Abstract
Second-line chemotherapy in patients with gemcitabine-refractory advanced pancreatic cancer has shown disappointing survival outcomes due to rapid disease progression and performance deterioration. The aim of this phase II trial was to evaluate the efficacy and safety of adoptive immunotherapy using ex vivo-expanded, cytokine-induced killer (CIK) cells in gemcitabine-refractory advanced pancreatic cancer. Patients with advanced pancreatic cancer who showed disease progression during gemcitabine-based chemotherapy were enrolled in this study. For generation of CIK cells, peripheral blood samples were collected from each patient and cultured with anti-CD3 monoclonal antibody and IL-2. Patients received CIK cells intravenously 10 times, every week for 5 weeks and then every other week for 10 weeks. Twenty patients were enrolled between November 2009 and September 2010. The disease control rate was 25 % (4/16 patients). The median progression-free survival (PFS) was 11.0 weeks (95 % CI 8.8–13.2), and the median overall survival (OS) was 26.6 weeks (95 % CI 8.6–44.6). Grade 3 toxicities included general weakness in two patients and thrombocytopenia in one patient. Grade 4 hematologic or non-hematologic toxicity was not observed. Patients showed improvement in pancreatic pain, gastrointestinal distress, jaundice, body image alterations, altered bowel habits, health satisfaction, and sexuality when assessing quality of life (QoL). Adoptive immunotherapy using CIK cells showed comparable PFS and OS to survival data of previous trials that assessed conventional chemotherapies while maintaining tolerability and showing encouraging results in terms of patient QoL in gemcitabine-refractory advanced pancreatic cancer (clinicalTrials.gov number NCT00965718).
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1566-3) contains supplementary material, which is available to authorized users.
Keywords: Adoptive immunotherapy, Cytokine-induced killer cells, Gemcitabine refractory, Pancreatic cancer
Introduction
Pancreatic cancer is one of the leading causes of cancer-related deaths in westernized countries. In Korea, pancreatic cancer accounts for the ninth highest cancer incidence and the fourth most common cause of cancer-related death [1]. Metastatic disease accounts for 60 % of all pancreatic cancer at the time of diagnosis and shows a median survival of 3–6 months [2]. Systemic chemotherapy plays a pivotal role in treating patients with pancreatic cancer. However, we still have limited chemotherapy regimens for pancreatic cancer. Currently, in metastatic pancreatic cancer, gemcitabine-based chemotherapy has been considered a standard therapy [3]; recently, combined therapy comprising gemcitabine and targeted agents has been evaluated as another treatment option [4–6]. Even though pancreatic cancer patients are administered with conventional chemotherapy regimens, one-half of patients experience progression of the cancer only after about 4 months.
Second-line chemotherapy in patients with gemcitabine-refractory advanced pancreatic cancer has shown disappointing survival outcomes. In a randomized phase III trial, patients treated with oxaliplatin, folinic acid, and 5-FU as a second-line chemotherapy showed a prolonged median second-line survival of 4.82 months compared with 2.30 months with supportive care alone [7]. However, no agents have emerged as a widely accepted, standard second-line treatment after gemcitabine failure in patients with advanced pancreatic cancer. In addition to the efficacy of second-line chemotherapy, tolerability is one of the most important factors in determining whether second-line chemotherapy is possible for patients who experienced progression after first-line chemotherapy, because most pancreatic cancer patients have poor performance status to withstand conventional chemotherapeutic agents.
Recently, immune cell-based cancer therapy has been attempted as an alternative treatment option for cancer therapy to eliminate cancer cells through the transfer of ex vivo-expanded active immune cells. Among various immune cell types studied as potential candidates for effective immunotherapy, cytokine-induced killer (CIK) cells, which are heterogeneous cell populations containing >20 % of CD3+ CD56+ cells, demonstrated potent cytolytic activity in a major histocompatibility complex (MHC)-unrestricted manner [8]. CIK cells have been evaluated for their antitumor effects in various cancers, including hepatocellular carcinoma (HCC), renal cell carcinoma, non-small cell lung cancer, gastric cancer, and colorectal cancer. Data with CIK cells have demonstrated convincing evidence of the feasibility and very high safety profile in several clinical trials [8–10]. Tolerability of immunotherapy with CIK cells is expected to be advantageous, especially in pancreatic cancer patients, most of whom cannot maintain a treatment regimen due to deteriorating performance status.
The aim of this single-center phase II trial was to evaluate the efficacy and safety of adoptive immunotherapy using ex vivo-expanded CIK cells in gemcitabine-refractory advanced pancreatic cancer.
