Abstract
Immunotherapeutic approaches are emerging as promising new treatment options for patients with solid cancers. The host immune system in cancer patients is dysfunctional due to a number of reasons. The level of immunosuppression is variable at the time of diagnosis and depends on the particular cancer entity, stage, and prior anti-cancer therapies. For many cancer entities, the immune alterations of the respective patient population have not been further characterized even though a patient’s immunophenotype may be prognostic for the course of the disease or predictive for clinical/biological response to immunotherapy. In this study, we used flow cytometry to determine the phenotype of peripheral blood mononuclear cells (PBMCs) from 30 patients with heavily pre-treated, advanced adenocarcinoma of the stomach and gastro-esophageal junction. The frequencies and activation status of relevant immune effector populations were determined in PBMCs and compared to those of healthy individuals. This report provides comprehensive immune phenotyping data of a patient population with a high medical need.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1596-x) contains supplementary material, which is available to authorized users.
Keywords: Gastric adenocarcinoma, Immune phenotyping, Anti-tumor immune response, Immunomonitoring
Introduction
Immunotherapeutic approaches, including vaccines and immune-modulating antibody drugs, are emerging as promising options for the treatment of solid tumors with high medical need. Therapeutic vaccination is designed to induce or enhance pre-existing antitumor responses. The dendritic cell (DC)-based vaccine sipuleucel-T was the first to be approved by the Food and Drug Administration (FDA) for the treatment of metastatic prostate cancer in 2010 [1]. Another successful strategy is the targeting of checkpoint inhibitors on immune cells, i.e., T-cell immunomodulatory monoclonal antibodies (mAbs), such as ipilimumab that blocks cytotoxic T-lymphocyte antigen-4 (CTLA-4) on T cells [2]. The consecutive increase in the frequencies of activated T cells leads to efficient clinical response in patients with metastatic melanoma [3]. Similarly, mAbs against programmed death-1 (PD-1) receptor or its ligand PD-L1 have been shown in early clinical trials to augment the T cell-mediated antitumor responses in non-small-cell lung cancer patients [4]. Whereas these immunotherapeutic concepts have only been recently validated by market approvals, mAbs against surface molecules on cancer cells have been clinically successful for many years. Most of them, such as the FDA-approved mAbs trastuzumab [5] and cetuximab [6], were engineered to primarily act as growth-factor-receptor antagonists. However, the clinical relevance of the activation of immune effector mechanisms by these mAbs, such as complement-dependent cytotoxicity (CDC) or antibody-dependent cell-mediated cytotoxicity (ADCC), has recently been recognized and is being optimized by, e.g., glycol-engineering [7–10].
Each of these immunotherapeutic concepts relies on or modifies specific immune effectors or functions of the patient’s immune system. The immune system of cancer patients is frequently altered compared to healthy individuals, especially during late-stage disease [11]; a variety of functional and quantitative defects in peripheral blood mononuclear cells (PBMCs) have been reported [12–15]. The mechanistic understanding of immune defects prevalent in patients with certain cancers may provide a rationale for the selection and combinations of suitable and optimized immunotherapeutic approaches. One surrogate for the immune competence of cancer patients and their potentially dysfunctional immune system is the frequency and phenotype of specific immune cell subsets which can be assessed in peripheral blood.
Gastric cancer is among the malignancies of highest unmet medical need [16, 17]. It is the fourth most common cancer and the second leading cause of cancer-related deaths worldwide [18]. The 5-year survival rate for gastric cancer is 20–25 % despite aggressive standard treatment regimens. Few publications exist with specific focus on monitoring the immune status of gastric cancer patients. This is particularly true for patient populations with late-stage disease, in which early-phase clinical studies for the development of new therapies for gastric cancer are usually launched.
IMAB362 is a first-in-class antibody drug against the cancer-associated surface molecule claudin18.2. We are evaluating the therapeutic effect of IMAB362 in patients with advanced heavily pre-treated adenocarcinoma of the stomach and the gastro-esophageal junction. Thus, we have a specific interest in a better understanding of the potential immune alterations in these patients. IMAB362 primarily acts through immune effector-mediated mechanisms such as ADCC and CDC. CDC is mediated by activation of the complement cascade in order to form the membrane attack complex and to subsequently eliminate the antibody-targeted tumor cells. ADCC is triggered through direct interaction of the Fc domain of human immunoglobulin (Ig)G, mostly subclass I (IgG1), with the corresponding receptors on immune effector cells such as natural killer (NK) cells, monocytes, macrophages, granulocytes, DCs, and γδ T cells. For a maximum effect of a therapeutic antibody such as IMAB362, desirable characteristics in cancer patients include the presence and the functional fitness of immune effectors combined with a lack of immunosuppressive mechanisms.
Here, we provide systematic immune phenotyping of patients with advanced heavily pre-treated gastric adenocarcinomas employing quality-controlled assays for the enumeration and phenotypic characterization of T cells, B cells, NK cells, Vδ2 T cells, regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs). Our data extends beyond existing studies in patients with gastric cancer due to the well-defined population, comprehensiveness of immune assessments, and the use of established multi-color flow cytometry with a well-standardized quality performance ensuring a robust quantification of up to 24 immune cell subpopulations.
Materials and methods
Patient/subject population and sample collection
Blood samples were collected prior to IMAB362 (study medication) treatment from patients with advanced heavily pre-treated adenocarcinoma of the stomach and gastro-esophageal junction enrolled into a multicenter, international phase II clinical trial (clinicaltrials.gov identifier: NCT01197885). The trial was conducted for testing single-agent activity and safety of the first-in-class anti-claudin18.2 therapeutic mAb IMAB362 [19].
The clinical trial was approved by regulatory authorities and independent local ethics committees, and signed informed consent was obtained from each patient. Blood samples from 30 patients enrolled at 12 sites in Germany (see Acknowledgements for full list of participating investigators) and blood samples from eight healthy donors (HDs) as comparators were subjected to immunophenotyping.
Anticoagulated (EDTA) peripheral whole-blood samples were used for immunophenotyping analysis. Patients’ blood samples were shipped by world courier at 2–8 °C (refrigerated, temperature controlled). Elapsed time between blood sampling and processing was less than 25 h (mean 7.92 h). Samples were stored at 2–8 °C until processing. Anticoagulated blood samples from HDs were stored at 2–8 °C for approximately 24 h until processing.
