Abstract
Human Vγ2 Vδ2-bearing T cells have recently received much attention in cancer immunotherapy. In this study, we conducted a phase I/II clinical trial of the adoptive transfer of γδ T cells to patients with advanced renal cell carcinoma. Eleven patients who had undergone nephrectomy and had lung metastasis were enrolled. Peripheral blood γδ T cells obtained from the patients were stimulated ex vivo with 2-methyl-3-butenyl-1-pyrophosphate (2M3B1PP), a synthetic pyrophosphomonoester antigen, and transferred in combination with zoledronic acid (Zol) and teceleukin (recombinant human interleukin-2). Expanded γδ T cells exhibited potent cytotoxic activity against tumor cells in vitro, and the proportion of peripheral blood γδ T cells among CD3+ cells typically peaked three to 5 days after transfer. Tumor doubling time was prolonged in all 11 patients, and the best overall responses were 1 CR, 5 SD, and 5 PD, as defined based on Response Evaluation Criteria in Solid Tumors (RECIST). Although ten patients developed adverse reactions of grade ≥3, they were likely to have been the result of the concomitant infusion of Zol and IL-2, and most symptoms swiftly reverted to normal during the course of treatment. In conclusion, this clinical trial demonstrated that our regimen for the adoptive transfer of γδ T cells in combination with Zol and IL-2 was well tolerated and that objective clinical responses could be achieved in some patients with advanced renal cell carcinoma.
Keywords: γδ T cell, Nitrogen-containing bisphosphonate, Pyrophosphomonoester, Isopentenyl pyrophosphate, Renal cell carcinoma, Cancer immunotherapy
Introduction
Human γδ T cells that express Vγ2 Vδ2 (also termed Vγ9 Vδ2)-bearing TCR recognize nonpeptide antigens derived from microbial pathogens such as mycobacteria [1–4] and exhibit natural cytolytic activity against a wide array of tumor cells in vitro [5, 6]. It is worth noting that γδ T cells exert specific cytolysis in a TCR-dependent manner when they encounter human tumor cells pulsed with nitrogen-containing bisphosphonates (N-BPs), such as pamidronate and zoledronic acid (Zol) [7, 8]. It is demonstrated that the inhibition of farnesyl pyrophosphate synthase by N-BPs results in the accumulation of isopentenyl pyrophosphate (IPP), a prenyl pyrophosphate intermediate, in tumor cells, which leads to the activation of γδ T cells [9, 10]. Although the elevated level of IPP is essential in the activation, it is not clear whether IPP per se is directly recognized by γδ T cells.
Based on these findings, a novel cancer immunotherapy using γδ T cells and N-BPs has been proposed [11–13]. It has recently been reported that the addition of Zol to first-line chemotherapy in the treatment of patients with multiple myeloma significantly improved disease-free survival [14]. Because Zol was administered every 3–4 weeks, the induction of tumor immunity, and especially the activation of γδ T cells, was considered to be one of the mechanisms by which Zol elicited beneficial effects on the clinical outcomes. In addition, we have previously observed that the proportion of renal cell carcinoma (RCC) patients whose peripheral blood γδ T cells were up-regulated increased as the patients progressed through the stages of the disease [15] and that the degree of the increase in γδ T cells in stage III patients correlated with their 10-year overall survival rate [16]. These clinical findings and in vitro observations encouraged us to develop a novel cancer immunotherapy.
We and others previously identified IPP-relating pyrophosphomonoesters and their nucleoside triphosphate γ-ester derivatives as antigens for human Vγ2 Vδ2-bearing T cells [2, 4]. Subsequently, it was demonstrated that (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate, a microbial metabolite, was the most potent stimulant of natural origin [17]. Because of the structural similarity between pyrophosphomonoesters and bisphosphonates, several N-BPs were examined for antigenicity in stimulating γδ T cells, leading to the discovery that N-BPs of the second and third generations effectively induced the expansion of human γδ T cells both in vitro and in vivo [7]. There are several differences between pyrophosphomonoesters and N-BPs. Whereas pyrophosphomonoesters can stimulate both unprimed and primed γδ T cells without accessory cells, N-BPs require monocyte lineage cells for efficient stimulation in primary γδ T-cell responses and human tumor cells for primed γδ T cells [8]. In terms of stability, pyrophosphomonoesters can be readily hydrolyzed by serum alkaline phosphatases, but N-BPs are generally resistant to serum enzymes.
Taking these immunological and pharmacological properties of nonpeptide antigens into account, we employed a strategy consisting of the adoptive transfer of γδ T cells to yield effector cells, followed by N-BP infusion to sensitize tumor cells in the present phase I/II clinical trial. During the course of this study, we evaluated the safety of γδ T-cell transfer concomitant with Zol and IL-2 infusion, the kinetics of γδ T cells in the peripheral blood, the prolongation of tumor doubling time, and the clinical outcomes based on RECIST.
Materials and methods
Patients and patient eligibility
Patients with histologically confirmed renal cell carcinoma (any T, any N, and M1, stage IV), who had undergone nephrectomy, with PS of 0, who had evaluable lung metastasis on computed tomography (CT) 3 months before the start of treatment, whose tumor doubling time before treatment was evaluable, whose age ranged from 20 to 80 years old, whose life expectancy was at least 6 months, whose major organs maintained function, whose lung metastatic lesions progressed even after treatment with IFN-α for at least 3 months, who met the laboratory test standards of our institution, and who voluntarily provided written consent to participate in this trial after having been thoroughly briefed and informed of its nature, were eligible for enrollment. Major exclusion criteria were a history of cancer other than RCC within 2 years, treatment with anticancer drugs, treatment with steroids, and serious complications.