Methods
Patient eligibility
Patients were required to meet the following inclusion criteria: older than 18 years and younger than or equal to 75 years; Eastern Cooperative Oncology Group performance status (ECOG-PS) [11] <2; cytologically or histologically proven metastatic adenocarcinoma originating from the pancreas that progressed after gemcitabine-based chemotherapy as a first-line treatment; adequate bone marrow (white blood cell count ≥3,500/µl, absolute neutrophil cell count ≥1,500/µl, platelet count ≥100,000/µl); adequate hepatic function (total bilirubin ≤2 × the upper limit of normal, serum alanine transaminase ≤3 × the upper limit of normal); adequate renal function serum creatinine ≤1.5 mg/dl); and adequate cardiopulmonary function. Patients were excluded if they had a concurrent malignancy other than pancreatic cancer; a serious, uncontrollable medical condition; or a psychiatric disorder. The study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. Written informed consent was obtained from each participant or responsible family member after possible complications of the diagnostic procedures had been fully explained. This study was approved by the Institutional Review Board of Yonsei University College of Medicine.
Generation of CIK and treatment plan
CIK cells were generated at central facility (GREENCROSSCELL Corp. Korea) as previously described [12–14]. Peripheral blood (>60 ml) was collected from each patient at least 2 weeks before administration. Mononuclear cells were separated and cultured for 2–3 weeks with interleukin-2 (Proleukin, Norvatis, Switzerland) and immobilized monoclonal antibody to CD3 (Orthoclone OKT3, Janssen, Belgium) at 37 °C. Cultivation was continued, preserving the cells as the source of the second to tenth infusions [12, 15, 16].
CIK cells were injected into all patients enrolled in this study for 1 h every week for 5 weeks and then every other week for 10 weeks; CIK cells were administered as an outpatient procedure. No other cancer therapy was administered during these treatments. Treatment cycles were repeated until either evidence of progressive disease (PD), significant clinical deterioration, or withdrawal of patient consent. Patients completing the full schedule of adoptive immunotherapy using CIK cells continued to receive maintenance therapy using another conventional chemotherapy regimen.
Phenotype analysis
Cellular phenotype was evaluated with fluorescence-activated cell sorting (FACS) analysis. Fresh peripheral blood mononuclear cells (PBMCs) and expanded CIK cells were analyzed with the appropriate monoclonal antibodies (CD3-FITC, CD8-PE, CD56-PE, CD14-PE, CD20-PE, Beckman Coulter, Fullerton, USA). FACS analyses were performed on a FC 500 Flow Cytometry System (Beckman Coulter, Fullerton, USA).
Assessment of data
Pretreatment evaluations included a complete medical history, physical examination, assessment of performance status, laboratory tests comprising complete blood count and differential, blood chemistry, carbohydrate antigen (CA) 19-9, and carcinoembryonic antigen. Pretreatment evaluations were performed 2 weeks before the initiation of adoptive immunotherapy using CIK cells. During the treatment, laboratory tests were performed every month. Tumor responses were assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) [17] based on high-resolution CT scans every 8 weeks. Quality of life (QoL) was assessed using the Quality of Life Questionnaire Core 30 (QLQ-C30) [18] and QoL questionnaire to supplement the QLQ-C30 in patients with pancreatic cancer (QLQ-PAN26 questionnaire) [19] developed by the European Organization for Research and Treatment of Cancer (EORTC), and changes in body weight and dose of pain control drugs were checked every 2 weeks. QoL changes between baseline and the last visit were analyzed, considering that the participation period varied per patients. QLQ-C30 constitutes a functional scale (physical, role, emotional, cognitive, and social functioning), symptom scores scale (fatigue, pain, nausea/vomiting, appetite loss, sleep disturbance, dyspnea, constipation, diarrhea, and financial impact), and global QoL scale [18]. QLQ-PAN26 includes 26 items related to disease symptoms, treatment side effects, and emotional issues specific to pancreatic cancer [19]. With the scores of all scales ranging from 0 to 100, a higher score indicates a better functional status as well as a worse symptom. Adverse events were recorded according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 3.0 [20].
Data analysis and statistical considerations
The primary end point was disease control rate (DCR), and the secondary end points were overall survival (OS), progression-free survival (PFS), changes in tumor markers and QoL, and evaluation of safety. OS was calculated from the date of enrollment until death from any cause. PFS was calculated from the initiation of treatment either until disease progression was confirmed on image study or death from any cause.
Twenty patients were enrolled, after accepting a type I error of 5 % and a power of 80 %, to test the null hypothesis based on 23.1 % DCR for pemetrexed for advanced pancreatic cancer [21] versus the alternative hypothesis of 58 % for gemcitabine/oxaliplatin chemotherapy [22], considering a 20 % dropout rate.