PBMC preparation
PBMCs were isolated by diluting blood 1:3 with phosphate-buffered saline (PBS) and a standard density-gradient separation by Ficoll-Hypaque (VWR/GE Healthcare). Isolated cells were counted by Guava ViaCount Reagent (Milipore) or Trypan Blue (GIBCO) and cryopreserved in cell culture medium (RPMI 1640; GIBCO) containing 50 % (v/v) human serum albumin (CSL Behring) and 10 % dimethyl sulfoxide (DMSO; Applichem). PBMCs were frozen in a control-rate freezer. Depending on cell harvest, between 0.9 × 106 and 9.5 × 106 cells/mL were frozen per cryovial, as the cells were aliquoted at a ratio of 1:1 as required per standard operation procedure. The cell densities during cryopreservation did neither affect cell viability nor recovery (data not shown). PBMC samples were stored in the gas phase of liquid nitrogen until the staining procedure was performed.
Shortly before staining, the cells were thawed in pre-warmed RPMI 1640 containing 10 % (v/v) human serum albumin in presence of 2 µg/mL DNase I (Roche). Cells were washed and counted using Tuerk solution. The mean viability was >96 %.
Staining protocol
PBMC staining was performed without prior resting of the freshly thawed cells using fluorochrome-labeled mAbs against the following antigens: CD3, CD4, CD11b, CD14, CD15, CD16, CD19, CD25, CD33, CD45RA, CD56, CD62L, CD69, CD103, CD124, CD127, CD195, CD197, FoxP3, HLA-DR, Vδ2, and Vγ9 (Supplementary Table 1; all Becton–Dickinson or Biolegend). Live/dead cells were discriminated by the LIVE/DEAD® Fixable Aqua Dead Cell Stain kit (Life Technologies). The mAbs were titrated and combined in four staining panels at the previously determined optimal concentrations to allow for comprehensive analysis of the major immune effector cells (Panel-1), Tregs (Panel-2), MDSCs (Panel-3), and Vδ2+ T cells (Panel-4; Supplementary Table 2).
A mean of 0.75 × 106 (median, 0.81 × 106) cells were stained according to standard procedures. Briefly, PBMCs were incubated with the LIVE/DEAD® Fixable Aqua Dead Cell Stain Kit for 30 min at 2–8 °C in the dark. Then, cells were washed and stained with the directly labeled mAbs at 2–8 °C for 20–30 min in the dark. Antibody mixtures were added to 50 µL staining buffer [PBS with 2 % fetal calf serum (FCS), 2 mol/L EDTA, and 0.02 % NaN3]. Fix/Perm buffer (eBiosciences) was used for intracellular FoxP3 staining. Washed samples were resuspended in Stabilizing Fixative (Becton–Dickinson) and stored at 2–8 °C in the dark. Samples were analyzed with the FACSCanto II with Diva software (Becton–Dickinson). In most of the experiments, analysis was done on singlet live lymphocytes or leukocytes [gating strategy using FlowJo 7.6.5 software (TreeStar) is depicted in Supplementary Figures 1–4]. For the identification of CD69 versus CD25 and of CD45RA versus CD62L gates on Vd2+ T cells, the original gates were set on CD3+ T cells and then transferred to the Vd2+ T cell population (Supplementary Figures 1d and 4d, respectively).
Baseline mean fluorescence intensity for unstained cells was set at ~2.5× above the electronic noise for all fluorochromes. Standardized application settings were used for all samples, daily adjustment runs, and automated performance checks (CS&T) of the cytometer. To control the reagents and guarantee a stable performance over time, pre-specified index donor samples were stained and tested before and after each patient cohort.
Statistical analyses
Statistical analysis was performed with GraphPad Prism software-version 5.00 (GraphPad Software Inc.). A two-sided Mann–Whitney test was used for statistical evaluation of differences between groups. Differences were considered significant, if p < 5 %.
For statistical analysis of the intra-assay variability, the mean of three (Panel-1, Panel-3, and Panel-4) or five (Panel-2) HD replicates was calculated (Supplementary Table 3). Cell frequencies of the target population were normalized to the calculated means of the HD replicates. Statistical analyses of inter-assay variability were performed by calculating the mean of three independent experiments. Each data point of this inter-assay triplicate reflects one intra-assay mean value. The coefficients of variance (CV) were based on the mean and standard deviation of normalized data.
General lab operation
The study was conducted following standard operating procedures, which were established for all steps including PBMC preparation (cell isolation, counting, freezing, storage, and thawing), the staining procedure, data acquisition, and the full gating strategy for analysis. The handling, storage times and conditions, as well as processing of analyzed PBMC samples were tightly controlled. All panels were tested for intra- and inter-assay variability and robustness (range of cells/test) of the target populations using samples from HDs. A cohort of eight HDs was used to characterize the normal distribution range of target cell populations. The results were assessed for plausibility by comparison to published distributions in the literature (e.g., percent T cells in lymphocytes), if available. The laboratory personnel regularly participate in proficiency panels addressing flow cytometry-associated topics organized by the Association for Cancer Immunotherapy (CIMT) and the Cancer Immunotherapy Consortium (CIC).
Results
Standardized immune monitoring to assess frequency and phenotype of immune cell subsets
We optimized and standardized different flow cytometry staining panels allowing a comprehensive exploratory analysis of the relative frequency and phenotype of 24 distinct immune cell subsets involved in immune competence, including B, T, and NK cells (Panel-1), of Tregs (Panel-2), MDSCs (Panel-3), and Vδ2+ T cells (Panel-4).
In order to standardize our flow cytometry-based immune phenotyping process, we determined intra- and inter-day variability. Samples from at least three HDs were independently processed in triplicates on the same day (intra-assay variability) and on three different time points (inter-assay or day-to-day variability), and the CV for each immune cell subset was determined (Supplementary Table 3). In order to establish reproducibility of intra- and inter-day runs of Panel-3 and thus the staining procedure applied to the six MDSC populations, we used cells from HDs expressing the same marker profile. However, it remains unclear whether healthy MDSC-like cells exert the same function as analogous cells in patients.
