Abstract
Adenovirus (Ad) endocytosis via αv integrins requires activation of the lipid kinase phosphatidylinositol-3-OH kinase (PI3K). Previous studies have linked PI3K activity to both the Ras and Rho signaling cascades, each of which has the capacity to alter the host cell actin cytoskeleton. Ad interaction with cells also stimulates reorganization of cortical actin filaments and the formation of membrane ruffles (lamellipodia). We demonstrate here that members of the Rho family of small GTP binding proteins, Rac and CDC42, act downstream of PI3K to promote Ad endocytosis. Ad internalization was significantly reduced in cells treated with Clostridium difficile toxin B and in cells expressing a dominant-negative Rac or CDC42 but not a H-Ras protein. Viral endocytosis was also inhibited by cytochalasin D as well as by expression of effector domain mutants of Rac or CDC42 that impair cytoskeletal function but not JNK/MAP kinase pathway activation. Thus, Ad endocytosis requires assembly of the actin cytoskeleton, an event initiated by activation of PI3K and, subsequently, Rac and CDC42.
Adenoviruses (Ad) are a significant cause of acute respiratory, ocular, and gastrointestinal diseases in humans. These diseases are generally self-limiting; however, severe disseminated Ad infections have been noted with increasing frequency in immunocompromised patients (21). Replication-defective Ad vectors are also currently being evaluated as vectors for gene delivery (51). While Ad vectors have the capacity to infect a broad range of different cell types, significant challenges to the efficient use of Ad vectors for in vivo gene delivery exist (5, 51). In particular, a lack of knowledge of the precise mechanisms by which Ad enters susceptible host cells exists. Recent studies have identified a 46-kDa host cell membrane protein (CAR) which serves as the primary receptor for Ad serotypes 2 and 5 as well as coxsackie B virus attachment to cells (4). Ad interaction with a second receptor, αv integrin, facilitates virus internalization into cells (50). Internalization is thought to occur by clathrin-mediated endocytosis (9, 40), a concept which is supported by recent studies of Ad uptake in cells expressing a mutant dynamin protein (49).
Ad endocytosis also requires activation of phosphatidylinositol-3-OH kinase (PI3K) (30). PI3K is a member of the lipid kinase family of enzymes which catalyzes the phosphorylation of PtdIns(4)P, and that of PtdIns(4,5)P2 at the D3 position of the inositol ring. These phospholipids are thought to act as second messengers for a number of important cell processes involving activation of protein kinase B (PKB/AKT) and the small GTP-binding proteins Ras, Rho, Rac, and CDC42 (19, 26, 47). Rho GTPases serve as molecular switches cycling between an inactive GDP-bound state and the active GTP-bound form. Ligation of both integrins and growth factors has been shown to stimulate activation of Ras or Rho GTPases and PI3K (11, 22, 23, 38). Activation of Rac and CDC42 induces polymerization of monomeric actin, resulting in the formation of a dense meshwork of actin filaments underlying the plasma membrane. Actin-rich regions form a variety of membrane extensions known as lamellipodia and filopodia. Overexpression of constitutively active Rac promotes the formation of membrane ruffling (lamellipodia), while CDC42 elicits the extension of hairlike structures known as filopodia (31). In contrast, activation of Rho is associated with the formation of actin-associated stress fibers (37, 41). In each of these processes, polymerized actin filaments maintain the architecture of the surface protrusions.
A growing body of work suggests that actin assembly plays an important role in a number of important biological and/or pathogenic processes, including directed cell migration (57), axonal guidance in neuronal cells (32), and cell invasion by a number of different bacteria (10, 20). Recent studies have indicated that actin polymerization also facilitates clathrin-mediated endocytosis (3, 29, 53). In addition to their role in reorganizing cell actin, Rho GTPases have been shown to regulate certain mitogen-activated protein (MAP) kinase pathways following integrin or growth factor ligation (11, 22, 38). Since earlier studies had also suggested that an intact actin cytoskeleton was required for Ad uptake and infection (40), we investigated whether assembly of the actin cytoskeleton via Ras or Rho family GTPases mediates Ad endocytosis.
MATERIALS AND METHODS
Ad, antibodies, and expression plasmids.
Ad type 2 (Ad2) (American Type Culture Collection) was propagated in A549 cells and isolated by ultracentrifugation on CsCl density gradients as previously described (16). For binding and internalization experiments, purified Ad2 virions were radiolabeled with 125I as previously described (30). A replication-defective Ad vector encoding β-galactosidase (Ad5.βgal) (46) was grown in 293 cells. The 9E10 anti-c-Myc monoclonal antibody was obtained from Invitrogen (Carlsbad, Calif.). Polyclonal antibodies to Rac1 and CDC42 were obtained from Santa Cruz Biotech, Inc. (Santa Cruz, Calif.). Anti-H-Ras antibody was kindly provided by J. Jackson (Scripps Research Institute).
The construction of plasmids encoding wild-type RhoA containing an influenza virus HA epitope tag (pCMV/RhoA), a constitutively-active (Q63L) or dominant-negative (T19N) Rac1, and CDC42 or Ras containing a c-Myc epitope (wild type, Q61L, and T17N in pcDNA3) has been previously described (34, 55). Effector domain mutants of Rac and CDC42 (Q61L,F37A and Q61L,Y40C) in pRK5myc (28) were kindly provided by Alan Hall (University College, London, England).
