Abstract
The cancer testis antigen Preferentially Expressed Antigen of Melanoma (PRAME) is overexpressed in many solid tumours and haematological malignancies whilst showing minimal expression in normal tissues and is therefore a promising target for immunotherapy. HLA-A0201-restricted peptide epitopes from PRAME have previously been identified as potential immunogens to drive antigen-specific autologous CTL responses, capable of lysing PRAME expressing tumour cells. CTL lines, from 13 normal donors and 10 melanoma patients, all of whom were HLA-A0201 positive, were generated against the PRAME peptide epitope PRA100−108. Specific killing activity against PRA100−108 peptide-pulsed targets was weak compared with CTL lines directed against known immunodominant peptides. Moreover, limiting dilution cloning from selected PRAME-specific CTL lines resulted in the generation of a clone of only low to intermediate avidity. Addition of the demethylating agent 5-aza-2′-Deoxycytidine (DAC) increased PRAME expression in 7 out of 11 malignant cell lines including several B lineage leukaemia lines and also increased class I expression. Pre-treatment of target cells was associated with increased sensitivity to antigen-specific killing by the low avidity CTL. When CTL, as well as of the target cells, were treated, the antigen-specific killing was further augmented. Interestingly, one HLA-A0201-negative DAC-treated line (RAJI) showed increased sensitivity to killing by clones despite a failure of expression of PRAME or HLA-A0201. Together these data point to a general increased augmentation of cancer immunogenocity by DAC involving both antigen-specific and non-specific mechanisms.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-011-1024-4) contains supplementary material, which is available to authorized users.
Keywords: PRAME, 5-Aza-2′-Deoxycytidine, Decitabine, CTL, Leukaemia, Cancer
Introduction
Preferentially expressed antigen of melanoma (PRAME) was initially isolated from melanoma-reactive cytotoxic T cells (CTL) [1] but has become a human tumour antigen of broader interest because of its wide expression in haematological malignancies and other solid tumours [1–10]. Furthermore, PRAME fulfils the criteria of a prototypic tumour antigen because it has a functional role in cancer progression involving its inhibition of retinoic acid induced differentiation and apoptosis, through binding to the retinoic acid receptor alpha [11, 12]. Previously, Melief and co-workers have identified HLA-A0201 binding peptide epitopes from PRAME and generated CTL against these epitopes in vitro [13, 14]. Subsequently, the applicability of these peptides as immunogens for cancer immunotherapy has been tested in patients with myeloid and lymphocytic leukaemias, and reactivity against cancer target cells or peptide-pulsed targets has been assessed by interferon gamma or granzyme B release assays, or cytotoxicity assays [15–17].
Previous work has identified a potential use of the demethylating agent 5-aza-2′-deoxycytidine (DAC, decitabine) to upregulate members of the cancer testis antigen family in cell lines derived from melanoma [18], Ovarian cancer [19] and acute myeloid leukaemia (AML) [20]. In most cases, DAC treatment has also upregulated surface MHC class I expression [19] and some studies have demonstrated enhanced sensitivity to lysis by CTL lines associated with this enhanced antigen expression [21]. DAC and other epigenetic modifiers designed to hypomethylate promoters to activate transcription of epigenetically silenced genes, has also been shown to have activity as a cytotoxic anticancer agent in its own right. A number of preclinical and clinical studies have identified a potentially useful role of DAC in the management of B cell leukaemias and lymphomas [22–24]. Therefore, there is a rationale for combining DAC with immunotherapy or pro-immunogenic chemotherapy in cancer.
In the current study, we have further investigated PRAME as a target for cancer immunotherapy by aiming to assess the strength and specificity of PRAME-specific CTL in the autologous repertoire of normal donors and patients with melanoma and by testing cytotoxicity against a range of leukaemia and solid tumour cell lines. We show evidence that PRAME-specific CTL in the autologous repertoire are limited to those of low avidity. A study of the use of DAC treatment in cancer cell lines shows upregulation of PRAME expression, which is associated with enhanced sensitivity to killing by low avidity PRAME-specific CTL. Surprisingly, prolonged exposure of PRAME-specific CTL to DAC also increases their antigen-specific killing. Moreover, DAC is also able to induce antigen-independent killing by the same CTL. Taken together, the data suggest the potential useful combination of DAC treatment and PRAME-directed immunotherapy for a range of cancer types.
Materials and methods
Peptides
Peptides were synthesised by Zinsser UK or Proimmune (UK), purified to more than 95% by reverse-phase high-performance liquid chromatography (HPLC), as confirmed by mass spectrometry. PRAME peptides have been described previously [13]. An immunodominant HLA-A0201-restricted peptide from Flu matrix (FLWGPRALV) was used as a positive control for HLA-A * 0201–binding ability. Irrelevant peptides were TLPGYPPHV (amino acids 311–320) from human PAX5, SLLMWITQV (amino acids 157–163) from NY-ESO-1 and TLGSCRERQPEFV from HA the minor histocompatibility antigen.
Cell lines
T2 (TAP-deficient lymphoblastoid cell line), K562 (chronic myelogenous leukaemia), HL60 (Acute promyelocytic leukaemia), Mott4 (acute T-cell leukaemia), REH, BV173, BJAB and Jekka (Pre-B leukaemia), Raji and Daudi (Burkitt’s lymphoma), U937 (histiocytic lymphoma), LAN-1, SKNAS and SH-SY5Y (neuroblastoma), SW116, colo205, SK-col-1, SW403 and SW480 (colon carcinoma), MDA-MB231, ZR-1-75-1 and MCF-7 (breast carcinoma), and TC32 (Ewing’s sarcoma) were all from American Type Culture Collection (ATCC); NALM1, NALM6, NALM-17, NALM-20 and NALM27 (Pre-B leukaemia) were kindly provided by Dr. Akira Harashima (Okayama, Japan). RH18 (rhabdomyosarcoma) and RH1 (Ewing family) were originally from Peter Houghton (St. Jude Children’s Research Hospital, Memphis, TN). Autologous B lymphoblastoid cell lines were established by incubation of peripheral blood mononuclear cells (PBMC) with supernatant from the EBV-producing marmoset cell line B95.8 (ATCC) as described [25]. G-7 is primary pre-B leukaemia cells isolated and cultured in vitro from pre-B leukaemia patient. Cell lines were maintained in RPMI 1640 or DMEM, supplemented with 10% foetal bovine serum (v/v), l-glutamine, nonessential amino acids, sodium pyruvate and gentamicin (complete medium). All culture materials were purchased from Life Technologies.
Transient transfection and quantitative RT-PCR
A Nucleofector® Device (Lonza, Wokingham, UK) was used to transfect HLA-A0201-positive PRAME-negative SW480 cells and HLA-A0201 negative, PRAME negative cell line Raji with a 1:10 ratio of a plasmid expressing GFP and a plasmid containing full-length PRAME coding sequence (1,529 bp) cloned into mammalian expression vector pcDNA3.1/Hygro(+) (Invitrogen, UK; kindly provided by Pierre Coulie Ludwig Institute for Cancer Research, Brussels, Belgium) according to manufacturer’s instruction. The transfection efficiency was between 75 and 95% (eGFP by flow cytometry). cDNA was generated using Trizol reagent and Superscript II (Invitrogen). Real-time PCR for PRAME was normalized to GAPDH using Applied Biosystems “Primers on demand” primer and probe sets, and data were collected using ABI 7900 PCR analyser. Relative quantitation was performed by the 2−δδCT method.
