Abstract
Cryoablation is a low-invasive surgical procedure for management of malignant tumors. Tissue destruction is obtained by repeated deep freezing and thawing and results in coagulative necrosis and in apoptosis. This procedure induces the release of tumor-associated antigens and proinflammatory factors into the microenvironment. Local administration of immature dendritic cells (DCs) potentiates the immune response induced by cryoablation. To further augment the induction of long-lasting antitumor immunity, we investigated the clinical value of combining cryoimmunotherapy consisting of cryoablation and inoculation of immature DCs with administration of the immune adjuvant, CpG oligodeoxynucleotides. Injection of the murine Lewis lung carcinoma, D122-luc-5.5 that expresses the luciferase gene, results in spontaneous metastases, which can be easily monitored in vivo. The local tumor was treated by the combined treatment. The clinical outcome was assessed by monitoring tumor growth, metastasis in distant organs, overall survival, and protection from tumor recurrence. The nature of the induced T cell responses was analyzed. Combined cryoimmunotherapy results in reduced tumor growth, low metastasis and significantly prolonged survival. Moreover, this treatment induces antitumor memory that protected mice from rechallenge. The underlying suggested mechanisms are the generation of tumor-specific type 1 T cell responses, subsequent induction of cytotoxic T lymphocytes, and generation of systemic memory. Our data highlight the combined cryoimmunotherapy as a novel antitumor vaccine with promising preclinical results. Adjustment of this technique into practice will provide the therapeutic benefits of both, ablation of the primary tumor and induction of robust antitumor and antimetastatic immunity.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1520-4) contains supplementary material, which is available to authorized users.
Keywords: Cryoablation, Dendritic cell, CpG-ODN, Tumor-associated antigens, Cytotoxic T lymphocytes
Introduction
Cryoablation of tumors offers a promising alternative for conventional surgical procedures. It has been used increasingly over the last few decades in a variety of human malignancies, such as prostate [1], renal [2], hepatic [3], esophageal [4], lung [5], bone, and soft tissue tumors [6]. Compared with surgical excision, the main advantages of this technique are the potential for minimal invasiveness, less damage to the surrounding tissues, reduced blood loss, low cost, and lower morbidity and mortality. Another advantage of cryoablation is the well-documented augmentation of the immune response [7]. Cryoablation induces cell death directly by damaging cellular membranes and organelles and indirectly by causing vascular compromise through microvascular thrombosis [8]. Cellular coagulation necrosis leads to the release of intracellular contents, including tumor-associated antigens (TAAs) and proinflammatory cytokines, into the tumor microenvironment. These serve as potent inducers of the antitumor response that is mediated through dendritic cells (DCs). Thus, cryosurgery has the potential to affect both as local and systemic treatment by direct ablation of the primary tumor, and by eradication of recurrence in distant organs, which is mediated by the immune arm [7]. However, many reported only a partial systemic response with no effect on metastasis [9–11], while others reported an immunosuppressive outcome following cryosurgery [12].
One of the main factors that affect the generation of the immune response following thermal tumor destruction is the availability of professional antigen-presenting cells (APCs) in the necrotic microenvironment. DCs are critical APCs that efficiently cross-present self-antigens on the MHC class I and II molecules and subsequently promote T cell priming and induce activation of TH1 helper cells and cytotoxic T cells (CTL). DCs potently capture antigens in their immature state and effectively cross-present them in their mature state [7]. Thus, we previously suggested a cryoimmunotherapy treatment that combines between cryoablation and subsequent intratumoral injection of ex vivo generated immature DCs. This modality produced robust, TH1-mediated, tumor-specific CTL responses and generated effector memory T cells that mediated protection from tumor rechallenge and reduced metastasis [13]. Although life extension was significantly improved when compared with cryoablation alone, the overall survival rate did not exceed 50 %. These findings prompted us to boost antitumor immunity by combining cryoimmunotherapy consisting of DCs administration with an immune adjuvant, CpG oligodeoxynucleotides (CpG-ODN).
Unmethylated CpG-ODNs are pathogen-expressed molecules, which are common in bacterial and viral DNA, but are suppressed and methylated in vertebrates. They signal through toll-like receptor 9 (TLR9), which is constitutively expressed in human and murine plasmacytoid DCs (pDCs) and B cells as well as in murine myeloid DCs [14]. Signal transduction, induced by CpG-ODN binding to TLR9, results in the activation of nuclear factor-κB (NF-κβ), cytokine production, upregulation of co-stimulatory molecules, and subsequent induction of antigen-specific humoral and cellular antitumor immunity [15]. Intratumoral administration of CpG-ODN following cryoablation of established melanoma tumors induced potent antitumor responses that protected mice from rechallenge [16].
Using a non-immunogenic highly metastatic murine lung carcinoma model, we sought to investigate the therapeutic potential of combined cryoimmunotherapy consisting of tumor cryoablation with subsequent intratumoral co-administration of ex vivo generated immature DCs and CpG-ODN. This treatment provides the factors that potentially synergize to induce robust antitumor immunity: a strong source for TAAs in the pro-inflammatory context; large amount of DCs in an immature state that is optimal for antigen uptake and activation; a potent immune modulator that further potentiates DC-activated responses; and shared spatial and temporal conditions. The combined modality was compared to single cryoimmunotherapies that include cryoablation in combination with either DCs or CpG-ODN. We studied the induction of functional antitumor immune responses and their persistence, indicative of memory induction, and evaluated the impact on tumor growth, metastasis, survival, and protection against tumor rechallenge.
Materials and methods
Mice and cell lines
Male C57BL/6 (H-2b) mice (8–10 weeks old) were purchased from Harlan Laboratories (Israel). Mice were maintained at the AAALAC-accredited, SPF facility of the Weizmann Institute. All animal procedures were approved and conducted under Institutional Animal Care and Use Committee guidelines.
