Abstract
High-level microsatellite-unstable (MSI-H) colorectal carcinomas (CRC) represent a distinct subtype of tumors commonly characterized by dense infiltration with cytotoxic T cells, most likely due to expression of MSI-H-related frameshift peptides (FSP). The contribution of FSP and classical antigens like MUC1 and CEA to the cellular immune response against MSI-H CRC had not been analyzed so far. We analyzed tumor-infiltrating and peripheral T cells from MSI-H (n = 4 and n = 14, respectively) and microsatellite-stable (MSS) tumor patients (n = 26 and n = 17) using interferon gamma ELISpot assays. Responses against 4 FSP antigens and peptides derived from MUC1 to CEA were compared with and without depletion of regulatory T cells, and the results were related to the presence of the respective antigens in tumor tissue. Preexisting FSP-specific T cell responses were detected in all (4 out of 4) tumor-infiltrating and in the majority (10 out of 14) of peripheral T cell samples from MSI-H CRC patients, but rarely observed in MSS CRC patients. Preexisting T cell responses in MSI-H CRC patients were significantly more frequently directed against FSP tested in the present study than against peptides derived from classical antigens MUC1 or CEA (p = 0.049). Depletion of regulatory T cells increased the frequency of effector T cell responses specific for MUC1/CEA-derived peptides and, to a lesser extent, T cell responses specific for FSP. Our data suggest that the analyzed FSP may represent an immunologically relevant pool of antigens capable of eliciting antitumoral effector T cell responses.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-012-1303-8) contains supplementary material, which is available to authorized users.
Keywords: Regulatory T cells, Frameshift peptides, High-level microsatellite instability, Colorectal cancer, Immune response
Introduction
Whereas the majority of colorectal cancers (CRC) show large chromosomal alterations, a subset of 10–15 % of CRC is characterized by high-level microsatellite instability (MSI-H) as a consequence of DNA mismatch repair deficiency [1, 2]. The accumulation of insertion/deletion mutations in coding microsatellites is generally regarded as one driving force of tumor development in MSI-H CRC [3]. Coding microsatellite mutations may result in translational frameshifts and the synthesis of truncated peptides [4]. The accumulation of these frameshift-derived peptides (FSP) most likely contributes to immune recognition and the dense lymphocyte infiltration observed in MSI-H tumors [5–7]. Cellular and humoral immune responses against FSP antigens can frequently be detected in the peripheral blood of MSI-H CRC patients and even healthy Lynch syndrome mutation carriers [4, 8–11].
Nevertheless, MSI-H tumors often grow out to large tumor masses, suggesting mechanisms of immune escape like loss of human leukocyte antigen class I and II expression which are frequently observed in MSI-H CRC [12–14]. An alternative mechanism to escape immunosurveillance is apparently mediated by the induction or recruitment of regulatory T cells (Treg cells) that can suppress antitumoral effector T cell responses in CRC patients. It is known from previous studies that MSI-H CRC show a pronounced infiltration with Foxp3-positive Treg cells [15–17].
Naturally occurring Treg cells develop in the thymus and play an important role in the maintenance of peripheral self-tolerance. Treg cells are defined through their ability to control the function of antigen-reactive effector T cells (reviewed in [18]) and are typically characterized by a CD4-positive CD25-high phenotype and expression of the transcription factor Foxp3 [19, 20]. The suppressive impact of Treg cells on antigen-specific effector T cells has been demonstrated ex vivo [16, 21], and interestingly, the depletion of Treg cells from peripheral blood samples of CRC patients led to enhanced effector T cell responses against a distinct set of CRC-associated tumor antigens, indicating the antigen-specific nature of Treg-cell-mediated suppression [21].
Up to now, there have been no comparative studies examining T cell responses directed against FSP and other cancer-associated antigens like MUC1 or CEA [22, 23] in CRC patients, and the antigen specificity and role of Treg cells for immune response and immune evasion in MSI-H CRC patients have been unknown. This prompted us to analyze effector and regulatory T cell reactivity against a set of four FSP antigens, HT001(-1), AIM2(-1), TAF1B(-1) and TGFBR2(-1), selected according to their high mutation frequency in MSI-H CRC [24, 25] and pronounced immunogenicity in vitro [8, 9, 26, 27] in comparison with the two classical antigens MUC1 and CEA, which are currently evaluated for CRC immunotherapy in clinical trials [22, 28, 29].
Materials and methods
Patients
Patients were recruited from the Department of General and Visceral Surgery, University Hospital, Heidelberg. Written consent was obtained from all participants. The protocol was approved by the Ethical Committee of the University of Heidelberg. A total number of 16 MSI-H and 32 MSS CRC patients were included in the study. Median age of patients at tumor resection was 46 (ranging from 33 to 77 years) for MSI-H and 65 years (ranging from 30 to 85) for MSS. UICC status was available for 9 MSI-H and 26 MSS CRC patients. Blood samples were drawn postoperatively with intervals between tumor resection and blood withdrawal ranging from 0.1 to 102 months. Patients’ characteristics are summarized in Online Resource Table 1.
Preparation of peripheral blood mononuclear cells (PBMCs)
Heparinized peripheral blood samples were obtained from 31 patients with CRC (14 MSI-H and 17 MSS) and 7 healthy donors without evidence for Lynch syndrome. Blood samples were subjected to density gradient centrifugation over lymphocyte separation medium (PAA Laboratories, Cölbe, Germany), and PBMCs at interphases were collected.
Preparation of tumor-infiltrating lymphocytes
CRC samples from 30 patients (4 MSI-H and 26 MSS) were transferred to our laboratory immediately after surgical resection and digested mechanically and enzymatically in RPMI medium (PAA Laboratories) containing 20 μg/ml DNase I, 1 mg/ml Blend collagenase N and 0.1 mg/ml hyaluronidase III (all purchased from Sigma-Aldrich) over night followed by a cell separation over a 100-μm cell strainer to obtain a single cell suspension. Tumor-infiltrating lymphocytes (TILs) were then isolated by a three-phase Percoll gradient (Amersham Biosciences, Uppsala, Sweden) and collected from the third interphase. The average number of TILs upon Percoll gradient isolation was 29 × 106 cells. However, numbers of isolated TILs was strongly dependent on the size of the tumor sample, ranging from 0.3 × 107 to 1.22 × 108 cells.
