Abstract
In the current study, we produced a novel fusion protein (melittin-mutant human interleukin 2, melittin-MhIL-2) comprising a mutant human interleukin 2 (Arg88/Ala125) genetically linked to melittin. The plasmid pET15b-melittin-MhIL-2 (Arg88/Ala125) was transformed into E. coli for protein expression. The expressed melittin-MhIL-2 protein was purified using a series of purification steps. The interleukin 2 (IL-2) activity of melittin-MhIL-2 fusion protein was compared with recombinant human interleukin 2 (rhIL-2) for its ability to induce CTLL-2 proliferation. Moreover, the fusion protein directly inhibits the growth of human ovarian cancer SKOV3 cells in vitro. In an in vivo initial experiment, the fusion protein inhibited tumor growth in ovarian cancer mice. In conclusion, we generated a novel melittin-MhIL-2 fusion protein that retained functional activity of IL-2 and melittin and inhibited tumor growth in vivo.
Keywords: Melittin, Interleukin 2, Mutant, Fusion protein, Human ovarian cancer SKOV3 cells, In vivo tumor growth
Introduction
For the treatment of ovarian cancer, surgery is principle therapy [1], and chemotherapy is also needed to remove the remaining cancer cells. Although cisplatin, carboplatin and paclitaxel are among the drugs that have been used to treat ovarian cancer, many patients develop resistance to these drugs [2–4]. Therefore, improvements of treatment modalities are needed to achieve cancer containment or elimination. Interleukin 2 (IL-2) has been used for immunotherapy of a variety of human malignancies, like melanoma and renal cancer [5, 6].
Cytokines, such as IL-2, are important mediators of immune response against cancer cells [7]. IL-2 is a potent growth factor produced mainly by activated T cells and could stimulate a number of immune cells (e.g., T cells and NK cells) [7, 8]. Human interleukin 2 (hIL-2) is an effective treatment against advanced cancers. However, therapeutic efficacy of hIL-2 is limited by severe systemic toxicity [9–11]. To improve therapeutic efficacy of IL-2, it is critical to develop new strategies without causing severe side effects. Several reports have shown that a series of mutant recombinant human IL-2 (MhIL-2) can increase antitumor activity and minimize systemic toxicity [11–13]. The disulfide bond between 2 cysteine residues in hIL-2 is required for biological activity [14]. The free cysteine at 125 is not involved in the recognition by IL-2 receptor and causes the formation of inclusion body [13]. Substitution of alanine with cysteine at 125 position attenuates the formation of protein aggregates [15]. A mutant hIL-2 (Asn88Arg) was ~3,000-fold more potent in stimulating proliferation of T cells in vitro compared with hIL-2. Therefore, we previously generated a functional mutant hIL-2 (Arg88/Ala125) using site-directed mutagenesis [16].
Melittin, the major component of European bee venom, possesses immunoregulatory and antitumor activities [17–24]. Bee venom can increase T lymphocytes esterase expression in S180 sarcoma mouse and enhance T lymphocyte function [17]. Melittin enhances immune function by enhancing Th1 cells function. Immunomodulatory effects of melittin can be used for the treatment of low immune function, cancer and viral infection [18]. Melittin can inhibit proliferation of hepatoma carcinoma (SMMC-7721, Bel-7402, Hep-3B) [19] and ovarian cancer cells [20], and kill HL-60 [21] and Hela cells [24]. Melittin targets are on both lymphocytes and tumor cells. IL-2 receptors are on lymphocytes. IL-2 could stimulate a number of immune cells (e.g., T cells and NK cells) [7, 8]. IL-2 targets also are on lymphocytes. Therefore, the melittin, as a fusion partner, would work well with IL-2.
In this study, we elected to create a novel fusion protein melittin-MhIL-2 and enhance IL-2 immune regulation and antitumor activity. The melittin-MhIL-2 fusion protein is comprised of a mutant hIL-2 (Arg88/Ala125) genetically linked to melittin. Our results suggest that the melittin-MhIL-2 fusion protein retained functional activity of IL-2 and melittin and inhibited the growth of human ovarian cancer SKOV3 cells in vitro and in vivo tumor growth. The fusion protein demonstrated additive or synergistic antitumor effects. The melittin-MhIL-2 fusion protein may be a potential agent for anticancer therapy.