Study design
The protocol was designed and written by the authors, in collaboration with staff from the Translational Research Informatics Center (TRI), Kobe, Hyogo, Japan, and reviewed and approved by the Tokyo Women’s Medical University Hospital Ethics Committee. This trial was a nonrandomized, uncontrolled, open-label, single-institutional study and is registered at http://www.clinicaltrials.gov as TRIC-CTR-GU-05-01. RCC patients who met the inclusion criteria were enrolled in this study.
Peripheral blood mononuclear cells were collected from each patient using an apheresis machine and then manually purified further with Ficoll-Paque™ PLUS (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). The cells were resuspended in 350 ml of the serum-free medium ALyS505 N (Cell Science & Technology Institute, Sendai, Miyagi, Japan) containing recombinant human interleukin-2 (rIL-2, Proleukin, Chiron, CA), 100 international units (IU)/ml, and stimulated with 2-methyl-3-butenyl-1-pyrophosphate (2M3B1PP), a pyrophosphomonoester, prepared at our institution as previously described [2] at a final concentration of 100 μM in an air-permeable culture bag (Nipro Corp., Kita-ku, Osaka, Japan) for 11 days at 37°C in a humidified atmosphere containing 5% CO2 at the Cell Processing Center of Tokyo Women’s Medical University Hospital, which is based upon GMP. The expanded γδ T cells were transferred into a sterile infusion bag (Nipro Corp.). The patients were infused with 4 mg of Zol in 100 ml saline over a period of 30 min. Then, γδ T cells were administered for 5 min starting 2 h after the completion of Zol infusion. Subsequently, rIL-2, 1.4 × 106 JRU (Teceleukin, Shionogi & Co., Ltd., Japan, 1 JRU: Japan reference unit = 1 IU), was administered every day for 5 days. This procedure was repeated six times, once every 4 weeks. The target lung legions were measured through standard CT imaging at −3, 0, 3, and 7 months after the start of treatment. Changes in laboratory test values were monitored to assess patient safety throughout the study, and immunological properties of the expanded γδ T cells and peripheral blood T cells were examined by means of flow cytometry. The standard cytotoxic assay was performed as described below.
The primary endpoints were the incidence of adverse events (AEs) and the increase in the proportion of peripheral blood γδ T cells. All AEs occurred during and within 1 month after completion of the treatment and were classified according to the NCI-Common Terminology Criteria for Adverse Events (NCI-CTCAE) ver. 3.0. The proportion of Vδ2-bearing γδ T cells among peripheral blood T cells was determined as described below.
The secondary endpoints were the prolongation of tumor doubling time and the best overall responses as defined by the RECIST criteria [18]. The target lesions were scanned using helical CT with a slice thickness of 5 mm. The tumor volume was calculated as ab(a + b)π/12, where a represents a major axis of the tumor ellipse and b a minor axis. The tumor doubling time was defined as log2(T 1−T 0)/(logV 1−logV 0), where V 0 and V 1 represent the tumor volumes at time T 0 and T 1, respectively. When two or more lesions were present in the lungs, the tumor volume was defined as the sum of the individual tumor volumes.
Immunological monitoring
The profiling of peripheral lymphocytes and cultured cells was examined by means of flow cytometry. Cells were treated with PC5-conjugated anti-CD3 mAb (SK7, Becton–Dickinson Immunocytometry Systems, San Jose, CA, USA), or phycoerythrin (PE)-conjugated anti-CD4, anti-CD8, or anti-CD25 mAbs (BD Pharmingen Inc., San Diego, CA, USA), and fluorescein isothiocyanate (FITC)-conjugated anti-Vδ2 mAb (Immunotech, Marseilles, France) at 2 × 105 cells/50 μl of phosphate buffered saline (PBS)/2% fetal calf serum (FCS) on ice for 30 min. After being washed three times with 200 μl of PBS/2% FCS, the cells were subjected to flow cytometry (EPICS XL, Coulter Electronics, Hialeah, FL, USA). The flow cytometric data were processed and analyzed using EXPO32 software (Coulter Electronics).
The serum cytokines, IL-2, IFN-γ, IL-4, IL-5, IL-10, and TNF-α, were measured using Cytometric Bead Array (CBA) Human Th1/Th2 Cytokine Kits (Becton–Dickinson) according to the manufacturer’s instructions.
To determine cytotoxic activity, the γδ T cell–sensitive Daudi (Burkitt’s lymphoma) line and the Caki-1 and VMRC-RCW (renal cell carcinomas) lines were treated with [51Cr]-sodium chromate solution (3.7 MBq) at 37°C for 1 h. After being washed with medium, the tumor cells were resuspended in the medium and placed in a round-bottom 96-well plate. To the suspension was added the expanded γδ T cells at the effector/target ratio of 40:1. The plate was briefly centrifuged and incubated at 37°C and 5% CO2. After 4 h of incubation, the supernatants were examined for [51Cr]-sodium chromate release using a γ-counter.
