Abstract
Natural killer (NK) cells hold promise for adoptive cancer immunotherapy but are dependent on cytokines such as interleukin (IL)-2 for growth and cytotoxicity. Here, we investigated the consequences of ectopic expression of IL-15 in human NK cells. IL-2 and IL-15 belong to the common γ chain family of cytokines and have overlapping activities. Transduction of clinically applicable NK-92 cells with lentiviral vectors encoding human IL-15 resulted in predominantly intracellular expression of the cytokine, and STAT5 activation, proliferation and cytotoxicity of the producer cells in the absence of IL-2. Growth of non-transduced bystander cells was not supported, allowing rapid enrichment of gene-modified cells solely by IL-2 withdrawal. This was also the case upon transduction of NK-92 and NKL cells with a bicistronic lentiviral vector encoding IL-15 and a chimeric antigen receptor (CAR) targeting the pancarcinoma antigen EpCAM. Effector cells co-expressing CAR and IL-15 continued to proliferate in the absence of exogenous cytokines and displayed high and selective cell-killing activity against EpCAM-expressing breast carcinoma cells that were resistant to the natural cytotoxicity of unmodified NK cells. This strategy facilitates rapid isolation and continuous expansion of retargeted NK cells and may extend their potential clinical utility.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-012-1212-x) contains supplementary material, which is available to authorized users.
Keywords: Interleukin-15, Natural killer cells, Lentiviral vector, scFv antibody, Chimeric antigen receptor, EpCAM
Introduction
Expression of chimeric antigen receptors (CAR) in cytotoxic lymphocytes constitutes a promising strategy for adoptive cancer immunotherapy with effector cells of defined specificity. Since the first description of chimeric receptors with antibody-based target-cell recognition and T-cell-receptor-like signaling capabilities more than 20 years ago [1], this approach has continuously been refined. Basic CARs usually consist of a tumor-specific scFv antibody fragment connected via a flexible spacer to the CD3 ζ chain for signaling [2]. While clinical application of T cells expressing such first-generation CARs has met limited success, current second-generation CARs that include CD3 ζ together with co-stimulatory signaling domains combined with improved retro- and lentiviral vectors for gene transfer are expected to yield genetically modified effector cells with more potent antitumoral activity and the ability to persist in vivo [3, 4]. Indeed, clinical activity and durable remissions have now been observed in lymphoma and leukemia patients treated with autologous T cells modified to express CD19-specific second-generation CARs [5].
Natural killer (NK) cells represent another valuable effector cell population suitable for the generation of tumor-specific variants by means of genetic modification [6–9]. NK cells are lymphocytes of the innate immune system. In contrast to naïve T cells, their natural cytotoxicity can be activated rapidly and is regulated by a complex balance of signals from germline-encoded activating and inhibitory cell surface receptors [10]. Donor-derived primary NK cells as well as continuously growing NK cell lines such as NK-92 are being developed for adoptive cancer immunotherapy [11]. General safety and tolerability of infusions of unmodified NK-92 cells has been established in initial phase I clinical studies, with some patients experiencing clinical responses [12, 13]. Utilizing retroviral transfer of CAR sequences, we previously generated NK-92 variants with specificity for different tumor-associated surface antigens. We demonstrated potent and highly selective antitumoral activity of such cells against otherwise NK-resistant targets derived from solid tumors [6, 14], as well as enhanced cytotoxicity against moderately NK-sensitive malignant cells of hematologic or neuroectodermal origins [15, 16].
For growth and cytotoxic activity, NK-92 like primary NK cells are dependent on interleukin (IL)-2, which cannot be produced endogenously, but must be provided exogenously during in vitro culture in recombinant form, or in vivo by accessory immune cells [17]. IL-2 and IL-15 belong to the common γ (γc) chain family of cytokines and share the IL-2Rβ and IL-2Rγ signaling subunits [18]. Both cytokines have overlapping activities and support growth, survival, and effector functions of T and NK cells [19–21]. Here, we investigated the consequences of ectopic expression of IL-15 on growth and activity of established NK cells, and NK cells co-expressing a tumor-specific chimeric antigen receptor. Transduction of established human NK cells with a lentiviral vector encoding human IL-15 resulted in predominantly intracellular expression of IL-15 and maintained proliferation and activity of the producer cells in the absence of IL-2. The growth of non-transduced bystander cells was not supported, allowing rapid enrichment of gene-modified cells solely by IL-2 withdrawal. This was also the case upon transduction of NK cells with a bicistronic lentiviral vector encoding IL-15 and a second-generation CAR targeting the pancarcinoma antigen epithelial cell adhesion molecule (EpCAM). CAR-expressing effector cells isolated from the resulting IL-2-independent cell pool continued to proliferate in the absence of exogenous cytokines and displayed high and selective cell-killing activity against EpCAM-expressing breast carcinoma cells that were resistant to the natural cytotoxicity of unmodified NK cells.
Materials and methods
Cells and culture conditions
Human K562 erythroleukemia and Raji Burkitt’s lymphoma cells, murine CTLL-2 cytotoxic T cells (all ATCC, Manassas, VA), human NKL natural killer cells [22], and murine HC11 mammary epithelial cells [23] were maintained in RPMI 1640 medium (Lonza, Köln, Germany). Human MDA-MB453 and MDA-MB468 mammary carcinoma, MDA-MB435 melanoma cells, and 293T cells (all ATCC) were cultured in DMEM (Lonza). All media were supplemented with 10% heat-inactivated FBS, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, in addition containing 50 μM β-mercaptoethanol and 50 IU/ml IL-2 (Proleukin; Novartis Pharma, Nürnberg, Germany) (CTLL-2), or 10% horse serum and 200 IU/ml IL-2 (NKL). Human NK-92 cells (ATCC) were propagated in X-VIVO 10 medium (Lonza) supplemented with 5% heat-inactivated human serum (Red Cross Blood Donor Service Baden-Württemberg—Hessen, Frankfurt, Germany) and 100 IU/ml IL-2.
IL-15 and CAR expression vectors
An IL-15 cDNA fragment carrying SacII restriction sites at its 5′ and 3′ ends was generated by PCR with oligonucleotide primers 5′-AAAACCGCGGATGAGAATTTCGAAACCACATTTGAGAAG-3′ and 5′-AAAACCGCGGTCAAGAAGTGTTGATGAACATTTGGAC-3′, using human full-length IL-15 cDNA (imaGenes, Berlin, Germany) as a template. The amplified DNA fragment was inserted into the SacII site of plasmid pHR’SIN-cPPT-SIEW (pSIEW) [24] upstream of IRES and EGFP sequences of the vector. The resulting construct pS-IL15-IEW allows co-expression of IL-15 and EGFP. To co-express IL-15 together with another gene of interest, IRES and IL-15 fragments were separately amplified by PCR, assembled stepwise in pBluescript SK (+), and the resulting IRES-IL-15 sequence was used to replace IRES-EGFP of pSIEW generating the vector pSI-IL15-W.
