Abstract
Hashimoto thyroiditis (HT) is the most frequent thyroid autoimmune disease, while papillary thyroid cancer (PTC) is one of the most common endocrine malignancies. A few patients with HT also develop PTC. The aim of this study was to analyze cytokine profiles in patients with PTC accompanied with autoimmune HT in comparison with those in patients with PTC alone or HT alone and healthy subjects. Cytokine levels were determined in supernatants obtained from phytohemagglutinin (PHA)-stimulated whole blood cultures in vitro. The concentrations of selected cytokines: Th1—interferon gamma (IFN-γ); Th2—interleukin 4 (IL-4), interleukin 5 (IL-5), interleukin 6 (IL-6), interleukin 10 (IL-10) and interleukin 13 (IL-13); Th9—interleukin 9 (IL-9); and Th17—interleukin 17 (IL-17A) were measured using multiplex cytokine detection systems for human Th1/Th2/Th9/Th17/Th22. We found that PTC patients with HT produced significantly higher concentrations of IL-4, IL-6, IL-9, IL-13 and IFN-γ than PTC patients without HT. In conclusion, autoimmune HT affects the cytokine profile of patients with PTC by stimulating secretion of Th1/Th2/Th9 types of cytokines. Th1/Th2 cytokine ratios in PTC patients with associated autoimmune HT indicate a marked shift toward Th2 immunity.
Keywords: Cytokines, Blood cells, Papillary thyroid cancer, Hashimoto thyroiditis
Introduction
As the most common autoimmune thyroid disease, Hashimoto thyroiditis (HT) is characterized by massive infiltration of the thyroid gland by immune cells, leading to destruction of thyroid follicles and hypothyroidism [1]. The majority of patients with HT have autoantibodies to thyroid antigens: thyroglobulin (TgAbs) and thyroperoxidase (TPOAbs). Low-grade chronic inflammation and an imbalance between pro- and anti-inflammatory cytokines have been proposed to play roles in the pathogenesis of HT. A prevalent T-helper-1 (Th1) and T-helper-17 (Th17) [2, 3] type of immune response has been described in patients with HT, while protective roles have been shown for cytokines produced by T-helper-2 (Th2) and regulatory T cells [4, 5].
Papillary thyroid cancer (PTC) is a common endocrine malignancy, with increasing incidence during the past decades [6]. This well-differentiated cancer derives from thyroid follicular cells and has a very slow growth rate. Although it metastasizes at a relatively high frequency, the prognosis is favorable [7]. Mutual interactions between PTC and immune cells are insufficiently investigated. Polymorphism of genes for some cytokines in patients with PTC has been examined [8, 9] and a possible role for them in thyroid cancerogenesis indicated. Recently, we showed that PHA-stimulated whole blood cells of patients with differentiated thyroid cancer produce significantly higher amounts of Th2/Th9 cytokines than controls [10]. Moreover, Stassi et al. [11] recorded autocrine production of Th2-type cytokines from thyroid cancer cells.
Although many studies demonstrated co-occurrence of HT and PTC, the association between these thyroid conditions remains controversial (reviewed in refs [12–14]). In population-based studies, the average prevalence of PTC in patients with HT was 1.2 %, while in a surgical series using thyroidectomy specimens, the average prevalence was 27.6 % [12]. Likewise, data in the literature on the impact of Hashimoto thyroiditis on the clinical course of thyroid cancer are inconsistent. While several studies suggest that patients with PTC and associated HT have favorable clinicopathologic characteristics and a higher survival rate [15–18], the results of other studies indicate that HT is not an independent prognostic factor [19, 20].
Studies examining cytokine production in patients with thyroid cancers accompanied with thyroid autoimmunity are still missing. In one investigation [21], lack of an increase in Th1 cytokine production was accompanied by enhanced Th2 cytokine secretion in patients with Hashimoto thyroiditis with elevated calcitonin levels. The aim of our study was to analyze cytokine production patterns in patients with papillary thyroid cancer accompanied with Hashimoto thyroiditis and to compare it with those in patients with papillary thyroid cancer without coexisting Hashimoto thyroiditis.
Materials and methods
The study population included 16 patients with PTC and 24 control subjects. Patients with PTC were divided into two groups: 12 PTC patients without coexisting Hashimoto thyroiditis (PTC group) and 4 PTC patients with Hashimoto thyroiditis (PTC + HT group). PTC was diagnosed histologically, according to the main principles of current WHO classification of thyroid tumors [22]. With the exception of two patients from the PTC group (T2N0M0 and T1N1M0) and one patient from the PTC + HT group (T2N0M0), all patients were in the T1N0M0 stage of disease. The mean TgAbs and TPOAbs concentrations in PTC + HT patients were 118 ± 70.2 and 893 ± 563 IU/ml, respectively. All patients with PTC entered this study 4–6 weeks after surgical total thyroidectomy. At the time of inclusion, the patients with PTC were hypothyroid after thyroid hormone withdrawal. Thyroid-stimulating hormone (TSH) concentrations in the PTC and PTC + HT groups of patients were as follows: 114 ± 86.1 and 111 ± 64.1 mIU/L, respectively. None of the participants had acute infections, chronic inflammatory or other conditions that could affect the tested parameters. Control subjects were also divided into two groups: 12 healthy controls (HC group) and 12 patients with newly onset Hashimoto thyroiditis (HT group). All individuals were evaluated for thyroid function and thyroid antibodies. The mean concentration of TSH in healthy controls was 2.1 ± 1.8 mIU/L, and thyroid antibodies were negative. Hashimoto thyroiditis was diagnosed on the basis of clinical presentation, high levels of antithyroid antibodies (851 ± 1922 IU/ml for TgAbs and 6753 ± 4786 IU/ml for TPO Abs) and TSH levels (57.8 ± 40.9 mIU/L). Both control groups were composed of subjects that had no acute infectious or other disease or condition that could affect the tested parameters. After venipuncture, blood samples (5 mL) from patients and control subjects were collected in tubes (Vacutainer).
