Abstract
Cancer vaccine therapy is one of the most attractive therapies as a new treatment procedure for pancreatic adenocarcinoma. Recent technical advances have enabled the identification of cytotoxic T lymphocyte (CTL) epitopes in various tumor-associated antigens (TAAs). However, little is known about which TAA and its epitope are the most immunogenic and useful for a cancer vaccine for pancreatic adenocarcinoma. We examined the expression of 17 kinds of TAA in 9 pancreatic cancer cell lines and 12 pancreatic cancer tissues. CTL responses to 23 epitopes derived from these TAAs were analyzed using enzyme-linked immunospot (ELISPOT), CTL, and tetramer assays in 41 patients, and factors affecting the immune responses were investigated. All TAAs were frequently expressed in pancreatic adenocarcinoma cells, except for adenocarcinoma antigens recognized by T cells 1, melanoma-associated antigen (MAGE)-A1, and MAGE-A3. Among the epitopes recognized by CTLs in more than two patients in the ELISPOT assay, 6 epitopes derived from 5 TAAs, namely, MAGE-A3, p53, human telomerase reverse transcriptase (hTERT), Wilms tumor (WT)-1, and vascular endothelial growth factor receptor (VEGFR)2, could induce specific CTLs that showed cytotoxicity against pancreatic cancer cell lines. The frequency of lymphocyte subsets correlated well with TAA-specific immune response. Overall survival was significantly longer in patients with TAA-specific CTL responses than in those without. P53, hTERT, WT-1, and VEGFR2 were shown to be attractive targets for immunotherapy in patients with pancreatic adenocarcinoma, and the induction of TAA-specific CTLs may improve the prognosis of these patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1529-8) contains supplementary material, which is available to authorized users.
Keywords: Epitope, Immunotherapy, Cytotoxic T lymphocyte (CTL), Enzyme-linked immunospot (ELISPOT)
Introduction
Pancreatic adenocarcinoma is the fourth leading cause of cancer death worldwide [1]. Despite recent advances in diagnostic techniques, pancreatic adenocarcinoma is diagnosed at an advanced stage in most patients and, consequently, the overall 5-year survival rate is <5 % [2]. Thus, the development of a new treatment option is needed to improve the prognosis of pancreatic cancer patients without toxicity.
Immunotherapy is one of the most attractive therapies as a new treatment procedure for melanoma and other solid tumors [3]. Recent technical advances have enabled the identification of various tumor-associated antigens (TAAs) [4–21]; however, few of their epitopes are inducers of cytotoxic T lymphocyte (CTL) responses against tumors [22]. Several kinds of epitope have also been identified in patients with pancreatic adenocarcinoma [23, 24]. However, previous studies focused on the identification and evaluation of a particular antigen, and different TAAs have not yet been compared simultaneously; therefore, little is known about which epitope is the most immunogenic and useful in eliciting clinical responses in pancreatic adenocarcinoma patients.
In the present study, we compared CTL responses with various TAA-derived epitopes in identical patients with pancreatic adenocarcinomas and examined the factors that affect immune responses. This approach provided information that is useful for selecting immunogenic TAAs and suitable patients and developing a new immunotherapy for pancreatic adenocarcinoma.
Materials and methods
Patients and clinical information
In this study, we examined 41 HLA-A24-positive patients with pancreatic adenocarcinoma and 14 healthy volunteers who were HLA-A24-positive, but did not have any cancers, as negative controls. Fine-needle biopsy, a surgical specimen, or autopsy was used for the pathological diagnosis of pancreatic adenocarcinoma in 18 patients. Diagnosis of the remaining 23 patients was achieved using the radiological findings of computed tomography and/or magnetic resonance imaging. We investigated patient background, treatment procedures, and outcomes.
Clinical information was obtained from the medical records of patients. We evaluated the tumor stage using TNM staging of the Union Internationale Contre Le Cancer (UICC) system (7th version) (UICC stage). The frequency of lymphocyte subsets was calculated by dividing the absolute lymphocyte count by the absolute leukocyte count. HLA typing of peripheral blood mononuclear cells (PBMCs) from patients and healthy volunteers was performed by the reverse sequence-specific oligonucleotide with polymerase chain reaction (PCR-RSSO). This study was approved by the Ethics Committees of Kanazawa University (No. 1237) and Kanazawa Medical Center (No. 17), and all patients gave written informed consent to participate in accordance with the Helsinki Declaration.
Synthetic peptides and preparation of PBMCs
The 23 epitopes derived from 17 different TAAs used in the present study are listed in Table 1. We selected epitopes that had previously been identified as HLA-A24-restricted and suggested to have immunogenicity in various cancers not restricted to pancreatic cancer [4–21]. Epitopes derived from the HIV envelope protein (HIV env584) [25] and cytomegalovirus (CMV) pp65 (CMVpp65328) [26] were also used to assess T cell responses. Peptides were synthesized at Mimotope (Melbourne, Australia), Sumitomo Pharmaceuticals (Osaka, Japan), COSMO BIO Co. (Tokyo, Japan), and Scrum Inc. (Tokyo, Japan). Purities were determined to be >80 % by analytical high-performance liquid chromatography (HPLC). PBMCs were separated as described below; heparinized venous blood was diluted in phosphate-buffered saline (PBS) and loaded on Ficoll-Histopaque (Sigma, St. Louis, MO) in 50-ml tubes. After centrifugation at 2,000 rpm for 20 min at room temperature, PBMCs were harvested from the interphase, resuspended in PBS, centrifuged at 1,400 rpm for 10 min, and finally resuspended in complete culture medium consisting of RPMI (GibcoBRL, Grand Island, NY), 10 % heat-inactivated FCS (Gibco BRL), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco BRL).
Table 1.
