Abstract
Tumor-associated macrophages (TAMs) derived from peripheral blood monocytes recruited into the renal cell carcinoma (RCC) microenvironment. In response to inflammatory stimuli, macrophages undergo M1 (classical) or M2 (alternative) activation. M1 cells produce high levels of inflammatory cytokines, such as tumor necrosis factor-α, interleukin (IL)-12, IL-23 and IL-6, while M2 cells produce anti-inflammatory cytokines, such as IL-10, thus contributing to RCC-related immune dysfunction. The presence of extensive TAM infiltration in RCC microenvironment contributes to cancer progression and metastasis by stimulating angiogenesis, tumor growth, and cellular migration and invasion. Moreover, TAMs are involved in epithelial–mesenchymal transition of RCC cancer cells and in the development of tumor resistance to targeted agents. Interestingly, macrophage autophagy seems to play an important role in RCC. Based on this scenario, TAMs represent a promising and effective target for cancer therapy in RCC. Several strategies have been proposed to suppress TAM recruitment, to deplete their number, to switch M2 TAMs into antitumor M1 phenotype and to inhibit TAM-associated molecules. In this review, we summarize current data on the essential role of TAMs in RCC angiogenesis, invasion, impaired anti-tumor immune response and development of drug resistance, thus describing the emerging TAM-centered therapies for RCC patients.
Keywords: Cancer, Clinical trials, Inflammation, Innate immunity, Renal cell carcinoma, Tumor-associated macrophage
Introduction
Renal cell carcinoma (RCC) has historically been considered highly resistant to both chemotherapy and radiation therapy, and for this reason, other therapeutic approaches have been investigated. In RCC, the ability of the immune system to recognize tumor antigens has been demonstrated, suggesting the possibility to effectively treat RCC patients with immunotherapeutic approaches. Rare cases of spontaneous tumor regression have been reported [1, 2], and diffuse tumor infiltrate consisting of T cells, natural killer cells (NK), dendritic cells (DCs), and macrophages has been observed [3, 4]. Despite being strongly infiltrated, RCC is generally not eliminated by different immune effector cells, and this immune dysfunction likely contributes to tumor evasion.
As recently reported, tumor microenvironment seems to be involved in the immune-escape mechanisms in RCC [5, 6]. Particularly, tumor-secreted factors such as CXCL8/interleukin 8, interleukin 6 (IL-6) and vascular endothelial growth factor (VEGF) play a crucial role in the intratumor alteration of DC differentiation, inducing a specific DC subset (ercDC), which co-expresses markers of DCs (CD209) and macrophages (CD14 and CD163). The ercDCs promote tumor cell proliferation by secreting high levels of metalloproteinase 9 (MMP-9) and by enhancing tumor-promoting tumor necrosis factor-α (TNF-α), while reducing specific chemokines, such as CXCL10 and CCL5 [5, 6].
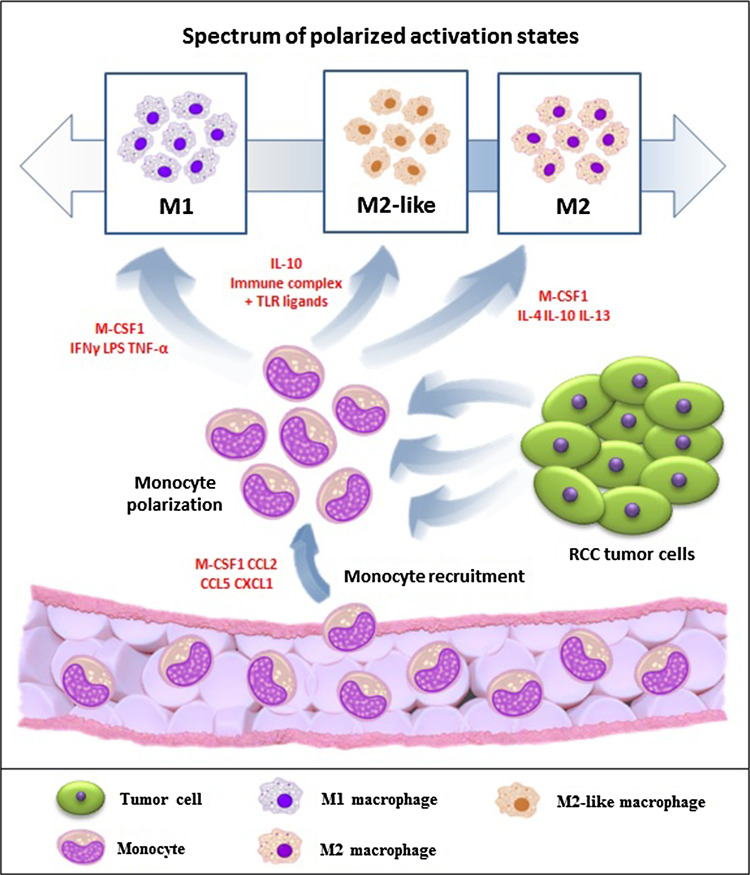
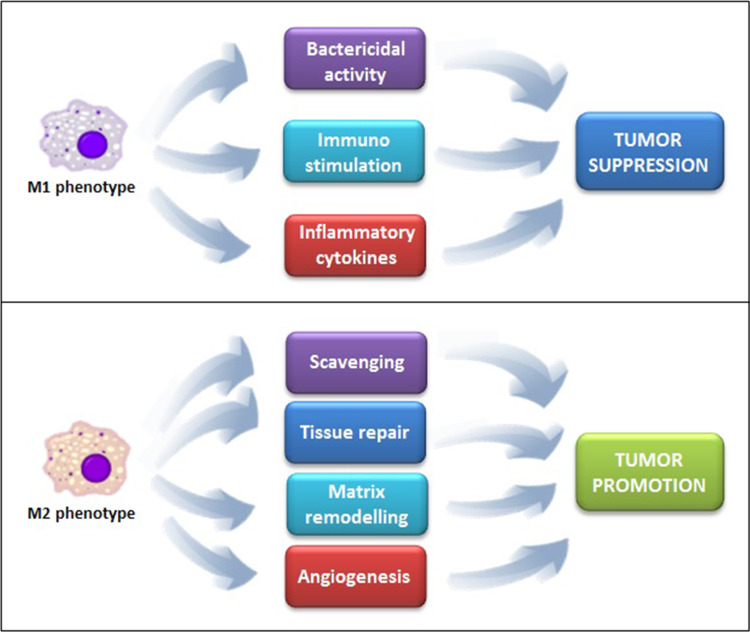
Tumor-associated macrophages (TAMs) represent a major leukocyte population infiltrating tumors. In many but not all human cancers, a high frequency of TAMs is associated with poor prognosis [7]. TAMs constitute a quantitative and functional important subpopulation in the RCC microenvironment that is able to induce the alternative activation and differentiation of TAMs. In fact, TAMs originate from circulating blood monocytes that differentiate into macrophages following their extravasation into tissues. Tissue microenvironmental signals, such as colony-stimulating factor (CSF)-1, act as monocyte chemoattractants and induce macrophage differentiation [8]. In response to various signals, macrophages can undergo classical (M1) or alternative (M2) activation (Fig. 1), which are the extremes of a wide spectrum of polarized activation states that differ in terms of receptors, cytokine and chemokine expression and effector functions (Fig. 2). Classical, or M1, macrophages are characterized by the expression of high amounts of iNOS and TNF-α, whereas, alternatively activated, M2 macrophages typically express arginase 1 (ARG1), but not the inducible nitric oxide synthase (iNOS) [9].