3LL Lewis lung carcinoma and B16 melanoma clones of C57BL/6 origin (H-2b) were maintained in DMEM supplemented with 10 % heat-inactivated FCS (HyClone, South Logan, UT, USA), 2 mM l-glutamine, 1 % sodium pyruvate, 1 % non-essential amino acids (all from Sigma, St. Louis, MO, USA), and combined antibiotics (Life Technologies, Grand Island, NY, USA). The D122 clone of 3LL is highly metastatic and poorly immunogenic [17]. These cells were stably transfected with a pCDNAIII-luciferase plasmid to produce D122-luc-5.5 and cultured in presence of 1,000 μg/ml G-418 (Life Technologies). The 3LL-Kb39.5, a Kb-transfected 3LL clone [18], was maintained in presence of 500 μg/ml G-418 (Life Technologies). Yac-1 was maintained in RPMI supplemented with 10 % heat-inactivated FCS, 2 mM l-glutamine, 5 × 10−5 M β-mercaptoethanol, and combined antibiotics. The F10.9 highly metastatic clone of the B16 melanoma [19] and Yac-1 were used as non-related targets in CTL assay.
Reagents
The CpG oligodeoxynucleotide 1826 (CpG-ODN1826, 5′-TCC ATG ACG TTC CTG ACG TT-3′) was phosphorothioated and synthesized by Sigma (Lyon, France). CpG-ODN1826 was reconstituted in sterile pyrogen-free water and then diluted in PBS. Murine granulocyte–macrophage colony-stimulating factor (GM-CSF) (Prospec, Rehovot, Israel) was reconstituted in 20 mM acetic acid and diluted in PBS (phosphate-buffered saline). Lipopolysaccharide (LPS) was purchased from Sigma (Saint Louis, MO, USA).
Generation of dendritic cells from murine bone marrow cells
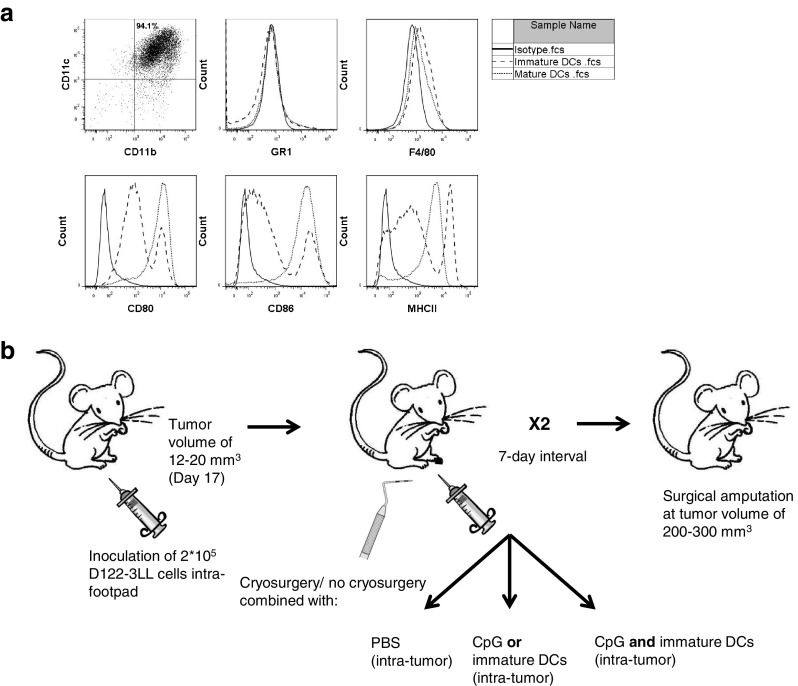
The procedure was previously described [13, 20] and used with minor modifications. On day 8 of the culture, non-adherent cells were collected, adjusted to 15 × 106 cells/ml, resuspended in fresh medium containing 100 U/ml GM-CSF, and seeded in 100-mm tissue culture plates for 24 h. On day 9, non-adherent cells were harvested, washed, and resuspended in PBS to 80 × 106 cells/ml before injection. To maturate DCs, 1 μg/ml of LPS was added to the medium for another 24 h. On day 10, non-adherent cells cultured with or without LPS were harvested and analyzed by FACS (LSR II, BD, San Jose, CA, USA) for cell surface markers characterizing mature and immature DCs (Fig. 1a).
Fig. 1.
Cryoimmunotherapy models. a Characterization of bone marrow-derived DCs generated ex vivo: expression of cell surface markers on naïve and LPS-treated cells. b Illustration of treatments and vaccination protocols
Treatment modes
For the D122 spontaneous metastasis model, 5–12 mice per group were inoculated intrafootpad (i.f.p.) with 2 × 105 cells/mouse of D122-luc-5.5. Tumors were measured with calipers, and when reached the volume of 12–20 mm3 (), mice were treated twice, at 7 days interval between two treatments. Anesthetized mice (ketamine/xylazine) underwent cryotherapy by applying mild pressure for 10 s with liquid nitrogen-frozen metal probe to the tumor-bearing foot. A 5-mm-sized iceball was created. To ensure complete thawing of the treated area before vaccination, mice were immunized 1 h later [13]. In the single cryoimmunotherapy mode, local cryotherapy was followed by intratumor (i.t.) injection of 2 × 106 immature DCs or 10–50 μg CpG, whereas, in the combined cryoimmunotherapy, it was followed by in situ co-administration of immature DC and CpG, in a volume of 50 μl. Control mice were injected i.t. with immature DC, CpG, or a combination of DC and CpG (immunotherapies) with PBS, in a volume of 50 μl (Fig. 1b). Buprenorphine (0.1 mg/kg, s.c., for analgesia) was given 1 h after the treatments and for 2 more consecutive days. When the tumor reached the volume of 200–300 mm3 (as measured with calipers), the tumor-bearing leg was amputated below the knee. Mice were monitored daily and killed when moribund or 100–120 days post-amputation. Lung metastatic load was assessed by lung weight and by in vivo imaging analysis (IVIS). Survival was defined as the day when mice were killed. In some experiments, mice that survived for 90 days post-amputation were rechallenged with 2 × 105 D122-luc-5.5 in the other foot and monitored for tumor growth kinetics and survival.
