DC vaccination in all cases and specifically in patients with adenocarcinoma (Table 2). Among treatment-related factors, DCR [11], simultaneous chemotherapy, and an erythema reaction at the injection site (threshold ≥30 mm in longitudinal diameter) [14] significantly correlated with patient survival from the time of first vaccination (Table 2).
Table 2.
Univariate analyses for predictors of survival time from the time of 1st vaccine
| All cases (N = 260) | Adenocarcinoma (N = 207) | ||||||
|---|---|---|---|---|---|---|---|
| Cases | MST | Log-rank | Cases | MST | Log-rank | ||
| A. Factors related to baseline and previous treatment | |||||||
| Sex | |||||||
| Male | 170 | 19 | 0.237 | 129 | 20 | 0.287 | |
| Female | 90 | 13 | 78 | 14 | |||
| BMI | |||||||
| ≥18 | 226 | 16 | <0.001* | 177 | 18 | <0.001* | |
| <18 | 33 | 8 | 29 | 9 | |||
| Operation | |||||||
| Y | 179 | 17 | 0.091 | 67 | 17 | 0.432 | |
| N | 81 | 13 | 140 | 14 | |||
| Subtype | |||||||
| Adenocarcinoma | 207 | 15 | 0.003* | – | – | ||
| Others | 53 | 9 | – | – | |||
| Brain metastasis | |||||||
| Y | 58 | 11 | 0.004* | 52 | 12 | <0.001* | |
| N | 200 | 15 | 153 | 19 | |||
| Bone metastasis | |||||||
| Y | 69 | 12 | 0.041* | 63 | 13 | 0.006* | |
| N | 189 | 14 | 142 | 17 | |||
| Liver metastasis | |||||||
| Y | 29 | 10 | 0.005* | 27 | 10 | <0.001* | |
| N | 229 | 14 | 178 | 14 | |||
| Pleural dissemination | |||||||
| Y | 70 | 10 | 0.058 | 64 | 12 | 0.023* | |
| N | 188 | 15 | 141 | 17 | |||
| EGFR mutation | |||||||
| Y | 88 | 17 | 0.454 | 78 | 17 | 0.834 | |
| N | 81 | 14 | 70 | 16 | |||
| Gefinitib or erlotinib before vaccine | |||||||
| Y | 82 | 14 | 0.530 | 80 | 14 | 0.200 | |
| N | 177 | 14 | 127 | 17 | |||
| B. Factors related to physical condition and laboratory data at the time of leukapheresis | |||||||
| ECOG-PS | |||||||
| 0–1 | 229 | 15 | 0.006* | 184 | 13 | 0.024* | |
| 2–4 | 31 | 8 | 23 | 6 | |||
| No. of lymphocytes | |||||||
| ≥1500 | 95 | 17 | 0.242 | 68 | 18 | 0.573 | |
| <1500 | 163 | 13 | 137 | 14 | |||
| NLR | |||||||
| ≥4 | 68 | 8 | <0.001* | 56 | 10 | <0.001* | |