Peptides used in this study
| Peptide No. | TAA | Amino acid sequence | Reference |
|---|---|---|---|
| 1 | ART1188 | EYCLKFTKL | [14] |
| 2 | ART4161 | AFLRHAAL | [11] |
| 3 | ART4899 | DYPSLSATDI | [11] |
| 4 | Cyp-B109 | KFHRVIKDF | [7] |
| 5 | Cyp-B315 | DFMIQGGDF | [7] |
| 6 | Lck208 | HYTNASDGL | [8] |
| 7 | Lck488 | DYLRSVLEDF | [8] |
| 8 | MAGE-A1135 | NYKHCFPEI | [6] |
| 9 | MAGE-A3195 | IMPKAGLLI | [16] |
| 10 | SART1690 | EYRGFTQDF | [12] |
| 11 | SART2899 | SYTRLFLIL | [13] |
| 12 | SART3109 | VYDYNCHVDL | [21] |
| 13 | Her-2/neu8 | RWGLLLALL | [17] |
| 14 | p53161 | AIYKQSQHM | [18] |
| 15 | p53204 | EYLDDRNTF | [5] |
| 16 | MRP3765 | VYSDADIFL | [20] |
| 17 | MRP3503 | LYAWEPSFL | [20] |
| 18 | hTERT461 | VYGFVRACL | [4] |
| 19 | hTERT324 | VYAETKHFL | [4] |
| 20 | WT-1235 | CMTWNQMNL | [15] |
| 21 | VEGFR2169 | RFVPDGNRI | [19] |
| 22 | VEGFR11084 | SYGVLLWEI | [10] |
| 23 | survivin2B80 | AYACNTSTL | [9] |
| 24 | HIV env584 | RYLRDQQLL | [25] |
| 25 | CMV pp65328 | QYDPVAALF | [26] |
Cell lines
The HLA-A*2402 gene-transfected C1R cell line (C1R-A24) was cultured in RPMI 1640 medium containing 10 % FCS and 500 μg/ml hygromycin B (Sigma, St. Louis, MO), and K562 was cultured in RPMI 1640 medium containing 10 % FCS [27]. MiaPaca2, AsPC1, BxPC3, Panc-1, CAPAN1, and CAPAN2 were purchased from the American Type Culture Collection (VA, USA). YPK-1 and YPK-2 were kind gifts from Prof. Oka and Dr. Yoshimura (Yamaguchi University Graduate School of Medicine, Yamaguchi, Japan). PK-1 was provided by the RIKEN BRC through the National Bio-Resource Project of MEXT, Japan. Human pancreatic cancer cell lines were cultured in DMEM (GibcoBRL) or RPMI 1640 medium containing 10 % fetal calf serum (FCS). All media contained 100 U/mL penicillin and 100 μg/mL streptomycin.
RNA preparation and real-time PCR
The expression of TAA messenger RNA (mRNA) in human pancreatic cancer cell lines and pancreatic adenocarcinoma tissues was analyzed by real-time polymerase chain reaction (PCR). Cell lines were harvested, centrifuged, and washed with PBS, and total RNA was then isolated using Quick-Gene (Fuji Film, Tokyo). Total RNA from frozen pancreatic adenocarcinoma samples was isolated using a GenElute Mammalian Total RNA Miniprep Kit (Sigma-Aldrich) according to the manufacturer’s protocol. cDNA was synthesized from 150 ng of total RNA using a high-capacity cDNA reverse transcription kit (PE Applied Biosystems, CA, USA) and was then mixed with TaqMan Universal Master Mix (PE Applied Biosystems) and each TaqMan probe. Primer pairs and probes for various TAAs and β-actin were obtained from the TaqMan assay reagents library. Thermal cycling conditions were 25 °C for 10 min, 37 °C for 120 min, and 85 °C for 1 min. cDNA was subjected to quantitative real-time PCR analyses targeting various TAAs and β-actin. Analyses were performed using the StepOne Real-Time PCR system and StepOne v2.0 software. Relative gene expression values were determined. Data are presented as fold differences in TAA expression normalized to the housekeeping gene β-actin as an endogenous reference.
Enzyme-linked immunospot assay (ELISPOT assay)
Ninety-six-well plates (Millititer, Millipore, Bedford, MA) were coated with anti-human interferon-γ (IFN-γ) (Mabtech, Nacka, Sweden) at 4 °C overnight and then washed 4 times with sterile PBS. The plates were then blocked with RPMI 1640 medium containing 5 % FCS for 2 h at room temperature. A total of 300,000 unfractionated PBMCs were added in duplicate cultures of RPMI 1640 containing 5 % FCS together with the peptides at 10 μg/ml. After 24 h, the plates were washed 8 times with PBS and incubated overnight with 100 μl of the biotin-conjugated anti-human IFN-γ antibody. After another 4 washes with PBS, streptavidin-AP was added for 2 h. Finally, the plates were washed again 4 times with PBS and developed with freshly prepared NBT/BCIP solution (Biorad, Hercules, CA). The reaction was stopped by washing with distilled water and drying at room temperature. Colored spots with fuzzy borders, which indicated the presence of IFN-γ-secreting cells, were counted. The number of specific spots was determined by subtracting the number of spots in the absence of the antigen. Responses were considered positive if 10 or more specific spots were detected and if the number of spots in the presence of an antigen was at least twofold than that in its absence.
Peptide-specific CTL induction and cytotoxicity assay
Synthetic peptide-specific T cells were expanded from PBMCs in 96-well round-bottom plates (NUNC, Naperville, IL). Four hundred thousand cells/well were stimulated with synthetic peptides at 10 μg/ml, 10 ng/ml rIL-7, and 100 pg/ml rIL-12 (Sigma) in RPMI 1640 supplemented with 10 % heat-inactivated human AB serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. The cultures were restimulated with 10 μg/ml peptide, 20 U/ml rIL-2 (Sigma), and 105 mitomycin C-treated autologous PBMCs as feeder cells on days 7 and 14. One hundred microliters of RPMI medium with 10 % human Ab serum and rIL-2 at a final concentration of 10 U/ml were added to each well on days 4, 11, and 18. The cytotoxicity assay was conducted on day 22.
The C1R-A24 and human pancreatic cancer cell lines were used as target cells for the 51Cr release assay. C1R-A24 cells were incubated overnight with 10 μg/ml synthetic peptides and labeled with 25 μCi of 51Cr for 1 h. Pancreatic cancer cell lines were also labeled with 25 μCi of 51Cr for 1 h without incubation with peptides. After three washes with PBS, target cells were plated at 3,000 cells/well in complete medium in round-bottom 96-well plates. Unlabeled K562 (120,000 cells/well) was added to reduce non-specific lysis. Peptide-stimulated PBMCs were added at various effector-to-target ratios as indicated. Maximum release was determined by the lysis of 51Cr-labeled targets with 5 % Triton X-100 (Sigma Chemical). Spontaneous release was <10 % of maximum release for all experiments, except for when it was <15 % when the target cells were human pancreatic cancer cell lines. Percent-specific cytotoxicity was determined using the following formula: 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release), and specific cytotoxic activity was calculated as follows: (cytotoxic activity in the presence of the peptide) − (cytotoxic activity in the absence of the peptide). Specific cytotoxicity of more than 10 % was considered to be positive.
