Abstract
Adoptive T cell therapy recently achieved impressive efficacy in early-phase clinical trials; this significantly raises the profile of immunotherapy in the fight against cancer. A broad variety of tumour cells can specifically be targeted by patients’ T cells, which are redirected in an antibody-defined, major histocompatibility complex–unrestricted fashion by endowing them with a chimeric antigen receptor (CAR). Despite promising results for some haematologic malignancies, the stroma of large, established tumours, the broad plethora of infiltrating repressor cells, and cancer cell variants that had lost the target antigen limit their therapeutic efficacy in the long term. This article reviews a newly described strategy for overcoming some of these shortcomings by engineering CAR T cells with inducible or constitutive release of IL-12. Once redirected, these T cells are activated, and released IL-12 accumulates in the tumour lesion where it promotes tumour destruction by at least two mechanisms: (1) induction of an innate immune cell response towards those cancer cells which are invisible to redirected T cells and (2) triggering programmatic changes in immune-suppressive cells. Given the enormous complexity of both tumour progression and immune attack, the upcoming strategies using CAR-redirected T cells for local delivery of immune-modulating payloads exhibited remarkable efficacy in pre-clinical models, suggesting their evaluation in clinical trials.
Keywords: Adoptive cell therapy, T cell, Chimeric antigen receptor, IL-12, Innate immunity, PIVAC 11
CAR-engineered T cells: the “T-body” strategy
Adoptive cell therapy (ACT) as a means of treating cancer is generating growing interest, and the adoptive transfer of ex vivo expanded tumour-infiltrating lymphocytes (TIL) has provided some encouraging results in the therapy of metastatic melanomas. T cells were isolated from tumour specimens, expanded in culture and re-infused in high numbers. To improve targeting specificity, strategies have been developed during the last decade to provide patients’ peripheral blood lymphocytes with a T cell receptor (TCR) or a chimeric antigen receptor (CAR) of defined specificity for targeting a tumour-associated antigen. The transgenic TCR consists of the α and β polypeptide chains, whereas the CAR is composed of one polypeptide chain combining the extracellular antigen-binding site of an antibody—generally a single-chain fragment of variable region (scFv) antibody—with the intracellular CD3ζ chain which links the CAR to the signalling machinery of the physiological TCR.
T cell receptor–modified T cells recognize antigen in a HLA-restricted fashion and thereby need the antigen correctly processed and presented on the target cell; both processes, however, are frequently deficient or defective in tumour cells. CARs overcome the limitation by having an antibody-derived binding domain, which mediates major histocompatibility complex (MHC)-unrestricted targeting and which enables targeting of a plethora of antigens, including proteins, carbohydrates, and gangliosides, as long as an antibody is available. In contrast to the protein processed and presented by the MHC and recognized by the TCR, the CAR-targeted antigen needs to be expressed on the surface of the target cell. Ligation of the binding domain with the cognate antigen on the targeted cell surface results in the secretion of cytokines, in particular IFN-γ and TNF-α, by the engineered T cells and redirected cytolysis of the target cells, predominantly via granzyme/perforin. Optimization of the CAR structure and addition of costimulatory signals have been a long-standing aspect of pre-clinical research into modified T cell therapy and have recently been thoroughly discussed [1, 2]. To date, clinical trials using CAR-engineered T cells in ACT have emerged as the most effective treatment for patients with CD19+ chronic B cell leukaemia [3] and GD2+ neuroblastoma, which confirms the potential of this strategy. A number of trials are currently exploring the use of CAR T cells for the treatment of other malignancies.
Despite some remarkable tumour regressions, to date most ACT trials have yielded marginal or transient tumour regression, in particular when applied to large, established tumours. As the disease progresses, cancer cells exhibit considerable phenotypic and functional variability, thus avoiding immune recognition or disabling effector cells in their anti-tumour attack; both of these factors facilitate a relapse [4, 5]. Defects in antigen processing and presentation frequently occur in progressing lesions, making cancer cells invisible to MHC-restricted T cell recognition; loss of antigen on the cell surface makes these cancer cells invisible to CAR-mediated recognition. When a powerful T cell anti-tumour response is elicited, escape of antigen-loss cancer cell variants is very likely to occur, which is prone to represent a problem for the development of effective immune therapeutic strategies to combat cancer. Moreover, immune repression accelerates during tumour progression with the accumulation of a plethora of repressor cells including regulatory T (Treg) cells, myeloid-derived suppressor cells (MDSCs), and macrophages, which together with other stroma cells strikingly counteract a T cell anti-tumour attack.
Current strategies in ACT using genetically engineered T cells with redirected tumour specificity have not yet overcome these challenges. Many research groups are attempting to increase T cell numbers or the affinity of the targeting CAR or to co-administer low- or medium-dose IL-2 to improve the T cell response. This review discusses an evolving approach that uses CAR-engineered T cells as targeting vehicles to accumulate IL-12 in the targeted tumour tissue as a response-modifying cytokine, which impacts the tumour environment in at least three ways: improving T cell cytolytic activity [6], activating and recruiting innate immune cells [7], and re-programming stroma-associated immune suppressor cells [8].