| <4 | 190 | 17 | 149 | 20 | |||
| Hemoglobin | |||||||
| ≥11.0 | 201 | 15 | 0.060 | 163 | 17 | 0.320 | |
| <11.0 | 59 | 8 | 44 | 12 | |||
| Albumin | |||||||
| ≥3.5 | 195 | 16 | <0.001* | 157 | 19 | <0.001* | |
| <3.5 | 42 | 7 | 33 | 7 | |||
| LDH | |||||||
| ≥240 | 184 | 14 | 0.031* | 51 | 14 | 0.117 | |
| <240 | 67 | 10 | 149 | 10 | |||
| CRP | |||||||
| ≥0.5 | 96 | 8 | <0.001* | 71 | 8 | <0.001* | |
| <0.5 | 142 | 17 | 120 | 17 | |||
| PNI | |||||||
| ≥40 | 201 | 15 | <0.001* | 161 | 17 | <0.001* | |
| <40 | 35 | 7 | 28 | 8 | |||
| C. Factors related to DC vaccination and related treatment | |||||||
| DCR at 3 M | |||||||
| CR + PR + SD | 105 | 28 | <0.001* | 89 | 30 | <0.001* | |
| PD | 106 | 8 | 79 | 9 | |||
| Simultaneous chemotherapy | |||||||
| Y | 185 | 16 | 0.018* | 155 | 17 | 0.042* | |
| N | 74 | 10 | 51 | 12 | |||
| Gefinitib or Erlotinib during vaccine | |||||||
| Y | 102 | 16 | 0.697 | 96 | 16 | 0.732 | |
| N | 158 | 12 | 111 | 15 | |||
| WT1 | |||||||
| Y | 214 | 13 | 0.549 | 170 | 15 | 0.816 | |
| N | 46 | 18 | 37 | 17 | |||
| OK-432 | |||||||
| Y | 177 | 14 | 0.858 | 116 | 23 | 0.687 | |
| N | 80 | 14 | 90 | 12 | |||
| Fever (°C) | |||||||
| ≥38 | 71 | 13 | 0.131 | 60 | 17 | 0.281 | |
| <38 | 186 | 16 | 146 | 15 | |||
| Erythema (cm) | |||||||
| ≥3 | 135 | 20 | <0.001* | 116 | 23 | <0.001* | |
| <3 | 122 | 9 | 90 | 11 | |||
MST median survival time
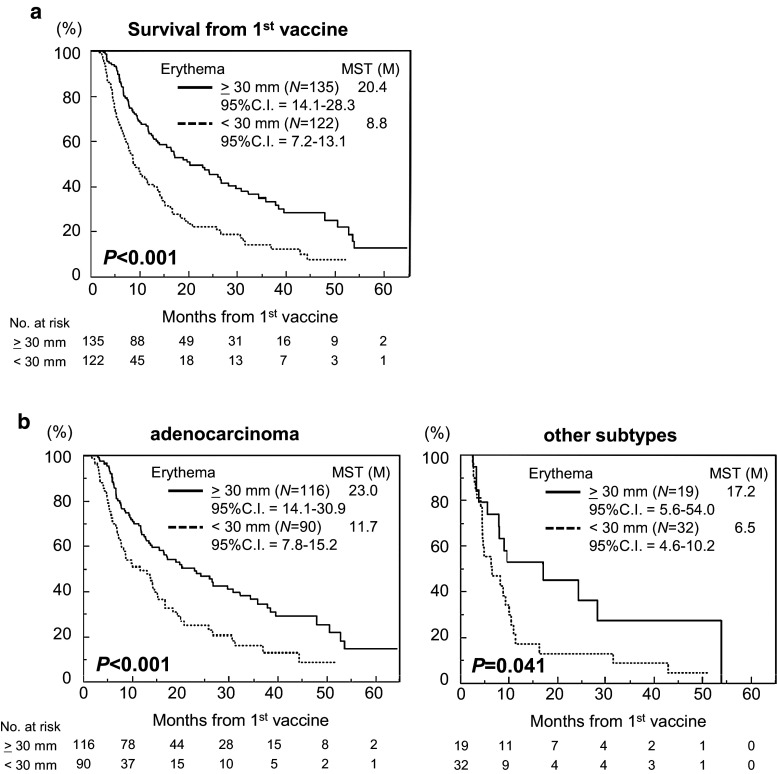
Erythema reaction is a good prognostic factor for longer survival from the time of first DC vaccination
Since our previous study revealed that erythema reaction at the injection site had prognostic value for advanced inoperable pancreatic cancer [14], we focused on this factor.
First, we identified factors related to the erythema reaction (Supplementary Table S2). Histological adenocarcinoma, ECOG-PS, NLR, Hb, CRP, DCR at 3 months, and fever after vaccinations correlated with erythema reactions for all cases, and these factors, except for Hb, also correlated with erythema reactions in the adenocarcinoma group specifically.