The chimeric antigen receptor sequence CAR 31.28.z was designed by in silico assembly of fragments encoding an immunoglobulin heavy chain signal peptide, the EpCAM-specific scFv(MOC31) antibody fragment [14, 25], a Myc-tag, the CD8 α hinge region (amino acid residues 105–165), transmembrane and intracellular domains of CD28 (residues 151–220), and the intracellular domain of CD3 ζ chain (residues 52–164), followed by synthesis of the codon-optimized fusion gene (GeneArt, Regensburg, Germany). Then, the complete CAR 31.28.z sequence was inserted as a SacII fragment 5′ of the IRES sequence into pSI-IL15-W, resulting in the vector pS-31.28.z-I-IL15-W. For comparison, a similar vector pS-31.TM-I-IL15-W was generated that encodes a truncated 31.TM CAR containing the transmembrane domain of CD28 with a short cytoplasmic tail (residues 151–185), but lacking intracellular CD28 and CD3 ζ chain signaling sequences.
Production of lentiviral vectors and transduction of NK and T cells
VSV-G pseudotyped lentiviral vector particles were produced by co-transfecting 293T cells with the respective lentiviral transfer plasmid together with packaging and envelope plasmids pCMVΔR8.91 and pMD2.G [26] by standard calcium phosphate transfection. For transduction, vector-containing supernatants were added to NK-92, NKL, or CTLL-2 cells in the presence of 8 μg/ml polybrene. Then, the samples were centrifuged for 60 min at 32°C and 1,800×g and incubated overnight at 37°C before replacing the medium with regular growth medium. CAR-expressing cells were identified by flow cytometry with Myc-tag-specific monoclonal antibody (mAb) 9E10 (1.5 μg/5 × 105 cells) (Sigma-Aldrich, Taufkirchen, Germany) followed by APC-coupled goat anti-mouse secondary antibody (Dianova, Hamburg, Germany), and EGFP-expressing cells by direct flow cytometry using FACScan, FACSCalibur, and FACSCanto II flow cytometers (BD Biosciences, Heidelberg, Germany). Data were analyzed with CELLQuest Pro and FACSDiva software (BD Biosciences). NK-92 single-cell clones expressing CAR 31.28.z and IL-15 were derived from pools of transduced cells enriched by IL-2 withdrawal, followed by single cell sorting after incubation with mAb 9E10 and APC-coupled secondary antibody using a FACSAria fluorescence-activated cell sorter (BD Biosciences).
Analysis of IL-15 expression and activity
Expression of IL-15 mRNA was analyzed by semi-quantitative RT-PCR using total RNA from NK-92/IL-15-EGFP or parental NK-92 cells as templates, and the IL-15 oligonucleotides indicated above as primers. The amount of IL-15 protein in culture supernatants collected after 3 days of culture of 2.5 × 105 IL-15-expressing HC11 cells in 2 ml of regular growth medium or 2 × 106 IL-15-expressing NK-92 cells in 10 ml of IL-2-free growth medium was quantified in triplicate samples using a human IL-15 ELISA kit (eBioscience, Frankfurt, Germany) following the manufacturer’s recommendations. Supernatants of non-transduced parental cells served as controls. For intracellular cytokine staining, 5 × 105 IL-15-producing NK-92 cells were harvested, washed once in DPBS containing 3% FBS, and fixed and permeabilized by adding 100 μl of fixation and permeabilization solution (BD Cytofix/Cytoperm™; BD Biosciences) for 20 min at 4°C. Then, cells were washed twice in washing buffer (BD Perm/Wash™; BD Biosciences) and incubated with anti-hIL-15 mAb 34559 (R&D Systems, Wiesbaden, Germany) for 1 h at 4°C, followed by APC-coupled goat anti-mouse secondary antibody (Dianova) for 30 min at 4°C and flow cytometric analysis with a FACSCalibur flow cytometer.
For detection of trans-presented IL-15 on the cell surface, IL-15-expressing NK-92 or parental NK-92 control cells were incubated with anti-hIL-15 mAb 34559 followed by APC-coupled goat anti-mouse secondary antibody and flow cytometric analysis with a FACSCalibur flow cytometer. For comparison, IL-15Rα-positive Raji cells were incubated with recombinant hIL-15 (PeproTech, Hamburg, Germany) for 1 h at 4°C. Then, the cells were washed, and surface-bound IL-15 was detected by flow cytometry as described above. To confirm bioactivity of secreted IL-15, CTLL-2 cells (1 × 104/well) were seeded in 96-well plates and tested in triplicates for their proliferative response to culture supernatants collected from IL-15-expressing NK-92 cells. Viability and proliferation of cells was analyzed in MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma-Aldrich) metabolization assays as described [15]. Likewise, proliferation of IL-15-expressing NK-92, NKL, and CTLL-2 cells in the presence or absence of exogenous IL-2 was tested in MTT metabolization assays with triplicate samples of 1 × 104 cells/well in 96-well plates.
Immunoblot analysis
For analysis of STAT5 activation, IL-15-expressing or unmodified NK-92 cells were starved overnight in medium without serum and cytokines. Then, the cells were treated either with 500 IU/ml IL-2 or 5 ng/ml IL-15 for 15 min. In addition, parental NK-92 from continuous culture in regular growth medium containing 100 IU/ml IL-2 and IL-15-expressing NK-92 cells from continuous culture in regular growth medium lacking IL-2 were included. Cells were collected by centrifugation and lysed for 20 min at 4°C in 100 μl of buffer containing 20 mM Tris–HCl, pH 7.3, 137 mM NaCl, 10% glycerol, 1% Triton-X-100, 2 mM EDTA, Protease Inhibitor Cocktail (Roche Diagnostics, Mannheim, Germany), and 1 mM sodium orthovanadate (Sigma-Aldrich). Cleared lysates were separated by SDS-PAGE and immunoblotted with anti-STAT5 antibody 9363 or anti-phospho-STAT5 antibody 9359 (Cell Signaling Technology, New England Biolabs, Frankfurt, Germany) followed by HRP-conjugated secondary antibody and chemiluminescent detection. As a loading control, blots were stripped and reprobed with γ-Tubulin-specific antibody (Sigma-Aldrich).
