Abstract
Background
Anti-tumor vaccination is a new frontier in cancer treatment applicable to immunogenic neoplasms such as prostate and renal cancers. GX301 is a vaccine constituted by four telomerase peptides and two adjuvants, Montanide ISA-51 and Imiquimod.
Objective
The aim of this study was to analyze safety and tolerability of GX301 in an open-label, phase I/II trial. Immunological and clinical responses were also evaluated as secondary endpoints.
Experimental design
GX301 was administered by intradermally injecting 500 μg of each peptide (dissolved in Montanide ISA-51) in the skin of the abdomen. Imiquimod was applied as a cream at the injection sites. The protocol included 8 administrations at days 1, 3, 5, 7, 14, 21, 35, 63. Eligible patients were affected with stage IV prostate or renal cancer resistant to conventional treatments. Patients were clinically and immunologically monitored up to 6 months from the first immunization.
Results
No grade 3–4 adverse events were observed. Evidence of vaccine-specific immunological responses was detected in 100 % of patients. Disease stabilization occurred in 4 patients. Prolonged progression-free survival and overall survival were observed in patients showing a full pattern of vaccine-specific immunological responses.
Conclusion
GX301 demonstrated to be safe and highly immunogenic. Further studies are needed to determine its clinical efficacy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1415-9) contains supplementary material, which is available to authorized users.
Keywords: Telomerase, Cancer vaccine, Adjuvants, Prostate cancer, Renal cancer
Introduction
Prostate cancer is a major medical problem being the most common cancer and a leading cause of death from cancer in men [1]. This tumor poses therapeutic problems when the disease is diagnosed either in young patients, since radical prostatectomy has poor tolerability in these individuals, or in advanced, castration-resistant patients, since actual standard therapeutic options have poor efficacy [2, 3].
Renal cell carcinoma incidence increases at a rate of 2 % per year. About 25–30 % of patients have advanced, metastatic disease at diagnosis, and actual therapeutic approaches have poor efficacy (23 % of metastatic patients survive after 5 years from diagnosis) [4].
Both prostate and renal cancers are immunogenic tumors [5, 6] so that could benefit from immunostimulating treatments. Several vaccination protocols are under evaluation for the treatment of these diseases [4, 7, 8], and Sipuleucel-T recently received FDA approval as treatment for prostate cancer [9]. Hence, we applied GX301, a new telomerase-based tumor vaccine, to the treatment of prostate and renal cancer patients.
Telomerase is the reverse transcriptase responsible for synthesis, elongation, and stability of the telomeric regions of chromosomes [10–13]. It is normally expressed by embryonic cells but not by adult somatic cells with few exceptions and re-expressed by tumor cells since essential for tumor immortalization [14–16]. Telomerase is immunogenic and telomerase-specific T cells were identified in both healthy subjects and cancer patients [17, 18]. In a previous study, we observed that about 90 % of cancer patients have telomerase-specific CTL in the circulation [19]. All together, these findings support the concept that telomerase may represent a universal tumor-associated antigen [20]. For this reason, several clinical trials were performed using telomerase as immunogenic agent in cancer patients in the last decade. In these trials, telomerase was administered in different molecular forms achieving variable degree of immunological and clinical responses [21–28]. Here, we report on the immunological and clinical effects of GX301 that was designed in order to increase the immunogenicity of telomerase vaccines and can be indifferently applied to a wide array of tumors including prostate and renal cancers.
Materials and methods
Patients
Between January, 2010, and April, 2012, a phase I/II trial was performed at the San Martino University Hospital (Genoa, Italy) to assess the safety and tolerability of GX301 vaccine administered to patients affected with stage IV prostate or renal cancer. Fourteen patients were enrolled, 11 with prostate cancer and 3 with renal cancer. Inclusion criteria were as follows: (a) Prostate cancer patients: clear disease progression, serologically (PSA increase in the last 3 months) and radiology assessed, and resistance to hormone therapy and at least one chemotherapy regimen; (b) Renal cancer patients: resistance to at least three lines of therapy, including biologic agents; (c) Eastern Cooperative Oncology Group performance status ≤2; (d) Acceptable hepatic, renal and hematological functions defined as total bilirubin, AST, ALT, alkaline phosphatase, creatinine ≤2 × upper normal limit (UNL), hemoglobin >7 g/l, leukocytes >3 × 109 cells/l, platelets >100 × 109 cells/l; (e) Expression of the HLA-A2 antigen. Patients with brain metastases were excluded from the study, although brain computerized tomography (CT) scan was mandatory only if clinically indicated. All enrolled patients had diffuse metastatic disease. Patients discontinued chemotherapy at least 30 days before the first immunization. A summary of patient’s characteristics is provided in Tables 1 and 2. The protocol was approved by the national institutional review board (Istituto Superiore di Sanità) and by the local ethics committee of the “Azienda Ospedaliera Universitaria San Martino di Genova”, which gave clear indications on both the dimension and clinical stage of the patient population to be treated. The study was performed in accordance with the Declaration of Helsinki and with Good Clinical Practice as defined by the International Conference on Harmonization. All patients gave voluntary, written informed consent. This study is registered with the European Union Drug Regulating Authorities Clinical Trials, EudraCT number 2009-011330-10 (URL: http://eudract.ema.europa.eu/).
Table 1.
Baseline patient characteristics
| Characteristics | Values |
|---|---|
| Age (years) | |
| All patients | 71.5 (49–81) |
| Prostate cancer patients | 74 (57–81) |
| Renal cancer patients | 63 (49–72) |
| Ethnic origin | |
| Caucasian | 14 (100 %) |
| Gleason score | |
| Gleason 4–6 | 3 (27 %) |
| Gleason 7 | 5 (45 %) |
| Gleason 8–10 | 3 (27 %) |
| Metastasis | |
| Bone | 14 (100 %) |
| Visceral | 13 (92 %) |
| PSA (μg/l) | 155 (10–400) |
| Hemoglobin (g/l) | 119 (89–153) |
| Creatinine (mg/dl) | 0.9 (0.6–1.4) |
| Lactate dehydrogenase (U/l) | 350.5 (216–1,934) |
| Alkaline phosphatase (U/l) | 239 (125–2,945) |
| C-reactive protein (mg/l) | 8.7 (4.8–73) |
Data are n (%) or median (range)
PSA prostate-specific antigen
Table 2.