Data were collected by the investigators at Tokyo Women’s Medical University Hospital and processed and analyzed by the staff of TRI.
Statistical analysis
Statistical analyses were conducted to test the differences between two items using the log-rank test, using the Stat View 5.0 J software package (Abacus Concepts, Inc, CA, USA).
Results
Patients’ profiles
A total of 11 patients who were diagnosed with metastatic renal cell carcinoma were recruited between January 2006 and March 2008. All patients underwent nephrectomy for RCC before enrollment. The final outcomes of all patients were assessed in October 2008, and the data were compiled and analyzed in December 2008. The patients’ profiles are presented in Table 1. Eight of the patients were men and three were women; at enrollment, the median age was 59.4 years and PS was 0. Memorial Sloan-Kettering Cancer Center (MSKCC) Risk status was assessed at the time of enrollment. Based on histological examination, 9 patients had been diagnosed with RCC of clear cell type, 1 with RCC of clear cell with sarcomatoid, and 1 with RCC of papillary cell type. All patients had lung metastasis and/or other site of distant metastasis and received IFN-α and/or IL-2 after the surgery.
Table 1.
Patients’ profile and clinical outcome
| Patient | Age/sex | MSKCCa risk group | Type of cellb | Metastatic lesion | Previous treatment | Treatment cycles | Overall response | Clinical Outcome |
|---|---|---|---|---|---|---|---|---|
| TR1 | 68/M | Intermediate | Clear | Lung | IFN-αc | 4 | SD | Death |
| TR2 | 52/M | Poor | Clear with salcomatoid | Lung/bone/lymph node | IFN-α/IL-2d | 2 | PD | Death |
| TR3 | 65/M | Intermediate | Clear | Lung | IFN-α | 6 | SD | Survival |
| TR4 | 56/F | Intermediate | Clear | Lung/liver | IFN-α/IL-2/metastasectomy | 1 | PD | Survival |
| TR5 | 61/M | Intermediate | Clear | Lung | IFN-α | 6 | CR | Survival |
| TR6 | 63/M | Poor | Clear | Lung/bone/pleura | IFN-α/metastasectomy | 1 | PD | Survival |
| TR7 | 59/F | Good | Clear | Lung/pleura | IFN-α/metastasectomy | 5 | PD | Survival |
| TR8 | 39/M | Intermediate | Clear | Lung/pleura | IFN-α | 3 | PD | Survival |
| TR9 | 61/M | Intermediate | Clear | Lung | IFN-α | 6 | SD | Survival |
| TR10 | 66/F | Intermediate | Clear | Lung/lymph nodes/retro peritoneal cavity/muscle/ascending colon | IFN-α | 6 | SD | Survival |
| TR11 | 63/M | Intermediate | Papillary | Lung/lymph nodes | IFN-α | 6 | SD | Survival |
a MSKCC Memorial Sloan-Kettering Cancer Center
bWorld Health Organization Classification of Tumors [44]
c IFN-α interferon-alpha
d IL-2 interleukin-2
After obtaining written informed consent, patients were treated for metastatic renal cell carcinoma in the Study Design section. Five patients completed the whole treatment schedule, whereas six eventually discontinued for various reasons: one patient due to a brain tumor after four cycles of the treatment, one patient due to a lack of efficacy after three cycles of treatment, two patients due to an investigator’s decision in response to the patients’ apprehension, and two patients through a withdrawal of informed consent.
Immunological responses
PBMC were stimulated with 2M3B1PP, and the resulting γδ T cells were collected and examined for immunological properties. As shown in Fig. 1a, there was no intrinsic difference in the proportion of CD3+ cells (lozenge) in each cycle of expansion. By contrast, the proportions of Vδ2+ cells among the CD3+ cells (rectangle) decreased over the course of the treatment, whereas those of CD4+ cells (cross) and CD8+ cells (triangle) gradually increased. The absolute numbers of Vδ2+ cells in transferred cells are measured in each cycle of the treatment in each patient. Whereas the number of Vδ2+ cells in the sixth cycle was higher than that in the first cycle in patients who achieved SD/CR, the number significantly decreased as the cycle progressed in 3 of 5 PD patients as shown in Fig. 3b, c.
Fig. 1.
a Relative proportions of T-cell subsets in transferred cells derived from patients who completed 6 cycles of the treatment. The proportions of CD3+ cells (lozenge) and those of Vδ2+ (rectangle), CD4+ (cross), and CD8+ cells (triangle) among CD3+ cells were measured in each cycle of the treatment. b/c The absolute numbers of Vδ2+, CD4+, and CD8+ cells among the transferred cells in each cycle of the treatment; SD/CR (b) and PD patients c
Fig. 3.
a Serum cytokine concentrations at different time points of the first cycle of the treatment. Dark gray line indicates cytokine concentrations in sera obtained from SD/CR patients and bright line PD patients. The cytokine concentrations are shown as the average ±SDs. Cytotoxicity exhibited by cultured cells against Daudi, Caki-1, and VWRC-RCW was measured by the standard 51Cr release assay. b Comparison of cytotoxic activity exhibited by cultured cells derived from SD/CR (dark gray column) and that from PD patients (bright gray column). c Comparison of cytotoxicity exhibited by cultured cells at the first and the sixth cycle of treatment; the first cycle (dark gray column) and the sixth cycle (bright gray column). The average and SDs of specific lysis were determined in SD/CR patients who had completed the six cycles of the treatment
We then analyzed the proportion of Vδ2-bearing T cells among CD3+ cells of peripheral blood before and after infusion of γδ T cells. Peripheral blood samples were examined for the expression of CD3, CD4, CD8, CD25, and Vδ2-TCR. In the first cycle of treatment, the proportions of peripheral blood Vδ2-bearing γδ T cells among CD3+ T cells in all patients increased with time to different degrees. Typically, the proportion of γδ T cells peaked 3–5 days after infusion, as shown in Fig. 2. On average, the Vδ2+/CD3+ ratio in the first infusion was 4.83% at the time of apheresis and 13.43% 1 week after infusion.