EpCAM expression and cytotoxicity assays
Expression of EpCAM on the surface of target cells was determined using mAb MOC31 [27] (kindly provided by U. Zangemeister-Wittke, University of Bern) followed by APC-coupled goat anti-mouse secondary antibody and flow cytometric analysis with a FACSCalibur flow cytometer.
Cytotoxic activity of NK cells toward target cells was analyzed in FACS-based assays as described [14]. Briefly, target cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) or calcein violet AM (both Molecular Probes, Invitrogen, Karlsruhe, Germany), washed, and co-cultured with effector cells at various effector to target (E/T) ratios for 2 h at 37°C. After co-culture, cells were washed, and 250 μl of a 1 μg/ml propidium iodide (PI) solution was added to each sample before flow cytometric analysis with FACScan, FACSCalibur, or FACSCanto II flow cytometers. Specific cytotoxicity was calculated using CellQuest Pro or FACSDiva software. Dead target cells were determined as CFSE or calcein violet AM, and PI double positive. Spontaneous target cell lysis was determined in samples only containing labeled target cells, and the number of spontaneously lysed target cells was subtracted.
Statistical analysis
Differences between values were evaluated using the two-tailed unpaired Student’s t test. Differences between groups were evaluated by two-way ANOVA followed by Bonferroni tests. p values < 0.05 were considered significant. Statistical calculations were done using Prism 5 software (GraphPad Software, La Jolla, CA).
Results
Generation of IL-15-expressing NK cells
Human IL-15 cDNA was inserted into the lentiviral transfer vector pSIEW that also encodes EGFP as a marker (Fig. 1a), and lentiviral particles were generated. After transduction of IL-2-dependent human NK-92 cells, up to 4% of EGFP-positive cells were obtained. The EGFP-expressing NK-92 cells were enriched by two rounds of flow cytometric cell sorting (data not shown). In this enriched NK-92/IL-15-EGFP cell population, IL-15 mRNA expression was verified by semi-quantitative RT-PCR analysis (Fig. 1b), and IL-15 protein expression was detected by intracellular cytokine staining (Fig. 1c). Nevertheless, NK-92/IL-15-EGFP cells secreted only low levels of IL-15 into the culture supernatant (<70 pg/ml) (Fig. 1d), which was insufficient to support the growth of IL-2/IL-15-dependent CTLL-2 cells (data not shown). In contrast, after transduction with the same lentiviral vector, established mammary epithelial cells used as a model for non-immune cells readily expressed and secreted relatively large amounts of biologically active IL-15 (>1.1 ng/ml) (see Supplementary Fig. 1 available on-line).
Fig. 1.
Expression of IL-15 in NK-92 cells. a Schematic representation of the lentiviral transfer vector pS-IL15-IEW that encodes under the control of the Spleen Focus Forming Virus promoter (SFFV) human IL-15, followed by an internal ribosome entry site (IRES) and cDNA encoding enhanced green fluorescent protein (EGFP) as a marker. After transduction with S-IL15-IEW vector particles, EGFP-expressing NK-92/IL-15-EGFP cells were enriched by flow cytometric cell sorting and tested for IL-15 expression. b IL-15 mRNA expression in NK-92/IL-15-EGFP cells grown in the presence and absence of exogenous IL-2 was verified by semi-quantitative RT-PCR. c The presence of IL-15 protein in NK-92/IL-15-EGFP cells was investigated by intracellular cytokine staining with anti-IL-15 antibody and flow cytometry (open area). Parental NK-92 cells served as a control (filled area). d The amount of IL-15 in cell lysate and conditioned culture supernatant was quantified by IL-15-specific ELISA. Parental NK-92 cells served as a control. Mean values ± SEM are shown (n = 3)
To investigate whether the IL-15 produced by NK-92/IL-15-EGFP was sufficient to activate IL-15 receptors in these cells, the cells were starved overnight in medium lacking serum and exogenous cytokines and then analyzed for activation of STAT5 as a signal transducer downstream of IL-2/IL-15 receptors. As expected, continuous culture of NK-92 cells in IL-2-containing medium and short-term activation of starved NK-92 or NK-92/IL-15-EGFP cells with recombinant IL-2 or IL-15 resulted in high and consistent levels of phospho-STAT5 (Fig. 2a, left panel, lanes 1, 3, 4; right panel, lanes 3 and 4). In the absence of cytokines, parental NK-92 cells displayed only marginal tyrosine phosphorylation of STAT5 (Fig. 2a, left panel, lane 2). In contrast, phospho-STAT5 was readily detectable in NK-92/IL-15-EGFP cells upon long-term culture in IL-2-free medium (Fig. 2a, right panel, lane 1), indicating activation of IL-15 receptor complexes by the ectopically expressed IL-15. To a lower extent, STAT5 activation was also detected in NK-92/IL-15-EGFP cells starved overnight in cytokine-free medium after continuous culture in IL-2-containing medium (Fig. 2a, right panel, lane 2). This suggests that the cells adapt during prolonged absence of IL-2 and utilize ectopically produced IL-15 more efficiently for activation of downstream signaling. To test whether the IL-15 levels produced can fully replace exogenous IL-2 and support normal growth of NK-92/IL-15-EGFP cells, NK-92/IL-15-EGFP and parental NK-92 were placed in growth medium containing or lacking IL-2, and proliferation was followed by MTT metabolization assays (Fig. 2b). Thereby, irrespective of the presence of exogenous IL-2, NK-92/IL-15-EGFP cells continued to proliferate with growth characteristics indistinguishable from parental NK-92 cells in IL-2-containing medium, while proliferation of unmodified NK-92 cells ceased without IL-2. Similar results were obtained for IL-2-dependent human NKL NK cells and murine CTLL-2 cytotoxic T lymphocytes upon transduction with IL-15 encoding lentiviral vectors (see Supplementary Fig. 2 available on-line).
Fig. 2.