In vivo optical imaging
Optical imaging of metastases in mice was performed by in vivo fluorescence imaging with an IVIS 200 small animal imaging system (Xenogen, Alameda, CA, USA). Identical illumination settings (lamp voltage, filters, f/stop, field of views, binning) were used for acquiring all images, and fluorescence emission was normalized to photons per second per centimeter squared per steradian (p s-1 cm-2 sr-1). Metastatic load was detected in mice following treatments. Mice were injected with 30 mg/ml of firefly d-luciferin (Xenogen, Alameda, CA, USA) i.p. in a volume of 100 μl and after 2 min were anaesthetized with mixture of 15 % xylazine HCL (20 mg/ml, V.M.D Arendonk, Belgium) and 85 % ketamin HCL (100 mg/ml, Fort Dodge, IA, USA) i.p. in a volume of 50 μl. The fur covering the chest area was shaved to reduce light absorbance and scattering. Images were acquired 8 and 10 min after firefly d-luciferin injection and analyzed using Living Image 3.1 software (Xenogen, Alameda, CA, USA).
FACS analysis
For cell surface markers and T regulatory cells staining, mice (3 per group) were killed 7 days after the second treatment. Single-cell suspensions from spleens and draining lymph nodes were prepared and adjusted to 1 × 106 cells/100 μl in staining buffer (PBS with 0.5 % BSA and 0.1 % sodium azide). Anti-CD16/32 (1 μg, e-Bioscience, San Diego, CA, USA) was added for 15 min at 4 °C, followed by fluorochrome-conjugated antibodies (CD4, CD8, CD11c, CD11b, Gr-1, CD44, CD62L, CD25, CD80, CD86, MHC class II, F4/80, Foxp3 eBioscience) for 30 min at 4 °C in the dark.
For intracellular staining of cytokines, cells (1 × 106) were incubated for 6 h at 37 °C, 5 % CO2 in lymphocyte medium supplemented with 50 ng/ml of phorbol 12-myristate 13-acetate (Sigma) and 750 ng/ml ionomycin (Sigma). Brefeldin A (GolgiPlug, 1 μg/ml, BD) was added for the last 4 h. Then, cells were harvested, suspended in staining buffer, and blocked with anti-CD16/32 as above. Anti-CD4 or CD8 antibodies were added, followed by washing and fixation with 4 % paraformaldehyde for 20 min at 4 °C. Further, cells were permeabilized in PBS containing 5 % FCS, 0.1 % saponin (Sigma), and 0.1 % sodium azide for 15 min at 4 °C and incubated with anti-IFN-γ, TNF-α, or IL-4 (eBioscience) for 30 min. Samples were acquired and electronically compensated on LSR II (BD) and exported for analysis in FlowJo (Tree Star, Ashland, OR, USA).
In vitro cytotoxicity assay
C57BL/6 mice surviving the indicated treatments and naïve control groups were rechallenged with 2 × 105 D122-luc-5.5 cells in the other footpad. At 40 days post-tumor inoculation, mice were killed and splenocytes were subjected to in vitro cytotoxicity assay previously described [13]. Briefly, splenocytes were seeded on monolayers of irradiated (7000RAD) mitomycin C (80 μg/ml, Sigma)-treated D122-luc-5.5 cells. The cultures were incubated for 5 days in complete lymphocyte medium (RPMI medium supplemented with 10 % FCS, 2 mM l-glutamine, 1 % sodium pyruvate, 1 % non-essential amino acids, 25 mM HEPES pH 7.4, 5 × 10−5 M β-mercaptoethanol, and combined antibiotics). Then, the cultures were harvested and separated on lympholyte-M gradients (Cedarlane Laboratories Ltd., Hornby, Ontario, Canada) by centrifugation at 2,400 rpm for 30 min at 18 °C. Viable cells were adjusted to 1 × 107 cell/ml and seeded at several concentrations in U-shaped microtiter wells.
Target cells (D122-luc-5.5, Kb39.5, B16-F10.9) were incubated with 10 μl 35S methionine (EASYTAG methionine, 10 mCi/ml, PerkinElmer, Waltham, MA, USA) over night. Then, 35S methionine-labeled cells were washed and adjusted to 5 × 104 cells/ml in complete lymphocyte medium. These were incubated with the prepared effector cultures for 5 and 18 h (at four effector-to-target ratios ranging from 100:1 to 12.5:1). Cultures were terminated by centrifugation. Supernatants were mixed with Microscint-20 scintillation fluid. Released 35S methionine was measured by β counter (PerkinElmer). Percentage of specific lysis was calculated as follows:
Statistical analysis
Statistical analyses of the lung weights, luciferase activity, and cell population analysis were conducted with 1-way ANOVA. Tumor growth kinetics and CTL assay analyses were conducted with 2-way ANOVA. Significance of the differences was assessed by Bonferroni multiple comparison post-test. Data presented as mean ± SEM. Survival curves were generated using product limit (Kaplan–Meier) method, and comparisons were conducted using log-rank (Mentel-Cox) test.
Results
Combined cryoimmunotherapy reduces tumor growth and metastasis
We tested whether combined cryoimmunotherapy, which consists of cryosurgery and co-administration of DCs and CpG, is more effective than a single mode of cryoimmunotherapy in eliciting antitumor response. We induced spontaneous metastases by inoculation of D122-Luc-5.5 cells i.f.p. Mice were treated immunotherapies or with a single (DCs or CpG) or combined (DCs and CpG) cryoimmunotherapy as described in materials and methods (Fig. 1b). The tumor-bearing feet were measured with calipers to monitor the kinetics of tumor growth. While single cryoimmunotherapy with CpG mildly inhibited tumor growth, the most significant decrease in tumor growth was detected in mice treated with combined cryoimmunotherapy. Minimal effects on tumor growth were observed in groups treated by immunotherapies alone (Fig. 2a). When tumors reached the volume of 200–300 mm3, tumor-bearing feet were amputated, and mice were monitored for their post-surgical survival. As shown in Fig. 2b, all groups treated with cryoimmunotherapy showed improved survival, when compared with other treatments. However, the most significant increase was detected in the group treated with combined cryoimmunotherapy (Fig. 2b).
Fig. 2.