Purification of B cells (Bc) and T cells
Whole CD3-positive T cells were isolated from PBMCs and TILs by depletion of non-T cells using the MACS Pan T cell Isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. T cells were cryopreserved at day 0 at −70 °C in human serum (PAA Laboratories) with 10 % dimethyl sulfoxide (DMSO) (Sigma-Aldrich). CD40-activated Bc were generated as described previously [8] by cultivation of non-T cells for at least 21 days on irradiated NIH3T3 feeder cells stably expressing CD154.
Three days before the ELISpot assays, T cells were thawed and cultivated in IMDM medium with 10 % human AB serum (both purchased from PAA Laboratories), 50 IU/ml interleukin-2, 10 IU/ml interleukin-7 (both from PromoCell, Heidelberg, Germany) and supplemented with insulin/transferring/selenium (1:100, Invitrogen, Karlsruhe, Germany).
Depletion of regulatory T cells
A total of 1.8 × 106 cells were separated from whole T cells as undepleted T cell fraction. Remaining T cells underwent a depletion of CD4/CD25 double-positive cells by using the CD4+CD25+ Regulatory T cell Isolation Kit according to manufacturer’s instructions (Miltenyi Biotec). CD8-positive T cells and CD4-positive CD25-negative T cells were mixed and applied as Treg-depleted T cell fraction. Treg cell depletion was performed with tumor-infiltrating T cells from 4 MSI-H to 18 MSS CRC patients as well as with peripheral T cells from 12 MSI-H to 11 MSS CRC patients.
Flow cytometry
The purity of peripheral and tumor-infiltrating T cells and Bc was characterized by flow cytometry analysis of cell surface markers using FITC-labeled CD4 (clone RPA-T4, 1:50) and CD8 (clone RPA-T8, 1:50) antibodies, PE-labeled CD19 (clone HIB19, 1:50) and CD25 antibodies (clone 4E3, 1:50) and unlabeled antibody against the Pan T cell marker CD3 (OKT3, undiluted) followed by FITC-labeled anti-mouse IgG secondary antibodies (1:50, eBioscience, San Diego, USA). Cells treated without primary antibody (for CD3) or treated with isotype-matched mouse IgG1 were used as negative control. All primary antibodies were purchased from BD Biosciences, Heidelberg, Germany.
Immunofluorescence was measured with a FACSCalibur (Becton–Dickinson) and evaluated with CellQuest software (Version 5.2, BD Bioscience).
Antigens
IFN-gamma ELISpot analysis was performed using a set of 4 MSI-H-specific FSP, one CEA- and 2 MUC1-derived peptides (Table 1). MUC1-derived peptides Muc20 and Muc100 represent the tandem repeat and the signal sequence of MUC1, respectively. FSP antigens were selected according to their high mutation frequency and in vitro immunogenicity in MSI-H CRC patients. For CEA and MUC1, peptides were selected that contained on the one hand validated HLA-A2-restricted epitopes and on the other hand a large variety of predicted HLA I- and II-restricted epitopes. These long peptides were also tested for recognition by antigen-specific T cells from CRC patients with multiple different HLA haplotypes, and those with the highest immunogenicity were selected. Peptides were obtained from the Peptide Synthesis Facility at the German Cancer Research Center. All peptides were purified by high-performance liquid chromatography and analyzed by mass spectrometry. They were dissolved to 5 mg/ml in DMSO and further diluted to 500 μg/ml in phosphate-buffered saline (PBS).
Table 1.
Predicted FSP (frameshift neosequences are underlined) with the number of deleted nucleotides in brackets and the length of the microsatellite, and classical antigens associated with CRC used in the ELISpot analysis
| Antigen | Repeat | Peptide sequence |
|---|---|---|
| HT001(-1) | A(11) | EIFLPKGRSNSKKKGRRNRIPAVLRTEGEPLHTPSVGMRETTGLGC |
| AIM2(-1) | A(10) | HSTIKVIKAKKKHREVKRTNSSQLV |
| TAF1B(-1) | A(11) | NTQIKALNRGLKKKTILKKAGIGMCVKVSSIFFINKQKP |
| TGFBR2(-1) | A(10) | LEDAASPKCIMKEKKSLVRLSSCVPVALMSAMTTSSSQKNITPAILTCC |
| MUC1 137–396 (Muc20) (tandem repeat) | (GVTSAPDTRPAPGSTAPPAH) × 5 | |
| MUC1 1–100 (Muc100) (signal sequence) | MTPGTQSPFFLLLLLTVLTVVTGSGHASSTPGGEKETSATQRSSVPSSTEKNA VSMTSSVLSSHSPGSGSSTTQGQDVTLAPATEPASGSAATWGQDVTS | |
| CEA 569–618 | YVCGIQNSVSANRSDPVTLDVLYGPDTPIISPPDSSYLGANLNLSCHSA |
IFN-gamma ELISpot
ELISpot assays using autologous Bc as antigen-presenting cells were performed as described previously [8] with slight modifications. Briefly, after depletion of Treg cells, Treg-undepleted and Treg-depleted tumor-infiltrating T cells and peripheral T cells were plated fourfold (0.5 × 105 cells per well) and coincubated with autologous tumor-infiltrating Bc or peripheral Bc (0.15 × 105 cells per well) and peptides (final concentration 10 μg/ml) in 200 μl IMDM with 10 % human serum for 24 h. As a negative control, no peptide was added to the respective wells. Addition of a PMA/ionomycin mix (20 nmol/l phorbol-12-myristate-13-acetatein and 350 nmol/l ionomycin) served as a positive control. Antigen-specific T cell reactivity was defined as significant, if spot numbers exceeded background reactivity after adjustment. In detail, spot numbers counted for a specific T cell sample and antigen were adjusted by subtraction of background (no peptide control) and standard deviations of peptide-specific value and no peptide value as described previously [8].