IL-15-expressing NK-92 cells proliferate in the absence of exogenous IL-2 and retain their cytotoxicity. a Cytokine-induced activation of STAT5. Cell lysates of parental NK-92 cells from continuous culture in IL-2-containing medium and of NK-92/IL-15-EGFP cells from continuous culture in medium lacking IL-2 (lanes 1) after starvation overnight in medium without serum or cytokines (lanes 2) or after activation of starved cells for 15 min with either 500 IU/ml IL-2 (lanes 3) or 5 ng/ml IL-15 (lanes 4) were analyzed by immunoblotting with phospho-STAT5-specific antibody as indicated. For comparison, also the levels of total STAT5 were determined. γ-Tubulin was analyzed as a loading control. b Growth of NK cells in the absence of exogenous cytokines. NK-92/IL-15-EGFP and parental NK-92 were placed in growth medium containing or lacking IL-2, and proliferation was followed for six consecutive days by MTT metabolization assays and determination of the absorbance at 595 nm as a measure for the relative number of viable cells. Mean values ± SEM are shown (n = 3); ***p < 0.0001. c Natural cytotoxicity of NK-92/IL-15-EGFP cells after culture in the presence or absence of IL-2 toward NK-sensitive K562 cells was determined in FACS-based cytotoxicity assays at different effector to target ratios (E/T). Parental NK-92 cells grown in the presence of IL-2 were included for comparison. Mean values ± SD are shown (n = 2); *p < 0.05; ns, p > 0.05
Next, we investigated whether ectopic IL-15 expression affects natural cytotoxicity of NK-92 cells. Cell-killing activity of NK-92/IL-15-EGFP cells against NK-sensitive K562 cells was assessed in FACS-based assays upon co-incubation of effector and target cells for 2 h at different ratios. While cytotoxicity of NK-92/IL-15-EGFP cells was somewhat less pronounced than that of parental NK-92, the IL-15 producing cells retained marked cell-killing activity irrespective of the presence or absence of exogenous IL-2 (Fig. 2c). Taken together these data demonstrate that expression of low levels of IL-15 in NK-92 cells is sufficient to activate IL-15 receptor signaling in the producer cells and render them independent from exogenous IL-2 for growth and natural cytotoxicity.
IL-15 expression facilitates selective enrichment of gene-modified NK-92 cells
In contrast to IL-2, which is mainly found as a soluble cytokine, IL-15 is often trans-presented on the cell surface in a complex with IL-15Rα to neighboring cells expressing IL-2 receptor β/γ heterodimers [28]. We investigated this possibility but did not detect surface-bound IL-15 on NK-92/IL-15-EGFP cells by flow cytometry (see Supplementary Fig. 3 available on-line). This indicates that the cells do not trans-present ectopically expressed IL-15 to neighboring cells in detectable amounts. Together with the finding that only low amounts of IL-15 are secreted into the culture supernatant by NK-92/IL-15-EGFP, these data suggest that the ectopically expressed cytokine predominantly activates IL-15 receptor complexes within the producer cells in an autocrine fashion.
To test whether this allows direct positive selection of IL-15-expressing cells, NK-92 were freshly transduced with S-IL15-IEW lentiviral particles in medium containing IL-2. Forty-eight hours after transduction, IL-2 was withdrawn, and the unsorted cells were allowed to grow for another 14 days in IL-2-free medium. Then, the relative proportion of gene-modified NK-92 was determined by flow cytometric analysis of EGFP expression. While transduction efficiency was relatively low yielding only 2% of EGFP-positive cells in the unsorted pool directly after transduction, withdrawal of IL-2 resulted in rapid and selective enrichment of cells co-expressing EGFP and IL-15, yielding >97% EGFP-positive cells after 14 days (Fig. 3). These data demonstrate that IL-15 functions as a selectable marker in cells that are otherwise dependent on exogenously supplied IL-2 or IL-15 and allows isolation of gene-modified cells that co-express another gene of interest such as EGFP used here as a marker.
Fig. 3.
IL-15 serves as a selectable marker for rapid enrichment of gene-modified NK cells. NK-92 cells were freshly transduced with S-IL15-IEW lentiviral vector, kept in IL-2-containing medium for 48 h, and then grown in medium without IL-2 for another 14 days. Non-transduced parental NK-92 control cells (a, c), unsorted NK-92 cells in IL-2-containing medium 48 h after transduction (b), and 14 days after IL-2 withdrawal (d) were analyzed for EGFP expression by flow cytometry. EGFP-negative (red) and EGFP-positive cell populations (green) are indicated
NK-92 cells co-expressing IL-15 and a chimeric antigen receptor
Expression of tumor-specific chimeric antigen receptors in NK cells facilitates efficient and selective killing of cancer cells expressing the respective target antigen on the cell surface [6, 15, 29]. To analyze the consequences of co-expression of IL-15 and a tumor-specific CAR in NK-92 cells, we first generated an optimized CAR targeted to the tumor-associated antigen EpCAM. Sequences encoding an immunoglobulin heavy chain signal peptide, the humanized EpCAM-specific scFv(MOC31) antibody fragment [14, 25], a Myc-tag, CD8 α hinge region, transmembrane and intracellular domains of CD28, and the intracellular domain of CD3 ζ chain were assembled in silico, and the respective CAR 31.28.z fusion gene was generated by de novo synthesis in a codon-optimized form. For co-expression with IL-15, internal ribosome entry site (IRES) and EGFP sequences in the lentiviral transfer vector pSIEW were replaced by a CAR-IRES-IL-15 cassette (Fig. 4a).
Fig. 4.
IL-2 withdrawal results in enrichment of NK-92 cells co-expressing IL-15 and a chimeric antigen receptor. a Schematic representation of the lentiviral transfer vector pS-31.28.z-I-IL15-W that encodes under the control of the Spleen Focus Forming Virus promoter (SFFV) the chimeric antigen receptor 31.28.z (CAR) that consists of an immunoglobulin heavy chain signal peptide (SP), the EpCAM-specific scFv(MOC31) antibody fragment (scFv), a Myc-tag (M), the CD8 α hinge region (CD8α), transmembrane and intracellular domains of CD28 (CD28), and the intracellular domain of CD3 ζ chain (CD3ζ), followed by an internal ribosome entry site (IRES) and cDNA encoding human IL-15. b After transduction with S-31.28.z-I-IL15-W vector particles, IL-15-expressing cells were selected by IL-2 withdrawal for 14 days. Then, proliferation of the selected cells in growth medium containing or lacking IL-2 was analyzed in MTT metabolization assays by determination of the absorbance at 595 nm as a measure for the relative number of viable cells. Parental NK-92 cells were included for comparison. Mean values ± SEM are shown (n = 3); ***p < 0.0001. NK-92/31.28.z-IL-15 cells with high CAR surface expression were isolated from the selected pool by flow cytometric cell sorting with Myc-tag-specific antibody 9E10. c The presence of IL-15 protein in sorted NK-92/31.28.z-IL-15 cells was investigated by intracellular cytokine staining with anti-IL-15 antibody and flow cytometry (open area). Parental NK-92 cells served as a control (gray area). d Homogeneous CAR surface expression in NK-92/31.28.z-IL-15 cells was confirmed by flow cytometry with 9E10 antibody (open area). Parental NK-92 cells were included for comparison (filled area)
NK-92 cells were transduced with lentiviral particles of the resulting S-31.28.z-I-IL15-W vector in medium containing IL-2, 48 h after transduction IL-2 was withdrawn, and successfully transduced IL-15-expressing cells were selected for 14 days in IL-2-free medium as described above. As already observed for NK-92/IL-15-EGFP cells (see Fig. 2b), irrespective of the presence of exogenous IL-2, the resulting NK-92/31.28.z-IL-15 cells displayed growth characteristics indistinguishable from parental NK-92 cells in IL-2-containing medium (Fig. 4b). While intracellular cytokine staining confirmed IL-15 expression (Fig. 4c), NK-92/31.28.z-IL-15 cells secreted IL-15 only in low amounts comparable to NK-92/IL-15-EGFP cells, which were insufficient to support the growth of non-transduced IL-2/IL-15-dependent bystander cells such as CTLL-2 (see Supplementary Fig. 4 available on-line and data not shown). These data demonstrate that ectopic IL-15 expression facilitates selective enrichment of genetically modified NK-92 cells that carry a therapeutically relevant effector gene. For further functional analysis, from the pool of NK-92/31.28.z-IL-15 cells selected by IL-2 withdrawal, single-cell clones with high and homogeneous CAR surface expression were derived by flow cytometric cell sorting with Myc-tag-specific antibody (Fig. 4d).