In order to identify cell surface markers, we established a consequent gating strategy by including HDs which was used throughout the entire study. The majority of immune cell subsets were quantified in a highly robust manner with CVs below 25 %. Inclusion of CD69 as an early activation marker for Vδ2+ T and NK cells, and CD25 as an activation marker for Vδ2+ T cells, coincided with a slightly increased assay variation with CVs up to 45.5 % which may be due to the typical appearance of these markers as smear, rather than clearly separated populations.
Patient characteristics
Thirty patients (26 male, 4 female) at a median age of 62 (range 42–77) with locally advanced, inoperable, and/or metastasized adenocarcinomas of the stomach (n = 13) or the gastro-esophageal junction (n = 17) were included in this analysis (Table 1). Median time since first diagnosis was 23 months (range 8–45 months) and patients had received a median of two prior therapies before enrollment into this trial (range 1–4), including antimetabolic compounds (e.g., 5-fluorouracil), platinum analogues (e.g., oxaliplatin), and taxanes (e.g., docetaxel) in various combinations. Median time since last cytotoxic treatment was 2 months (range 1–18 months). A total of eight HDs, including three female and five male volunteers with a median age of 40.5 years (range 29–48 years), served as control group.
Table 1.
Baseline characteristics of patients at baseline
| Number (total N = 30) | % | |
|---|---|---|
| Median age (years) | 62 | |
| Range | 42–77 | |
| Missing | 1 | 3.3 |
| Sex | ||
| Male | 26 | 86.7 |
| Female | 4 | 13.3 |
| Median time interval since first diagnosis (months) | 23 | |
| Range | 8–45 | |
| Median time interval since last chemotherapy (months) | 2 | |
| Range | 1–18 | |
| Missing | 2 | 6.7 |
| Median number of previous chemotherapy regimens | 2 | |
| Range | 1–4 | |
| Missing | 2 | 6.7 |
| Median number of previous chemotherapy cycles | 8 | |
| Range | 2–21 | |
| Missing | 6 | 20.0 |
| Location of primary tumor | ||
| Stomach | 13 | 43.3 |
| Gastro-esophageal junction | 17 | 56.7 |
| Histological type of primary tumor | ||
| Adenocarcinoma | 30 | 100 |
| Diffuse | 9 | 30.0 |
| Intestinal | 9 | 30.0 |
| Signet ring | 4 | 13.3 |
| Adenocarcinoma NOS | 8 | 26.7 |
| T-stage | ||
| T1/2 | 7 | 23.3 |
| T3/4 | 13 | 43.3 |
| Missinga | 10 | 33.4 |
| N-stage | ||
| N0 | 4 | 13.3 |
| N+ | 18 | 60.0 |
| Missinga | 8 | 26.7 |
| M-stage | ||
| M0 | 3 | 10.0 |
| M1 | 20 | 66.7 |
| Missinga | 7 | 23.3 |
M metastatasis, N node, NOS not otherwise specified, T tumor
aMissing includes values that could not be determined (i.e., Tx, Gx Nx, Mx)
T cells, B cells, and NK cells
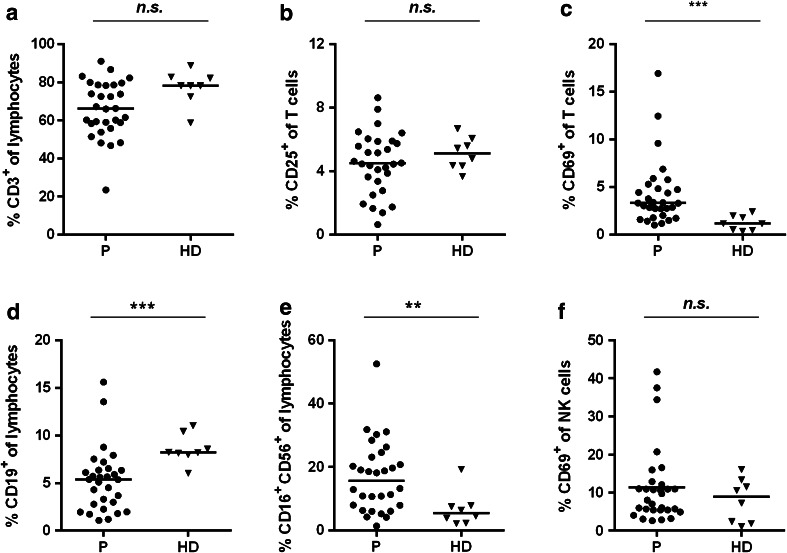
All samples were analyzed by flow cytometry to discriminate CD3+ T cells, B cells, and NK cells. Activation of T cells was measured by the expression of CD25 and CD69 and activation of CD16+CD56+ NK cells by expression of CD69 alone. We observed a trend toward reduced T cell frequencies in patients with gastric cancer versus HDs, which was not significant (Fig. 1a). Frequencies of activated CD3+ T cells based on CD25 expression were comparable in patients and HDs, but the frequency of T cells expressing the early activation marker CD69 was significantly increased by 2.4-fold with 3.3 % (range 1.0–16.9 %) of patient-derived compared to 1.1 % (range 0.4–2.4 %) of HD-derived T cells (Fig. 1b, c).
Fig. 1.
Immune effector cells in gastric cancer patients are not generally suppressed (staining Panel-1). PBMCs from 30 gastric cancer patients (filled circles) and eight healthy donor samples (filled triangles) were analyzed with regard to frequency of CD3+ T cells (a), CD25+ of CD3+ T cells (b), CD69+ of CD3+ T cells (c), CD19+ B cells (d), CD16+CD56+ NK cells (e), and the activation marker CD69 on NK cells (f). The horizontal black lines represent median. p values were obtained with a Mann–Whitney test, **p < 0.01; ***p < 0.001. HD healthy donors, NK cells, natural killer cells, n.s. not significant, P patients, PBMCs peripheral blood mononuclear cells
B and NK cell frequencies significantly differed between patients and HDs with lower median B cell frequency (patients: 5.4 %, range 1.1–15.6 %; HDs: 8.2 %, range 6.0–11.0 %) and a higher median NK cell frequency (patients: 15.7 %, range 1.4–52.5 %; HDs: 5.5 %, range 2.1–19.3 %) in the patient group (Fig. 1d, e). Activation status of NK cells determined by CD69 expression was comparable between both groups (Fig. 1f). Notably, we identified three patients showing considerably high up-regulation of CD69 in >34 % of NK cells.