Fig. 2.
Time course of Vδ2-bearing γδ T-cell circulation in the peripheral blood of patients with advanced RCC after infusion of 2M3B1PP-stimulated PBMC together with IL-2 and Zol. Before and after the infusion, PBMCs were examined for the expression of Vδ2 and CD3
Serum cytokine contents before and after infusion in the first cycle of treatment were measured by Cell Beads Assay. Levels of serum IFN-γ, IL-5, and IL-10 concentrations are shown in Fig. 3a at different time points. IFN-γ peaked 1–2 days after infusion and gradually decreased to the levels that had existed before infusion in 7 days and IL-5 3–5 days after infusion and then swiftly decreased. There are, however, no statistically significant differences between the SD/CR patients (dark gray line) and the PD patients (bright gray line). We also measured IL-4, TNF-α, and IL-2, but no remarkable differences were observed between the two groups. In terms of cytotoxicity, SD/CR patients exhibited a slightly higher specific lysis against Daudi, Caki-1, and VMRC-RCW (dark gray bar) than PD patients (bright gray bar), though there was no statistically significant difference between the two groups, as shown in Fig. 3b. In SD/CR patients, tumoricidal activity in the sixth cycle appears to be lower than that in the first cycle, as depicted in Fig. 3c.
Clinical responses
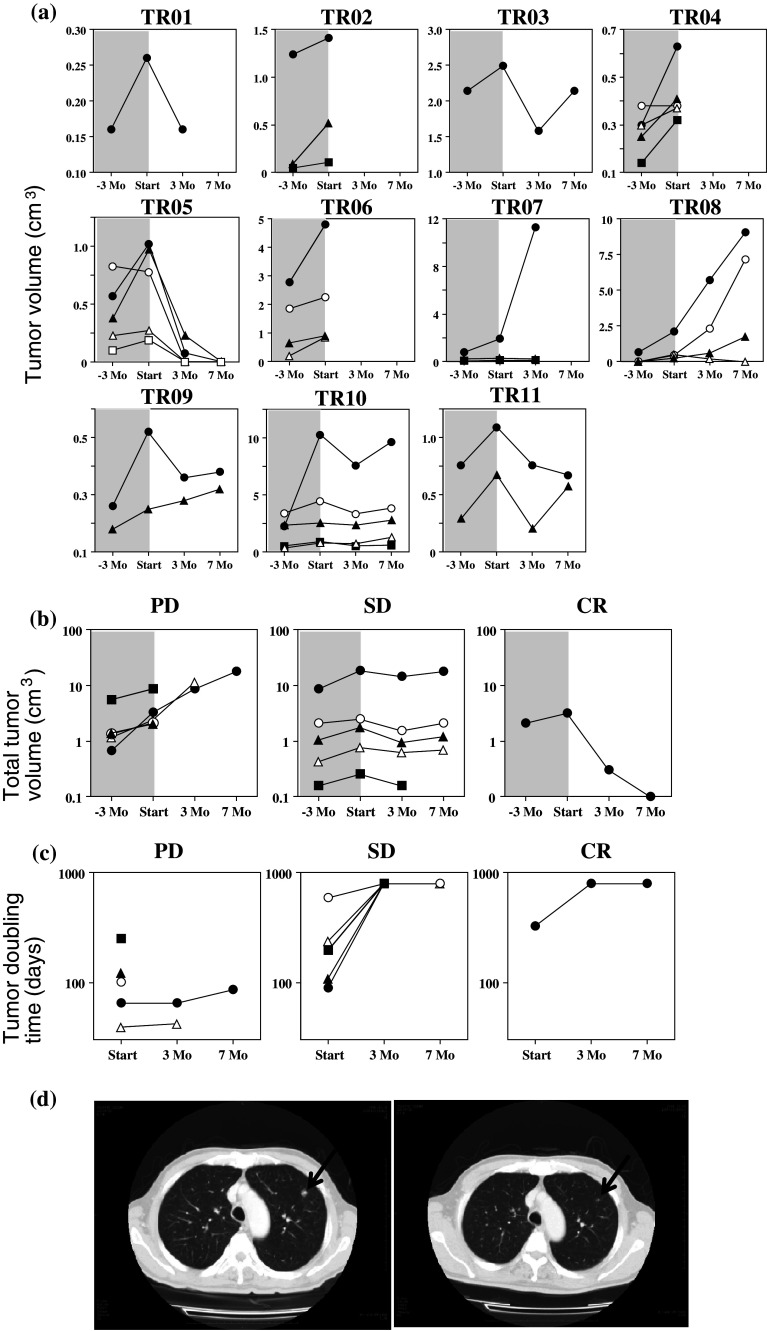
In order to assess the secondary endpoints, tumor volumes were determined through CT −3 Mo, 0 Mo, +3 Mo, and +7 Mo after the start of treatment. No newly appearing lesions in the lungs were observed in any of the patients during the course of this study. As summarized in Fig. 4a, the tumor volumes of most metastatic lesions increased steeply in the baseline period from −3 months to the beginning of treatment in all patients. After the start of treatment, the tumor volumes of some metastatic lesions began to decrease or continued to increase but at a more moderate rate. As depicted in the upper panels of Fig. 4b, the total tumor volumes in the lungs increased moderately in patients TR07 and TR08, whereas those in TR01, TR03, TR09, TR10, and TR11 did not change significantly. Remarkably, metastatic lesions disappeared within 7 months in TR05. Because the treatment was discontinued in TR02, TR04, and TR06, the total tumor volumes were not measured in this study. A moderate to significant increase in the tumor doubling time was observed in all patients as shown in the lower panels of Fig. 4b. Based on these results, one patient was considered to exhibit CR, five patients SD, and five patients PD (Table 1). Two patients had died as a result of malignant tumors by the end of the study in October 2008 (Table 1). It is encouraging to note that TR05, the patient who exhibited CR, has remained disease-free to date (more than 36 months after the completion of the treatment). Figure 4d demonstrates representative CT images of the metastatic lesions in TR05 at the beginning of the treatment and 3 months later; it was during this 3-month interval that the two lesions macroscopically disappeared.