The study was planned according to ethical guidelines following the Declaration of Helsinki. The institutional review committee approved our study protocol according to local biomedical research regulations. All patients and control subjects gave informed consent prior to enrollment in the investigation.
Whole blood culture
Whole blood (0.5 ml per subject) was added to 5 ml RPMI-based complete medium containing fetal bovine serum, l-glutamine and phytohemagglutinin (PHA) (GIBCO™ PB-MAX™ karyotyping medium, Invitrogen, California, USA, lot no 01930 K11) and incubated at 37 °C for 72 h. The supernatant was harvested by centrifugation (1400g for 12 min) and then stored at −20 °C until required.
Cytokine measurements
Cytokines were determined in supernatants obtained from whole blood cultures of all patients and control subjects. After thawing, the supernatant samples were analyzed with multiplex cytokine detection systems for human Th1/Th2/Th9/Th17/Th22 13plex, FlowCytomix Multiplex (ebioscience Cat. No. BMS817FF) according to the manufacturer’s instructions. All samples were acquired and analyzed on a FC500 Beckman Coulter flow cytometer. Collected data were evaluated using FlowCytomix™ Pro Software.
Statistical analysis
All values were expressed as mean ± standard deviation (SD). The commercial SPSS version 10.0 for Windows was used for statistical analysis. Student’s t test was employed for comparison of paired samples. For non-normal variables, differences between two independent groups were determined by the Mann–Whitney U test, while the Wilcoxon test was used for dependent groups. The observed variables were compared by the bivariate correlation test and Spearman coefficient. Probability (P) values <0.05 were considered to be statistically significant.
Results
We analyzed cytokine production in peripheral blood cells from two groups of patients with PTC (with or without HT), a group of patients with HT alone and a group of healthy control subjects. Cytokine production was measured in the supernatants of 72-h PHA-stimulated whole blood cultures in vitro. The results are shown in Tables 1 and 2 and Figs. 1, 2 and 3.
Table 1.
Cytokine concentrations in PHA-stimulated whole blood cultures of healthy controls (HC), patients with Hashimoto thyroiditis (HT patients), patients with papillary thyroid cancer (PTC patients) and patients with papillary thyroid cancer and associated Hashimoto thyroiditis (PTC + HT patients)
| Cytokine | HC (n = 12) x ± SD | HT patients (n = 12) x ± SD | PTC patients (n = 12) x ± SD | PTC + HT patients (n = 4) x ± SD |
|---|---|---|---|---|
| IL-13 | 144 ± 171 | 301 ± 137 | 609 ± 321d,g | 4212 ± 3224i |
| IL-4 | 91.6 ± 108 | 85.9 ± 53.7 | 181 ± 94.6h | 404 ± 221j |
| IL-5 | 282 ± 314 | 440 ± 267 | 857 ± 613e | 1906 ± 1015 |
| IL-9 | 11.9 ± 20.7 | 40 ± 24.9 | 47.7 ± 23.1f | 199 ± 167k |
| IFN-γ | 7284 ± 6699 | 14647 ± 3516a | 9916 ± 3699 | 14521 ± 718l |
| IL-6 | 1355 ± 1658 | 4968 ± 3348b | 1897 ± 1651 | 6038 ± 2288m |
| IL-17A | 1521 ± 1393 | 3487 ± 2793 | 2965 ± 1652 | 3673 ± 458 |
| IL-10 | 203 ± 198 | 739 ± 358c | 547 ± 752 | 671 ± 516 |
a significant difference between HT patients and HC, p = 0.030
b significant difference between HT patients and HC, p = 0.017
c significant difference between HT patients and HC, p = 0.005
d significant difference between PTC patients and HC, p = 0.005
e significant difference between PTC patients and HC, p = 0.047
f significant difference between PTC patients and HC, p = 0.010
g significant difference between PTC patients and HT patients, p = 0.038
h significant difference between PTC patients and HT patients, p = 0.040
i significant difference between PTC + HT patients and PTC patients, p = 0.033
j significant difference between PTC + HT patients and PTC patients, p = 0.047
k significant difference between PTC + HT patients and PTC patients, p = 0.017
l significant difference between PTC + HT patients and PTC patients, p = 0.016
m significant difference between PTC + HT patients and PTC patients, p = 0.011
Table 2.