Increased survival of mice treated with combined cryoimmunotherapy. Mice were inoculated i.f.p. with D122-luc-5.5 and then treated as described in materials and methods. a Tumor growth kinetics measured with calipers (tumor volume, mm3). b Post-surgical survival of mice treated with different protocols. Combined results of five independent experiments (n = 5–8 mice per group per experiment). *P < 0.05; **P < 0.001; ***P < 0.0001
Next, we studied whether the increased survival of mice treated with combined cryoimmunotherapy stems from the anti-metastatic effect induced by this treatment. Mice were treated as described in Fig. 1b and were subjected to in vivo optical imaging analysis (IVIS) of lung metastases, 57 and 72 days post-tumor inoculation. On day 57, most of the mice in the control groups suffered from progressive lung metastases, while in mice treated with combined cryoimmunotherapy metastases were undetectable by IVIS (Fig. 3a, b). By day 72, surviving mice had fewer metastases or metastases undetectable by IVIS, as can be appreciated from the fluorescence in the lung area (Fig. 3a, c). To further validate the results obtained by IVIS, lung weights were measured, as an indication for metastatic spread. In accordance with the results obtained by IVIS, only combined treatment statistically reduced metastatic loads and resulted in normal lung weights (Fig. 3d). Immunotherapies alone showed a mild antimetastatic effect (Fig. 3b–d and supplementary Fig. 1).
Fig. 3.
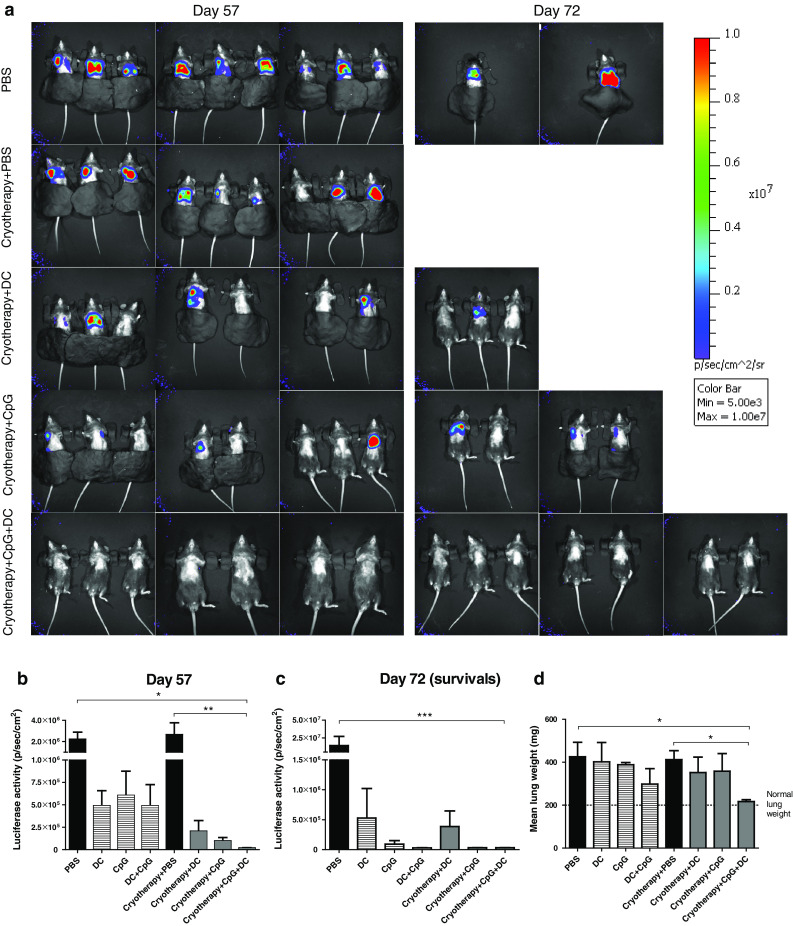
Combined cryoimmunotherapy induces anti-metastatic immunity. a Representative images of in vivo D122-luc-5.5 tumor metastasis monitored 57 and 72 days following tumor inoculation (captured with an IVIS 200 imaging system). Luciferase activity 57 (b) and 72 (c) days following tumor inoculation. d Lungs weight, as an indication for metastatic load, was detected 100–120 days post-amputation or when mice were moribund. The data are presented as mean lungs weight (mg). Lungs weight of naïve 25-week-old mouse is 200 mg. Combined results of five independent experiments (n = 5–8 mice per group per experiment). *P < 0.05; **P < 0.001; ***P < 0.0001
Combined cryoimmunotherapy modulates immune responses
To study the mechanisms involved in the anti-tumoral activity induced by combined cryoimmunotherapy, we analyzed the immune cell populations residing in the tumor-draining lymph node (dLN) 7 days following the second treatment. Initially, we tested the activation status of T lymphocytes, as manifested by cell surface expression of CD44 and CD62L. Relatively to control treatments and to single cryoimmunotherapy, combined cryoimmunotherapy markedly increased the percentage of effector CD4+ (Fig. 4a and supplementary Fig. 2a) and CD8+ (supplementary Fig. 2b) T cells, as was reflected by high and low expression levels of CD44 and CD62L, respectively. Moreover, we detected an elevation in CD4+ and CD8+ T effector cell populations also in spleens of mice treated with combined cryoimmunotherapy, indicative of the systemic immunity induced by this treatment (supplementary Fig. 3).
Fig. 4.