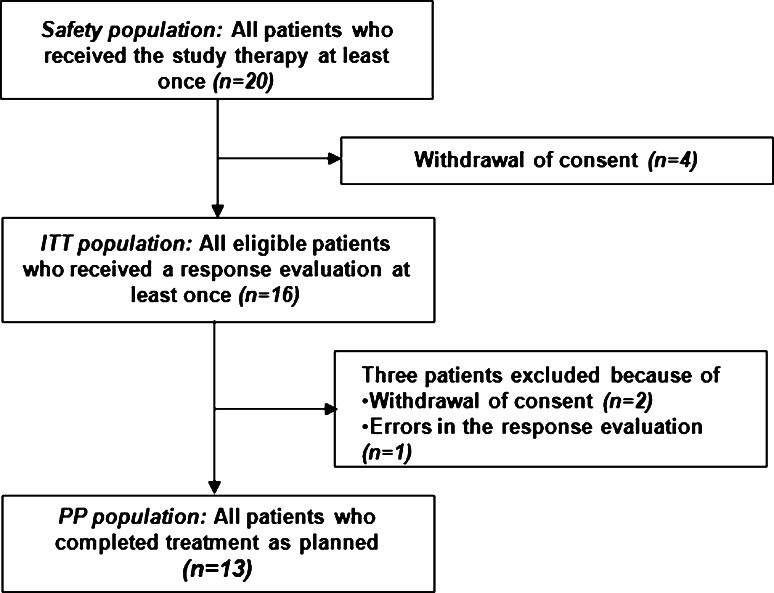
All patients who received the study treatment at least once were included in the safety population for toxicity analysis. Patients who underwent response evaluation at least once were included in the intention-to-treat (ITT) population, and patients who completed treatment as planned were included in the per-protocol (PP) population (Fig. 1). All efficacy analyses were based on the ITT analysis. PFS and OS were estimated using Kaplan–Meier methods with 95 % confidence intervals (CIs). When comparing data at baseline with the final observation point, the paired t test was used for normally distributed data and the Wilcoxon signed rank test was used for non-normally distributed data. All analyses were performed with the SPSS statistical program (version 12.0; SPSS Inc., Cary, NC). A P value <0.05 was considered statistically significant.
Fig. 1.
Diagram of patient flow
Results
Baseline characteristics of patients and tumors
Twenty patients were enrolled between November 2009 and September 2010. The median age at the time of diagnosis was 59.2 years, with a range from 41 to 69 years. Males made up 60 % of the sample, and all patients had ECOG-PS < 2 at the start of the study treatment. All patients were treated with gemcitabine-based chemotherapy as a first-line therapy and showed disease progression before participating in this trial. The median duration since diagnosis at the start of study treatment was 9.2 months (range 3.8–94.6), and the median period of prior chemotherapy was 5.2 months (range 2.0–13.9). All enrolled patients had stage IV disease with accompanying distant metastasis. The liver and lungs were the most common metastatic sites: 45 % of patients had metastasis to the liver, 30 % of patients had metastasis to the lungs, 25 % of patients had metastasis to the lymph node, 5 % of patients had metastasis to the peritoneum, and 5 % of patients had metastasis to the kidney (Table 1).
Table 1.
Baseline characteristics
| Characteristic | N = 20 | % |
|---|---|---|
| Age (years) | ||
| Median | 59.5 (41–69) | |
| 40–49 | 1 | 5 |
| 50–59 | 9 | 45 |
| 60–69 | 10 | 50 |
| Sex | ||
| Male | 12 | 60 |
| Female | 8 | 40 |
| ECOG-PS | ||
| 0 | 12 | 60 |
| 1 | 7 | 35 |
| 2 | 1 | 5 |
| Duration since diagnosis (months) | ||
| 9.2 (3.8–94.6) | ||
| Period of prior chemotherapy (months) | ||
| 5.2 (2.0–13.9) | ||
| Site of metastasis | ||
| Liver | 9 | 45 |
| Lung | 6 | 30 |
| Lymph node | 5 | 25 |
| Peritoneum | 1 | 5 |
| Kidney | 1 | 5 |
| Prior treatment | ||
| Operation | ||
| CCRTx | ||
| CTx | ||
ECOG-PS Eastern Cooperative Oncology Group performance status, CCRTx concurrent chemoradiotherapy, CTx chemotherapy
Tumor responses and survival
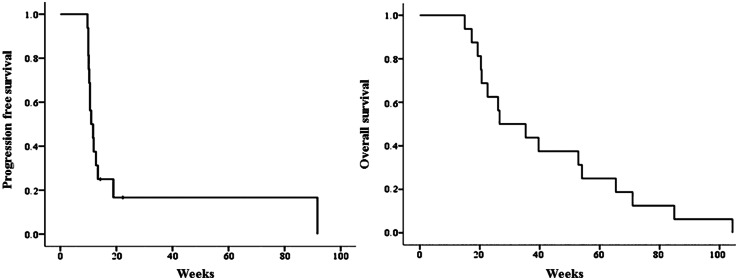
Of the 16 patients in the ITT population, stable disease (SD) was confirmed in 4 patients and PD was confirmed in 12 patients. By ITT analysis, DCR was 25 %. During the follow-up period, all 16 patients died. Figure 2 shows the Kaplan–Meier curve for PFS and OS of all patients. The median PFS was 11.0 weeks (95 % CI 8.8–13.2 weeks), and the median OS was 26.6 weeks (95 % CI 8.6–44.6 weeks). The survival rate was 60.0 % at 6 months from the date of enrollment.