IL-12 bridges innate and adaptive immunity
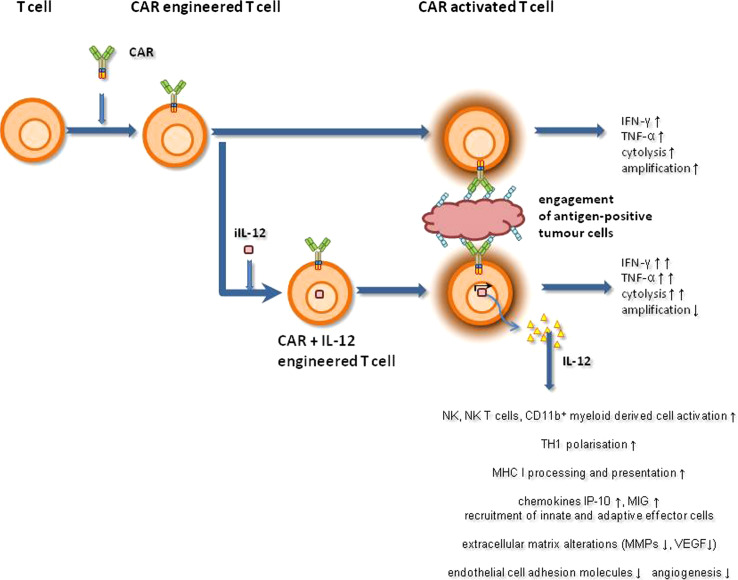
IL-12 is a heterodimeric protein composed of p35 and p40 subunits, which can be linked in a recombinant single-chain (p40-p35) IL-12 molecule with retained biological activity. As a multifunctional cytokine, IL-12 bridges innate and adaptive immunity. The general model of its biological role predicts that IL-12 will act as a regulator of cell-mediated immune responses and will be required for resistance to bacterial infections and for the establishment of auto-immunity. The main physiological producers of IL-12 are phagocytes and dendritic cells; its production occurs in response to pathogens, to T and NK cell signals, and to components of the inflammatory extracellular matrix [9]. By binding to the IL-12 receptor, IL-12 triggers the JAK–STAT signalling pathway, predominantly mediated by STAT4. In response to bacteria, macrophage-produced IL-12 drives the adaptive immune response by polarizing naive CD4+ helper T cells towards the T helper-1 (TH1) phenotype [10]. As a master regulator of adaptive TH1 cell-mediated immunity, which is critical for an effective anti-tumour response, IL-12 has additionally generated interest as a co-modulator of a redirected T cell attack against cancer. This interest is sustained by various pre-clinical models [11, 12], which demonstrate IL-12-modulated anti-tumour responses at various levels (Fig. 1), for example through the induction of TH1 cell differentiation, re-programming dysfunctional TH2 CD4+ T cells into a TH1 phenotype [13], and sustaining survival and re-activation of CD4+ T memory cells [14]. IL-12 improves cellular immunity by enhancing IFN-γ release, by improving granzyme and perforin production, and by boosting amplification of T and natural killer (NK) cells [15, 16]. IL-12 counteracts neo-angiogenesis by inducing IFN-γ-inducible genes and by modulating lymphocyte–endothelial crosstalk. Furthermore, the anti-tumour effects induced by CD8+ T cell and macrophage infiltration—vessel damage and necrosis—could be improved by co-administrated IL-2 or IL-18 [17] or by CD28 co-stimulation of effector T cells [18].
Fig. 1.
Schematic presentation of effector functions mediated by CAR-redirected T cells with or without co-expressed transgenic IL-12
In contrast to a number of pre-clinical studies, early clinical trials using IL-12 as a single agent or as vaccine adjuvant produced only limited efficacy in most instances. However, trials have revealed some TH1-type responses in the treated tumour lesion and infiltration of both NK cells and macrophages into it [19, 20]. In addition, an increase in the frequency of circulating and tumour-infiltrating CD8+ T cells [21] with some improvement in the clinical outcome has been observed. However, achievement of therapeutic concentrations in the tumour lesion remains elusive because systemic IL-12 administration is limited by dose-dependent toxicity [22], including toxicity in hematopoietic, intestinal, hepatic, and pulmonary tissues [23]; such toxicity is probably mediated by inducing high IFN-γ levels. Consequently, clinical trials have explored strategies to deliver IL-12 to the tumour lesion in a locally controlled manner. However, most metastatic cancer lesions are not accessible to direct IL-12 application. This situation favours delivery systems producing the cytokine at the tumour site in situ while avoiding increase in serum concentrations. Virus-based and gene-modified cell-based systems were evaluated in this context. IL-12-modified fibroblasts and tumour cells, when injected peri-tumourally, and intra-tumoural injection of IL-12 expression plasmid DNA proved to be innocuous and exhibited some clinical responses [22, 24–29].
The capability of modulating an ongoing T cell response and of re-programming polarized CD4+ T cells, promoting CD8+ T cell differentiation, and interfering with angiogenesis provides a strong rationale for exploiting IL-12 as adjuvant for the adoptive T cell therapy of cancer. This rationale is supported by pre-clinical models which revealed potent anti-tumour activity of IL-12 that is produced at the tumour site by engineered neoplastic cells [30]. Strategies are therefore currently being developed to produce IL-12 by CAR-redirected T cells in a loco-regionally restricted manner when T cells engage their targeted tumour tissue.
