Abstract
We previously reported that substantial amounts of IL-10, an immunomodulatory cytokine, are produced by cell suspensions of fresh human metastatic melanoma tissues. Production diminished with continuous culturing of cells, which suggests a pivotal interactive role between melanoma cells and the tumor microenvironment. In this study, we found that the culture media obtained from LPS-stimulated peripheral blood mononuclear cells induced IL-10 production by metastatic melanoma cells. Of the multiple cytokines present in the conditioned culture media, IL-6 was identified as the inducer of IL-10 production. A neutralizing antibody against IL-6 completely blocked the conditioned medium-induced IL-10 production. Metastatic melanoma cells that constitutively produce low amount of IL-10 increased IL-10 production in response to recombinant human IL-6 in a dose-dependent fashion. The response to exogenously added IL-6 was less significant in melanoma cells that produced high amounts of IL-6, probably due to pre-existing autocrine stimulation of IL-10 by endogenous IL-6. On the other hand, metastatic melanoma cells that do not constitutively produce IL-10 protein did not respond to exogenous IL-6. In IL-6-responsive melanoma cells, IL-6 increased STAT3 phosphorylation and inhibition of STAT3 signaling using siRNA or inhibitors for JAKs diminished IL-6-induced IL-10 production. In addition, inhibition of MEK and PI3K, but not mTOR, interfered with IL-10 production. Taken together, the data suggest that blocking of these signals leading to IL-10 production is a potential strategy to enhance an anti-melanoma immune response in metastatic melanoma.
Keywords: Melanoma, Metastasis, IL-10, IL-6, STAT3
Introduction
Specific cytokines or growth factors produced in the tumor microenvironment by tumor-related immune cells are key factors in suppressing the anti-tumor immune response, often to favor tumor growth [1]. Indeed, tumor cells themselves can secrete factors that inhibit maturation of professional antigen presenting cells (APC) [2]. In the absence of the appropriate maturation signals, professional APC such as dendritic cells do not become fully activated and can induce tolerance rather than immunity against cancer cells. One of the key cytokines that is frequently detected in the tumor microenvironment and that is reported to impair APC function is interleukin 10 (IL-10) [3].
IL-10 is an immunomodulatory cytokine that plays an important role in negative regulation of cellular immune responses. It suppresses the function of APCs and T cells by inhibiting pro-inflammatory cytokine production and by down-regulation of co-stimulatory molecules and major histocompatibility complex (MHC) class II expression [3–5]. We have reported that mRNA for IL-10 was present in most metastatic melanoma tissues [6]. Indeed, single-cell suspensions obtained from melanoma metastases produced IL-10 protein, and the major source of intralesional IL-10 production was the melanoma cells themselves [7]. Furthermore, melanoma patients with clinically evident metastases showed elevations of circulating IL-10, the level of which correlated with the progression of disease [7].
IL-10 is also produced by many other different types of tumor cells including basal cell and squamous cell carcinomas of the skin [8], colon cancer [9], renal cell carcinoma [10], and breast cancer [11]. Elevated serum levels of IL-10 have been reported in cancer patients including malignant melanoma [12], diffuse large B-cell lymphoma [13], and non-Hodgkin’s lymphoma [14]. IL-10 is secreted by tumors or tumor-infiltrating immune cells; this could allow malignant cells to escape immune surveillance [15].
Interestingly, IL-10 production by freshly isolated metastatic melanoma cells was progressively reduced during the culture period. Only a limited number of established melanoma cell lines continued indefinitely to produce IL-10 [7].
In this study, we first found that stimulated peripheral blood mononuclear cells (PBMC) produce factors that can induce IL-10 production by human melanoma cells. We surveyed cytokines and found that interleukin 6 (IL-6) was one of the cytokines that increased IL-10 production from melanoma cells in vitro. Mechanisms underlying IL-6-induced IL-10 production were further investigated using multiple synthetic inhibitors of key signal pathways.
Materials and methods
Freshly isolated metastatic melanoma cell suspensions
Discarded specimens of lymph node metastases harvested in the courses of associated clinical studies for patients with stage III and stage IV disease were collected [16, 17]. The tissue specimens were dissected with a disposable scalpel and dissociated with collagenases (SIGMA, St. Louis, MO). The single melanoma cell suspensions were aliquoted, frozen in a controlled-rate freezing machine, and stored in liquid nitrogen until needed [18]. The protocols for clinical studies and tissue collection were reviewed and approved by the Institutional Review Board of Thomas Jefferson University.
Cell lines and cell culture
Melanoma cell lines (CM005, Cr, Ho88, DIL. Cla, and Beu) were established in our laboratory from freshly isolated metastatic melanoma cell suspensions described above. A long-term cultured cell line, CM005, derived from a lymph node metastasis, was used for the majority of experiments. It was assayed for the following parameters: (1) expression of mRNA for IL-10, (2) production of IL-10 in the culture medium (80–150 pg/ml/24 h), (3) lack of constitutive production of IL-6, and (4) dose-dependent production of IL-10 in response to exogenous IL-6. Tumor cell lines and PBMC were cultured with RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 4 mM l-glutamine, 10 mM HEPES, 1% non-essential amino acids, penicillin G at 100 U/ml, and streptomycin at 100 μg/ml.