Fig. 1.
Renal cell carcinoma (RCC) microenvironment induces monocyte recruitment and polarization
Fig. 2.
Different functions exerted by M1 and M2 macrophage phenotypes
In this paper, we will focus on the role of TAMs and associated molecules in RCC tumor angiogenesis, invasion and development of drug resistance, thus underlying their potential as therapeutic targets in RCC patients.
Role of TAMs in RCC tumor angiogenesis and invasion
TAMs are a key component of the RCC tumor microenvironment and orchestrate various aspects of cancer, such as tumor cell growth, invasion, metastasis, angiogenesis and immunoregulation. In RCC, the number of TAMs significantly correlates with tumor microvessel density and VEGF level [10]. Accordingly, the presence of E- and P-selectin-positive RCC tumor microvessels correlates with the amount of TAMs, and the expression of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) on neoplastic epithelia is associated with an increased density of macrophages and a minor degree of tumor differentiation [11].
TAMs significantly contribute to tumor angiogenesis by producing a wide array of growth factors such as platelet-derived growth factor (PDGF) and transforming growth factors β (TGF-β) [12, 13], and consequently stimulating several crucial signaling pathways, including the VEGF/VEGFR-1 pathway. Li and colleagues reported that VEGF in clear cell RCC is mainly produced by tumor stromal cells instead of the tumor cells themselves. The critical role of TAMs in the regulation of angiogenesis in RCC is suggested also by the evidence that knockdown of VEGFR-1 expression significantly attenuates macrophage tumor infiltration and inhibits the expression of monocyte chemoattractant protein-1 (MCP-1). Therefore, a reduction in VEGF production and tumor microvessel density has been reported [14].
The TAM production of placental growth factor (PIGF) that is a homolog of VEGF and is able to bind to VEGFR1 may also contribute to stimulate tumor angiogenesis [15] and may in part explain the resistance to VEGFR-targeted therapies [16]. Moreover, in RCC, TAMs and microvessels express simultaneously gastrin-relasing peptide (GRP) and its receptor suggesting the existence of an autocrine or paracrine loop within the tumor that regulates TAM recruitment, tumor growth and neoangiogenesis [17].
TAMs can adapt to the hypoxia status that characterizes RCC, resulting in an enhanced expression of pro-angiogenic genes. Indeed, hypoxia promotes the activation of the hypoxia inducible factor-1 (HIF-1) and hypoxia inducible factor-2 (HIF-2), which induce the expression of various protumoral genes in TAMs, such as VEGF and IL-8, thus significantly supporting angiogenesis, tumor growth and invasion [18].
Role of TAMs and associated molecules in RCC growth and metastasis
Macrophages are dynamic and heterogeneous cells. This is due to different mechanisms governing their differentiation, tissue distribution and responsiveness to stimuli. Mosser and Edwards proposed to classify macrophages on the basis of their functions in three distinct subgroups: classically activated macrophages involved in host defense, wound healing macrophages involved in tissue repair and remodeling, and regulatory macrophages that play a role in immunoregulation [19]. Based on the evidence that wound healing and regulatory macrophages are basically variations of the M2 state, Mantovani et al. suggested the presence of three macrophage polarization states: M1 (=classical activation), M2 (=alternative activation) and M2-like (that incorporate all the other variations of M2 state) [20]. M1 macrophages stimulate cell-mediated responses via the production of pro-inflammatory cytokines IL-1, IL-6, IL-12, IL-23, TNF-α and high levels of IL-1 receptor type I (IL-1RI). On the other hand, M2 macrophages stimulate humoral responses, tissue remodeling and angiogenesis through the production of anti-inflammatory cytokines (IL-10 and TGF-β) and high levels of decoys that antagonize IL-1, such as IL1RII and IL-1 receptor antagonist [21]. M2 macrophages include at least three subsets: M2a induced by IL-4 or IL-13; M2b induced by immune complexes and agonists of TLRs or IL-1Rs; and M2c induced by IL-10 and glucocorticoid hormones [22].
M1 and M2 macrophages are also distinct for their chemokine expression profiles [23]. Indeed, M1 macrophages express inflammatory chemokines such as CXCL9 and CXCL10, whereas M2 macrophages express non-inflammatory chemokines CCL17, CCL18, CCL22 and CCL24 [20]. Furthermore, M1 and M2 macrophage phenotypes also show distinct metabolic features relating to glucose, amino acid, lipid and iron metabolism [24]. Notably, several differences have been shown between mouse and human polarized macrophages, such as the repertoire of surface receptors and arginine metabolism [25].
The presence of TAMs and high serum levels of these cytokines represents poor prognostic factors in RCC patients [26]. Accordingly, Yanase et al. reported that treatment with IL-1β, TNF-α or IL-6 increases in vitro the invasiveness of RCC cells. These effects are inhibited in the presence of an anti-VCAM-1 monoclonal antibody (mAb) [27].
A retrospective analysis by Dannenmann and colleagues has shown that clear cell RCC can progressively attract macrophages and promote their skewing into immunosuppressive M2 TAMs. The analysis of TAM-related transcripts reveals that the M2 but not M1 phenotype is associated with reduced survival and advanced tumor stage [28].
Furthermore, Komohara et al. have investigated the role of an anti-inflammatory macrophage phenotype M2 in clear cell RCC patients using CD163 and CD204 as markers. The number of CD163(+) cells was significantly associated with age, sex, nuclear grade and TNM classification. In addition, in vitro direct co-culture of RCC cells with macrophages led to stronger activation of signal transducers and activators of transcription-3 (STAT3) in RCC cancer cells. Interestingly, STAT3 activation was suppressed by down-regulating the membrane-type macrophage colony-stimulating factor (mM-CSF), thus suggesting for a potential contribute of mM-CSF to cancer cell activation [4].
Macrophages are the major producers of TNF-α and interestingly are also highly responsive to TNF-α. Indeed, TNF-α induces the activation of the MAPK cascade in a c-Raf-1 and Raf-B-independent fashion [29]. In addition, low doses of TNF-α, produced by RCC cancer cells and stromal cells, promote tumor growth and metastasis [30, 31]. Notably, IL-4 inhibits TNF-α-induced proliferation of RCC [32]. In addition, Ho et al. have shown that TNF-α induces epithelial–mesenchymal transition (EMT) and promotes tumorigenicity of RCC by repressing E-cadherin, up-regulating vimentin, and enhancing MMP9 expression and invasion. TNF-α-mediated tumor promotion of RCC is associated with TNF-α-induced inhibition of glycogen synthase kinase-3β (GSK-3β) activity through serine-9 phosphorylation mediated by the phosphatidylinositol 3-kinase/protein kinase B (PI3 K/Akt) pathway [33].
Sarcomatoid RCC often has an aggressive clinical course characterized by rapid disease progression. The sarcomatoid conversion of clear cell RCC can be associated with the process of EMT. In this context, TGF-β1 seems to play a major role during the sarcomatoid transdifferentiation of clear cell RCC [34].