Fluorescence analysis of Ad-induced morphologic changes and actin polymerization.
Subconfluent monolayers of A549 cells were grown on glass coverslips and serum-starved for 24 h by culturing in Dulbecco’s modified Eagle medium (DMEM) prior to assaying actin assembly. Cells were then infected at a multiplicity of infection of 100 PFU of Ad2 per cell or were incubated with 100 ng of epidermal growth factor (EGF) (Sigma, St. Louis, Mo.)/ml at 37°C for various times. The cells were briefly washed and then fixed with 3% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min. Following additional washes, the cells were permeabilized by the addition of 0.2% Triton X-100 in PBS for 2 min. The cells were then washed, and the nonspecific binding sites were blocked by incubation with 0.2% gelatin in PBS. Polymerized actin was then detected by incubation of the cells for 60 min with a 1:1,000 dilution of rhodamine-labeled phalloidin (Sigma) in PBS-gelatin. After washing the cells, the coverslips were mounted (Dako mounting solution; Carpinteria, Calif.) and examined for actin rearrangement and membrane ruffling by using an Olympus BX-40 photomicroscope.
Cell transfection, Ad internalization, and gene delivery assays.
To examine the effect of Rho or Ras GTPases on virus entry, 30% confluent monolayers of SW480 cells in 100-mm plastic tissue culture plates (Costar) were transfected with 50 μl of cationic lipids (Lipofectamine; GIBCO/BRL) with 10 μg of plasmid DNA in accordance with the manufacturer’s protocol. The transfected cells were then cultured in complete medium (DMEM) containing 10% fetal bovine serum 8 h posttransfection. Under these conditions, transient transfection efficiency in these cells was 50 to 70%, as determined by the use of a reporter plasmid (pCMVβ; Clontech). Western blotting, virus internalization, and gene delivery assays were performed 40 to 48 h posttransfection. Internalization of 125I-labeled Ad2 into SW480 cells was quantitated by resistance to trypsin digestion, as previously described (50). Briefly, 105 cpm of radiolabeled Ad2 was incubated with 106 cells for 60 min at 4°C. The unbound virus was removed by washing with ice-cold PBS, and the cells were warmed to 37°C for 10 min prior to trypsinization. Virus uptake was determined by dividing the percentage of trypsin-resistant counts by the total number of specifically bound counts. In certain experiments, cells were pretreated with various amounts of recombinant Clostridium difficile toxin B (25) in serum-free medium for 5 h at 37°C or in the presence of various amounts of cytochalasin D (Sigma) for 90 min prior to measuring virus internalization. For gene delivery studies, SW480 cells were incubated with various amounts of Ad5.βgal for 48 h prior to measuring β-galactosidase activity in solubilized cells as previously described (30).
Kinase assays for PAK1 and JNK/MAP kinase activation.
p65/PAK1 activation was assayed as previously described (15). Briefly, serum-starved SW480 cells were incubated with 100 PFU of Ad2 per cell for 5 to 20 min or with 10 μg of insulin/ml for 5 min at 37°C. SW480 cells were also transfected with a constitutively active form (T423E) of PAK1 (45) prior to assaying kinase activity. The cells were then lysed in 50 mM Tris-HCl containing 150 mM NaCl, 0.5% Nonidet P-40, 0.1% sodium dodecyl sulfate (SDS), 0.25% sodium deoxycholate, 1 mM NaVO3, and protease inhibitors (phenylmethylsulfonyl fluoride, aprotinin, and leupeptin). Cell lysates were then electrophoresed on an 8% SDS gel containing 0.5 mg of histone-H4 (Sigma)/ml. Following separation, the gel was prefixed by soaking in 20% isopropanol in Tris-HCl (pH 7.5), and the proteins were denatured by incubation in 6 M guanidine (pH 7.0) for 2 h and then renatured in 50 mM Tris-HCl (pH 7.5) containing 5 mM β-mercaptoethanol–0.04% Tween 20 at 4°C for 18 h. After washing once with 10 mM HEPES buffer containing 1 mM EDTA, 0.1 mM EGTA, and 5 mM β-mercaptoethanol at 22°C and then at 30°C, the gel was incubated for 30 min in 10 ml of kinase assay buffer (50 mM HEPES, 10 mM MgCl2, 2 mM MnCl2, 1 mM dithiothreitol, 15 mM ATP, and 10 μCi of [γ-32P]ATP). The gel was then washed five times with 5% trichloroacetic acid containing 1% sodium pyrophosphate, dried, and analyzed by PhosphorImager densitometry with ImageQuant software (Molecular Dynamics).
To assay cells for JNK activation, cell lysates were incubated for 2 h at 4°C with recombinant glutathione S-transferase (GST)–c-Jun (amino acid residues 1 to 79) (2, 55) immobilized on glutathione-Sepharose 4B beads (Pharmacia). As a positive control, cells were treated with anisomycin (10 μg/ml), a known activator of JNK/MAP kinase (2). The immobilized complexes were washed and assayed for JNK activity by incubation at 22°C for 20 min in 30 μl of kinase buffer (pH 7.6) containing 20 μM ATP, 5 μCi of [γ-32P]ATP, 20 mM MgCl2, 25 mM HEPES, 20 mM glycerophosphate, 20 mM p-nitrophenylphosphate, and 0.1 mM NaVO3. The kinase reaction was terminated by addition of SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer, and the proteins were separated by 15% polyacrylamide SDS–PAGE. Incorporation of 32P into GST–c-Jun was analyzed by PhosphorImager densitometry.