Fig. 4.
a Time course of individual tumor volumes in the lungs of advanced RCC patients after infusion of 2M3B1PP-stimulated PBMC with IL-2 plus Zol. The tumor size of each lesion was measured by means of CT imaging −3 Mo, 0 Mo, 3 Mo, and 7 Mo after the start of the treatment. b Time course of total tumor volumes and c tumor doubling time. Total lung tumor volumes of each patient were calculated and plotted against time separately for three patient subgroups: PD (closed triangle: TR02, open circle: TR04, closed square: TR06, open triangle: TR07, and closed circle: TR08), SD (closed square: TR01, open circle: TR03, open triangle: TR09, closed circle, TR10, and closed triangle, TR11), and CR (closed circle: TR05). d CT images of metastatic lung tumors in an advanced RCC patient. Five metastatic lung lesions were observed through CT imaging in TR05; one metastatic site depicted here was measured at 1.26 × 1.08 cm (not shown), 1.33 × 0.97 cm (left), and 0.00 × 0.00 cm (right) at −3 Mo, 0 Mo, and 3 Mo after the start of treatment, respectively
Adverse events
In order to assess the safety of this protocol as the first primary endpoint, we monitored PS scores, laboratory test values, and adverse reactions throughout the study. Although the patient referred to as TR02 had a PS of 0 at pre-enrollment, his PS rose to 1 at the first apheresis through the second infusion of γδ T cells, and he dropped out of the study after completing the second cycle. TR07, who had also had a PS of 0 at pre-enrollment, had developed a PS of 1 by the fifth γδ T-cell transfer and dropped out of the study after completing the fifth cycle. The PS scores of the other patients were 0 at pre-enrollment and remained unchanged throughout the study. All patients developed adverse reactions of grade 1 or 2, including fatigue and fever, and ten patients developed adverse reactions of grade 3 or 4, as summarized in Table 2, including lymphopenia, hyponatremia, hypopotassemia, an increase in serum alanine aminotransferase, an increase in serum aspartate aminotransferase, an increase in serum creatinine, and a decrease in hemoglobin. All the deviated laboratory values reverted to normal during the course of the treatment. Patients, TR06 and TR10, whose serum creatinine increased should have been treated with rehydration therapy, but the symptom disappeared without treatment in a week. Almost all AEs occurred in the first cycle, and the frequency of these AEs decreased over the course of treatment.
Table 2.
Adverse events
| Grade >3 | Number of patients/frequency (%) | |||||
|---|---|---|---|---|---|---|
| 1 course (n = 11) | 2 course (n = 9) | 3 course (n = 8) | 4 course (n = 7) | 5 course (n = 6) | 6 course (n = 5) | |
| Lymphopenia | 10 (91%) | 3 (30%) | 1 (13%) | 1 (14%) | 0 (0%) | 0 (0%) |
| Neutropenia | 0 (0%) | 0 (0%) | 0 (0%) | 1 (14%) | 1 (17%) | 0 (0%) |
| Hyponatremia | 2 (18%) | 0 (0%) | 1 (13%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hypopotassemia | 0 (0%) | 1 (11%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (20%) |
| AST | 1 (9%) | 0 (0%) | 0 (0%) | 1 (14%) | 0 (0%) | 0 (0%) |
| ALT | 1 (9%) | 1 (11%) | 2 (25%) | 1 (14%) | 0 (0%) | 0 (0%) |
| Creatinine | 1 (9%) | 0 (0%) | 1 (13%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hemoglobin | 0 (0%) | 0 (0%) | 1 (13%) | 0 (0%) | 0 (0%) | 0 (0%) |
Discussion
Kidney cancer is one of the leading cancer types among estimated new cancer cases and deaths [19]. Historically, metastatic RCC was relatively unresponsive to traditional chemotherapy, and biological response modifiers such as IL-2 and IFN-α were the favorable treatments for patients with advanced RCC [20]. The recent development of targeted agents has changed the management of metastatic RCC [21]. These agents have distinct modes of action and include multitargeted receptor tyrosine kinase inhibitors, mTOR inhibitors, and vascular endothelial growth factor signaling blockers [22–24]. Although the prognosis for patients with metastatic RCC has improved significantly because of advances in these treatments, it should be noted that the primary achievement of the target therapies is the induction of SD, not CR [21]. IL-2 remains the only agent known to produce durable complete responses [25], but its use is accompanied by severe side effects, including hypotension, capillary leak syndrome, renal insufficiency, and the like, and is limited to good responders to the lymphokines [20]. Notwithstanding the availability of a variety of therapeutics, no satisfactory regimen for advanced RCC has yet been defined.