CAR T cells with engineered IL-12
The ability to achieve therapeutic IL-12 levels at the tumour site probably represents a major barrier for treatment. The strategy of modifying T cells for transgenic IL-12 release attempts to combine the profound immunomodulatory activity of IL-12 with the migratory and targeting capacity of T cells in order to accumulate the cytokine in the T cell-targeted lesion. In this context, engineered T cells are used as targeted factories that release the cytokine either constitutively or upon activation; both strategies are intended to increase in situ T cell effector functions, to recruit an innate immune response, and to re-programme suppressor cells to support the redirected cytotoxic T cell attack.
Improving a cytolytic T cell anti-tumour attack
When T cells bind to target antigens during their passage through tissues, they remain in contact with the antigen for longer periods of time while executing their effector functions. To accumulate transgenic IL-12 in the same targeted environment, antigen-specific T cells were modified with an expression vector for the constitutive release of single-chain p40-p35 IL-12 [8]. These T cells released IL-12 in large quantities in the tissue where T cells were trapped by antigen recognition, thus producing a substantially improved anti-tumour response. A single dose of 104 IL-12-modified T cells was more therapeutically effective against established tumours in a pre-clinical model than a high dose of 2 × 107 T cells without IL-12. In these targeted tumour lesions, the number of NK cells greatly increased, which probably contributed to an improved anti-tumour effect in addition to an elevated cytotoxicity of redirected T cells. The conclusion is supported by the observation that tumour destruction exhibited delayed kinetics. T cell-delivered IL-12 was also more effective than other means of delivery as shown by the fact that high doses of systemically applied IL-12 did not reproduce the effect. Compared to other strategies of administering IL-12 to the tumour lesion (including intra-tumoural injections or cytokine-modified tumour cells, fibroblasts, or dendritic cells), CAR- and IL-12-modified T cells have the advantage of preferentially delivering IL-12 to the targeted tissue in a controlled manner irrespective of whether the lesion is detectable by imaging and can be reached by injection. Similar to tumour-targeted T cells, Epstein–Barr virus (EBV)-specific T cells were engineered to constitutively express single-chain p40-p35 IL-12 in an attempt to deliver IL-12 to the targeted EBV+ Hodgkin’s lymphoma [31]. Compared to EBV-specific T cells, engineering with a CAR has the advantage of allowing redirection of IL-12-producing T cells towards any target which can be defined by a CAR.
However, T cells that constitutively produced IL-12 showed several disadvantages, which limited their therapeutic efficacy, for example supra-therapeutic levels of produced IL-12, off-target IL-12 delivery, and pronounced inhibition of T cell expansion due to Fas-/FasL-mediated apoptosis ex vivo [32, 33]. This situation can be circumvented by induced IL-12 (iIL-12) expression under control of the nuclear factor of activated T cell (NFAT)-derived minimal promoter that initiates IL-12 transcription upon TCR- or CAR-mediated T cell activation [7, 32]. In the process, IL-12 is released on CAR signalling in engineered T cells intended to accumulate to high levels in the targeted solid tumour lesion while avoiding substantial increase in serum IL-12. By using CAR-redirected T cells to produce IL-12, the concept exploits the migratory and tissue-penetrating capacities of T cells. Pre-clinical models confirmed that modified T cells migrated to the targeted tumour tissue where redirected T cell activation occurred. Antigen engagement by the TCR or CAR—both of which utilize the CD3ζ signalling domain for downstream signalling—activated the NFAT-responsive elements, resulting in subsequent IL-12 transcription and triggered IL-12 release. By activating the inducible IL-12 (iIL-12) pathway, individual CAR T cells produced IL-12 release at the cognate tumour lesion but not at other sites during their passage through tissues. To our knowledge, these are the first reports on the use of T cells as inducible, TCR-triggered producers of transgenic cytokines in a pre-defined, targeted environment.
In contrast to constitutive IL-12 expression, iIL-12 has the advantage of only delivering the cytokine when engineered T cells engage the cognate antigen. Such controlled IL-12 augmentation in the tumour environment by CAR-redirected T cells will be beneficial to the patient for several reasons. First, the systemically high IL-12 levels which are associated with severe toxicity are avoided. Second, the extraordinary tissue-penetrating capabilities of T cells are assumed to allow local IL-12 accumulation in any targeted tumour lesion which is otherwise not accessible, for instance brain metastases or multiple lesions in inner organs. Third, as long as they are activated, engineered T cells continuously produce IL-12, providing consistently high cytokine levels in the targeted organ. However, IL-12 production ceases when T cells no longer engage the CAR-defined antigen.
Pre-clinical models indicate that inducible IL-12 apparently does not generate toxic cytokine levels. Apart from a transient body weight loss, no other side effect was observed. Adoptive transfer of 3 × 106 T cells with iIL-12 was tolerated without substantial toxicity, which is in striking contrast to severe toxicity upon transfer of >5 × 105 cells with constitutive IL-12 [6, 7, 32].