Importantly, Kaplan–Meier curve comparisons by log-rank test demonstrated superior survival in erythema-positive cases (MST 20.4 months, 95 % CI 14.1–28.3 months, n = 135) compared to erythema-negative cases (MST 8.8 months, 95 % CI 7.2–13.1 months, n = 122) (Fig. 2a, P < 0.001), and in both the groups of patients with adenocarcinoma (erythema-positive vs. -negative: MST 23.0 vs. 11.7 months, 95 % CI 14.1–30.9 vs. 7.7–15.2 months, n = 115 vs. 90; P < 0.001) and other subtypes (MST 17.2 vs. 6.5 months, 95 % CI 5.6–54.0 vs. 4.6–10.2 months, n = 19 vs. 32; P = 0.041). However, the response rate of patients with erythema amongst those with adenocarcinoma was significantly higher than that for patients with other subtypes (56.3 vs. 37.3 %, P = 0.014), suggesting that adenocarcinoma might be more susceptible to DC vaccines.
Fig. 2.
Erythema at the injection site as a treatment-related prognostic factor for OS in the histological subtypes. a Kaplan–Meier plots of the OS times for all 260 patients with or without ≥30-mm erythema from the time of the first DC vaccine. b Kaplan–Meier plots of the OS times of patients with adenocarcinoma (n = 206, left) and those with other subtypes (n = 51) with and without ≥30-mm erythema from the time of first DC vaccine (P < 0.001 and P = 0.041, respectively)
Finally, a multivariate analysis using a Cox proportional hazards regression model was performed to confirm the role of survival-related factors related to DC vaccination (Table 3). Of interest, BMI was an independent prognostic factor irrespective of histological subtype. The presence of brain or liver metastasis and decreased serum albumin were predictors of poor prognosis in patients with adenocarcinoma, but this was not true for patients with other subtypes. Importantly, erythema reaction, but not simultaneous chemotherapy, was an independent prognostic factor for better prognosis in the adenocarcinoma group, but inversely, simultaneous chemotherapy, but not erythema reaction, was an independent prognostic factor for patients with other subtypes.
Table 3.
Multivariate analyses of predictors of survival from the time of 1st vaccine
| All cases (N = 260) | Adenocarcinoma (N = 207) | Other subtypes (N = 53) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95 % CI | P values | Hazard ratio | 95 % CI | P values | Hazard ratio | 95 % CI | P values | |
| A. Factors related to the baseline and previous treatment | |||||||||
| Sex | |||||||||
| Female | 0.757 | 0.517 | 0.135 | 0.817 | 0.542 | 0.317 | 1.152 | 0.332 | 0.811 |
| Male | 1.000 | −1.089 | 1.000 | −1.211 | 1.000 | −3.466 | |||
| BMI | |||||||||
| <18 | 1.884 | 1.12 | 0.017* | 1.898 | 1.079 | 0.027* | 14.871 | 2.724 | 0.004* |
| ≥18 | 1.000 | –73.049 | 1.000 | −3.230 | 1.000 | −68.377 | |||
| Operation | |||||||||
| Y | 0.740 | 0.504 | 0.109 | 0.965 | 0.627 | 0.870 | 0.361 | 0.087 | 0.092 |
| N | 1.000 | −1.068 | 1.000 | −1.463 | 1.000 | −1.167 | |||
| Brain metastasis | |||||||||
| Y | 1.568 | 1.027 | 0.038* | 1.808 | 1.148 | 0.011* | 2.898 | 0.563 | 0.186 |
| N | 1.000 | −2.341 | 1.000 | −2.789 | 1.000 | −12.361 | |||
| Bone metastasis | |||||||||
| Y | 0.934 | 0.622 | 0.734 | 1.158 | 0.733 | 0.526 | 0.938 | 0.194 | 0.929 |
| N | 1.000 | −1.378 | 1.000 | −1.806 | 1.000 | −3.491 | |||
| Liver metastasis | |||||||||
| Y | 1.911 | 1.109 | 0.021* | 2.067 | 1.165 | 0.014* | 1.365 | 0.073 | 0.780 |