Human CTL line generation
Fresh peripheral blood was centrifuged over Ficoll-Hypaque to obtain peripheral blood mononuclear cells (PBMC). Adherent cells (1.5 × 106/well of 6-well plate) were cultured in 10% AB serum, IL-4 (30 ng/ml) and GM-CSF (100 ng/mL) for 7 days, with replenishment on days 3 and 5. On day 6, DCs were matured with LPS (200nM/mL), CD40L at 500 ng/ml (Biosource, UK) and IFN-γ at 500U/ml (Peprotech). T cells were stimulated two times at weekly intervals by autologous peptide (10 μM)-pulsed DCs, and a further two stimulations using autologous CD40-activated B cells as an alternative source of highly efficient antigen-presenting cells. Briefly, on day 7, mature DCs were pulsed with 10 μM of peptide for 4 h, γ-irradiated and cocultured with autologous T cells. 1 × 106 T cells were incubated with autologous mature, peptide-pulsed DCs in the presence of IL-12 (20 U/ml) and IL-7 (10 ng/ml) with a ratio of T cells:DCs of 10:1. T cells were restimulated for a second week with autologous mature peptide-pulsed DC in the presence of IL-12, IL-7 and IL-2. Two further weekly stimulations using autologous peptide-loaded (10 μM) CD40-activated B cells were used to maintain the specific T-cell line. T cells were harvested after four rounds of stimulation and were further cloned by limiting dilution methods (at 0.4 and 1 cell/well) using γ-irradiated allogeneic peripheral blood leukocytes (PBLs) at 1 × 105 cells/well (25 Gy) and B-LCL at 1x103/well (75 Gy) as feeder cells in RPMI medium, containing 250 IU IL-2 and 1 μg/ml PHA. Different types of starter T-cell populations were used in different expansions; these were CD3 positively selected, CD8 positively selected or CD8 with 10% add back of the CD8 negative population. All T-cell populations were obtained by immunomagnetic positive selection (MIltenyi, Bergisch Gladbach, Germany). After 12 days, growing T-cell clones were tested by 51Cr cytotoxic assay and subsequently expanded in RPMI medium.
Generating human autologous B cells as APC
Autologous CD40-activated B cells were generated as previously described [26]. Briefly, CD40L stably transfected mouse fibroblast cells (t-CD40L- cells, from Dr John Gordon) were γ-irradiated at 100 Gy, plated at 3.5 × 105 cells/well in six-well plates in basal iscove’s medium + 10% FCS and incubated overnight. CD8 T cells depleted PBMC were added at 1–2 × 106 cells/mL in the presence of 0.55 μM Cyclosporin A (Sandoz Pharmaceutical) for 4 days. Fresh irradiated t-CD40L cells were added every 4 days. B cells generated were 75% CD19 positive.
Peptide-binding assay
T2 cells were used to determine binding of peptides to HLA-A0201 as described previously [27]. Briefly, 2 × 105 T2 cells/well in 96-well plates were incubated for 18 h with 0–100 μM of each synthetic peptide, washed twice in PBS, and stained in the dark at 4°C for 30 min with 2 μg/ml PE-conjugated anti-HLA-A0201 (BB7.2, BD PharMingen) or PE-conjugated mouse IgG2 isotype control (BD PharMingen). Fluorescence index (FI) was calculated as the mean fluorescence intensity (MFI).
Treatment of cell lines and CTL with 5-aza-2′-Deoxycytidine
DAC was added to 5 × 105 cells in a 25 cm2 flask. After 72 h, the cells were harvested and used for experiments. We determined an optimal concentration of DAC by investigating its IC-50 for four representative malignant B cell lines by 72 h MTS assay (Promega) according to manufacturer’s instructions.
Cytotoxicity assays
CTL lines or clones were used as effector cells. Target cells were labelled with 100 μCi Na512CrO4 in cell culture medium containing 10% FCS for 60 min at 37°C. The cells were washed twice in culture medium. When required, the targets were pulsed with the specific or non-specific control peptides (0.0001–10 μM) for 1 h, washed once in culture medium and re-suspended at 50,000 cells/ml. After 4–18 h incubation, 25 μl of the assay supernatant was placed into a 96-well Lumaplate (Packard). Scintillant (100 μl) was added and radioactivity was counted using a Microbeta scintillation counter (PerkinElmer). The results are expressed as % specific lysis calculated as (Experimental release − Spontaneous release/Total release−Spontaneous release) × 100. For blocking assays, target cells were incubated for 1 h at room temperature with human anti-HLA-A0201 monoclonal antibody (clone BB7.2, purified from hybridoma, ATCC) or isotype control prior to coculture with effector cells.
Results
The cancer testis antigen PRAME is expressed in a broad range of human cancers
The cancer testis antigen PRAME has been identified as an excellent potential target for tumour immunotherapy by virtue of its overexpression and oncogenic role in human cancers [11], and low expression in normal tissues [1]. We analysed PRAME mRNA levels in a panel of human normal tissues. In contrast with the high level of transcript expression of the K562 leukaemia cell line, PRAME was virtually undetectable in 15 normal human tissues with the exception of low-level expression seen in kidney and testis (Table 1). In contrast, and in keeping with published results, of 29 human cancer cell lines, high-level expression (defined as 5 × the level seen in testis) was measured in 11 lines including the previously described targets leukaemia, breast cancer, colon cancer and neuroblastoma [1, 5] as well as the paediatric cancer lines rhabdomyosarcoma and Ewing sarcoma (Table 1).
Table 1.
Expression of PRAME mRNA in human cancer cell lines and normal tissues, and HLA-A0201 protein expression by flow cytometry in cell lines used in this study
| Tissues and tumour cell line | % of PRAME mRNA expression (Standard deviation) relative to RH18 | Type of cell line | HLA-A0201 expression |
|---|---|---|---|
| Cancer cell lines | |||
| BV173 | 1.5 (0.6) | Pre-B leukaemia | ++ |
| BJAB | 99 (3) | Pre-B leukaemia | ++ |
| Jekka | 20 (0.8) | Pre-B leukaemia | ++ |
| NALM-1 | 0.1 (0.001) | Pre-B leukaemia | + |
| NALM-6 | 0.2 (0.001) | Pre-B leukaemia | + |
| NALM-17 | 2 (0.2) | Pre-B leukaemia | + |
| NALM-20 | 3 (0.3) | Pre-B leukaemia | + |
| NALM-27 | 1.2 (0.3) | Pre-B leukaemia | + |
| REH | 32 (0.2) | Pre-B leukaemia | − |
| K562 | 312000 (20) | Chronic myelogenous leukaemia | − |
| Mott 4 | 132 (3.6) | Acute T-cell leukaemia, | − |
| HL60 | 110 (6) | Acute promyelocytic leukaemia | − |
| Daudi | 14.9 (1) | Burkitt’s lymphoma | − |
| Raji | 0.3 (0.1) | Burkitt lymphoma | − |
| U937 | 70.8 (0.2) | Histiocytic lymphoma | − |
| MDA-MB-231 | 1.9 (0.1) | Breast adenocarcinoma | +++ |
| ZR-1-75-1 | 1.4 (0.1) | Breast adenocarcinoma | − |
| MCF-7 | 145 (5.6) | Breast adenocarcinoma | ++ |
| SHSY5Y | 241 (5) | Neuroblastoma | − |
| LAN-1 | 110 (6) | Neuroblastoma | − |
| SK-N-AS | 0.1 (0.1) | Neuroblastoma | − |
| TC32 | 21 (2.1) | Ewing sarcoma | ++ |
| RH1 | 26 (1.5) | Ewing sarcoma | + |
| RH18 | 100 (1) | Rhabdomyosarcoma | ++ |
| SW480 | 1.2 (0.2) | Colon adenocarcinoma | ++ |
| SW1116 | 8.9 (0.5) | Colon adenocarcinoma | − |