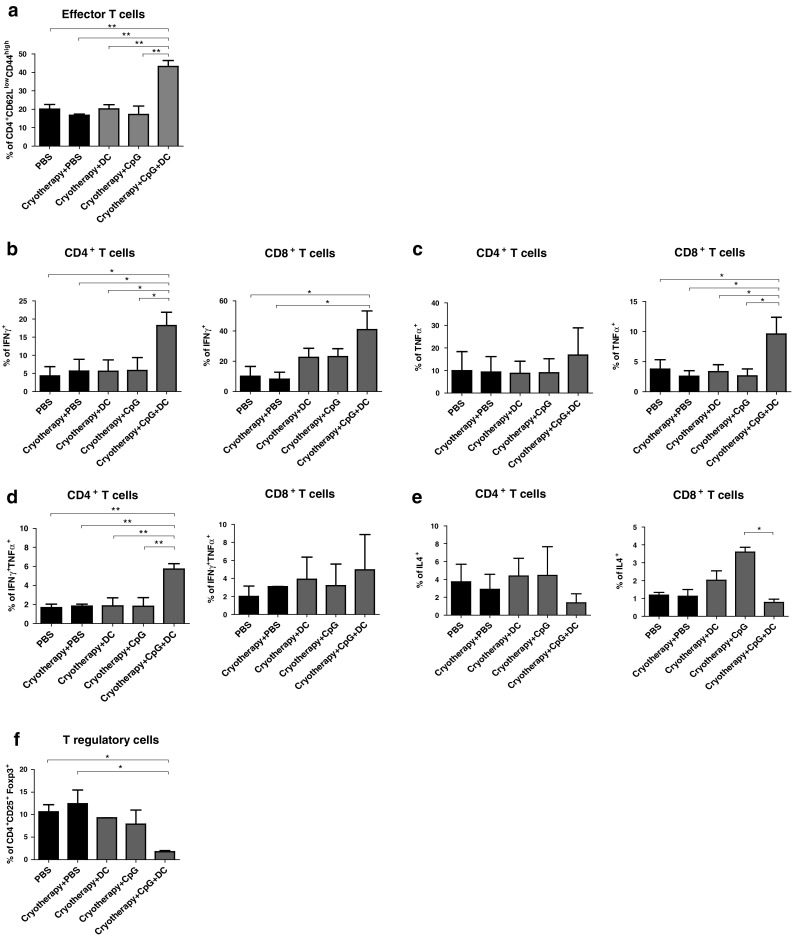
Immunocyte populations in the dLNs of tumor-bearing mice following treatments. Mice were inoculated i.f.p. with D122-luc-5.5 and further treated as described in materials and methods. Eight days post-second treatment, the dLNs were excised and analyzed by flow cytometry. a Percentages of CD4+ effector T cells (CD62Llow CD44high). b–e Percentages of CD4+ and CD8+ T cells secreting IFNγ (b), TNFα (c), IFNγ and TNFα (d), IL4 (e). f Percentages of Treg (CD4+CD25+Foxp3+) cells. Data presented as mean ± SEM, combined results of three independent experiments (n = 5–8 mice per group per experiment). *P < 0.05; **P < 0.001
To further elucidate the antitumor mechanism, we studied the T helper subsets that were induced by the different treatments. Types 1 and 2 immune responses are characterized by the patterns of cytokines they produce. Cytokine-releasing profiles of CD4+ and CD8+ T cells were characterized on day 8 post-second treatment by flow cytometric analysis. The percentages of CD4+ and CD8+ T cells that produced IFNγ were significantly higher in mice treated with combined cryoimmunotherapy, when compared with single cryoimmunotherapy or control treatments (Fig. 4b, supplementary Fig. 4a). Additionally, this treatment resulted in increased levels of TNFα (Fig. 4c, supplementary Fig. 4a) and double positive (IFNγ and TNFα) (Fig. 4d, supplementary Fig. 4a) secreting CD4+ and CD8+ T cells, indicating induction of CD8+ CTL and CD4+ T helper 1 (TH1) tumor-specific effector cells. TH2 CD4+ T cells, that predominantly secret high levels of IL4, alter adaptive immunity by inducing T cell anergy, inhibiting T cell-mediated cytotoxicity as well as promoting humoral immune responses directed by B cells [21]. While single cryoimmunotherapy with DC or CpG increased the percentage of IL4 secreting CD4+ and CD8+ T cells, the proportion of predominantly expressing IL4 cells in the combined treatment was similar to that detected in control treatments (Fig. 4e). Taken together, these findings suggest that type 1 rather than type 2 responses, promoting antitumor immunity, were induced by combined cryoimmunotherapy.
Regulatory T cells (Tregs), key mediators of immune tolerance toward self-antigens, potently suppress antitumor immunity and are often responsible for the failure of immunotherapeutic regimens [22–24]. Combined cryoimmunotherapy resulted in a significant decrease in CD4+CD25+Foxp3+ Treg population residing in the tumor dLN, relatively to control and single cryoimmunotherapy treatments (Fig. 4f, supplementary Fig. 4b). This indicates that the combined therapy induces local inhibition of this suppressive cell population, which augments the antitumor response.
Cryoimmunotherapy induces potent systemic antitumor immunity
To analyze whether cryoimmunotherapy induces systemic antitumor immunity, we inoculated D122-Luc-5.5 cells into mice that were treated as described previously. Eighty to 95 days following amputation of the primary tumor-bearing foot, surviving mice, and a group of naïve mice were rechallenged with 2 × 105 D122-Luc-5.5 cells in the other foot. All mice, in both single and combined cryoimmunotherapy treated groups, rejected the second tumor as opposed to the control group (Fig. 5a). This result points to a specific antitumor memory that was induced by cryoimmunotherapy.
Fig. 5.
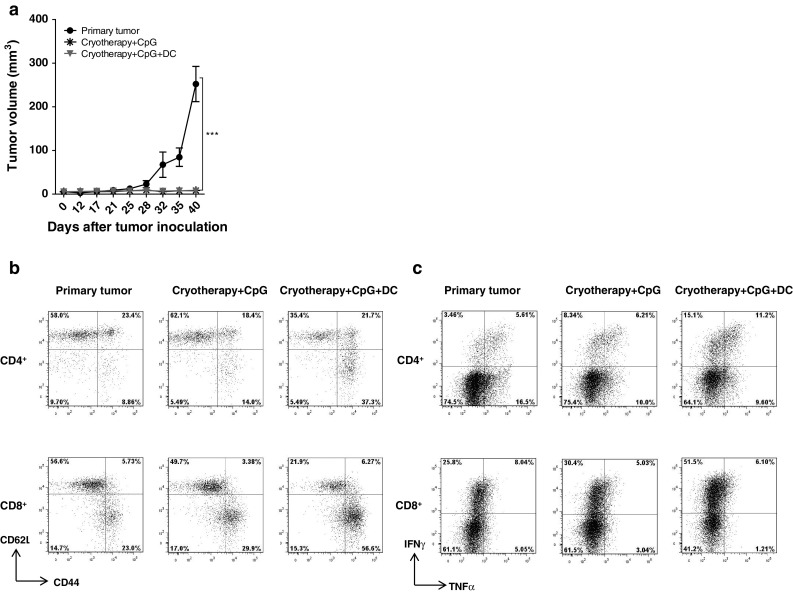
Protection against parental tumor rechallenge in mice that rejected the primary tumor. Mice were inoculated with D122-luc-5.5 cells and treated as previously described. Surviving mice (80–95 days following amputation of the primary tumor-bearing foot) and a control group of six age-matched naïve mice were rechallenged with 2 × 105 D122-luc-5.5 cells in the other foot. a Tumor growth kinetics measured with calipers (tumor volume, mm3). Forty-five days after tumor rechallenge, cells were isolated from spleens and analyzed for: b percentages of CD4+ and CD8+ effector T cells (CD62Llow CD44high), c percentages of IFNγ and TNFα secreting CD4+ and CD8+ T cells. Combined data of two independent experiments (n = 5–8 mice per group per experiment). ***P < 0.0001
At 45 days after tumor rechallenge, splenocytes were isolated and analyzed for T cell activation and helper subsets. As shown in Fig. 5b, mice that received combined therapy showed a significant increase in both CD4+ and CD8+ T effector cell populations. Moreover, this treatment resulted in induction of TH1 and CTL responses, as reflected by the elevation of IFNγ producing CD4+ and CD8+ T lymphocytes (Fig. 5c).