Cytotoxic activity of NK-92/31.28.z-IL-15 cells and specificity of cell killing
As a prerequisite for the analysis of CAR functionality and activity of retargeted NK-92 cells, first expression of EpCAM on the surface of a panel of human tumor cell lines was investigated by flow cytometry using EpCAM-specific monoclonal antibody MOC31 [27]. Thereby, high EpCAM expression was found for MDA-MB453 and MDA-MB468 breast carcinoma cells, and erythroleukemic K562 cells. MDA-MB435 melanoma cells were EpCAM-negative (Fig. 5a).
Fig. 5.
NK-92/31.28.z-IL-15 cells display targeted cytotoxicity in the absence of exogenous cytokines. a Expression of EpCAM on the surface of erythroleukemic K562 cells, MDA-MB468 and MDA-MB453 breast carcinoma cells, and MDA-MB435 melanoma cells was determined by flow cytometry with EpCAM-specific MOC31 antibody (filled areas). Cells only treated with APC-coupled secondary antibody served as controls (open areas). b Cytotoxicity of NK-92/31.28.z-IL-15 cells grown in the absence of exogenous IL-2 toward K562, MDA-MB468, MDA-MB453, and MDA-MB435 cells was determined in FACS-based cytotoxicity assays at different effector to target ratios (E/T) as indicated (open squares). Parental NK-92 cells grown in the presence of IL-2 were included for comparison (filled circles). Cytotoxicity of NK-92/31.TM-IL-15 cells that express IL-15 and an EpCAM-specific CAR that lack signaling domains was analyzed using EpCAM-expressing MDA-MB468 and MDA-MB453 cells (gray squares). c Specificity of target cell recognition and cell killing by NK-92/31.28.z-IL-15 cells was analyzed in FACS-based cytotoxicity assays with K562 cells at an effector to target ratio of 5:1. Cells were co-incubated in the absence of competitor (filled bar), or in the presence of EpCAM-specific antibody MOC31 (gray bar) or isotype-matched control antibody (open bar)
Next, cytotoxicity of clonal NK-92/31.28.z-IL-15 cells grown in the absence of IL-2 against K562, MDA-MB468, MDA-MB453, and MDA-MB435 cells during co-culture at different E/T ratios was analyzed in FACS-based assays. Parental NK-92 cells in the presence of IL-2 were included for comparison (Fig. 5b). As expected, K562 cells displayed already pronounced sensitivity to parental NK-92 cells (32% specific lysis at an E/T ratio of 5:1) but were much more potently killed by CAR-expressing NK-92/31.28.z-IL-15 cells (77% specific lysis) (Fig. 5b, top left panel). EpCAM-expressing MDA-MB468 and MDA-MB453 cells were resistant to lysis by unmodified NK-92, but highly sensitive to NK-92/31.28.z-IL-15 cells (61 and 64% specific lysis at an E/T ratio of 5:1, respectively) (Fig. 5b, top right and bottom left panels). This cytotoxicity was completely abolished when NK-92/31.TM-IL-15 cells were used as effectors (Fig. 5b, gray boxes). NK-92/31.TM-IL-15 co-express IL-15 together with a truncated transmembrane CAR that contains the EpCAM-specific scFv(MOC31) antibody fragment for cell recognition but lacks intracellular signaling domains, thereby displaying CAR surface expression comparable to NK-92/31.28.z-IL-15 cells (data not shown). EpCAM-negative MDA-MB435 melanoma cells remained unaffected by co-culture with NK-92 or NK-92/31.28.z-IL-15 cells, demonstrating selectivity of the 31.28.z CAR- and IL-15-expressing variant for target-antigen-expressing tumor cells (Fig. 5b, bottom right panel). When we blocked EpCAM on the surface of K562 cells with anti-EpCAM antibody before co-culture with NK-92/31.28.z-IL-15 cells, cell killing was reduced to levels observed for unmodified NK-92, confirming that the enhanced activity of CAR-expressing NK cells was dependent on the accessibility of EpCAM on the tumor cell surface (Fig. 5c).
These results demonstrate that expression of EpCAM-specific 31.28.z CAR on NK-92 triggers markedly enhanced cell-killing activity toward EpCAM expressing but otherwise NK-resistant tumor cells, which is dependent on CAR-mediated recognition of EpCAM on the target cell surface and the presence of intracellular signaling domains within the CAR. Thereby, ectopic co-expression of IL-15 bypasses the requirement for exogenous IL-2 to achieve high and sustained effector activity.
Selective enrichment and targeted cytotoxicity of NKL cells co-expressing IL-15 and EpCAM-specific CAR 31.28.z
Functionality of IL-15 for selective enrichment of gene-modified cells was not restricted to NK-92 cells but also established for other IL-2/IL-15-dependent cytotoxic lymphocytes such as NKL and CTLL-2 cells (see above and Supplementary Fig. 2). To test whether co-expression of a tumor-specific CAR and IL-15 is feasible in effector cells other than NK-92, human NKL cells were transduced with S-31.28.z-I-IL15-W vector particles and selected by IL-2 withdrawal for 14 days as described above for NK-92. This resulted in rapid enrichment of a pool of IL-2-independent NKL cells, which showed growth properties very similar to those of parental NKL in the presence of IL-2 (Fig. 6a). From the selected cell pool, NKL cells with high and homogeneous 31.28.z CAR surface expression could easily be isolated by flow cytometric cell sorting with Myc-tag-specific antibody (Fig. 6b). Analysis of the cytotoxic activity of the resulting NKL/31.28.z-IL-15 cells in co-culture experiments with EpCAM-expressing MDA-MB453 and EpCAM-negative MDA-MB435 cells revealed high cell-killing activity against antigen-positive target cells in the absence of IL-2, whereas target cells lacking EpCAM remained unaffected (Fig. 6c). As in the case of unmodified NK-92 cells, both target cell lines were resistant to parental NKL in the presence of exogenous IL-2. These data confirm our findings with NK-92 cells and indicate that enhanced cytotoxicity is not a result of increased natural cytotoxicity due to autocrine stimulation with IL-15 but mediated by specific recognition of EpCAM by the 31.28.z CAR.