CD4+ T cells and FoxP3+CD25hiCD127− Tregs
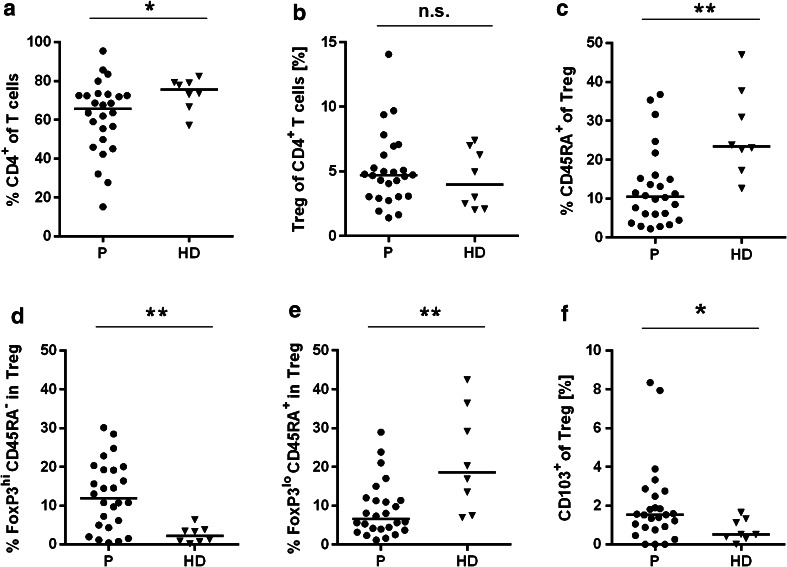
We detected a significantly decreased total CD4+ T cell pool in 7 of 26 gastric cancer patients (Fig. 2a). Less than 50 % of viable lymphocytes were CD4+ T cells, and in three patients, even less than 35 % of viable lymphocytes were CD4+ T cells. We did not formally determine the CD4/CD8 T cell ratio, but as CD3+CD4− T cells represent mainly CD8+ T cells, we speculate that the CD4/CD8 T cell ratio is likely reduced in this patient population.
Fig. 2.
Regulatory T cells in gastric cancer patients are activated and have the potential to infiltrate tumor tissue (staining Panel-2). PBMCs from 26 gastric cancer patients (filled circles) and eight healthy donor samples (filled triangles) were analyzed with regard to frequency of CD4+ T cells (a) and Tregs of CD4+ T cells (b). Resting Tregs were determined by CD45RA positivity (c). Fine dissection of Tregs shows activated FoxP3hiCD45RA− (d) and resting FoxP3loCD45RA+ (e) subpopulations. Tumor-infiltrating Tregs were identified by cell surface marker staining CD103 (f). The horizontal black lines represent median. p values were obtained with a Mann–Whitney test, *p < 0.05; **p < 0.01. HD healthy donors, n.s. not significant, P patients, Treg T regulatory cells, PBMCs peripheral blood mononuclear cells
Next, we analyzed the frequency and phenotype of Tregs. In both groups, a median of 4–5 % of CD4+ T cells were Tregs with the phenotype CD4+Foxp3+CD25hiCD127lo (patients: 4.7 %, range 1.4–14.0 %; HDs: 4.0 %, range 2.0–7.4 %) (Fig. 2b). The median percentage of resting CD45RA+ Tregs was significantly reduced by 2.2-fold in gastric cancer patients (10.5 %) compared to HDs (23.4 %; Fig. 2c). We further dissected the Treg subpopulations according to the Treg classification including differential Foxp3 expression levels proposed by Miyara et al. [20]. This analysis also confirmed that the frequency of resting CD4+Foxp3loCD25hiCD127loCD45RA+ cells was decreased, whereas the frequency of activated CD4+Foxp3hiCD25hiCD127loCD45RA− cells was significantly increased in patients compared to HDs (Fig. 2d, e). Noteworthy, the frequency of CD103+ Tregs was significantly increased in patients compared to HDs (Fig. 2f).
MDSC subpopulations
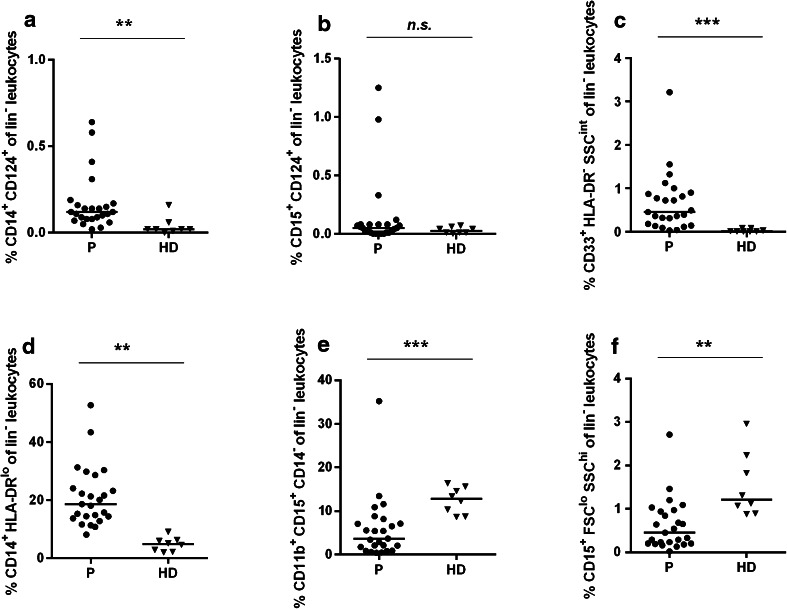
Immature myeloid cells with various MDSC-related phenotypes comprise a low percentage of the isolated PBMC fraction. We characterized selected MDSC subpopulations using six different cell surface marker combinations (Panel-3). All, except the MDSC2 subpopulation (CD15+CD124+lin− leukocytes; Fig. 3b), differed significantly between patients and HDs (Fig. 3). The CD14+CD124+ subpopulation (MDSC1; Fig. 3a), the CD33+HLA-DR−SSCint subpopulation (MDSC3; Fig. 3c), and the CD14+HLA-DRlo subpopulation (MDSC4; Fig. 3d) were significantly increased, whereas the CD11b+CD15+CD14− (MDSC5; Fig. 3e) as well as the CD15+FSCloSSChi subpopulations (MDSC6; Fig. 3f) were significantly decreased in patients compared to HDs.