Fig. 2.
Progression-free survival and overall survival. Kaplan–Meier analysis of overall survival after initiation of adoptive immunotherapy using ex vivo-expanded cytokine-induced killer cells. The median progression-free survival was 11.4 weeks, and the median overall survival was 26.6 weeks
Characteristic of CIK cells and delivery of drugs
Table 2 shows the absolute number and composition of total cells after 2 weeks of culture. A total of 127 times of ex vivo-expanded CIK cell therapy were delivered. Four patients completed 10 times of ex vivo-expanded CIK cell therapy as planned. The CIK cell agent contained a total of 6.73 (±2.32) × 109 cells including 1.67 (±1.14) × 109 of CIK cells in 200 ml of fluid; CD3+, CD8+, and CD56+ cells were 98.7, 86.2, and 24.1 %, respectively, each FACS analysis. The mean viability of the cells was 97.8 %.
Table 2.
Phenotype of cells per patient
| Patient | Total infusion/patient | Average cell counts/infusion (×109) | Viability (%) | Phenotype of cell product | Best response | Survival time | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|
| CD3 (%) | CD8 (%) | CD56 (%) | PFS (weeks) | OS (weeks) | ||||||
| 1 | 8 | 69 | 97.2 | 97.7 | 93.8 | 13.9 | PD | 12.7 | 22.6 | Dead |
| 2 | 7 | 44.5 | 98 | 99.3 | 83.2 | 28.7 | PD | 11.9 | 20.6 | Dead |
| 3 | 7 | 58.5 | 98.2 | 99.4 | 88.2 | 20.1 | PD | 10.6 | 54.1 | Dead |
| 4 | 10 | 95.8 | 96.8 | 96.8 | 92.3 | 47.2 | SD | 18.9 | 84.9 | Dead |
| 5 | 7 | 55.2 | 97.6 | 98.5 | 87.4 | 18.5 | PD | 11.0 | 26.6 | Dead |
| 6 | 10 | 59.3 | 97.6 | 96.4 | 82.3 | 28.5 | SD | 22.3 | 52.9 | Dead |
| 7 | 10 | 68.2 | 97.5 | 98.2 | 86.1 | 22.3 | SD | 91.7 | 104.3 | Dead |
| 8 | 8 | 62.2 | 97.6 | 97 | 91 | 11.8 | PD | 13.4 | 71.0 | Dead |
| 9 | 7 | 45.5 | 97.2 | 99.1 | 89.8 | 27.5 | PD | 11.7 | 15.0 | Dead |
| 10 | 7 | 42.3 | 98.4 | 99.9 | 92 | 38.4 | PD | 10.6 | 39.6 | Dead |
| 11 | 7 | 46.6 | 97.7 | 99.6 | 80.4 | 36.9 | PD | 9.7 | 26.1 | Dead |
| 12 | 7 | 37.7 | 98 | 99.2 | 90.1 | 16.7 | PD | 10.0 | 35.3 | Dead |
| 13 | 7 | 42.9 | 98.4 | 99.5 | 90.1 | 19.2 | PD | 10.0 | 20.4 | Dead |
| 14 | 10 | 48.2 | 97.9 | 99.2 | 87.7 | 19.3 | SD | 14.3 | 19.3 | Dead |
| 15 | 8 | 40.2 | 97.5 | 99.5 | 72.9 | 21.4 | PD | 10.3 | 17.3 | Dead |
| 16 | 8 | 41.9 | 98.5 | 99.7 | 72.2 | 15.3 | PD | 10.1 | 65.4 | Dead |
PFS progression-free survival, OS overall survival, PD progress disease, SD stable disease
Changes in QoL
Global health status scores of the EORTC QLQ-C30 worsened after ex vivo-expanded CIK cell therapy but was not statistically significant (P = 0.123). In general, most functional scores showed worsening but were not statistically significant. Among symptom scores, pain and insomnia were significantly worse (P = 0.004 and P = 0.002). Only marginal worsening was observed in diarrhea, nausea, vomiting, and other functional difficulties (Supplemental Table 1).