It is well known that RCC sometimes evokes immune responses that lead to complete tumor remissions [20]. We recently conducted a pilot study investigating the safety and feasibility of the adoptive transfer of γδ T cells concomitantly with IL-2 [26]. γδ T cells exert tumoricidal activity via two distinct mechanisms, natural killer-like activity and γδ TCR-dependent cytotoxicity [27]. γδ TCR is involved in the antitumor activity that results when tumor cells are treated with N-BPs [5]. Whereas it is most likely that IPP per se or its derivatives, which are accumulated in the tumor cells via the inhibition of farnesyl pyrophosphate synthase by N-BPs, are presented to γδ T cells, the translocation or induction of antigenic proteinaceous entities to the cell surface is also plausible [28]. Generally, γδ TCR-dependent cytotoxicity is more potent than natural killer-like activity. We therefore examined the safety and clinical outcomes of the infusion of γδ T cells plus IL-2 and Zol in this study.
Regarding primary endpoints, we successfully observed an increase in the proportion of Vγ2 Vδ2-bearing γδ T cells in peripheral blood after the infusion of γδ T cells. There was a lag of several days between the infusion and the observed increase in the number of peripheral blood γδ T cells. This is probably because γδ T cells were trapped immediately after infusion in the reticuloendothelial system and the lungs. It is worth noting that the proportions of γδ T cells among the CD3+ cells cultured with 2M3B1PP and IL-2 decreased as the number of treatment cycles increased, from 77.54% in the first cycle to 33.54% in the sixth cycle. Consequently, the tumoricidal activity of the cultured cells decreased, from 36.4 and 42.9% in the first cycle against Caki-1 and VMRC-RCW, respectively, to 15.2 and 21.0% in the sixth cycle. The lower proportion of γδ T cells in the cultured cells of the sixth cycles compared to those of the first cycles may account for the reduced cytotoxicity. The proportion of γδ T cells among the peripheral blood CD3+ cells after infusion also decreased significantly. We did not observe such a decrease in the pilot study, in which expanded γδ T cells and IL-2 were infused without Zol [26]. Thus, the induced unresponsiveness or hyporesponsiveness of γδ T cells to 2M3B1PP is attributable to the prior infusion of Zol. This finding may lead to a future improvement in the current protocol. An improved regimen could consist of the collection of peripheral blood by apheresis, the storage of purified PMBC aliquots until use, and the expansion of cryopreserved γδ T cells. Because our initial objective was to increase the number of γδ T cells in peripheral blood by adoptive transfer of ex vivo expanded γδ T cells, this new regimen would further our aims. In addition, we should determine the optimum dose of Zol in the future regimen because the current dose of Zol, 4 mg, was originally chosen for the treatment of patients with hypercalcimea, but not with malignant tumors.
Regarding another primary endpoint, we observed some AEs of grades 3 and 4. In our previous pilot study comprising γδ T-cell transfer plus IL-2 infusion without Zol, AEs of these magnitudes did not occur during treatment [26]. Because Zol infusion itself is also well tolerated in clinical settings [29–37], the combination of Zol, IL-2, and γδ T cells may have caused the AEs. Regardless, the symptoms were manageable and disappeared swiftly during the course of treatment, indicating that the regimen used in this study was well tolerated. In the pioneering study on N-BPs, AEs were induced after the initial infusion of N-BPs and essentially no AEs were observed in the subsequent infusions [38–40]. It is thus possible to reduce the dose of Zol in the initial infusion in order to reduce the severity of AEs. Furthermore, the interval between Zol administration and γδ T-cell infusion should be optimized to minimize AEs in future regimens.
It is worth noting that tumor doubling time was successfully increased in all patients. In TR07, for instance, there were three metastatic tumors in the lungs, one of which grew consistently, while the other two remained stable. Summing the volumes of the individual tumors revealed that the tumor doubling time was prolonged even in this PD case. In TR05, there were five metastatic tumors, three of which disappeared within 3 months and two within 7 months. To date, this patient has not developed any evidence of recurrent tumors during the nearly three-year period since the completion of the treatment.
In addition, several new lesions that had not been present in the CT images recorded 3 months before the start of the treatment were detected at the start of immunotherapy, but no new lesions were observed in the lungs after the start of immunotherapy. This finding suggests that the present regimen may prevent micrometastasis of renal cell carcinoma. It has recently been reported that the addition of Zol to the standard regimen for the treatment of breast cancer improved disease-free survival times [41]. It is therefore necessary to identify the mechanism by which Zol elicits its therapeutic effects [42].