Patient treatments before GX301 administration
| Patient code | Surgery | Hormone therapy | Chemotherapy | Radiotherapy |
|---|---|---|---|---|
| TEL01Pa | No | Yes | Cyclophosphamide | No |
| TEL04P | No | Yes | Docetaxel, carboplatin, cyclophosphamide | Yes |
| TEL05R | No | N/A | Sunitinib, sorafenib | Yes |
| TEL06P | Yes | Yes | Docetaxel | Yes |
| TEL07P | No | Yes | Cyclophosphamide, docetaxel | Yes |
| TEL09P | Yes | Yes | Estramustine | Yes |
| TEL10P | Yes | Yes | Cyclophosphamide, vinorelbine | No |
| TEL11R | Yes | N/A | Bevacizumab, interferon, sunitinib | No |
| TEL14P | No | Yes | Docetaxel | No |
| TEL15P | No | Yes | Cyclophosphamide | Yes |
| TEL16P | Yes | Yes | Docetaxel, ketoconazole | Yes |
| TEL22P | Yes | Yes | Docetaxel, cisplatin | Yes |
| TEL25R | No | N/A | Sunitinib, bevacizumab | Yes |
| TEL28P | Yes | Yes | Docetaxel, estramustine | Yes |
N/A not applicable
aP in patient code relates to prostate cancer while R to renal cancer
Treatment
GX301 vaccine is constituted by 4 telomerase peptides (peptide540–548, peptide611–626, peptide672–686, peptide766–780) and two adjuvants, Montanide-ISA51 (Seppic Italia s.r.l., Milan, Italy) and Imiquimod (Aldara, Meda Pharma spa, Milan, Italy). The peptide540–548 was chosen because highly immunogenic and HLA-A2-restricted [29] and the other peptides were selected because of their high HLA promiscuity [26, 30, 31]. Peptides were purchased from Bachem AG (Bubendorf, Switzerland). Each peptide (500 μg) was dissolved in sterile physiological saline (300 μl) and emulsified in Montanide-ISA51 (300 μl). The obtained emulsion (≈550 μl due to the dead volume of the syringe) was administered by intradermal injection in contiguous sites of the abdominal skin. To this end, a circle divided into four quadrants was designed on the abdomen skin by a marking pencil: each peptide was injected in only one of the four quadrants for all the repeated administrations. Hence, it was possible to monitor separately the local response to each peptide. Immediately after peptide administration, Imiquimod, in the form of cream, was applied to the sites of vaccine administrations. The schedule of vaccination included administrations on days 1, 3, 5, 7, 14, 21, 35, 63.
Clinical evaluation
Patients were monitored by physical examination and laboratory tests of serum chemistry, complete blood count, lymphocyte subset immunophenotype, and PSA levels on days 0, 7, 14, 21, 35, and 63, then monthly up to 180 days. Radiological studies included bone scan and chest, abdomen and pelvis CT scan on days 0, 90, and 180. Disease progression was established according to RECIST criteria [32].
Immunological evaluation
Evaluation of the immunological response to GX301 was the secondary endpoint of the study. We performed a panel of immunological tests to analyze phenotype of circulating T lymphocytes, as well as frequency, IFNγ secretion, proliferation, and cytotoxic activity of vaccine-specific T cells. To this aim, peripheral blood mononuclear cells (PBMC) were purified from heparinized blood samples by centrifugation on Ficoll density gradient. Immunological analyses were performed at baseline and 14, 30, 90, 180 days after the first immunization. When a limited number of cells were available, a restricted panel of tests was performed. Each procedure was performed as here described.
Lymphocyte subset count
The absolute number of lymphocyte subpopulations was determined as follows. One hundred microlitres of whole blood, collected in Vacutainers containing tetrasodium EDTA, was stained with a mix of the following monoclonal antibodies (mAbs) conjugated with fluorochromes as specified: anti-CD45-fluorescein isothiocyanate (FITC), anti-CD4 R-phycoerythrin (RD1), anti-CD8-phycoerythrin-Texas Red (ECD), anti-CD3-R-phycoerythrin-cyanin5 (PC5), and a mix of anti-CD45-FITC, anti-CD56-RD1, anti-CD19-ECD, and anti-CD3-PC5 (Coulter, Hialeah, FL) for 15 min at room temperature. After fixation and red cell lysis by an automated cell preparator (TQ-Prep Coulter), 100 μl of fluorospheres Flow-Count (Coulter) was added to samples for the determination of lymphocyte subset absolute number. The acquisition and analysis were performed on a CYTOMIX™FC500 cytometer (Coulter) using a TetraCXP™ (Coulter).
Phenotypic analyses
Cell expression of membrane antigens was analyzed by immunofluorescence incubating 100 μl of whole blood, collected in Vacutainers containing tetrasodium EDTA, with membrane antigen-specific mAbs. In particular, the mAbs used were as follows: anti-CD25-FITC and anti-CD45RA-FITC (BD Biosciences, San Josè, CA, USA), anti-CCR7-phycoerythrin (PE) and anti-CD127-PE (BD Biosciences), anti-CD25-PE (Miltenyi, Bergisch Gladbach, Germany), anti-CD28-Peridinin Chlorophyll Protein Complex-cyanin 5.5 (PerCPCy5.5) (e-Biosciences, San Diego, CA, USA), anti-CD8-PECy7 or anti-CD8-APC (Biolegend, San Diego, CA, USA), anti-CD45RA-allophycocyanin (APC) and anti-CD45RO-APC (BD Biosciences), anti-CD3-APC-Cy7 (Biolegend, San Diego, CA, USA) or anti-CD3-PerCPCy5.5 (e-Biosciences, San Diego, CA, USA). The samples were treated with 1 ml of BD FACS lysing solution (BD) and washed once with PBS. The acquisition and analysis were performed by a FACSCanto flow cytometer using FACSDIVA software (BD).
Proliferation assay
The assessment of proliferative response was performed by dye dilution assay in flow cytometry, as already described [33]. PBMC were stimulated by a mix of 20 μg/ml final concentration of each of the four GX301 vaccine peptides or 1 μg/ml pokeweed (PKW, Sigma-Aldrich) as positive control for 4 days. The cultures were acquired on a FACSCanto cytometer using FACS DIVA software and 5 × 104 live lymphocytes were collected for each sample. The results were expressed by stimulation index (SI), as the ratio between percent of CFSEdim cells in stimulated samples and percent of CFSEdim cells in unstimulated sample. The value of SI ≥2 was referred as positive proliferative response.
Pentamer staining
For pentamer staining, PBMC were incubated with A*02:01/ILAKFLHWL-PE Pro5® MHC Pentamer of Telomerase (hTERT, Proimmune) following manufacturer instructions. Pentamer loaded with the HIV peptide A0201/*SLYNTVATL-A was used as a negative control. Cells were stained with pentamers for 10 min at room temperature, washed in washing buffer, and stained with anti-CD3APC-Cy7 and anti-CD8 PerCP-Cy5.5 mAbs (Biolegend) at room temperature for 20 min. Samples were washed once with PBS and acquired on a FACSCanto cytometer using FACS DIVA software. Gate was set on CD3+ T lymphocytes and the results were expressed as percent of pentamer+CD3+CD8+ T cells.