| N | 1.000 | −3.199 | 1.000 | −3.578 | 1.000 | −7.683 | |||
| B. Factors related to physical condition and laboratory data at the time of leukapheresis | |||||||||
| Albumin | |||||||||
| <3.5 | 1.963 | 1.273 | 0.003* | 2.287 | 1.408 | 0.001* | 1.938 | 0.552 | 0.285 |
| ≥3.5 | 1.000 | −2.951 | 1.000 | −3.609 | 1.000 | −6.017 | |||
| LDH | |||||||||
| ≥240 | 1.450 | 0.971 | 0.069 | 1.147 | 0.713 | 0.563 | 2.645 | 0.950 | 0.063 |
| <240 | 1.000 | −2.129 | 1.000 | −1.795 | 1.000 | −7.299 | |||
| C. Factors related to DC vaccination and combined treatment | |||||||||
| Simultaneous chemotherapy | |||||||||
| N | 1.590 | 1.067 | 0.023* | 1.424 | 0.881 | 0.146 | 2.586 | 1.086 | 0.032* |
| Y | 1.000 | −2.335 | 1.000 | −2.249 | 1.000 | −6.162 | |||
| WT1 | |||||||||
| Y | 1.469 | 0.887 | 0.139 | 1.188 | 0.684 | 0.555 | 2.098 | 0.408 | 0.394 |
| N | 1.000 | −2.570 | 1.000 | −2.205 | 1.000 | −14.157 | |||
| Erythema (cm) | |||||||||
| ≥3 | 0.545 | 0.385 | <0.001* | 0.583 | 0.340 | 0.009* | 0.695 | 0.291 | 0.392 |
| <3 | 1.000 | −0.771 | 1.000 | −0.875 | 1.000 | −1.592 | |||
Taking these results together, we concluded that adenocarcinoma was more susceptible to DC vaccination, and patients with NSCLC who were diagnosed with adenocarcinoma and treated with DC vaccines had better prognoses.
Discussion
The main goal of this study was to identify essential factors that are related to the survival of patients with inoperative or postoperatively recurrent NSCLC who were treated with DC vaccines. To do this, we performed an exploratory analysis of 260 Japanese patients with advanced NSCLC who were vaccinated with synthetic WT1 and/or MUC1 peptide-pulsed DCs at six medical institutions. There were three key findings from this study. First, previously identified prognostic factors known to be related to advanced NSCLCs, i.e., BMI, distant metastasis (pleural dissemination, brain, bone, and liver), ECOG-PS, NLR, Alb, CRP, and PNI, were confirmed to be significantly prognostic in this study as well. Second, similar to our previous study using a data set of patients with inoperable pancreatic cancer, erythema at the forearm injection site at the time of vaccination was an independent prognostic factor for survival of both patients with adenocarcinoma and patients with other subtypes. Third, patients with adenocarcinoma showed both better survival and a higher response rate for erythema reaction, suggesting that adenocarcinoma might be more susceptible to DC-based cancer vaccines. To the best of our knowledge, this is the first report of a multicenter clinical study using intradermal DC vaccines to treat patients with advanced NSCLC with well-organized and well-controlled autologous cell preparations and a similar treatment regimen.
The most important finding of this study is the correlation between erythema reaction and survival of patients with advanced NSCLC who received a DC vaccine, which was similar to the prognostic value of erythema previously revealed in our patients with inoperable pancreatic cancer [14]. Delayed-type hypersensitivity (DTH)-based local responses suggest in vivo T cell function and tumor antigen-specific T cells [36]. Moreover, DTH has also been correlated with clinical responses [37, 38]. The erythema reaction observed in the present study was not equivalent to the DTH in these earlier studies. However, it may be reasonable to propose that these two methods reflect similar responses.