Assessment of FSP mutational status
MSI analysis was performed as described previously [30]. PCR primer sequences for multiplex PCR of FSP-related coding microsatellite-bearing genes were obtained from a publicly accessible database containing information about microsatellites in the human genome (www.seltarbase.org). The following primers were used for amplification: TGFBR2 (sense 5′ GCTGCTTCTCCAAAGTGCAT 3′; antisense 5′ CAGATCTCAGGTCCCACACC 3′), TAF1B (sense 5′ ACCCAAATAAAAGCCCTCAAC 3′; antisense 5′ CTACTTAAAATTCCATTCCATGTCC 3′), AIM2 (sense 5′ TTCTCCATCCAGGTTATTAAGGC 3′; antisense 5′ TTAGACCAGTTGGCTTGAATTG 3′) and HT001 (ASTE1) (sense 5′ ATATGCCCCCGCTGAAATA 3′; antisense 5′ TTGGTGTGTGCAGTGGTTCT 3′). Sense (TGFBR2 and TAF1B) or antisense primers (AIM2 and HT001) were labeled with fluorescein isothiocyanate (FITC). Primers were used at a final concentration of 7.5 μM with 0.25 mM dNTPs (Invitrogen), 1× PCR buffer, 1.5 mM MgCl2 and 0.05 U/μl Hot FirePol DNA polymerase (all purchased from Solis Biodyne, Tartu, Estonia). Hi-Di/Rox buffer (12 μl, Applied Biosystems, Warrington, UK) was added to 2 μl PCR product. Analysis was performed in an ABI3100 (Applied Biosystems) and evaluated with the GeneScan™ software.
Immunohistochemistry
Sections from formalin-fixed, paraffin-embedded tissue were mounted on aminopropylsilane-coated slides (SuperFrost, Menzel, Braunschweig, Germany), and sections were deparaffinized, rehydrated and subsequently heated for 15 min in 10 mM citrate buffer (pH6) to retrieve antigen epitopes. Detection of the expression of CEA and the hypoglycosylated form of MUC1 was performed using the avidin/biotin complex (ABC) method following standard procedures as described previously [15]. Mouse monoclonal antibodies specific for hypoglycosylated human MUC1 (1:200 dilution, clone VU-4H5, Acris Antibodies, Herford, Germany) and human CEA (1:100 dilution, clone Col-1, Abcam, Cambridge, UK) were applied at 4 °C overnight.
Treg cell specificity assay
Treg cell specificity was feasible for 4 of the patients from whom sufficient numbers of Treg cells could be isolated. Treg cell specificity assay was performed as described previously [21] with the following modifications: Bc were incubated in IMDM medium containing 10 % human serum and loaded with the respective peptide (10 μg/ml) or no peptide as control. Treg cells (2.5 × 104) were added to the pulsed Bc. Treg-cell-depleted T cells were incubated with CD3/CD28-Dynabeads (Invitrogen) (ratio 1:1). After 14 h, Dynabeads were removed and T cells (2.5 × 104) were added to the Bc and Treg cells. 3H-thymidine incorporation, measurement and evaluation were performed as described previously [21]. Antigen-specific Treg cell reactivity was defined as significant, if DPM of an antigen was below background DPM after adjustment. DPM for a specific T cell sample and antigen was adjusted by the addition of background (no peptide control) and standard deviations of peptide-specific value and no peptide value. Percent of growth inhibition was evaluated for T cell/antigen samples with a significant reduction of T cell proliferation by calculating the percentage of the DPM difference between the respective sample and the no peptide control to the DPM of the no peptide control.
Results
Preexisting antigen-specific tumor-infiltrating T cell responses in MSI-H and MSS CRC patients
T cell responses directed against 4 FSP antigens and peptides derived from the classical CRC-associated antigens MUC1 and CEA were analyzed by IFN-gamma ELISpot assay. FSP-specific T cell responses were measured separately for peripheral and tumor-infiltrating CD3-positive T cells from MSI-H (peripheral n = 14, tumor-infiltrating n = 4) and MSS CRC patients (peripheral n = 17, tumor infiltrating n = 26) using autologous CD19-positive Bc as antigen-presenting cells. Among T cell samples categorized as positive, no significant differences in absolute spot numbers were observed between FSP antigens and MUC1- and CEA-derived peptides (not shown). Additionally, we did not observe differences in background spot numbers between responding and non-responding T cell samples.
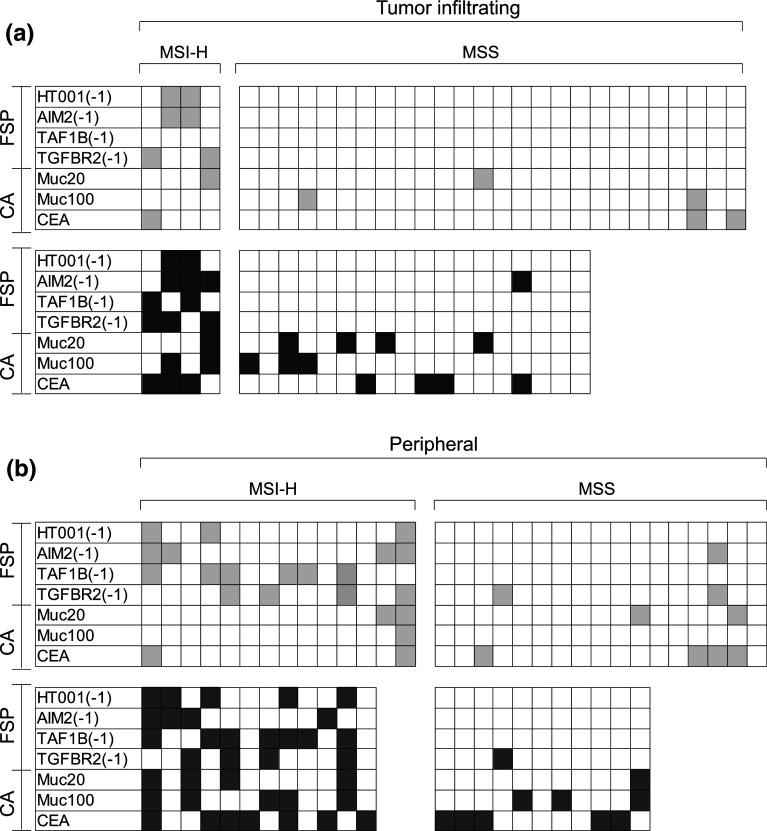
Representative primary data for tumor-infiltrating T cells are displayed in Fig. 1a. Tumor-infiltrating T cell samples from 4 MSI-H CRC patients predominantly showed reactivity against FSP antigens, with reactivity against at least one FSP detectable in all four tested specimens. No FSP-specific reactivity was detected in tumor-infiltrating T cell samples from 26 MSS CRC patients. Four out of 26 tumor-infiltrating T cell samples from MSS patients (15.4 %) were detected with responses against at least one classical peptide. Significant T cell responses are summarized in Fig. 2a. In tumor-infiltrating T cells from MSS CRC patients, peptide-specific response rates were lower than 10 % for each of the tested peptides derived from the classical antigens MUC1 and CEA.
Fig. 1.