Materials and methods
Reagents and materials
Recombinant plasmid pGEX-4T-2/Melittin-IL-2 (88Arg) was constructed and stored in our laboratory. The E. coli BL21 (DE3), expression vector pET15b, PCR agent and DNA Clean-up kit were purchased from Promega (USA). Restriction enzymes NdeI and BamHI were purchased from TAKARA (Dalian, China). The oligonucleotide fragments and primers were synthesized by Sangon (Shanghai, China). The rabbit antihuman IL-2 monoclonal antibody was purchased from CHEMICON international, Inc. (USA). The goat antirabbit IgG was purchased from Southern Biotech Associates, Inc. (USA). Recombinant human IL-2 (rhIL-2) was (specific activity: 1.0 × 107 IU/mg) from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). CTLL-2 cell line, an IL-2-dependent T cell line, was cultured in RPMI 1640 supplemented with 10 % fetal bovine serum (FBS) and IL-2. The human ovarian cancer SKOV3 cells were purchased from the American Type Culture Collection (USA). The SKOV3 cells were cultured in RPMI-1640 medium supplemented with 10 % FBS and penicillin/streptomycin (100 U/ml). Melittin was purchased from Nanning Innovation and Technology Pharmaceutical Company (Guangxi, China). BALB/C nude mice were purchased from The National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China).
Construction of expression plasmid pET15b-melittin-MhIL-2 (Arg88/Ala125)
The recombinant plasmid pGEX-4T-2/Melittin-IL-2 (88Arg) as a template was amplified in a reaction system using the primer (P1) 5′-GGACATATGGGAATTGGAGCAGTTCTGAAGG-3′, the primer (P2) 5′-CGGGATCCTCAAGTTAGTGTTGAGATGATGCTTTGAGCAAAGGTAATCCATCTGT-3′. PCR conditions were as follows: pre-denaturation at 94 °C for 5 min; denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 90 s, reacting for 35 cycles; extension at 72 °C for 10 min. The PCR product was digested with Nde I and BamH I and cloned into the same sites of pET15b to get pET15b-melittin-MhIL-2 (Arg88/Ala125). The fragment was validated by restriction enzyme digestion with Nde I and BamH I and by DNA sequencing.
Expression and extraction of protein
The plasmid pET15b-melittin-MhIL-2 (Arg88/Ala125) was transformed into E. coli BL21 (DE3) for protein expression, followed by enrichment for transformed colonies by growth in Luria–Bertani (LB) medium supplemented with ampicillin (100 mg/L). The transformants were grown overnight at 37 °C in LB medium supplemented with ampicillin. The bacteria were subcultured for 16 h when grown to OD600 = 0.6 at 27 °C and added by isopropy-β-D-thiogalactoside (IPTG) (0.6 mM). The best yield of full-length protein was obtained when cultures were harvested 8 h after IPTG induction. Approximately, 6 g wet weight cells were obtained per liter of culture. The bacterial pellets were rinsed with buffer consisting of 20 mM Tris–HCl (pH 8.0), recentrifuged, snap-frozen in liquid nitrogen and stored at −80 °C.
Protein purification
The melittin-MhIL-2 (Arg88/Ala125) protein was purified using the HiTrap™ Chelating column (Amersham Biosciences), the HiTrap™ Desalting column (Amersham Biosciences), the enterokinase digesting and Superdex 75 gel filtration column (Amersham Biosciences). The purity of the fusion protein was identified by UV/colorimetric analysis. The yield of purified protein was quantified using a MicroBCA kit and densitometric analysis of SDS-PAGE.
Western blot analysis
The purified melittin-MhIL-2 (Arg88/Ala125) fusion protein was subjected to 10 % SDS-PAGE and electroblotted onto a polyvinylidene difluoride (PVDF) membrane (Schleicher&Schuell, Germany). The PVDF membrane was probed with a 1:500 dilution of monoclonal rabbit antihuman hIL-2 antibody (CHEMICON international Co., USA) and detected with a 1:4,000 dilution of goat antirabbit IgG (Southern Biotech Associates Co., USA) conjugated to HRP and diaminobenzidine (DAB) color development reagent.