| Colo205 | 1.3 (0.1) | Colon adenocarcinoma | + |
| SK-col-1 | 107 (3) | Colon adenocarcinoma | − |
| SW403 | 184 (7.9) | Colon adenocarcinoma | ++ |
| Non-malignant cell line | |||
| T2 | 0.2 (0.01) | TAP-deficient Lymphoblastoid cell | ++ |
| Primary cells | |||
| G-7 | 2 (0.4) | Acute lymphoblastic leukaemia | ++ |
| Normal tissue | |||
| Testis | 17.20 (0.01) | ||
| Kidney | 13.4 (0.031) | ||
| Bone marrow | 5.95 (0.025) | ||
| Liver | 2.35 (0.001) | ||
| Salivary gland | 2.35 (0.001) | ||
| Prostate | 2.3 (0.013) | ||
| Spleen | 2.3 (0.1) | ||
| Brain | 1.2 (0.01) | ||
| Skeletal muscle | 1.2 (0.01) | ||
| Small intestinal | 1.18 (0.012) | ||
| Stomach | 0.7 (0.021) | ||
| Colon | 0.66 (0.001) | ||
| Lung | 0.6 (0.022) | ||
| Heart | 0.1 (0.011) | ||
| Placenta | 0 (0.001) | ||
Percentage PRAME expression is relative to RH18
CTL lines against PRAME can be readily generated from normal donors or melanoma patients, but have low specific killing activity
Previous authors have identified 4 immunogenic HLA-A0201-restricted peptide epitopes from PRAME (PRA100−108, PRA142−151, PRA300−309, PRA425−433) [13]. We tested the ability of these peptides to stabilise HLA-A0201 expression on TAP-deficient T2 cells, as a surrogate for HLA-A0201 binding avidity. Of the four peptides, PRA100−108 showed comparable HLA-A0201 stabilisation compared with the immunodominant HLA-A0201-restricted flu matrix peptide (Fig. 1a). We therefore selected PRA100−108 peptide to generate anti-PRAME CTL lines in vitro from HLA-A0201-positive blood donors and HLA-A0201-positive patients with melanoma (known to express PRAME in over 90% of cases [1]). CTL lines against PRA100−108 were generated from the peripheral blood of 13 independent blood donors and 10 melanoma patients using a common stimulation protocol but with three different starting cell populations (CD8 purified, CD3 purified or CD8 purified with 10% add back of CD8 negative cells to provide additional T cell help; Table 2). Although it was possible to generate PRA100−108-specific CTL lines from all donors tested, the degree of target killing was only modest (range of 0–40% specific killing at E:T ratio of 30:1, Table 2) and background killing or irrelevant peptide-pulsed targets was relatively high (range 25–60% at E:T ratio 30:1) (representative data shown in Supplementary Figure 1A). Because melanoma is an immunogenic PRAME-expressing tumour, we reasoned that there might be higher precursor frequency in PRAME-specific CTL in peripheral blood of melanoma patients with active disease. However, using the same CTL expansion strategy, PRA100−108-specific CTL with killing activity more than 5% above background were only observed in 5 out of 10 patients, and the percent-specific killing was similar to that in normal blood donors (representative data showed in Supplementary Figure 1A and Table 2). In contrast, in parallel expansions from the same donors, higher killing activity was seen in T-cell lines generated against two other HLA-A0201-restricted peptide epitopes; the immunogenic flu matrix peptide (Supplementary Figure 1B) and an immunodominant peptide from the PAX5 transcription factor [28] (Supplementary Figure 1C). We also generated CTL lines against the epitopes PRA142−151, PRA300−309 and PRA425−433 using the same T-cell expansion methodology from the same donors and melanoma patients. Similar low levels of specific killing of PRAME peptide-pulsed targets were observed (data not shown). Taken together these data are suggestive of tolerance against PRAME epitopes in T cells derived from peripheral blood of normal donors and cancer patients.
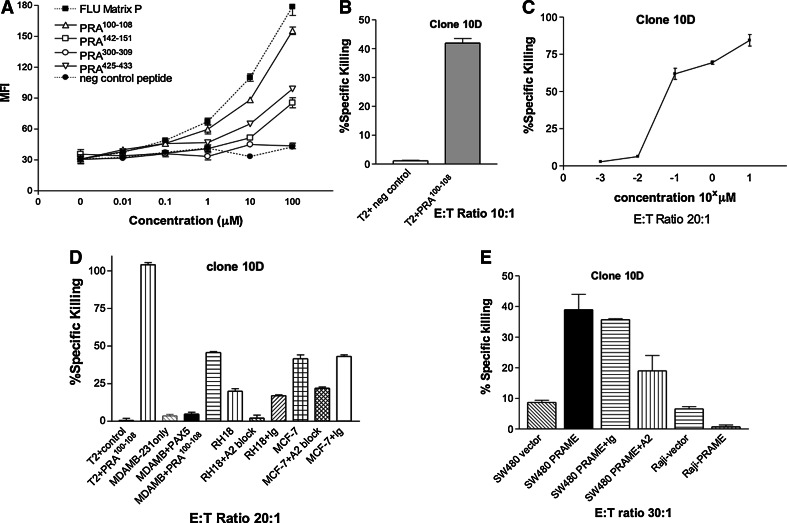
Fig. 1.
A PRAME100−108 CTL clone effectively lyses PRAME expressing target cells. a T2 cell binding assay to demonstrate stabilisation of HLA-A0201 on surface of T2 cells by named peptides with immunodominant Flu matrix peptide as positive control. b PRA100−108-specific clone 10D shows high specific killing of peptide-pulsed T2 cells. E:T ratio 10:1. c CTL clone 10D shows relatively low avidity as determined by killing of T2 cells pulsed with increasing concentrations of PRA100−108 peptide. d Killing by clone 10D as determined by chromium release assay, of cell lines pulsed with indicated peptides or pretreated with HLA-A0201 blocking antibody (A2). Ig denotes isotype control. T2 cells pulsed with PRA100−108 as positive control. e 18 h chromium release killing of SW480 and Raji cells lines transfected with PRAME or empty vector in presence of specific HLA-A0201 blocking antibody (A2) or isotype control (Ig). Error bars denote mean ± SEM. All chromium assays were performed over 4 h
Table 2.
Summary of killing activity of CTL lines directed against the PRA100−108 peptide antigen
| Normal donors | Starting responder cell population for generating CTL lines against PRA100−108 | % Specific killing (30:1) (T2 + Peptide) |
|---|---|---|
| BC1 | PBMC | 5–10 |
| BC6 | CD8 | 15–25 |
| BC7 | PBMC | 10–20 |
| BC17 | CD8 | 20–40 |
| BC21 | CD8+10% non-CD8 fraction | 5–20 |
| BC23 | CD8 | 5–30 |
| BC24 | CD8 + 10% non-CD8 fraction | 10–20 |
| BC26 | CD3 | 0–10 |
| BC38 | CD8 | 10–20 |
| BC42 | CD8 + 10% non-CD8 fraction | 0–5 |
| BC48 | CD8 + 10% non-CD8 fraction | 5–10 |
| BC49 | CD8 + 10% non-CD8 fraction | 0–5 |
| BC52 | CD8 + 10% non-CD8 fraction | 15–25 |
| Melanoma patients | ||
| Patient 1 | CD8 | 20–30 |
| Patient 2 | CD8 | 0–5 |
| Patient 3 | CD8 | 0–5 |
| Patient 4 | CD8 | 5–10 |
| Patient 5 | CD8 | 5–10 |
| Patient 6 | CD8 | 0–5 |
| Patient 7 | CD8 | 10–20 |
| Patient 8 | CD8 | 0–5 |
| Patient 9 | CD8 | 10–20 |
| Patient 10 | CD8 | 0–5 |
Several independent CTL lines were generated from each donor and the range of specific killing activities from each donor is indicated. Specific killing was defined as killing of PRA100−108-pulsed T2 cells minus killing of unpulsed T2 cells
CTL clones against PRA100−108 peptide from the autologous repertoire are of low avidity
Attempts to generate clones from PRA142−151, PRA300−309 and PRA425−433 by limiting dilution of CTL lines were unsuccessful (approximately 50,000 clones screened; data not shown). Moreover, we were able to generate only one CTL clone (termed 10D from normal donor BC23) that specifically recognised PRA100−108 (the epitope with the best HLA-A0201 binding profile) from approximately 6,000 clones screened. By way of contrast, using the same CTL generation and cloning technique in parallel, we successfully generated 6 high-avidity clones from a single blood donor against a novel epitope of PAX5, as we have recently reported [28]. The clonal nature of 10D was validated by demonstrating its homogeneous staining with CD3 and absence of NK markers and by showing a single V beta specificity by TCR spectrotyping (supplementary Figure 2A). Clone 10D had very high specific killing of peptide-pulsed T2 cells (Fig. 1b) but the avidity of the clone was only moderate (between 10 and 100 nM; Fig. 1c.