Fig. 3.
Subpopulations of myeloid-derived suppressor cells are increased in gastric cancer patients (staining Panel-3). PBMCs from 25 gastric cancer patients (filled circles) and eight healthy donor samples (filled triangles) were analyzed with regard to frequency of various MDSC subpopulations determined as: MDSC1 CD14+CD124+ (a), MDSC2 CD15+CD124+ (b), MDSC3 CD33+HLA-DR−SSCint (c), MDSC4 CD14+HLA-DRlo (d), MDSC5 CD11b+CD15+CD14− (e) and MDSC6 CD15+FSCloSSChi cells (f). The horizontal black lines represent median. p values were obtained with a Mann–Whitney test, **p < 0.01; ***p < 0.001. HD healthy donors, MDSCs myeloid-derived suppressor cells, n.s. not significant, P patients, PBMCs peripheral blood mononuclear cells
Vδ2+ T cells
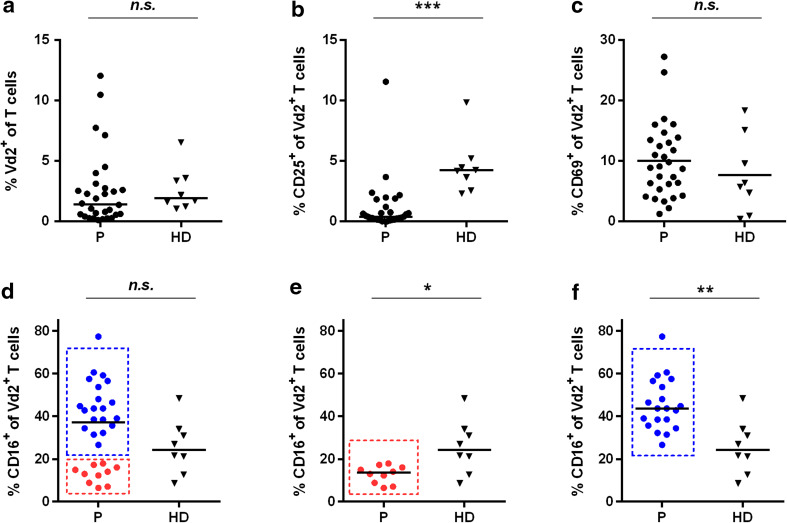
Regarding Vδ2+ T cells, we found similar frequencies in patients with gastric cancer and HDs demonstrating that the disease did not alter Vδ2+ T cell frequency (Fig. 4a). The median frequency of CD25+Vδ2+ T cells was decreased by 90.5 % in gastric cancer patients versus HDs (patients: 0.4 %, range 0.0–11.5 %; HDs: 4.2 %, range 2.3–9.8 %; Fig. 4b). However, the activation status (CD69 expression) of Vδ2+ T cells in patients was comparable to HDs (Fig. 4c). A substantial proportion of Vδ2+ T cells expressed FcγRIII (CD16+) at comparable levels in both patients and HDs (Fig. 4d). Although the frequency of total CD16+Vδ2+ cells did not differ between patients and HDs, the patient population split into two subpopulations one with significantly increased (n = 20: median, 43.7 %; range 26.6–77.3 %;) the other with decreased (n = 10: median, 13.6 %; range 6.5–17.9 %) CD16+Vδ2+ cell frequencies compared to HDs (median, 24.3 %; range 8.6–48.4 %) (Fig. 4e, f).
Fig. 4.
Vδ2+ T cell activation in gastric cancer patients is impaired (staining Panel-1). PBMCs from 30 gastric cancer patients (filled circles) and eight healthy donor samples (filled triangles) were analyzed with regard to Vδ2+ T cells in terms of overall frequency by determining Vδ2+ T cells (a), the activation status with regard to CD25 (b) and CD69 (c) and the CD16-positive Vδ2+ T cell subpopulation for the whole patient population (d), and patients with a low percentage of CD16+ Vδ2 T cells (e) and with an increased percentage of CD16+ Vδ2+ T cells (f). The horizontal black lines represent median. p values were obtained with a Mann–Whitney test, *p < 0.05; **p < 0.01; ***p < 0.001. HD healthy donors, n.s. not significant, P patients, PBMCs peripheral blood mononuclear cells
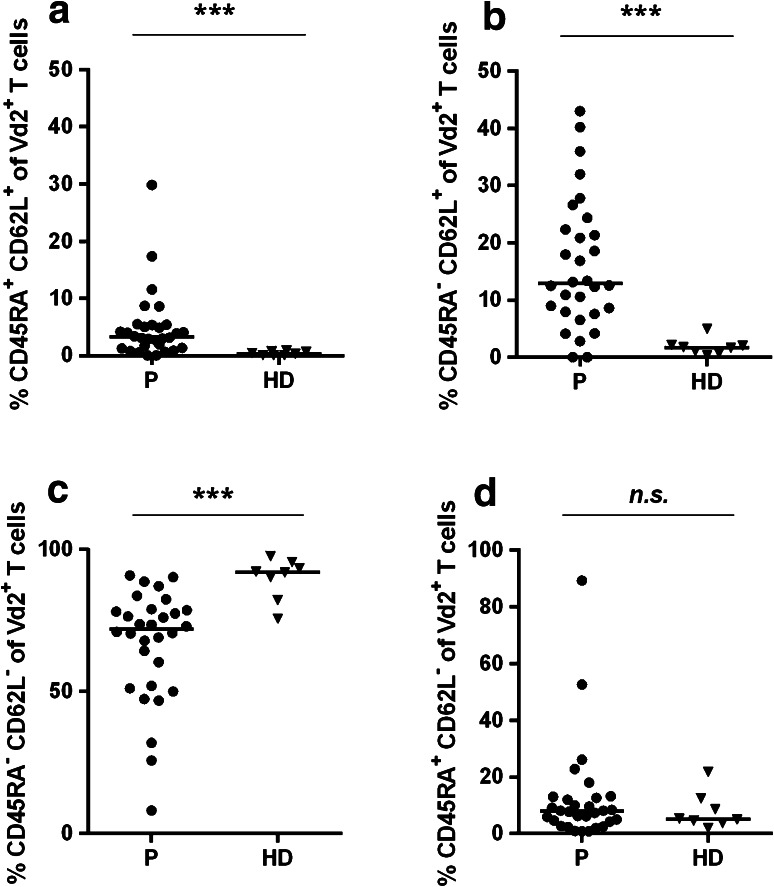
In addition to activation markers, we focused on two additional phenotypic markers to characterize Vδ2+ T cells, namely CD45RA and CD62L. Both CD45RA+CD62L+ and CD45RA−CD62L+Vδ2+ T cells were significantly increased in gastric cancer patients compared to HDs [CD45RA+CD62L+: patients, median 3.2 %, range 0.0–29.8 %; HDs: median 0.4 %, range 0.0–0.9 % (Fig. 5a) and CD45RA−CD62L+: patients, median 12.9 %, range 0.0–43.0 %; HDs: median 1.7 %, range 0.3–4.9 % (Fig. 5b)]. In contrast, a significantly lower median fraction of CD45RA−CD62L−Vδ2+ T cells was detected in gastric cancer patients [patients: 72.1 %, range 8.1–90.9 %; HDs: 92.1 %, range 75.7–97.7 % (Fig. 5c)]. Regarding CD45RA+CD62L−Vδ2+ T cells, no significant differences between patients and HDs were observed (Fig. 5d).
