Abstract
The abscopal effect, which is the spontaneous regression of tumors or metastases outside the radiation field, occurs rarely in cancer patients. Interestingly, radiotherapy (RT) triggers an immunogenic cell death (ICD) that is able to generate tumor-specific cytotoxic CD8+ T cells that are efficient in killing cancer cells. The key question is: why is this “abscopal effect” so uncommon in cancer patients treated with RT? Most probably, the main reason may be related to the highly immunosuppressive tumor microenvironment of well-established tumors that constantly antagonizes the anti-tumor immune responses triggered by RT. In this case, additional or combinatorial immunotherapy is needed to attenuate these immunosuppressive networks and, therefore, substantially increases the efficacy of RT. Here, we describe a potentially promising synergistic radio-immunotherapy “in situ tumor vaccination” protocol by antagonizing the tumor-immunosuppressive microenvironment with a combinatorial approach using local RT and IL-12-based TH1 response augmentation.
Keywords: Abscopal effect, In situ tumor vaccination, Radio-immunotherapy, Combinatorial approach, IL-12
The immunogenic tumor cell death and immune modulation induced by radiotherapy
Until recently, it had been accepted that the effectiveness of radiation therapy (RT) depends on the “quantity” of RT-induced tumor cell death. Other reports have highlighted the possibility that apoptosis in tumor cells may act as a driver of oncogenic progression especially by promoting angiogenesis and replacement of the dying cells, as well as by eliciting anti-inflammatory responses [1, 2]. However, other data have shown that the effectiveness of RT is also related to the “quality” of the RT-induced tumor cell death. It has been shown that, under some circumstances, local RT triggers an immunogenic cell death (ICD) that is able to eradicate established distant tumors through the generation of tumor-specific cytotoxic CD8+ T cells [3]. The ICD, which is a “good death” for tumor immunogenicity, can enhance both the priming and the effector phase of the anti-tumor immune response, and it is mainly mediated by damage-associated molecular patterns (DAMPs) that include surface-exposed calreticulin (CRT) [4] and ERP57 [5, 6], ATP secretion that activates the NLRP3 inflammasome in dendritic cells (DCs) [7], and the secretion of high mobility group protein B1 (HMGB1) [8]. In addition, this antigen-specific T-cell immunity was TLR4 dependent as RT could not lead to the rejection of inoculated live tumors in the TLR4−/− mice, whereas it did so in the wild-type mice [9]. The effectiveness of RT was suppressed in immunodeficient mice or after the depletion of CD8+ or CD4+ T cells [4].
Moreover, RT seems to impact multiple aspects of anti-tumor immunity, such as priming and effector phases, and of crosstalk between the immune system and the tumor [10]. In particular, low-dose irradiation induces macrophage differentiation to an iNOS+/M1 phenotype, which is a key element in eliciting effective T-cell immunotherapy [11]. More remarkably, the depletion of Treg significantly ameliorates the outcome of RT, mainly through the enhancement of radiation-mediated anti-tumor immunity [12].
Recently, the occurrence of ICD has been reported in cancer patients. Interestingly, the majority of ICD characteristics, such as tumor antigen-specific T-cell responses, elevated serum levels of HMGB1 and the expression of cell surface CRT, have been documented in patients with esophageal squamous cell carcinoma (ESCC) after RT [13]. More interestingly, the level of secreted HMGB1 was correlated with patient survival and clinic outcomes [13].
The “abscopal effect”
In rare cases, spontaneous regressions of tumors or metastases that were not included in the radiation field have been reported. This type of distant effect was qualified historically as the “abscopal effect”. This systemic effect has been reported clinically in multiple cancer types, such as hepatocellular carcinoma [14, 15], chronic lymphocytic leukemia [16], renal cell carcinoma [17], malignant lymphomas [18] and melanomas [19, 20], and, in patients treated concomitantly with RT and granulocyte–macrophage colony-stimulating factor; objective abscopal responses were observed in some patients with metastatic solid tumors [21]. Moreover, another report suggests that in some advanced melanoma patients who were given RT after progression under ipilimumab, the abscopal effect was observed, which was associated with prolonged survival [22].