Fig. 6.
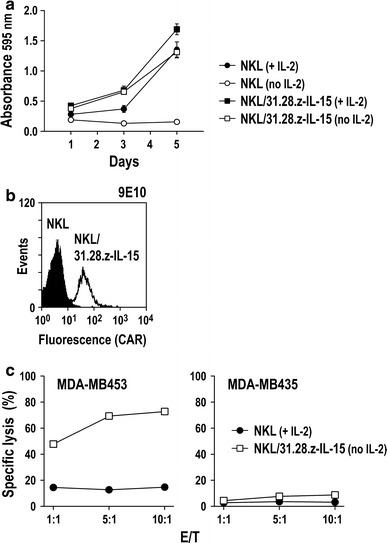
Selective enrichment and targeted cytotoxicity of NKL cells co-expressing IL-15 and EpCAM-specific CAR 31.28.z. a Human NKL cells were transduced with S-31.28.z-I-IL15-W vector particles and selected by IL-2 withdrawal for 14 days. Then, proliferation of the selected cells in growth medium containing or lacking IL-2 was analyzed in MTT metabolization assays by determination of the absorbance at 595 nm as a measure for the relative number of viable cells. Parental NKL cells were included for comparison. Mean values ± SEM are shown (n = 3). b NKL/31.28.z-IL-15 cells with high CAR surface expression were isolated from the selected pool by flow cytometric cell sorting with Myc-tag-specific antibody 9E10 (open area). Parental NKL cells were included for comparison (filled area). c Cytotoxicity of NKL/31.28.z-IL-15 cells grown in the absence of IL-2 toward EpCAM-positive MDA-MB453 and EpCAM-negative MDA-MB435 cells was determined in FACS-based cytotoxicity assays at different effector to target ratios (E/T) as indicated (open squares). Parental NKL cells grown in the presence of IL-2 were included for comparison (filled circles)
Discussion
Genetically modified cytotoxic lymphocytes with specificity for defined tumor-associated surface antigens hold promise for adoptive cancer immunotherapy [3–5]. While initially developed as an approach for retargeting of T cells, expression of chimeric antigen receptors in NK cells also results in potent and specific antitumoral effectors [6–9]. To further modulate their activity, retargeted T cells have been modified to ectopically express cytokines such as IL-15 or IL-12 [30, 31]. Here, we investigated the activities of established human NK cells transduced with a bicistronic lentiviral vector encoding IL-15 together with a humanized CAR that targets the epithelial cell adhesion molecule. Ectopic expression of IL-15 in NK-92 and NKL cells mediated continued proliferation in the absence of IL-2 and facilitated high natural cytotoxicity against NK-sensitive targets as well as potent CAR-mediated cell-killing activity against EpCAM-positive but otherwise NK-resistant tumor cells.
In previous studies, transfection and electroporation of NK-92 and NKL cells with an IL-15 encoding plasmid did not render these cells fully independent from exogenous IL-2, despite enhanced proliferation and effector functions of the gene-modified cells in response to low IL-2 concentrations [20, 32]. In contrast, here NK-92 and NKL NK cells, and CTLL-2 cytotoxic T lymphocytes all acquired growth completely independent from exogenous cytokines upon transduction with mono- or bicistronic lentiviral vectors encoding wild-type human IL-15. IL-15 mRNA was readily detected in transduced NK-92 cells, but only very low amounts of IL-15 were secreted into the culture medium, which were insufficient to support the growth of non-transduced bystander cells or IL-2/IL-15-dependent CTLL-2 indicator cells. Similar results were obtained upon transduction of NKL and CTLL-2 cells, and irrespective of whether pools or clones of cells transduced with IL-15-IRES-EGFP or CAR-IRES-IL-15 vectors were investigated. This rules out the possibility that the position of IL-15 relative to the promoter and the IRES within the bicistronic constructs or a particular vector integration site negatively affected IL-15 expression levels and instead suggests that the complex intracellular routing previously reported for IL-15 in IL-15 receptor-expressing immune cells may constrict effective secretion of the cytokine [33, 34].
While not present in culture supernatants in high amounts, IL-15 produced by the gene-modified NK cells triggered signal transduction via the IL-2/IL-15 receptor complex indicated by STAT5 phosphorylation and supported growth of the producer cells in the absence of IL-2. In flow cytometric analysis, we did not find IL-15 associated with the cell surface (see supplementary data), making trans-presentation of ectopically expressed IL-15 in complex with IL-15Rα to neighboring cells unlikely [28]. Instead, stimulation by IL-15 occurred in a strictly autocrine fashion, likely involving functional interaction between the cytokine and its receptors in the secretory pathway, but prior to secretion. A similar mechanism was previously suggested for ectopically expressed unmodified IL-3 [35] and ectopically expressed IL-2 modified to contain an endoplasmic reticulum retention signal [36].
The ability of ectopically expressed IL-15 to fully replace exogenous IL-2 and restriction of its growth-promoting activity to the producer cells allowed us to employ the cytokine for selective enrichment of gene-modified cells solely by IL-2 withdrawal. Selection in IL-2-free medium after transduction with a bicistronic IL-15 and EGFP encoding vector yielded NK cell populations almost entirely consisting of cells that expressed the EGFP marker gene, and displaying high natural cytotoxicity in the absence of IL-2. Similarly, IL-2 withdrawal allowed selective enrichment of NK-92 and NKL cells co-expressing IL-15 and a chimeric antigen receptor that recognizes the epithelial cell adhesion molecule. Single-cell clones derived from these populations continued to proliferate in the absence of exogenous IL-2 and displayed stable CAR surface expression over several months in continuous culture.