The IL-2 activity assay of melittin-MhIL-2 (Arg88/Ala125)
The biological activity of IL-2 of the melittin-MhIL-2 (Arg88/Ala125) fusion protein was determined by its ability to stimulate proliferation of IL-2-dependent CTLL-2 cells. Serial dilutions (8.0, 2.0, 0.5, 0.125 μM) of the fusion protein were incubated for 48 h with 2 × 104 CTLL-2 cells that had been starved of IL-2. One micro Ci of [3H] thymidine was added to the medium for the last 18 h, and cell proliferation was measured by [3H] thymidine incorporation. The same dilutions of recombinant hIL-2 were used as standard.
Growth inhibition assay of human ovarian cancer cells SKOV3
The human ovarian cancer cells SKOV3 were cultured in RPMI-1640 medium supplemented with 10 % heat-inactivated fetal bovine serum (FBS) and penicillin/streptomycin (100 U/ml). Cell cultures were then maintained at 37 °C in a humidified atmosphere with 5 % CO2. Cells were plated on T-25 tissue culture flasks, and on the second day, the medium was replaced with medium containing different concentration of melittin-MhIL-2 (Arg88/Ala125) fusion protein (0, 1, 2 and 4 μM). We used the same molar concentration of rhIL-2 and melittin as experimental control. All tests were carried out for 24, 48 and 72 h, and each was performed three times and in triplicate. Attached cells were trypsinized, and the number of living cells in each flask was determined by the trypan blue dye exclusion test: Aliquots of cells resuspended in PBS were briefly mixed with an equal volume of 0.4 % trypan blue in 0.85 % saline and counted using a hematocytometer.
Animal model
Human ovarian cancer SKOV3 cells, 2 × 105/ml cells in 500 μl buffer, were injected subcutaneously into the in-house bred BALB/C nude mice. After 2 weeks, tumor was generated in mice. Tumor-bearing mice were randomly divided into four groups. The experimental procedures and the animal use and care protocols were approved by the Committee on Ethical Use of Animals of *** University, in compliance with the National Committee for Animal Experiments guidelines on animal welfare.
Results
Expression of recombinant melittin-MhIL-2 (Arg88/Ala125) fusion protein
The plasmid pET15b-melittin-MhIL-2 (Arg88/Ala125) was validated by restriction enzyme digestion with Nde I and BamH I and by DNA sequencing. The recombinant melittin-MhIL-2 (Arg88/Ala125) fusion protein was highly expressed after induction at 37 °C with 0.6 mM IPTG. SDS-PAGE of cell lysates followed by scanning with the Amersham Pharmacia Image Master VDS image analysis system revealed a major protein band of the expected 37 kDa size (Fig. 1a). Under the condition of 0.6 mM IPTG, 27 °C culture temperature and 8 h inducing, the soluble recombinant melittin-MhIL-2 (Arg88/Ala125) fusion protein expressed by E. coli. in the supernatant of cell lysate (Fig. 1b). The main part of the recombinant melittin-MhIL-2 (Arg88/Ala125) fusion protein produced by E. coli. was in soluble form.
Fig. 1.
SDS-PAGE analysis of melittin-MhIL-2 (Arg88/Ala125) fusion protein in E. coli. a Lane 1 uninduced whole cell lysate of pET15b-melittin-MhIL-2 (Arg88/Ala125)/BL21 at 37 °C. Lane 2 whole cell lysate of pET15b-melittin-MhIL-2 (Arg88/Ala125)/BL21 induced at 37 °C. Lane 3 uninduced whole cell lysate of pET15b/BL21 at 37 °C. Lane 4 whole cell lysate of pET15b/BL21 induced at 37 °C. Lane M protein molecular weight markers. b Lane 1 supernatan of cell lysate of pET15b-melittin-MhIL-2 (Arg88/Ala125)/BL21 induced at 27 °C. Lane 2 sediments t of cell lysate of pET15b-melittin-MhIL-2 (Arg88/Ala125)/BL21 induced at 27 °C. Lane M protein molecular weight markers
Purification and characterization of melittin-MhIL-2 (Arg88/Ala125) fusion protein
The melittin-MhIL-2 (Arg88/Ala125) fusion protein was efficiently purified to greater than 95 % purity by a combination of HiTrap™ Chelating column chromatography, HiTrap™ Desalting column chromatography, enterokinase cleavage and Superdex 75 gel filtration column chromatography. The concentration of the purified melittin-MhIL-2 (Arg88/Ala125) fusion protein was approximately 100 mg/L. The melittin-MhIL-2 (Arg88/Ala125) had an apparent molecular weight of 19 kDa (Fig. 2a). The identity of melittin-MhIL-2 (Arg88/Ala125) was confirmed with immuno-reactivity with a rabbit antihuman hIL-2 antibody in Western blot analysis (Fig. 2b). The results were consistent with our expected.