Moreover, Kominsky and colleagues have demonstrated that TGF-β promotes the establishment of RCC bone metastasis [35]. TGF-β1 stimulation of RCC bone metastasis cells resulted in the initiation of tumor-promoting paracrine interactions between tumor cells and the bone microenvironment, thus promoting tumor growth and subsequent osteolysis in vivo. In addition, an extensive cross-talk between the Notch and TGF-β signaling pathways in clear cell RCC that is associated with the aggressiveness of this disease has been reported [36].
IL-6 has been implicated in the osteoclastic bone resorption and hypercalcemia associated with metastatic RCC [37]. The results published by Fitzgerald et al. have shown that enhanced levels of IL-6 and IL-8 result in RCC cell invasion and that activation of AMP-activated protein kinase (AMPK) reduces the expression of the NADPH oxidase isoform Nox4, IL-6 and IL-8 production and RCC cell invasion [38]. Furthermore, IL-6-induced proliferation of RCC cells is mediated by increased DNA binding activity of STAT3 and, to a lesser extent, of STAT1 [39]. Recently, Porta et al. have reported that progression in RCC patients is preceded by a significant increase in pro-angiogenic cytokines other than VEGF, such as IL-6, bFGF and HGF [40].
Role of TAMs and associated molecules in RCC-related immune dysfunction
TAMs isolated from RCC tumors mediate their immunosuppressive activity by a number of mechanisms, including the secretion of inhibitory cytokines, such as IL-1β, TNF-α, TGF-β and IL-6 [41], the generation of reactive oxygen species and the promotion of Treg development. Furthermore, TAMs increase the production of IL-10 by T cells and enhance the expression of the co-inhibitory molecules programmed death 1 (PD-1) and T-cell immunoglobulin mucin 3 (TIM-3) [28].
Moreover, RCC TAMs secrete IL-10, and this release can be prevented by inhibition of lipoxygenase activity in accordance with their high levels of 15-lipoxygenase-2 (15-LOX2) expression [42]. Furthermore, RCC TAMs can induce the pivotal T regulatory cell transcription factor forkhead box P3 (FOXP3) and the inhibitory cytotoxic T-lymphocyte antigen 4 (CTLA-4) coreceptor in a 15-LOX2 independent manner [42]. Taken together, these data suggest that RCC TAMs contribute to RCC-related inflammation, immunosuppression and malignant progression by activating the 15-LOX2-dependent pathway.
Bone morphogenetic proteins (BMPs) are multi-functional growth factors that belong to the TGF-β superfamily. In the context of RCC, BMP-4, -6 and -7 are often overexpressed [43, 44], whereas BMP antagonist sclerostin domain containing 1 (SOSTDC1) is down-regulated [45]. BMP-6 has been shown to inhibit B- and T-cell proliferation [46, 47], and it regulates the proliferation and gene expression profile of macrophages [48, 49]. In addition, Lee et al. have reported that human RCC cells frequently have a loss of expression of BMP receptors [43], suggesting a paracrine role of BMP-6 in RCC [50]. BMP-6 mediates IL-10 expression in macrophages via Smad5 and STAT3, thus leading to M2 polarization of TAMs [50].
Role of macrophage autophagy in RCC
Autophagy is a catabolic process conserved in all eukaryotes that involves the delivery of unnecessary or dysfunctional cytoplasmatic elements to the lysosome or vacuole for definitive degradation and recycling [51, 52].
Autophagic process is activated in various pathological situations involving the immune system such as cancer, pathogen infections and autoimmune diseases [53]. By using human peripheral blood monocytes exposed to CSF-1 and consequently differentiated in M2-polarized macrophages, Jacquel and co-workers demonstrated that autophagy is triggered during macrophage differentiation. The stimulation of CSF-1 receptor induces characteristic autophagic features such as LC3-II increased expression and acidic vesicle accumulation [54]. They also demonstrated by inhibiting autophagy with the use of pharmacological inhibitors, siRNA approaches and using ATG7−/− mice as experimental model that autophagy is an essential process for a proper differentiation of monocytes into macrophages and for the acquisition of normal phagocytic functions.
Furthermore, it has been recently reported that sorafenib can suppress the activation of human macrophages by inducing autophagy. Sorafenib inhibits the surface antigen expression and the function of macrophages, and is accompanied by morphological changes characteristic of autophagy. Moreover, in this study, sorafenib was found to reduce macrophage secretion of IL-10, but not IL-6, TNF-α or TGF-β [55].
Role of TAMs in modulating RCC response to treatment
Histamine inhibits the formation and release of phagocyte-derived reactive oxygen species, and thereby protects NK and T cells against oxidative damage. Donskov et al. have investigated the potential role of histamine in improving the efficacy of IL-2 in metastatic RCC patients. Patients with high number of peripheral blood monocytes and neutrophils had very poor survival with either IL-2 alone or IL-2/histamine treatment. While the number of blood NK cells positively correlated with cytotoxicity, that of blood monocytes and neutrophils negatively correlated with cytotoxicity. Treatment with IL-2 alone resulted in a significantly higher number of circulating monocytes and intratumoral macrophages, while no changes as compared with baseline were observed in patients treated with IL-2/HDC [56].
TAMs produce large amounts of VEGF and MMP9 and may be responsible for the tumor angiogenic switch [14]. Of interest, TNF-α and MMP-9 have been proposed as potential baseline predictive serum markers for the outcome of metastatic RCC patients treated with first-line sunitinib. In this study, TNF-α and MMP-9 baseline levels were significantly increased in non-responders and significantly associated with reduced overall survival (OS) and time to progression, respectively [57].
Using an orthotopic model of RCC, Weiss et al. have observed that IL-2/α-CD40 induces IFN-γ- and NO-dependent decrease in matrix MMP expression and activity, concomitantly with increased levels of the tissue inhibitor of metalloproteinase (TIMP) 1 and E-cadherin expression within tumors. Treatment with the NO donor JS-K significantly reduces the metastatic spread. The reduced MMP9 activity implicates M1-polarized macrophages within the tumor microenvironment as critical components of therapeutic response [58].
Furthermore, TAM depletion enhances sorafenib-induced inhibition of tumor progression, angiogenesis and lung metastasis in a metastatic liver cancer mouse model [59]. Notably, sorafenib was also found to potentially reverse the immunosuppressive cytokine profile of TAMs, rendering the tumor microenvironment more conducive to an antitumor immune response [60]. Currently, no evidence is available on the effect of mTOR inhibitors on TAM polarization and activity in RCC.
Finally, an association of IL-6, IL-8, VEGF, osteopontin, E-selectin and HGF with continuous tumor shrinkage or PFS has been reported in RCC patients treated with pazopanib [61].
Relationship between RCC myeloid-derived suppressor cells and TAMs
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of cells that expand during cancer, inflammation and infection, and that have a remarkable ability to suppress T-cell responses and to promote angiogenesis [62]. Two main subsets are described and belong to granulocytic or monocytic lineages. MDSC originate in the bone marrow from common myeloid precursors (CMP) and often differentiate into CD11b+ Gr1med F4/80low/− IL-4Rα+ cells.