Fig. 5.
Vδ2+ T cells in gastric cancer patients shift toward CD45RA+CD62L+ and CD45RA−CD62L+ T cell phenotype (staining Panel-4). PBMCs from 30 gastric cancer patients (filled circles) and eight healthy donor samples (filled triangles) were analyzed with regard to CD45RA+CD62L+ Vδ2+ T cells (a), CD45RA−CD62L+ Vδ2+ T cells (b), CD45RA−CD62L− Vδ2+ T cells (c), CD45RA+CD62L− Vδ2+ T cells (d). The horizontal black lines represent median. p values were obtained with a Mann–Whitney test, ***p < 0.001. HD healthy donors, P patients, PBMCs peripheral blood mononuclear cells
Discussion
Our aim was to monitor the immune status of a well-defined patient population with heavily pre-treated, advanced adenocarcinoma of the stomach and the gastro-esophageal junction by mapping frequencies and activation status of relevant immune cell populations versus HDs. We applied standardized multi-color flow cytometry with documented performance characteristics, ensuring a sensitive and robust detection of up to 24 different immune cell subsets. To our knowledge, this is the first comprehensive and systematic analysis of multiple immune cell subsets in this patient population.
One of the key finding is that both the frequency and activation status of total T and NK cells (as the major antigen-specific immune effector populations addressed by vaccine-based therapies) were comparable between cancer patients and HDs. We observed a trend toward lower total CD3+ T-lymphocyte populations in these patients which was likely caused by the proportional decrease of the CD4+ T cell pool. It is noteworthy to highlight that the reduction of CD4+ T cells in about 25 % of patients may be indicative for the generally poor prognosis of patients with late-stage gastric cancer, as a high CD4/CD8 T cell ratio has been demonstrated to be an important factor with prognostic value. Interestingly, CD69+ T cell levels were increased in patients indicating a potentially higher activation status. Interestingly, the frequencies of CD25+ T cells remained comparable between patients and HDs. This “asymmetric” expression pattern could be explained by the differential IL-2 dependence of activated T cell subsets; however, this was not investigated in this study. In line with previous reports [13], B cell frequency was significantly reduced in patients.
Another key finding is the increased frequency and activation status of regulatory T cell subsets. Tregs effectively impair immune effector function, proliferation of T cells, and NK cell-mediated cytotoxicity in cancer patients [21, 22]. In other cancer types, an increased Treg frequency correlates with poor clinical outcome [21, 23–25]. This may originate from differences in the studied tumor entities and/or patient populations. However, we cannot exclude that a possible difference in cell handling, storage, staining with antibodies, and the analysis of cytometric data account for this discrepancy. The percentage of Tregs in our patients with gastric cancer does not significantly differ compared to HDs. However, we found that patients with gastric cancer have a significantly higher frequency of activated Tregs in comparison to HDs, whereas the frequency of resting Tregs is decreased. Importantly, we observed a parallel increase in the frequencies of CD103+ Treg cells that have been shown to possess tumor-infiltrating potential in mice [26]. These findings together strongly support the concept that in these patients, Treg cells actively contribute to global and/or local (i.e., tumor microenvironment) immune suppression that is often observed in cancer patients with poor prognosis [27, 28].
MDSCs are a heterogenous, immunosuppressive population of early progenitor cells, immature granulocytes, macrophages, and DCs at different differentiation stages. We investigated six different MDSC subpopulations (MDSC1-6) identified by combinations of granulocytic or monocytic markers, based on Walter et al. [29–33]. MDSC1, MDSC3, and MDSC4 were significantly increased in our patient population. Previous studies already reported an increase of MDSC4 cells in patients with renal cell carcinoma (RCC) [29, 34], breast, or colon cancer [35] and an increase in HLA-DR−CD33+CD11b+ MDSC (here MDSC3) frequency in patients with pancreatic, esophageal, and gastric cancer [36]. Interestingly, we observed significant decreases of MDSC5 and MDSC6 subpopulations, which have been reported to be elevated in patients with RCC, breast, and colon cancer [29, 34, 35]. Although the role of the rare MDSC pool in cancer patients as well as the potential influence of transport and processing of blood samples on these populations remains to be further investigated in more detail, our data indicate that gastric cancer patients may profit from immunotherapies that counterbalance MDSC-mediated tolerogenic mechanisms.
We found particularly interesting NK cells and γδ T cells which are FcR positive and thus important effectors mediating the mAb-triggered ADCC response. Accordingly, NK cells have been shown to prevent the dissemination of metastatic tumors [37], and their presence in tumors has been associated with better prognosis [38]. In contrast to earlier reports in gastric cancer patients [13, 39], NK cell frequencies in our cohort were not decreased, and we detected significantly higher frequencies of CD16+CD56+ cells with an activation status comparable to HDs (CD69 expression).