Fig. 2.
The SDS-PAGE and Western blot analysis of the purified melittin-MhIL-2 (Arg88/Ala125). The melittin-MhIL-2 (Arg88/Ala125) had an apparent molecular weight of 19 kDa. The identity of melittin-MhIL-2 (Arg88/Ala125) was confirmed with immuno-reactivity with a rabbit antihuman hIL-2 antibody in Western blot analysis. The results were consistent with our expected. a Lane 1 purified melittin-MhIL-2 (Arg88/Ala125). Lane 2 supernatant of total cell lysate. Lane M protein molecular weight markers. b Lane 1 melittin-MhIL-2 (Arg88/Ala125) and antihuman hIL-2 antibody reaction. Lane 2 uninduced control. Lane M protein molecular weight markers
IL-2 activity of melittin-MhIL-2 (Arg88/Ala125)
The IL-2 activity of melittin-MhIL-2 (Arg88/Ala125) was compared with rhIL-2 for its ability to induce the proliferation of an IL-2-dependent T cell line, CTLL-2. As shown in Fig. 3, the IL-2 activity of melittin-MhIL-2 (Arg88/Ala125) is comparable to rhIL-2.
Fig. 3.
The biological activity of IL-2 of the melittin-MhIL-2 (Arg88/Ala125) fusion protein was determined by its ability to stimulate proliferation of IL-2-dependent CTLL-2 cells. Serial dilutions (8.0, 2.0, 0.5, 0.125 μM) of the fusion protein were incubated for 48 h with 2 × 104 CTLL-2 cells that had been starved of IL-2. One micro Ci of [3H] thymidine was added to the medium for the last 18 h, and cell proliferation was measured by [3H] thymidine incorporation. The same dilutions of recombinant hIL-2 were used as standard; Error bars represent SD; *p < 0.05 compared to negative control
Melittin-MhIL-2 (Arg88/Ala125) directly inhibits the growth of human ovarian cancer SKOV3 cells in vitro
To assess the inhibitory effect of melittin-MhIL-2 (Arg88/Ala125) on cell growth of ovarian cancer SKOV3 cells, we analyzed cell viability by direct cell counting. The cells were treated with several concentrations of melittin-MhIL-2 (Arg88/Ala125) (0, 1, 2 and 4 μM) for 24 h. As shown in Fig. 4, melittin-MhIL-2 (Arg88/Ala125) and melittin inhibited cell proliferation of ovarian cancer SKOV3 cells in a concentration-dependent manner; rhIL-2 did not inhibit cell proliferation of ovarian cancer SKOV3 cells.
Fig. 4.
Effect of melittin-MhIL-2 (Arg88/Ala125) on cell viability in ovarian cancer SKOV3 cells. Relative cell survival rate was determined by counting live and dead cells. The results were expressed as a percentage of viable cells. Columns, means of three experiments, with triplicates of each experiment; Error bars represent SD; *p < 0.05 compared to untreated control cells. The most effective inhibitory melittin-MhIL-2 (Arg88/Ala125) concentration was 4 μM, causing growth inhibition of 77 %
We examined the growth curves of SKOV3 cells, following incremental increases in concentration of melittin-MhIL-2 (Arg88/Ala125) for 24, 48 and 72 h. In all examined concentrations (0, 1, 2 and 4 μM), treatment for 48 h was most effective. Leaving the medication for a total of 72 h did not add to its effectiveness as measured by the decrease in the number of cancer cells. Therefore, any further examination of the effect of the medication on the ovarian cell line was examined for 48 h only. Figure 4 shows the significant growth inhibiting effects of melittin-MhIL-2 (Arg88/Ala125) in increasing concentrations, as mean of three different experiments, each of which is composed of a triplicate. The number of cells is presented as a percentage of those cells of the control (0 μM melittin-MhIL-2 (Arg88/Ala125)) cases. The most effective inhibitory melittin-MhIL-2 (Arg88/Ala125) concentration was 4 μM, causing growth inhibition of 77 %.