In the mouse, MDSCs and TAMs in the mouse share several characteristics, such as the expression of the monocyte and macrophage marker CD11b. Mounting evidence suggests that, upon entering tumor tissues, MDSCs can differentiate into TAMs. This process is mediated primarily by hypoxia via HIF-1α [63]. In addition, the differentiation of MDSCs into TAMs can lead to elevated IL-10 production, inhibition of T-cell responses and promotion of angiogenesis [64]. However, the mechanism behind regulation of MDSC differentiation remains unclear [9, 64, 65]. MDSCs can oscillate between M1 and M2 phenotypes depending on the stimulation they receive. In addition, with respect to the status of polarization, some differences exist between mouse and human MDSCs [66].
The presence of MDCSs has been reported in RCC-bearing patients and mice and can account for their impaired immune responses [67]. Both monocytic and granulocytic MDSCs have been described in murine tumor models, whereas granulocytic MDSCs are the prevalent population in the blood of RCC patients [67].
Treatment with sunitinib was reported to result in significant reduction in both monocytic and granulocytic spleen MDSCs in tumor-bearing mice. This inhibition correlated with reversal of suppression on type 1 T-cell-mediated IFN-γ production [62]. Similarly, in metastatic RCC patients, treatment with sunitinib results in MDSC reduction that is associated with reversal of regulatory T-cell increase and of type 1 T-cell suppression [68]. Of interest, the development of sunitinib resistance has been found to be partially mediated by the survival of MDSCs intratumorally leading to sustained immunosuppresion and angiogenesis [69].
TAMs and associated molecules as targets for RCC cancer therapy
The role of macrophages in tumor microenvironment and the observation that the presence of TAMs is associated with advanced tumor stages and poor prognosis in RCC lead to the option of targeting these cells therapeutically. Tyrosine kinase and mTOR inhibitors have been reported to influence host immune response [70]. Recent studies suggest that medications with proven clinical benefit exert part of their action through macrophage inhibition or depletion. Zoledronic acid combined with sorafenib or other pharmacological drugs such as bevacizumab, thalidomide and linomide has been shown to inhibit macrophage infiltration and to reduce or neutralize pro-angiogenic factors [59, 71–73].
Several promising strategies have been proposed to affect TAM functions: they include suppression of TAM recruitment, TAM depletion, switch of M2 to M1 antitumor phenotype and suppression of TAM-induced tumor angiogenesis. Table 1 summarizes these promising strategies.
Table 1.
Emerging TAM-centered strategies
| Strategies | Mechanism or targets | Effects on TAMs |
|---|---|---|
| CSF-1 antisense oligonucleotides [15] | CSF-1 blockade | Decrease accumulation |
| Carlumab (CNTO 888) [85] | Human anti-CCL2 IgG1κ mAb | Decrease TAM accumulation |
| Bindarit [87] | Selective inhibitor of CCL2, CCL7 and CCL8 | Decrease TAM accumulation |
| Recombinant type I IFN-α [89] | CSF-1 blockade | Suppress TAM generation and accumulation |
| Trabectedin [90] | Exhibits cytotoxic activity against TAMs and reduces the production of CCL2 and IL-6 | Selectively eradicate TAMs |
| Toll-like receptor 9 ligand CpG and anti-IL-10 antibody [93] | Revert tumor-induced immunosuppression | M2-to-M1 phenotype switch |
| HRG [94] | Down-regulates PDGF | M2-to-M1 phenotype switch |
| CT-011 [32] | Anti-PD-1 mAb | Restore antitumor immune response |
| IFN-γ, IL-4, IL-6, or TNF-α genes [95] | Utilize TAMs as gene delivery vector | Enhance antitumor activity |
| Silibinin [96] | Suppress NF-κB and STAT3 phosphorylation | Suppress TAM-induced tumor angiogenesis |
| WP1066 [97] | STAT3 inhibitor | Suppress the expression of Bcl-2, induces apoptosis, and inhibits VEGF secretion |
| Infliximab [98] | Anti-TNF-α mAb | Suppress TNF-α-induced effects |
| Legumain-based DNA minigene vaccine [101] | Reduce TAM density | Suppress TAMs-induced tumor angiogenesis |
| LCL-PLP [102] | Reduce of TAMs mediated production of pro-angiogenic factors | Suppress TAM accumulation and related tumor angiogenesis |
CCL2 CSF-1 colony-stimulating factor-1, HRG histidine-rich glycoprotein, IFN interferon, IL-4 interleukin-4, IL-6 interleukin-6, IL-10 interleukin-10, LCL–PLP prednisolone phosphate (PLP) encapsulated in long-circulating liposomes (LCLs) (LCL-PLP), mAb monoclonal antibody, PD-1 programmed death-1, PDGF platelet-derived growth factor, STAT3 signal transducers and activators of transcription-3, TAM tumor-associated macrophage, TNF-α tumor necrosis factor-α, VEGF vascular endothelial growth factor
Decrease in TAM recruitment and accumulation
The generation of TAMs is positively regulated by several chemotactic cytokines, such as CSF-1 and CCL2. CSF-1 and its receptor CSF-1R contribute to monocyte recruitment and induction of macrophage differentiation [8], and their co-expression has been associated with RCC tumor growth [74]. Aharinejad et al. [75] have shown that CSF-1 blockade by antisense oligonucleotides suppresses tumor growth in mice xenografted with CSF-1 receptor (c-fms)-positive or CSF-1-negative human malignant embryonic or colon cancer cells. These data suggest that CSF-1 blockade could be tested in treatment for RCC patients.
TAMs isolated from human RCC produce substantial amounts of the pro-inflammatory chemokine CCL2 [42]. Daurkin et al. have shown that 15-LOX2 is involved in the regulation of CCL2 production, and may potentially represent a valuable strategy to limit the effects of CCL2 and to attenuate TAM-induced immunosuppression.
Recently, a human anti-CCL2 IgG1κ mAb, carlumab (CNTO 888), has been demonstrated to be well tolerated with evidence of transient-free CCL2 suppression and antitumor activity in a phase I study of 44 patients with solid tumors [76].
TWIST1 is a basic helix-loop-helix transcription factor expressed in newly formed mesenchymal cells. Low-Marchelli et al. reported that TWIST1 promotes angiogenesis and tumor progression without increasing the secretion of VEGF but rather inducing the expression of the macrophage chemoattractant CCL2. Indeed, the inhibition of endogenous TWIST1 in vivo blocks macrophage recruitment and angiogenesis [77].
Bindarit is an original compound with anti-inflammatory activity due to selective inhibition of monocyte chemotactic proteins CCL2, CCL7 and CCL8 [78]. In syngeneic Balb/c mice injected under the mammary gland with murine breast cancer cells (4T1-Luc cells), bindarit treatment significantly decreases the infiltration of TAMs and MDSCs [79]. Presently, the efficacy and safety of bindarit has not been investigated in RCC patients.
Furthermore, U’Ren and colleagues have revealed that type I IFNs generated in tumors inhibit the macrophage stimulatory effects of CSF-1 and suppress the generation of TAMs [80].
Drug-mediated inhibition of TAMs
The monocytes/macrophages-selective cytotoxicity of antitumor agents represents an emerging focus in cancer research. Germano et al. have demonstrated that macrophage depletion is essential for the antitumor activity of trabectedin, a licensed and commercially available anticancer agent. They found that trabectedin is selectively cytotoxic for TAMs in four different mouse tumor models [81]. Furthermore, trabectedin impairs the production of cancer-derived CCL2 and IL-6 from cancer cells to further decrease TAM recruitment [81].