To demonstrate that clone PRA100−108 10D could kill cells that naturally processed PRA100−108, we showed killing of RH18 rhabdomyosarcoma and MCF7 breast cancer cells known to express both HLA-A0201 and PRAME (Table 1), and the killing was blocked by an HLA-A0201-specific antibody, but Clone 10D showed no killing of PRAME negative or HLA-A0201 negative cancer cell lines (Fig. 1d, Supplementary Figure 3). To demonstrate further that the CTL clone was capable of killing endogenously expressed PRAME in tumour cells, we transiently transfected the PRAME negative lines Raji (HLA-A0201 negative) and SW480 (HLA-A0201 positive) with PRAME cDNA or empty vector. Transfection efficiency was shown to be 80-90% by co-transfection of eGFP (data not shown). SW480, but not Raji, cells acquired new sensitivity to clone PRA100−108 10D killing, which was inhibited by an HLA-A0201 blocking antibody (Fig. 1e).
Therefore, clonal CTL with specificity for PRAME-expressing cells can be generated and might be of use in cancer immunotherapy, but the precursor frequency in the autologous repertoire is very low suggesting that vaccine approaches or short-term bulk culture stimulation approaches are unlikely to be successful.
5-Aza-2′ Deoxycytidine treatment of B leukaemia cells increases PRAME expression and sensitises to specific killing by a low avidity CTL clone
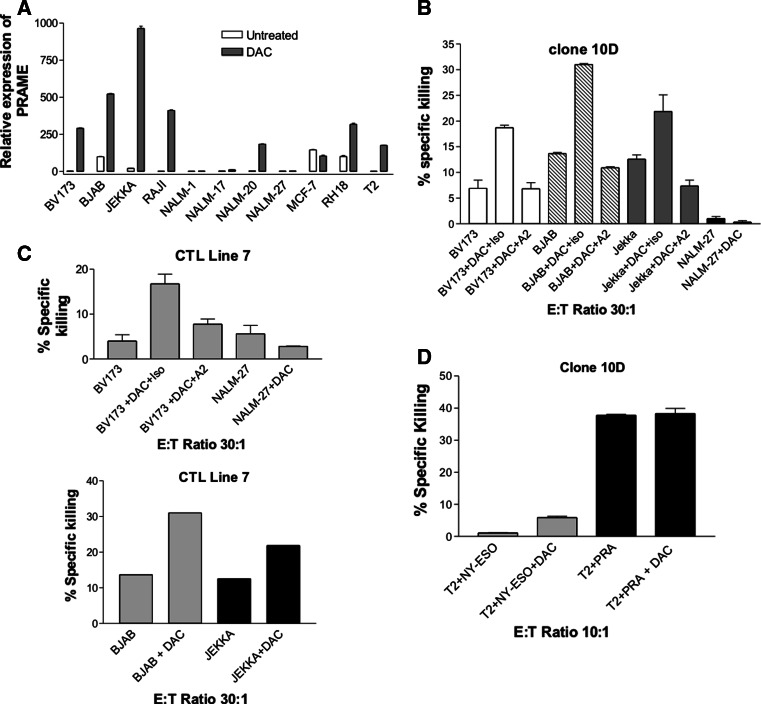
We speculated that the relatively poor in vitro killing could be improved by use of epigenetic modifying drugs to improve antigen presentation by the tumour targets. We made use of the demethylating agent 5 aza-2′-DeoxyCytidine (DAC) which has previously been reported to increase expression of cancer testis antigens including PRAME [20, 29]. We first determined an optimal concentration of DAC by investigating its IC50 for four representative malignant B cell lines by 72 h MTS assay. BJAB and NALM16 were relatively sensitive with IC50 at approximately 2 μM, whereas Raji and Jekka were resistant up to 10 μM. 1–4 μM of DAC has been previously used to induce promoter hypomethylation [19, 20, 30, 31] and is not significantly greater than the lowest IC50 values. We therefore reasoned that 3 μM would be minimally cytotoxic to the majority of cell lines but effective at demethylation, and we therefore used 3 μM in all subsequent experiments.
Using quantitative RT-PCR, we found that PRAME mRNA expression was increased by more than 2-fold (range 2.5–12,500) following 3 μM DAC treatment for 72 h in 7 out of 11 cell lines, including 5 lines which had almost complete silencing of PRAME in the absence of DAC (Fig. 2a). We next analysed CTL clone killing of DAC-treated tumour cell lines. Because of the minimal direct cytotoxic effects of DAC on tumour cells, background chromium release (in the absence of added T cells) was subtracted from all cytotoxicity results (range of background killing 5–30%). We selected three cell lines known to be HLA-A0201 positive (BV173, BJAB and Jekka) in which DAC pre-treatment resulted in high-level expression of PRAME. In all three lines, specific killing by clone 10D was increased approximately 2 fold and was HLA-A0201 restricted (Fig. 2b). In contrast, in NALM-27 cells, DAC treatment did not induce PRAME gene expression and did not induce any sensitivity to CTL clone-induced killing (Fig. 2b). Moreover, we repeated the experiments pre-treating BV173 and NALM-27 cells with 3 μM DAC but using a different PRA100−108-specific T-cell line-termed CTL Line 7 and provided by Professor Melief, Leiden. CTL Line 7 stained homogeneously with CD3 and CD8 and was negative for NK markers (supplementary Figure 2) but showed evidence of polyclonality on TCR spectrotyping (data not shown). CTL Line 7 showed the same pattern of enhanced killing of BV173, which was specifically inhibited by an HLA-A2 blocking antibody (Fig. 2c upper panel) and, in an independent experiment, with BJAB and Jekka (Fig. 2c lower panel). To provide further evidence that the increased killing induced by DAC was due to enhanced antigen presentation, we analysed the effects of the drug on TAP-deficient T2 cells which have no endogenous class I antigen presentation function. DAC did not increase killing of T2 cells pulsed with an irrelevant peptide, whereas pulsing of 10 μM of PRA100−108 peptide to produce high level of PRA100−108 antigen presentation resulted in a high-level killing which was not increased further by addition of DAC to the targets (Fig. 2d). Taken together these data suggest a role of DAC in enhancing antigen presentation and, in T2 cells, there is no evidence that addition of DAC increases sensitivity to CTL clone killing by non-antigen-specific mechanisms.
Fig. 2.