Tetramer staining and flow cytometry
TAA-specific tetramers were purchased from Medical Biological Laboratories Co., Ltd. (Nagoya, Japan). Tetramer staining was performed as described below. One million isolated PBMCs or peptide-specific CTLs pulsed with TAA-derived peptides were washed, resuspended in 200 μl of PBS without calcium or phosphate, and stained with 40 μg/ml tetrameric complexes and monoclonal antibodies against cell surface proteins for 30 min at room temperature. The following monoclonal antibodies were used: anti-CD8-APC (BD PharMingen, San Diego, CA), anti-CCR7-FITC, anti-CD45RA-PerCP, and tetramer-PE. Cells were washed, fixed with 0.5 % paraformaldehyde/PBS, and analyzed on a Becton–Dickinson FACSAria II system.
Statistical analysis
Fisher’s exact test and unpaired Student’s t test were used to analyze the effect of variables on immune responses in pancreatic cancer patients. Overall survival was calculated from the day of pancreatic cancer diagnosis until the date of death or the last day of the follow-up period. Cumulative survival proportions were calculated using the Kaplan–Meier method, and any differences were evaluated using the log-rank test. A p value of <0.05 was considered to be significant, and all the tests were two-sided. All statistical analyses were performed using the SPSS statistical software program package (SPSS version 11.0 for Windows).
Results
Patients
Patient characteristics are summarized in the Supplementary Table. The median age of patients was 72 years, and patients included 24 males (59 %). The main localization of the tumors was the pancreatic head in 39 % of patients and the pancreatic body or tail in 61 %. The majority of patients (93 %) had advanced-stage cancer, namely, UICC stage III or IV. Therapeutic procedures mainly involved chemotherapy consisting of protocols such as gemcitabine monotherapy, S-1 monotherapy, or a combination of both drugs. Only 11 patients received the best supportive therapy to relieve physical and spiritual pain. A total of 61 % of patients had died by the last day of the follow-up period, and the median overall survival time of patients was 7.2 months.
TAA expression in pancreatic cancer cell lines and human cancer tissues
We evaluated the expression of 17 different TAAs in 9 human pancreatic cancer cell lines using real-time PCR. Although differences were observed from cell to cell, TAAs were expressed in more than 40 % of pancreatic adenocarcinoma cell lines, except for adenocarcinoma antigens recognized by T cells (ART)1 (11 %) and ART4 (33 %) (Table 2). We then investigated TAA expression in 7 surgical and 5 autopsy specimens. The expression of most TAAs in pancreatic adenocarcinoma specimens was similar to or more frequent than that in human pancreatic cancer cell lines, except for melanoma-associated antigen (MAGE)-A1 and MAGE-A3 (Table 2).
Table 2.
Expression of various TAAs mRNA in pancreatic cancer cell lines and pancreatic cancer tissues measured by real-time PCR
| TAA | Primer | Positive cell lines/cell lines tested | Positive specimens/specimens tested |
|---|---|---|---|
| n (%) | n (%) | ||
| ART1 | Hs00188841_m1 | 1/9 (11) | 5/12 (42) |
| ART4 | Hs00221465_m1 | 3/9 (33) | 11/12 (92) |
| CypB | Hs00168719_m1 | 9/9 (100) | 12/12 (100) |
| Lck | Hs00178427_m1 | 8/9 (89) | 11/12 (92) |
| MAGEA1 | Hs00607097_m1 | 4/9 (43) | 1/12 (8) |
| MAGEA3 | Hs00366532_m1 | 4/9 (43) | 1/12 (8) |
| SART1 | Hs00193002_m1 | 9/9 (100) | 12/12 (100) |
| SART2 | Hs00203441_m1 | 9/9 (100) | 12/12 (100) |
| SART3 | Hs00206829_m1 | 9/9 (100) | 12/12 (100) |
| HER2/neu | Hs00170433_m1 | 9/9 (100) | 12/12 (100) |
| p53 | Hs00153340_m1 | 9/9 (100) | 12/12 (100) |
| MRP3 | Hs00358656_m1 | 9/9 (100) | 12/12 (100) |
| hTERT | Hs00162669_m1 | 9/9 (100) | 9/12 (75) |
| WT-1 | Hs00240913_m1 | 5/9 (56) | 9/12 (75) |
| VEGFR2 | Hs00911700_m1 | 5/9 (56) | 11/12 (92) |
| VEGFR1 | Hs01052961_m1 | 6/9 (67) | 12/12 (100) |
| Survivin | Hs00153353_m1 | 9/9 (100) | 12/12 (100) |
Detection of TAA-specific T cells by IFN-γ ELISPOT analysis
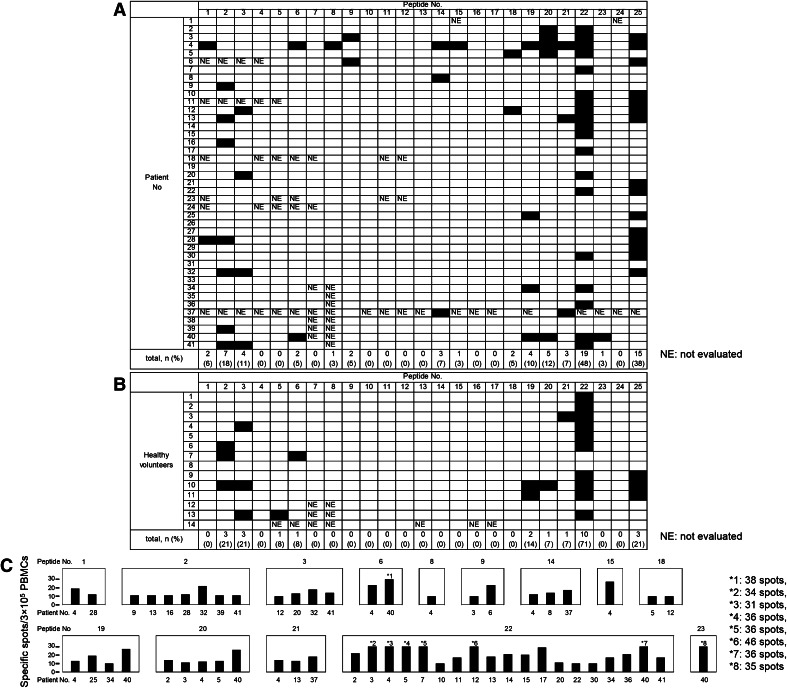
IFN-γ ELISPOT responses were evaluated with PBMCs to determine how frequently T cells respond to TAA-derived peptides and control peptides in patients with pancreatic adenocarcinoma (Fig. 1a). Positive responses to at least one TAA-derived peptide were observed in 28 of 41 (68 %) patients. On the other hand, 14 of 23 (61 %) peptides were recognized by T cells obtained from at least one patient. ART1188, ART4161, ART4899, lymphocyte-specific protein tyrosine kinase (Lck)208, MAGE-A3195, p53161, human telomerase reverse transcriptase (hTERT)461, hTERT324, Wilms tumor (WT)-1235, vascular endothelial growth factor receptor (VEGFR)2169, and VEGFR11084 were recognized in more than two patients, which suggested that these peptides have the potential to be immunogenic. Peptides 24 (HIVenv584) and 25 (CMVpp65328) were recognized in 0 and 38 % of patients, respectively.