Mean values (X ± SD) of Th1/Th2 cytokine ratios for healthy controls (HC), patients with Hashimoto thyroiditis (HT), patients with papillary thyroid cancer (PTC) and patients with papillary thyroid cancer accompanied with Hashimoto thyroiditis (PTC + HT)
| Th1/Th2 | HC (n = 12) (X ± SD) | HT (n = 12) (X ± SD) | PTC (n = 12) (X ± SD) | PTC + HT (n = 4) (X ± SD) |
|---|---|---|---|---|
| IFN-γ/IL-4 | 39.16 ± 45.23 | 134.46 ± 70.16a | 72.59 ± 62.65 | 50.38 ± 40.02 |
| IFN-γ/IL-5 | 22.93 ± 23.38 | 40.57 ± 17.34 | 19.18 ± 14.55 | 10.40 ± 7.93c |
| IFN-γ/IL-6 | 5.78 ± 3.70 | 6.18 ± 6.28 | 10.79 ± 7.96 | 2.75 ± 1.37d |
| IFN-γ/IL-10 | 31.37 ± 23.91 | 22.44 ± 6.92 | 45.14 ± 33.31 | 29.37 ± 15.11 |
| IFN-γ/IL-13 | 24.89 ± 27.85 | 59.58 ± 30.08b | 23.60 ± 17.46 | 6.77 ± 7.21e |
a HT versus HC, IFN-γ/IL-4, p = 0.011
b HT versus HC, IFN-γ/IL-13, p = 0.045
c PTC + HT versus HT, IFN-γ/IL-5, p = 0.023
d PTC + HT versus PTC, IFN-γ/IL-6, p = 0.037
e PTC + HT versus HT, IFN-γ/IL-13, p = 0.020
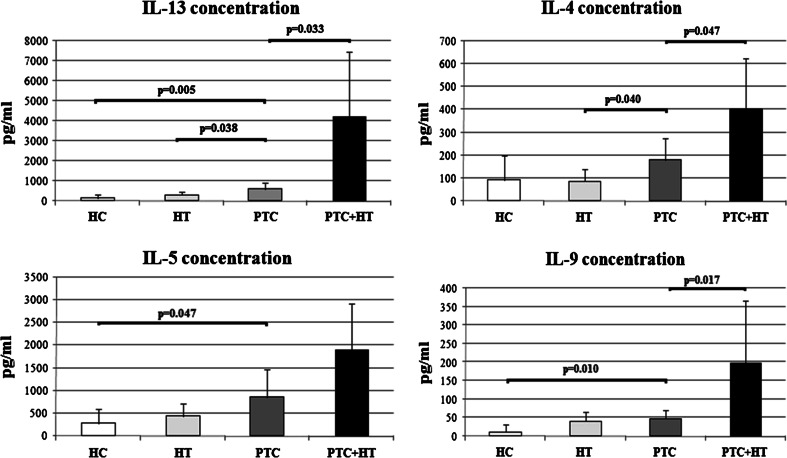
Fig. 1.
Mean concentrations of IL-4, IL-5, IL-13 and IL-9 secreted by PHA-stimulated whole blood cultures from healthy control subjects (HC, n = 12) and patients with Hashimoto thyroiditis (HT, n = 12) or papillary thyroid cancer (PTC, n = 12) or both papillary thyroid cancer and Hashimoto thyroiditis (PTC + HT, n = 4)
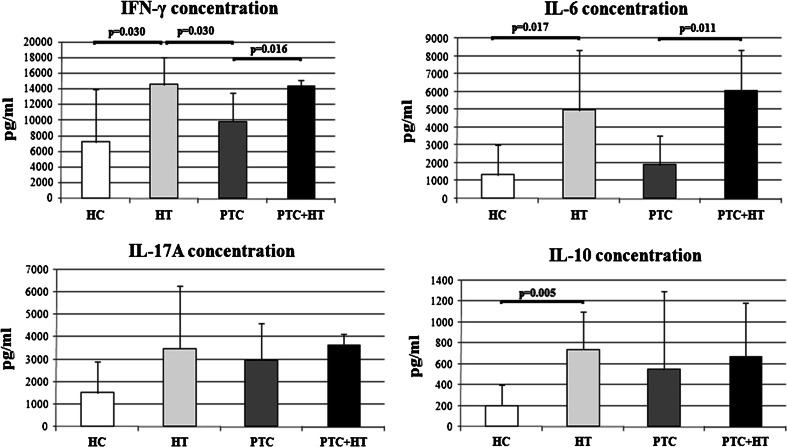
Fig. 2.
Mean concentrations of IFN-γ, IL-6, IL-17A and IL-10 secreted by PHA-stimulated whole blood cultures from healthy control subjects (HC, n = 12) and patients with Hashimoto thyroiditis (HT, n = 12) or papillary thyroid cancer (PTC, n = 12) or both papillary thyroid cancer and Hashimoto thyroiditis (PTC + HT, n = 4)
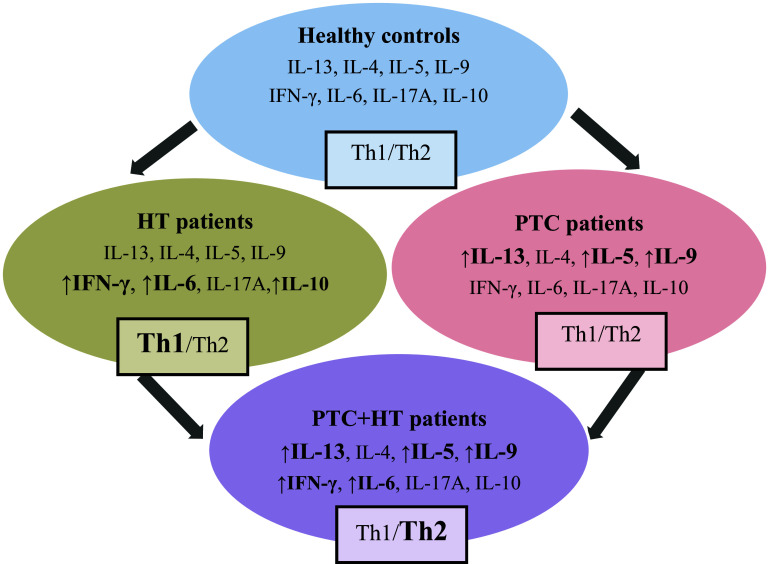
Fig. 3.