Combined cryoimmunotherapy induces in vitro CTL activity
To evaluate the CTL response in vitro, splenocytes from rechallenged mice were tested for their CTL activity against relevant and irrelevant tumor targets. D122-Luc5.5 and the 3LL-Kb39.5, a Kb-transfected 3LL-D122 clone, served as specific targets, B16-F10.9 cells served as unrelated target, and Yac-1 cells were used for detecting natural killer (NK) cell activity. Only splenocytes from groups treated with the combined treatment showed significant killing activity against the specific Lewis lung carcinoma targets (Fig. 6a, b). These CTLs were tumor specific, since no killing of the unrelated melanoma clone or Yac-1 cells was detected (Fig. 6c, supplementary Fig. 5). These findings suggest that though single cryotherapy managed to induce specific antitumor memory, only combined therapy resulted in the generation of effector memory cells. Noteworthy, 10 μg of CpG were sufficient to induce CTL activity when they were co-administrated with DCs subsequently to cryosurgery. Single cryoimmunotherapy with 50 μg of CpG also induced mild CTL antitumor response, when compared with the group with primary tumor. However, the combined treatment with co-administration of 50 μg of CpG and DCs resulted in markedly increased CTL activity (Fig. 6d).
Fig. 6.

Induction of systemic antitumor immunity by combined cryoimmunotherapy. Mice were inoculated with D122-luc-5.5 cells and treated as previously described. Surviving mice (80–95 days following amputation of the primary tumor-bearing foot) and a control group of six age-matched naïve mice were rechallenged with 2 × 105 D122-luc-5.5 cells in the other foot. Spleens were removed 45 days later, and cells were stimulated with irradiated D122-luc-5.5 cells for 5 days. Further, they were incubated with the following 35S-methionine-labeled target cells: D122-luc-5.5 (a), Kb39.5 (b), and B16-F10.9 (c). CTL assays were carried out at four effector:target ratios ranging from 100:1 to 12.5:1, in triplicates. d Specific killing of D122-luc-5.5 at effector:target ratio of 100:1 is shown for the different CpG-ODN concentrations. n = 5–8 mice per group; *P < 0.05; **P < 0.001; ***P < 0.0001
Discussion
Minimally invasive therapies are an alternative approach to surgical intervention in the treatment of malignant diseases. In situ cryoablation of malignant tissues leads to cellular necrosis and apoptosis that stimulate innate immune response by generating a supportive immune platform for DC activation, subsequently inducing antigen-specific adaptive immunity [25]. However, to achieve a reliable clinical response, there is a necessity to augment this process [8]. Local administration of immune adjuvants that activate DCs is a common vaccination strategy that induces tumor-associated inflammation and protective immunity [26]. One of the frequently used adjuvants in anti-cancer treatments is synthetic ODN that contain unmethylated cytosine-phosphate-guanine (CpG) motifs. CpG-ODN was shown to potently induce activation and maturation of DCs by engaging TLR9 [14]. Upon activation through TLR9 signaling, DCs secrete type-1 IFNs, express increased levels of co-stimulatory molecules CD80 and CD86, and subsequently initiate cell-mediated TH1 and CTL responses [14]. Preclinical studies in a variety of murine tumor models demonstrate antitumor activity of CpG-ODN, not only as a monotherapy but also in combination with other therapies [27, 28]. den Brok et al. [16] demonstrated that TLR stimulation by CpG, combined with cryoablation, eradicated primary and recurrent murine melanoma tumors by modulating DCs in vivo. However, when using immune adjuvants, it is both difficult to assure the directed migration of DCs to the tumor microenvironment and to control their quantity and quality. The DCs maturation state is of great importance when considering effective induction of immunity, since immature rather than mature DCs are considered to be efficient in antigen ingestion and processing [29]. These obstacles could be surpassed by the use of ex vivo generated DCs. Numerous studies showed that ex vivo generated DCs potently evoke specific antitumor responses [30, 31]. The selection of tumor antigens to be presented by these cells, as well as the provision of DC maturation and activation signals, is important parameters in successful induction of immunity [32, 33]. Previously, we demonstrated, in mouse models of lung carcinoma and melanoma, that intratumoral injections of immature DCs following cryosurgery resulted in generation of robust antitumor responses and reduced lung metastasis [13]. However, only 50 % survival was achieved.
This study aimed to improve the therapeutic value of cryoimmunotherapy by applying a novel immunization protocol. Herein, we report that in situ tumor destruction by cryoablation combined with co-administration of immune stimulatory TLR agonist, CpG, and ex vivo generated immature DCs induced tumor-specific immune responses that enhanced robust systemic immunity, increased survival (Fig. 2), and inhibited lung metastasis (Fig. 3). Our immunization strategy incorporates a combination of factors, including a strong source for tumor antigens in a supportive pro-inflammatory context, ex vivo generated antigen-unloaded immature DCs, and a potent DCs modulator, CpG-ODN. The route and the timing of CpG inoculation were shown to be of great influence on the clinical outcome. Only simultaneous cryosurgery and peritumoral CpG injection were shown to induce long-lasting potent antitumor responses due to the prerequisite for colocalization of antigen uptake by DCs and their concurrent stimulation [34, 35]. Co-administration of DCs and CpG immediately following cryosurgery ensures that CpG remains in close proximity to the TAAs and to the DCs. Activated DCs induce robust TH1-type and CTL responses, as was shown by an elevation in IFNγ and TNFα secreting effector CD4+ and CD8+ T cells (Fig. 4a–d), which control tumor expansion and initiate persistent systemic immunity. Although intratumoral administration of DC and CpG without cryoablation shows mild reduction in tumor metastasis, the overall survival shows a significant improvement of combined cryoimmunotherapy (Fig. 2b).