Treatment of cancer cell lines with 5-Aza -2′-Deoxycytidine enhances CTL killing, which correlates with upregulation of PRAME target antigen. a Expression of PRAME mRNA as determined by q-RT-PCR following treatment of indicated cell lines with 3 μM DAC for 72 h. b, c and d Specific killing by PRA100−108-specific CTL clones 10D (b and d) and CTL Line 7 (c) of indicated cell lines pretreated with 3 μM DAC for 72 h. In some experiments, the indicated blocking monoclonal antibodies were added to the chromium assay where A2 indicates HLA-A0201 blocking antibody and ISO is isotype control. In all panels, error bars denote SEM of triplicate estimates of a single experiment. All chromium assays were performed over 4 h
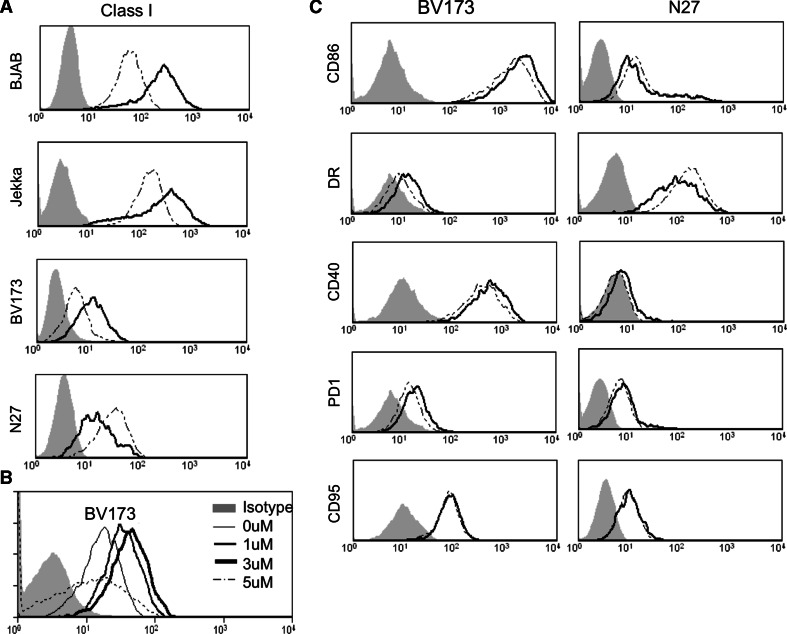
5-aza-2′-deoxycytidine treatment can upregulate class I MHC expression on tumour cells but does not increase costimulation
We speculated whether the increased sensitivity to CTL killing after treatment with DAC might also be associated with increased antigen presentation and/or co-stimulation of CTL by tumour targets. BV173, BJAB and Jekka cells were all rendered more sensitive to MHC-restricted antigen-specific killing following 3 μM DAC and this was associated with upregulation of MHC class I in all 3 cell lines. In contrast, NALM-27 cells, which remained resistant to antigen-specific killing, did not upregulate class I (Fig. 3a). A titration of DAC indeed showed a dose-dependent upregulation of class I in BV173 cells (Fig. 3b). To test whether DAC was also increasing co-stimulation by tumour cells or altering expression of death receptors or inhibitory receptors, we also measured CD86, HLA-DR, CD95, CD40 and PD1 following treatment of BV173 with increasing dosages of DAC but expression of none of these was significantly altered by DAC (Fig. 3c). Therefore, DAC can increase sensitivity to CTL killing by improving antigen presentation on class I MHC.
Fig. 3.
Treatment of cancer cell lines with 5-Aza -2′-Deoxycytidine increases expression of MHC class I but not costimulatory molecules. a MHC class I expression in the presence (dark line) and absence (dotted line) of 3 μM DAC. Cell lines were treated for 72 h and the filled histogram denotes an isotype control antibody. b Dose dependency of DAC treatment in BV173 cells. At 5 μM, the agent was cytotoxic and MHC I was not upregulated. c Failure of upregulation of the indicated surface molecules following 72 h treatment with 3 μM DAC. Data are representative of 3 independent experiments
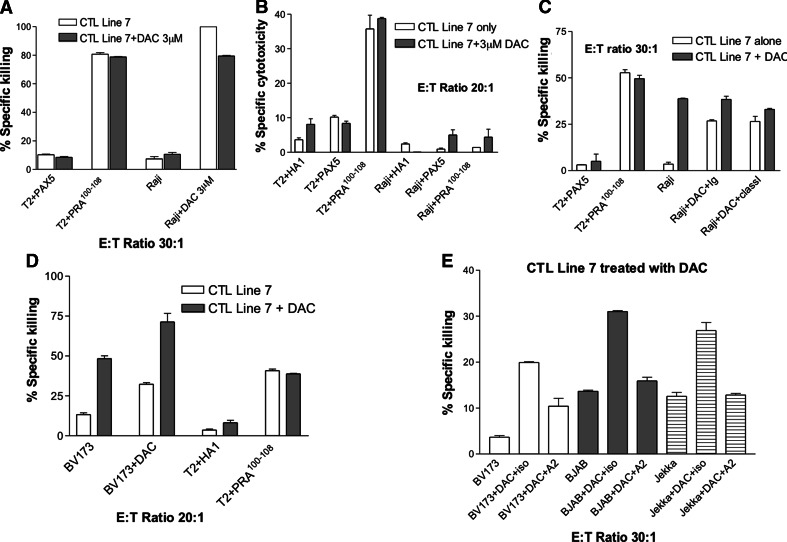
We next analysed 4 cell lines, which were HLA-A0201 negative, to determine the frequency by which DAC might also increase non-specific killing by CTL. Although there was no effect of DAC on the killing of K562, Daudi and SH-SY5Y (data not shown), Raji cells were rendered more sensitive to killing by the anti-PRA100−108 CTL line 7. The enhanced sensitivity was most dramatic with Raji cells (range from 25 to 95%) but was observed only following pre-treatment of the Raji cells, and not following treatment of the CTL (Fig. 4a). We could find no evidence of HLA-A0201 expression on Raji but confirmed that the killing of Raji was not due to presentation of the PRA100−108 peptide on low-level HLA-A0201 because pulsing of the cells with peptide did not render them sensitive to killing (Fig. 4b). Moreover, the enhanced killing following DAC treatment of Raji was not inhibited to any extent by an HLA-A0201 blocking antibody (Fig. 4c). Contaminating feeder cells, including NK cells, in the CTL clone population could not be held attributable for the killing by CTL line 7 because co-culture of feeder cells with DAC-treated Raji did not induce any significant killing (Supplementary Figure 4). Moreover, the CTL Line 7 stained negatively for NK markers. Therefore, with certain target cancer cell lines, DAC can beneficially enhance CTL killing by mechanisms independent of enhanced antigen presentation.
Fig. 4.
Effects of pretreatment of CTL clones with DAC on antigen-specific killing. a–e Chromium release cytotoxicity assays in which target cells and/or PRA100−108-specific CTL Line 7 were pretreated with DAC for 72 h. Cell lines were pulsed with peptides (HA1, PAX5 or PRA100−108) HA1 and PAX5 are non-specific HLA-A0201 binding peptides, class I denotes pretreatment of target cells with a pan-class-I blocking antibody, whereas Ig denotes its isotype control. All chromium assays performed over 4 h apart from a) (18 h)
5-aza-2′-deoxycytidine increases antigen-specific killing activity of CTL clones independent of its effects on target cells
It follows from these initial observations that there is a rationale for combining DAC treatment with immunotherapies such as adoptively transferred CTL. It was important therefore also to determine the effect of prolonged DAC administration on CTL in terms of their killing ability. To this end, we treated PRAME CTL line 7 directed against PRA100−108 with DAC for 72 h prior to assessing specific killing activity against BV173 cells treated or not with 3 μM DAC. Exposure of the CTL clone to DAC further increased specific killing of BV173 cells and the maximal killing was seen when both CTL and target cell were pre-treated. In contrast, the DAC-treated CTL did not acquire non-specific killing activity against T2 cells pulsed with irrelevant peptide, and killing of T2 cells saturated with 10 μM PRA100−108 was not further increased by the DAC (Fig. 4d). This enhanced killing by the DAC-treated CTL line 7 was inhibited by an HLA-A0201 blocking antibody for three independent target cells BV173, Jekka and BJAB (Fig. 4e). Moreover, identical results were obtained with an independent PRA100−108-specific clone 10D (supplementary figure 5).