We must also address the differences in results between the previous single-center study (n = 62) [11] and the present multicenter study (n = 260). The majority of the prognostic factors identified in both studies were assessed by univariate log-rank tests, i.e., histological identification as adenocarcinoma, ECOG-PS, Alb, CRP, DCR, and erythema reaction. However, the use of WT1 peptides, which was also an independent prognostic factor affecting patient survival in the previous study [11], was not identified as a prognostic factor in the present study. The exact reason for this discrepancy is unclear, but it could be due to the differences in patient conditions among the six centers. For example, when we used the data sets from only two of the six centers, one of which was used in a previous study [14], the use of WT1 peptides was still a significant prognostic factor, but use of WT1 peptides was not prognostic in the data sets from the other four centers (data not shown). DC vaccinations were initiated later for patients at the other four centers. Therefore, further prospective studies will be needed to clarify whether the use of WT1 peptides is truly effective for patients with advanced NSCLC.
Second, the reason why the present patients with adenocarcinoma were more susceptible to DC vaccines compared to the patients with other subtypes should be investigated. The responder (erythema-positive) rate in the adenocarcinoma group was significantly higher than that in the group of patients with other subtypes (Fig. 2b; Table 3), suggesting that the patients with NSCLCs that are not adenocarcinomas (the majority were SCCs in this study) may be in a more immunosuppressive state than those with adenocarcinomas.
There are a number of limitations in this study, including its retrospective nature and therefore heterogeneous patient population as well as the loss of data regarding specific immunological responses evoked by DC vaccination under well-validated methodology. Therefore, it is still difficult to draw any definitive conclusions in regard to the clinical benefit of the current DC vaccine. However, we believe that the identification of significant prognostic factors related to patient survival will be very useful for the selection of potential inclusion criteria in future prospective clinical trials.
In summary, we found that erythema after DC vaccination was a good prognostic factor for patients with advanced NSCLC, especially those with adenocarcinoma, which agreed with our previous observation of erythema as a favorable prognostic factor for patients with advanced pancreatic cancer. These findings seem reasonable and encouraging. However, because of the retrospective and exploratory nature of this investigation, the results need to be further addressed in well-controlled prospective randomized trials.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This report is dedicated to the patients who participated in our studies and to their primary oncology doctors. We also thank the present and former staff of each participating institution.
Abbreviations
- Alb
Albumin
- BMI
Body mass index
- CI
Confidence interval
- CPC
Cell processing center
- CR
Complete response
- CRP
C-reactive protein
- CTCAE
Common terminology criteria for adverse events
- CU
Clinical unit(s)
- DCR
Disease control rate
- DTH
Delayed-type hypersensitivity
- ECOG-PS
Eastern Cooperative Oncology Group performance status
- Hb
Hemoglobin
- IRB
Institutional review board
- J-SICT
The Japanese society of immunotherapy and cell therapy
- MST
Median survival time
- MUC1
Mucin 1
- NLR
Neutrophil to lymphocyte ratio
- NSCLC
Non-small cell lung cancer
- PBMC
Peripheral blood mononuclear cell
- PNI
Prognostic nutritional index
- PR
Partial response
- SCC
Squamous cell carcinoma
- SD
Stable disease
- SOP
Standard operating procedure
- WT1
Wilms’ tumor gene 1
Compliance with ethical standards
Funding
No funding supported this study.
Conflict of interest
Professor Y. Yonemitsu was a previous scientific advisor at tella, Inc., and Prof. M. Okamoto, who was excluded from data analyses, was a previous stockholder of tella, Inc. All remaining authors declare no conflicts of interest.