Assessment of vaccine-specific IFN-γ+ T-cell frequency
PBMC were stimulated by a mix of 20 μg/ml final concentration of each of the four GX301 vaccine peptides in the presence of recombinant human interleukin (rhIL)-7 (10 ng/ml) (Peprotech), as previously described [34]. At day 3, rhIL-2 (10 U/ml) was added to the cultures. After 10 days, the cells were re-stimulated overnight with vaccine peptides as above. Ten μg/ml of Brefeldin A was added during the last 3-h incubation. Non-re-stimulated T cells were used as negative controls. Then, the cells, washed and stained sequentially with LIVEDEAD (Invitrogen), anti-CD3APC-Cy7, and anti-CD8-PerCP-Cy5.5 mAbs, were permeabilized using the Cytofix/Cytoperm kit (BD Bioscience) according to the manufacturer’s instruction and incubated with an anti-IFN-γ-FITC mAb (BD Bioscience). Finally, cells were resuspended in FACSL solution (BD Bioscience) and acquired on a FACSCanto cytometer using FACS DIVA software. The results were expressed as frequency of IFN-γ producing cells in CD3+CD8+ or in CD3+CD8− alive lymphocytes after subtracting the frequency of unstimulated T cells spontaneously secreting IFNγ.
Cytotoxic assay
Cytotoxic activity was analyzed by flow cytometry as previously described [35]. TAP-deficient, HLA-A2+ T2 target cells (1 × 107 cells) (a kind gift from Prof R. De Palma, University of Naples, Italy) [36] were resuspended in 1 ml PBS containing CFSE (5 μM) for 5 min at room temperature and then washed twice in PBS-1 % fetal calf serum at 4 °C. Labeled cells (5 × 105) were pulsed (or not) with 10 μg of telomerase peptide540–548 for 90 min at 37 °C. After washings, 5x103 target cells were incubated at 37 °C in 5 % CO2 for 6 h with patient PBMC at a 20:1 effector to target ratio in round-bottom microtiter plates. Negative control was performed with non-pulsed target cells. Then, the cells were collected and 5 μl of 7-amino-actinomycin D (7-AAD 0.25 μg; BD) was added to each cell culture before the flow cytometry acquisition. The percentage of specific lysis was calculated as
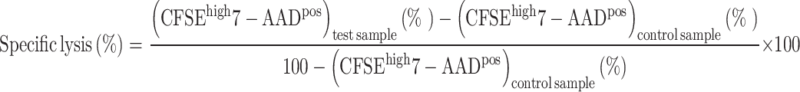 |
Since cell purification procedures usually cause loss of several cells, in order to save cells for executing the widest possible panel of tests, cytotoxic assays were performed using patient PBMC and then normalizing achieved values to the percentage of CD3+CD8+ T cells detected among PBMC by phenotypic analysis.
Assessment of IFN-γ production by ELISPOT
ELISPOT assay was performed as described [37]. T2 cells (1 × 104/well) were incubated with 2 × 105/well PBMC in the presence or not of GX301 vaccine peptides (10 μg/ml each) for 20 h at 37 °C. Spots were enumerated using the automated analyzer Bioreader 2000 (BIO-SYS GmbH, Karben, Germany). The mean number of spots was calculated and net results (corrected for background signals detected in samples with medium alone) were expressed as the number of spot-forming cells (SFC) per 105 PBMCs.
Statistical analyses
Statistically significant differences between mean values of immunologic parameters were analyzed by the Wilcoxon matched pairs test or by the Mann–Whitney test for nonparametric values. Time-to-event endpoints such as progression-free survival and overall survival were estimated with the Kaplan–Meier product limit method. Differences were considered statistically significant when P < 0.05. All statistical analyses were performed using the GraphPad Prism 4.0 Software (GraphPad Software Inc, La Jolla, CA, USA).
Results
Safety and adverse events
A total of 106 immunizations were performed (Table 3). Immunizations were well tolerated by all the patients and no severe (grade 3 or 4) adverse events were observed. The most frequently observed adverse events were as follows: (1) local erythema and granulomata formation at the injection site (all patients) (Fig. 1a); (2) moderate fever (≤38.5 °C) within the first 24 h from immunization (five patients); (3) bone pain (nine patients). Noteworthy, neither reduction in hemoglobin, reticulocyte levels and of B-lymphocyte or T-lymphocyte percentages in the peripheral blood, nor mucositis were observed, dispelling any concerns related to a possible toxicity on actively proliferating cells. No signs of autoimmune disease were observed.
Table 3.
Overview of immunological responses, number of immunizations, and status of the different patients
| Patient code | Proliferationa | Cytotoxicityb | ELISPOTc | Intracyto IFNγd | Pentamerse | Administered doses | Status |
|---|---|---|---|---|---|---|---|
| TEL01Pf | 1.7 (14 days) | 8 (14 days) | 0 | NDg | 2.5 (14 days) | 5 | Dead |
| TEL04P | 1 (14 days) | 19 (14 days) | 0 | ND | ND | 7 | Dead |
| TEL05R | 3.3 (180 days) | 66 (180 days) | 41 (180 days) | 5.3 (90 days) | 4.5 (180 days) | 8 | Dead |
| TEL06P | 1.3 (14 days) | 0 | 52 (30 days) | 10 (30 days) | 0.7 (30 days) | 8 | Dead |
| TEL07P | 8.4 (90 days) | 8 (14 days) | 35 (180 days) | 6.2 (90 days) | 0.8 (180 days) | 8 | Dead |
| TEL09P | 1.8 (90 days) | 48 (14 days) | 42 (14 days) | 0.2 (30 days) | 0.7 (90 days) | 8 | Dead |
| TEL10P | 11.6 (90 days) | 8 (180 days) | 40 (90 days) | 2.1 (180 days) | 0.2 (180 days) | 8 | Dead |
| TEL11R | 8.6 (90 days) | 0 | 26 (90 days) | 1.9 (90 days) | 0 | 8 | Dead |
| TEL14P | 2.3 (90 days) | 65 (30 days) | 34 (30 days) | 0.3 (30 days) | 1.5 (90 days) | 8 | Alive |
| TEL15P | 3.6 (30 days) | 29 (30 days) | ND | 3.6 (14 days) | 2.4 (30 days) | 8 | Alive |
| TEL16P | 2.6 (90 days) | 84 (90 days) | ND | 0.5 (90 days) | 1.8 (30 days) | 8 | Alive |
| TEL22P | 2 (14 days) | 7 (14 days) | ND | 0.4 (14 days) | 0 | 6 | Dead |
| TEL25R | 1.2 (30 days) | 44 (90 days) | ND | 6.1 (30 days) | 0 | 8 | Deadh |
| TEL28P | 2 (30 days) | 18 (30 days) | ND | 0.2 (14 days) | 1.2 (14 days) | 8 | Alive |
aData are expressed as stimulation index; values ≥2 were considered as positive
b Data are expressed as percentages of specific lysis; values ≥15 were considered as positive
c Data are expressed as number of spots/105 PBMC, values ≥20 were considered as positive