RESULTS
Ad-induced actin polymerization and morphologic changes in human epithelial cells.
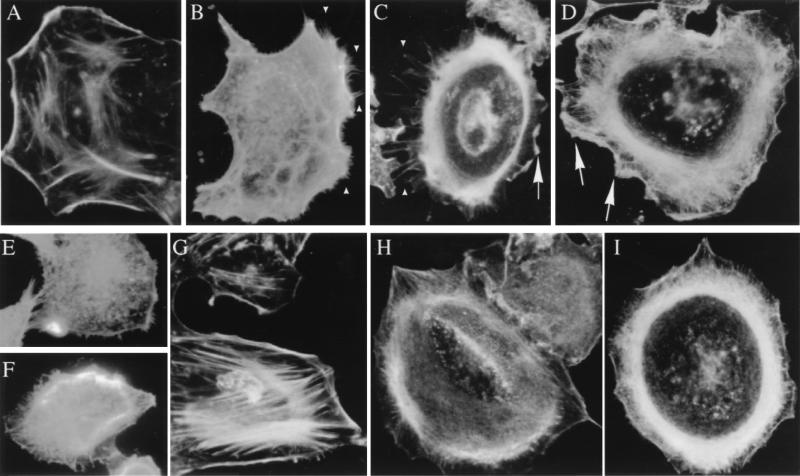
Previous studies have indicated that PI3K activation can promote reorganization of the actin cytoskeleton. Since Ad interaction with cells induces PI3K activation (30), we examined whether Ad interaction with adherent A549 epithelial cells also induced polymerization of cortical actin filaments. Untreated A549 cells exhibited strong peripheral F-actin staining along the cell edge, indicative of cortical actin fibers, as well as stress fiber formation in a large portion of the cells (Fig. 1A and G). However, the addition of Ad caused a rapid polymerization of actin within the cell, and this was associated with the extension of hair-like filopodia within 10 min (Fig. 1B and C). These processes were accompanied by cellular ruffling, with the lamellipodia eventually filling the regions between the filopodia (Fig. 1C) resulting in overall advancement of the leading edge of the membrane away from the perinuclear actin belt. After 25 min, few filopodia were still visible (Fig. 1D). These results mimicked those which occur after the addition of EGF, a mitogenic growth factor which facilitates actin polymerization through the action of the small GTPases CDC42 (initial filopodial extension [Fig. 1E]) and rac (lamellipodia advance [Fig. 1F]) (23). Interestingly, these downstream effects of the EGF receptor require PI3K, the heterodimeric lipid kinase previously implicated in Ad entry. Treatment of A549 cells with wortmannin, a selective inhibitor of PI3K activity, virtually abrogated filopodial extension in response to Ad (Fig. 1H) or EGF, although it did not inhibit actin assembly per se (Fig. 1I). Altogether, these results demonstrated that Ad interaction with cells could modulate actin assembly downstream of PI3K. They also suggested that Ad interactions with cells involve the small GTPases Rac and CDC42.
FIG. 1.
Ad interaction with cells induces morphologic changes and reorganization of the actin cytoskeleton. Serum-starved A549 epithelial cells were incubated for various times with different ligands at 37°C, permeabilized, and stained for polymerized actin with rhodamine-labeled phalloidin. (A and G) Unstimulated control cells. (B and C) Cells incubated at 37°C for 5 to 10 min with 100 Ad2 particles/cell. (D) Cells incubated with Ad2 for 25 min at 37°C. (E and F) Cells incubated with 100 ng of EGF/ml for 5 min at 37°C. (H and I) Cells treated with 100 nM wortmannin followed by incubation with 100 Ad2 particles/cell for 10 min. Arrowheads indicate sites of membrane filopodia, while the arrows indicate sites of lamellipodia.
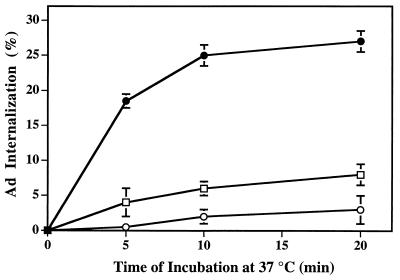
In further studies, we asked whether an intact actin cytoskeleton was required for virus entry into cells. As shown in Fig. 2, treatment of cells with cytochalasin D, a fungal metabolite which disrupts cortical actin filaments, caused dose-dependent inhibition of Ad2 internalization. Treatment of cells with cytochalasin D did not impair Ad2 activation of PI3K (data not shown), suggesting that virus-induced signaling events precede actin polymerization. Together, these studies indicate that virus-induced activation of PI3K results in the assembly of actin filaments, an event required for virus internalization.
FIG. 2.