An overview of cytokine productions and Th1/Th2 cytokine ratios in PHA-stimulated blood cells from healthy controls, patients with Hashimoto thyroiditis (HT), patients with papillary thyroid cancer (PTC) and patients with papillary thyroid cancer and associated Hashimoto thyroiditis (PTC + HT). In patients with HT, the production of IFN-γ, IL-6 and IL-10 were increased, with marked shift toward Th1 immunity (Th1/Th2). In patients with PTC, the production of IL-13, IL-5 and IL-9 were increased, without a marked shift toward Th2 immunity (Th1/Th2). In patients with PTC + HT, the production of IL-13, IL-5, IL-9, IFN-γ and IL-6 were increased, with marked shift toward Th2 immunity (Th1/Th2)
The cytokine concentrations in supernatants from PHA-stimulated whole blood cultures are shown in Table 1.
We noticed that some cytokines had similar patterns of production, and so the analyzed cytokines were divided into two sets.
Figure 1 gives the concentrations of four cytokines: IL-13, IL-4, IL-5 and IL-9. Peripheral blood cells of PTC patients accompanied with HT produced more of the following cytokines: IL-13 (p = 0.033), IL-4 (p = 0.047) and IL-9 (p = 0.017) than PTC patients without HT. In addition, production of IL-13 (p = 0.005), IL-5 (p = 0.047) and IL-9 (p = 0.010) was significantly higher in PTC patients without HT than in healthy subjects. Moreover, PTC patients without HT produced more of certain cytokines in this set than patients with HT alone, as follows: IL-13 (p = 0.038) and IL-4 (p = 0.040).
Figure 2 shows the concentrations of four more cytokines: IFN-γ, IL-6, IL-17A and IL-10. The PHA-stimulated peripheral blood cells of PTC + HT patients produced more IFN-γ (p = 0.016) and IL-6 (p = 0.011) than patients with PTC alone. Production of IFN-γ was significantly higher in HT patients than in healthy subjects (p = 0.030) and PTC patients without HT (p = 0.030). Significantly more IL-6 was produced by HT patients than healthy controls (p = 0.017), while the difference in mean IL-6 production between HT and PTC patients without HT did not reach statistical significance (p = 0.058).
Mean production of IL-17A tended to be higher in HT patients and PTC patients with HT than in PTC patients without HT, but the differences were not significant (p = 0.902 and p = 0.499, respectively). The PHA-stimulated peripheral blood cells from healthy controls produced less IL-10 than all three groups of patients, but significantly less only in comparison with the HT group (p = 0.005). The lack of significant differences in production of these two cytokines between PTC and PTC + HT patients might be the consequence of the small sample size and high individual variations within patient groups.
Cytokine ratios (Th1/Th2) were calculated for each healthy control (HC), each patient with Hashimoto thyroiditis (HT), each patient with papillary thyroid cancer (PTC) and each patient with papillary thyroid cancer and associated Hashimoto thyroiditis (PTC + HT). Mean values for each group are shown in Table 2. There were statistically significant differences for Th1/Th2 ratios between HT patients and healthy controls (IFN-γ/IL-5, p = 0.011 and IFN-γ/IL-13, p = 0.045), PTC + HT patients and HT patients (IFN-γ/IL-5, p = 0.023 and IFN-γ/IL-13, p = 0.020) and PTC + HT patients and PTC patients (IFN-γ/IL-6, p = 0.037).
The results of this study are summarized in Fig. 3.
Discussion
The aim of this study was to evaluate cytokine production patterns in patients with PTC accompanied with HT and to compare it with those in patients with PTC alone. Cytokine concentrations were measured in the supernatants of PHA-stimulated whole blood cultures in vitro. We used PHA as a strong nonspecific stimulant to obtain relatively high cytokine levels to enable detection of any differences in their profiles. We showed that blood cells of PTC patients associated with HT produced significantly higher amounts of Th2/Th9 cytokines (IL-4, IL-6, IL-9 and IL-13) and the Th1 cytokine, IFN-γ, than blood cells from patients with PTC alone.
According to the secretion profile, the examined cytokines were divided into two sets. The first consisted of four Th2/Th9 cytokines (IL-13, IL-4, IL-5 and IL-9), where secretion was higher in PTC patients with accompanying HT than in PTC patients without HT, while patients with PTC alone produced more of these cytokines than healthy controls. The second set of cytokines included the Th1 cytokine, IFN-γ, the Th17 cytokine, IL-17A and two Th2 cytokines: IL-6 and IL-10, where production was also higher in patients with PTC+HT than in PTC patients without HT, but the HT patients produced more of these cytokines than healthy subjects. It seems that coexistence of HT and PTC contributed to the increase in production of cytokines from both groups.