d Data are expressed as percentage of vaccine-specific CD4+ IFNγ+ T cells, values ≥0.2 % were considered as positive
e Data are expressed as percentage of CD8+pentamer+ T cells, values ≥0.2 % were considered as positive. For all values, the timing of the assessment (days after baseline) is indicated in parentheses; for each patient, the highest value registered in the different assessments is reported
f P in patient code relates to prostate cancer while R to renal cancer
g ND: not done
h Patient TEL25R died after 87 days from the first immunization
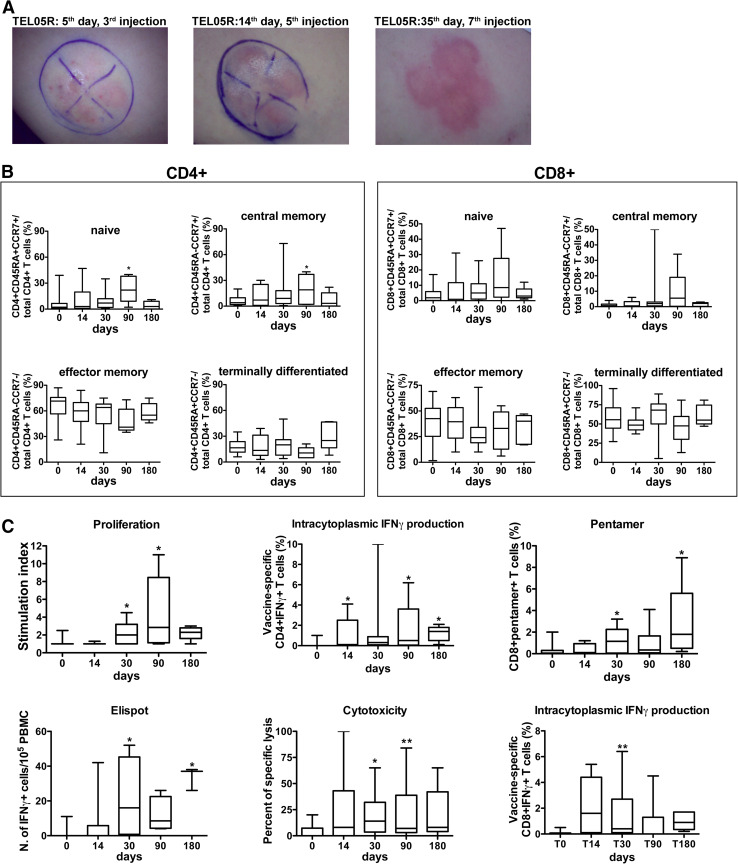
Fig. 1.
GX301-specific T-cell responses. a Local skin reaction at the site of peptide injections at different time-points of GX301 administration to patient TEL05R. A clockwise dial was designed on the abdominal skin: telomerase peptide540–548, peptide611–626, peptide672–686, peptide766–780 were separately injected in the upper left, upper right, lower left, and lower right quadrants, respectively. b Phenotypic analyses on circulating CD4+ (left panel) and CD8+ (right panel) T lymphocytes. Percent concentration of naïve, central memory, effector memory, and terminally differentiated T-cell subpopulations (T cells), analyzed by flow cytometry, are shown. Data, collected at baseline and after 14, 30, 90 and 180 days from the first immunization, are expressed as min–max boxes ± standard deviation for 14 patients. *P = 0.05 versus the baseline value. c Functional analyses on circulating T lymphocytes of treated patients. The panel of immunological tests included the analyses of: GX301-specific proliferation (proliferation); GX301-specific cytotoxicity (cytotoxicity); frequency in the PBMC of T cells secreting IFNγ in response to vaccine peptides analyzed by ELISPOT (ELISPOT); frequency of circulating vaccine-specific CD4+IFNγ+ T cells analyzed by intracytoplasmic immunofluorescence (intracytoplasmic IFNγ production); frequency of circulating CD8+ T cells binding HLA-A2 pentamers loaded with the telomerase peptide540–548 (Pentamer staining ). Data are expressed as min–max boxes ± standard deviation for 14 patients. *P = 0.05 versus the baseline value; **P = 0.01 versus the baseline value
Concerning the local reaction at the injection site, its initial signs (faint redness) invariably appeared in 7–14 days from the first administration. However, the timing of appearance differed among the four quadrants where the peptides were separately injected, likely suggesting a different timing of induction of the immune response against each peptide. The order of appearance of the local reaction against each peptide differed also among patients, indicating a diverse immune status and predisposition to specific reactivity among the patients, as expected. All patients showed an evident local response (redness and cell infiltration) in the four quadrants after 3–4 weeks from the first administration. In surviving patients, the local reaction lasted for several months. However, no patients complained for pain, discomfort, or ulceration phenomena.
Immunologic response
Phenotypic analyses
No significant changes of both absolute and percent concentrations of circulating total lymphocytes as well as T, B, and NK cells were observed during the 6 months following the first immunization (not shown). Similarly, no significant variations of total CD4+ and CD8+ T cell numbers were detected. However, the analysis of CD4+ and CD8+ T cell subsets, namely naïve (CD45RA+ CCR7+), central memory (CD45RA− CCR7+), effector memory (CD45RA− CCR7−), and terminally differentiated (CD45RA+ CCR7−) subpopulations [38, 39], showed progressive increase in both naïve and central memory subsets during the initial 3 months after the first immunization, followed by their return to values comparable to baseline and by the expansion of terminally differentiated CD4+ T cells at the 6-month assessment (Fig. 1b).
The frequency of circulating CD4+ CD25+ CD127− regulatory T cells (Treg) was evaluated at baseline showing increased values with respect to that of 10 healthy donors (Supplementary Fig. S1).
Proliferation activity
Vaccine-specific proliferation activity of circulating T lymphocytes was analyzed stimulating patient’s PBMC with the 4 vaccinating peptides. A test was considered positive when the stimulation index (SI) (i.e., the ratio between the proliferative response of stimulated PBMCs and that of unstimulated PBMCs) was ≥2. No patients showed vaccine-specific T-cell proliferation at the baseline. However, 9 patients (64 %) showed a specific proliferation response at different time-points after vaccination. Subsequently, the mean SI at the time-points comprised between 30 and 180 days was significantly higher than the basal one (Fig. 1c, proliferation panel). A representative analysis performed on cells from TEL11R patient is shown in Supplementary Fig. S2.
Cytotoxic function
Vaccine-specific cytotoxic activity of circulating T lymphocytes was analyzed by flow cytometry incubating patient PBMC with CFSE-labeled T2 cells, pulsed or not with a pool of the vaccinating peptides. Vaccine-specific cytotoxic values ≥15 % were considered as positive. PBMC from 8 patients (57 %) exerted peptide-specific cytotoxic activity at various time-points from the first vaccination. Figure 1c (cytotoxicity panel) shows that the mean cytotoxic activity at all time-points following the first immunization was higher than that at baseline, with statistically significant differences registered after 30 and 90 days. A representative analysis performed on cells from TEL14P patient is shown in Supplementary Fig. S3.
ELISPOT analysis
The ELISPOT analysis was performed to calculate the frequency of circulating total T cells secreting IFNγ after PBMC exposure to the 4 immunizing peptides. Analyses were performed on PBMC from 9 patients (Table 3). Seven out of 9 (77 %) tested patients showed increased frequency of vaccine-specific IFNγ-secreting cells after the beginning of vaccination so that the mean frequency at 30-, 90-, and 180-day time-points was significantly higher than that at baseline (Fig. 1c, ELISPOT panel).