Footnotes
J-SICT was formerly the Japan Society of Innovative Cell Therapy.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Langer CJ, Manola J, Bernardo P, Kugler JW, Bonomi P, Cella D, et al. Cisplatin-based therapy for elderly patients with advanced non-small-cell lung cancer: implications of Eastern Cooperative Oncology Group 5592, a randomized trial. J Natl Cancer Inst. 2002;94:173–181. doi: 10.1093/jnci/94.3.173. [DOI] [PubMed] [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Eastern Cooperative Oncology Group Comparison of four chemotherapy regimens for advanced non–small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 4.Ohe Y, Ohashi Y, Kubota K, Tamura T, Nakagawa K, Negoro S, et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: four-Arm Cooperative Study in Japan. Ann Oncol. 2007;18:317–323. doi: 10.1093/annonc/mdl377. [DOI] [PubMed] [Google Scholar]
- 5.Paz-Ares LG, Biesma B, Heigener D, von Pawel J, Eisen T, Bennouna J, et al. Phase III, randomized, double-blind, placebo-controlled trial of gemcitabine/cisplatin alone or with sorafenib for the first-line treatment of advanced, nonsquamous non-small-cell lung cancer. J Clin Oncol. 2012;30:3084–3092. doi: 10.1200/JCO.2011.39.7646. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 7.Perroud MW, Jr, Honma HN, Barbeiro AS, Gilli SC, Almeida MT, Vassallo J, et al. Mature autologous dendritic cell vaccines in advanced non-small cell lung cancer: a phase I pilot study. J Exp Clin Cancer Res. 2011 doi: 10.1186/1756-9966-30-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morse MA, Clay TM, Hobeika AC, Osada T, Khan S, Chui S, et al. Phase I study of immunization with dendritic cells modified with fowlpox encoding carcinoembryonic antigen and costimulatory molecule. Clin Cancer Res. 2005;11:3017–3024. doi: 10.1158/1078-0432.CCR-04-2172. [DOI] [PubMed] [Google Scholar]
- 9.Chang GC, Lan HC, Juang SH, Wu YC, Lee HC, Hung YM, et al. A pilot clinical trial of vaccination with dendritic cells pulsed with autologous tumor cells derived from malignant pleural effusion in patients with late-stage lung carcinoma. Cancer. 2005;103:763–771. doi: 10.1002/cncr.20843. [DOI] [PubMed] [Google Scholar]
- 10.Hirschowitz EA, Foody T, Kryscio R, Dickson L, Sturgill J, Yannelli J. Autologous dendritic cell vaccines for non-small-cell lung cancer. J Clin Oncol. 2004;22:2808–2815. doi: 10.1200/JCO.2004.01.074. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi H, Okamoto M, Shimodaira S, Tsujitani S, Nagaya M, Ishidao T, DC-vaccine study group at the Japan Society of Innovative Cell Therapy (J-SICT) et al. Impact of dendritic cell vaccines pulsed with Wilms’ tumour-1 peptide antigen on the survival of patients with advanced non-small cell lung cancers. Eur J Cancer. 2013;49:852–859. doi: 10.1016/j.ejca.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Kimura Y, Tsukada J, Tomoda T, Takahashi H, Imai K, Shimamura K, et al. Clinical and immunologic evaluation of dendritic cell-based immunotherapy in combination with gemcitabine and/or S-1 in patients with advanced pancreatic carcinoma. Pancreas. 2012;41:195–205. doi: 10.1097/MPA.0b013e31822398c6. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi M, Sakabe T, Abe H, Tanii M, Takahashi H, Chiba A, DC-vaccine study group at the Japan Society of Innovative Cell Therapy (J-SICT) et al. Dendritic cell-based immunotherapy targeting synthesized peptides for advanced biliary tract cancer. J Gastrointest Surg. 2013;17:1609–1617. doi: 10.1007/s11605-013-2286-2. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi M, Shimodaira S, Nagai K, Ogasawara M, Takahashi H, Abe H, DC Vaccine Study Group at the Japan Society of Innovative Cell Therapy (J-SICT) et al. Prognostic factors related to add-on dendritic cell vaccines on patients with inoperable pancreatic cancer receiving chemotherapy: a multicenter analysis. Cancer Immunol Immunother. 2014;63:797–806. doi: 10.1007/s00262-014-1554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi M, Chiba A, Izawa H, Yanagida E, Okamoto M, Shimodaira S, DC-vaccine study group at the Japan Society of Innovative Cell Therapy (J-SICT) et al. The feasibility and clinical effects of dendritic cell-based immunotherapy targeting synthesized peptides for recurrent ovarian cancer. J Ovarian Res. 2014;7:48. doi: 10.1186/1757-2215-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi M, Sakabe T, Chiba A, Nakajima A, Okamoto M, Shimodaira S, DC-vaccine study group at the Japan Society of Innovative Cell Therapy (J-SICT) et al. Therapeutic effect of intratumoral injections of dendritic cells for locally recurrent gastric cancer: a case report. World J Surg Oncol. 2014;12:390. doi: 10.1186/1477-7819-12-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito S, Yanagisawa R, Yoshikawa K, Higuchi Y, Koya T, Yoshizawa K, et al. Safety and tolerability of allogeneic dendritic cell vaccination with induction of Wilms tumor 1-specific T cells in a pediatric donor and pediatric patient with relapsed leukemia: a case report and review of the literature. Cytotherapy. 2015;17:330–335. doi: 10.1016/j.jcyt.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Sakai K, Shimodaira S, Maejima S, Udagawa N, Sano K, Higuchi Y, et al. Dendritic cell-based immunotherapy targeting Wilms’ tumor 1 in patients with recurrent malignant glioma. J Neurosurg. 2015;123:989–997. doi: 10.3171/2015.1.JNS141554. [DOI] [PubMed] [Google Scholar]
- 19.Oji Y, Miyoshi S, Maeda H, Hayashi S, Tamaki H, Nakatsuka S, et al. Overexpression of the Wilms’ tumor gene WT1 in de novo lung cancers. Int J Cancer. 2002;100:297–303. doi: 10.1002/ijc.10476. [DOI] [PubMed] [Google Scholar]
- 20.Giatromanolaki A, Koukourakis MI, Sivridis E, O’Byrne K, Cox G, Thorpe PE, et al. Coexpression of MUC1 glycoprotein with multiple angiogenic factors in non-small cell lung cancer suggests coactivation of angiogenic and migration pathways. Clin Cancer Res. 2000;6:1917–1921. [PubMed] [Google Scholar]
- 21.Okamoto M, Furuichi S, Nishioka Y, Oshikawa T, Tano T, Ahmed SU, et al. Expression of toll-like receptor 4 on dendritic cells is significant for anticancer effect of dendritic cell-based immunotherapy in combination with an active component of OK-432, a streptococcal preparation. Cancer Res. 2004;64:5461–5470. doi: 10.1158/0008-5472.CAN-03-4005. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto M, Oshikawa T, Tano T, Ohe G, Furuichi S, Nishikawa H, et al. Involvement of Toll-like receptor 4 signaling in interferon-gamma production and antitumor effect by streptococcal agent OK-432. J Natl Cancer Inst. 2003;95:316–326. doi: 10.1093/jnci/95.4.316. [DOI] [PubMed] [Google Scholar]
- 23.Dahlberg SE, Schiller JH, Bonomi PB, Sandler AB, Brahmer JR, Ramalingam SS, et al. Body mass index and its association with clinical outcomes for advanced non-small-cell lung cancer patients enrolled on Eastern Cooperative Oncology Group clinical trials. J Thorac Oncol. 2013;8:1121–1127. doi: 10.1097/JTO.0b013e31829cf942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung CC, Lam TH, Yew WW, Chan WM, Law WS, Tam CM. Lower lung cancer mortality in obesity. Int J Epidemiol. 2011;40:174–182. doi: 10.1093/ije/dyq134. [DOI] [PubMed] [Google Scholar]
- 25.Attaran S, McShane J, Whittle I, Poullis M, Shackcloth M. A propensity-matched comparison of survival after lung resection in patients with a high versus low body mass index. Eur J Cardiothorac Surg. 2012;42:653–658. doi: 10.1093/ejcts/ezs135. [DOI] [PubMed] [Google Scholar]