Reversal of TAM-related immunosuppression
The potential to “re-educate” TAMs may be an effective and novel therapeutic approach for cancer. Several studies have been performed testing the possibility to switch M2 TAMs to the antitumor M1 phenotype. The transcription factor NF-κB plays a crucial role in the activation of TAMs. Thus, the activation of macrophages in response to M1 stimuli, such as TLR ligands, TNF-α or IL-1β, is regulated primarily by NF-κB [22]. Moreover, the transcription of several tumor-promoting genes, such as VEGF, IL-6, TNF-α and COX2, is partly regulated by NF-κB [82]. In this regard, Hagemann et al. [83] have observed that the inhibition of IκB kinase (IKK)β, the major activator of NF-κB, reversed TAM tumor-promoting activity and promoted the switch to M1 phenotype.
Furthermore, the combined use of Toll-like receptor 9 ligand CpG and anti-IL-10 Ab has been shown to induce the switch from M2 to M1 phenotype, and this is associated with an increase in cytotoxic function [84]. Additionally, Rolny et al. [15] have reported that treatment with host-generated histidine-rich glycoprotein (HRG) results in the down-regulation of PDGF and contributes to redirect M2 TAMs into M1 phenotype.
Concerning PD-1, it is a member of the CD28 family of receptors that includes CD28, cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), inducible costimulator (ICOS), and B and T-lymphocyte attenuator. The evidence that TAMs induce the skewing of autologous, blood-derived CD4+ T cells toward a more immunosuppressive phenotype as shown by increased PD-1 expression [28], provides the rationale for targeting this pathway in RCC patients. In this regard, a phase II study is ongoing to evaluate CT-011 a humanized anti-PD-1 mAb, alone or in combination with DC/RCC fusion vaccine in RCC patients (NCT01441765). Moreover, the anti-PD-1 mAb BMS-936558 is under evaluation in a phase I study (NCT01472081) in combination with sunitinib, pazopanib, or ipilimumab and anti-CTLA-4 mAb, and in comparison with everolimus in metastatic clear cell RCC patients who have received prior anti-angiogenic therapy (NCT01668784).
Role of TAMs as functional vehicles
The use of macrophages as vehicles to deliver gene therapy in regions of tumor hypoxia is a promising approach for cancer therapy. In this regard, Nishihara and co-workers have used retroviral vectors to engineer a macrophage cell line to express IFN-γ, IL-4, IL-6 or TNF-α. They have shown increased doubling times and in vitro and in vivo tumoricidal activity by transfected macrophages against the TNF-sensitive fibrosarcoma line WEHI 164 and the TNF-alpha-resistant cell lines B16 melanoma and C1300 neuroblastoma [85].
Suppression of TAM-induced tumor angiogenesis
Angiogenic cytokines released by TAMs regulate angiogenesis by activating NF-κB and STAT3 transcription factors. As mentioned above, STAT3 is involved in the activation of RCC cancer cells mediated by macrophages. Thus, blockade of STAT3 signaling pathways may be considered to be potentially useful as a novel therapeutic approach for RCC. Horiguchi et al. evaluated the in vitro and in vivo efficacy of STAT3 inhibitor WP1066 in RCC cell lines and on murine xenografts. They found that WP1066 suppresses the in vitro expression of Bcl-2, induces apoptosis and inhibits the basal and hypoxia-induced expression of HIF1alpha and HIF2alpha, as well as VEGF secretion. The pathological analysis of xenografts of WP1066-treated mice showed decreased immunostaining of phosphorylated STAT3 and reduced length of CD34-positive vessels [86]. At present, the STAT 3 inhibitors ISIS 481464 (NCT01563302) and OPB-31121 (NCT00955812) are under evaluation in patients with advanced solid tumors including RCC. Similarly, silibinin, a natural polyphenolic flavonoid, has been demonstrated to suppress NF-κB p65 and STAT3 ser727 phosphorylation and to increase the expression of the endogenous angiogenesis inhibitors Ang-2 and ang-receptor tyrosine kinase (Tie-2) [87].
As mentioned above, TNF-α can be associated with in vitro invasiveness of RCC cells [27]. Maisey et al. [88] have evaluated the efficacy of anti-TNF-α mAb infliximab in patients with previously treated advanced RCC and have observed a response rate of 16 % with a further 16 % of patients with stable disease. In 2010, Larkin et al. led a phase I/II trial of sorafenib and infliximab in advanced RCC patients, without registering clinical benefits as compared to sorafenib alone. This combination was also characterized by increased toxicity, with 75 % of the patients requiring at least one dose reduction and 81 % requiring at least one dose delay of sorafenib [89]. Recently, the rLj-RGD3, a recombinant RGD (Arg-Gly-Asp)-toxin protein has been shown to inhibit the TNF-α-induced MMP-9 secretion, proliferation, migration and invasion of human RCC cells [90].
Legumain is a member of the asparaginyl endopeptidase family and is overexpressed in TAMs [101]. In 2008, Lewēn and his colleagues constructed a legumain-based DNA minigene vaccine that induced a specific CD8+ T-cell response against Legumain+ TAMs and reduced tumor angiogenesis in a breast tumor model [91].
The antitumor activity of prednisolone phosphate (PLP) encapsulated in long-circulating liposomes (LCLs) (LCL–PLP) has been also evaluated. LCL–PLP is likely primarily caused by its suppressive effect on the TAM-mediated production of pro-angiogenic factors in tumors. Moreover, LCL–PLP strongly reduced the production of GM-CSF, M-CSF, G-CSF and MCP1, thus affecting TAM functions and recruitment into tumor tissues [92].
Discussion
Until a few years ago, cytokines, particularly IL-2 and IFN-α were the only available therapeutic options with promising antitumor activity in advanced RCC [93–96]. Unfortunately, patients suffered from acute toxicity, and the complete response rate was low [97, 98].
The introduction in clinical practice of several targeted agents has dramatically change the therapeutic scenario in RCC, improving the prognosis and greatly increasing the therapeutic options [99–107]. Nevertheless, tumors often develop resistance to these drugs. To date, we are unable to early select the patients who will benefit most from the treatment, lacking predictive biomarkers of response.
Macrophages are an essential component of the host defense system and have critical roles in both innate and adaptive immune responses [108]. TAM undergo a wide spectrum of polarized activation states and have the potential both to elicit tumor and tissue destructive reactions and to promote cancer progression and metastasis by stimulating angiogenesis, tumor growth, migration and invasion in RCC. Based on these data, several TAM-centered strategies have been proposed to target these cells. Reducing the numbers and eliminating TAMs are alternatives to their re-education. However, these approaches have not been compared yet in terms of efficacy and safety for RCC patients.
Identification of the pathways responsible for the skewing of TAM functions provides the rational for macrophage-targeted therapies complementary to cytoreductive approaches,and for exploiting the prognostic role of TAMs.
In spite of the notion that TAM frequency is associated with poor prognosis in many human tumors [109], there are notable exceptions, such as in colorectal cancer [110]. The reasons of this divergence remain still unclear.
Thus, TAMs may represent a promising and effective target for RCC cancer therapy. A better dissection of the functional diversity of TAMs and further knowledge of the exact molecular mechanism of TAM-induced angiogenesis and metastasis, and of the interaction between TAMs and RCC microenvironment, may open the way to innovative therapeutic strategies for RCC patients.