Intracytoplasmic IFNγ secretion
The frequency in patient PBMC of IFNγ secreting CD4 + and CD8 + T cells after incubation with the vaccination peptides was evaluated by intracytoplasmic immunofluorescence analyses. Analyses could be performed on 12 patients. No patients showed vaccine-specific IFNγ production by any T-cell subtype at baseline. Conversely, all tested patients showed both CD4 + and CD8 + T cells specifically responding to immunizing peptides at various time-points following vaccination (considering ≥0.2 % of IFNγ secreting cells as positive value). Figure 1c (Intracytoplasmic IFNγ secretion panel) shows that the mean frequency of vaccine-specific CD4+ IFNγ+ T cells increased after vaccination reaching a statistically significant difference versus baseline after the 30-day time-point. A representative analysis performed on cells from TEL07P patient is shown in Supplementary Fig. S4.
Pentamer staining
The frequency of telomerase peptide540–548-specific CD8+ T cells was analyzed by pentamer staining in flow cytometry using HLA-A2 pentamers loaded either with the peptide540-548 or with the HLA-A2 HIV-1 gag p24 peptide19–27 as control in association with anti-CD8 and CD3 mAbs. Patient TEL04P could not be analyzed. Increased frequency of CD8+pentamer+ T cells in patient PBMC was observed in 10 out of 13 tested patients (76 %) starting from 30 days after vaccination (Fig. 1c, Pentamer panel). A representative analysis performed on cells from TEL09P patient is shown in Supplementary Fig. S5.
Collectively, 100 % of examined patients showed evidence of specific immunological response to the vaccine in at least 1 test (Table 3).
Clinical evaluation: progression-free survival (PFS) and overall survival (OS)
In order to determine PFS, PSA doubling time (PSAdt) and CT scan analyses were considered. Data on PSAdt were available on 9 of the 11 prostate cancer patients, due to the early death of patients TEL01P and TEL22P. For each patient, repeated PSA measurements were performed in order to generate pre- and post-vaccination slopes and to calculate the PSAdt. No significant differences of PSAdt mean values were observed between pre- and post-vaccination measurements (not shown).
CT scans were performed at baseline and after 3 and 6 months from the first immunization; the 4 patients dead before the 3-month assessment (Table 3) could not be analyzed. Multiple target lesions were identified for each patient, and the sum of the longest diameter of each target lesion was comparatively analyzed at the different time-points in order to verify the disease behavior according to RECIST criteria [32]. Four patients (TEL05R, TEL10P, TEL16P, TEL28P) (3 with prostate cancer and 1 with renal cancer) showed stable disease up to the 6-month assessment.
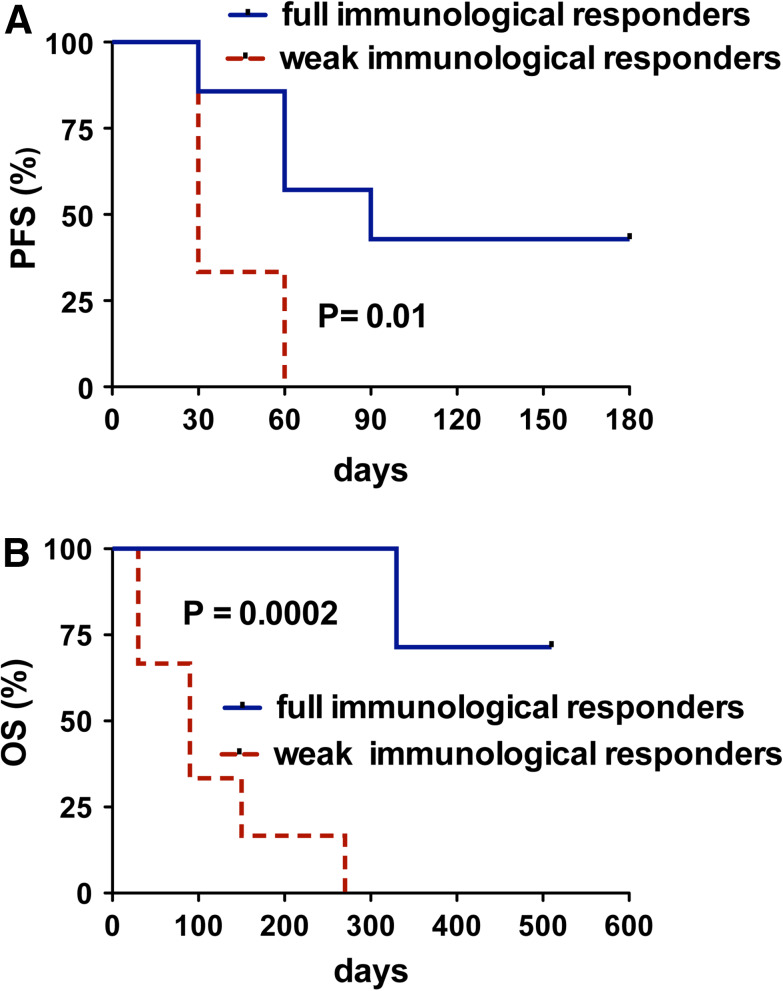
To verify the existence of correlation between immunological response and clinical outcome, all patients were retrospectively divided into two groups: the high immunological responders (8 patients who showed positive specific responses in ≥4 immunological tests at any time-point) and the low responders (6 patients who showed positive specific responses in <4 immunological tests at any time-point). Notably, at baseline, the two groups did not differ concerning disease stage and activity, as demonstrated by the absence of significant differences related to serologic markers of disease activity or to number/site (bone and/or visceral) of metastatic involvement. Figure 2a, b shows that both PFS and OS significantly differed between the two groups, suggesting a close correlation between the immunological and the clinical outcome after GX301 vaccination.
Fig. 2.
Clinical effects of GX301 vaccination. Comparative analysis of PFS (a) and OS (b) between patients showing a full immunological response (≥4 positive immunological tests) (patients TEL05R, TEL07P, TEL09P, TEL10P, TEL14P, TEL15P, TEL16P, TEL28P) (blue solid line) or a weak immunological response (<4 positive immunological tests) (patients TEL01P, TEL04P, TEL06P, TEL11R, TEL22P, TEL25R) (red dashed line)
OS in prostate or renal cancer patients who completed the treatment (11 out of 14) is shown in Table 4. Interestingly, actual OS of prostate cancer patients (four patients are still alive) is longer than that reported in the literature for prostate cancer patients with comparable disease undergoing a second line of chemotherapy [40, 41].
Table 4.