The strategy of modifying T cells with transgenic IL-12 release provides an approach to improving the cytolytic T cell attack by local auto-stimulation via topically therapeutic concentrations of secreted IL-12. Arrested T cell targeting at the tumour site appears to be required since IL-12-engineered T cells without tumour-specific recognition did not demonstrate an improved anti-tumour attack. Consequently, TCR- or CAR-modified T cells with transgenic IL-12 expression are expected to exhibit anti-tumour action at lower cell numbers than modified T cells without IL-12. Indeed, CAR-mediated T cell anti-tumour attack is still antigen-restricted in the presence of IL-12 [7]. Redirected cytolysis was not altered, but antigen engagement of engineered T cells increased IFN-γ release. The therapeutic efficacy in tumour eradication in pre-clinical models was substantially enhanced by using iIL-12-modified T cells; tumourous mice had prolonged survival compared to control groups. Although IL-12 was reported to directly stimulate the cytotoxic activity of CD8+ T cells [34], the redirected cytolytic activity, as measured by a 2-day in vitro cytotoxicity assay, was not altered in the presence of IL-12. This is in accordance with the observation made by Zhang et al. [32], who reported on unaltered CD107a and granzyme B staining upon IL-12 induction. However, the impact of T cell-secreted iIL-12 on the local host’s innate immune response is probably more pronounced than on T cell-mediated cytotoxicity itself. For instance, NKp46+ lymphoid tissue–induced cells are stimulated by IL-12; this induces a local anti-tumour immunity [29]. Interestingly, increasing T cell dose above the threshold value did not increase anti-tumour effect [32], which indicates a strictly indirect mechanism. Once iIL-12 concentration in the tumour environment has reached a therapeutic level, a secondary immune response is induced without further increase in efficacy with increasing IL-12 concentrations. This phenomenon became apparent when recording the antigen-independent elimination of antigen-loss cancer cells in mixed tumour tissues [7] as discussed below.
The power of attracting an innate immune response: eliminating cancer cells which are invisible to CAR T cells
Antigen-loss cancer cells are not visible to CAR T cells; hence, they survive and amplify in the presence of specifically redirected T cell therapy. The situation highlights a conceptual deficit of specifically antigen-redirected T cells that cannot be solved by improving the CAR itself or by substitution with low or medium doses of IL-2. In a recent paper [7], we proposed overcoming this situation by recruiting and activating innate immune cells in situ in the tumour tissue by inducible IL-12 co-expression, which is intended to recruit a second wave of immune response mediated by innate immune cells, in particular macrophages that eliminate antigen-loss cancer cell variants in the tumour lesion (Fig. 2). Elimination of those tumour cells is loco-regionally restricted where CAR engagement and thus IL-12 induction occurs; engineered T cells which do not engage cognate antigen do not release iIL-12. The effect is loco-regionally restricted since distant tumour lesions which did not mediate CAR engagement were not affected; this indicates the lack of a general immune cell activation by systemically elevated IL-12 levels. On the other hand, homogeneous antigen-loss tumours are not eradicated due to the lack of T cell activation by their CAR.
Fig. 2.
Transplanted tumours were treated by adoptive transfer of T cells engineered with anti-CEA CAR and inducible IL-12 (iIL-12). a T cells (1 × 106) were engineered with a CEA-specific CAR, iIL-12, and GLuc+ (Gaussia luciferase) and tested for luciferase activity in vitro compared to non-modified T cells in serial dilutions. A CBLuc+ (click beetle luciferase) CEA+ tumour was induced by s.c. transplantation in NIH-III mice that were deficient in NK, T, and B cells, and the established tumour recorded by bioluminescence imaging (left panel). Engineered T cells were intravenously injected into the same tumour-bearing mice and recorded by bioluminescence using a GLuc-specific substrate (right panel). Engineered T cells accumulated at the tumour lesion, in the spleen and lymph nodes. b Subcutaneous co-injection of anti-CEA CAR+ iIL-12+ T cells together with CEA+ tumour cells caused swelling at the injection site (up to 96 h), which was not observed after injection of CAR T cells or non-modified T cells (w/o CAR) together with tumour cells. c Mice co-injected with CAR+ iIL-12+ T cells and CEA+ tumour cells (T cell/tumour cell ratio 1:4.5) showed reduced tumour growth in contrast to mice treated with T cells expressing the CEA CAR+ only
Previous studies have shown improved efficacy of IL-12-modified T cells towards large, established tumours induced by the transplantation of a cancer cell line [32]. Such experimental tumours are homogenous with respect to the cell type and expression of the targeted antigen. The substantial advantage of iIL-12 for the adoptive therapy of cancer becomes obvious when recruitment of an innate immune response is required to mediate elimination of antigen-loss cancer cell variants. Tumours which harbour a substantial number of cancer cells that lack the targeted antigen more closely mimic the clinical situation than tumours grown from a homogenous cell line.