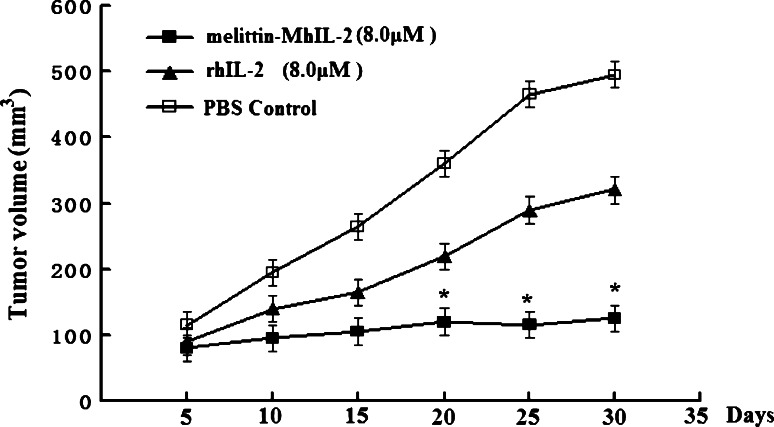
Melittin-MhIL-2 (Arg88/Ala125) inhibits tumor growth in human ovarian cancer SKOV3 mice model and exhibits enhanced antitumor activity compared to rhIL-2
Mice were intraperitoneally injected with the fusion protein melittin-MhIL-2 or rhIL-2 or PBS control. A group was as PBS control. In the other two groups, mice were, respectively, treaded with melittin-MhIL-2 (8.0 μM) or rhIL-2 (8.0 μM). The treatment was repeated every day for 10 days. Tumor volume was measured on day 1 of the treatment. Subsequent measurement was once every 5 day. After the treatment, mice were continuously observed 3 weeks. Reduced tumor volume was observed (Fig. 5) and, as shown in the figure, the tumors in the melittin-MhIL-2-treated group increased from 80 to 125 mm3 in 25 days compared to 5 days in the rhIL-2 group, suggesting that the melittin-MhIL-2 inhibited tumor growth in human ovarian cancer SKOV3 mice model.
Fig. 5.
Effect of melittin-MhIL-2 (Arg88/Ala125) on tumor growth in vivo. Mice were intraperitoneally injected with the fusion protein melittin-MhIL-2 or rhIL-2 or PBS control. A group was as PBS control. In the other two groups, mice were, respectively, treaded with melittin-MhIL-2 (8.0 μM) or rhIL-2 (8.0 μM). The treatment was repeated every day for 10 days. Tumor volume was measured on day 1 of the treatment. Subsequent measurement was once every 5 day. Error bars represent SD; *p < 0.05 compared to rhIL-2
Discussion
In this study, the mutant hIL-2 (Arg88/Ala125) was genetically fused with the coding sequence of melittin. Our data clearly demonstrated that the fusion protein melittin-MhIL-2 (Arg88/Ala125) maintained the functions of both IL-2 and melittin and inhibited tumor growth in vivo.