Up-to-date OS (days) of patients who completed the treatment
| All patients | Prostate cancer patients | Renal cancer patients | |
|---|---|---|---|
| No. 11 | No. 8 | No. 3 | |
| Mean (median) | 369 (341) | 397 (398) | 294 (270) |
| Range | 87–715 (4 still alive) | 158–715 (4 still alive) | 87–527 |
Discussion
Our study shows that treatment with GX301 is safe, well tolerated by the patients, and with potential immunologic and clinical efficacy. When we designed the protocol, our ambitious aim was the generation of a highly immunogenic vaccine easy to administer and manage, not requiring particular facilities (i.e., cell factory) and/or expensive procedures for its preparation, potentially covering all tumor histological types as well as the majority of human haplotypes. For these reasons, the initial choice was a peptide vaccine, due to the relatively low-cost production, the high stability, the easy preparation and administration, and the high safety profile of peptides [42]. On this regard, our study confirmed our anticipated safety and tolerability profile of peptide vaccination since no grade 3–4 adverse events were observed and vaccination was well accepted and tolerated by all the patients. The choice of telomerase as immunogenic antigen derives by its wide expression in tumors that makes it a universal tumor-associated antigen [20]. Moreover, telomerase molecule contains both HLA class I and II restricted epitopes. Accordingly, GX301 contains peptides able to bind to different HLA class I and II allelic molecules [18, 26, 30, 31]. This circumvents the immunogenicity issue related to HLA restriction and allows activation of both CD4 + and CD8 + T cells subsets, as required for optimal immune responses [43]. Importantly, telomerase peptide540–548 was also included in GX301 because of its demonstrated high immunogenicity in HLA-A2+ individuals, who constitute almost the 50 % of Caucasian population [29, 44]. For this reason, our trial was restricted to HLA-A2+ cancer patients. Concerning this peptide, although its presentation by tumor cells was questioned by studies in which the lack of recognition of tumor cells by telomerase peptide540–548-specific CTL was reported [27, 45], findings from our and other groups seem to assess its suitability for immunotherapy protocols [19, 29, 46–48]. On this regard, it is likely that the inclusion in the GX301 vaccine of two adjuvants and of other peptides able to elicit CD4+ T-cell reactivity may exert a synergic effect able to stimulate and sustain the development of effective telomerase peptide540–548-specific CTL responses. Indeed, how important is the choice of the adjuvant in the induction of telomerase-specific T-cell responses was shown by a study in which GM-CSF, but not tuberculin, demonstrated to be an effective adjuvant for telomerase peptide611–626 immunization [22]. Noteworthy, physiologic activation of adaptive immune responses occurs after the induction of innate immune responses [49]. GX301 includes two adjuvants, Montanide ISA-51 and Imiquimod. The former allows dissolution of peptides in a water-in-oil emulsion that protects peptides from tissue proteases and favors their uptake by local dendritic cells (more represented in the dermis than in the subcutaneous tissue, thus justifying the intradermal way of administration); moreover, it is a strong IFNγ inducer [50]. The latter is a potent activator of the Toll-like receptor 7, inducing activation and maturation of dendritic cells [51]. The finding of 100 % vaccine-specific immune responses in our series of patients, associated with the evidences of generation of vaccine-specific long-lasting T-cell responses, is quite surprising if related to the compromised patient’s clinical conditions and to the multiple chemotherapy regimens to which patients were subjected before receiving GX301 vaccination. Concerning the immunologic efficacy of GX301 vaccine, our results are in line with those already shown immunizing with the dual-peptide GV1001 vaccine [21, 22, 26] and suggest that increasing the number of available TAA-immunizing epitopes may represent a useful strategy for enhancing vaccine immunogenicity. Indeed, it would be of great interest to verify in future studies whether GX301 immunogenicity could be further potentiated by combination with agents blocking immune-regulatory circuits.
Importantly, the study enrolled patients affected with an extremely advanced disease refractory to all available treatments: this kind of patients may not represent the optimal target for a vaccine since the advanced disease and the previous treatments may have deeply hampered their immune competence. In the attempt to counteract a likely status of immunodeficiency, we tried to “force” the onset of the immune response through repeated vaccine administrations during the first week. We are aware that this strategy could be a double-edged sword since repeated administrations may be detrimental for the clinical outcome of vaccination [52]. Indeed, future studies with GX301 vaccine will need to specifically address this important issue in order to identify the optimal schedule of immunization. Notwithstanding the extremely advanced disease, eight patients (57 %) in our series showed longer survival (ranging from 332 to 715 days, with 4 patients still alive at the time of analysis) than expected for patients with their clinical conditions [40, 41]. It is of interest that prolonged PFS and OS in our study were observed in patients showing a full pattern of vaccine-specific immune activation, as demonstrated by their positive responses to a panel of 4 or more different immunologic analyses. This observation correlates the immune to the clinical response, envisaging a reciprocal inter-dependence. Notably, none of the immunological parameters considered in the study correlated per se with the clinical outcome. However, the stratification of patients on the basis of their responsivity to various immunological tests, exploring different aspects of the vaccine-specific immune response (frequency of antigen-specific T-cell clones as well as vaccine-specific proliferation, cytotoxicity and IFNγ secretion), allowed the identification of two patient’s subpopulations showing either favorable or unfavorable clinical outcome, respectively. Although this way of stratification is arbitrary, our results may suggest that the application of a wide panel of tests (investigating different immune functions) may be of predictive value when monitoring the immune response to a cancer vaccine. This approach could be further implemented by the analysis of patient responsiveness to each single peptide, an analysis that we could not perform in our study due to the limited number of cell sample achieved from our patients. Since the identification of effective immune-related response criteria is an actual issue in the follow-up of cancer immunotherapy [53], our approach will deserve appropriate confirmation analyses in wider clinical trials.
The results here reported have to be considered preliminary due to the small number of treated patients. Indeed, only a study enrolling a wider series of patients could provide clear evidence of the effects (and eventual efficacy) of this new strategy of vaccination based on the association of multiple telomerase peptides and dual adjuvants. However, this study, other than necessary for demonstrating the safety of GX301 components, was conceived to provide a proof-of-concept on the potential immunogenicity of the vaccine. For this reason, multiple aspects of patient’s immunological responses were evaluated. The results seem to support the opportunity to carry on the studies performing phase II/III trials on prostate and renal cancer patients (at both early and late stages, alone or in association with existing treatments), as well as testing GX301 in patients affected with different tumors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported by grants from Genovax s.r.l. and Compagnia di San Paolo, Torino. Neither of the two sponsors of the study had any role in the study design, data collection, analysis, data interpretation, or writing of the manuscript.
Conflict of interest
GX301 is patented by Genovax s.r.l. Domenico Criscuolo is the President of Genovax; Gilberto Filaci, Francesco Indiveri, and Daniela Fenoglio are the stockholders of Genovax, while Francesco Boccardo is the member of the advisory board of Genovax; however, they worked at the project as academics so that they did not receive any payment by Genovax. All the other authors do not have any conflict of interest to declare.