This is the first study showing elevated production of Th2/TH9 cytokines in patients with PTC accompanied with autoimmune HT when compared to patients without concomitant autoimmune thyroiditis. Recently, we recorded that peripheral blood cells of patients with differentiated thyroid cancers produce more Th2/Th9 cytokines than healthy controls [10]. The role of Th2/Th9 responses in tumor immunity and immunosurveillance has not been sufficiently examined. While it is generally accepted that Th2 cytokines downregulate cell-mediated immunity, providing a microenvironment conducive to disease progression [23, 24], some studies indicate a protective role for them in anti-tumor immunity [25]. Namely, it was shown that tumor-specific CD4+ T lymphocytes with a Th2 cytokine profile can remove an established melanoma metastasis in lungs [26]. Low-grade chronic inflammation and imbalance between pro- and anti-inflammatory cytokines have been proposed to play a role in the pathogenesis of HT, with prevalence of Th1 and Th17 [2, 3] types of immune response. Th1/Th2 cytokine ratios in our patients with Hashimoto thyroiditis indicate a marked shift toward Th1-type immunity. But, we have demonstrated here that inflammatory conditions associated with Hashimoto thyroiditis additionally augment the production of Th2/Th9 cytokines in peripheral blood cells of PTC patients. Th1/Th2 cytokine ratios calculated in this study indicate that Hashimoto thyroiditis contributes to a marked shift toward the Th2 type of immune response in patients with papillary thyroid cancer. Our results are in agreement with those of Schuetz et al. [21] who found increased Th2 cytokine production in patients with Hashimoto thyroiditis with elevated calcitonin levels. The higher calcitonin levels were caused by hyperplasia of thyroid C cells. It is important to note that C cell hyperplasia may precede the occurrence of medullary thyroid cancer [27].
In addition, we found that PHA-stimulated blood cells of PTC patients with accompanying HT produced significantly more of the Th1 cytokine, IFN-γ, and the Th2 cytokine, IL-6, than PTC patients without HT. The primary cellular source of IFN-γ is CD4+ Th1 lymphocytes, but other cells can also produce IFN-γ (for example, CD8+ cytotoxic lymphocytes, natural killer cells, NKT cells, B cells, dendritic cells and macrophages) (reviewed in ref: [28]). It was earlier shown that IFN-γ may contribute to autoimmune pathology, including Hashimoto thyroiditis [29]. Regarding tumor immunity, it is generally accepted that IFN-γ has an important role in tumor immunosurveillance, but there are studies suggesting that under certain circumstances it may have pro-tumorigenic effects (reviewed in ref: [30]). The biological activity of IFN-γ is conventionally associated with cytostatic/cytotoxic and anti-tumor mechanisms during cell-mediated immune responses. IFN-γ has been used in therapy of a variety of malignancies, but the results have been mixed and side effects can be severe (reviewed in Ref. [30, 31]). In a clinical study, Hossain et al. [32] showed that expression of IFN-γ in peripheral mononuclear cells of patients with thyroid cancer (with and without PMA/ionomycin stimulation) was not significantly different from IFN-γ expression in cells of healthy controls. However, functional profiling of cell subpopulations revealed a markedly impaired ability of CD8+ T cells from thyroid cancer patients to produce IFN-γ following nonspecific in vitro stimulation, suggesting possible immune exhaustion of these cells in patients with active disease. We found no difference in production of IFN-γ between patients with thyroid cancer and healthy controls [10]. If thyroid cancer patients have impaired cytotoxic T cell function [32], the increase in production of IFN-γ in those with accompanying HT might indicate that the autoimmune condition could be beneficial.
IL-6 is a pleiotropic cytokine involved in the regulation of diverse physiological and pathological processes [33]. Together with transforming growth factor beta (TGF-β), IL-6 preferentially induces differentiation of Th17 cells, but inhibits differentiation of Treg cells. This results in an immunological imbalance between Treg and Th17 subsets, which is believed to be an important pathological mechanism in the development of autoimmune and chronic inflammatory diseases [33, 34]. Polymorphism of the gene for IL-6, in association with other genes for cytokines, has been suggested to contribute to susceptibility to Hashimoto thyroiditis [35]. Although it is classified in the Th2 cytokine group, IL-6 can be secreted by many other cell types. Thus, IL-6 production was shown for thyroid follicular cells and intrathyroid inflammatory cells [36, 37]. Elevated serum IL-6 in patients with HT has also been observed [38, 39]. On the other hand, IL-6 has already been attributed a possible role in the regulation of growth, progression and metastasis of malignant tumors [40, 41]. Zhang et al. [42] observed that a lack of IL-6 completely ablated pancreatic cancer progression, while in another study, it was recorded that serum IL-6 concentration could be a useful prognostic indicator for survival in patients with lung cancer [43].
Production of IFN-γ and IL-6 in PTC + HT patients was approximately equal to that in HT patients. However, if we take into account the finding that antithyroid antibody concentrations were several times higher in HT than in PTC + HT patients, it seems that coexistence of PTC and autoimmune HT contributes to the additional increase in production of these two cytokines. The increase in IL-6 seems to be especially interesting, because the IFN-γ/IL-6 ratio was significantly lower in PTC + HT patients, indicating a marked shift toward Th2 immunity in PTC patients with autoimmune HT.
The Th17 type of immune response with IL-17 production occurs in patients with autoimmune diseases, including HT [3]. In addition, a correlation between chronic lymphocytic infiltration of PTC, including Th17 cells, and improved outcome or less aggressive disease was observed recently [20, 44]. In our study, peripheral blood cells from all three patient groups tended to produce more IL-17A than those from healthy controls, but the differences were not statistically significant.
PHA-stimulated blood cells of HT patients secreted more IL-10 than controls. This result is consistent with earlier findings of augmented production of the anti-inflammatory cytokine IL-10 in patients with autoimmune diseases [45, 46]. IL-10 inhibits secretion of Th1-cytokines [47], but not Th2-cytokines. After generation of a pro-inflammatory immune response, IL-10 may contribute to limitation of inflammation deleterious to the host [48].
If we accept that the Th1/Th17 type of response is dominant in patients with HT [2, 3], one might expect that coexistence of autoimmune HT in patients with PTC leads to enhanced production of Th1 and/or Th17 cytokines. Indeed, our results indicate that autoimmune conditions contribute to elevated production of IFN-γ (a Th1 type of cytokine) and IL-17A (a Th17 type of cytokine), although the difference in IL-17A levels did not attain statistical significance. Nevertheless, autoimmune HT in PTC patients led to significantly enhanced production of IL-4, IL-6, IL-9 and IL-13 (Th2/Th9 cytokines).