Disruption of cortical actin filaments with cytochalasin D inhibits Ad internalization. A549 cells were incubated in the presence of 0.5 μM (squares) or 2 μM (open circles) cytochalasin D at 37°C for 90 min or in medium containing 0.1% dimethyl sulfoxide (closed circles). Internalization of 125I-labeled Ad2 particles was subsequently measured by resistance to trypsin treatment as described in Materials and Methods.
Rho GTPases selectively promote Ad internalization.
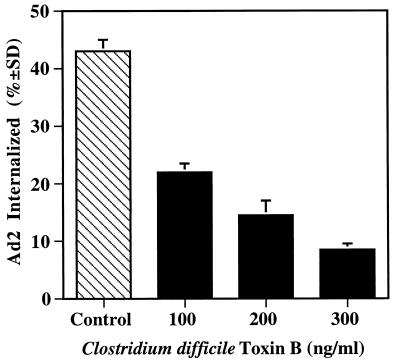
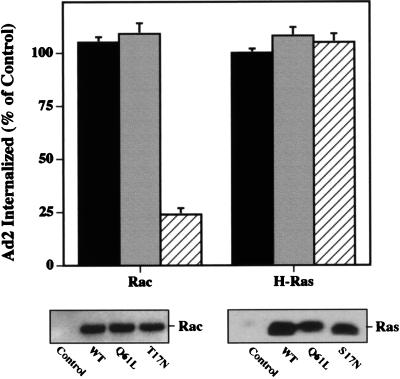
The Rho and Ras families of small GTP binding proteins are each known to be involved in PI3K activation, and direct interactions between these proteins and PI3K have been previously reported (6, 42, 48, 56). To further define the role of these molecules in Ad2 internalization, we treated cells with C. difficile toxin B. This agent selectively inactivates the Rho subfamily GTPases by glucosylating the threonine at position 37 in Rho or at the corresponding position in Rac and CDC42 (25). Treatment of cells with toxin B caused a dose-dependent inhibition of Ad2 internalization (Fig. 3). The inhibitory concentrations of this agent also abrogated EGF- and Ad2-induced actin polymerization as assessed by incorporation of rhodamine-labeled phalloidin (data not shown). These findings strongly suggested that Rho rather than Ras family GTPases regulate Ad2 internalization via assembly of the actin cytoskeleton. To substantiate these findings, we transiently transfected SW480 cells with a cDNA plasmid encoding wild-type, constitutively active, or dominant-negative point mutants of Rac or H-Ras GTPases. Transfected cells expressed similar levels of the recombinant proteins as ascertained by Western blotting; however, only the dominant-negative Rac (i.e., not the wild-type or constitutively active form of Rac or Ras) inhibited Ad2 internalization (Fig. 4). Similar results were obtained with a dominant-negative CDC42 (data not shown). In contrast, expression of a dominant-negative H-Ras failed to alter virus uptake (Fig. 4). These findings indicate further that Rho family GTPases selectively mediate Ad2 internalization.
FIG. 3.
Inhibition of Ad internalization by C. difficile toxin B. A549 cells were incubated for 5 h in serum-free medium containing various amounts of recombinant toxin B, a specific inhibitor of Rho family GTPases, prior to measuring Ad internalization by resistance to trypsin treatment at 10 min postwarming.
FIG. 4.
Ad internalization is specifically inhibited by overexpression of dominant-negative Rac mutant protein. SW480 cells were transiently transfected with a control plasmid lacking a transgene or with plasmids encoding a wild-type (solid bars), a constitutively active (Q61L) (shaded bars), or a dominant-negative (17N) (cross-hatched bars) form of Rac or H-Ras. Ad internalization was assayed 45 h posttransfection. The lower panels show Western blots of transfected cell lysates probed for Rac or Ras expression. WT, wild type.
Ad internalization into wortmannin-treated cells is restored by expression of constitutively active Rac.
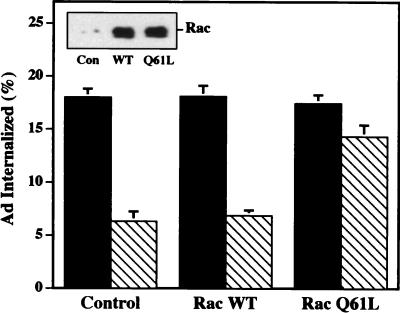
Although Rho GTPases are known to modulate PI3K activity (6, 42, 48, 56), it is uncertain whether Rho GTPases act upstream or downstream of PI3K activation to promote virus uptake. To address this question, we transfected cells with a plasmid encoding wild-type Rac or the constitutively active form of Rac (Q61L) or a control plasmid lacking a cDNA. Forty hours later, transfected cells were incubated in the presence or absence of the PI3K inhibitor, wortmannin, and then assayed for virus internalization. As shown in Fig. 5, each of the transfected cells supported similar levels of Ad internalization in the absence of wortmannin; however, only cells expressing the constitutively active Rac protein (Q61L) partially restored Ad internalization in wortmannin treated cells. These findings suggest that Rac acts downstream of PI3K to facilitate Ad internalization.
FIG. 5.