Vδ2+ T cells comprise 1–6 % of circulating T cells in the healthy population [40]. In contrast to previous findings reporting an increase of the Vδ2+ T cell pool in gastric cancer patients [41], our data suggests that Vδ2+ T cell frequency does not differ in gastric cancer patients versus HDs [13]. Furthermore, we found a similar frequency of CD69+Vδ2+ T cells but a significantly lower frequency of CD25+Vδ2+ T cells as compared to HDs. In women with breast cancer, the γδ T cell-induced immune modulation associated with good prognosis is correlated with an increase in CD69+ γδ T cells [42]. Accordingly, the progressive disease status in our patient population may account for the unaltered (CD69) and decreased (CD25) activation of Vδ2+ T cells. We detected a median of 61 events for the CD69+Vδ2+ T cells subpopulation. Such rather low event counts should be regarded with caution if only small differences are observed between different donors or time points of interest. Nevertheless, the observed tenfold decrease in the CD25+Vδ2+ cell population in patients versus HD represents such a strong signal that we consider this difference a real effect.
Interestingly, based on the frequency of CD16+Vδ2+ T cells, gastric cancer patients can be divided into two subpopulations, both significantly different from HDs. CD16+ γδ T cells have a high cytolytic potency and are enriched in peripheral blood of cancer patients [43]. Vδ2+ T cells seem to undergo phenotypic changes following antigen exposure, i.e., the CD45RA+CD62L+ T cell phenotype shifts toward a CD45RA−CD62L+ phenotype, and subsequent stimulation seems to induce a shift from CD45RA−CD62L− to CD45RA+CD62L− phenotype. Patients with advanced gastric cancer appear to have significantly higher frequencies of CD45RA+CD62L+ and CD45RA−CD62L+ Vδ2+ T cells, whereas CD45RA−CD62L− Vδ2+ T cells were clearly reduced compared to HDs. In breast and prostate cancer patients with stable disease or partial regression, the opposite has been observed [42, 44]. Our data suggests that most Vδ2+ T cells in patients with advanced gastric cancer have not yet encountered antigen or remain in an inactivated state following antigen exposure and traffic to the lymph nodes, but lack immediate effector function. Patients with late-stage disease are clearly impaired regarding their highly potent IFNγ-producing effector memory cells. It remains to be clarified whether the reduced CD45RA−CD62L− Vδ2+ T cell frequency is due to increased tissue migration or a result of potentially tumor-induced alteration of cell differentiation of CD45RA+CD62L+ and CD45RA−CD62L+ Vδ2+ T cells. Other factors, such as age, have been described to influence Vδ2+ T cell populations [45] proposing further studies in cancer patients and control groups with predefined age groups. In addition, the detected events for selected sub-populations were rather low and should therefore be interpreted with caution and require additional experiments acquiring more events. Notably, the observed expression pattern of CD62L and CD45RA on Vd2+ cells closely resembles the pattern detected by Roux et al. [45], suggesting that the acquired data set indeed reflects true biological findings.
In conclusion, this study provides a comprehensive and systematic analysis of the immune status of patients with gastric cancer. We observed differences between patients and HDs in frequency, phenotype, and activation status both of rare immune cell subsets that have not been studied extensively so far and of major immune cell subsets such as B cells and NK cells. We observed signs of ongoing antitumor immune response indicated by higher T cell activation and the enhanced frequencies of highly potent effector cells such as CD16+ NK and Vδ2+ T cells in parts of the patient population. Importantly, our results thus demonstrate that while patients enrolled in this study might have an altered immune system, they were not generally immunosuppressed. Despite the apparently ongoing antitumor response, we also demonstrated the presence of patient-specific local/general suppressive mechanisms that likely hinder the immune system to exert a fully effective antitumor response. Furthermore, we provide important data about different cell populations of the patient´s immune system which might allow the future development of novel immunomodulatory treatments to improve antitumor responses while in parallel overcoming disease-specific tolerogenic mechanisms. Moreover, our findings are valuable for the determination of patient prognostic and immunotherapy response predictive markers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We wish to thank Helene Schroeder and Nicole Bidmon for sample processing and Daniela Kirsch and Richard Rae for their technical assistance with performing flow cytometry. We thank Marc Roller, Marlene Knippenberg, and Vilmos Posevitz for excellent scientific writing support. The majority of samples were provided by clinical trial centers represented by authors of this manuscript as part of their participation in the phase II clinical trial testing the safety and single-agent activity of the therapeutic antibody IMAB362. We wish to acknowledge also all investigators and clinical trial centers, who have provided single patient samples: Ulrike Helbig, Hospital Braunschweig, Wolff Schmiegel, University Hospital Bochum, Salah-Eddin Al-Batran, Hospital Nordwest Frankfurt, Joern Ruessel, University Hospital Halle a. d. Saale, Susanna Hegewisch-Becker, Hematologisch-Onkologische Praxis Eppendorf, Hamburg and Albrecht Hoffmeister, University Hospital Leipzig (all Germany).
Conflict of interest
M.-C. Kuehnle and Ö. Türeci are employees of Ganymed Pharmaceuticals AG; U. Sahin and Ö. Türeci are inventors of patents on claudin18.2 and hold stock of Ganymed Pharmaceuticals AG. U. Sahin is consultant of Ganymed Pharmaceuticals AG. S. Attig, C. M. Britten, H. Schulze-Bergkamen, F. Lordick, G. von Wichert, P. Thuss-Patience, A. Stein, M. Schuler, and F. Bassermann declare no conflict of interests.
Abbreviations
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- CDC
Complement-dependent cytotoxicity
- CIC
Cancer Immunotherapy Consortium
- CIMT
Association for Cancer Immunotherapy
- CTLA-4
Cytotoxic T-lymphocyte antigen-4
- CV
Coefficients of variance
- DC
Dendritic cell
- DMSO
Dimethyl sulfoxide
- FDA
Food and Drug Administration
- FoxP3
Forkhead box protein 3
- HD
Healthy donors
- IgG1
Immunoglobulin G subclass I
- M
Metastasis
- mAb
Monoclonal antibody
- MDSCs
Myeloid-derived suppressor cells
- N
Node
- NK cells
Natural killer cells
- n.s.