Our results indicate that, in response to a nonspecific stimulus, peripheral blood cells of PTC patients with accompanying HT are directed to produce more Th1/Th2/Th9 cytokines than those from PTC patients without HT. However, we cannot yet conclude about the cytokine profiles in the PTC microenvironment or the potential significance of these findings. If we assume that in vitro produced cytokines reflect the cytokine production patterns of the immune cells present in the thyroid microenvironment, this would be only part of a very complex picture. Namely, tumor-associated inflammation can induce diverse molecules expressed by the tumors themselves or surrounding cells to create a microenvironment that potentially inhibits or promotes cancer development [49–53].
Some studies indicate a favorable clinical course in patients with PTC and associated HT [15–18], while others failed to find a connection [19, 20]. Although our research was not designed to analyze associations of any individual cytokine or groups of cytokines with the clinical course of PTC, we will try to discuss it briefly. One possibility is that the Th2/Th9 type of immune response has a protective role in tumor surveillance and anti-tumor immunity against PTC, as has been shown in studies with some other tumor types [25, 26]. Another possibility is that the favorable clinical course in patients with PTC and associated HT occurs as a result of the action of numerous other cytokines released in the autoimmune microenvironment of the thyroid cancer, not only the Th2/Th9 type. In this regard, IFN-γ, shown to be increased in our study, could have a role. Finally, factors outside the immune system might be responsible for the favorable clinical course in patients with PTC and associated HT. Namely, expression of some molecules or gene rearrangements possibly associated with tumorigenesis occurred in similar relative amounts in patients with PTC and patients with HT (reviewed in ref [12]).
In conclusion, autoimmune HT was found to affect the cytokine profile in patients with PTC by stimulating secretion of Th1-/Th2-/Th9-type cytokines. Th1/Th2 cytokine ratios in PTC patients with associated autoimmune HT indicate a marked shift toward Th2 immunity. As most cytokines might have many contradictory functions in tumor immune surveillance and tumor immunity, additional studies are needed to determine the significance of these findings.
Acknowledgments
The authors would like to thank the anonymous reviewers whose remarks and suggestions have highly improved the final version of our paper. The study was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant Nos. III41010 and ON175069) and Faculty of Medical Sciences University of Kragujevac, Serbia (JP 06-12).
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- Abs
Antibodies
- HT
Hashimoto thyroiditis
- IFN-γ
Interferon gamma
- IL
Interleukin
- PHA
Phytohemagglutinin
- PTC
Papillary thyroid cancer
- Tg
Thyroglobulin
- Th1
T-helper-1
- Th17
T-helper-17
- Th2
T-helper-2
- Th9
T-helper-9
- TPO
Thyroperoxidase
References
- 1.Dayan CM, Daniels GH. Chronic autoimmune thyroiditis. N Engl J Med. 1996;335:99–107. doi: 10.1056/NEJM199607113350206. [DOI] [PubMed] [Google Scholar]
- 2.Mazziotti G, Sorvillo F, Naclerio C, et al. Type-1 response in peripheral CD4+ and CD8+ T cells from patients with Hashimoto’s thyroiditis. Eur J Endocrinol. 2003;148:383–388. doi: 10.1530/eje.0.1480383. [DOI] [PubMed] [Google Scholar]
- 3.Figueroa-Vega N, Alfonso-Pérez M, Benedicto I, et al. Increased circulating proinflammatory cytokines and Th17 lymphocytes in Hashimoto’s thyroiditis. J Clin Endocrinol Metab. 2010;2010(95):953–962. doi: 10.1210/jc.2009-1719. [DOI] [PubMed] [Google Scholar]
- 4.Skapenko A, Niedobitek GU, Kalden JR, et al. Generation and regulation of human Th1-biased immune responses in vivo: a critical role for IL-4 and IL-10. J Immunol. 2004;172:6427–6434. doi: 10.4049/jimmunol.172.10.6427. [DOI] [PubMed] [Google Scholar]
- 5.Marazuela M, García-López MA, Figueroa-Vega N, et al. Regulatory T cells in human autoimmune thyroid disease. J Clin Endocrinol Metab. 2006;91:3639–3646. doi: 10.1210/jc.2005-2337. [DOI] [PubMed] [Google Scholar]
- 6.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 7.Riesco-Eizaguirre G, Santisteban P. New insights in thyroid follicular cell biology and its impact in thyroid cancer therapy. Endocr Relat Cancer. 2007;14:957–977. doi: 10.1677/ERC-07-0085. [DOI] [PubMed] [Google Scholar]
- 8.Ozgen AG, Karadeniz M, Erdogan M, et al. The (-174) G/C polymorphism in the interleukin-6 gene is associated with risk of papillary thyroid carcinoma in Turkish patients. J Endocrinol Invest. 2009;32:491–494. doi: 10.1007/BF03346494. [DOI] [PubMed] [Google Scholar]