Fig. 1.
T cell responses to TAA-derived peptides and control peptides in pancreatic adenocarcinoma patients a and healthy volunteers b. T cell responses were evaluated by the IFN-γ ELISPOT assay. Responses were considered positive if 10 or more specific spots were detected and if the number of spots in the presence of an antigen was at least twofold that in its absence. Black boxes indicate positive responses. c The frequency of TAA-specific IFN-γ-producing T cells evaluated by the ELISPOT assay. Black bars indicate the response of one patient
Peptides ART4161, ART4899, Cyclophilin B (Cyp-B)315, Lck208, hTERT324, and VEGFR11084 were recognized in more than one healthy volunteer, and/or the percentage of positive responses was higher in healthy volunteers than in pancreatic adenocarcinoma patients, which indicated that the responses to these peptides were not specific to T cells from patients with pancreatic adenocarcinoma (Fig. 1b). In other words, peptides ART1188, MAGE-A3195, p53161, hTERT461, WT-1235, and VEGFR2169 have specific immunogenic potential in patients with pancreatic adenocarcinoma.
The number of peptide-specific IFN-γ-producing T cells was counted to examine the frequency of T cells responsive to TAA-derived peptides. A range of 10–46 T cells per 300,000 PBMCs in patients with pancreatic adenocarcinoma produced IFN-γ (Fig. 1c).
TAA-specific CTL induction and cytotoxic activity
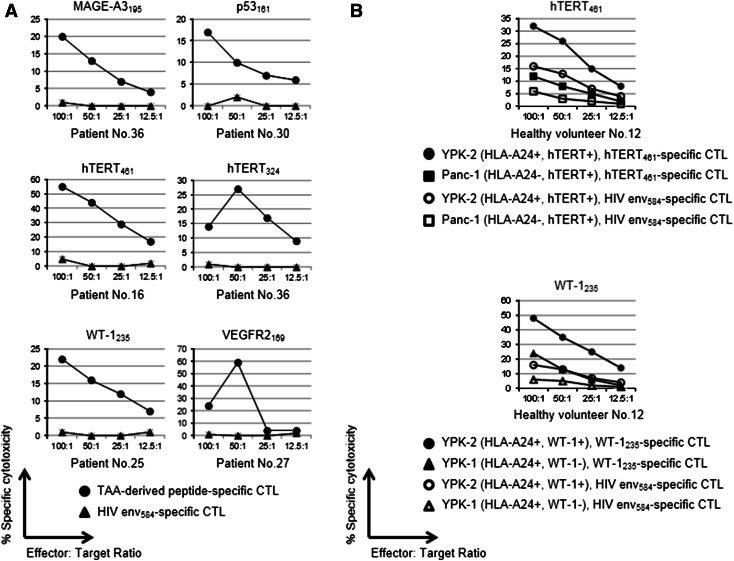
We attempted to induce peptides specific to CTLs from the PBMCs of pancreatic adenocarcinoma patients. Cytotoxicity assays were performed in more than five patients for each peptide. Of the 11 peptides recognized in more than two patients in the IFN-γ ELISPOT assay, 6 peptides (MAGE-A3195, p53161, hTERT461, hTERT324, WT-1235, and VEGFR2169) could induce their specific CTLs, which were confirmed to be able to respond to C1RA24 cells pulsed with corresponding peptides by the cytotoxicity assay, as shown in Fig. 2a.
Fig. 2.
a T cell responses to peptides evaluated by the cytotoxicity assay. Peptide-specific CTL induction and cytotoxicity assays were performed on the PBMCs from at least five patients, and representative data are shown when peptide-specific CTLs were induced in one or more patients. A percent-specific cytotoxicity of more than 10 % was considered to be positive. Six peptides: 9, 14, 18, 19, 20, and 21, could induce their specific CTLs, and these could respond to C1RA24 cells pulsed with the corresponding peptides in the cytotoxicity assay. b Cytotoxic activity against the pancreatic carcinoma cell lines of TAA-specific CTLs from healthy volunteers evaluated by the cytotoxicity assay. Cytotoxicity was stronger against pancreatic carcinoma cells that were HLA-A24-restricted and expressed corresponding TAAs than against those not HLA-A24-restricted or not expressing corresponding TAAs
We conducted a cytotoxicity assay to determine whether peptide-specific CTLs from healthy volunteers could show their cytotoxic activity against pancreatic carcinoma cell lines. P53161-, hTERT461-, and hTERT324-specific CTLs showed cytotoxicity against YPK-2 (HLA-A24-, p53-, and hTERT-positive), but not against Panc-1 (HLA-A24-negative, p53- and hTERT-positive). MAGE-A3195-, WT-1235-, and VEGFR2169-specific CTLs also showed cytotoxic activity against YPK-2 (HLA-A24-, MAGE-A3-, WT-1-, and VEGFR2-positive), but not against YPK-1 (HLA-A24-positive, MAGE-A3-, WT-1-, and VEGFR2-negative). Representative data are shown in Fig. 2b.
Phenotypic analysis of TAA-derived peptides specific to T cells
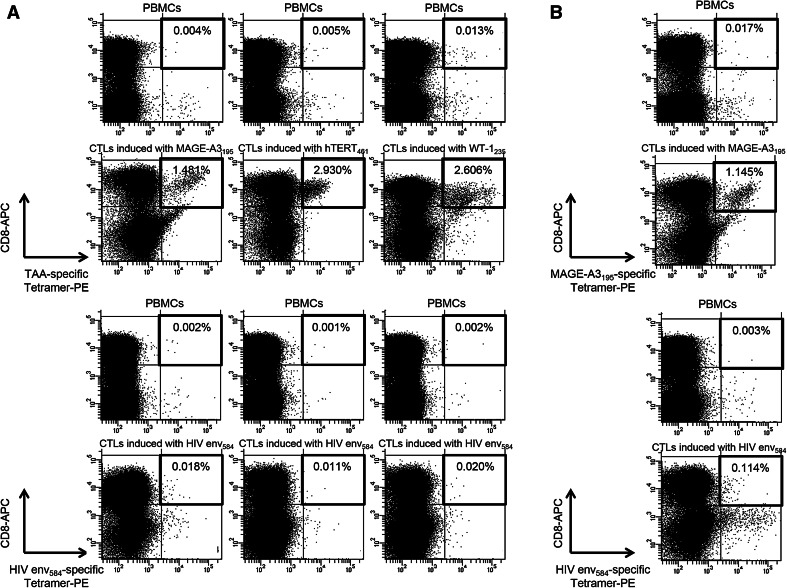
To analyze the characteristics of TAA-derived peptides specific to T cells and select the appropriate epitope for immunotherapy in patients with pancreatic adenocarcinoma, we performed phenotypic analysis by tetramer staining and FACS analysis. We first attempted to detect MAGE-A3195-, hTERT461-, and WT-1235-specific tetramer-positive T cells in PBMCs and CTLs induced by the corresponding peptides in healthy volunteers. The ratio of tetramer-positive T cells was increased in CTLs and their frequencies were 1.481–2.930 % of CD8+ T cells, suggesting that these tetramers work well (Fig. 3a). We also conducted similar assays in pancreatic adenocarcinoma patients and detected tetramer-positive T cells in CTLs (Fig. 3b).