Antitumor immune responses might be suppressed by several immunoregulatory populations. Treg cells can recognize self-antigens, including tumor-associated antigens with high avidity, and when properly stimulated, to suppress effector T cell function, directly through IL-10 and TGFβ secretion and indirectly by altering DCs maturation and activation [36]. Tregs constitutively express cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), and their immunosuppressive function was shown to be dependent on its signaling [37]. Disruptive radiofrequency ablation (RFA) of murine melanoma, in conjunction with CTLA-4 blockade, was shown to induce antitumor immunity [25], presumably due to suppression of Tregs. Likewise, it was shown that only co-injection of anti-CTLA-4 together with CpG depleted tumor-infiltrating Tregs and resulted in tumor eradication [38]. Thus, immunization protocols that reduce Treg expansion and activation would be the most beneficial when considered to be combined with cryotherapy. Only combined cryoimmunotherapy succeeded in overcoming the immunosuppressive tumor microenvironment and resulted in a significant reduction in CD4+CD25+Foxp3+ Treg population residing in the tumor dLN (Fig. 4f).
TH2 cells are another population that may suppress the generation of effective CTLs by secreting IL-4, which alters their capacity to kill and to infiltrate into tumors [39]. Indeed, our findings indicate that when compared with single cryoimmunotherapies, the combined treatment reduced the suppressive TH2 population (Fig. 4e).
One of the major goals of tumor immune vaccines is to induce durable and long-lasting functional CTLs. Properly stimulated DCs will efficiently cross-present the processed antigens to CD8+ T cells and prime the differentiation of these cells into effector CTLs [40]. In this regard, the combined cryoimmunotherapy protected mice that had survived primary tumors from rechallenge (Fig. 5a). High levels of IFNγ secreting CD8+ T cells that express CD44high and CD62Llow on the cell surface were abundant in spleens of the rechallenged mice, primarily treated with the combined treatment (Fig. 5c, b). These cells seem to underlie the detected protection, since they transacted strong tumor-specific CTL responses, indicating on the induction of long-term systemic memory (Fig. 6).
Cryoablation combined with co-administration of DCs and CpG shows promising results in the murine lung carcinoma model. However, in order to apply this vaccination regimen to human trials, some adjustments may be considered. While using CpG to induce DC-mediated antitumor immunity has shown promising results in mouse pre-clinical studies, the clinical application of CpG in humans has thus far showed only partial clinical success [41]. This may stem from the differential expression of TLR9 on DC subsets between humans and mice. While in mice, TLR9 is expressed on both pDC and myeloid (mDC) DCs, human mDCs express all TLRs except TLR7 and TLR9, which are selectively expressed by pDCs [42]. Recent studies suggest that pDCs and mDCs should cooperate synergistically to induce potent antitumor responses, especially when stimulated by cryoablation [43]. Thus, in order to adjust the suggested immunization protocol into clinical practice, TLRs other than TLR9 should be considered as targets for stimulation. Moreover, a simultaneous stimulation of several TLRs may be even more beneficial.
In conclusion, although some adjustments are needed in order to apply this therapeutic mode for use in humans, this study introduces a novel and promising vaccination approach for the treatment of solid tumors and the prevention of metastases development.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank Hagai Tavori for generating the D122-luc-5.5 clone. Israel Science Foundation, the Lewis Family Charitable Trust, and a research grant from the estate of John Hunter (to L. Eisenbach). L. Eisenbach is the incumbent of the George F. Duckwitz Chair of Cancer Research.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Tsivian M, Polascik TJ. Focal cryotherapy for prostate cancer. Curr Urol Rep. 2010;11:147–151. doi: 10.1007/s11934-010-0100-1. [DOI] [PubMed] [Google Scholar]
- 2.Rioja J, Tzortzis V, Mamoulakis C, Laguna MP. Cryotherapy for renal tumors: current status and contemporary developments. Actas Urol Esp (English Edition) 2010;34:309–317. doi: 10.1016/S2173-5786(10)70076-7. [DOI] [PubMed] [Google Scholar]
- 3.Ng KM, Chua TC, Saxena A, Zhao J, Chu F, et al. Two decades of experience with hepatic cryotherapy for advanced colorectal metastases. Ann Surg Oncol. 2012;19:1276–1283. doi: 10.1245/s10434-011-2025-4. [DOI] [PubMed] [Google Scholar]
- 4.Greenwald BD, Dumot JA, Abrams JA, Lightdale CJ, David DS, et al. Endoscopic spray cryotherapy for esophageal cancer: safety and efficacy. Gastrointest Endosc. 2010;71:686–693. doi: 10.1016/j.gie.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S-H, Choi W-J, Sung S-W, Kim Y-K, Kim C-H, et al. Endoscopic cryotherapy of lung and bronchial tumors: a systematic review. Korean J Intern Med. 2011;26:137–144. doi: 10.3904/kjim.2011.26.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishida H, Yamamoto N, Tanzawa Y, Tsuchiya H. Cryoimmunology for malignant bone and soft-tissue tumors. Int J Clin Oncol. 2011;16:109–117. doi: 10.1007/s10147-011-0218-2. [DOI] [PubMed] [Google Scholar]
- 7.Sabel MS. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology. 2009;58:1–11. doi: 10.1016/j.cryobiol.2008.10.126. [DOI] [PubMed] [Google Scholar]
- 8.Sidana A, Chowdhury WH, Fuchs EJ, Rodriguez R. Cryoimmunotherapy in urologic oncology. Urology. 2010;75:1009–1014. doi: 10.1016/j.urology.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Redondo P, del Olmo J, López-Diaz de Cerio A, Inoges S, Marquina M, et al. Imiquimod enhances the systemic immunity attained by local cryosurgery destruction of melanoma lesions. J Investig Dermatol. 2007;127:1673–1680. doi: 10.1038/sj.jid.5700777. [DOI] [PubMed] [Google Scholar]