- 9.Cunha LL, Aj Tincani, Assumpcao LV, et al. Interleukin-10 but not interleukin-18 may be associated with the immune response against well-differentiated thyroid cancer. Clinics (Sao Paulo) 2011;66:1203–1208. doi: 10.1590/S1807-59322011000700014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonovic SZ, Mihaljevic O, Majstorovic I, et al. Cytokine production in peripheral blood cells of patients with differentiated thyroid cancer: elevated Th2/Th9 production before and reduced Th2 cytokine production after radioactive iodine therapy. Cancer Immunol Immunother. 2015;64:75–82. doi: 10.1007/s00262-014-1619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stassi G, Todaro M, Zerilli M, et al. Thyroid cancer resistance to chemotherapeutic drugs via autocrine production of interleukin-4 and interleukin-10. Cancer Res. 2003;63:6784–6790. [PubMed] [Google Scholar]
- 12.Jankovic B, Le KT, Hershman JM. Hashimoto’s thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab. 2013;98:474–482. doi: 10.1210/jc.2012-2978. [DOI] [PubMed] [Google Scholar]
- 13.Weber F. Lymphocytes and thyroid cancer: more to it than meets the eye? Endocr Relat Cancer. 2014;21:C1–C5. doi: 10.1530/ERC-14-0229. [DOI] [PubMed] [Google Scholar]
- 14.Cunha LL, Marcello MA, Ward LS. The role of the inflammatory microenvironment in thyroid carcinogenesis. Endocr Relat Cancer. 2014;21:R85–R103. doi: 10.1530/ERC-13-0431. [DOI] [PubMed] [Google Scholar]
- 15.Kashima K, Yokoyama S, Noguchi S, et al. Chronic thyroiditis as a favorable prognostic factor in papillary thyroid carcinoma. Thyroid. 1998;8:197–202. doi: 10.1089/thy.1998.8.197. [DOI] [PubMed] [Google Scholar]
- 16.Singh B, Shaha AR, Trivedi H, et al. Coexistent Hashimoto’s thyroiditis with papillary thyroid carcinoma: impact of presentation, management and outcome. Surgery. 1999;126:1070–1077. doi: 10.1067/msy.2099.101431. [DOI] [PubMed] [Google Scholar]
- 17.Kim EY, Kim WG, Kim WB, et al. Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2009;71:581–586. doi: 10.1111/j.1365-2265.2009.03537.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Kim Y, Choi JW, et al. The association between papillary thyroid carcinoma and histologically proven Hashimoto’s thyroiditis: a meta-analysis. Eur J Endocrinol. 2013;168:343–349. doi: 10.1530/EJE-12-0903. [DOI] [PubMed] [Google Scholar]
- 19.Kebebew E, Treseler P, Ituarte P, Clark O. Coexisting chronic lymphocytic thyroiditis and papillary thyroid cancer revisited. World J Surg. 2001;25:632–637. doi: 10.1007/s002680020165. [DOI] [PubMed] [Google Scholar]
- 20.Cunha LL, Ward LS. Concurrent lymphocytic thyroiditis is associated to less aggressive papillary thyroid carcinomas. Eur Arch Otorhinolaryngol. 2012;269:699–700. doi: 10.1007/s00405-011-1764-y. [DOI] [PubMed] [Google Scholar]
- 21.Schuetz M, Duan H, Wahl K, et al. T Lymphocyte cytokine production patterns in Hashimoto patients with elevated calcitonin levels and their relationship to tumor initiation. Anticancer Res. 2006;26:4591–4596. [PubMed] [Google Scholar]
- 22.Nikiforov YE. Thyroid tumors: classification, staging, and general considerations. In: Nikiforov YE, Biddinger PW, Thompson LDR, editors. Diagnostic pathology and molecular genetics of the thyroid. 2. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2012. pp. 108–119. [Google Scholar]
- 23.Budhu A, Wang XW. The role of cytokines in hepatocellular carcinoma. J Leukoc Biol. 2006;80:1197–1213. doi: 10.1189/jlb.0506297. [DOI] [PubMed] [Google Scholar]
- 24.Bodelon C, Polley MY, Kemp TJ, et al. Circulating levels of immune and inflammatory markers and long versus short survival in early-stage lung cancer. Ann Oncol. 2013;24:2073–2079. doi: 10.1093/annonc/mdt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellyard JI, Simson L, Parish CR. Th2-mediated anti-tumour immunity: friend or foe? Tissue Antigens. 2007;70:1–11. doi: 10.1111/j.1399-0039.2007.00869.x. [DOI] [PubMed] [Google Scholar]
- 26.Mattes J, Hulett M, Xie W, et al. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J Exp Med. 2003;197:387–393. doi: 10.1084/jem.20021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy M, Chen H, Sippel RS. Current understanding and management of medullary thyroid cancer. Oncologist. 2013;18:1093–1100. doi: 10.1634/theoncologist.2013-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schroder K, Hertzog PJ, Ravasi T, et al. Interferon-Υ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 29.Del Prete GF, Tiri A, Mariotti S, et al. Enhanced production of gamma-interferon by thyroid-derived T cell clones from patients with Hashimoto’s thyroiditis. Clin Exp Immunol. 1987;69:323–331. [PMC free article] [PubMed] [Google Scholar]
- 30.Zaidi MR, Merlino G. The two faces of interferon-Υ in cancer. Clin Cancer Res. 2011;17:6118–6124. doi: 10.1158/1078-0432.CCR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller CH, Maher SG, Young HA. Clinical use of interferon-gamma. Ann N Y Acad Sci. 2009;1182:69–79. doi: 10.1111/j.1749-6632.2009.05069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hossain MS, Bhimani C, Zhengjia C et al (2011) Profiling counter-regulatory and cytotoxic immune pathways with cellular biomarkers in thyroid cancer patients. [abstract]. In: Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research; 2011 Apr 2–6; Orlando, FL. Philadelphia (PA): AACR; Cancer Res 71(8 Suppl):Abstract nr 5525. doi:10.1158/1538-7445.AM2011-5525