There has been a very promising report that concomitant RT with the blockade of CTLA-4 was able to elicit an abscopal effect in mouse models [23]. Similar distant effects have been frequently described in many mouse models of tumor after local RT [24–26] without the successful determination of the exact molecular and cellular mechanisms behind them, although some speculations have been made about the implication of immune mediation [27]. Overall, despite this missing information, accumulating scientific evidence underlines the ability of local RT-induced immuno-modulation to trigger a systemic immune reaction.
Why does such a phenomenon occur rarely in cancer patients?
Why does the “abscopal effect” occur rarely in patients treated with RT and why does RT not result in tumor rejection more often? It is likely that one of the main reasons is due to the highly immunosuppressive tumor microenvironment of well-established tumors that constantly antagonizes the anti-tumor immune responses that are triggered by RT. In this situation, additional or combinatorial immunotherapy may be needed to overcome the immunosuppressive networks and, therefore, enhance the systemic immune impact of RT.
The main challenge of such a combinatorial therapy is to find the appropriate immunotherapy (IT) that is most synergistic with RT
In general, IT could be directed to target one or multiple steps of the immune activation pathway. In particular, the following steps can be targeted: DC presentation and T-cell priming, T-cell activation and anti-tumor effector functions, T-cell differentiation into memory T cells, and tumor microenvironment antagonism. As far as we know, many negative regulatory molecules are expressed on the surface of activated T cells. The essential role of these regulatory molecules is to avoid auto-immune complications through an over-activation of T cells. Many such molecules, called immune checkpoints, exist such as CTLA4 (cytotoxic T lymphocyte-associated antigen 4) and programmed cell death protein 1 (PD1).
In the preclinical setting, the association of RT and immunotherapy has shown encouraging signs of synergistic improvements in different tumor models. Radio-immunotherapy with PD1 blockade induced an increase in the secretion of TNF-α by CD8+ T cells and the suppression of MDSCs, and consequently caused a decrease in tumor volume [28], higher TILs and lower Treg densities with higher survival rates in a glioblastoma model [29]. In addition, radio-immunotherapy with CTLA4 blockade induced lower Treg densities and higher T-cell receptor diversity [30]. Interestingly, adding RT to autologous T-cell infusion induced long lasting remission and improved survival [31].
An excellent clinical outcome was obtained with immune checkpoint inhibitors such as in patients with metastatic melanoma after CTLA-4 inhibition by ipilimumab [32] or in many others cancers types after the inhibition of PD-1 by nivolumab where the tumors shrank by about half or more in 31% of melanoma, 29% of kidney cancer and 17% of lung cancer patients [33, 34]. The benefit was additive when a combination of nivolumab and ipilimumab was administered in patients with advanced melanoma, and at least 53% of patients had an objective response, all with tumor reduction of 80% or more [35]; similar to NSCLC lung cancer patients, a 1-year overall survival rate was observed in 69% of the patients [36]. Even in more aggressive lung cancer, such as recurrent SCLC, combination IT conferred an objective response in 42% of patients compared with the 10% obtained with nivolumab alone [37]. Other immune checkpoint inhibitors showed similar benefits in their outcomes and safety profiles, such as the anti-PD-L1 antibody durvalumab and the anti-CTLA-4 antibody tremelimumab [38].
Altogether, these clinical data showing combination benefits in different cancer types suggest that combination or sequential immunotherapies that target distinct immune pathways may be an effective strategy with the potential to further enhance the magnitude of the anti-tumor immune response over single agents.
In spite of these outstanding advantages, an important limitation of immune checkpoint inhibitor-based IT is the small proportion of patients who achieve a complete clinical response. Similar limitations have been observed with dendritic cell (DC) activation-based IT [39].