Acknowledgments
Many thanks to Professor Alberto Mantovani, Scientific Director of Istituto Clinico Humanitas, Milan, Italy, for his collaboration during the preparation of this manuscript. Particular thanks to Francesca Tartari for her precious help in drafting the manuscript.
Conflict of interest
We declare to have no conflict of interest.
References
- 1.Oliver RT, Nethersell AB, Bottomley JM. Unexplained spontaneous regression and alpha-interferon as treatment for metastatic renal carcinoma. Br J Urol. 1989;63:128–131. doi: 10.1111/j.1464-410x.1989.tb05147.x. [DOI] [PubMed] [Google Scholar]
- 2.Vogelzang NJ, Priest ER, Borden L. Spontaneous regression of histologically proved pulmonary metastases from renal cell carcinoma: a case with 5-year follow-up. J Urol. 1992;148:1247–1248. doi: 10.1016/s0022-5347(17)36874-x. [DOI] [PubMed] [Google Scholar]
- 3.Alexander RB, Fitzgerald EB, Mixon A, Carter CS, Jakobsen M, Cohen PA, et al. Helper T cells infiltrating human renal cell carcinomas have the phenotype of activated memory-like T lymphocytes. J Immunother Emphas Tumor Immunol. 1995;17:39–46. doi: 10.1097/00002371-199501000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Komohara Y, Hasita H, Ohnishi K, Fujiwara Y, Suzu S, Eto M, et al. Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci. 2011;102:1424–1431. doi: 10.1111/j.1349-7006.2011.01945.x. [DOI] [PubMed] [Google Scholar]
- 5.Noessner E, Brech D, Mendler AN, Masouris I, Schlenker R, Prinz PU. Intratumoral alterations of dendritic-cell differentiation and CD8+ T-cell anergy are immune escape mechanisms of clear cell renal cell carcinoma. Oncoimmunology. 2012;1:1451–1453. doi: 10.4161/onci.21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figel AM, Brech D, Prinz PU, Lettenmeyer UK, Eckl J, Turqueti-Neves A, et al. Human renal cell carcinoma induces a dendritic cell subset that uses T-cell crosstalk for tumor-permissive milieu alterations. Am J Pathol. 2011;129:436–451. doi: 10.1016/j.ajpath.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemmerlein B, Markus A, Wehner M, Kugler A, Zschunke F, Radzum HJ. Expression of acute and late-stage inflammatory antigens, c-fms, CSF-1, and human monocytic serine esterase 1, in tumor-associated macrophages of renal cell carcinomas. Cancer Immunol Immunother. 2000;49:485–492. doi: 10.1007/s002620000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 10.Toge H, Inagaki T, Kojimoto Y, Shinka T, Hara I. Angiogenesis in renal cell carcinoma: the role of tumor-associated macrophages. Int J Urol. 2009;16:801–807. doi: 10.1111/j.1442-2042.2009.02377.x. [DOI] [PubMed] [Google Scholar]
- 11.Hemmerlein B, Scherbening J, Kugler A, Radzun HJ. Expression of VCAM-1, ICAM-1, E- and P-selectin and tumour-associated macrophages in renal cell carcinoma. Histopathology. 2000;37:78–83. doi: 10.1046/j.1365-2559.2000.00933.x. [DOI] [PubMed] [Google Scholar]
- 12.Ruffel B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C, Liu B, Dai Z, Tao Y. Knockdown of VEGF receptor-1 (VEGFR-1) impairs macrophage infiltration, angiogenesis and growth of clear cell renal cell carcinoma. Cancer Biol Ther. 2011;12:872–880. doi: 10.4161/cbt.12.10.17672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PIGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, et al. Anti-PIGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 17.Bedke J, Hemmerlein B, Perske C, Gross A, Heuser M. Tumor-associated macrophages in clear cell renal cell carcinoma express both gastrin-releasing peptide and its receptor: a possible modulatory role of immune effectors cells. World J Urol. 2010;28:335–341. doi: 10.1007/s00345-009-0492-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol. 2013 doi: 10.1007/s00281-013-0367-7. [DOI] [PubMed] [Google Scholar]
- 21.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2007;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 24.Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell Metab. 2012;15:432–437. doi: 10.1016/j.cmet.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida N, Ikemoto S, Narita K, Sugimura K, Wada S, Yasumoto R, et al. Interleukin-6, tumour necrosis factor alpha and interleukin-1beta in patients with renal cell carcinoma. Br J Cancer. 2002;86:1396–1400. doi: 10.1038/sj.bjc.6600257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yanase M, Tsukamoto T, Kumamoto Y. Cytokines modulate in vitro invasiveness of renal cell carcinoma cells through action on the process of cell attachment to endothelial cells. J Urol. 1995;153:844–848. [PubMed] [Google Scholar]
- 28.Dannenmann SR, Thielicke J, Stöckli M, Matter C, von Boehmer L, Cecconi V, et al. Tumor-associated macrophages subvert T-cell function and correlate with reduced survival in clear cell renal cell carcinoma. Oncoimmunology. 2013;2:e23562. doi: 10.4161/onci.23562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riches DW, Chan ED, Winston BW. TNF-alpha-induced regulation and signalling in macrophages. Immunobiology. 1996;195:477–490. doi: 10.1016/s0171-2985(96)80017-9. [DOI] [PubMed] [Google Scholar]
- 30.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25:409–416. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 31.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 32.Falkensammer C, Jöhrer K, Gander H, Ramoner R, Putz T, Rahm A, et al. IL-4 inhibits the TNF-alpha induced proliferation of renal cell carcinoma (RCC) and cooperates with TNF-alpha to induce apoptotic and cytokine responses by RCC: implications for antitumor immune responses. Cancer Immunol Immunother. 2006;55:1228–1237. doi: 10.1007/s00262-006-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho MY, Tang SJ, Chuang MJ, Cha TL, Li JY, Sun GH, et al. TNF-α induces epithelial-mesenchymal transition of renal cell carcinoma cells via a GSK3β-dependent mechanism. Mol Cancer Res. 2012;10:1109–1119. doi: 10.1158/1541-7786.MCR-12-0160. [DOI] [PubMed] [Google Scholar]
- 34.Boström AK, Möller C, Nilsson E, Elfving P, Axelson H, Johansson ME. Sarcomatoid conversion of clear cell renal cell carcinoma in relation to epithelial-to-mesenchymal transition. Hum Pathol. 2012;43:708–719. doi: 10.1016/j.humpath.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Kominsky SL, Doucet M, Brady K, Weber KL. TGF-beta promotes the establishment of renal cell carcinoma bone metastasis. J Bone Miner Res. 2007;22:37–44. doi: 10.1359/jbmr.061005. [DOI] [PubMed] [Google Scholar]
- 36.Sjölund J, Boström AK, Lindgren D, Manna S, Moustakas A, Ljungberg B, et al. The notch and TGF-β signaling pathways contribute to the aggressiveness of clear cell renal cell carcinoma. PLoS One. 2011;6:e23057. doi: 10.1371/journal.pone.0023057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paule B. Interleukin-6 and bone metastasis of renal cancer: molecular bases and therapeutic implications. Prog Urol. 2001;11:368–375. [PubMed] [Google Scholar]