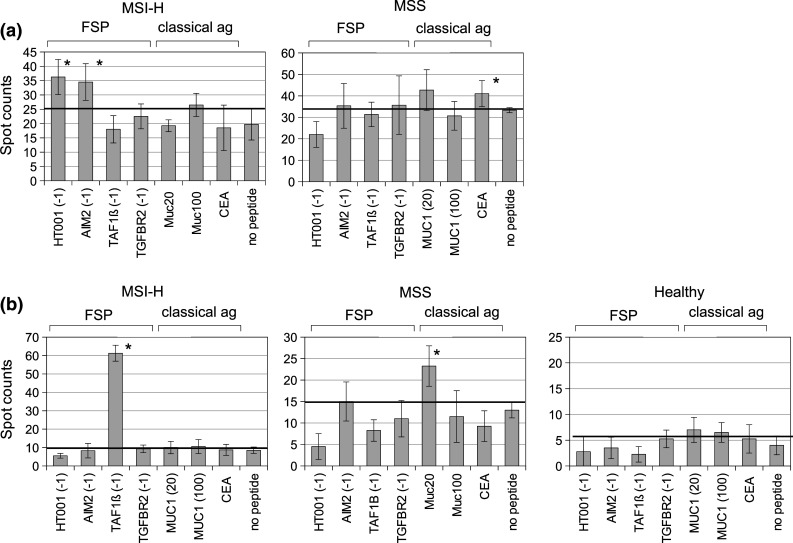
IFN-gamma ELISpot spot counts per 5 × 104 T cells for tumor-infiltrating (a) and peripheral (b) T cell samples from representative MSI-H and MSS CRC patients as well as one healthy donor. Bars represent mean spot numbers ± standard deviation. Significant elevated spot numbers compared to the negative control are marked with asterisk
Fig. 2.
Response pattern of all tested tumor-infiltrating (a) and peripheral (b) T cell samples from MSI-H and MSS CRC patients. Significant responses without Treg cell depletion are marked in light gray and responses in Treg-cell-depleted T cells are marked in dark gray. T cell samples for which Treg cell depletion was not feasible are not shown in the −Treg panel
Preexisting peripheral T cell responses against FSP and classical antigens
Fig. 1b shows representative primary data for peripheral T cell responses of one MSI-H and one MSS CRC patient as well as one healthy control. Ten out of 14 MSI-H CRC patients (71.4 %) were detected with a peripheral T cell response significantly above background against at least one FSP. Five of them (35.7 %) showed responses against 2 or more FSP antigens. The FSP antigen with the highest response rate was TAF1B(-1) with responses in 6 out of 14 T cell samples (42.9 %). Peripheral T cell responses against the FSP AIM2(-1) and TGFBR2(-1) were also observed in 1 (5.8 %) and 2 (11.7 %) out of 17 MSS CRC patients, respectively. Responses against the classical peptides derived from MUC1 to CEA were more frequent in MSS compared to MSI-H CRC patients (5 out of 17 patients, 29.4 % vs. 3 out of 14, 21.4 %). Highest response rate for MSS CRC patients’ peripheral T cell samples was observed against CEA (23.5 %) (Fig. 2).
Among MSI-H CRC patients, peripheral FSP-specific T cell responses were detectable in 10 out of 14 patients, whereas responses against MUC1 or CEA were detectable in 3 out of 14 patients (Fig. 3). Comparison of FSP antigens with classical antigens MUC1/CEA revealed a significantly higher prevalence of preexisting FSP-specific T cell responses compared to T cell responses specific for antigens derived from MUC1 or CEA (17 out of 56, 30.4 % vs. 5 out of 42, 11.9 %, p = 0.049, two-tailed Fisher’s exact test).
Fig. 3.
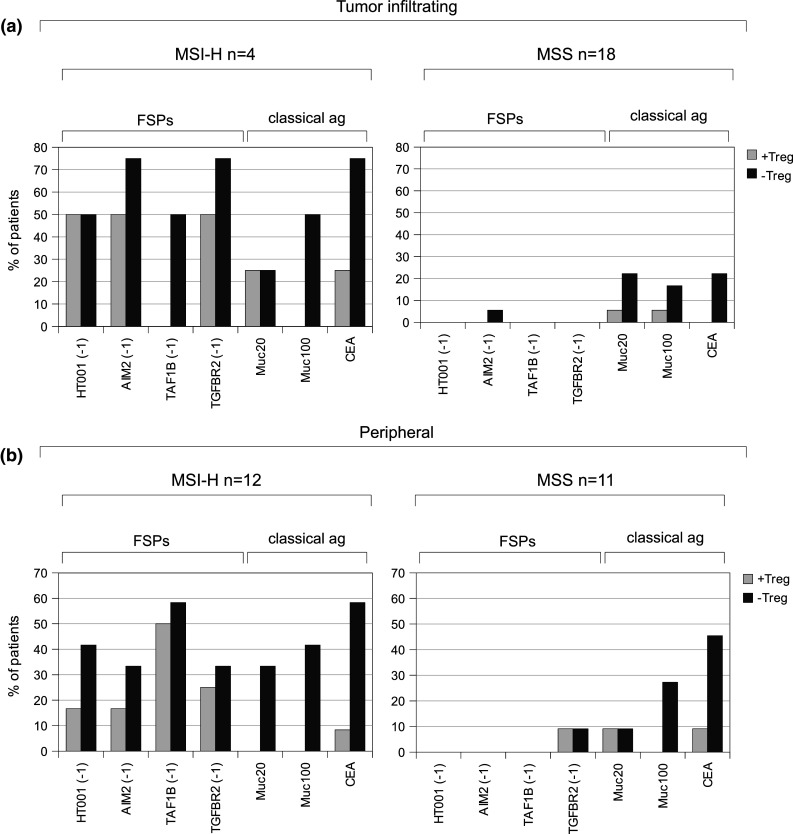
Response rates of tumor-infiltrating (a) and peripheral (b) T cell samples from MSI-H (tumor infiltrating n = 4, peripheral n = 12) and MSS CRC patients (tumor infiltrating n = 18, peripheral n = 11) without (light gray) and with (dark gray) Treg cell depletion against the analyzed antigens. T cell samples from healthy donors did not display T cell responses against the tested antigens with or without Treg cell depletion and are therefore not shown
Enhanced frequency of T cell responses upon Treg cell depletion
The presence of CD25-high CD4-positive Treg cells in tumor-infiltrating and peripheral T cell samples from CRC patients was assessed by flow cytometry. CD25-high CD4-positive cells accounted for 2–10 % of total CD3-positive T cell numbers. Depletion of cells with CD25-high CD4-positive phenotype from CD3-positive T cells by magnetic bead isolation resulted in a decrease in CD25-high CD4-positive T cells as determined by flow cytometry (Online Resource Figure 1).