Thus, these IT strategies may not be completely effective in overcoming the immunosuppressive networks that are associated with the tumor. Consequently, there is a critical need to develop more potent immunotherapies. This may be achieved by therapies that decrease the tumor-derived immunosuppression. Tumors deploy very complex immunosuppressive mechanisms that act at each of the steps required for immune activation. Principally, this occurs through the secretion of suppressive molecules, such as the inhibitory cytokines IL-10 and transforming growth factor-β (TGF-β), the presence of regulatory lymphocytes (Treg) or myeloid-derived suppressor cells (MDSCs), and many other mechanisms [40].
In situ tumor vaccination: IL-12-based TH1 immunotherapy combined with RT
Ideally, the optimal IT will be one that will dampen many levels of the tumor-associated immunosuppression. One of the rare candidates with such an ability is interleukin-12 (IL-12). Because it is polyvalent and one of the rare cytokines connecting innate and adaptive immunity, it can co-activate both NK cells and cytotoxic CD8+ T lymphocytes. IL-12 is a major factor in tumor rejection by polarizing the naive CD4+ helper T cells toward the T helper-1 (TH1) phenotype. In addition, IL-12 can modulate the anti-tumor response at different levels: by increasing antigen presentation through IFN-ɣ secretion and leading to MHC class I/II upregulation and by improving cellular immunity through boosting both the proliferation of cytotoxic CD8+ T and NK cells as well as their cytolytic activity [41]. Moreover, a radiosensitizing effect of combined IR and IL-12 treatment has already been demonstrated that probably incorporates long term immune memory, as the cured animals were resistant to secondary challenge. The work indirectly implied a possible abscopal effect of the treatment combination [42].
After the excellent results obtained in animal models, there have been multiple clinical trials aimed at evaluating systemic recombinant IL-12 in cancer patients. Unfortunately, the clinical outcomes were very modest and often accompanied with unacceptable levels of adverse events, which tempered the enthusiasm for the use of this recombinant cytokine in patients. In fact, only a few studies reported promising results with recombinant IL-12. After a meticulous analysis of all these clinical studies, we and other teams have noted that the major limiting factor was the low levels of IL-12 infiltrating into the tumor even in patients with high IL-12 exposure and toxicities. In contrast, in tumor biopsies of the few cases of objective complete responses obtained for patients with Kaposi’s sarcoma and B-cell non-Hodgkin lymphoma, notable tumor levels of IL-12 were detected.
Recently, we showed that IL-12 is the main mediator of the Immunogenic Cell Death. The depletion of IL-12 abolished the immunity mediated by RT. Local RT combined with local IL-12-based TH1 therapy triggers a massive reduction of CT26 colon cancer cells in tumor-bearing mice (Fig. 1a), glioblastoma, melanoma, lung cancer, and castration-resistant prostate cancer (Obeid et al., in preparation). Moreover, this in situ vaccination and combinatorial approach conferred a longer survival of mice with CT26 colon cancer compared to mice treated with RT or intratumoral IL-12 alone (Fig. 1b.) Our preliminary results showed that this radio-immunotherapy combination mediates tumor immuno-modulation that causes a systemic anti-tumor response through the augmentation of TILs (tumor-infiltrating lymphocytes) (Fig. 2). These results confirmed a powerful synergy between local RT and local IL-12-based immunotherapy by promoting anti-tumor immunity of T cells to overcome tumor-derived immunosuppression in a highly immunosuppressive cancer such as glioblastoma (GB), among others (Fig. 2). GB is an incurable cancer and one of the most aggressive cancers known because of the high tumor-associated immunosuppressive microenvironment. An existing study reported that intratumoral IL-12 therapy combined with CTLA-4 inhibition-induced T-cell-mediated glioma rejection [43].
Fig. 1.