Local IL-12 supplement recruits and activates innate immune cells that mediate an antigen-independent anti-tumour reaction in the targeted lesion; this results in the elimination of antigen-loss tumour cells. The therapeutic effect of IL-12 is widely attributed to the enhancement of the cytolytic activity of NK and CD8+ T cells and to the stimulation of a subset of NKp46+ cells [29, 35, 36]. We recently revealed that the effect is additionally mediated by tumour-infiltrating macrophages, which accumulate in treated tumour lesions in a substantial number but not during T cell therapy without IL-12. Tumours treated with iIL-12-engineered CAR T cells were infiltrated with high numbers of activated macrophages, as indicated by the presence of IL-12 receptor β1 chain, and increased CD80 and CD86 expression. Elimination of antigen-loss cancer cells required the presence of these macrophages since their depletion abrogated the therapeutic efficacy towards antigen-loss cancer cells in vivo; add-back of purified macrophages reconstituted the effect. Elimination of target cells by macrophages occurred in a TNF-α-mediated process; TNF-α neutralization substantially reduced anti-tumour activity in vivo [7]. Because of the synergistic effect of IL-12 and T cell-secreted IFN-γ, we assume that the T cell efficacy towards cognate tumour cells is also improved compared to T cells without IL-12. In addition to macrophages, other innate cells including NK and NK-T cells are targets for iIL-12 and probably contribute to TNF-α secretion and elimination of antigen-loss tumour cells.
Re-programming suppressor cells in the tumour stroma
Stromal cells provide an optimal inflammatory, vascular, and nutritive support for triggering tumour cell expansion; multiple cells in the stromal environment mediate potent immune repression involving regulatory T cells, activated macrophages, immature dendritic cells, and myeloid-derived suppressor cells (MDSCs). In the stroma of large, established tumours, the activation and maturation of these cells are impaired; this supports a suppressive environment and results in resistance to cytotoxic T cells. Overcoming immune repression remains a crucial step for the success in combating cancer by adoptive T cell therapy. IL-12 delivered by redirected, engineered T cells to the tumour environment is intended to re-programming these suppressor cells; reversing their dysfunction is assumed to stimulate tumour-specific T cells as most recently shown in a pre-clinical model [37]. To initiate the process and to enable crosstalk between T cells and antigen-presenting cells, the immune-suppressive environment has to be converted to a more acute inflammatory environment. A recent report by Lu et al. [37] indicates that reversion of immune suppression by dysfunctional MDSCs, dendritic cell, and macrophages—all of which are potential antigen-presenting cells—can occur via IL-12 supplementation in the tumour tissue. Transfer of IL-12-engineered T cells remodelled the tumour microenvironment and resulted in the disappearance of MDSCs, macrophages, and dendritic cells in regressing lesions. Mechanistically, IL-12 at the tumour site re-programmed immune suppressor cells towards antigen-presenting cells which cross-present antigen and then are targeted by transferred CD8+ T cells recognizing cognate antigen. As a result, IL-12 supplementation improved recognition of antigen by tumour-infiltrating cytotoxic T cells in an indirect manner. The conclusion is supported by the observation that a low number of transferred engineered T cells induced regression of large tumour mass. However, neither altered differentiation or regression of those suppressor cells from the tumour lesion nor so-called bystander killing can be ruled out [38].
Perspective: CAR T cells as trucks—vehicles to deliver immune response modifiers to the tumour stroma
Chimeric antigen receptor-redirected T cells with inducible or constitutive expression of transgenic IL-12 provide a strategy for depositing therapeutic levels of the cytokine in the targeted tumour, irrespective of its localization or size. However, when using CAR T cells with iIL-12, activation and IL-12 release require at least some target cells that are recognized by the CAR in the lesion; tumour lesions without target cells do not induce T cell accumulation and iIL-12 expression [7]. This is a significant issue underlining the safety of the strategy in terms of restricted IL-12 expression; however, it also indicates the importance of targeting the appropriate antigen.
Producing IL-12 in a locally restricted fashion may produce more severe tissue damage and local toxicity than redirected T cells without transgenic production of immune modulators. Although pre-clinical models have not revealed systemic or local toxicities of IL-12-modified T cells thus far, high-dose application of modified cells, at least with constitutive IL-12 expression, probably induces treatment-related toxicities. T cell toxicity due to local supra-therapeutical IL-12 levels may be the underlying mechanism determining why modified T cells with constitutive IL-12 expression did not show long-term persistence in a pre-clinical model [6]. Although supra-therapeutic IL-12 levels eliminate T cells and concurrently limit IL-12 production, induced release usually produces lower IL-12 levels and is probably less toxic. The expression vector for inducible and, more relevantly, for constitutive IL-12 release therefore has to be optimized to produce low therapeutic levels. Since iIL-12 release is currently driven by the NFAT minimal promoter, which is also triggered by physiological TCR engagement, IL-12 is also produced upon physiological TCR engagement. Antigen-unrestricted T cell activation, moreover, probably produces iIL-12 release in potentially toxic levels. In this regard, an iIL-12 expression cassette driven exclusively by the CAR would be desirable. Apart from that, a careful dose-escalation scheme is required during translation into clinical practice to determine the maximum-tolerated cell number and IL-12 dose.