Fig. 3.
Detection of TAA-specific, HLA-A24-tetramer+, and CD8+ lymphocytes in PBMCs from healthy volunteers and pancreatic adenocarcinoma patients. a Tetramer analyses were performed on eight healthy volunteers for each peptide (MAGE-A3195, hTERT461, and WT-1235). Tetramer+ and CD8+ T cells were detectable in both PBMCs and CTLs induced by their corresponding peptides in at least one healthy volunteer, and representative data are shown in cases in which the ratio of tetramer+ and CD8+ T cells to CD8+ T cells was higher in CTLs induced with each TAA-derived peptide than in PBMCs. b Tetramer analyses were performed on pancreatic adenocarcinoma patients using PBMCs and CTLs, which were induced with TAA-derived peptides and showed cytotoxicity against pancreatic cancer cell lines in cytotoxicity assay. Levels of tetramer+ and CD8+ T cells were higher in CTLs induced with TAA-derived peptides than in PBMCs. Representative data are shown in cases in which the ratio of tetramer+ and CD8+ T cells to CD8+ was 0.017 % in PBMCs and 1.145 % in MAGE-A3195-specific CTLs
We then examined the naïve/effector/memory phenotype of tetramer-positive cells in the PBMCs of patients. The memory phenotype was investigated by the criterion of CD45RA/CCR7 expression [28]. In tetramer analysis, the frequencies of MAGE-A3195-, hTERT461-, and WT-1235-specific tetramer-positive T cells were 0.003–0.044, 0.006–0.053, and 0.030–0.191 % of CD8+ T cells, respectively. The frequency of CD45RA−/CCR7+ (central memory), CD45RA−/CCR7− (effector memory), and CD45RA+/CCR7− (effector) T cells in tetramer-positive cells depended on the patient and all phenotypes were observed in all patients, except for patients 1, 8, 28, 29, and 4 (Supplementary Fig. 1).
TAA-specific T cell responses and clinical features of pancreatic cancer patients
In the present study, we analyzed the clinical features that can affect TAA-specific immune responses. When we divided patients into two groups based on their frequencies of lymphocyte subsets in peripheral leukocytes (<24 %, the median value among all patients, or equal to or more than 24 %) and the strength of TAA-specific immune responses into three groups according to the frequency of TAA-specific T cells (<10 specific spots on ELISPOT assays, no response; 10–19 specific spots, weak response; equal to or more than 20 specific spots, strong response), the patients with more lymphocyte subsets in peripheral leukocytes showed stronger TAA-specific T cell responses (Supplementary Fig. 2). On the other hand, we could not find any relationship between TAA-specific immune responses and other clinical characteristics such as age, sex, tumor marker levels, UICC stage, or metastasis status.
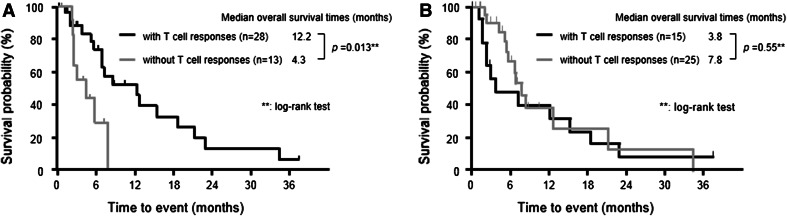
We also analyzed the correlation between T cell responses and the prognosis of pancreatic cancer patients. The median overall survival time of patients with T cell responses to at least one TAA-derived peptide evaluated by the ELISPOT assay was 12.2 months, which was significantly longer than that without T cell responses (4.3 months) (p = 0.013) (Fig. 4a). On the other hand, no correlation was observed between positive T cell responses and CMV-derived peptides and clinical outcomes (Fig. 4b), suggesting that TAA-specific T cell responses, but not the general immune response, is a prognostic factor in patients with pancreatic adenocarcinoma. The frequencies of regulatory T cells or the ratio of regulatory T cells to CD8+ T cells had no impact on the outcomes of patients in this study.
Fig. 4.
Kaplan-Meier plot of the overall survival of pancreatic cancer patients according to a TAA-specific T cell responses and b T cell responses to CMV-derived peptides. a TAA-specific T cell responses were defined as positive if 10 or more specific spots to at least one TAA-derived peptide were detected on the ELISPOT assay. The overall survival time of patients with TAA-specific T cell responses was significantly longer than that of patients without TAA-specific T cell responses. b T cell responses to CMV-derived peptides were defined as positive if 10 or more specific spots to CMV-derived peptides were detected on ELISPOT assays. No correlation was observed between positive T cell responses to CMV-derived peptides and the clinical outcomes of patients
Discussion
Immunotherapy is considered to be a fourth treatment procedure for cancer following surgical resection, radiotherapy, and chemotherapy [29]. Cancer vaccine therapy was previously shown to convey survival benefits to prostate cancer patients in a clinical phase III trial [30], and some candidates of other cancers have been identified and separately evaluated to determine whether a CTL response can be elicited, with the subsequent elimination of cancer cells and improvement in outcomes. Although a successful clinical response depends on how much tumor antigens elicit their specific CTLs, which are the most important effector cells for antitumor immune responses, to the best of our knowledge, no studies have attempted to identify which epitopes are optimal for peptide vaccine therapy in patients with pancreatic adenocarcinoma. Therefore, we simultaneously compared peptide-specific T cell responses among various TAAs in 41 identical patients with pancreatic adenocarcinoma under the same experimental conditions.