- 26.Jiang L, Jiang S, Lin Y, Yang H, Zhao Z, Xie Z, et al. Combination of body mass index and oxidized low density lipoprotein receptor 1 in prognosis prediction of patients with squamous non-small cell lung cancer. Oncotarget. 2015;6:22072–22080. doi: 10.18632/oncotarget.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandrekar SJ, Schild SE, Hillman SL, Allen KL, Marks RS, Mailliard JA, et al. A prognostic model for advanced stage nonsmall cell lung cancer. Pooled analysis of North Central Cancer Treatment Group trials. Cancer. 2006;107:781–792. doi: 10.1002/cncr.22049. [DOI] [PubMed] [Google Scholar]
- 28.Luo J, Chen YJ, Narsavage GL, Ducatman A. Predictors of survival in patients with non-small cell lung cancer. Oncol Nurs Forum. 2012;39:609–616. doi: 10.1188/12.ONF.609-616. [DOI] [PubMed] [Google Scholar]
- 29.Arslan D, Tural D, Koca T, Tastekin D, Kaymak Cerkesli A, et al. Prognostic factors in clinical stage T4N2 locally advanced non-small cell lung cancer. J BU ON. 2015;20:573–579. [PubMed] [Google Scholar]
- 30.Ulas A, Turkoz FP, Silay K, Tokluoglu S, Avci N, Oksuzoglu B, et al. A laboratory prognostic index model for patients with advanced non-small cell lung cancer. PLoS ONE. 2014;9:e114471. doi: 10.1371/journal.pone.0114471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yildirim M, Yildiz M, Duman E, Goktas S, Kaya V. Prognostic importance of the nutritional status and systemic inflammatory response in non-small cell lung cancer. J BU ON. 2013;18:728–732. [PubMed] [Google Scholar]
- 32.Gu XB, Tian T, Tian XJ, Zhang XJ. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. Sci Rep. 2015;5:12493. doi: 10.1038/srep12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kos FT, Hocazade C, Kos M, Uncu D, Karakas E, Dogan M, et al. Assessment of prognostic value of “neutrophil to lymphocyte ratio” and “prognostic nutritional index” as a sytemic inflammatory marker in non-small cell lung cancer. Asian Pac J Cancer Prev. 2015;16:3997–4002. doi: 10.7314/APJCP.2015.16.9.3997. [DOI] [PubMed] [Google Scholar]
- 34.Jin Y, Sun Y, Shi X, Zhao J, Shi L, Yu X. Prognostic value of circulating C-reactive protein levels in patients with non-small cell lung cancer: a systematic review with meta-analysis. J Cancer Res Ther. 2014;10(Suppl):C160–C166. doi: 10.4103/0973-1482.145854. [DOI] [PubMed] [Google Scholar]
- 35.Liao C, Yu Z, Guo W, Liu Q, Wu Y, Li Y, et al. Prognostic value of circulating inflammatory factors in non-small cell lung cancer: a systematic review and meta-analysis. Cancer Biomark. 2014;14:469–481. doi: 10.3233/CBM-140423. [DOI] [PubMed] [Google Scholar]
- 36.de Vries IJ, Bernsen MR, Lesterhuis WJ, Scharenborg NM, Strijk SP, Gerritsen MJ, et al. Immunomonitoring tumor-specific T cells in delayed-type hypersensitivity skin biopsies after dendritic cell vaccination correlates with clinical outcome. J Clin Oncol. 2005;23:5779–5787. doi: 10.1200/JCO.2005.06.478. [DOI] [PubMed] [Google Scholar]
- 37.Hersey P, Menzies SW, Halliday GM, Nguyen T, Farrelly ML, DeSilva C, et al. Phase I/II study of treatment with dendritic cell vaccines in patients with disseminated melanoma. Cancer Immunol Immunother. 2004;53:125–134. doi: 10.1007/s00262-003-0429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishida S, Koido S, Takeda Y, Homma S, Komita H, Takehara A, et al. Wilms tumor gene (WT1) peptide-based cancer vaccine combined with gemcitabine for patients with advanced pancreatic cancer. J Immunother. 2014;37:105–114. doi: 10.1097/CJI.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.