Pancreatic pain, gastrointestinal distress, jaundice, body image alternations, altered bowel habit scores, health satisfaction, and sexuality scale scores of QLQ-PNA26 all improved. Among these improvements, pancreatic pain and altered bowel habits were statistically significant (P = 0.012 and P = 0.003). Among separate questions, worsening bloated pain and ability to plan ahead were observed but were not statistically significant (P = 0.016 and P = 0.027) (Supplemental Table 2). In patients showing treatment responses, improvements were observed in most of the QoL questionnaire items with increasing time of visits. This trend was also true for patients who completed 10 times of ex vivo-expanded CIK cell therapy.
Average pain measured using the visual analog scale (VAS) was 1.13 ± 1.89 at baseline and 3.19 ± 2.43 at the final time point, which was a significantly worsening trend (2.06 ± 2.82, P = 0.01).
Adverse events
The most common adverse events reported by the attending physicians are listed in Table 3. A total of 136 adverse events occurred. Common adverse events observed in more than 20 % of patents included weight loss, asthenia, nausea, abdominal pain, diarrhea, back pain, hypophagia, and dyspepsia. Weight loss was observed in 14 patients (70 % of the safety population) but was classified as a grade 1 adverse event. Of a total of 136 adverse events, serious adverse events occurred in seven patients (35 %). All adverse events were regarded as unrelated to therapy by the investigators. There were no life-threatening or disabling adverse events or deaths related to adverse events. No therapy was discontinued due to treatment-related complications. Hematologic toxicities included grade 1 thrombocytopenia in one patient. Analysis of changes in laboratory tests between before and after therapy showed that the mean prothrombin time [international normalized ratio (INR)] significantly increased from 0.98 ± 0.13 to 1.09 ± 0.27 (P = 0.002), and there were no other laboratory tests showing statistically significant changes. No cases of cytokine storm or anaphylactic reactions were observed during this clinical trial.
Table 3.
Adverse events
| N = 20 | Intensity (CTC grade) [19] | |||
|---|---|---|---|---|
| n (%) | 1, n (%) | 2, n (%) | 3, n (%) | |
| Weight loss | 14 (70) | 14 (70) | 0 (0) | 0 (0) |
| Asthenia | 7 (35) | 4 (20) | 2 (10) | 1 (5) |
| Vomiting | 7 (35) | 7 (35) | 0 (0) | 0 (0) |
| Nausea | 7 (35) | 7 (35) | 0 (0) | 0 (0) |
| Abdominal pain | 5 (25) | 2 (10) | 2 (10) | 1 (5) |
| Diarrhea | 5 (25) | 5 (25) | 0 (0) | 0 (0) |
| Back pain | 4 (20) | 3 (15) | 1 (5) | 0 (0) |
| Hypophagia | 4 (20) | 2 (10) | 2 (10) | 0 (0) |
| Dyspepsia | 4 (20) | 4 (20) | 0 (0) | 0 (0) |
CTC common toxicity criteria
Discussion
Recently, immune cell-based cancer therapy has been attempted as an alternative treatment option for various cancers to eliminate cancer cells through the transfer of ex vivo-expanded active immune cells. Tolerability of immune cell-based cancer therapy is expected to be advantageous, especially in pancreatic cancer patients, most of whom cannot maintain a treatment regimen due to deteriorating performance status. Among various immune cell types used in immune cell-based therapy, CIK cells exert anti-tumor activity by the secretion of cytotoxic molecules such as granzyme and perforin, the activation of the Fas signaling pathway of tumor cells, and the production of multiple cytokines that regulate immune responses [8]. Their biologic features, together with potent ex vivo expansibility and wide MHC-unrestricted tumor killing, meet important clinical requirements in terms of simplicity and effectiveness. Such simplicity is a key issue that will hopefully facilitate the transition of CIK cells into a clinical therapy used by many cancer centers [9]. The anti-tumor activity of ex vivo-expanded CIK cells against HCC and lung cancer has been evaluated in vitro and in a nude mouse xenograft model [13, 14]. CIK cells were shown to destroy one-third of SNU-354 human HCC cells in vitro [13]. In addition, a dose of 1 × 106 CIK cells per mouse inhibited 60 % of SNU-354 tumor growth in irradiated nude mice [13]. CIK cells were shown to destroy almost all NCI-H460 human lung cancer cells in vitro [14]. Furthermore, CIK cells at doses of 3 and 30 million cells per mouse inhibited 57 and 77 % of NCI-H460 tumor growth in a nude mouse xenograft assay [14].