Intratumor administration of IL-12 and concomitant daily radiotherapy (RT) reduces the tumor volume (a) and extends the lifespan (b) of BALB/c mice-bearing CT26 colon cancer cells. Untreated CT26 tumors (seeded with 3 × 105 cells) were established in 6-week-old female BALB/c mice, as described previously [6]). The intratumoral administration of IL-12 was delivered at 10 µg/day for a period of 7 days, in the presence or absence of radiotherapy (RT) delivered to the tumor site at 2 Gy/day for a period of 7 days only. a Tumors treated concomitantly with IL-12 and RT were significantly smaller (Student’s t test; P < 0.001) than untreated tumors (treated with PBS, co) or those treated with either IL-12 or RT alone. b Mice treated concomitantly with IL-12 and RT survived longer than the untreated mice (co) or those treated with IL-12 or RT alone, as shown by a Kaplan–Meier survival plot (n = 14 animals/group; P < 0.05, log-rank test)
Fig. 2.
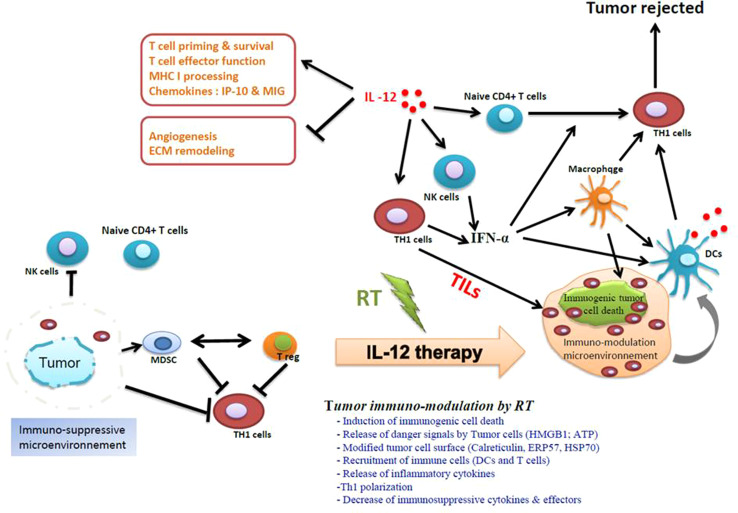
Generation of a potent anti-tumor immune response by an in situ vaccination approach that combines local radiotherapy and intratumoral IL-12-based immunotherapy. Antagonizing the immunosuppressive microenvironment in the tumors with IL-12 substantially increases the immune reaction generated by ICD-induced RT. The tumor immuno-modulation by RT is a very complex process and goes through multiple steps. The tumor ICD exposes an “eat-me signal”, such as CRT, which is a required step for the phagocytosis by DCs. The phagocytosis of ICD by DCs is accompanied by the release of other mediators such as the HMGB1 that is required for DC maturation and consequently for cross presentation into the draining lymph nodes and generation of tumor-specific effector T cells. In well-established tumors, the cytotoxic CD8+ T-cell response generated by RT is not enough to overcome the immunosuppressive nature of the tumor and consequently to reject the tumors. One of the possible solutions is to dampen the immunosuppressive microenvironment in the tumors by bridging innate and adaptive immunity. IL-12 is a multifunctional cytokine. IL-12 can act in many levels of the tumor-associated immunosuppressive network. IL-12 directly activates both NK cells and cytotoxic-T cells, which can cause high levels of IFN-ɣ secretion and TH1 polarization, two crucial steps for the generation of potent anti-tumor immunity. IL-12 can also cause macrophages to produce several anti-angiogenic cytokines such as CXCL9 and CXCL10. By inducing the upregulation of MHC I and MHC II, IL-12 enhances cross presentation, which is an essential step for eliciting a strong anti-tumor immune response. IL-12 is able to reprogram the immune suppressor cells of the tumor stroma, including regulatory T cells, immature dendritic cells, and myeloid-derived suppressor cells (MDSCs). Reversing the dysfunction of these cells is required to stimulate tumor-specific T cells, as shown in a preclinical model [49]. Specifically, the immunosuppressive environment will be converted from a TH2-dominated environment to a more TH1-like response with reduced IL-4 and IL-5 levels. Thus, this radio-immunotherapy (RIT) in situ tumor vaccination protocol, which combines RT with IL-12-based TH1 immunotherapy, allows for a strong synergy and is able to generate potent anti-tumor immunity