Discussion
The human cancer testis antigen PRAME has been considered a promising target for immunotherapy for several years but so far no PRAME-directed immunotherapy approaches have been introduced into clinical trials. We confirm the results of other workers, that PRAME gene expression at the RNA level is minimal in normal adult human tissues, confirming it as a promising specific target for immunotherapy. Although PRAME has been shown to have growth-promoting functions in cancer cell lines, the relative lack of expression in normal tissues suggests that its normal function might be restricted to a developmental role. Gene targeting studies would be of interest in defining such a role. It is also of note that the level of expression at the RNA level in the tissues where it can be detected (for example, testis and kidney) is about an order of magnitude lower than in the expressing cancer cell lines (Table 1). A detailed study of PRAME protein expression in tumour and normal tissue sections could be very informative in determining whether transcript levels correlate with protein. However, there are no published immunohistochemical studies on PRAME expression and our own experiments with formalin-fixed PRAME-transfected cells have shown lack of specificity of staining (data not shown).
The list of cancer types in which PRAME is overexpressed and/or plays an oncogenic role has also increased since its initial description in melanoma [1], leukaemia [2, 9, 10, 15] and other cancers [1]. It has more recently been reported as expressed in medulloblastoma [4], renal cell carcinoma [7] and Wilms tumour [8], and is expressed and associated with poor prognosis in neuroblastoma [5] and breast cancer [3, 6]. We have now shown evidence of expression in cell lines derived from rhabdomyosarcoma and Ewing sarcoma. Despite the strong clinical justification for targeting PRAME in immunotherapy in terms of its wide and specific expression profile, no clinical immunotherapy trials attempting to harness natural immunity against PRAME through a vaccine approach have been published.
From the existing literature, it is unclear as to the strength of natural immunity against PRAME. Whereas several groups have shown convincing evidence for circulating PRAME-specific CTL by tetramer staining, there was little evidence that tetramer-positive cells were significantly more abundant or activated in patients with PRAME expressing cancers compared with normal donors [14, 16, 17]. Our findings of relatively weak PRAME CTL avidity in patients and normal donors would be consistent with a high degree of tolerance. The reason for the restriction of the PRAME CTL repertoire to low to intermediate avidity cells is not known; the possibility of killing of high avidity peptide-specific CTL by neighbouring CTL at the tumour immune synapse is an unexplored possibility [32]. We therefore investigated whether an epigenetic modifying drug 5-Aza 2′-deoxycytidine could significantly improve killing by naturally occurring PRAME-specific CTL. This approach is therapeutically attractive because DAC is FDA approved for the treatment of myelodysplastic syndromes (MDS) [33] following its evaluation in phase III studies [34, 35] and has been evaluated in numerous phase 2 clinical studies in leukaemia and other malignancies [36–38]. Its mechanisms of action as a cytotoxic agent have been widely reviewed but are thought to derive in part from reactivation of epigenetically silenced tumour suppressor genes, specifically as a result of demethylation of hypermethylated DNA regions associated with transcriptional repression. Another documented function of hypermethylation in cancer is suppression of antigen presentation and suppression of MHC expression has been shown to be reversible by DAC in a number of cancer models [31, 39]. For the purposes of combination therapy, it has recently been reported that the use of DAC prior to allogeneic transplantation is safe and feasible [40, 41].
We have shown that DAC, at a physiologically attainable concentration, can increase sensitivity of pre-B leukaemia cell lines to MHC-I-restricted killing by a PRAME-specific CTL clone and that the increased sensitivity correlates with upregulation of both PRAME mRNA expression and MHC Class I surface staining. This observation is consistent with a number of other reports in the literature in other disease types. For example, Adair and Hogan have demonstrated that DAC can upregulate cancer testis antigens in ovarian cancer cell lines [19] whilst Weber et al. [30] have shown upregulation of MAGE, and Natsume and coworkers have shown a similar pattern of upregulation of NY-ESO in glioma [31]. Specific upregulation of PRAME by DAC in AML has been reported, leading to the speculation that epigenetic mechanisms largely control silencing of PRAME in the majority of normal tissues [20]. This conclusion has been supported in other cell lines, and a hypermethylated region of the PRAME promoter associated with gene silencing has been identified [42].
We investigated the effect of DAC on CTL clone killing assays because we were interested in combination cancer therapy involving antigen-specific CTL and epigenetic modifiers, and because most previous studies have investigated killing by CTL lines where the antigen specificity and MHC restriction of killing is harder to demonstrate. Use of a CTL clone enabled us to differentiate antigen-specific and non-specific mechanisms. Similar results to ours demonstrating increased antigen-specific killing induced by DAC have recently been described in an NY-ESO TCR gene transfer model [43].
Interestingly, although the DAC increased sensitivity to a number of cell lines through a mechanism that was largely reversible by blocking MHC class I, we additionally identified 1 out of 4 cell lines where the DAC sensitising of target cells was antigen independent. The implication of this finding is that the CTL clone has antigen-independent killing pathways and that DAC increases target cell sensitivity to those pathways. We speculate that mechanisms such as death receptors or downregulation of inhibitory receptors on target cells could account for this antigen-independent pathway but further studies will be required to dissect the key mechanisms. It is of note that DAC pretreatment of the CTL clone increased antigen-specific killing but had no effect on antigen-independent killing. The mechanism by which the CTL is modified by DAC to enhance antigen-specific killing is unknown but one possibility is increased perforin expression induced by demethylation of the perforin promoter [44]. Our results must also be balanced against other workers using primary T cells who have shown an inhibitory effect of DAC on proliferation and activation [45, 46].
The additive effect of DAC treating both CTL and tumour target is supportive of exploratory studies of combination therapy in which tumour-specific CTL are infused into DAC-treated patients. However, the effect of DAC on induction of primary or memory immune responses against cancer antigens would be need to be carefully evaluated to provide a rationale for combining DAC with vaccine-based immunotherapy. One encouraging study has shown an increased expression of tumour antigen from a DNA vaccine administered following pre-treatment of mice with DAC [47].
In conclusion, PRAME remains a highly attractive potential target for cancer immunotherapy. However, low precursor frequency of PRAME-reactive CTL in the autologous repertoire of both normal donors and melanoma patients suggests tolerance and limits the likely clinical benefit of vaccine approaches. Harnessing high avidity T cells from allogeneic sources as a source of T-cell receptors for gene transfer is promising approach for PRAME-directed immunotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Supported by research grants from SPARKS, the Leukaemia Research Fund (UK), Children With Leukaemia and RICC.
Contributor Information
Owen Williams, Email: owen.williams@ich.ucl.ac.uk.
John Anderson, Email: j.anderson@ich.ucl.ac.uk.