Footnotes
Daniela Fenoglio and Paolo Traverso contributed equally to this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Rigamonti N, Bellone M. Prostate cancer, tumor immunity and a renewed sense of optimism in immunotherapy. Cancer Immunol Immunother. 2012;61:453–468. doi: 10.1007/s00262-012-1216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich A, Pfister D (2012) Treatment decisions for metastatic castration-resistant prostate cancer progressing after docetaxel chemotherapy: the role of cabazitaxel in the continuum of care. Eur Urol. doi:10.1016/j.eururo.2012.08.048 [DOI] [PubMed]
- 4.Figlin R, Sternberg C, Wood CG. Novel agents and approaches for advanced renal cell carcinoma. J Urol. 2012;18:707–715. doi: 10.1016/j.juro.2012.04.108. [DOI] [PubMed] [Google Scholar]
- 5.Kwek SS, Cha E, Fong L. Unmasking the immune recognition of prostate cancer with CTLA4 blockade. Nat Rev Cancer. 2012;12:289–297. doi: 10.1038/nrc3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perut F, Cenni E, Unger RE, Kirkpatrick CJ, Giunti A, Baldini N. Immunogenic properties of renal cell carcinoma and the pathogenesis of osteolytic bone metastases. Int J Oncol. 2009;34:1387–1393. [PubMed] [Google Scholar]
- 7.Gerritsen WR. The evolving role of immunotherapy in prostate cancer. Ann Oncol. 2012;23(Supplement 8):viii22–viii27. doi: 10.1093/annonc/mds259. [DOI] [PubMed] [Google Scholar]
- 8.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, Hilf N, Schoor O, Fritsche J, Mahr A, Maurer D, Vass V, Trautwein C, Lewandrowski P, Flohr C, Pohla H, Stanczak JJ, Bronte V, Mandruzzato S, Biedermann T, Pawelec G, Derhovanessian E, Yamagishi H, Miki T, Hongo F, Takaha N, Hirakawa K, Tanaka H, Stevanovic S, Frisch J, Mayer-Mokler A, Kirner A, Rammensee HG, Reinhardt C, Singh-Jasuja H. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med doi. 2012 doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 9.Sheikh NA, Petrylak D, Kantoff PW, Dela Rosa C, Stewart FP, Kuan LY, Whitmore JB, Trager JB, Poehlein CH, Frohlich MW, Urdal DL (2012) Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. (Epub ahead of print) PMID: 22865266 [DOI] [PMC free article] [PubMed]
- 10.Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 11.Greider CW. Mammalian telomere dynamics: healing, fragmentation shortening and stabilization. Curr Opin Genet Dev. 1994;4:203–211. doi: 10.1016/S0959-437X(05)80046-2. [DOI] [PubMed] [Google Scholar]
- 12.Nugent CI, Lundblad V. The telomerase reverse transcriptase: components and regulation. Genes Dev. 1998;12:1073–1185. doi: 10.1101/gad.12.8.1073. [DOI] [PubMed] [Google Scholar]
- 13.Gomez DE, Armando RG, Farina HG, Menna PL, Cerrudo CS, Ghiringhelli PD, Alonso DF. Telomere structure and telomerase in health and disease (review) Int J Oncol. 2012;41:1561–1569. doi: 10.3892/ijo.2012.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2013. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 15.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 16.Dhaene K, Van Marck E, Parwaresch R. Telomeres, telomerase and cancer: an up-date. Virchows Arch. 2000;437:1–16. doi: 10.1007/s004280000189. [DOI] [PubMed] [Google Scholar]
- 17.Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–679. doi: 10.1016/S1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 18.Minev B, Hipp J, Firat H, Schmidt JD, Langlade-Demoyen P, Zanetti M. Cytotoxic T cell immunity against telomerase reverse transcriptase in humans. Proc Natl Acad Sci. 2000;97:4796–4801. doi: 10.1073/pnas.070560797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filaci G, Fravega M, Setti M, Traverso P, Millo E, Fenoglio D, Negrini S, Ferrera F, Romagnoli AR, Basso M, Contini P, Rizzi M, Ghio M, Benatti U, Damonte G, Ravetti JL, Carmignani G, Zanetti M, Indiveri F. Frequency of telomerase-specific CD8 + T lymphocytes in patients with cancer. Blood. 2006;107:1505–1512. doi: 10.1182/blood-2005-01-0258. [DOI] [PubMed] [Google Scholar]
- 20.Beatty GL, Vonderheide RH. Telomerase as a universal tumor antigen for cancer vaccines. Expert Rev Vaccines. 2008;7:881–887. doi: 10.1586/14760584.7.7.881. [DOI] [PubMed] [Google Scholar]
- 21.Brunsvig PF, Kyte JA, Kersten C, Sundstrøm S, Møller M, Nyakas M, Hansen GL, Gaudernack G, Aamdal S. Telomerase peptide vaccination in NSCLC: a phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a phase I/II trial. Clin Cancer Res. 2011;17:6847–6857. doi: 10.1158/1078-0432.CCR-11-1385. [DOI] [PubMed] [Google Scholar]
- 22.Hunger RE, Kernland Lang K, Markowski CJ, Trachsel S, Møller M, Eriksen JA, Rasmussen AM, Braathen LR, Gaudernack G. Vaccination of patients with cutaneous melanoma with telomerase-specific peptides. Cancer Immunol Immunother. 2011;60:1553–1564. doi: 10.1007/s00262-011-1061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rittig SM, Haentschel M, Weimer KJ, Heine A, Muller MR, Brugger W, Horger MS, Maksimovic O, Stenzl A, Hoerr I, Rammensee HG, Holderried TA, Kanz L, Pascolo S, Brossart P. Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients. Mol Ther. 2011;19:990–999. doi: 10.1038/mt.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berntsen A, Trepiakas R, Wenandy L, Geertsen PF, thor Straten P, Andersen MH, Pedersen AE, Claesson MH, Lorentzen T, Johansen JS, Svane IM. Therapeutic dendritic cell vaccination of patients with metastatic renal cell carcinoma: a clinical phase 1/2 trial. J Immunother. 2008;31:771–780. doi: 10.1097/CJI.0b013e3181833818. [DOI] [PubMed] [Google Scholar]
- 25.Cortez-Gonzalez X, Zanetti M. Telomerase immunity from bench to bedside: round one. J Transl Med. 2007;5:12. doi: 10.1186/1479-5876-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunsvig PF, Aamdal S, Gjertsen MK, Kvalheim G, Markowski-Grimsrud CJ, Sve I, Dyrhaug M, Trachsel S, Møller M, Eriksen JA, Gaudernack G. Telomerase peptide vaccination: a phase I/II study in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2006;55:1553–1564. doi: 10.1007/s00262-006-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parkhurst MR, Riley JP, Igarashi T, Li Y, Robbins PF, Rosenberg SA. hTERT: 540–548 peptide induces peptide-reactive T lymphocytes that do not recognize tumors endogenously expressing telomerase. Clin Cancer Res. 2004;10:4688–4698. doi: 10.1158/1078-0432.CCR-04-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su Z, Dannull J, Heiser A, Yancey D, Pruitt S, Madden J, Coleman D, Niedzwiecki D, Gilboa E, Vieweg J. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 2003;63:2127–2133. [PubMed] [Google Scholar]