Therefore, we believe that IL-12 remains a very promising candidate for tumor immunotherapy, but the systemic administration strategy should be undoubtedly replaced by a tumor-targeting approach to maximize efficacy by increasing IL-12 expression in the tumors while improving the safety profile by reducing systemic exposure. Hence, an IL-12-targeted tumor-delivery system is required that will allow an acceptable safety profile and a stronger dampening of the tumor-associated immunosuppressive microenvironment. Recently, several IL-12-based therapies with intratumoral/local delivery systems have been evaluated clinically. There are three pilot strategies: the first is based on intratumoral delivery of the IL-12 gene by electroporation [44, 45], the second is based on intratumoral injections of IL-12-expressing adenovirus vector in combination with oral activator ligand [46], and the third is based on intralesional administration of IL-12 combined with the human monoclonal antibody fragment L19 [47].
The first two approaches showed excellent safety profiles during phase I trials, and the recently updated phase II interim data show very promising results with the IL-12-expressing adenovirus vector. In fact, 78 and 45% of the treated lesions resulted in a durable response at 3 and 6 months, respectively. Biopsies of the injected lesions showed necrotic areas and lymphocyte infiltrations [44]. The electroporation-based DNA approach showed a robust response and better performance in comparative studies with high and long lasting levels of serum IL-12. The results from the third targeting approach were very encouraging as well, with the detection of systemic activity of IL-12 following intralesional immuno-stimulatory treatment. In fact, 7/13 (53.8%) of the non-injected lesions in patients with stage IIIC and IVM1a metastatic melanoma exhibited a complete response. Accordingly, the intralesional administration of L19-IL2 and L19-TNF may represent an effective method for the local control of inoperable melanoma lesions.
Optimistically, a nice proof of concept report has shown that the combination of local ablative techniques with peritumoral IL-2 gene therapy is effective [48]. In fact, in dogs with mastocytoma, the rate of complete response (CR) to electrochemotherapy combined with peritumoral IL-12 electrotransfer was increased to 72% of all the treated tumors and even to 100% in tumors smaller than 2 cm3. Very interestingly, some very large tumors (10 cm3) were also cured and the authors reported the absence of recurrence over a very long period of at least 40 months. These data clearly show the benefit of combining intratumoral IL-12 therapy with the conventional treatment. The appropriate delivery strategy may depend on the clinical situation as well as the tumor depth and the topography.
The relevance and potential contribution of a combinatorial approach to cancer treatment
The last 3 years have marked a turning point in cancer immunotherapy. We have experienced complete remission in metastatic melanoma patients and excellent clinical responses in many other advanced cancers. Unfortunately, this “breakthrough” was not observed in all patients, as many of them experienced a low or no clinical response. The reason for these discrepancies is not well understood, but accumulating evidence suggests that one of the major impediments could be the “powerful” immunosuppressive tumor microenvironment. Tumor-derived immunosuppression constantly alienates anti-tumor immune responses, and accordingly, immunotherapies that are optimally designed to reverse this solid network need to overcome the tumor-derived immunosuppression and provide benefits for the cancer treatment.
Targeted IL-12-based TH1 therapy offers great promise for both boosting immune responses and dampening immunosuppression. IL-12 is a master regulator of adaptive TH1 cell-mediated immunity. Optimally boosting tumor IL-12 with a targeted strategy will impact the tumor environment in at least three ways: by improving the T-cell cytotoxic activity, by activating and recruiting innate immune cells, and by re-programming the stroma-associated immune suppressor cells. The second main rationale for this local-immune intervention is to avoid the toxic effects of systemic IL-12 administration.