IL-2 is a well-characterized growth factor for immune effector cells which play critical roles in tumor control and rejection. However, the major drawback of rhIL-2 therapy is its severe systemic toxicity [25]. Several reports have shown that a series of mutant recombinant human IL-2 (MhIL-2) can increase antitumor activity and decrease toxicity [12–15]. Therefore, we previously generated a functional mutant hIL-2 (Arg88/Ala125) using site-directed mutagenesis [16]. In the present study, the IL-2 activity of melittin-MhIL-2 (Arg88/Ala125) was compared with rhIL-2 for its ability to induce CTLL-2 proliferation. The results revealed that the fusion protein melittin-MhIL-2 (Arg88/Ala125) retained its IL-2 functional activity. In the present study, we attempted to assess PBMC cytotoxicity against SKOV3 cells after treatment with the fusion protein or recIL-2. However, the effect of the fusion protein on PBMC may be cytotoxic or trigger NK blasts. Also, melittin itself is a cytotoxic peptide. Thus, the LDH release could result from direct killing of melittin, not from PBMC cytotoxicity, and the LDH release read-out may not be an appropriate. In the future study, we will apply more appropriate methods to determine the effect of the fusion protein on PBMC.
Melittin, a small linear peptide consisting of 26 amino acids, is the major component of bee venom [26]. Bee venom has been used as a traditional medicine to treat back pain, rheumatism and skin diseases by its antibacterial, antiviral and anti-inflammatory effects [26–28]. Moreover, several studies have demonstrated that bee venom and/or melittin have anticancer effects including prostate [28], liver [29], breast, cervical, renal [27] and ovarian cancer cells [20]. Our finding is consistent with previous studies showing that melittin inhibits cell growth of ovarian cancer cell lines [20, 30]. In addition, both the fusion protein melittin-MhIL-2 (Arg88/Ala125) and melittin directly inhibited cell proliferation of ovarian cancer SKOV3 cells in a concentration-dependent manner; rhIL-2 did not directly inhibit cell proliferation of SKOV3 cells in vitro. The most effective inhibitory melittin-MhIL-2 (Arg88/Ala125) concentration was 4 μM, causing growth inhibition of 77 %. The maximum inhibitory effect of the fusion protein melittin-MhIL-2 (Arg88/Ala125) on cell proliferation was seen after 48 h. Our experiments demonstrated that the fusion protein also retained its melittin functional activity.
According to the reports, some novel fusion proteins containing IL-2 were generated and used in some other experimental study [31, 32]. In this study, we constructed a novel fusion protein in which mutant hIL-2 was genetically fused with melittin. The in vivo data from the current study suggest that the fusion protein melittin-MhIL-2 (Arg88/Ala125) inhibited tumor growth in human ovarian cancer SKOV3 mice model and exhibited enhanced antitumor activity compared to rhIL-2. The fusion protein may demonstrate additive or synergistic antitumor effects. IL-2 can stimulate T cells, B cells, monocytes, macrophages, LAK cells and NK cells [33, 34]. Our initial tumors studies were carried out in nude mice lacking T cells, thus the antitumor effects of the fusion protein melittin-MhIL-2 (Arg88/Ala125) are likely due to cells of the innate immune system and/or the effect of melittin. Further experiments are being carried out.
In summary, we generated a novel fusion protein melittin-MhIL-2 (Arg88/Ala125). It directly inhibits the growth of human ovarian cancer SKOV3 cells in vitro and in vivo tumor growth. We plan to first improve the production of melittin-MhIL-2 (Arg88/Ala125) and optimize the method of purification. More animal studies are planned to test the antitumor effect and mechanism of this fusion protein in vivo.
Conflict of interest
The authors declare that there are no conflict of interest.