At present, there is no recognized standard of anticancer therapy for patients who experience PD after gemcitabine-based chemotherapy. Beyond first-line therapy, options for metastatic pancreatic cancer become less clear, as patients often demonstrate rapid clinical deterioration and are no longer suitable candidates for additional treatment beyond supportive care. A number of small prospective single-arm studies have evaluated both cytotoxic and targeted agents in the setting of gemcitabine-refractory disease, generally demonstrating low response rates, PFS, and OS of 10–20 %, 6–8, and 15–20 weeks, respectively [21, 23–26]. Results from one of the largest studies conducted to date for the second-line treatment of advanced pancreatic cancer (CONKO-003) randomized 165 patients to receive either oxaliplatin, folinic acid, and 5-FU (OFF), or 5-FU/folinic acid alone [7]. Patients receiving the oxaliplatin-containing combination demonstrated significantly improved outcomes in terms of both PFS (13 vs. 9 weeks, P = 0.012) and OS (26 vs. 13 weeks, P = 0.014), leading to the adoption of the OFF regimen as a standard of care in the salvage setting [7]. When we evaluated the efficacy of adoptive immunotherapy using ex vivo-expanded CIK cells in gemcitabine-refractory advanced pancreatic cancer, DCR, defined as showing CR, PR, and SD of the best overall response, was 25 % (4/16 of the ITT population), the median estimated PFS was 11.0 weeks, and the median estimated OS was 26.6 weeks, which were comparable to results of previous studies [7, 21, 23–26]. Mean serum levels of CA19-9 were 2,620.4 ± 5,392.8 U/ml at baseline and 5,315.5 ± 8,373.3 U/ml at the last visit, which was a statistically significant 2,695.1 ± 5,364.9 U/ml increase (P = 0.001). These results may be due to the fact that all enrolled in this trial were advanced pancreatic cancer patients who failed conventional therapy and most patients had PD at the last visit.
Up-to-date study on QoL changes throughout the treatment process for pancreatic cancer is lacking. For objective measurements of physical, mental, social, and subjective QoL in patients with pancreatic cancer, EORTC QLQ-C30 and QLQ-PAN26 were utilized to assess changes during the test period in our study. Patients showed improvement in pancreatic pain, gastrointestinal distress, jaundice, body image alterations, altered bowel habits, health satisfaction, and sexuality scale scores of QLQ-PNA26. However, no improvement was observed in global health status, functional health status, or symptoms scale scores of QLQ-C30. Since the patients were relatively old and suffered from advanced cancer, their already-existing physical abilities and the pain and discomfort of the previous treatment itself might have contributed to these results. We considered that improvement in some QoL items had clinical meanings, especially for pancreatic cancer patients expected to have only marginally prolonged survival with any treatment. In previous study including 21 patients who underwent from first- to second-line conventional chemotherapy for pancreatic and bile duct cancer, most patients showed significant impairments with regard to physical functioning, global QoL, fatigue, dyspnea, diarrhea, and taste alterations scale scores of QLQ-C30, which was consistent with results of our study [27]. But they did not assess QLQ-PAN26 including items specific to pancreatic cancer, so comparison between conventional chemotherapies and adoptive immunotherapy using ex vivo-expanded CIK cells in terms of QoL changes during treatment for pancreatic cancer is not feasible. In referring to QoL questionnaire data for each visit in Supplemental Tables 3 and 4, most subjects who showed a response to treatment demonstrated an increase in QoL questionnaire items at each visit, especially at week 16 by which 10 times of therapy had been completed.
To assess clinical status, pain and change in weight were measured. Baseline pain (VAS) was 1.13 ± 1.89 and end point pain was 3.19 ± 2.43. Therefore, the pain increased by 2.06 ± 2.82, which was statistically significant. Considering that 12 patients were evaluated as having PD after treatment among 16 patients of the ITT population, this worsening in pain score is likely due to an increase in tumor size or progression of cancer causing invasion of the nerve ganglion. However, in patients showing treatment responses, pain scores remained unchanged. Pain-related items in the QoL questionnaire showed an increase in overall pain but improvements in other cancer-related pain items. At baseline, the average weight was 57.8 ± 12.3 kg and the weight at the last visit was 56.3 ± 11.6 kg, a statistically significant difference (P = 0.026). This weight loss may reflect various aspects of cancer progression, such as digestive disturbances, anorexia, nausea, vomiting, and malnutrition. Body weight decreased significantly and pain increased significantly; both were statistically significant changes. A possible explanation for these is that since most subjects showed PD, cancer progression may play a role. Other factors to consider are the high percentage of patients with comorbidities associated with digestive disease, anorexia nervosa, or food intake difficulties.