Non-myeloablative lymphodepletion and IL-2 substitution are integral parts of nearly every clinical protocol in adoptive T cell therapy. The effect of lymphodepletion prior to adoptive cell transfer and the requirement for low- or medium-dose IL-2 after transfer of IL-12-modified T cells remain to be elucidated in detail. The ability of host cells to function as “sinks”, which limits homoeostatic expansion of adoptively transferred T cells, is well documented; lymphodepletion, along with the elimination of regulatory T cells and other suppressor cells, allows expansion of engineered T cells at the targeted tumour site [39]. In the situation of IL-12 supplementation, however, we assume that the multiple changes in the local host immune response induced by accumulated IL-12 favour T cell expansion and execution of effector functions, thus making lymphodepletion and IL-2 substitution dispensable.
Instead of IL-12, application of other members of the IL-12 family, in particular IL-23 and IL-27 [40], is worth evaluating due to their considerable potential in cancer therapy. Similar to IL-12, these cytokines affect IFN-γ production by T and NK cells. IL-23 stimulates IL-6, IL-17, IL-22, and IL-25 production by TH4/TH17 cells. Since IL-12, IL-23, and IL-27 promote priming and re-activation of polarized T cell responses, these cytokines are good candidates to be explored by CAR- or TCR-induced expression in a redirected T cell attack. Both IL-12 and IL-27 impact priming of the TH1 response with some effects on regulatory T cell survival; IL-12 and IL-23 enhance memory T cell responses [41]. However, IL-23 is a less favourable candidate since it sustains inflammatory reactions and thereby promotes tumour progression [42]. IL-27 limits inflammatory T cell reaction, promotes TH1 development, and suppresses TH4/TH17 development [40]. IL-18 in concert with T cell-secreted IFN-γ may be of benefit with respect to recruiting and activating innate immune cells [43, 44]. In contrast to IL-12, IL-18 promotes both TH1 and TH2 responses and, in synergy with IL-12, enhances innate immune cell activity and IFN-γ production. NK cells and macrophages express the corresponding receptors for both IL-12 and IL-18 and produce IFN-γ in response to those cytokines; this provides a rationale for engineering T cells with inducible IL-18 in order to activate an innate immune response.
T cell-released IL-12 will convert a TH2-dominated immunosuppressive environment to a more TH1-like response with reduced IL-4 and IL-5 levels. The therapeutic effects due to such a conversion of the tumour environment may be further improved if T cells are administered together with other cytokines, such as IL-2 and IL-18, or with tumour cells exhibiting costimulatory ligands to boost T cell activation. On the other hand, IL-12 contributes to immune repression via the induction of IL-10 [45]; this can be counteracted by application of a soluble IL-10 receptor or of IL-10-neutralizing antibodies.
IL-12-mediated tumour regression resulting from the activation of T, NK, and NKT cells [29, 34, 35] is defined as the anti-tumour role of macrophage-produced cytotoxic metabolites IFN-γ and TNF-α [7, 8]. More controversial is the specific role of nitric oxide (NO), a major macrophage effector molecule, in post-IL-12 tumour regression. Egilmez et al. [46] have been investigating the IFN-γ-dependent nitric oxide synthase (iNOS) expression in CD11b(+) F4/80(+) macrophages with respect to anti-tumour immunity. Blockage of iNOS activity with N-nitro-l-arginine methyl ester (l-NAME) enhanced tumour suppression; this revealed the inhibitory impact of NO on IL-12-based anti-tumour effect [46]. These results provide a strong argument for the use of l-NAME as an adjuvant in IL-12 cancer therapy.
Engineering redirected T cells so that they are capable of altering the tightly controlled immune milieu in the targeted tumour stroma may substantially improve future trials and enable current strategies to achieve their full therapeutic efficacy. A NIH trial is currently recruiting in which patients with metastatic melanoma are to receive IL-12-engineered tumour-infiltrating lymphocytes subsequent to lymphodepletion [47]. Based on the results of our pre-clinical studies, we suggest exploration of the anti-tumour efficacy of CAR-redirected T cells with inducible IL-12 in a thoroughly controlled dose-escalation study, which is directed towards generating an innate immune response that attacks a variety of cancer cell variants and the tumour stroma, which are invisible to CAR T cells.