Undeniably, combined immunotherapy with the conventional treatment results in a potent synergy that may significantly improve the clinical outcomes. Our results and many recent reports support the fact that local immuno-modulation by radiation therapy can synergize with immunotherapy to promote systemic anti-tumor immunity, leading to the spontaneous regression of tumors and metastases that are outside the radiation field. This radio-immunotherapy approach may bring new hope for patients with very limited therapeutic options, especially for brain tumors and for tumors deep within the body.
Acknowledgements
This work was supported by a Grant from INSERM (Institut National de la santé et de la recherche médicale) and faculté de médécine Paris VI.
Abbreviations
- ATP
Adenosine triphosphate
- CRT
Calreticulin
- CTLA4
Cytotoxic-T lymphocyte-associated antigen 4
- CXCL10/IP-10
Interferon-inducible protein 10
- CXCL9/Mig
Monokine induced by interferon gamma
- DAMPs
Damage-associated molecular patterns
- DCs
Dendritic cells
- ESCC
Esophageal squamous cell carcinoma
- GB
Glioblastoma
- HMGB1
High mobility group protein B1
- ICD
Immunogenic cell death
- iNOS/M1
Macrophage M1 inducible nitric oxide synthase
- MDCSs
Myeloid-derived suppressor cells
- NSCLC
Non-small cell lung cancer
- PD1
Programmed cell death protein 1
- RT
Radiotherapy
- TGF-β
Transforming growth factor-β
- TH1
Type I T helper
- TILs
Tumor-infiltrating lymphocytes
- TLR4
Toll like receptor 4
- Treg
Regulatory T cell(s)
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the European Union “European directive 86/609/EEC” and the French National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the University of Pierre et Marie Curie, Paris, France (Permit Number: A751301).
References
- 1.Gregory CD. Inflammation and cancer revisited: an hypothesis on the oncogenic potential of the apoptotic tumor cell. Autoimmunity. 2013;46(5):312–316. doi: 10.3109/08916934.2012.755961. [DOI] [PubMed] [Google Scholar]
- 2.Gregory CD, Pound JD. Cell death in the neighbourhood: direct microenvironmental effects of apoptosis in normal and neoplastic tissues. J Pathol. 2011;223(2):177–194. doi: 10.1002/path.2792. [DOI] [PubMed] [Google Scholar]
- 3.Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, Zitvogel L, Kroemer G. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14(10):1848–1850. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 4.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 5.Obeid M. ERP57 membrane translocation dictates the immunogenicity of tumor cell death by controlling the membrane translocation of calreticulin. J Immunol. 2008;181(4):2533–2543. doi: 10.4049/jimmunol.181.4.2533. [DOI] [PubMed] [Google Scholar]
- 6.Obeid M. Anticancer activity of targeted proapoptotic peptides and chemotherapy is highly improved by targeted cell surface calreticulin-inducer peptides. Mol Cancer Ther. 2009;8(9):2693–2707. doi: 10.1158/1535-7163.MCT-09-0228. [DOI] [PubMed] [Google Scholar]
- 7.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Genin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, Andre F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15(10):1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 8.Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, Andre F, Tursz T, Kroemer G, Zitvogel L. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 9.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 10.Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1(9):1325–1332. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- 11.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, Klapproth K, Schakel K, Garbi N, Jager D, Weitz J, Schmitz-Winnenthal H, Hammerling GJ, Beckhove P. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Son CH, Bae JH, Shin DY, Lee HR, Jo WS, Yang K, Park YS. Combination effect of regulatory T-cell depletion and ionizing radiation in mouse models of lung and colon cancer. Int J Radiat Oncol Biol Phys. 2015;92(2):390–398. doi: 10.1016/j.ijrobp.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki Y, Mimura K, Yoshimoto Y, Watanabe M, Ohkubo Y, Izawa S, Murata K, Fujii H, Nakano T, Kono K. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res. 2012;72(16):3967–3976. doi: 10.1158/0008-5472.CAN-12-0851. [DOI] [PubMed] [Google Scholar]