Second-generation CARs that include a costimulatory moiety enhance functionality of retargeted lymphocytes [8, 37]. In this study, we employed a codon-optimized EpCAM-specific antigen receptor (CAR 31.28.z) for tumor targeting, which consists of a humanized single-chain antibody fragment derived from EpCAM-specific murine antibody MOC31 [25], linked via the CD8 α hinge region to transmembrane and intracellular domains of CD28 followed by the intracellular fragment of CD3 ζ. EpCAM is a pan-epithelial differentiation antigen that is expressed on almost all carcinomas and has endogenous oncogenic potential [38, 39]. NK-92 and NKL cells co-expressing the EpCAM-specific CAR 31.28.z and IL-15 displayed high and selective cytotoxic activity in the absence of IL-2 against otherwise NK-resistant breast carcinoma cells, which correlated with the EpCAM expression levels on the targets. Irrespective of the presence of exogenous IL-2, EpCAM-negative MDA-MB435 melanoma cells were neither affected by parental nor by CAR-expressing NK-92 and NKL cells. Deletion of the intracellular CD28 and CD3 ζ domains completely abolished cytotoxicity of NK-92 cells co-expressing this 31.TM CAR and IL-15 against EpCAM-positive and otherwise NK-resistant targets, demonstrating that the observed cell-killing activity requires CAR signaling and is not just mediated by IL-15 and/or improved adhesion via the membrane-anchored scFv molecule. In the case of EpCAM-positive but intrinsically NK-sensitive K562 cells, we observed markedly increased cell-killing activity of NK-92 cells co-expressing CAR and IL-15. This enhanced activity was strictly dependent on specific recognition of the target antigen and abrogated by blocking of EpCAM on the target cell surface with EpCAM-specific antibody. Homing of NK-92 cells carrying an EpCAM-specific first-generation CAR to human prostate cancer xenografts was previously demonstrated in in vivo imaging experiments [14]. Thereby, no concurrent IL-2 treatment was provided, limiting the lifespan of the infused effector cells. This could be different for NK-92/31.28.z-IL-15 cells, and as a next step, persistence and antitumoral activity of these cells will be investigated in suitable in vivo models.
In immunodeficient SCID mice, neither injection of parental NK-92 nor NK-92 ectopically expressing IL-2 resulted in lymphoma formation [40]. Nevertheless, autocrine self-stimulation and factor-independence are typical characteristics of neoplastic cells, and infusion of cytokine-gene transduced lymphocytes may result in unwanted expansion and an increased risk for malignant transformation. These safety concerns need to be addressed before potential clinical application. In the case of NK-92/31.28.z-IL-15, only low amounts of IL-15 were secreted into the surroundings, making it unlikely that in vivo application of these cells would result in adverse effects due to high systemic IL-15 concentrations. However, in vivo expansion of the cells may have to be actively controlled. In clinical studies evaluating unmodified NK-92 cells, the effector cells were irradiated as a safety measure prior to infusion into cancer patients. This prevented permanent engraftment, while cells remained viable and retained cytotoxicity for several days [12, 13]. Likewise, gene-modified NK-92 cells transiently retained CAR expression and selective cytotoxicity upon irradiation [6, 16]. A similar strategy appears feasible for therapeutic application of continuously expanding NK cells that co-express a chimeric antigen receptor and IL-15. Alternatively, a suicide gene could be included as a safety measure, as it has been demonstrated for T cells genetically modified to express IL-15 [30].
Isolation, genetic modification and expansion of primary NK and T cells are labor intensive and may yield variable results. Clinically applicable cytotoxic cell lines such as NK-92 could bypass the need for separate genetic modification of effector cells for each individual patient, especially in cases where autologous effector cells cannot be employed and suitable donors are not available. Co-expression of an EpCAM-specific CAR together with IL-15 allowed rapid isolation and long-term maintenance of NK cells that demonstrated durable antigen-specific cytotoxicity. This strategy may therefore complement existing approaches for GMP-compliant large-scale production of established lines such as NK-92 and extend the potential clinical utility of these effector cells for a wider range of disease indications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported in part by grants from the Bundesministerium für Bildung und Forschung (BMBF) FKZ 01GU0805, the Deutsche Forschungsgemeinschaft (DFG) GRK1172, and the LOEWE Center for Cell and Gene Therapy Frankfurt (CGT). We thank Dr. Uwe Zangemeister-Wittke, University of Bern, for MOC31 antibody, Dr. Manuel Grez, Georg-Speyer-Haus, for helpful discussions and for providing lentiviral vectors and packaging constructs, Dr. Stefan Stein and Mr. Tevik Merovci, Georg-Speyer-Haus for help with flow cytometric cell sorting, and Mrs. Annemarie Schimpf, Georg-Speyer-Haus, for excellent technical assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uherek C, Groner B, Wels W. Chimeric antigen receptors for the retargeting of cytotoxic effector cells. J Hematother Stem Cell Res. 2001;10:523–534. doi: 10.1089/15258160152509136. [DOI] [PubMed] [Google Scholar]
- 3.Hombach A, Abken H. Costimulation tunes tumor-specific activation of redirected T cells in adoptive immunotherapy. Cancer Immunol Immunother. 2007;56:731–737. doi: 10.1007/s00262-006-0249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ngo MC, Rooney CM, Howard JM, Heslop HE. Ex vivo gene transfer for improved adoptive immunotherapy of cancer. Hum Mol Genet. 2011;20:R93–R99. doi: 10.1093/hmg/ddr102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uherek C, Tonn T, Uherek B, Becker S, Schnierle B, Klingemann HG, Wels W. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood. 2002;100:1265–1273. [PubMed] [Google Scholar]
- 7.Wels W, Biburger M, Müller T, Dälken B, Giesübel U, Tonn T, Uherek C. Recombinant immunotoxins and retargeted killer cells: employing engineered antibody fragments for tumor-specific targeting of cytotoxic effectors. Cancer Immunol Immunother. 2004;53:217–226. doi: 10.1007/s00262-003-0482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pegram HJ, Kershaw MH, Darcy PK. Genetic modification of natural killer cells for adoptive cellular immunotherapy. Immunotherapy. 2009;1:623–630. doi: 10.2217/imt.09.36. [DOI] [PubMed] [Google Scholar]
- 10.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klingemann HG. Natural killer cell-based immunotherapeutic strategies. Cytotherapy. 2005;7:16–22. doi: 10.1080/14653240510018000. [DOI] [PubMed] [Google Scholar]