Acknowledgments
Work in the author’s laboratory was supported by the Deutsche Forschungsgemeinschaft, Bonn, the Deutsche Krebshilfe, Bonn, and the Wilhelm Sander-Stiftung, München.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Eleventh International Conference on Progress in Vaccination against Cancer (PIVAC 11), held in Copenhagen, Denmark, 10th–13th October 2011. It is part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.Bridgeman JS, Hawkins RE, Hombach AA, Abken H, Gilham DE. Building better chimeric antigen receptors for adoptive T cell therapy. Curr Gene Ther. 2010;10:77–90. doi: 10.2174/156652310791111001. [DOI] [PubMed] [Google Scholar]
- 2.Hombach AA, Abken H. Costimulation by chimeric antigen receptors revisited the T cell antitumour response benefits from combined CD28-OX40 signalling. Int J Cancer. 2011;129:2935–2944. doi: 10.1002/ijc.25960. [DOI] [PubMed] [Google Scholar]
- 3.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumour effects and can establish memory in patients with advanced Leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Croci DO, Zacarías Fluck MF, Rico MJ, Matar P, Rabinovich GA, Scharovsky OG. Dynamic cross-talk between tumour and immune cells in orchestrating the immunosuppressive network at the tumour microenvironment. Cancer Immunol Immunother. 2007;56:1687–1700. doi: 10.1007/s00262-007-0343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 6.Kerkar SP, Muranski P, Kaiser A, Boni A, Sanchez-Perez L, Yu Z, Palmer DC, Reger RN, Borman ZA, Zhang L, Morgan RA, et al. Tumour-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res. 2010;70:6725–6734. doi: 10.1158/0008-5472.CAN-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumour cells that have shut down tumour antigen expression. Cancer Res. 2011;71:5697–5706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 8.Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM, Trinchieri G, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumours. J Clin Invest. 2011;121:4746–4757. doi: 10.1172/JCI58814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/S1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 11.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumour immune response. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tatsumi T, Huang J, Gooding WE, Gambotto A, Robbins PD, Vujanovic NL, Alber SM, Watkins SC, Okada H, Storkus WJ. Intratumoural delivery of dendritic cells engineered to secrete both interleukin (IL)-12 and IL-18 effectively treats local and distant disease in association with broadly reactive Tc1-type immunity. Cancer Res. 2003;63:6378–6386. [PubMed] [Google Scholar]
- 13.Wesa A, Kalinski P, Kirkwood JM, Tatsumi T, Storkus WJ. Polarized type-1 dendritic cells (DC1) producing high levels of IL-12 family members rescue patient TH1-type antimelanoma CD4+ T cell responses in vitro. J Immunother. 2007;30:75–82. doi: 10.1097/01.cji.0000211316.15278.6e. [DOI] [PubMed] [Google Scholar]
- 14.Yoo JK, Cho JH, Lee SW, Sung YC. IL-12 provides proliferation and survival signals to murine CD4+ T cells through phosphatidylinositol 3-kinase/Akt signaling pathway. J Immunol. 2002;169:3637–3643. doi: 10.4049/jimmunol.169.7.3637. [DOI] [PubMed] [Google Scholar]
- 15.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–567. doi: 10.1016/S0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 17.Coughlin CM, Salhany KE, Wysocka M, Aruga E, Kurzawa H, Chang AE, Hunter CA, Fox JC, Trinchieri G, Lee WM. Interleukin-12 and interleukin-18 synergistically induce murine tumour regression which involves inhibition of angiogenesis. J Clin Invest. 1998;101:1441–1452. doi: 10.1172/JCI1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zitvogel L, Robbins PD, Storkus WJ, Clarke MR, Maeurer MJ, Campbell RL, Davis CG, Tahara H, Schreiber RD, Lotze MT. B7.1 costimulation markedly enhances IL 12-mediated antitumour immunity in vivo. Eur J Immunol. 1996;26:1335–1341. doi: 10.1002/eji.1830260624. [DOI] [PubMed] [Google Scholar]
- 19.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 20.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, et al. Type, density, and location of immune cells within human colorectal tumours predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 21.Mortarini R, Borri A, Tragni G, Bersani I, Vegetti C, Bajetta E, Pilotti S, Cerundolo V, Anichini A. Peripheral burst of tumour-specific cytotoxic T lymphocytes and infiltration of metastatic lesions by memory CD8+ T cells in melanoma patients receiving interleukin 12. Cancer Res. 2000;60:3559–3568. [PubMed] [Google Scholar]
- 22.van Herpen CM, van der Laak JA, de Vries IJ, van Krieken JH, de Wilde PC, Balvers MG, Adema GJ, De Mulder PH. Intratumoural recombinant human interleukin-12 administration in head and neck squamous cell carcinoma patients modifies locoregional lymph node architecture and induces natural killer cell infiltration in the primary tumour. Clin Cancer Res. 2005;11:1899–1909. doi: 10.1158/1078-0432.CCR-04-1524. [DOI] [PubMed] [Google Scholar]
- 23.Car BD, Eng VM, Lipman JM, Anderson TD. The toxicology of interleukin-12: a review. Toxicol Pathol. 1999;27:58–63. doi: 10.1177/019262339902700112. [DOI] [PubMed] [Google Scholar]