To determine the effect of Treg cell depletion, we compared IFN-gamma ELISpot response rates for Treg-cell-undepleted and Treg-cell-depleted T cell samples for all antigens. Reactivity patterns for all analyzed samples from CRC patients with and without Treg cell depletion are summarized in Fig. 2. Figure 3 provides a graphical display of the response rates only for T cell samples from which Treg cell depletion was feasible, to compare antigen-specific T cell responses with and without Treg cell depletion. T cell samples from healthy donors did not display T cell reactivity in Treg-cell-undepleted or Treg-cell-depleted samples and are therefore not shown.
Depletion of Treg cells from tumor-infiltrating T cells of MSI-H CRC increased the number of recognized antigens for all 4 tested T cell samples. In tumor-infiltrating T cells from MSS CRC patients, response rates for the peptides derived from MUC1 and CEA increased after depletion of Treg cells. Reactivity against AIM2(-1) was observed in T cells from 1 out of 18 (5.6 %) patients when Treg cells were depleted. The number of T cell samples recognizing at least one antigen was increased from 2 out of 18 (11.1 %) samples without, to 10 out of 18 (55.6 %) samples with Treg cell depletion.
In peripheral T cell samples from MSI-H CRC patients, Treg cell depletion resulted in an increase of response rates for all of the tested antigens with a particularly pronounced increase for the MUC1-derived peptides Muc20 (from 0 to 33.3 %) and Muc100 (from 0 to 41.7 %) and for the CEA-derived peptide (from 8.3 to 58.3 %). All Treg-cell-depleted samples showed responses against at least one of the tested antigens. In MSS patients, Treg cell depletion also increased the number of peripheral T cell samples that showed reactivity against at least one of the tested antigens (3 out of 11, 27.3 % without Treg cell depletion, 9 out of 11, 81.8 % with Treg cell depletion).
In peripheral T cells from MSI-H CRC patients, Treg cell depletion led to a more pronounced increase of effector T cell responses directed against MUC1- or CEA-derived peptides (1 out of 36 responses without Treg cell depletion, 16 out of 36 responses with Treg cell depletion) compared to effector T cell responses directed against FSP antigens (13 out of 48 responses without Treg cell depletion, 20 out of 48 with Treg cell depletion, p = 0.070). A similar trend was observed for tumor-infiltrating T cells isolated from MSI-H CRC lesions (MUC1/CEA: 2 out of 12 possible responses without Treg cell depletion, 6 out of 12 responses with Treg cell depletion; FSP: 6 out of 16 possible responses without Treg cell depletion, 10 out of 16 responses with Treg cell depletion).
T cell reactivity, FSP mutational status and MUC1/CEA expression
Frameshift mutation analysis was performed to assess the FSP mutational status of MSI-H tumors, because the analyzed FSP are supposed to result from the presence of a mutation resulting in the deletion of one nucleotide at a coding microsatellite of the corresponding gene. Consequently, the relation between T cell responses in IFN-gamma ELISpot analysis and the coding microsatellite mutation status of the respective genes was examined. Mutations predicted to result in the generation of FSP antigens analyzed in the present study were not always associated with a T cell response directed against the respective FSP antigen, whereas a subset of patients presented with T cell responses directed against an FSP antigen without the respective mutation detectable in the tumor. Frequency of FSP-specific T cell responses was not statistically associated with mutation status, both in Treg-undepleted and in Treg-depleted samples (Online Resource Figure 2).
Additionally, the amount of tumor cells expressing CEA and the immunogenic hypoglycosylated MUC1 antigen was evaluated by immunohistochemical staining of paraffin-embedded tissue specimens. Tumor samples were scored from 1 to 4 according to the number of stained tumor cells (representative stainings in Online Resource Figure 3). Expression of the antigens was detected in all samples at varying abundance. Most of the tumors, irrespective of their microsatellite instability status, expressed the analyzed antigens in more than 50 % of the tumor cells (score 3 or 4). Comparison of T cell responses directed against the peptides derived from MUC1 to CEA and the tumor scoring according to the proportion of tumor cells expressing CEA and the hypoglycosylated form of MUC1 did not reveal a significant correlation (not shown, Online Resource Figures 2 and 4).
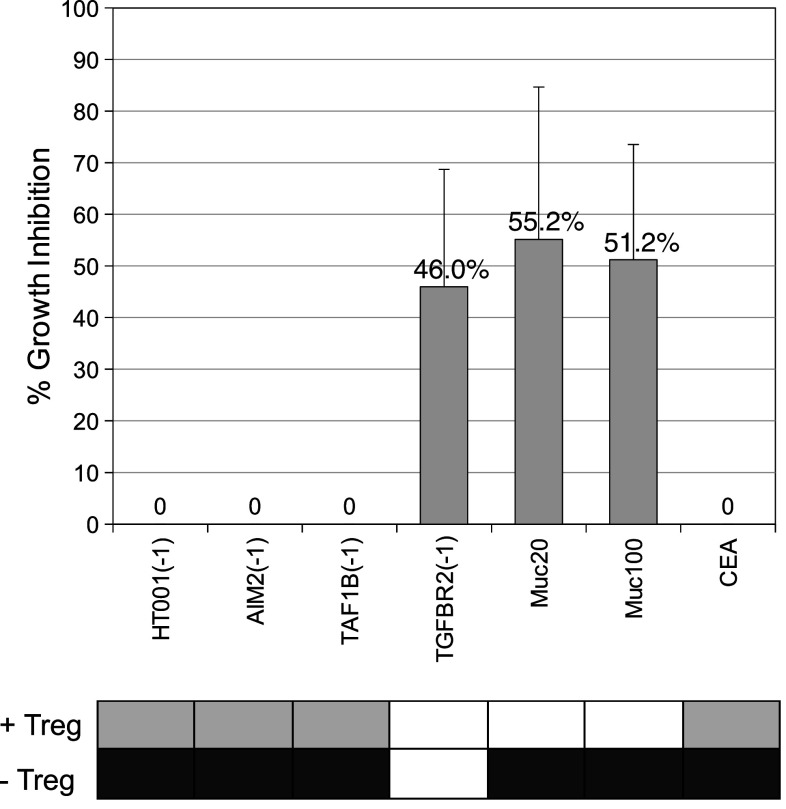
Treg cell specificity assay
Treg cell specificity assay could be performed for a subset of patients (n = 4). We compared individual patients for their Treg cell pattern obtained from Treg specificity assay and their antigen recognition pattern with and without Treg cell depletion from ELISpot analysis (Fig. 4). Treg cell influence in ELISpot assay was determined as antigen-specific T cell responses observed in Treg-cell-depleted samples, but not in samples without Treg cell depletion. T cell samples with an influence of Treg cells observed in ELISpot assay also displayed significantly decreased effector T cell proliferation in Treg specificity assay. Additionally, Treg cell specificities could be detected for antigens, for which no T cell responses either without or with Treg cell depletion were observed, suggesting the existence of antigen-specific Treg cells, but no preexisting antigen-specific IFN-gamma-secreting effector T cells in the respective patients.