Therapeutic function is the most important factor to consider when determining the usefulness of cancer antigens for peptide vaccine therapy. However, it is very difficult to compare the efficacy of more than one epitope, especially in patients with pancreatic adenocarcinoma whose survival time is very short. Under such circumstances, immunogenicity, specificity, oncogenicity, expression levels, % of positive cells, and the number of patients with antigen-positive cancer are considered to be alternative criteria [31]. On the basis of our results, p53161, hTERT461, WT-1235, and VEGFR2169 are considered the most optimal epitopes that satisfy all of the above criteria for peptide vaccine therapy in pancreatic adenocarcinoma patients. Although MAGE-A3195 showed immunogenicity, its expression did not appear to be high in pancreatic adenocarcinoma tissue [32]. Therefore, it may be a candidate for cancer vaccine therapy when MAGE-A3 is confirmed to be overexpressed in pancreatic cancer tissue.
A mutation in the p53 gene and overexpression of the p53 protein have been reported previously in pancreatic adenocarcinoma [33], and all pancreatic cancer cell lines and specimens used in our study expressed p53. Some strategies targeting p53 have been proposed over the last decade [34]. As peptide vaccine therapy, the wild-type p53 peptide is well preserved in mutant p53 because most mutations in the p53 gene are missense mutations, and are considered to be one of the attractive targets as a cancer antigen. The frequencies of the CTL response against HLA-A24-restricted p53161 investigated by the ELISPOT assay in head and neck carcinoma and hepatocellular carcinoma were shown to be 35 and 10 %, respectively [35, 36]. Although the frequency of 7 % in our study is lower, given the difference according to the primary tumor site or balance between sensitivity and specificity, induced CTLs showed cytotoxic activity against pancreatic adenocarcinoma cell lines, which suggested that p53 may be an attractive target in patients with pancreatic cancer.
hTERT is widely overexpressed in various cancer cells including pancreatic cancer [37], which is consistent with our results. A clinical trial demonstrated that GV1001, a HLA class II epitope corresponding to the hTERT (611–626) fragment, was immunogenic in pancreatic cancer patients [38]. Another previous study evaluating T cell responses to several hTERT epitopes in patients with hepatocellular carcinoma [39] demonstrated that hTERT461- and hTERT324-specific CTLs were induced in 5 (6.9 %) and 9 (12.5 %) of 72 patients, respectively. In the current study, these frequencies were equivalent and the killing of pancreatic cancer cell lines was demonstrated, which suggested that these epitopes also had immunogenicity in pancreatic cancer patients.
Peptide vaccine therapies using WT-1235 and VEGFR2169 combined with gemcitabine have already been conducted in pancreatic adenocarcinoma patients [23, 24]. We clarified that WT-1235- and VEGFR2169-specific CTLs induced from PBMCs showed cytotoxicity for human pancreatic cancer cell lines, and the results of further investigations are anticipated.
We performed phenotypic analysis of TAA-derived epitope-specific T cells to determine the most appropriate epitope for immunotherapy in patients with pancreatic adenocarcinoma. Epitope-specific tetramer+ cells in PBMCs were also found in patients without IFN-γ ELISPOT responses, which was consistent with the findings of previous studies [39, 40] and suggested the existence of dysfunctional epitope-specific T cells. Epitope-specific tetramer+ cells were also identified at a very low frequency in PBMCs from healthy volunteers and increased in CTLs induced with TAA-derived peptides, which was also consistent with previous studies in which TAA-specific tetramer+ T cells were detectable in samples from healthy donors [41] or the in vitro stimulation of PBMCs with the epitopes derived from TAA could induce TAA-specific CTLs in healthy volunteers [42], even though the precise mechanism has not yet been clarified. Phenotypic analysis showed that the frequency of T cells with each memory and effector phenotype depended on the patient and also that peptide-specific memory T cells existed in PBMCs of patients with pancreatic adenocarcinoma. Because T cells with the memory phenotype exert stronger antitumor effects by secondary stimulation with the antigen, our results suggest that an additional immunological approach such as that consisting of a TAA-derived protein or peptide, recombinant virus, and engineered tumor cells to boost T cell function may be useful to enhance host antitumor immune responses.
Another purpose of this study was to identify the factors influencing immune responses. Our results suggested that the frequencies of the lymphocyte subsets in peripheral leukocytes were very important in the induction of TAA-specific CTLs. Although the relationship between cancer, inflammation, and immunity has already been documented [43], the precise mechanism has yet to be fully understood. One of the speculated reasons why PBMC from patients with lymphocytopenia could not induce a good immune response in our study is that the release of inhibitory immunological cytokines such as transforming growth factor β or IL-10 from pancreatic adenocarcinoma tissue decreases lymphocyte counts and impairs the function of lymphocytes both systemically and in the microenvironment [44]. It was also reported that lymphocyte counts and CTL responses were prognostic markers in advanced cancer cases receiving peptide vaccine therapy [45, 46]. Our results showing a correlation between the T cell response and outcomes in pancreatic adenocarcinoma patients corresponded to these previous findings, which indicate that restricting the objective to those with an adequate lymphocyte subset could lead to a clinical trial with favorable outcomes.
A limitation of this study was the lack of data for the clinical response. Tumor shrinkage or survival benefits are not always observed in all patients who exhibit immune responses. Further, clinical studies using peptides that could induce TAA-specific CTLs are needed to confirm our findings.
In conclusion, we simultaneously compared T cell responses to various TAA-derived epitopes in patients with pancreatic adenocarcinomas; our results suggested that p53161, hTERT461, WT-1235, and VEGFR2169 were the most suitable epitopes for cancer vaccine therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank Kazumi Fushimi, Maki Kawamura, Nami Nishiyama, and Mikiko Nakamura for their technical assistance.
Conflict of interest
The authors do not have any conflict of interest.