References
- 1.Ramirez I, Chon HS, Apte SM. The role of surgery in the management of epithelial ovarian cancer. Cancer Control. 2011;18:22–30. doi: 10.1177/107327481101800104. [DOI] [PubMed] [Google Scholar]
- 2.Al-Alem L, Southard RC, Kilgore MW, Curry TE. Specific thiazolidinediones inhibit ovarian cancer cell line proliferation and cause cell cycle arrest in a PPARγ independent manner. PLoS ONE. 2011;6:e16179. doi: 10.1371/journal.pone.0016179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Bahlani S, Fraser M, Wong AY, Sayan BSR, Melino G, Tsang BK. P73 regulates cisplatin-induced apoptosis in ovarian cancer cells via a calcium/calpain-dependent mechanism. Oncogene. 2011;30:4219–4230. doi: 10.1038/onc.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain A, Dubashi B, Reddy KS, Jain P. Weekly paclitaxel in ovarian cancer-the latest success story. Curr Oncol. 2011;18:16–17. doi: 10.3747/co.v18i1.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reisfeld RA, Becker JC, Gillies SD. Immunocytokines: a new approach to immunotherapy of melanoma. Melanoma Res. 1997;7(Suppl 2):S99–106. [PubMed] [Google Scholar]
- 6.Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228:307–319. doi: 10.1097/00000658-199809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- 8.Henney CS, Kuribayashi K, Kern DE, Gillis S. Interleukin 2 augments natural killer cell activity. Nature. 1981;291:335–338. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- 9.Ortaldo JR, Mason AT, Gerard JP, Henderson LE, Farrar W, Hopkins RF, 3rd, Herberman RB, Rabin H. Effects of natural and recombinant IL 2 on regulation of IFN gamma production and natural killer activity: lack of involvement of the Tac antigen for these immunoregulatory effects. J Immunol. 1984;133:779–783. [PubMed] [Google Scholar]
- 10.Siegel JP, Puri RK. Interleukin-2 toxicity. J Clin Oncol. 1991;9:694–704. doi: 10.1200/JCO.1991.9.4.694. [DOI] [PubMed] [Google Scholar]
- 11.Maas RA, Dullens HF, Den Otter W. Interleukin-2 in cancer treatment: disappointing or (still) promising? A review. Cancer Immunol Immunother. 1993;36:141–148. doi: 10.1007/BF01741084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanafelt AB, Lin Y, Shanafelt MC, Forte CP, Dubois-Stringfellow N, Carter C, Gibbons JA, Cheng SL, Delaria KA, Fleischer R, Greve JM, Gundel R, Harris K, Kelly R, Koh B, Li Y, Lantz L, Mak P, Neyer L, Plym MJ, Roczniak S, Serban D, Thrift J, Tsuchiyama L, Wetzel M, Wong M, Zolotorev A. A T-cell-selective interleukin-2 mutein exhibits potent antitumor activity and is well tolerated in vivo. Nat Biotechnol. 2000;6:1197–1202. doi: 10.1038/81199. [DOI] [PubMed] [Google Scholar]
- 13.Ju G, Collins L, Kaffka KL, Tsien W, Simpson R. Structure-function analysis of human interleukin-2. Identification of amino acid residues required for biological activity. J Biol Chem. 1987;262:5723–5731. [PubMed] [Google Scholar]
- 14.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 15.Moya G, González LJ, Huerta V, García Y, Morera V, Pérez D, Breña F, Araña M. Isolation and characterization of modified species of a mutated (Cys125-Ala) recombinant human interleukin-2. J Chromatogr A. 2002;971:129–142. doi: 10.1016/S0021-9673(02)00845-2. [DOI] [PubMed] [Google Scholar]
- 16.Liu M, Wang B, Sun G, Qian D, Yan Z, Song X, Ding S. Expression, purification, and characterization of a functional mutant recombinant human interleukin-2. Protein Pept Lett. 2010;17:1280–1284. doi: 10.2174/092986610792231474. [DOI] [PubMed] [Google Scholar]
- 17.Zhu X, Wang J, Wang R, Zhang X. Immunomodulatory effects of bee venom on S180 sarcoma mice. Pharmacol Clin Chin Materia Medica. 2000;16:24–25. [Google Scholar]
- 18.Wang Q, Lu Y, Zang Y, Zhang Y. Study on the immune-regulating mechanism of the bee venom. Chin J Immunol. 2000;16:542–544. [Google Scholar]
- 19.Liu L, Ling C, Huang X. Study on purification of melittin and its effect on anti-tumor in vitro. Chin J Biochem Pharm. 2003;24:163–166. [Google Scholar]