A total of 127 transfusions were performed for the 16 patients in the ITT population, and no severe adverse effects at or higher than grade IV were observed. Grade III adverse effects occurred in 19 cases, and all other adverse effects were grade I or II, which were self-limiting and required no treatment. Although all subjects reported adverse events, none could be considered as treatment related. No cases of immunotherapy-related adverse events, such as “cytokine storm” or “anaphylaxis,” were reported [28]. The most frequent adverse events reported after ex vivo-expanded CIK cell infusion were “gastrointestinal disorders.” All infusions were successfully done without toxicity or adverse events. Prothrombin time (INR) was the only laboratory test that showed statistically significant changes from baseline. At baseline, INR was 0.98 ± 0.13 and INR was 1.09 ± 0.27 at the last visit, a statistically significant increase (P = 0.002). However, these changes were mostly within the normal range (INR 0.91–1.16) and lacked clinical meaning. Disease progression and adverse events, such as biliary obstruction caused by liver metastasis, were thought to affect INR in some patients. No other laboratory results showed significant changes, which is consistent with reports from a previous study evaluating adoptive immunotherapy [16].
Immunotherapy would be more beneficial when residual tumors are minimal, such as in cases of postoperative adjuvant treatment [29]. However, this study included patients with advanced pancreatic cancer who failed conventional chemotherapy. This consideration is particularly important for designing future trials. In the future, instead of utilizing a single immunotherapy, studies exploring a combination of immunotherapy with gemcitabine-based chemotherapy are predicted to have a much better outcome. A previous study in patients with glioblastoma showed that a combination treatment of human CIK cells and temozolomide further increased tumor cell apoptosis and decreased tumor cell proliferation and vessel density, creating a more potent therapeutic regimen compared with temozolomide single therapy [8].
In summary, adoptive immunotherapy using ex vivo-expanded CIK cells showed comparable results in terms of efficacy to conventional cytotoxic chemotherapy. Significant adverse events related to treatment were not observed, and no adverse events appeared to be related to cytotoxicity. Therefore, adoptive immunotherapy using ex vivo-expanded CIK cells can be considered as an option with a good safety profile in patients with gemcitabine-refractory advanced pancreatic cancer. In addition, considering that after failing first-line chemotherapy, patients with advanced pancreatic cancer quickly deteriorate due to rapid disease progression and treatment toxicity, adoptive immune cell therapy using ex vivo-expanded CIK cells shows encouraging results in terms of patient QoL.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- CIK cells
Cytokine-induced killer cells
- CA 19-9
Carbohydrate antigen 19-9
- CIs
Confidence intervals
- DCR
Disease control rate
- ECOG-PS
Eastern Cooperative Oncology Group performance status
- EORTC
European Organization for Research and Treatment of Cancer
- FACS
Fluorescence-activated cell sorting
- HCC
Hepatocellular carcinoma
- INR
International normalized ratio
- ITT
Intention-to-treat
- MHC
Major histocompatibility complex
- OFF
Oxaliplatin, folinic acid, and 5-FU
- OS
Overall survival
- PBMCs
Peripheral blood mononuclear cells
- PD
Progressive disease
- PFS
Progression-free survival
- PP
Per-protocol
- QLQ-C30
Quality of Life Questionnaire Core 30
- QLQ-PAN26
Quality of Life Questionnaire Core 30 in patients with pancreatic cancer
- QoL
Quality of life
- SD
Stable disease
- VAS
Visual analog scale
References
- 1.National Cancer Information Center (2013) Cancer Information Service National Cancer Information Center. http://www.cancer.go.kr/mbs/cancer/subview.jsp?id=cancer_040201000000. Accessed 12 Aug 2013
- 2.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68–72. doi: 10.1016/S0002-9610(05)80406-4. [DOI] [PubMed] [Google Scholar]
- 3.Colucci G, Labianca R, Di Costanzo F, Gebbia V, Cartenì G, Massidda B, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol. 2010;28:1645–1651. doi: 10.1200/JCO.2009.25.4433. [DOI] [PubMed] [Google Scholar]
- 4.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 6.Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605–3610. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pelzer U, Schwaner I, Stieler J, Adler M, Seraphin J, Dörken B, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer. 2011;47:1676–1681. doi: 10.1016/j.ejca.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Jin J, Joo KM, Lee SJ, Jo M, Kim Y, Jin Y, et al. Synergistic therapeutic effects of cytokine-induced killer cells and temozolomide against glioblastoma. Oncol Rep. 2011;25:33–39. [PubMed] [Google Scholar]