Overexpression of a constitutively active Rac protein restores Ad internalization into wortmannin-treated cells. SW480 cells were transfected with a control plasmid or a plasmid encoding a wild-type (WT) or constitutively active (Q61L) Rac. Forty-eight hours posttransfection, the cells were incubated in the presence (cross-hatched bars) or absence (solid bars) of wortmannin for 10 min at 4°C prior to measuring Ad internalization. The inset shows a Western blot of transfected cell lysates probed for Rac expression.
Expression of effector domain mutants of Rac/CDC42 define the pathway of Ad internalization.
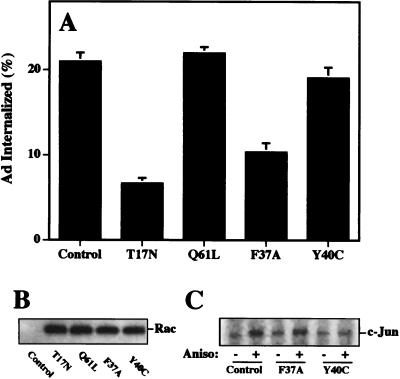
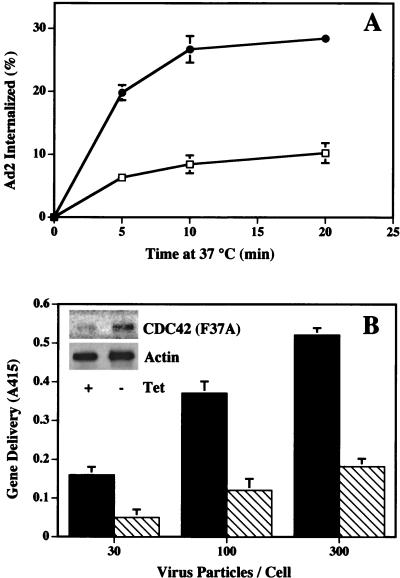
In addition to their ability to regulate assembly of the actin cytoskeleton, Rac and CDC42 also promote activation of the Jun N-terminal (JNK) MAP kinase pathway (13, 34). While the downstream targets of Rac are not well defined, mutational analysis of Rac and CDC42 has partially delineated the pathways controlling actin assembly and MAP kinase signaling (24, 28). For example, a point mutation in Rac or CDC42 (F37A) blocks actin-associated lamellipodia formation without altering JNK/MAP kinase activation. In contrast, another point mutation (Y40C) selectively inhibits JNK/MAP kinase activity (24, 28). We therefore transiently transfected cells with plasmids encoding these mutant Rac proteins to identify the signaling pathway of Ad2 internalization. Transient expression of either wild-type Rac or the constitutively active (Q61L) protein did not significantly alter virus uptake (Fig. 6A). In contrast, expression of a dominant-negative Rac (S17N) or the cytoskeleton-associated Rac mutant (F37A) significantly decreased Ad internalization. Importantly, expression of the Y40C mutant did not alter virus internalization (Fig. 6A) even though it downregulated JNK/MAP kinase activation by anisomycin (Fig. 6C). Consistent with these findings, Ad2 interaction with host cells also did not activate the JNK/MAP kinase pathway as measured by phosphorylation of c-Jun (see Fig. 8B). These findings indicate that reorganization of the actin cytoskeleton rather than activation of the JNK/MAP kinase pathway plays the major role in Ad internalization. To further confirm these findings, we examined Ad internalization and gene delivery into HeLa cells stably transfected with the CDC42 F37A mutant under control of the tetracycline-inducible promoter (17). Expression of the F37A CDC42 protein by removal of tetracycline from the culture medium inhibited both virus internalization (Fig. 7A) and gene delivery (Fig. 7B). These findings provide further evidence that Ad2 internalization specifically requires the host cell actin cytoskeleton which is regulated by activation of Rac and/or CDC42 GTPases.
FIG. 6.
Expression of an effector domain mutant of Rac that impairs cytoskeletal function but not JNK/MAP kinase activation inhibits Ad internalization. (A) SW480 cells were transiently transfected with a control plasmid, a wild-type Rac, or Rac mutants as indicated. Ad internalization was measured 45 h posttransfection. (B) Western blot of transfected cell lysates with an anti-c-Myc antibody. (C) JNK activation by transfected cells. Aniso, anisomycin.
FIG. 8.
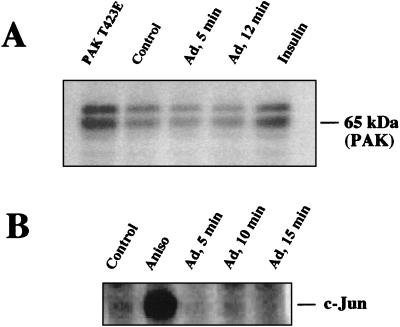
Ad internalization does not involve activation of p65PAK or JNK/MAP kinase. Serum-starved SW480 cell lysates derived from cells incubated with Ad were probed for p65PAK (A) or JNK activation (B) as described in Materials and Methods. Cells were treated with insulin or anisomycin (Aniso) or transfected with a dominant active PAK1 (T432E) as positive controls for kinase activation.
FIG. 7.