Fig. 4.
Representative T cell sample for comparison of antigen-specific T cell responses in Treg-cell-undepleted (light gray) and Treg-cell-depleted T cells (dark gray) with percentage of growth inhibition in Treg specificity assay (calculated as the difference between mean DPM no peptide control and mean DPM antigen, divided by mean DPM of the no peptide control) including standard deviation
Discussion
MSI-H CRC are highly immunogenic and characterized by a dense infiltration with cytotoxic T cells [5, 6]. The immunogenicity of MSI-H cancers is commonly assumed to result from the abundance of MSI-induced FSP neo-antigens that can be recognized as foreign by the host’s immune system. The present study is the first to directly compare T cell responses against selected FSP antigens and the classical CRC-associated antigens MUC1 and CEA. We observed preexisting peripheral T cell reactivity against all of the tested antigens, FSP and classical, in peripheral T cell samples from MSI-H CRC patients, with a significantly higher prevalence of responses directed against the tested FSP compared to MUC1/CEA. These data and similar results obtained in tumor-infiltrating T cells isolated from MSI-H CRC support the hypothesis that FSP represent an immunologically relevant pool of antigens in MSI-H CRC. However, it has to be conceded that responses against non-FSP antigens other than the analyzed peptides derived from MUC1/CEA may behave differently; for example, recent studies indicated that modification of MUC1 peptide by partial glycosylation led to an increased frequency of immune responses [31].
In MSS CRC patients, preexisting FSP-specific T cell reactivity was rarely observed and restricted to the antigens AIM2(-1) and TGFBR2(-1). The presence of FSP-specific T cell responses in the peripheral blood of MSS CRC patients might result from cross-reactivity of lymphocytes specific for an epitope contained within a different immunogenic peptide, sharing sequence similarity with FSP, though peptide BLAST analysis did not reveal significant sequence identities (data not shown). Alternatively, coding microsatellite mutations may also occur at a low frequency in MMR-proficient cells, thus giving rise to the generation of an FSP antigen. However, none of the T cell samples from the healthy donors analyzed in this study showed FSP-specific responses significantly above background, suggesting that FSP-specific T cells were limited to CRC patients in the present experimental setting. Response rates of MSS CRC patients against the peptides derived from MUC1 to CEA ranged from 4 to 8 % in the tumor-infiltrating T cell samples and from 11 to 22 % in the T cell samples from peripheral blood of MSS patients. This is comparable with response rates reported in a previous study with 14 % for MUC1 and 20 % for CEA [21].
MSI-H CRC lesions commonly display dense infiltration with FOXP3-positive regulatory T cells. Therefore, we asked whether regulatory T cells had a functional impact on T cell responses against the selected FSP antigens and on responses against the classical antigens MUC1 and CEA. Depletion of Treg cells led to increased response rates for all analyzed antigens in from the peripheral blood of MSI-H CRC patients. Additionally, Treg cell depletion increased the average number of antigens, FSP as well as MUC1- and CEA-derived antigens, recognized by tumor-infiltrating and peripheral T cell samples from MSI-H CRC patients. For the group of MSS patients, the MUC1- and CEA-specific response rates were also elevated upon Treg cell depletion. This observation suggests that preexisting IFN-gamma-secreting antigen-specific T cells might be suppressed by Treg cells.
Interestingly, T cell responses against TAF1B(-1), which was the antigen most frequently recognized by peripheral T cells, were only observed in tumor-infiltrating samples upon Treg cell depletion. However, matched pairs of peripheral and tumor-infiltrating T cells could only be analyzed from two MSI-H CRC patients, so the difference of TAF1B(-1)-specific responses between the two compartments might result from small sample numbers.
Although the presence of FSP-specific Treg cells and Treg cell suppression of effector T cell responses directed against FSP was demonstrated in the present study, one might speculate that an enhanced frequency of Treg cells against the analyzed peptides from MUC1 to CEA might be responsible for the lower frequency of preexisting IFN-gamma-secreting MUC1- and CEA-specific effector T cells, potentially resulting from the fact that CEA and MUC1 are, in contrast to FSP antigens, not induced by mutation [31–33].
FSP antigens are neopeptides supposed to be specific for mismatch repair-deficient cells like MSI-H cancer lesions. FSP antigens might therefore rather elicit an effector T cell response in the absence of preexisting FSP-specific Treg-cell-mediated tolerance. However, Treg cells specific for other neopeptides including viral antigens derived from HPV have been reported in the literature [34]. These differences might be related to the different potential of distinct antigens to induce CD4-positive T cells. We assume that the FSP antigens used in our study can be recognized by CD4-positive T cells because they are predicted or have been shown to encompass HLA class II epitopes [10, 35, 36]. The low frequency of Treg-cell-mediated suppression of FSP-specific effector T cell responses is therefore most likely not the result of lacking CD4-positive T cell recognition. It will be an interesting task for future studies to elucidate the difference between antigens inducing or not inducing Treg cell suppression, and to examine whether this difference translates to differences in the clinical outcome of vaccinated patients.
The generation of FSP antigens in MMR-deficient cells results from insertion/deletion mutations affecting microsatellites located in the coding region of the respective genes. Therefore, we here analyzed whether FSP-specific T cell responses were related to the presence of at least one mutated allele of the respective gene. However, like in a previous study [8], no such correlation was observed. Besides from the possibility of false-positive results, lack of correlation might result from the fact that coding microsatellite instability is heterogeneously distributed in MSI-H CRC [37] and only selected tumor regions can be analyzed for coding microsatellite mutation status after routine pathology workup. Alternatively, one might speculate that cells carrying an FSP-inducing mutation and thus expressing an immunogenic peptide have been successfully attacked and eliminated from the pool of tumor cells by cytotoxic T cells according to the concept of immunosurveillance or immunoediting [38, 39].