Based on the present results and those of other clinical trials, it is most likely that γδ T cells exert cytotoxic activity against malignant tumors in vivo. The effector roles of γδ T cells in vivo are still controversial, however. Thus, it is imperative that the immunological properties of γδ T cells in tumor-infiltrating lymphocytes be examined and the results applied to the development of efficacious regimens for malignant tumors.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Culture, Sports, and Technology of Japan (MEXT) and by Special Coordination Funds for Promoting Science and Technology from MEXT and Astellas Pharma Inc. as part of the “Formation of Innovation Center for Fusion of Advanced Technologies” program. The authors wish to acknowledge the support provided by the staff from the Translational Research Informatics Center (TRI), Kobe, Hyogo, Japan, and would like to thank Ms Clare Dover for providing English correction prior to paper submission.
Footnotes
Part of this study was previously published as a case report [43].
References
- 1.Hayday AC. γδ T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka Y, Morita CT, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka Y, Sano S, Nieves E, De Libero G, Rosa D, Modlin RL, Brenner MB, Bloom BR, Morita CT. Nonpeptide ligands for human γδ T cells. Proc Natl Acad Sci U S A. 1994;91:8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, Fournie JJ. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 5.Kato Y, Tanaka Y, Miyagawa F, Yamashita S, Minato N. Targeting of tumor cells for human γδ T cells by nonpeptide antigens. J Immunol. 2001;167:5092–5098. doi: 10.4049/jimmunol.167.9.5092. [DOI] [PubMed] [Google Scholar]
- 6.Bukowski JF, Morita CT, Tanaka Y, Bloom BR, Brenner MB, Band H. Vγ2 Vδ2 TCR-dependent recognition of non-peptide antigens and Daudi cells analyzed by TCR gene transfer. J Immunol. 1995;154:998–1006. [PubMed] [Google Scholar]
- 7.Kunzmann V, Bauer E, Wilhelm M. γ/δ T-cell stimulation by pamidronate. N Engl J Med. 1999;340:737–738. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- 8.Miyagawa F, Tanaka Y, Yamashita S, Minato N. Essential requirement of antigen presentation by monocyte lineage cells for the activation of primary human γδ T cells by aminobisphosphonate antigen. J Immunol. 2001;166:5508–5514. doi: 10.4049/jimmunol.166.9.5508. [DOI] [PubMed] [Google Scholar]
- 9.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jauhiainen M, Monkkonen H, Raikkonen J, Monkkonen J, Auriola S. Analysis of endogenous ATP analogs and mevalonate pathway metabolites in cancer cell cultures using liquid chromatography-electrospray ionization mass spectrometry. J Chromatography B. 2009;877:2967–2975. doi: 10.1016/j.jchromb.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Bonneville M, Scotet E. Human Vγ9 Vδ2 T cells: promising new leads for immunotherapy of infections and tumors. Curr Opin Immunol. 2006;18:539–546. doi: 10.1016/j.coi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Kabelitz D, Wesch D, He W. Perspectives of γδ T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 13.Kabelitz D, Wesch D, Pitters E, Zoller M. Potential of human γδ T lymphocytes for immunotherapy of cancer. Int J Cancer. 2004;112:727–732. doi: 10.1002/ijc.20445. [DOI] [PubMed] [Google Scholar]
- 14.Morgan GJ, Davies FE, Gregory WM, Cocks K, Bell SE, Szubert AJ, Navarro-Coy N, Drayson MT, Owen RG, Feyler S, Ashcroft AJ, Ross F, Byrne J, Roddie H, Rudin C, Cook G, Jackson GH, Child JA, On behalf of the National Cancer Research Institute Haematological Oncology Clinical Study Group First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376:1989–1999. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi H, Tanaka Y, Yagi J, Toma H, Uchiyama T. γ/δ T cells provide innate immunity against renal cell carcinoma. Cancer Immunol Immunother. 2001;50:115–124. doi: 10.1007/s002620100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi H, Tanaka Y, Nakazawa H, Yagi J, Minato N, Tanabe K (2011) A new indicator of a favorable progress in locally advanced renal cell carcinomas: γδ T-Cells in peripheral blood. Anticancer Res (in press) [PubMed]
- 17.Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK, Beck E, Wiesner J, Eberl M, Jomaa H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli . FEBS Lett. 2001;509:317–322. doi: 10.1016/S0014-5793(01)03191-X. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Reeves DJ, Liu CY. Treatment of metastatic renal cell carcinoma. Cancer Chemother Pharmacol. 2009;64:11–25. doi: 10.1007/s00280-009-0983-z. [DOI] [PubMed] [Google Scholar]
- 20.Atkins MB. Treatment selection for patients with metastatic renal cell carcinoma: identification of features favoring upfront IL-2-based immunotherapy. Med Oncol. 2009;26(Suppl 1):18–22. doi: 10.1007/s12032-008-9148-x. [DOI] [PubMed] [Google Scholar]
- 21.Bellmunt J. Future developments in renal cell carcinoma. Ann Oncol. 2009;20(Suppl 1):113–117. doi: 10.1093/annonc/mdp074. [DOI] [PubMed] [Google Scholar]