- 10.Udagawa M, Kudo-Saito C, Hasegawa G, Yano K, Yamamoto A, et al. Enhancement of immunologic tumor regression by intratumoral administration of dendritic cells in combination with cryoablative tumor pretreatment and Bacillus Calmette-Guerin cell wall skeleton stimulation. Clin Cancer Res. 2006;12:7465–7475. doi: 10.1158/1078-0432.CCR-06-1840. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann NE, Coad JE, Huot CS, Swanlund DJ, Bischof JC. Investigation of the mechanism and the effect of cryoimmunology in the Copenhagen rat. Cryobiology. 2001;42:59–68. doi: 10.1006/cryo.2001.2305. [DOI] [PubMed] [Google Scholar]
- 12.Shibata T, Suzuki K, Yamashita T, Takeichi N, Mark M, Hosokawa M, Kobayashi HAM. Immunological analysis of enhanced spontaneous metastasis in WKA rats following cryosurgery. Anticancer Res. 1998;18(4A):2483–2486. [PubMed] [Google Scholar]
- 13.Machlenkin A, Goldberger O, Tirosh B, Paz A, Bar-haim E, et al. Combined dendritic cell cryotherapy of tumor induces systemic antimetastatic immunity. Clin Cancer Res. 2005;11:4955–4961. doi: 10.1158/1078-0432.CCR-04-2422. [DOI] [PubMed] [Google Scholar]
- 14.Krieg AM. Therapeutic potential of toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 15.Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009;61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 16.den Brok MHMGM, Sutmuller RPM, Nierkens S, Bennink EJ, Toonen LWJ, et al. Synergy between in situ cryoablation and TLR9 stimulation results in a highly effective in vivo dendritic cell vaccine. Cancer Res. 2006;66:7285–7292. doi: 10.1158/0008-5472.CAN-06-0206. [DOI] [PubMed] [Google Scholar]
- 17.Eisenbach L, Hollander N, Greenfeld L, Yakor H, Segal S, et al. The differential expression of H-2 K versus H-2D antigens, distinguishing high- metastatic from low- metastatic clones, is correlated with the immunogenic properties of the tumor cells. Int J Cancer. 1984;34:567–573. doi: 10.1002/ijc.2910340421. [DOI] [PubMed] [Google Scholar]
- 18.Plaksin D, Gelber C, Feldman M, Eisenbach L. Reversal of the metastatic phenotype in Lewis lung carcinoma cells after transfection with syngeneic H-2 Kb gene. Proc Natl Acad Sci USA. 1988;85:4463–4467. doi: 10.1073/pnas.85.12.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porgador A, Feldman M, Eisenbach L. H-2 Kb transfection of B16 melanoma cells results in reduced tumorigenicity and metastatic competence. J Immunogenet. 1989;16:291–303. doi: 10.1111/j.1744-313X.1989.tb00475.x. [DOI] [PubMed] [Google Scholar]
- 20.Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/S0022-1759(98)00204-X. [DOI] [PubMed] [Google Scholar]
- 21.DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29:309–316. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosmaczewska A, Ciszak L, Potoczek S, Frydecka I. The significance of Treg cells in defective tumor immunity. Arch Immunol Ther Exp (Warsz) 2008;56:181–191. doi: 10.1007/s00005-008-0018-1. [DOI] [PubMed] [Google Scholar]
- 23.Curiel T. Tregs and rethinking cancer immunotherapy. J Clin Investig. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 25.Den Brok MHMGM, Sutmuller RPM, van der Voort R, Bennink EJ, Figdor CG, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024–4029. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- 26.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 27.Krieg AM. Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene. 2008;27:161–167. doi: 10.1038/sj.onc.1210911. [DOI] [PubMed] [Google Scholar]
- 28.Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4:249–258. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 29.Figdor CG, de Vries IJM, Lesterhuis WJ, Melief CJM. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 30.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueno H, Schmitt N, Klechevsky E, Pedroza-Gonzalez A, Matsui T, et al. Harnessing human dendritic cell subsets for medicine. Immunol Rev. 2010;234:199–212. doi: 10.1111/j.0105-2896.2009.00884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parmiani G, De Filippo A, Novellino L, Castelli C. Unique human tumor antigens: immunobiology and use in clinical trials. J Immunol. 2007;178:1975–1979. doi: 10.4049/jimmunol.178.4.1975. [DOI] [PubMed] [Google Scholar]
- 33.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S-I, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nierkens S, den Brok MH, Roelofsen T, Wagenaars JA, Figdor CG, et al. Route of administration of the TLR9 agonist CpG critically determines the efficacy of cancer immunotherapy in mice. PLoS ONE. 2009;4:e8368. doi: 10.1371/journal.pone.0008368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nierkens S, den Brok MH, Sutmuller RPM, Grauer OM, Bennink E, et al. In vivo colocalization of antigen and CpG [corrected] within dendritic cells is associated with the efficacy of cancer immunotherapy. Cancer Res. 2008;68:5390–5396. doi: 10.1158/0008-5472.CAN-07-6023. [DOI] [PubMed] [Google Scholar]
- 36.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell RC, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Investig. 2013;123:2447–2463. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sasaki K, Pardee AD, Okada H, Storkus WJ. IL-4 inhibits VLA-4 expression on Tc1 cells resulting in poor tumor infiltration and reduced therapy benefit. Eur J Immunol. 2008;38:2865–2873. doi: 10.1002/eji.200838334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuertes MB, Kacha AK, Kline J, Woo S-R, Kranz DM, et al. Host type I IFN signals are required for antitumor CD8 + T cell responses through CD8{alpha} + dendritic cells. J Exp Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt C. Clinical setbacks for toll-like receptor 9 agonists in cancer. Nat Biotechnol. 2007;25:825–826. doi: 10.1038/nbt0807-825. [DOI] [PubMed] [Google Scholar]
- 42.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::AID-IMMU3388>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 43.Nierkens S, den Brok MH, Garcia Z, Togher S, Wagenaars J, et al. Immune adjuvant efficacy of CpG oligonucleotide in cancer treatment is founded specifically upon TLR9 function in plasmacytoid dendritic cells. Cancer Res. 2011;71:6428–6437. doi: 10.1158/0008-5472.CAN-11-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.