References
- 1.Ikeda H, Lethe B, Lehmann F, van Baren N, Baurain JF, de Smet C, Chambost H, Vitale M, Moretta A, Boon T, Coulie PG. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6(2):199–208. doi: 10.1016/S1074-7613(00)80426-4. [DOI] [PubMed] [Google Scholar]
- 2.van Baren N, Chambost H, Ferrant A, Michaux L, Ikeda H, Millard I, Olive D, Boon T, Coulie PG. PRAME, a gene encoding an antigen recognized on a human melanoma by cytolytic T cells, is expressed in acute leukaemia cells. Br J Haematol. 1998;102(5):1376–1379. doi: 10.1046/j.1365-2141.1998.00982.x. [DOI] [PubMed] [Google Scholar]
- 3.Van ‘t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415(6871):530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 4.Boon K, Edwards JB, Siu IM, Olschner D, Eberhart CG, Marra MA, Strausberg RL, Riggins GJ. Comparison of medulloblastoma and normal neural transcriptomes identifies a restricted set of activated genes. Oncogene. 2003;22(48):7687–7694. doi: 10.1038/sj.onc.1207043. [DOI] [PubMed] [Google Scholar]
- 5.Oberthuer A, Hero B, Spitz R, Berthold F, Fischer M. The tumor-associated antigen PRAME is universally expressed in high-stage neuroblastoma and associated with poor outcome. Clin Cancer Res. 2004;10(13):4307–4313. doi: 10.1158/1078-0432.CCR-03-0813. [DOI] [PubMed] [Google Scholar]
- 6.Epping MT, Hart AA, Glas AM, Krijgsman O, Bernards R. PRAME expression and clinical outcome of breast cancer. Br J Cancer. 2008;99(3):398–403. doi: 10.1038/sj.bjc.6604494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neumann E, Engelsberg A, Decker J, Storkel S, Jaeger E, Huber C, Seliger B. Heterogeneous expression of the tumor-associated antigens RAGE-1, PRAME, and glycoprotein 75 in human renal cell carcinoma: candidates for T-cell-based immunotherapies? Cancer Res. 1998;58(18):4090–4095. [PubMed] [Google Scholar]
- 8.Li CM, Guo M, Borczuk A, Powell CA, Wei M, Thaker HM, Friedman R, Klein U, Tycko B. Gene expression in Wilms’ tumor mimics the earliest committed stage in the metanephric mesenchymal-epithelial transition. Am J Pathol. 2002;160(6):2181–2190. doi: 10.1016/S0002-9440(10)61166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinbach D, Viehmann S, Zintl F, Gruhn B. PRAME gene expression in childhood acute lymphoblastic leukemia. Cancer Genet Cytogenet. 2002;138(1):89–91. doi: 10.1016/S0165-4608(02)00582-4. [DOI] [PubMed] [Google Scholar]
- 10.Radich JP, Dai H, Mao M, Oehler V, Schelter J, Druker B, Sawyers C, Shah N, Stock W, Willman CL, Friend S, Linsley PS. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci USA. 2006;103(8):2794–2799. doi: 10.1073/pnas.0510423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epping MT, Wang L, Edel MJ, Carlee L, Hernandez M, Bernards R. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122(6):835–847. doi: 10.1016/j.cell.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Passeron T, Valencia JC, Namiki T, Vieira WD, Passeron H, Miyamura Y, Hearing VJ. Upregulation of SOX9 inhibits the growth of human and mouse melanomas and restores their sensitivity to retinoic acid. J Clin Invest. 2009;119(4):954–963. doi: 10.1172/JCI34015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kessler JH, Beekman NJ, Bres-Vloemans SA, Verdijk P, van Veelen PA, Kloosterman-Joosten AM, Vissers DC, ten Bosch GJ, Kester MG, Sijts A, Wouter Drijfhout J, Ossendorp F, Offringa R, Melief CJ. Efficient identification of novel HLA-A(*)0201-presented cytotoxic T lymphocyte epitopes in the widely expressed tumor antigen PRAME by proteasome-mediated digestion analysis. J Exp Med. 2001;193(1):73–88. doi: 10.1084/jem.193.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffioen M, Kessler JH, Borghi M, van Soest RA, van der Minne CE, Nouta J, van der Burg SH, Medema JP, Schrier PI, Falkenburg JH, Osanto S, Melief CJ. Detection and functional analysis of CD8 + T cells specific for PRAME: a target for T-cell therapy. Clin Cancer Res. 2006;12(10):3130–3136. doi: 10.1158/1078-0432.CCR-05-2578. [DOI] [PubMed] [Google Scholar]
- 15.Greiner J, Schmitt M, Li L, Giannopoulos K, Bosch K, Schmitt A, Dohner K, Schlenk RF, Pollack JR, Dohner H, Bullinger L. Expression of tumor-associated antigens in acute myeloid leukemia: Implications for specific immunotherapeutic approaches. Blood. 2006;108(13):4109–4117. doi: 10.1182/blood-2006-01-023127. [DOI] [PubMed] [Google Scholar]
- 16.Rezvani K, Yong AS, Tawab A, Jafarpour B, Eniafe R, Mielke S, Savani BN, Keyvanfar K, Li Y, Kurlander R, Barrett AJ. Ex vivo characterization of polyclonal memory CD8 + T-cell responses to PRAME-specific peptides in patients with acute lymphoblastic leukemia and acute and chronic myeloid leukemia. Blood. 2009;113(10):2245–2255. doi: 10.1182/blood-2008-03-144071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quintarelli C, Dotti G, De Angelis B, Hoyos V, Mims M, Luciano L, Heslop HE, Rooney CM, Pane F, Savoldo B. Cytotoxic T lymphocytes directed to the preferentially expressed antigen of melanoma (PRAME) target chronic myeloid leukemia. Blood. 2008;112(5):1876–1885. doi: 10.1182/blood-2008-04-150045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiser TS, Ohnmacht GA, Guo ZS, Fischette MR, Chen GA, Hong JA, Nguyen DM, Schrump DS. Induction of MAGE-3 expression in lung and esophageal cancer cells. Ann Thorac Surg. 2001;71(1):295–301. doi: 10.1016/S0003-4975(00)02421-8. [DOI] [PubMed] [Google Scholar]
- 19.Adair SJ, Hogan KT. Treatment of ovarian cancer cell lines with 5-aza-2′-deoxycytidine upregulates the expression of cancer-testis antigens and class I major histocompatibility complex-encoded molecules. Cancer Immunol Immunother. 2009;58(4):589–601. doi: 10.1007/s00262-008-0582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roman-Gomez J, Jimenez-Velasco A, Agirre X, Castillejo JA, Navarro G, Jose-Eneriz ES, Garate L, Cordeu L, Cervantes F, Prosper F, Heiniger A, Torres A. Epigenetic regulation of PRAME gene in chronic myeloid leukemia. Leuk Res. 2007;31(11):1521–1528. doi: 10.1016/j.leukres.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Weiser TS, Guo ZS, Ohnmacht GA, Parkhurst ML, Tong-On P, Marincola FM, Fischette MR, Yu X, Chen GA, Hong JA, Stewart JH, Nguyen DM, Rosenberg SA, Schrump DS. Sequential 5-Aza-2′ deoxycytidine-depsipeptide FR901228 treatment induces apoptosis preferentially in cancer cells and facilitates their recognition by cytolytic T lymphocytes specific for NY-ESO-1. J Immunother. 2001;24(2):151–161. doi: 10.1097/00002371-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Blum KA, Liu Z, Lucas DM, Chen P, Xie Z, Baiocchi R, Benson DM, Devine SM, Jones J, Andritsos L, Flynn J, Plass C, Marcucci G, Chan KK, Grever MR, Byrd JC. Phase I trial of low dose decitabine targeting DNA hypermethylation in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: dose-limiting myelosuppression without evidence of DNA hypomethylation. Br J Haematol. 2010;150(2):189–195. doi: 10.1111/j.1365-2141.2010.08213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blum W, Klisovic RB, Hackanson B, Liu Z, Liu S, Devine H, Vukosavljevic T, Huynh L, Lozanski G, Kefauver C, Plass C, Devine SM, Heerema NA, Murgo A, Chan KK, Grever MR, Byrd JC, Marcucci G. Phase I study of decitabine alone or in combination with valproic acid in acute myeloid leukemia. J Clin Oncol. 2007;25(25):3884–3891. doi: 10.1200/JCO.2006.09.4169. [DOI] [PubMed] [Google Scholar]