- 9.Mesiano G, Todorovic M, Gammaitoni L, Leuci V, Giraudo Diego L, Carnevale Schianca F, et al. Cytokine-induced killer (CIK) cells as feasible and effective adoptive immunotherapy for the treatment of solid tumors. Expert Opin Biol Ther. 2012;12:673–684. doi: 10.1517/14712598.2012.675323. [DOI] [PubMed] [Google Scholar]
- 10.Hontscha C, Borck Y, Zhou H, Messmer D, Schmidt-Wolf IG. Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC) J Cancer Res Clin Oncol. 2011;137:305–310. doi: 10.1007/s00432-010-0887-7. [DOI] [PubMed] [Google Scholar]
- 11.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991;174:139–149. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HM, Lim J, Yoon YD, Ahn JM, Kang JS, Lee K, et al. Anti-tumor activity of ex vivo expanded cytokine-induced killer cells against human hepatocellular carcinoma. Int Immunopharmacol. 2007;7:1793–1801. doi: 10.1016/j.intimp.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Kim HM, Lim J, Park S, Kang JS, Lee CW, Lee KH, et al. Antitumor activity of cytokine-induced killer cells against human lung cancer. Int Immunopharmacol. 2007;7:1802–1807. doi: 10.1016/j.intimp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Sekine T, Shiraiwa H, Yamazaki T, Tobisu K, Kakizoe T. A feasible method for expansion of peripheral blood lymphocytes by culture with immobilized anti-CD3 monoclonal antibody and interleukin-2 for use in adoptive immunotherapy of cancer patients. Biomed Pharmacother. 1993;47:73–78. doi: 10.1016/0753-3322(93)90294-U. [DOI] [PubMed] [Google Scholar]
- 16.Sun Z, Shi L, Zhang H, Shao Y, Wang Y, Lin Y, et al. Immune modulation and safety profile of adoptive immunotherapy using expanded autologous activated lymphocytes against advanced cancer. Clin Immunol. 2011;138:23–32. doi: 10.1016/j.clim.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 19.Fitzsimmons D, Johnson CD, George S, Payne S, Sandberg AA, Bassi C, et al. Development of a disease specific quality of life (QoL) questionnaire module to supplement the EORTC core cancer QoL questionnaire, the QLQ-C30 in patients with pancreatic cancer. EORTC Study Group on Quality of Life. Eur J Cancer. 1999;35:939–941. doi: 10.1016/S0959-8049(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 20.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 21.Boeck S, Weigang Köhler K, Fuchs M, Kettner E, Quietzsch D, Trojan J, et al. Second-line chemotherapy with pemetrexed after gemcitabine failure in patients with advanced pancreatic cancer: a multicenter phase II trial. Ann Oncol. 2007;18:745–751. doi: 10.1093/annonc/mdl463. [DOI] [PubMed] [Google Scholar]
- 22.Demols A, Peeters M, Polus M, Marechal R, Gay F, Monsaert E, et al. Gemcitabine and oxaliplatin (GEMOX) in gemcitabine refractory advanced pancreatic adenocarcinoma: a phase II study. Br J Cancer. 2006;94:481–485. doi: 10.1038/sj.bjc.6602966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulke MH, Blaszkowsky LS, Ryan DP, Clark JW, Meyerhardt JA, Zhu AX, et al. Capecitabine plus erlotinib in gemcitabine-refractory advanced pancreatic cancer. J Clin Oncol. 2007;25:4787–4792. doi: 10.1200/JCO.2007.11.8521. [DOI] [PubMed] [Google Scholar]
- 24.Oh SY, Kim HJ, Kim TH, Lee G, Jeong C, Kwon H, et al. Pilot study of irinotecan/oxaliplatin (IROX) combination chemotherapy for patients with gemcitabine- and 5-fluorouracil-refractory pancreatic cancer. Invest New Drugs. 2010;28:343–349. doi: 10.1007/s10637-009-9265-1. [DOI] [PubMed] [Google Scholar]
- 25.O’Reilly EM, Niedzwiecki D, Hall M, Hollis D, Bekaii Saab T, Pluard T, et al. A Cancer and Leukemia Group B phase II study of sunitinib malate in patients with previously treated metastatic pancreatic adenocarcinoma (CALGB 80603) Oncologist. 2010;15:1310–1319. doi: 10.1634/theoncologist.2010-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko AH, Tempero MA, Shan YS, Su WC, Lin YL, Dito E, et al. A multinational phase 2 study of nanoliposomal irinotecan sucrosofate (PEP02, MM-398) for patients with gemcitabine-refractory metastatic pancreatic cancer. Br J Cancer. 2013;109:920–925. doi: 10.1038/bjc.2013.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zabernigg A, Giesinger JM, Pall G, Gamper EM, Gattringer K, Wintner LM, et al. Quality of life across chemotherapy lines in patients with cancers of the pancreas and biliary tract. BMC Cancer. 2012;12:390. doi: 10.1186/1471-2407-12-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ly LV, Sluijter M, Versluis M, Luyten GP, van Stipdonk MJ, van der Burg SH, et al. Peptide vaccination after T-cell transfer causes massive clonal expansion, tumor eradication, and manageable cytokine storm. Cancer Res. 2010;70:8339–8346. doi: 10.1158/0008-5472.CAN-10-2288. [DOI] [PubMed] [Google Scholar]
- 29.Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802–807. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.