- 29.Wenandy L, Sørensen RB, Sengeløv L, Svane IM, thor Straten P, Andersen MH. The immunogenicity of the hTERT540-548 peptide in cancer. Clin Cancer Res. 2008;14:4–7. doi: 10.1158/1078-0432.CCR-07-4590. [DOI] [PubMed] [Google Scholar]
- 30.Schroers R, Huang XF, Hammer J, Zhang J, Chen SY. Identification of HLA DR7-restricted epitopes from human telomerase reverse transcriptase recognized by CD4+ T-Helper cells. Cancer Res. 2002;62:2600–2605. [PubMed] [Google Scholar]
- 31.Schroers R, Shen L, Rollins L, Rooney CM, Slawin K, Sonderstrup G, Huang XF, Chen SY. Human telomerase reverse transcriptase-specific T-helper responses induced by promiscuous major histocompatibility complex class II-restricted epitopes. Clin Cancer Res. 2003;9:4743–4755. [PubMed] [Google Scholar]
- 32.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 33.Durando P, Fenoglio D, Boschini A, Ansaldi F, Icardi G, Sticchi L, Renzoni A, Fabbri P, Ferrera A, Parodi A, Bruzzone B, Gabutti G, Podda A, Del Giudice G, Fragapane E, Indiveri F, Crovari P, Gasparini R. Safety and immunogenicity of two influenza virus subunit vaccines, with or without MF59 adjuvant, administered to human immunodeficiency virus type 1-seropositive and -seronegative adults. Clin Vaccine Immunol. 2008;15:253–259. doi: 10.1128/CVI.00316-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O’Neill D, Pavlick A, Escalon JB, Cruz CM, Angiulli A, Angiulli F, Mears G, Vogel SM, Pan L, Jungbluth AA, Hoffmann EW, Venhaus R, Ritter G, Old LJ, Ayyoub M. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci USA. 2007;104:8947–8952. doi: 10.1073/pnas.0703395104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Godoy-Ramirez K, Mäkitalo B, Thorstensson R, Sandström E, Biberfeld G, Gaines H. A novel assay for assessment of HIV-specific cytotoxicity by multiparameter flow cytometry. Cytometry A. 2005;68:71–80. doi: 10.1002/cyto.a.20189. [DOI] [PubMed] [Google Scholar]
- 36.Young NT, Mulder A, Cerundolo V, Claas FH, Welsh KI. Expression of HLA class I antigens in transporter associated with antigen processing (TAP)-deficient mutant cell lines. Tissue Antigens. 1998;52:368–373. doi: 10.1111/j.1399-0039.1998.tb03057.x. [DOI] [PubMed] [Google Scholar]
- 37.Czerkinsky C, Andersson G, Ekre HP, Nilsson LA, Klareskog L, Ouchterlony O. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988;110:29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- 38.Amyes E, McMichael AJ, Callan MF. Human CD4+ T cells are predominantly distributed among six phenotypically and functionally distinct subsets. J Immunol. 2005;175:5765–5773. doi: 10.4049/jimmunol.175.9.5765. [DOI] [PubMed] [Google Scholar]
- 39.Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM, Corthesy P, Devevre E, Speiser DE, Rufer N. Four functionally distinct populations of human effector–memory CD8+ T lymphocytes. J Immunol. 2007;178:4112–4119. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- 40.Oh WK, Manola J, Babcic V, Harnam N, Kantoff PW. Response to second-line chemotherapy in patients with hormone refractory prostate cancer receiving two sequences of mitoxantrone and taxanes. Urology. 2006;6:1235–1240. doi: 10.1016/j.urology.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Michels J, Montemurro T, Murray N, Kollmannsberger C, Nguyen Chi K. First- and second-line chemotherapy with docetaxel or mitoxantrone in patients with hormone-refractory prostate cancer: does sequence matter? Cancer. 2006;106:1041–1046. doi: 10.1002/cncr.21695. [DOI] [PubMed] [Google Scholar]
- 42.Parmiani G, Castelli C, Rivoltini L, Casati C, Tully GA, Novellino L, Patuzzo A, Tosi D, Anichini A, Santinami M. Immunotherapy of melanoma. Semin Cancer Biol. 2003;13:391–400. doi: 10.1016/j.semcancer.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–594. doi: 10.1016/S0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez-Viña MA, Falco M, Sun Y, Stastny P. DNA typing for HLA class I alleles: I. Subsets of HLA-A2 and of -A28. Hum Immunol. 1992;33:163–173. doi: 10.1016/0198-8859(92)90068-X. [DOI] [PubMed] [Google Scholar]
- 45.Ayyoub M, Migliaccio M, Guillaume P, Liénard D, Cerottini JC, Romero P, Lévy F, Speiser DE, Valmori D. Lack of tumor recognition by hTERT peptide 540–548-specific CD8(+) T cells from melanoma patients reveals inefficient antigen processing. Eur J Immunol. 2001;31:2642–2651. doi: 10.1002/1521-4141(200109)31:9<2642::AID-IMMU2642>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 46.Lev A, Denkberg G, Cohen CJ, Tzukerman M, Skorecki KL, Chames P, Hoogenboom HR, Reiter Y. Isolation and characterization of human recombinant antibodies endowed with the antigen-specific, major histocompatibility complex-restricted specificity of T cells directed toward the widely expressed tumor T-cell epitopes of the telomerase catalytic subunit. Cancer Res. 2002;62:3184–3194. [PubMed] [Google Scholar]
- 47.Vonderheide RH, Domchek SM, Schultze JL, George DJ, Hoar KM, Chen DY, Stephans KF, Masutomi K, Loda M, Xia Z, Anderson KS, Hahn WC, Nadler LM. Vaccination of cancer patients against telomerase induces functional antitumor CD8+ T lymphocytes. Clin Cancer Res. 2004;10:828–839. doi: 10.1158/1078-0432.CCR-0620-3. [DOI] [PubMed] [Google Scholar]
- 48.Dupont J, Latouche JB, Ma C, Sadelain M. Artificial antigen-presenting cells transduced with telomerase efficiently expand epitope-specific, human leukocyte antigen-restricted cytotoxic T cells. Cancer Res. 2005;65:5417–5427. doi: 10.1158/0008-5472.CAN-04-2991. [DOI] [PubMed] [Google Scholar]
- 49.Moser M, Leo O. Key concepts in immunology. Vaccine. 2010;28(Suppl 3):C2–C13. doi: 10.1016/j.vaccine.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 50.Aucouturier J, Dupuis L, Deville S, Ascarateil S, Ganne V. Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev Vaccines. 2002;1:111–118. doi: 10.1586/14760584.1.1.111. [DOI] [PubMed] [Google Scholar]
- 51.Johnston D, Bystryn JC. Topical imiquimod is a potent adjuvant to a weakly-immunogenic protein prototype vaccine. Vaccine. 2006;24:1958–1965. doi: 10.1016/j.vaccine.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 52.Church SE, Jensen SM, Twitty CG, Bahjat K, Hu HM, Urba WJ, Fox BA. Multiple vaccinations: friend or foe. Cancer J. 2011;17:379–396. doi: 10.1097/PPO.0b013e3182346320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.