- 38.Fitzgerald JP, Nayak B, Shanmugasundaram K, Friedrichs W, Sudarshan S, Eid AA, et al. Nox4 mediates renal cell carcinoma cell invasion through hypoxia-induced interleukin 6- and 8- production. PLoS One. 2012;7:e30712. doi: 10.1371/journal.pone.0030712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horiguchi A, Oya M, Marumo K, Murai M. STAT3, but not ERKs, mediates the IL-6-induced proliferation of renal cancer cells, ACHN and 769P. Kidney Int. 2002;61:926–938. doi: 10.1046/j.1523-1755.2002.00206.x. [DOI] [PubMed] [Google Scholar]
- 40.Porta C, Paglino C, Imarisio I, Ganini C, Sacchi L, Quaglini S, et al. Changes in circulating pro-angiogenic cytokines, other than VEGF, before progression to sunitinib therapy in advanced renal cell carcinoma patients. Oncology. 2013;84:115–122. doi: 10.1159/000342099. [DOI] [PubMed] [Google Scholar]
- 41.Ikemoto S, Yoshida N, Narita K, Wada S, Kishimoto T, Sugimura K, et al. Role of tumor-associated macrophages in renal cell carcinoma. Oncol Rep. 2003;10:1843–1849. [PubMed] [Google Scholar]
- 42.Daurkin I, Eruslanov E, Stoffs T, Perrin GQ, Algood C, Gilbert SM, et al. Tumor-associated macrophages mediate immunosuppression in the renal cancer microenvironment by activating the 15-lipoxygenase-2 pathway. Cancer Res. 2011;71:6400–6409. doi: 10.1158/0008-5472.CAN-11-1261. [DOI] [PubMed] [Google Scholar]
- 43.Kim IY, Lee DH, Lee DK, Kim BC, Kim HT, Leach FS, et al. Decreased expression of bone morphogenetic protein (BMP) receptor type II correlates with insensitivity to BMP-6 in human renal cell carcinoma cells. Clin Cancer Res. 2003;9:6046–6051. [PubMed] [Google Scholar]
- 44.Kwak C, Park YH, Kim IY, Moon KC, Ku JH. Expression of bone morphogenetic proteins, the subfamily of the transforming growth factor-beta superfamily, in renal cell carcinoma. J Urol. 2007;178:1062–1107. doi: 10.1016/j.juro.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Blish KR, Wang W, Willingham MC, Du W, Birse CE, Krishnan SR, et al. A human bone morphogenetic protein antagonist is down-regulated in renal cancer. Mol Biol Cell. 2008;19:457–464. doi: 10.1091/mbc.E07-05-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sivertsen EA, Huse K, Hystad ME, Kersten C, Smeland EB, Myklebust JH. Inhibitory effects and target genes of bone morphogenetic protein 6 in Jurkat TAg cells. Eur J Immunol. 2007;37:2937–2948. doi: 10.1002/eji.200636759. [DOI] [PubMed] [Google Scholar]
- 47.Kersten C, Sivertsen EA, Hystad ME, Forfang L, Smeland EB, Myklebust JH. BMP-6 inhibits growth of mature human B cells; induction of Smad phosphorylation and upregulation of Id1. BMC Immunol. 2005;6:9. doi: 10.1186/1471-2172-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong JH, Lee GT, Lee JH, Kwon SJ, Park SH, Kim SJ, et al. Effect of bone morphogenetic protein-6 on macrophages. Immunology. 2009;128:e442–e450. doi: 10.1111/j.1365-2567.2008.02998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon SJ, Lee GT, Lee JH, Kim WJ, Kim IY. Bone morphogenetic protein-6 induces the expression of inducible nitric oxide synthase in macrophages. Immunology. 2009;128:e758–e765. doi: 10.1111/j.1365-2567.2009.03079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JH, Lee GT, Woo SH, Ha YS, Kwon SJ, Kim WJ, et al. BMP-6 in Renal Cell Carcinoma Promotes Tumor Proliferation through IL-10-Dependent M2 Polarization of Tumor-Associated Macrophages. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-12-4563. [DOI] [PubMed] [Google Scholar]
- 51.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signalling regulation. Curr Opin Cell Biol. 2010;22:124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y, Klionsky DJ. The regulation of autophagy—unanswered questions. J Cell Science. 2011;124:161–170. doi: 10.1242/jcs.064576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacquel A, Obba S, Solary E, Auberger P. Proper macrophagic differentiation requires both autophagy and caspase activation. Autophagy. 2012;8:1141–1143. doi: 10.4161/auto.20367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin JC, Liu CL, Lee JJ, Liu TP, Ko WC, Huang YC, et al. Sorafenib induces autophagy and suppresses activation of human macrophage. Int Immunopharmacol. 2013;15:333–339. doi: 10.1016/j.intimp.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donskov F, Hokland M, Marcussen N, Torp Madsen HH, von der Maase H. Monocytes and neutrophils as ‘bad guys’ for the outcome of interleukin-2 with and without histamine in metastatic renal cell carcinoma–results from a randomised phase II trial. Br J Cancer. 2006;94:218–226. doi: 10.1038/sj.bjc.6602937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perez-Gracia JL, Prior C, Guillén-Grima F, Segura V, Gonzalez A, Panizo A, et al. A. Identification of TNF-alpha and MMP-9 as potential baseline predictive serum markers of sunitinib activity in patients with renal cell carcinoma using a human cytokine array. Br J Cancer. 2009;101:1876–1883. doi: 10.1038/sj.bjc.6605409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiss JM, Ridnour LA, Back T, Hussain SP, He P, Maciag AE, et al. Macrophage-dependent nitric oxide expression regulates tumor cell detachment and metastasis after IL-2/anti-CD40 immunotherapy. J Exp Med. 2010;207:2455–2467. doi: 10.1084/jem.20100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang PY, Xu HX, et al. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16:3420–3430. doi: 10.1158/1078-0432.CCR-09-2904. [DOI] [PubMed] [Google Scholar]
- 60.Edwards JP, Emens LA. The multikinase inhibitor sorafenib reverses the suppression of IL-12 and enhancement of IL-10 by PGE2 in murine macrophages. Int Immunopharmacol. 2010;10:1220–1228. doi: 10.1016/j.intimp.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tran HT, Liu Y, Zurita AJ, Lin Y, Baker-Neblett KL, Martin AM, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol. 2012;13:827–837. doi: 10.1016/S1470-2045(12)70241-3. [DOI] [PubMed] [Google Scholar]
- 62.Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD, et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70:3526–3536. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, et al. Expansion of myeloid immune suppressor Gr+ CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 65.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 66.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Najjar YG, Finke JH. Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front Oncol. 2013;3:49. doi: 10.3389/fonc.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 69.Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol. 2011;11:856–861. doi: 10.1016/j.intimp.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santoni M, Rizzo M, Burattini L, Farfariello V, Berardi R, Santoni G, et al. Recent Pat Antiinfect Drug Discov. 2012;7:104–110. doi: 10.2174/157489112801619719. [DOI] [PubMed] [Google Scholar]
- 71.Rogers TL, Holen I. Tumor macrophages as potential target of bisphosphonates. J Transl Med. 2011;9:177. doi: 10.1186/1479-5876-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vukanovic J, Isaacs JT. Linomide inhibits angiogenesis, growth, metastasis, and macrophage infiltration within rat prostatic cancers. Cancer Res. 1995;55:1499–1504. [PubMed] [Google Scholar]