- 24.Dubovsky JA, McNeel DG, Powers JJ, Gordon J, Sotomayor EM, Pinilla-Ibarz JA. Treatment of chronic lymphocytic leukemia with a hypomethylating agent induces expression of NXF2, an immunogenic cancer testis antigen. Clin Cancer Res. 2009;15(10):3406–3415. doi: 10.1158/1078-0432.CCR-08-2099. [DOI] [PubMed] [Google Scholar]
- 25.Kozbor D, Roder JC. Requirements for the establishment of high-titered human monoclonal antibodies against tetanus toxoid using the Epstein-Barr virus technique. J Immunol. 1981;127(4):1275–1280. [PubMed] [Google Scholar]
- 26.Schultze JL, Michalak S, Seamon MJ, Dranoff G, Jung K, Daley J, Delgado JC, Gribben JG, Nadler LM. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997;100(11):2757–2765. doi: 10.1172/JCI119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10(6):673–679. doi: 10.1016/S1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 28.Yan M, Himoudi N, Pule M, Sebire N, Poon E, Blair A, Williams O, Anderson J. Development of cellular immune responses against PAX5, a novel target for cancer immunotherapy. Cancer Res. 2008;68(19):8058–8065. doi: 10.1158/0008-5472.CAN-08-0153. [DOI] [PubMed] [Google Scholar]
- 29.Sigalotti L, Fratta E, Coral S, Tanzarella S, Danielli R, Colizzi F, Fonsatti E, Traversari C, Altomonte M, Maio M. Intratumor heterogeneity of cancer/testis antigens expression in human cutaneous melanoma is methylation-regulated and functionally reverted by 5-aza-2′-deoxycytidine. Cancer Res. 2004;64(24):9167–9171. doi: 10.1158/0008-5472.CAN-04-1442. [DOI] [PubMed] [Google Scholar]
- 30.Weber J, Salgaller M, Samid D, Johnson B, Herlyn M, Lassam N, Treisman J, Rosenberg SA. Expression of the MAGE-1 tumor antigen is up-regulated by the demethylating agent 5-aza-2′-deoxycytidine. Cancer Res. 1994;54(7):1766–1771. [PubMed] [Google Scholar]
- 31.Natsume A, Wakabayashi T, Tsujimura K, Shimato S, Ito M, Kuzushima K, Kondo Y, Sekido Y, Kawatsura H, Narita Y, Yoshida J. The DNA demethylating agent 5-aza-2′-deoxycytidine activates NY-ESO-1 antigenicity in orthotopic human glioma. Int J Cancer. 2008;122(11):2542–2553. doi: 10.1002/ijc.23407. [DOI] [PubMed] [Google Scholar]
- 32.Leisegang M, Wilde S, Spranger S, Milosevic S, Frankenberger B, Uckert W, Schendel DJ. MHC-restricted fratricide of human lymphocytes expressing survivin-specific transgenic T cell receptors. J Clin Invest. 2010;120(11):3869–3877. doi: 10.1172/JCI43437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCormack SE, Warlick ED. Epigenetic approaches in the treatment of myelodysplastic syndromes: clinical utility of azacitidine. Onco Targets Ther. 2010;3:157–165. doi: 10.2147/ott.s5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, Klimek V, Slack J, de Castro C, Ravandi F, Helmer R, III, Shen L, Nimer SD, Leavitt R, Raza A, Saba H. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106(8):1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 35.Atallah E, Kantarjian H, Garcia-Manero G. The role of decitabine in the treatment of myelodysplastic syndromes. Expert Opin Pharmacother. 2007;8(1):65–73. doi: 10.1517/14656566.8.1.65. [DOI] [PubMed] [Google Scholar]
- 36.Jain N, Rossi A, Garcia-Manero G. Epigenetic therapy of leukemia: an update. Int J Biochem Cell Biol. 2009;41(1):72–80. doi: 10.1016/j.biocel.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Momparler RL. Epigenetic therapy of cancer with 5-aza-2′-deoxycytidine (decitabine) Semin Oncol. 2005;32(5):443–451. doi: 10.1053/j.seminoncol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Oki Y, Issa JP. Review: recent clinical trials in epigenetic therapy. Rev Recent Clin Trials. 2006;1(2):169–182. doi: 10.2174/157488706776876490. [DOI] [PubMed] [Google Scholar]
- 39.Coral S, Sigalotti L, Colizzi F, Spessotto A, Nardi G, Cortini E, Pezzani L, Fratta E, Fonsatti E, Di Giacomo AM, Nicotra MR, Natali PG, Altomonte M, Maio M. Phenotypic and functional changes of human melanoma xenografts induced by DNA hypomethylation: immunotherapeutic implications. J Cell Physiol. 2006;207(1):58–66. doi: 10.1002/jcp.20540. [DOI] [PubMed] [Google Scholar]
- 40.Lubbert M, Bertz H, Ruter B, Marks R, Claus R, Wasch R, Finke J. Non-intensive treatment with low-dose 5-aza-2′-deoxycytidine (DAC) prior to allogeneic blood SCT of older MDS/AML patients. Bone Marrow Transplant. 2009;44(9):585–588. doi: 10.1038/bmt.2009.64. [DOI] [PubMed] [Google Scholar]
- 41.Czibere A, Bruns I, Kroger N, Platzbecker U, Lind J, Zohren F, Fenk R, Germing U, Schroder T, Graf T, Haas R, Kobbe G. 5-Azacytidine for the treatment of patients with acute myeloid leukemia or myelodysplastic syndrome who relapse after allo-SCT: a retrospective analysis. Bone Marrow Transplant. 2010;45(5):872–876. doi: 10.1038/bmt.2009.266. [DOI] [PubMed] [Google Scholar]
- 42.Schenk T, Stengel S, Goellner S, Steinbach D, Saluz HP. Hypomethylation of PRAME is responsible for its aberrant overexpression in human malignancies. Genes Chromosom Cancer. 2007;46(9):796–804. doi: 10.1002/gcc.20465. [DOI] [PubMed] [Google Scholar]
- 43.Wargo JA, Robbins PF, Li Y, Zhao Y, El-Gamil M, Caragacianu D, Zheng Z, Hong JA, Downey S, Schrump DS, Rosenberg SA, Morgan RA. Recognition of NY-ESO-1 + tumor cells by engineered lymphocytes is enhanced by improved vector design and epigenetic modulation of tumor antigen expression. Cancer Immunol Immunother. 2009;58(3):383–394. doi: 10.1007/s00262-008-0562-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu Q, Wu A, Ray D, Deng C, Attwood J, Hanash S, Pipkin M, Lichtenheld M, Richardson B. DNA methylation and chromatin structure regulate T cell perforin gene expression. J Immunol. 2003;170(10):5124–5132. doi: 10.4049/jimmunol.170.10.5124. [DOI] [PubMed] [Google Scholar]
- 45.Goodyear O, Agathanggelou A, Novitzky-Basso I, Siddique S, McSkeane T, Ryan G, Vyas P, Cavenagh J, Stankovic T, Moss P, Craddock C. Induction of a CD8 + T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood. 2010;116(11):1908–1918. doi: 10.1182/blood-2009-11-249474. [DOI] [PubMed] [Google Scholar]
- 46.Sanchez-Abarca LI, Gutierrez-Cosio S, Santamaria C, Caballero-Velazquez T, Blanco B, Herrero-Sanchez C, Garcia JL, Carrancio S, Hernandez-Campo P, Gonzalez FJ, Flores T, Ciudad L, Ballestar E, Del Canizo C, San Miguel JF, Perez-Simon JA. Immunomodulatory effect of 5-azacytidine (5-azaC): potential role in the transplantation setting. Blood. 2010;115(1):107–121. doi: 10.1182/blood-2009-03-210393. [DOI] [PubMed] [Google Scholar]
- 47.Lu D, Hoory T, Monie A, Wu A, Wang MC, Hung CF. Treatment with demethylating agent, 5-aza-2′-deoxycytidine enhances therapeutic HPV DNA vaccine potency. Vaccine. 2009;27(32):4363–4369. doi: 10.1016/j.vaccine.2009.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.