- 24.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumours. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 25.Kang WK, Park C, Yoon HL, Kim WS, Yoon SS, Lee MH, Park K, Kim K, Jeong HS, Kim JA, Nam SJ, et al. Interleukin 12 gene therapy of cancer by peritumoural injection of transduced autologous fibroblasts: outcome of a phase I study. Hum Gene Ther. 2001;12:671–684. doi: 10.1089/104303401300057388. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Jurgovsky K, Moller P. Vaccination with IL-12 gene-modified autologous melanoma cells: preclinical results and a first clinical phase I study. Gene Ther. 1998;5:481–490. doi: 10.1038/sj.gt.3300619. [DOI] [PubMed] [Google Scholar]
- 27.Heinzerling L, Burg G, Dummer R, Maier T, Oberholzer PA, Schultz J, Elzaouk L, Pavlovic J, Moelling K. Intratumoural injection of DNA encoding human interleukin-12 into patients with metastatic melanoma: clinical efficacy. Hum Gene Ther. 2005;16:35–48. doi: 10.1089/hum.2005.16.35. [DOI] [PubMed] [Google Scholar]
- 28.Curti A, Parenza M, Colombo MP. Autologous and MHC class I-negative allogeneic tumour cells secreting IL-12 together cure disseminated A20 lymphoma. Blood. 2003;101:568–575. doi: 10.1182/blood-2002-03-0991. [DOI] [PubMed] [Google Scholar]
- 29.Eisenring M, vom Berg J, Kristiansen G, Saller E, Becher B. IL-12 initiates tumour rejection via lymphoid tissue-inducer cells bearing the natural cytotoxicity receptor NKp46. Nat Immunol. 2010;11:1030–1038. doi: 10.1038/ni.1947. [DOI] [PubMed] [Google Scholar]
- 30.Cavallo F, Signorelli P, Giovarelli M, Musiani P, Modesti A, Brunda MJ, Colombo MP, Forni G. Antitumour efficacy of adenocarcinoma cells engineered to produce interleukin 12 (IL-12) or other cytokines compared with exogenous IL-12. J Natl Cancer Inst. 1997;89:1049–1058. doi: 10.1093/jnci/89.14.1049. [DOI] [PubMed] [Google Scholar]
- 31.Wagner HJ, Bollard CM, Vigouroux S, Huls MH, Anderson R, Prentice HG, Brenner MK, Heslop HE, Rooney CM. A strategy for treatment of Epstein–Barr virus-positive Hodgkin’s disease by targeting interleukin 12 to the tumour environment using tumour antigen-specific T cells. Cancer Gene Ther. 2004;11:81–91. doi: 10.1038/sj.cgt.7700664. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Kerkar SP, Yu Z, Zheng Z, Yang S, Restifo NP, Rosenberg SA, Morgan RA. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumour environment. Mol Ther. 2011;19:751–759. doi: 10.1038/mt.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan H, Walters CS, Dunston GM, Tackey R. IL-12 plays a significant role in the apoptosis of human T cells in the absence of antigenic stimulation. Cytokine. 2002;19:126–137. doi: 10.1006/cyto.2002.1958. [DOI] [PubMed] [Google Scholar]
- 34.Mehrotra PT, Wu D, Crim JA, Mostowski HS, Siegel JP. Effects of IL-12 on the generation of cytotoxic activity in human CD8+ T lymphocytes. J Immunol. 1993;151:2444–2452. [PubMed] [Google Scholar]
- 35.Dowell AC, Oldham KA, Bhatt RI, Lee SP, Searle PF (2011) Long-term proliferation of functional human NK cells, with conversion of CD56(dim) NK cells to a CD56 (bright) phenotype, induced by carcinoma cells co-expressing 4-1BBL and IL-12. Cancer Immunol Immunother. doi:10.1007/s00262-011-1122-3 [DOI] [PMC free article] [PubMed]
- 36.Díaz-Montero CM, Naga O, Zidan AA, Salem ML, Pallin M, Parmigiani A, Walker G, Wieder E, Komanduri K, Cole DJ, Montero AJ, et al. Synergy of brief activation of CD8 T-cells in the presence of IL-12 and adoptive transfer into lymphopenic hosts promotes tumour clearance and anti-tumour memory. Am J Cancer Res. 2011;1:882–896. [PMC free article] [PubMed] [Google Scholar]
- 37.Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, Pisarev V, Sherman S, Sporn MB, Gabrilovich D. Tumour-infiltrating myeloid cells induce tumour cell resistance to cytotoxic T cells in mice. J Clin Invest. 2011;121:4015–4029. doi: 10.1172/JCI45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumours. Nat Med. 2004;10:294–298. doi: 10.1038/nm999. [DOI] [PubMed] [Google Scholar]
- 39.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumour-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 41.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/S1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 42.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 43.Ju DW, Yang Y, Tao Q, Song WG, He L, Chen G, Gu S, Ting CC, Cao X. Interleukin-18 gene transfer increases antitumour effects of suicide gene therapy through efficient induction of antitumour immunity. Gene Ther. 2000;7:1672–1679. doi: 10.1038/sj.gt.3301291. [DOI] [PubMed] [Google Scholar]
- 44.Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durrant DM, Metzger DW. IL-12 can alleviate Th17-mediated allergic lung inflammation through induction of pulmonary IL-10 expression. Mucosal Immunol. 2010;3:301–311. doi: 10.1038/mi.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Egilmez NK, Harden JL, Virtuoso LP, Schwendener RA, Kilinc MO. Nitric oxide short-circuits interleukin-12-mediated tumour regression. Cancer Immunol Immunother. 2011;60:839–845. doi: 10.1007/s00262-011-0998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenberg SA (2011) Cell therapy for metastatic melanoma using CD8 enriched tumour infiltrating lymphocytes. http://www.ClinicalTrials.gov. NCT01236573