- 14.Ohba K, Omagari K, Nakamura T, Ikuno N, Saeki S, Matsuo I, Kinoshita H, Masuda J, Hazama H, Sakamoto I, Kohno S. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut. 1998;43(4):575–577. doi: 10.1136/gut.43.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okuma K, Yamashita H, Niibe Y, Hayakawa K, Nakagawa K. Abscopal effect of radiation on lung metastases of hepatocellular carcinoma: a case report. J Med Case Rep. 2011;5:111. doi: 10.1186/1752-1947-5-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sham RL. The abscopal effect and chronic lymphocytic leukemia. Am J Med. 1995;98(3):307–308. doi: 10.1016/S0002-9343(99)80380-5. [DOI] [PubMed] [Google Scholar]
- 17.Wersall PJ, Blomgren H, Pisa P, Lax I, Kalkner KM, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol. 2006;45(4):493–497. doi: 10.1080/02841860600604611. [DOI] [PubMed] [Google Scholar]
- 18.Antoniades J, Brady LW, Lightfoot DA. Lymphangiographic demonstration of the abscopal effect in patients with malignant lymphomas. Int J Radiat Oncol Biol Phys. 1977;2(1–2):141–147. doi: 10.1016/0360-3016(77)90020-7. [DOI] [PubMed] [Google Scholar]
- 19.Kingsley DP. An interesting case of possible abscopal effect in malignant melanoma. Br J Radiol. 1975;48(574):863–866. doi: 10.1259/0007-1285-48-574-863. [DOI] [PubMed] [Google Scholar]
- 20.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, Sedrak C, Jungbluth AA, Chua R, Yang AS, Roman RA, Rosner S, Benson B, Allison JP, Lesokhin AM, Gnjatic S, Wolchok JD. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, Friedman K, Ponzo F, Babb JS, Goldberg J, Demaria S, Formenti SC. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16(7):795–803. doi: 10.1016/S1470-2045(15)00054-6. [DOI] [PubMed] [Google Scholar]
- 22.Grimaldi AM, Simeone E, Giannarelli D, Muto P, Falivene S, Borzillo V, Giugliano FM, Sandomenico F, Petrillo A, Curvietto M, Esposito A, Paone M, Palla M, Palmieri G, Caraco C, Ciliberto G, Mozzillo N, Ascierto PA. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014;3:e28780. doi: 10.4161/onci.28780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akutsu Y, Matsubara H, Urashima T, Komatsu A, Sakata H, Nishimori T, Yoneyama Y, Hoshino I, Murakami K, Usui A, Kano M, Ochiai T. Combination of direct intratumoral administration of dendritic cells and irradiation induces strong systemic antitumor effect mediated by GRP94/gp96 against squamous cell carcinoma in mice. Int J Oncol. 2007;31(3):509–515. [PubMed] [Google Scholar]
- 25.Shiraishi K, Ishiwata Y, Nakagawa K, Yokochi S, Taruki C, Akuta T, Ohtomo K, Matsushima K, Tamatani T, Kanegasaki S. Enhancement of antitumor radiation efficacy and consistent induction of the abscopal effect in mice by ECI301, an active variant of macrophage inflammatory protein-1alpha. Clin Cancer Res. 2008;14(4):1159–1166. doi: 10.1158/1078-0432.CCR-07-4485. [DOI] [PubMed] [Google Scholar]
- 26.Takeshima T, Chamoto K, Wakita D, Ohkuri T, Togashi Y, Shirato H, Kitamura H, Nishimura T. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res. 2010;70(7):2697–2706. doi: 10.1158/0008-5472.CAN-09-2982. [DOI] [PubMed] [Google Scholar]