Expression of an effector domain mutant of CDC42 (Q61,F37A) inhibits Ad internalization and gene delivery. (A) A stably transfected cell line expressing CDC42 (Q61L,F37A) was assayed for its ability to support Ad internalization 40 h after the removal of tetracycline (open squares) or in the continued presence of tetracycline (filled circles). (B) Transfected cells cultured in the absence (cross-hatched bars) or presence (solid bars) of tetracycline were also assayed for susceptibility to Ad-mediated delivery of a reporter gene (β-galactosidase). The inset shows Western blot analysis of the CDC42 mutant protein and actin expressed in cells cultured in the presence or absence of tetracycline.
Analysis of PAK1 activation during Ad internalization.
A member of the p-21 serine-threonine kinase family, PAK1, contains a high-affinity binding site for the GTP-bound form of Rac and CDC42 (33). Binding of Rho GTPases to PAK1 results in autophosphorylation and activation of the kinase, and this can lead to reorganization of the actin cytoskeleton (14, 45). However, an absolute requirement for PAK1 for actin polymerization and alterations in membrane structure has not been firmly established in all cell types (28). To examine the role of PAK1 in Ad2 internalization, we assayed cell lysates derived from cells incubated with Ad particles or with insulin for PAK1 activity by using an in-gel kinase assay (15). p65/PAK1 activity was induced in cells treated with insulin or by overexpressing a constitutively active PAK1 protein (T423E) (Fig. 8A). In contrast, Ad interaction with cells failed to stimulate PAK1 activation. These studies suggest that activation of PAK1, a known downstream effector of Rac and CDC42, is not a prerequisite for Ad internalization.
DISCUSSION
While almost all nonenveloped viruses characterized to date exploit integrins or other cell adhesion molecules (52), it is not clear how these receptors promote virus internalization and/or membrane penetration. Our current studies demonstrate that Ad interaction with cells induces polymerization of cortical actin filaments (Fig. 1) and that an intact actin cytoskeleton is required for virus entry into cells (Fig. 2). The precise role that actin filaments play in Ad internalization is not yet clear; however, polymerized actin could provide the mechanical force that helps sever coated pit vesicles from the cell plasma membrane (43). A growing number of studies indicates not only that the actin cytoskeleton is required for cell motility and cell shape (8) but also that it plays a significant role in receptor-mediated endocytosis. The actin cytoskeleton is required for receptor-mediated endocytosis in yeast (35, 36) and has recently been reported to facilitate ligand internalization in mammalian cells (29). Earlier morphologic studies had also suggested that actin filaments promote entry of certain enveloped viruses into polarized epithelial cells (18) as well as Ad uptake (40).
Integrin-mediated actin assembly may also provide a stable platform for the intracellular assembly of cell-signaling complexes. Clustering of cell integrins by extracellular matrix proteins leads to activation of signaling pathways that influence cell adhesion, cell spreading, and cell motility (11). The cytoplasmic domains of integrins have been shown to recruit several different cytoskeletal proteins (39) as well as signal transduction molecules responsible for transmitting the signals that induce specific alterations in cell morphology and function (11). Our previous studies have indicated that Ad endocytosis via ligation of αv integrins requires a cell-signaling pathway involving PI3K activation (30). Previous studies have demonstrated that the Rho family of small GTP-binding proteins serve as regulators of PI3K activation, and direct interactions between PI3K and Rho GTPases have been reported (6, 42, 48, 56). Our current studies indicate that Rho GTPases also regulate Ad internalization. Virus uptake was blocked by pretreatment of cells with a specific inhibitor of Rho GTPases, C. difficile toxin B, and by transient expression of dominant-negative forms of Rho GTPases. Since expression of constitutively active Rac was capable of restoring Ad internalization in wortmannin-treated cells (Fig. 5), we deduce that the Rho GTPases function downstream of PI3K. Importantly, expression of an effector domain mutant of Rac/CDC42 that prevents cytoskeletal reorganization also blocked virus uptake and gene delivery, while mutants which lack this capacity did not. These studies indicate that the principal role of Rho GTPases in Ad cell entry is assembly of the actin cytoskeleton. Previous studies have also indicated that Rho GTPases are associated with cytoskeletal function, including formation of focal adhesion complexes following integrin clustering (11, 34), cell invasion by certain bacteria (10), and the morphologic changes in the actin-rich membrane protrusions known as lamellipodia and filopodia (37, 41).
A major gap in the understanding of Rho family signaling pathways is the identity of downstream effector molecules that carry out specific host cell functions (7, 19). A previously identified target for Rac and CDC42 is a member of the p21-serine/threonine kinases, PAK1. Although PAK1 has recently been shown to be localized to pinocytic vesicles and cortical actin filaments in fibroblasts (14), our current studies indicate that this kinase is not activated during virus entry. Further studies are needed to determine whether other related members of this kinase family (44) or a distinct signaling molecule(s) acts downstream of Rac/CDC42 to promote Ad internalization. In this regard, Rho kinase, a previously identified effector of Rho GTPases (1, 27), has recently been reported to associate with clathrin-coated vesicles (53). However, this signaling molecule has not yet been linked to ligand internalization.
Our current findings provide further knowledge of the signaling pathway involved in Ad endocytosis. A better understanding of the mechanisms involved in Ad entry may help improve the transduction of cells lacking the appropriate cell receptors (54). Augmentation of integrin signaling processes to promote virus internalization may also allow lower doses of viral vectors to be used in certain clinical settings to reduce the host immune response to either primary or repeated administration of Ad vectors (12).