- 33.Kang S, Tanaka T, Kishimoto T. Therapeutic uses of anti-interleukin-6 receptor antibody. Int Immunol. 2015;27:21–29. doi: 10.1093/intimm/dxu081. [DOI] [PubMed] [Google Scholar]
- 34.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 35.Baki M, Akman FE, Vural P, et al. The combination of interleukin-10 -1082 and tumor necrosis factor α -308 or interleukin-6 -174 genes polymorphisms suggests an association with susceptibility to Hashimoto’s thyroiditis. Int Immunopharmacol. 2012;12:543–546. doi: 10.1016/j.intimp.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Weetman AP, Bright-Thomas R, Freeman M. Regulation of interleukin-6 release by human thyrocytes. J Endocrinol. 1990;127:357–361. doi: 10.1677/joe.0.1270357. [DOI] [PubMed] [Google Scholar]
- 37.Ajjan RA, Watson PF, Weetman AP. Cytokines and thyroid function. Adv Neuroimmunol. 1996;6:359–386. doi: 10.1016/S0960-5428(97)00027-7. [DOI] [PubMed] [Google Scholar]
- 38.Ruggeri RM, Sciacchitano S, Vitale A, et al. Serum hepatocyte growth factor (HGF) is increased in Hashimoto’s thyroiditis whether or not it is associated with nodular goiter as compared with healthy non-goitrous individuals. J Endocrinol Invest. 2009;32:465–469. doi: 10.1007/BF03346487. [DOI] [PubMed] [Google Scholar]
- 39.Sieminska L, Wojciechowska C, Kos-Kudla B, et al. Serum concentrations of leptin, adiponectin, and interleukin-6 in postmenopausal women with Hashimoto’s thyroiditis. Endokrynol Pol. 2010;61:112–116. [PubMed] [Google Scholar]
- 40.Sehgal PB. Interleukin 6 in infection and cancer. Proc Soc Exp Biol Med. 1990;195:183–191. doi: 10.3181/00379727-195-43129D. [DOI] [PubMed] [Google Scholar]
- 41.Kura Y, De Velasco MA, Kobayashi Y, et al (2013) Interleukin-6 (IL-6) as a therapeutic target in prostate cancer. [abstract]. In: Proceedings of the 104th Annual Meeting of the American Association for Cancer Research; 2013 Apr 6–10; Washington, DC. Philadelphia (PA): AACR; Cancer Res 73(8 Suppl):Abstract nr 1226. doi:10.1158/1538-7445.AM2013-1226
- 42.Zhang Y, Yan W, Collins MA, et al. Interleukin-6 is required for pancreatic cancer progression by promoting MAPK signaling activation and oxidative stress resistance. Cancer Res. 2013;73:6359–6374. doi: 10.1158/0008-5472.CAN-13-1558-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ujiie H, Tomida M, Akiyama H, et al. Serum hepatocyte growth factor and interleukin-6 are effective prognostic markers for non-small cell lung cancer. Anticancer Res. 2012;32:3251–3258. [PubMed] [Google Scholar]
- 44.Huang BY, Hseuh C, Chao TC, et al. Well-differentiated thyroid carcinoma with concomitant Hashimoto’s thyroiditis present with less aggressive clinical stage and low recurrence. Endocr Pathol. 2011;22:144–149. doi: 10.1007/s12022-011-9164-9. [DOI] [PubMed] [Google Scholar]
- 45.Ng TH, Britton GJ, Hill EV, et al. Regulation of adaptive immunity; the role of interleukin-10. Front Immunol. 2013;4:129–140. doi: 10.3389/fimmu.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smilek DE, Ehlers MR, Nepom GT. Restoring the balance: immunotherapeutic combinations for autoimmune disease. Dis Model Mech. 2014;7:503–513. doi: 10.1242/dmm.015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Waal-Malefyt R, Yssel H, de Vries JE. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993;150:4754–4765. [PubMed] [Google Scholar]
- 48.Cyktor JC, Turner J. Interleukin-10 and immunity against prokaryotic and eukaryotic intracellular pathogens. Infect Immunol. 2011;79:2964–2973. doi: 10.1128/IAI.00047-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franks AL, Slansky JE. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res. 2012;32:1119–1136. [PMC free article] [PubMed] [Google Scholar]
- 50.Luheshi N, Davies GC, Poon E et al (2013) Th1 and Th2 cytokines determine how CD40 activation changes human macrophage function in vitro. [abstract]. In: Proceedings of the 104th Annual Meeting of the American Association for Cancer Research; 2013 Apr 6–10; Washington, DC. Philadelphia (PA): AACR; Cancer Res 73(8 Suppl):Abstract nr 1542. doi:10.1158/1538-7445.AM2013-1542
- 51.Becker JC, Andersen MH, Schrama D, et al. Immune-suppressive properties of the tumor microenvironment. Cancer Immunol Immunother. 2013;62:1137–1148. doi: 10.1007/s00262-013-1434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Atsumi T, Singh R, Sabharwal L, et al. Inflammation amplifier, a new paradigm in cancer biology. Cancer Res. 2014;74:8–14. doi: 10.1158/0008-5472.CAN-13-2322. [DOI] [PubMed] [Google Scholar]
- 53.Murray JI, West NR, Murphy LC, et al. Intratumoral inflammation and endocrine resistance in breast cancer. Endocr Relat Cancer. 2014;22:R51–R67. doi: 10.1530/ERC-14-0096. [DOI] [PubMed] [Google Scholar]