- 27.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58(3):862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, Durham N, Meyer C, Harris TJ, Albesiano E, Pradilla G, Ford E, Wong J, Hammers HJ, Mathios D, Tyler B, Brem H, Tran PT, Pardoll D, Drake CG, Lim M. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filatenkov A, Baker J, Muller AM, Ahn GO, Kohrt H, Dutt S, Jensen K, Dejbakhsh-Jones S, Negrin RS, Shizuru JA, Engleman EG, Strober S. Treatment of 4T1 metastatic breast cancer with combined hypofractionated irradiation and autologous T-cell infusion. Radiat Res. 2014;182(2):163–169. doi: 10.1667/RR13471.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, Ready NE, Gerber DE, Chow LQ, Juergens RA, Shepherd FA, Laurie SA, Geese WJ, Agrawal S, Young TC, Li X, Antonia SJ. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18(1):31–41. doi: 10.1016/S1470-2045(16)30624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antonia SJ, Lopez-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, Jager D, Pietanza MC, Le DT, de Braud F, Morse MA, Ascierto PA, Horn L, Amin A, Pillai RN, Evans J, Chau I, Bono P, Atmaca A, Sharma P, Harbison CT, Lin CS, Christensen O, Calvo E. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883–895. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 38.Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, Narwal R, Steele K, Gu Y, Karakunnel JJ, Rizvi NA. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17(3):299–308. doi: 10.1016/S1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 40.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13(2):155–168. doi: 10.1016/S1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 42.Sedlar A, Kranjc S, Dolinsek T, Cemazar M, Coer A, Sersa G. Radiosensitizing effect of intratumoral interleukin-12 gene electrotransfer in murine sarcoma. BMC Cancer. 2013;13:38. doi: 10.1186/1471-2407-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vom Berg J, Vrohlings M, Haller S, Haimovici A, Kulig P, Sledzinska A, Weller M, Becher B. Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell-mediated glioma rejection. J Exp Med. 2013;210(13):2803–2811. doi: 10.1084/jem.20130678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, Munster PN, Sullivan DM, Ugen KE, Messina JL, Heller R. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008;26(36):5896–5903. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sersa G, Teissie J, Cemazar M, Signori E, Kamensek U, Marshall G, Miklavcic D. Electrochemotherapy of tumors as in situ vaccination boosted by immunogene electrotransfer. Cancer Immunol Immunother. 2015;64(10):1315–1327. doi: 10.1007/s00262-015-1724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linette GP, Hamid O, Whitman ED, Nemunaitis JJ, Chesney J, Agarwal SS, Starodub A, Barrett JA, Marsh A, Martel LA, Cho A, Reed TD, Youssoufian H, Vergara-Silva A (2013) A phase I open-label study of Ad-RTS-hIL-12, an adenoviral vector engineered to express hIL-12 under the control of an oral activator ligand, in subjects with unresectable stage III/IV melanoma. J Clin Oncol, 2013 ASCO Annual Meeting Abstracts Vol 31, No 15_suppl (May 20 Supplement)
- 47.Danielli R, Patuzzo R, Di Giacomo AM, Gallino G, Maurichi A, Di Florio A, Cutaia O, Lazzeri A, Fazio C, Miracco C, Giovannoni L, Elia G, Neri D, Maio M, Santinami M. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunol Immunother. 2015;64(8):999–1009. doi: 10.1007/s00262-015-1704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cemazar M, Ambrozic Avgustin J, Pavlin D, Sersa G, Poli A, Krhac Levacic A, Tesic N, Lampreht Tratar U, Rak M, Tozon N. Efficacy and safety of electrochemotherapy combined with peritumoral IL-12 gene electrotransfer of canine mast cell tumours. Vet Comp Oncol. 2016 doi: 10.1111/vco.12208. [DOI] [PubMed] [Google Scholar]
- 49.Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, Pisarev V, Sherman S, Sporn MB, Gabrilovich D. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest. 2011;121(10):4015–4029. doi: 10.1172/JCI45862. [DOI] [PMC free article] [PubMed] [Google Scholar]