Not significant
- P
Patient
- PBMCs
Peripheral blood mononuclear cells
- PBS
Phosphate-buffered saline
- PD-1
Programmed death-1
- PDL-1
Programmed death ligand-1
- RCC
Renal cell carcinoma
- T
Tumor
- Tregs
Regulatory T cells
References
- 1.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 2.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–5283. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 3.Weber JS, Hamid O, Chasalow SD, et al. Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma. J Immunother. 2012;35:89–97. doi: 10.1097/CJI.0b013e31823aa41c. [DOI] [PubMed] [Google Scholar]
- 4.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnould L, Gelly M, Penault-Llorca F, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259–267. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurai J, Chikumi H, Hashimoto K, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res. 2007;13:1552–1561. doi: 10.1158/1078-0432.CCR-06-1726. [DOI] [PubMed] [Google Scholar]
- 7.Bibeau F, Lopez-Crapez E, Di Fiore F, et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27:1122–1129. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 8.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.V99.3.754. [DOI] [PubMed] [Google Scholar]
- 9.Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 10.Racila E, Link BK, Weng WK, et al. A polymorphism in the complement component C1qA correlates with prolonged response following rituximab therapy of follicular lymphoma. Clin Cancer Res. 2008;14:6697–6703. doi: 10.1158/1078-0432.CCR-08-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun DP, Harris JE. Relationship of leukocyte numbers, immunoregulatory cell function, and phytohemagglutinin responsiveness in cancer patients. J Natl Cancer Inst. 1981;67:809–814. [PubMed] [Google Scholar]
- 12.Hong WS, Kim CM, Lee JO, Kang TW, Yun TK, Kim CY. Natural killer and lymphokine-activated killer activities in stomach cancer patients with special emphasis on the effect of 5-fluorouracil, adriamycin and mitomycin-C chemotherapy. Jpn J Clin Oncol. 1990;20:87–93. [PubMed] [Google Scholar]
- 13.Hong WS, Min YI, Son YS, Hong SI. Peripheral blood lymphocyte subsets in patients with stomach cancer. J Korean Med Sci. 1995;10:164–168. doi: 10.3346/jkms.1995.10.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dillman RO, Koziol JA, Zavanelli MI, et al. Immunoincompetence in cancer patients. Assessment by in vitro stimulation tests and quantification of lymphocyte subpopulations. Cancer. 1984;53:1484–1491. doi: 10.1002/1097-0142(19840401)53:7<1484::AID-CNCR2820530710>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 15.Kaszubowski PA, Husby G, Tung KS, Williams RC., Jr T-lymphocyte subpopulations in peripheral blood and tissues of cancer patients. Cancer Res. 1980;40:4648–4657. [PubMed] [Google Scholar]
- 16.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 17.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 18.Parkin DM. International variation. Oncogene. 2004;23:6329–6340. doi: 10.1038/sj.onc.1207726. [DOI] [PubMed] [Google Scholar]
- 19.Schuler MH, Zvirbule Z, Lordick F et al (2013) Safety, tolerability and efficacy of the first-in-class antibody IMAB362 as evaluated in a phase I single dose and phase II multiple dose study in patients with advanced gastroesophageal adenocarcinomas. J Clin Oncol 31:Suppl; abstr 4080
- 20.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
- 22.Li L, Wu CY. CD4+ CD25+ Treg cells inhibit human memory gammadelta T cells to produce IFN-gamma in response to M tuberculosis antigen ESAT-6. Blood. 2008;111:5629–5636. doi: 10.1182/blood-2008-02-139899. [DOI] [PubMed] [Google Scholar]
- 23.Wing JB, Sakaguchi S. Multiple treg suppressive modules and their adaptability. Front Immunol. 2012;3:178. doi: 10.3389/fimmu.2012.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 25.Liotta F, Gacci M, Frosali F, et al. Frequency of regulatory T cells in peripheral blood and in tumour-infiltrating lymphocytes correlates with poor prognosis in renal cell carcinoma. BJU Int. 2011;107:1500–1506. doi: 10.1111/j.1464-410X.2010.09555.x. [DOI] [PubMed] [Google Scholar]
- 26.Anz D, Mueller W, Golic M, et al. CD103 is a hallmark of tumor-infiltrating regulatory T cells. Int J Cancer. 2011;129:2417–2426. doi: 10.1002/ijc.25902. [DOI] [PubMed] [Google Scholar]
- 27.Carreras J, Lopez-Guillermo A, Fox BC, et al. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108:2957–2964. doi: 10.1182/blood-2006-04-018218. [DOI] [PubMed] [Google Scholar]
- 28.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walter S, Weinschenk T, Stenzl A, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 30.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- 32.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 33.Gallina G, Dolcetti L, Serafini P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pohla H, Buchner A, Stadlbauer B, et al. High immune response rates and decreased frequencies of regulatory T cells in metastatic renal cell carcinoma patients after tumor cell vaccination. Mol Med. 2013;18:1499–1508. doi: 10.2119/molmed.2012.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solito S, Falisi E, Diaz-Montero CM, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118:2254–2265. doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci USA. 2000;97:2731–2736. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coca S, Perez-Piqueras J, Martinez D, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–2328. doi: 10.1002/(SICI)1097-0142(19970615)79:12<2320::AID-CNCR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 39.Levy EM, Roberti MP, Mordoh J. Natural killer cells in human cancer: from biological functions to clinical applications. J Biomed Biotechnol. 2011;2011:676198. doi: 10.1155/2011/676198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanier LL, Ruitenberg J, Bolhuis RL, Borst J, Phillips JH, Testi R. Structural and serological heterogeneity of gamma/delta T cell antigen receptor expression in thymus and peripheral blood. Eur J Immunol. 1988;18:1985–1992. doi: 10.1002/eji.1830181218. [DOI] [PubMed] [Google Scholar]
- 41.Lee AJ, Kim SG, Chae HD, Lee GH, Shin IH. gammadelta T cells are increased in the peripheral blood of patients with gastric cancer. Clin Chim Acta. 2012;413:1495–1499. doi: 10.1016/j.cca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 42.Meraviglia S, Eberl M, Vermijlen D, et al. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol. 2010;161:290–297. doi: 10.1111/j.1365-2249.2010.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tokuyama H, Hagi T, Mattarollo SR, et al. V gamma 9 V delta 2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs—rituximab and trastuzumab. Int J Cancer. 2008;122:2526–2534. doi: 10.1002/ijc.23365. [DOI] [PubMed] [Google Scholar]
- 44.Dieli F, Vermijlen D, Fulfaro F, et al. Targeting human gamma}delta T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roux A, Mourin G, Larsen M, et al. Differential impact of age and cytomegalovirus infection on the gammadelta T cell compartment. J Immunol. 2013;191:1300–1306. doi: 10.4049/jimmunol.1202940. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.