Abbreviations
- CTL
Cytotoxic T lymphocyte
- TAA
Tumor-associated antigen
- ELISPOT
Enzyme-linked immunospot
- MAGE
Melanoma-associated antigen
- hTERT
Human telomerase reverse transcriptase
- WT-1
Wilms tumor-1
- VEGFR
Vascular endothelial growth factor receptor
- PBMC
Peripheral blood mononuclear cells
- PCR
Polymerase chain reaction
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 3.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arai J, Yasukawa M, Ohminami H, Kakimoto M, Hasegawa A, Fujita S. Identification of human telomerase reverse transcriptase-derived peptides that induce HLA-A24-restricted antileukemia cytotoxic T lymphocytes. Blood. 2001;97:2903–2907. doi: 10.1182/blood.V97.9.2903. [DOI] [PubMed] [Google Scholar]
- 5.Ferries E, Connan F, Pages F, Gaston J, Hagnere AM, Vieillefond A, Thiounn N, Guillet J, Choppin J. Identification of p53 peptides recognized by CD8(+) T lymphocytes from patients with bladder cancer. Hum Immunol. 2001;62:791–798. doi: 10.1016/S0198-8859(01)00266-X. [DOI] [PubMed] [Google Scholar]
- 6.Fujie T, Tahara K, Tanaka F, Mori M, Takesako K, Akiyoshi T. A MAGE-1-encoded HLA-A24-binding synthetic peptide induces specific anti-tumor cytotoxic T lymphocytes. Int J Cancer. 1999;80:169–172. doi: 10.1002/(SICI)1097-0215(19990118)80:2<169::AID-IJC1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 7.Gomi S, Nakao M, Niiya F, Imamura Y, Kawano K, Nishizaka S, Hayashi A, Sobao Y, Oizumi K, Itoh K. A cyclophilin B gene encodes antigenic epitopes recognized by HLA-A24-restricted and tumor-specific CTLs. J Immunol. 1999;163:4994–5004. [PubMed] [Google Scholar]
- 8.Harashima N, Tanaka K, Sasatomi T, Shimizu K, Miyagi Y, Yamada A, Tamura M, Yamana H, Itoh K, Shichijo S. Recognition of the Lck tyrosine kinase as a tumor antigen by cytotoxic T lymphocytes of cancer patients with distant metastases. Eur J Immunol. 2001;31:323–332. doi: 10.1002/1521-4141(200102)31:2<323::AID-IMMU323>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Hirohashi Y, Torigoe T, Maeda A, Nabeta Y, Kamiguchi K, Sato T, Yoda J, Ikeda H, Hirata K, Yamanaka N, Sato N. An HLA-A24-restricted cytotoxic T lymphocyte epitope of a tumor-associated protein, survivin. Clin Cancer Res. 2002;8:1731–1739. [PubMed] [Google Scholar]
- 10.Ishizaki H, Tsunoda T, Wada S, Yamauchi M, Shibuya M, Tahara H. Inhibition of tumor growth with antiangiogenic cancer vaccine using epitope peptides derived from human vascular endothelial growth factor receptor 1. Clin Cancer Res. 2006;12:5841–5849. doi: 10.1158/1078-0432.CCR-06-0750. [DOI] [PubMed] [Google Scholar]
- 11.Kawano K, Gomi S, Tanaka K, Tsuda N, Kamura T, Itoh K, Yamada A. Identification of a new endoplasmic reticulum-resident protein recognized by HLA-A24-restricted tumor-infiltrating lymphocytes of lung cancer. Cancer Res. 2000;60:3550–3558. [PubMed] [Google Scholar]
- 12.Kikuchi M, Nakao M, Inoue Y, Matsunaga K, Shichijo S, Yamana H, Itoh K. Identification of a SART-1-derived peptide capable of inducing HLA-A24-restricted and tumor-specific cytotoxic T lymphocytes. Int J Cancer. 1999;81:459–466. doi: 10.1002/(SICI)1097-0215(19990505)81:3<459::AID-IJC21>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Nakao M, Shichijo S, Imaizumi T, Inoue Y, Matsunaga K, Yamada A, Kikuchi M, Tsuda N, Ohta K, Takamori S, Yamana H, Fujita H, Itoh K. Identification of a gene coding for a new squamous cell carcinoma antigen recognized by the CTL. J Immunol. 2000;164:2565–2574. doi: 10.4049/jimmunol.164.5.2565. [DOI] [PubMed] [Google Scholar]
- 14.Nishizaka S, Gomi S, Harada K, Oizumi K, Itoh K, Shichijo S. A new tumor-rejection antigen recognized by cytotoxic T lymphocytes infiltrating into a lung adenocarcinoma. Cancer Res. 2000;60:4830–4837. [PubMed] [Google Scholar]
- 15.Ohminami H, Yasukawa M, Fujita S. HLA class I-restricted lysis of leukemia cells by a CD8(+) cytotoxic T-lymphocyte clone specific for WT1 peptide. Blood. 2000;95:286–293. [PubMed] [Google Scholar]
- 16.Tanaka F, Fujie T, Tahara K, Mori M, Takesako K, Sette A, Celis E, Akiyoshi T. Induction of antitumor cytotoxic T lymphocytes with a MAGE-3-encoded synthetic peptide presented by human leukocytes antigen-A24. Cancer Res. 1997;57:4465–4468. [PubMed] [Google Scholar]
- 17.Tanaka H, Tsunoda T, Nukaya I, Sette A, Matsuda K, Umano Y, Yamaue H, Takesako K, Tanimura H. Mapping the HLA-A24-restricted T-cell epitope peptide from a tumour-associated antigen HER2/neu: possible immunotherapy for colorectal carcinomas. Br J Cancer. 2001;84:94–99. doi: 10.1054/bjoc.2000.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umano Y, Tsunoda T, Tanaka H, Matsuda K, Yamaue H, Tanimura H. Generation of cytotoxic T cell responses to an HLA-A24 restricted epitope peptide derived from wild-type p53. Br J Cancer. 2001;84:1052–1057. doi: 10.1054/bjoc.2000.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wada S, Tsunoda T, Baba T, Primus FJ, Kuwano H, Shibuya M, Tahara H. Rationale for antiangiogenic cancer therapy with vaccination using epitope peptides derived from human vascular endothelial growth factor receptor 2. Cancer Res. 2005;65:4939–4946. doi: 10.1158/0008-5472.CAN-04-3759. [DOI] [PubMed] [Google Scholar]
- 20.Yamada A, Kawano K, Koga M, Matsumoto T, Itoh K. Multidrug resistance-associated protein 3 is a tumor rejection antigen recognized by HLA-A2402-restricted cytotoxic T lymphocytes. Cancer Res. 2001;61:6459–6466. [PubMed] [Google Scholar]
- 21.Yang D, Nakao M, Shichijo S, Sasatomi T, Takasu H, Matsumoto H, Mori K, Hayashi A, Yamana H, Shirouzu K, Itoh K. Identification of a gene coding for a protein possessing shared tumor epitopes capable of inducing HLA-A24-restricted cytotoxic T lymphocytes in cancer patients. Cancer Res. 1999;59:4056–4063. [PubMed] [Google Scholar]
- 22.Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol Immunother. 2005;54:187–207. doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaida M, Morita-Hoshi Y, Soeda A, Wakeda T, Yamaki Y, Kojima Y, Ueno H, Kondo S, Morizane C, Ikeda M, Okusaka T, Takaue Y, Heike Y. Phase 1 trial of Wilms tumor 1 (WT1) peptide vaccine and gemcitabine combination therapy in patients with advanced pancreatic or biliary tract cancer. J Immunother. 2011;34:92–99. doi: 10.1097/CJI.0b013e3181fb65b9. [DOI] [PubMed] [Google Scholar]