ACKNOWLEDGMENTS
We express our gratitude to Joan Fernandez and Swati Lad Brown for expert technical assistance and to Luraynne Sanders for assistance in the PAK activity assay. We also thank Alan Hall for plasmids encoding Rho GTPase effector domain mutants, Fred Hofmann and Klaus Aktories for their generous gift of C. difficile toxin B, and Catalina Hope and Joan Gausepohl for preparation of the manuscript.
This work was supported by NIH grants EY11431, HL54352, and GM44428.
REFERENCES
- 1.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 2.Bagrodia S, Derijard B, Davis R J, Cerione R A. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- 3.Benmerah A, Lamaze C, Begue B, Schmid S L, Dautry-Varsat A, Cerf-Bensussan N. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J Cell Biol. 1998;140:1055–1062. doi: 10.1083/jcb.140.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 5.Blau H, Khavari P. Gene therapy: progress, problems, prospects. Nat Med. 1997;3:612–613. doi: 10.1038/nm0697-612. [DOI] [PubMed] [Google Scholar]
- 6.Bokoch G M, Vlahos C J, Wang Y, Knaus U G, Traynor-Kaplan A E. Rac GTPase interacts specifically with phosphatidylinositol 3-kinase. Biochem J. 1996;315:775–779. doi: 10.1042/bj3150775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burbelo P D, Drechsel D, Hall D. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J Biol Chem. 1995;270:29071–29074. doi: 10.1074/jbc.270.49.29071. [DOI] [PubMed] [Google Scholar]
- 8.Chant J, Stowers L. GTPase cascades choreographing cellular behavior: movement, morphogenesis and more. Cell. 1995;81:1–4. doi: 10.1016/0092-8674(95)90363-1. [DOI] [PubMed] [Google Scholar]
- 9.Chardonnet Y, Dales S. Early events in the interaction of adenoviruses with HeLa cells. I. Penetration of type 5 and intracellular release of the DNA genome. Virology. 1970;40:462–477. doi: 10.1016/0042-6822(70)90189-3. [DOI] [PubMed] [Google Scholar]
- 10.Chen L-M, Hobbie S, Galan J E. Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science. 1996;274:2115–2118. doi: 10.1126/science.274.5295.2115. [DOI] [PubMed] [Google Scholar]
- 11.Clark E A, Brugge J S. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 12.Connelly S, Smith T A G, Dhir G, Gardner J M, Mehaffey M G, Zaret K S, McClelland A, Kaleko M. In vivo gene delivery and expression of physiological levels of functional human factor VIII in mice. Hum Gene Ther. 1995;6:185–193. doi: 10.1089/hum.1995.6.2-185. [DOI] [PubMed] [Google Scholar]
- 13.Coso O A, Chiariello M, Yu J-C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 14.Dharmawhardane S, Sanders L C, Martin S S, Daniels R H, Bokoch G M. Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. J Cell Biol. 1997;138:1265–1278. doi: 10.1083/jcb.138.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding J, Knaus U G, Lian J P, Bokoch G M, Badwey J A. The renaturable 69- and 63-kDa protein kinases that undergo rapid activation in chemoattractant-stimulated guinea pig neutrophils are p21-activated kinases. J Biol Chem. 1996;271:24869–24873. doi: 10.1074/jbc.271.40.24869. [DOI] [PubMed] [Google Scholar]
- 16.Everitt E, Meador S A, Levine A S. Synthesis and processing of the precursor to the major core protein of adenovirus type 2. J Virol. 1977;21:199–214. doi: 10.1128/jvi.21.1.199-214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottlieb T A, Ivanov I E, Adesnik M, Sabatini D D. Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J Cell Biol. 1993;120:695–709. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 20.Hordijk P L, Klooster J P, van der Kammen R A, Michiels F, Oomen L C, Collard J G. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science. 1998;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- 21.Horwitz M S. Adenovirus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2149–2171. [Google Scholar]
- 22.Hotchin N, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular Rho/Rac GTPases. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotchin N A, Hall A. Regulation of the actin cytoskeleton, integrins and cell growth by the Rho family of small GTPases. Cancer Surv. 1996;27:311–322. [PubMed] [Google Scholar]
- 24.Joneson T, McDonough M, Bar-Sagi D, Aelst L. RAC regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science. 1998;274:1374–1378. doi: 10.1126/science.274.5291.1374. [DOI] [PubMed] [Google Scholar]
- 25.Just I, Selzer J, Wilm M, von Eichel-Streiber C, Mann M, Aktories K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 26.Karlund J K, Guilherme A, Holik J J, Virbasius J V, Chawla A, Czech M P. Signaling by phosphoinositide-3,4,5,-trisphosphate through proteins containing pleckstrin and Sec7 homology domains. Science. 1997;275:1927–1930. doi: 10.1126/science.275.5308.1927. [DOI] [PubMed] [Google Scholar]
- 27.Kimura K, Fukata Y, Matsuoka Y, Bennett V, Matsuura Y, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of the association of adducin with actin filaments by Rho-associated kinase (Rho-kinase) and myosin phosphatase. J Biol Chem. 1998;273:5542–5548. doi: 10.1074/jbc.273.10.5542. [DOI] [PubMed] [Google Scholar]