- 22.Feldman DR, Baum MS, Ginsberg MS, Hassoun H, Flombaum CD, Velasco S, Fischer P, Ronnen E, Ishill N, Patil S, Motzer RJ. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1432–1439. doi: 10.1200/JCO.2008.19.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escudier B, Szczylik C, Hutson TF, Demkow T, Staehler M, Rolland F, Negrier S, Laferriere N, Scheuring UJ, Cella D, Shah S, Bukowski RM. Randomized phase II trial of first-line treatment with sorafenib versus interferon α-2a in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1280–1289. doi: 10.1200/JCO.2008.19.3342. [DOI] [PubMed] [Google Scholar]
- 24.Motzer RJ, Hudes GR, Curti BD, McDermott DF, Escudier BJ, Negrier S, Duclos B, Moore L, O’Toole T, Boni JP, Dutcher JP. Phase I/II trial of temsirolimus combined with interferon α for advanced renal cell carcinoma. J Clin Oncol. 2007;25:3958–3964. doi: 10.1200/JCO.2006.10.5916. [DOI] [PubMed] [Google Scholar]
- 25.McDermott DF. The application of high-dose interleukin-2 for metastatic renal cell carcinoma. Med Oncol. 2009;26(Suppl 1):13–17. doi: 10.1007/s12032-008-9152-1. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T, Minato N, Toma H. Safety profile and anti-tumor effects of adoptive immunotherapy using γδ T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother. 2007;56:469–476. doi: 10.1007/s00262-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, Golan DE, Brenner MB. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 28.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 29.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of γδ T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–394. [PubMed] [Google Scholar]
- 30.Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP. γδ T cells for immune therapy of patients with lymphoid malignancies Blood. 2003;102:200–206. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 31.Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D’Asaro M, Gebbia N, Salerno A, Eberl M, Hayday AC. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunzmann V, Wilhelm M. Anti-lymphoma effect of γδ T cells. Leuk Lymphoma. 2005;46:671–680. doi: 10.1080/10428190500051893. [DOI] [PubMed] [Google Scholar]
- 33.Santini D, Martini F, Fratto ME, Galluzzo S, Vincenzi B, Agrati C, Turchi F, Piacentini P, Rocci L, Manavalan JS, Tonini G, Poccia F. In vivo effects of zoledronic acid on peripheral γδ T lymphocytes in early breast cancer patients. Cancer Immunol Immunother. 2009;58:31–38. doi: 10.1007/s00262-008-0521-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamb LS., Jr γδ T cells as immune effectors against high-grade gliomas. Immunol Res. 2009;45:85–95. doi: 10.1007/s12026-009-8114-9. [DOI] [PubMed] [Google Scholar]
- 35.Laggner U, Lopez JS, Perera G, Warbey VS, Sita-Lumsden A, O’Doherty MJ, Hayday A, Harries M, Nestle FO. Regression of melanoma metastases following treatment with the n-bisphosphonate zoledronate and localised radiotherapy. Clin Immunol. 2009;131:367–373. doi: 10.1016/j.clim.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Viey E, Fromont G, Escudier B, Morel Y, Da Rocha S, Chouaib S, Caignard A. Phosphostim-activated γδ T cells kill autologous metastatic renal cell carcinoma. J Immunol. 2005;174:1338–1347. doi: 10.4049/jimmunol.174.3.1338. [DOI] [PubMed] [Google Scholar]
- 37.Sicard H, Ingoure S, Luciani B, Serraz C, Fournie JJ, Bonneville M, Tiollier J, Romagne F. In vivo immunomanipulation of Vγ9 Vδ2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol. 2005;175:5471–5480. doi: 10.4049/jimmunol.175.8.5471. [DOI] [PubMed] [Google Scholar]
- 38.Buckler HM, Mercer SJ, Davison CE, Hollis S, Richardson PC, Anderson DG. Evaluation of adverse experiences related to pamidronate infusion in Paget’s disease of bone. Ann Rheum Dis. 1998;57:572. doi: 10.1136/ard.57.9.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adami S, Bhalla AK, Dorizzi R, Montesanti F, Rosini S, Salvagno G, Lo Cascio V. The acute-phase response after bisphosphonate administration. Calcif Tissue Int. 1987;41:326–331. doi: 10.1007/BF02556671. [DOI] [PubMed] [Google Scholar]
- 40.Purohit OP, Anthony C, Radstone CR, Owen J, Coleman RE. High-dose intravenous pamidronate for metastatic bone pain. Br J Cancer. 1994;70:554–558. doi: 10.1038/bjc.1994.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C, Jakesz R, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 42.Bivi N, Romanello M, Harrison R, Clarke I, Hoyle DC, Moro L, Ortolani F, Bonetti A, Quadrifoglio F, Tell G, Delneri D. Identification of secondary targets of N-containing bisphosphonates in mammalian cells via parallel competition analysis of the barcoded yeast deletion collection. Genome Biol. 2009;10:R93.1–R93.11. doi: 10.1186/gb-2009-10-9-r93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobayashi H, Tanaka Y, Shimmura H, Minato N, Tanabe K. Complete remission of lung metastasis following adoptive immunotherapy using activated autologous γδ T-cells in a patient with renal cell carcinoma. Anticancer Res. 2010;30:575–580. [PubMed] [Google Scholar]
- 44.Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. World Health Organization Classification of Tumors. IRAC Press, Lyon: Pathology and Genetics of Tumors of the Urinary System and Male Genital Organs; 2004. [Google Scholar]