Similarly, no correlation was observed between expression of CEA or hypoglycosylated MUC1 and antigen-specific T cell reactivity. The amount of tumor cells expressing CEA and hypoglycosylated MUC1 was highly variable in the different samples, but was not related to the presence of antigen-specific T cell reactivity, suggesting that the detection of T cells recognizing MUC1 and CEA is not directly correlated with overexpression of the antigens by cancer cells.
The observed trend toward a more pronounced increase of effector T cell responses directed against MUC1/CEA than against the tested FSP upon Treg cell depletion is in line with previous reports of a high variability of Treg cell suppression among distinct tumor antigens [21]. Consequently, antigen selection for immune therapy approaches is expected to have critical impact on their efficacy, potentially related to the antigens’ capability of inducing effective T cell cytotoxicity, but no immune regulatory response.
In summary, we here demonstrate in a comparative study that T cell responses in patients with MSI-H CRC are frequently directed against selected microsatellite instability-induced FSP. Moreover, our results suggest that the high frequency of preexisting FSP-specific effector T cell responses in MSI-H CRC patients may result from differences in Treg cell suppression between FSP and classical CRC antigens MUC1 and CEA. Preselected microsatellite instability-induced FSP therefore represent promising targets for immunotherapy approaches, and clinical trials will have to show whether the differences in the regulation of antigen-specific T cell responses will translate into different outcomes in patients vaccinated with CRC antigens.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The excellent technical assistance of Beate Kuchenbuch, Petra Höfler and Daniel Baumgärtner is gratefully acknowledged. The study was funded in part by the Deutsche Krebshilfe (German Cancer Aid) and the Deutsche Forschungsgemeinschaft (KFO 227).
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- Bc
B lymphocytes
- CRC
Colorectal carcinoma
- FSP
Frameshift-derived peptides
- IFN
Interferon
- MSI-H
High-level microsatellite instability
- MSS
Microsatellite stable
- PBMC
Peripheral blood mononuclear cells
- TIL
Tumor-infiltrating lymphocytes
- Treg cells
Regulatory T cells
References
- 1.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 2.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348(10):919–932. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 3.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87(2):159–170. doi: 10.1016/S0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 4.Saeterdal I, Bjorheim J, Lislerud K, Gjertsen MK, Bukholm IK, Olsen OC, Nesland JM, Eriksen JA, Moller M, Lindblom A, Gaudernack G. Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci USA. 2001;98(23):13255–13260. doi: 10.1073/pnas.231326898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001;91(12):2417–2422. doi: 10.1002/1097-0142(20010615)91:12<2417::AID-CNCR1276>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 6.Dolcetti R, Viel A, Doglioni C, Russo A, Guidoboni M, Capozzi E, Vecchiato N, Macri E, Fornasarig M, Boiocchi M. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999;154(6):1805–1813. doi: 10.1016/S0002-9440(10)65436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips SM, Banerjea A, Feakins R, Li SR, Bustin SA, Dorudi S. Tumour-infiltrating lymphocytes in colorectal cancer with microsatellite instability are activated and cytotoxic. Br J Surg. 2004;91(4):469–475. doi: 10.1002/bjs.4472. [DOI] [PubMed] [Google Scholar]
- 8.Schwitalle Y, Kloor M, Eiermann S, Linnebacher M, Kienle P, Knaebel HP, Tariverdian M, Benner A, von Knebel Doeberitz M. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology. 2008;134(4):988–997. doi: 10.1053/j.gastro.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Reuschenbach M, Kloor M, Morak M, Wentzensen N, Germann A, Garbe Y, Tariverdian M, Findeisen P, Neumaier M, Holinski-Feder E, von Knebel Doeberitz M. Serum antibodies against frameshift peptides in microsatellite unstable colorectal cancer patients with Lynch syndrome. Fam Cancer. 2010;9(2):173–179. doi: 10.1007/s10689-009-9307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saeterdal I, Gjertsen MK, Straten P, Eriksen JA, Gaudernack G. A TGF betaRII frameshift-mutation-derived CTL epitope recognised by HLA-A2-restricted CD8+ T cells. Cancer Immunol Immunother. 2001;50(9):469–476. doi: 10.1007/s002620100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikawa T, Fujita T, Suzuki Y, Okabe S, Yuasa Y, Iwai T, Kawakami Y. Tumor-specific immunological recognition of frameshift-mutated peptides in colon cancer with microsatellite instability. Cancer Res. 2003;63(17):5564–5572. [PubMed] [Google Scholar]
- 12.Kloor M, Michel S, Buckowitz B, Ruschoff J, Buttner R, Holinski-Feder E, Dippold W, Wagner R, Tariverdian M, Benner A, Schwitalle Y, Kuchenbuch B, von Knebel Doeberitz M. Beta2-microglobulin mutations in microsatellite unstable colorectal tumors. Int J Cancer. 2007;121(2):454–458. doi: 10.1002/ijc.22691. [DOI] [PubMed] [Google Scholar]
- 13.Michel S, Linnebacher M, Alcaniz J, Voss M, Wagner R, Dippold W, Becker C, von Knebel Doeberitz M, Ferrone S, Kloor M. Lack of HLA class II antigen expression in microsatellite unstable colorectal carcinomas is caused by mutations in HLA class II regulatory genes. Int J Cancer. 2010;127(4):889–898. doi: 10.1002/ijc.25106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kloor M, Becker C, Benner A, Woerner SM, Gebert J, Ferrone S, von Knebel Doeberitz M. Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res. 2005;65(14):6418–6424. doi: 10.1158/0008-5472.CAN-05-0044. [DOI] [PubMed] [Google Scholar]