- 28.Lamarche N, Tapon N, Stowers L, Burbelo P D, Aspenström P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65pak and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- 29.Lamaze C, Fujimoto L M, Yin H L, Schmid S L. The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J Biol Chem. 1997;272:20332–20335. doi: 10.1074/jbc.272.33.20332. [DOI] [PubMed] [Google Scholar]
- 30.Li E, Stupack D, Klemke R, Cheresh D, Nemerow G. Adenovirus endocytosis via αv integrins requires phosphoinositide-3-OH-kinase. J Virol. 1998;72:2055–2061. doi: 10.1128/jvi.72.3.2055-2061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo L, Hensch T K, Ackerman L, Barbel S, Jan L Y, Jan Y N. Differential effects of the Rac GTPase on purkinje cell axons and dendritic trunks and spines. Nature. 1996;379:837–840. doi: 10.1038/379837a0. [DOI] [PubMed] [Google Scholar]
- 32.Luo L, Liao J, Jan L Y, Jan Y N. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- 33.Manser E, Leung T, Salihuddin H, Zhao Z, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 34.Minden A, Lin A, Claret F X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 35.Moreau V, Madania A, Martin R P, Winsor B. The Saccharomyces cerevisiae actin-related protein Arp2 is involved in the actin cytoskeleton. J Cell Biol. 1996;134:117–132. doi: 10.1083/jcb.134.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulholland J, Preuss D, Moon A, Wong A, Drubin D, Botstein D. Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J Cell Biol. 1994;125:381–391. doi: 10.1083/jcb.125.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nobes C D, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 38.Nobes C D, Hawkins P, Stephens L, Hall A. Activation of the small GTP-binding proteins Rho and Rac by growth factor receptors. J Cell Science. 1995;108:225–233. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- 39.Parsons J T. Integrin-mediated signaling: regulation by protein tyrosine kinases and small GTP-binding proteins. Curr Opin Cell Biol. 1996;8:146–152. doi: 10.1016/s0955-0674(96)80059-7. [DOI] [PubMed] [Google Scholar]
- 40.Patterson S, Russell W C. Ultrastructural and immunofluorescence studies of early events in adenovirus-HeLa cell interactions. J Gen Virol. 1983;64:1091–1099. doi: 10.1099/0022-1317-64-5-1091. [DOI] [PubMed] [Google Scholar]
- 41.Ridley A J, Hall A. The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Viciana P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M J, Waterfield M D, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 43.Roos J, Kelly R B. Is dynamin really a ‘pinchase’? Trends Cell Biol. 1997;7:257–259. doi: 10.1016/S0962-8924(97)01068-4. [DOI] [PubMed] [Google Scholar]
- 44.Sells M A, Chernoff J. Emerging from the Pak: the p21-activated protein kinase family. Trends Cell Biol. 1997;7:162–167. doi: 10.1016/S0962-8924(97)01003-9. [DOI] [PubMed] [Google Scholar]
- 45.Sells M A, Knaus U G, Bagrodia S, Ambrose D M, Bokoch G M, Chernoff J. Human p21-activated kinase (PAK1) regulates actin organization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- 46.Stratford-Perricaudet L D, Makeh I, Perricaudet M, Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest. 1992;90:626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- 48.Tolias K F, Cantley L, Carpenter C L. Rho family GTPases bind to phosphoinositide kinases. J Biol Chem. 1995;270:17656–17659. doi: 10.1074/jbc.270.30.17656. [DOI] [PubMed] [Google Scholar]
- 49.Wang K, Huang S, Kapoor-Munshi A, Nemerow G. Adenovirus internalization and infection require dynamin. J Virol. 1998;72:3455–3458. doi: 10.1128/jvi.72.4.3455-3458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 51.Wilson J M. Adenoviruses as gene-delivery vehicles. N Engl J Med. 1996;334:1185–1187. doi: 10.1056/NEJM199605023341809. [DOI] [PubMed] [Google Scholar]
- 52.Wimmer E, editor. Cellular receptors for animal viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. Introduction and overview; pp. 1–13. [Google Scholar]
- 53.Witke W, Podtelejnikov A V, DiNardo A, Sutherland J D, Gurniak C B, Dotti C, Mann M. In mouse brain profilin I and profilin II associate with regulators of the endocytic pathway and actin assembly. EMBO J. 1998;17:967–976. doi: 10.1093/emboj/17.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zabner J, Freimuth P, Puga A, Fabrega A, Welsh M J. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J Clin Invest. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang S, Han J, Sells M A, Chernoff J, Knaus U G, Ulevitch R J, Bokoch G M. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J Biol Chem. 1995;270:23934–23936. doi: 10.1074/jbc.270.41.23934. [DOI] [PubMed] [Google Scholar]
- 56.Zheng Y, Bagrodia S, Cerione R A. Activation of phosphoinositide 3-kinase activity by Cdc42Hs binding to p85. J Biol Chem. 1994;269:18727–18730. [PubMed] [Google Scholar]
- 57.Zigmond S H. Signal transduction and actin filament organization. Curr Biol. 1996;8:66–73. doi: 10.1016/s0955-0674(96)80050-0. [DOI] [PubMed] [Google Scholar]