- 73.Dineen SP, Lynn KD, Holloway SE, Miller AF, Sullivan JP, Shames DS, et al. Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res. 2008;68:4340–4346. doi: 10.1158/0008-5472.CAN-07-6705. [DOI] [PubMed] [Google Scholar]
- 74.Menke J, Kriegsmann J, Schimanski CC, Schwartz MM, Schwarting A, Kelley VR. Autocrine CSF-1 and CSF-1 receptor coexpression promotes renal cell carcinoma growth. Cancer Res. 2012;72:187–200. doi: 10.1158/0008-5472.CAN-11-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aharinejad S, Abraham D, Paulus P, Abri H, Hofmann M, Grossschmidt K, et al. Colony-stimulating factor-1 antisense treatment suppresses growth of human tumor xenografts in mice. Cancer Res. 2002;62:5317–5324. [PubMed] [Google Scholar]
- 76.Ping YF, Yao XH, Jiang JY, Zhao LT, Yu SC, Jiang T, et al. The chemokine CXCL12 and its receptor CXCR4 promote glioma stem cell-mediated VEGF production and tumour angiogenesis via PI3K/AKT signalling. J Pathol. 2011;224:344–354. doi: 10.1002/path.2908. [DOI] [PubMed] [Google Scholar]
- 77.Bajetto A, Barbieri F, Dorcaratto A, Barbero S, Daga A, Porcile C, et al. Expression of CXC chemokine receptors 1-5 and their ligands in human glioma tissues: role of CXCR4 and SDF1 in glioma cell proliferation and migration. Neurochem Int. 2006;49:423–432. doi: 10.1016/j.neuint.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 78.Cioli V, Ciarniello MG, Guglielmotti A, Luparini MR, Durando L, Martinelli B, et al. A new protein antidenaturant agent, bindarit, reduces secondary phase of adjuvant arthritis in rats. J Rheumatol. 1992;19:1735–1742. [PubMed] [Google Scholar]
- 79.Zollo M, Di Dato V, Spano D, De Martino D, Liguori L, Marino N, et al. Targeting monocyte chemotactic protein-1 synthesis with bindarit induces tumor regression in prostate and breast cancer animal models. Clin Exp Metastasis. 2012;29:585–601. doi: 10.1007/s10585-012-9473-5. [DOI] [PubMed] [Google Scholar]
- 80.U’Ren L, Guth A, Kamstock D, Dow S. Type I interferons inhibit the generation of tumor-associated macrophages. Cancer Immunol Immunother. 2010;59:587–598. doi: 10.1007/s00262-009-0776-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23:249–262. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 82.Hagemann T, Biswas SK, Lawrence T, Sica A, Lewis CE. Regulation of macrophage function in tumors: the multifaceted role of NF-κB. Blood. 2009;113:3139–3146. doi: 10.1182/blood-2008-12-172825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, et al. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 85.Nishihara K, Barth RF, Wilkie N, Lang JC, Oda Y, Kikuchi H, et al. Increased in vitro and in vivo tumoricidal activity of a macrophage cell line genetically engineered to express IFN-gamma, IL-4, IL-6, or TNF-alpha. Cancer Gene Ther. 1995;2:113–124. [PubMed] [Google Scholar]
- 86.Horiguchi A, Asano T, Kuroda K, Sato A, Asakuma J, Ito K, et al. STAT3 inhibitor WP1066 as a novel therapeutic agent for renal cell carcinoma. Br J Cancer. 2010;102:1592–1599. doi: 10.1038/sj.bjc.6605691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tyagi A, Singh RP, Ramasamy K, Raina K, Redente EF, Dwyer-Nield LD, et al. Growth inhibition and regression of lung tumors by silibinin: modulation of angiogenesis by macrophage-associated cytokines, and NF-κB and STAT3. Cancer Prev Res. 2009;2:74–83. doi: 10.1158/1940-6207.CAPR-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maisey N. Antitumor necrosis factor (TNF-a) antibodies in the treatment of renal cell cancer. Cancer Invest. 2007;25:589–593. doi: 10.1080/07357900701359700. [DOI] [PubMed] [Google Scholar]
- 89.Larkin JM, Ferguson TR, Pickering LM, Edmonds K, James MG, Thomas K, et al. A phase I/II trial of sorafenib and infliximab in advanced renal cell carcinoma. Br J Cancer. 2010;103:1149–1153. doi: 10.1038/sj.bjc.6605889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jin M, Xiao R, Wang J, Liu X, Liu Y, Xue Z, et al. Low concentrations of the recombinant toxin protein rLj-RGD3 suppress TNF-α-induced human renal carcinoma cell invasion. Acta Biochim Biophys Sin (Shanghai) 2013;45:377–382. doi: 10.1093/abbs/gmt015. [DOI] [PubMed] [Google Scholar]
- 91.Lewēn S, Zhou H, Hu HD, Cheng T, Markowitz D, Reisfeld RA, et al. Legumain-based minigene vaccine targets the tumor stroma and suppresses breast cancer growth and angiogenesis. Cancer Immunol Immunother. 2008;57:507–515. doi: 10.1007/s00262-007-0389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Banciu M, Schiffelers RM, Fens MH, Metselaar JM, Storm G. Anti-angiogenic effects of liposomal prednisolone phosphate on B16 melanoma in mice. J Control Release. 2006;113:1–8. doi: 10.1016/j.jconrel.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 93.Medical Research Council Renal Cancer Collaborators Interferon-alpha and survival in metastatic renal carcinoma: early results of randomised controlled trial. Lancet. 1999;353:14–17. [PubMed] [Google Scholar]
- 94.Rosenberg SA, Lotze MT, Yang JC, Topalian SL, Chang AE, Schwartzentruber DJ, et al. Prospective randomized trial of high-dose interleukin 2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J Natl Cancer Inst. 1993;85:622–632. doi: 10.1093/jnci/85.8.622. [DOI] [PubMed] [Google Scholar]
- 95.Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 96.McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 97.Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shablak A, Sikand K, Shanks JH, Thistlethwaite F, Spencer-Shaw A, Hawkins RE. High-dose interleukin-2 can produce a high rate of response and durable remissions in appropriately selected patients with metastatic renal cancer. J Immunother. 2011;34:107–112. doi: 10.1097/CJI.0b013e3181fb659f. [DOI] [PubMed] [Google Scholar]
- 99.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 100.Motzer RJ, Hutson TE, Tomczak P, Michaelson D, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 101.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 102.Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomized, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 103.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind randomised placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 104.Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou SS, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 106.Rini BI, Escudier B, Tomczack P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 107.Motzer RJ, Nosov D, Eisen T, Bondarenko I, Lesovoy V, Lipatov O, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: results from a phase III randomized, open-label, multicenter trial. J Clin Oncol. 2012;30:4501. doi: 10.1200/JCO.2012.47.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 109.Bingle L, Brown NJ, Lewis CE. The role of tumourassociated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 110.Edin S, Wikberg ML, Dahlin AM, Rutegård J, Öberg Å, Oldenborg PA, Palmqvist R. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One. 2012;7:e47045. doi: 10.1371/journal.pone.0047045. [DOI] [PMC free article] [PubMed] [Google Scholar]