- 15.Michel S, Benner A, Tariverdian M, Wentzensen N, Hoefler P, Pommerencke T, Grabe N, von Knebel Doeberitz M, Kloor M. High density of FOXP3-positive T cells infiltrating colorectal cancers with microsatellite instability. Br J Cancer. 2008;99(11):1867–1873. doi: 10.1038/sj.bjc.6604756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke SL, Betts GJ, Plant A, Wright KL, El-Shanawany TM, Harrop R, Torkington J, Rees BI, Williams GT, Gallimore AM, Godkin AJ. CD4+ CD25+ FOXP3+ regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS One. 2006;1:e129. doi: 10.1371/journal.pone.0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 18.Knutson KL, Disis ML, Salazar LG. CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunol Immunother. 2007;56(3):271–285. doi: 10.1007/s00262-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 20.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 21.Bonertz A, Weitz J, Pietsch DH, Rahbari NN, Schlude C, Ge Y, Juenger S, Vlodavsky I, Khazaie K, Jaeger D, Reissfelder C, Antolovic D, Aigner M, Koch M, Beckhove P. Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J Clin Invest. 2009;119(11):3311–3321. doi: 10.1172/JCI39608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lepisto AJ, Moser AJ, Zeh H, Lee K, Bartlett D, McKolanis JR, Geller BA, Schmotzer A, Potter DP, Whiteside T, Finn OJ, Ramanathan RK. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6(B):955–964. [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng C, Feng J, Lu D, Wang P, Xing S, Coll JL, Yang D, Yan X. A novel anti-CEACAM5 monoclonal antibody, CC4, suppresses colorectal tumor growth and enhances NK cells-mediated tumor immunity. PLoS One. 2011;6(6):e21146. doi: 10.1371/journal.pone.0021146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woerner SM, Benner A, Sutter C, Schiller M, Yuan YP, Keller G, Bork P, Doeberitz MK, Gebert JF. Pathogenesis of DNA repair-deficient cancers: a statistical meta-analysis of putative real common target genes. Oncogene. 2003;22(15):2226–2235. doi: 10.1038/sj.onc.1206421. [DOI] [PubMed] [Google Scholar]
- 25.Woerner SM, Kloor M, von Knebel Doeberitz M, Gebert JF. Microsatellite instability in the development of DNA mismatch repair deficient tumors. Cancer Biomark. 2006;2(1–2):69–86. doi: 10.3233/cbm-2006-21-208. [DOI] [PubMed] [Google Scholar]
- 26.Linnebacher M, Gebert J, Rudy W, Woerner S, Yuan YP, Bork P, von Knebel Doeberitz M. Frameshift peptide-derived T-cell epitopes: a source of novel tumor-specific antigens. Int J Cancer. 2001;93(1):6–11. doi: 10.1002/ijc.1298. [DOI] [PubMed] [Google Scholar]
- 27.Schwitalle Y, Linnebacher M, Ripberger E, Gebert J, von Knebel Doeberitz M. Immunogenic peptides generated by frameshift mutations in DNA mismatch repair-deficient cancer cells. Cancer Immun. 2004;4:14. [PubMed] [Google Scholar]
- 28.Ullenhag GJ, Frodin JE, Jeddi-Tehrani M, Strigard K, Eriksson E, Samanci A, Choudhury A, Nilsson B, Rossmann ED, Mosolits S, Mellstedt H. Durable carcinoembryonic antigen (CEA)-specific humoral and cellular immune responses in colorectal carcinoma patients vaccinated with recombinant CEA and granulocyte/macrophage colony-stimulating factor. Clin Cancer Res. 2004;10(10):3273–3281. doi: 10.1158/1078-0432.CCR-03-0706. [DOI] [PubMed] [Google Scholar]
- 29.Lesterhuis WJ, De Vries IJ, Schreibelt G, Schuurhuis DH, Aarntzen EH, De Boer A, Scharenborg NM, Van de Rakt M, Hesselink EJ, Figdor CG, Adema GJ, Punt CJ. Immunogenicity of dendritic cells pulsed with CEA peptide or transfected with CEA mRNA for vaccination of colorectal cancer patients. Anticancer Res. 2010;30(12):5091–5097. [PubMed] [Google Scholar]
- 30.Findeisen P, Kloor M, Merx S, Sutter C, Woerner SM, Dostmann N, Benner A, Dondog B, Pawlita M, Dippold W, Wagner R, Gebert J, von Knebel Doeberitz M. T25 repeat in the 3′ untranslated region of the CASP2 gene: a sensitive and specific marker for microsatellite instability in colorectal cancer. Cancer Res. 2005;65(18):8072–8078. doi: 10.1158/0008-5472.CAN-04-4146. [DOI] [PubMed] [Google Scholar]
- 31.Ryan SO, Vlad AM, Islam K, Gariepy J, Finn OL. Tumor-associated MUC1 glycopeptide epitopes are not subject to self-tolerance and improve responses to MUC1 peptide epitopes in MUC1 transgenic mice. Biol Chem. 2009;390(7):611–618. doi: 10.1515/BC.2009.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall J. Carcinoembryonic antigen-based vaccines. Semin Oncol. 2003;30(3 Suppl 8):30–36. doi: 10.1016/S0093-7754(03)00233-1. [DOI] [PubMed] [Google Scholar]
- 33.Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9(2):67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 34.Molling JW, de Gruijl TD, Glim J, Moreno M, Rozendaal L, Meijer CJ, van den Eertwegh AJ, Scheper RJ, von Blomberg ME, Bontkes HJ. CD4 (+) CD25hi regulatory T-cell frequency correlates with persistence of human papillomavirus type 16 and T helper cell responses in patients with cervical intraepithelial neoplasia. Int J Cancer. 2007;121(8):1749–1755. doi: 10.1002/ijc.22894. [DOI] [PubMed] [Google Scholar]
- 35.Speetjens FM, Lauwen MM, Franken KL, van Janssen-Rhijn CM, van Duikeren S, Bres SA, van de Velde CJ, Melief CJ, Kuppen PJ, van der Burg SH, Morreau H, Offringa R. Prediction of the immunogenic potential of frameshift-mutated antigens in microsatellite instable cancer. Int J Cancer. 2008;123(4):838–845. doi: 10.1002/ijc.23570. [DOI] [PubMed] [Google Scholar]
- 36.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50(3–4):213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 37.Barnetson R, Jass J, Tse R, Eckstein R, Robinson B, Schnitzler M. Mutations associated with microsatellite unstable colorectal carcinomas exhibit widespread intratumoral heterogeneity. Genes Chromosomes Cancer. 2000;29(2):130–136. doi: 10.1002/1098-2264(200010)29:2<130::AID-GCC1023>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 38.Urban JL, Kripke ML, Schreiber H. Stepwise immunologic selection of antigenic variants during tumor growth. J Immunol. 1986;137(9):3036–3041. [PubMed] [Google Scholar]
- 39.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.