- 20.Jo M, Park MH, Kollipara PS, An BJ, Song HS, Han SB, Kim JH, Song MJ, Hong JT. Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol Appl Pharmacol. 2012;258:72–81. doi: 10.1016/j.taap.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Saini SS, Chopra AK, Peterson JW. Melittin activates endogenous phospholipase D during cytolysis of human monocytic leukemia cells. Toxicon. 1999;37:1605–1619. doi: 10.1016/S0041-0101(99)00110-5. [DOI] [PubMed] [Google Scholar]
- 22.Arora AS, de Groen PC, Croall DE, Emori Y, Gores GJ. Hepatocellular carcinoma cells resist necrosis during anoxia by preventing phospholipase-mediated calpain activation. J Cell Physiol. 1996;167:434–442. doi: 10.1002/(SICI)1097-4652(199606)167:3<434::AID-JCP7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 23.Kubo H, Loegering DA, Adophson CR, Gleich GJ. Cytotoxic properties of eosinophil granule major basic protein for tumor cells. Int Arch Allergy Immunol. 1999;118:426–428. doi: 10.1159/000024154. [DOI] [PubMed] [Google Scholar]
- 24.Lazarev VN, Parfenova TM, Gularyan SK, Misyurina OY, Akopian TA, Govorun VM. Induced expression of melittin, an antimicrobial peptide, inhibits infection by Chlamydia trachomatis and Mycoplasma hominis in a Hela cell line. Int J Antimicrob Agents. 2002;19:133–137. doi: 10.1016/S0924-8579(01)00479-4. [DOI] [PubMed] [Google Scholar]
- 25.Tarhini AA, Agarwala SS. Interleukin-2 for the treatment of melanoma. Curr Opin Investig Drugs. 2005;6:1234–1239. [PubMed] [Google Scholar]
- 26.Wang C, Chen T, Zhang N, Yang M, Li B, Lü X, Cao X, Ling C. Melittin, a major component of Bee venom, sensitizes human hepatocellular carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by activating caMKII-TAK1-JNK/p38 and inhibiting iκBα kinase-NFκB. J Biol Chem. 2009;284:3804–3813. doi: 10.1074/jbc.M807191200. [DOI] [PubMed] [Google Scholar]
- 27.Park JH, Jeong YJ, Park KK, Cho HJ, Chung IK, Min KS, Kim M, Lee KG, Yeo JH, Park KK, Chang YC. Melittin suppresses PMA-induced tumor cell invasion by inhibiting NF-kappaB and AP-1-dependent MMP-9 expression. Mol Cells. 2010;29:209–215. doi: 10.1007/s10059-010-0028-9. [DOI] [PubMed] [Google Scholar]
- 28.Park MH, Choi MS, Kwak DH, Oh KW, Yoon DY, Han SB, Song HS, Song MJ, Hong JT. Anti-cancer effect of bee venom in prostate cancer cells through activation of caspase pathway via inactivation of NF-ΚB. Prostate. 2011;71:801–812. doi: 10.1002/pros.21296. [DOI] [PubMed] [Google Scholar]
- 29.Liu S, Yu M, He Y, Xiao L, Wang F, Song C, Sun S, Ling C, Xu Z. Melittin prevents liver cancer cell metastasis through inhibition of the Rac1-dependent pathway. Hepatology. 2008;47:1964–1973. doi: 10.1002/hep.22240. [DOI] [PubMed] [Google Scholar]
- 30.Holle L, Song W, Holle E, Wei Y, Wagner T, Yu X. A matrix metalloproteinase 2 cleavable melittin/avidin conjugate specifically targets tumor cells in vitro and in vivo. Int J Oncol. 2003;22:93–98. [PubMed] [Google Scholar]
- 31.Belmont HJ, Price-Schiavi S, Liu B, Card KF, Lee HI, Han KP, Wen J, Tang S, Zhu X, Merrill J, Chavillaz PA, Wong JL, Rhode PR, Wong HC. Potent antitumor activity of a tumor-specific soluble TCR/IL-2 fusion protein. Clin Immunol. 2006;121:29–39. doi: 10.1016/j.clim.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Penafuerte C, Bautista-Lopez N, Boulassel MR, Routy JP, Galipeau J. The human ortholog of granulocyte macrophage colony-stimulating factor and interleukin-2 fusion protein induces potent ex vivo natural killer cell activation and maturation. Cancer Res. 2009;69:9020–9028. doi: 10.1158/0008-5472.CAN-09-2322. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, Linehan WM, Robertson CN, Lee RE, Rubin JT, Seipp CA, Simpson CG, White DE. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316:889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- 34.Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155:1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]