- 24.Miyazawa M, Ohsawa R, Tsunoda T, Hirono S, Kawai M, Tani M, Nakamura Y, Yamaue H. Phase I clinical trial using peptide vaccine for human vascular endothelial growth factor receptor 2 in combination with gemcitabine for patients with advanced pancreatic cancer. Cancer Sci. 2010;101:433–439. doi: 10.1111/j.1349-7006.2009.01416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda-Moore Y, Tomiyama H, Miwa K, Oka S, Iwamoto A, Kaneko Y, Takiguchi M. Identification and characterization of multiple HLA-A24-restricted HIV-1 CTL epitopes: strong epitopes are derived from V regions of HIV-1. J Immunol. 1997;159:6242–6252. [PubMed] [Google Scholar]
- 26.Kuzushima K, Hayashi N, Kimura H, Tsurumi T. Efficient identification of HLA-A*2402-restricted cytomegalovirus-specific CD8(+) T-cell epitopes by a computer algorithm and an enzyme-linked immunospot assay. Blood. 2001;98:1872–1881. doi: 10.1182/blood.V98.6.1872. [DOI] [PubMed] [Google Scholar]
- 27.Oiso M, Eura M, Katsura F, Takiguchi M, Sobao Y, Masuyama K, Nakashima M, Itoh K, Ishikawa T. A newly identified MAGE-3-derived epitope recognized by HLA-A24-restricted cytotoxic T lymphocytes. Int J Cancer. 1999;81:387–394. doi: 10.1002/(SICI)1097-0215(19990505)81:3<387::AID-IJC12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 28.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 29.Dodson LF, Hawkins WG, Goedegebuure P. Potential targets for pancreatic cancer immunotherapeutics. Immunotherapy. 2011;3:517–537. doi: 10.2217/imt.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 31.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitz-Winnenthal FH, Galindo-Escobedo LV, Rimoldi D, Geng W, Romero P, Koch M, Weitz J, Krempien R, Niethammer AG, Beckhove P, Buchler MW, Z’Graggen K. Potential target antigens for immunotherapy in human pancreatic cancer. Cancer Lett. 2007;252:290–298. doi: 10.1016/j.canlet.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Singh P, Srinivasan R, Wig JD. Major molecular markers in pancreatic ductal adenocarcinoma and their roles in screening, diagnosis, prognosis, and treatment. Pancreas. 2011;40:644–652. doi: 10.1097/MPA.0b013e31821ff741. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki K, Matsubara H. Recent advances in p53 research and cancer treatment. J Biomed Biotechnol. 2011;2011:978312. doi: 10.1155/2011/978312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizukoshi E, Nakamoto Y, Arai K, Yamashita T, Sakai A, Sakai Y, Kagaya T, Honda M, Kaneko S. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology. 2011;53:1206–1216. doi: 10.1002/hep.24149. [DOI] [PubMed] [Google Scholar]
- 36.Sakakura K, Chikamatsu K, Furuya N, Appella E, Whiteside TL, Deleo AB. Toward the development of multi-epitope p53 cancer vaccines: an in vitro assessment of CD8(+) T cell responses to HLA class I-restricted wild-type sequence p53 peptides. Clin Immunol. 2007;125:43–51. doi: 10.1016/j.clim.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suehara N, Mizumoto K, Tanaka M, Niiyama H, Yokohata K, Tominaga Y, Shimura H, Muta T, Hamasaki N. Telomerase activity in pancreatic juice differentiates ductal carcinoma from adenoma and pancreatitis. Clin Cancer Res. 1997;3:2479–2483. [PubMed] [Google Scholar]
- 38.Bernhardt SL, Gjertsen MK, Trachsel S, Moller M, Eriksen JA, Meo M, Buanes T, Gaudernack G. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: a dose escalating phase I/II study. Br J Cancer. 2006;95:1474–1482. doi: 10.1038/sj.bjc.6603437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizukoshi E, Nakamoto Y, Marukawa Y, Arai K, Yamashita T, Tsuji H, Kuzushima K, Takiguchi M, Kaneko S. Cytotoxic T cell responses to human telomerase reverse transcriptase in patients with hepatocellular carcinoma. Hepatology. 2006;43:1284–1294. doi: 10.1002/hep.21203. [DOI] [PubMed] [Google Scholar]
- 40.Shang XY, Chen HS, Zhang HG, Pang XW, Qiao H, Peng JR, Qin LL, Fei R, Mei MH, Leng XS, Gnjatic S, Ritter G, Simpson AJ, Old LJ, Chen WF. The spontaneous CD8+ T-cell response to HLA-A2-restricted NY-ESO-1b peptide in hepatocellular carcinoma patients. Clin Cancer Res. 2004;10:6946–6955. doi: 10.1158/1078-0432.CCR-04-0502. [DOI] [PubMed] [Google Scholar]
- 41.van den Ancker W, Ruben JM, Westers TM, Wulandari D, Bontkes HJ, Hooijberg E, Stam AG, Santegoets SJ, Ossenkoppele GJ, de Gruijl T, van de Loosdrecht A. Priming of PRAME- and WT1-specific CD8+ T cells in healthy donors but not in AML patients in complete remission: implications for immunotherapy. Oncoimmunology. 2013;2(4):e23971. doi: 10.4161/onci.23971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ho WY, Nguyen HN, Wolfl M, Kuball J, Greenberg PD. In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naïve repertoire. J Immunol Methods. 2006;310:40–52. doi: 10.1016/j.jim.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 43.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bellone G, Smirne C, Mauri FA, Tonel E, Carbone A, Buffolino A, Dughera L, Robecchi A, Pirisi M, Emanuelli G. Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: implications for survival. Cancer Immunol Immunother. 2006;55:684–698. doi: 10.1007/s00262-005-0047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noguchi M, Mine T, Komatsu N, Suekane S, Moriya F, Matsuoka K, Yutani S, Shichijo S, Yamada A, Toh U, Kawano K, Azuma K, Uemura H, Okuno K, Matsumoto K, Yanagimoto H, Yamanaka R, Oka M, Todo S, Sasada T, Itoh K. Assessment of immunological biomarkers in patients with advanced cancer treated by personalized peptide vaccination. Cancer Biol Ther. 2010;10:1266–1279. doi: 10.4161/cbt.10.12.13448. [DOI] [PubMed] [Google Scholar]
- 46.Laheru D, Lutz E, Burke J, Biedrzycki B, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, Hege K, Jaffee E. Allogenic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Cin Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.