- 12.Tonn T, Becker S, Esser R, Schwabe D, Seifried E. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J Hematother Stem Cell Res. 2001;10:535–544. doi: 10.1089/15258160152509145. [DOI] [PubMed] [Google Scholar]
- 13.Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, Klingemann H. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10:625–632. doi: 10.1080/14653240802301872. [DOI] [PubMed] [Google Scholar]
- 14.Tavri S, Jha P, Meier R, Henning TD, Müller T, Hostetter D, Knopp C, Johansson M, Reinhart V, Boddington S, Sista A, Wels WS, Daldrup-Link HE. Optical imaging of cellular immunotherapy against prostate cancer. Mol Imaging. 2009;8:15–26. [PubMed] [Google Scholar]
- 15.Müller T, Uherek C, Maki G, Chow KU, Schimpf A, Klingemann HG, Tonn T, Wels WS. Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol Immunother. 2008;57:411–423. doi: 10.1007/s00262-007-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esser R, Müller T, Stefes D, Kloess S, Seidel D, Gillies SD, Aperlo-Iffland C, Huston JS, Uherek C, Schönfeld K, Tonn T, Huebener N, Lode HN, Koehl U, Wels WS (2011) NK cells engineered to express a GD(2) -specific antigen receptor display built-in ADCC-like activity against tumor cells of neuroectodermal origin. J Cell Mol Med (in press). doi:10.1111/j.1582-4934.2011.01343.x [DOI] [PMC free article] [PubMed]
- 17.Maki G, Klingemann HG, Martinson JA, Tam YK. Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J Hematother Stem Cell Res. 2001;10:369–383. doi: 10.1089/152581601750288975. [DOI] [PubMed] [Google Scholar]
- 18.Overwijk WW, Schluns KS. Functions of gammaC cytokines in immune homeostasis: current and potential clinical applications. Clin Immunol. 2009;132:153–165. doi: 10.1016/j.clim.2009.03.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamasaki S, Maeda M, Ohshima K, Kikuchi M, Otsuka T, Harada M. Growth and apoptosis of human natural killer cell neoplasms: role of interleukin-2/15 signaling. Leuk Res. 2004;28:1023–1031. doi: 10.1016/j.leukres.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Sun R, Wei H, Tian Z. Characterization of interleukin-15 gene-modified human natural killer cells: implications for adoptive cellular immunotherapy. Haematologica. 2004;89:338–347. [PubMed] [Google Scholar]
- 21.Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol. 2005;86:209–239. doi: 10.1016/S0065-2776(04)86006-1. [DOI] [PubMed] [Google Scholar]
- 22.Robertson MJ, Cochran KJ, Cameron C, Le JM, Tantravahi R, Ritz J. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp Hematol. 1996;24:406–415. [PubMed] [Google Scholar]
- 23.Ball RK, Friis RR, Schoenenberger CA, Doppler W, Groner B. Prolactin regulation of beta-casein gene expression and of a cytosolic 120-kd protein in a cloned mouse mammary epithelial cell line. EMBO J. 1988;7:2089–2095. doi: 10.1002/j.1460-2075.1988.tb03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C, Grez M, Thrasher AJ. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther. 2002;13:803–813. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]
- 25.Willuda J, Honegger A, Waibel R, Schubiger PA, Stahel R, Zangemeister-Wittke U, Plückthun A. High thermal stability is essential for tumor targeting of antibody fragments: engineering of a humanized anti-epithelial glycoprotein-2 (epithelial cell adhesion molecule) single-chain Fv fragment. Cancer Res. 1999;59:5758–5767. [PubMed] [Google Scholar]
- 26.Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann S, Wels W, Froesch BA, Gerstmayer B, Stahel RA, Zangemeister-Wittke U. A novel immunotoxin recognising the epithelial glycoprotein-2 has potent antitumoural activity on chemotherapy-resistant lung cancer. Cancer Immunol Immunother. 1997;44:1–9. doi: 10.1007/s002620050348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stonier SW, Schluns KS. Trans-presentation: a novel mechanism regulating IL-15 delivery and responses. Immunol Lett. 2010;127:85–92. doi: 10.1016/j.imlet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boissel L, Betancur M, Wels WS, Tuncer H, Klingemann H. Transfection with mRNA for CD19 specific chimeric antigen receptor restores NK cell mediated killing of CLL cells. Leuk Res. 2009;33:1255–1259. doi: 10.1016/j.leukres.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, Heslop HE, Rooney CM, Brenner MK, Dotti G. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71:5697–5706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 32.Jiang W, Zhang J, Tian Z. Functional characterization of interleukin-15 gene transduction into the human natural killer cell line NKL. Cytotherapy. 2008;10:265–274. doi: 10.1080/14653240801965156. [DOI] [PubMed] [Google Scholar]
- 33.Kurys G, Tagaya Y, Bamford R, Hanover JA, Waldmann TA. The long signal peptide isoform and its alternative processing direct the intracellular trafficking of interleukin-15. J Biol Chem. 2000;275:30653–30659. doi: 10.1074/jbc.M002373200. [DOI] [PubMed] [Google Scholar]
- 34.Bergamaschi C, Jalah R, Kulkarni V, Rosati M, Zhang GM, Alicea C, Zolotukhin AS, Felber BK, Pavlakis GN. Secretion and biological activity of short signal peptide IL-15 is chaperoned by IL-15 receptor alpha in vivo. J Immunol. 2009;183:3064–3072. doi: 10.4049/jimmunol.0900693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Browder TM, Abrams JS, Wong PM, Nienhuis AW. Mechanism of autocrine stimulation in hematopoietic cells producing interleukin-3 after retrovirus-mediated gene transfer. Mol Cell Biol. 1989;9:204–213. doi: 10.1128/mcb.9.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konstantinidis KV, Alici E, Aints A, Christensson B, Ljunggren HG, Dilber MS. Targeting IL-2 to the endoplasmic reticulum confines autocrine growth stimulation to NK-92 cells. Exp Hematol. 2005;33:159–164. doi: 10.1016/j.exphem.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Hombach A, Wieczarkowiecz A, Marquardt T, Heuser C, Usai L, Pohl C, Seliger B, Abken H. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol. 2001;167:6123–6131. doi: 10.4049/jimmunol.167.11.6123. [DOI] [PubMed] [Google Scholar]
- 38.Munz M, Baeuerle PA, Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2010;69:5627–5629. doi: 10.1158/0008-5472.CAN-09-0654. [DOI] [PubMed] [Google Scholar]
- 39.van der Gun BT, Melchers LJ, Ruiters MH, de Leij LF, McLaughlin PM, Rots MG. EpCAM in carcinogenesis: the good, the bad or the ugly. Carcinogenesis. 2010;31:1913–1921. doi: 10.1093/carcin/bgq187. [DOI] [PubMed] [Google Scholar]
- 40.Tam YK, Miyagawa B, Ho VC, Klingemann HG. Immunotherapy of malignant melanoma in a SCID mouse model using the highly cytotoxic natural killer cell line NK-92. J Hematother. 1999;8:281–290. doi: 10.1089/106161299320316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