Enzyme-linked immunosorbent assays (ELISA)
Human interleukin 1β (IL-1β), interleukin 2 (IL-2), IL-6, interleukin 8 (IL-8), IL-10, interleukin 12 (IL-12) p40, interferon α (IFN-α), interferon γ (IFN-γ), granulocyte macrophage colony-stimulating factor (GM-CSF), and tumor necrosis factor α (TNF-α) were measured with commercially available ELISA kits (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. Human soluble IL-6 receptor alpha (sIL-6Ra) was measured by ELISA kit (R&D systems, Minneapolis, MN). The above human cytokines and sIL-6Ra were not detectable in the medium that was used for cell culture.
Flow cytometry
Expressions of IL-6 receptors, CD126 (gp80) and CD130 (gp130), on the surface of melanoma cells were tested by flow cytometry (EPICS MCL-XL, Beckman Coulter, Inc., Brea, CA) using anti-human IL-6 receptor antibodies, conjugated to fluorescein isothiocyanate (FTIC). Isotype-matched mouse IgG FITC was used as the negative control. Antibodies for flow cytometric analysis were purchased from R&D Systems.
mRNA for IL-6 receptors
mRNA for IL-6 receptors (gp80 and gp130) was assayed by RT–PCR. In brief, total RNA was extracted from 106 melanoma cells, according to the manufacturer’s instructions for the use of RNeasy Mini kit (QIAGEN, Valencia, CA). Reverse transcription was performed with the Super Script System (Invitrogen) for first-strand cDNA synthesis. RT–PCR was performed using a GENE Amp PCR System 9700 (Applied Biosystems Inc, Foster City, CA). One μg of cDNA was amplified for 26 cycles. Each cycle consisted of the following: 1 min at 94°C for denaturation, 30 s at 55°C for annealing, and 1 min for primer extension. Oligonucleotide primers for gp80 (ligand-binding subunit of IL-6 receptor) and gp130 (signal-transducing subunit of IL-6 receptor) were as follows:
gp80: F: 5′-CATTGCCATTGTTCTGAGGTT-3′; R: 5′-AGTAGTCTGTATTGCTGATGT-3′,
gp130: F: 5′-CATGCTTTGGGTGGAATGGAC-3′; R: 5′-CATCAACAGGAAGTTGGTCCC-3′.
A house-keeping gene, β-actin, was used as a reference.
Western blotting
Melanoma cells were fixed with 10% trichloroacetic acid (TCA) on ice for 10 min, and then, the debris was scraped into tubes and washed with diethylether to remove TCA. The protein precipitates were dissolved with a buffer containing 1% sodium dodecyl sulfate (SDS), 1.2 mM sodium orthovanadate, 1 mM EDTA, and 50 mM Tris–HCl, pH 8.0. Protein concentration was determined by a BCA protein assay kit (Pierce, Rockford, IL). Equal amounts of protein were subjected to Western blotting. Primary antibodies used were as follows: anti-signal transducers and activators of transcription 3 (STAT3) antibody (Cell Signaling, Danvers, MA), anti-phospho-STAT3 (Y705)-antibody (Cell Signaling), and anti-phospho-ERK1/2-antibody (Sigma). The primary antibodies on the membranes were detected using secondary antibodies coupled with horseradish peroxidase and SuperSignal™ West Pico chemiluminescent substrate (Thermo Scientific, Rockford, IL). Chemiluminescence signal on the membrane was quantified using a CCD camera imaging system (Alpha-Innotech, Santa Clara, CA).
Knockdown of STAT3 by small interfering RNA (siRNA)
Melanoma cells were seeded into a 6-well plate and transfected with 200 pmol of the silencer pre-designed siRNA fragment for STAT3 or negative control (Dharmacon, Thermo Scientific) using Lipofectamine 2000 (Invitrogen). After 3 days of siRNA treatment, 2 ng/ml of recombinant human IL-6 (rhIL-6) was added. After 24 h of culture with rhIL-6, the supernatant was collected for IL-10 production. Melanoma cells were also collected for examination of total and phosphorylated STAT3 by the Western blotting.
Blocking of IL-6 signal transduction pathway
Melanoma cells in 35-mm dishes were treated with pharmacological inhibitors of individual signal pathways listed in Table 1 for 1 h and then stimulated with 2 ng/ml of rhIL-6. After 24 h, melanoma cells were harvested and washed twice with PBS. The cells were fixed and lysed, and then phosphorylation of key signal molecules was checked by Western blotting. Janus kinases (JAKs) inhibitor, Pyrdone 6 (Py6); JAK2 inhibitor, 1,2,3,4,5,6-hexabromocyclohexane (Br6CH); c-RAF inhibitor, GW5074; mammalian target of rapamycin (mTOR) inhibitor, Rapamycin (Rap); and Rho-associated, coiled coil-containing protein kinases (ROCKs) inhibitor, H1152, were purchased from Carbiochem-EMD4Biosciences (San Diego, CA). MAPK/ERK kinase (MEK)1/2 inhibitor, U0126; Src inhibitor, PP2 and epidermal growth factor receptor (EGFR) inhibitor, AG1478, were purchased from Biomol-Enzo Life Sciences (Farmingdale, NY). Phosphoinositide 3-kinase (PI3K) inhibitor, LY294002, and protein kinases C (PKCs) inhibitor, GFx, were purchased from LC Laboratories (Woburn, MA) (Table 1).
Table 1.
Pharmacological inhibitors of individual signal pathways
| Abbrev. | Conc. | Target | Company | |
|---|---|---|---|---|
| Ctl | Control | |||
| Py6 | Pyrdone 6 | 1 µM | JAKs | Calbiochem |
| Br6CH | 1,2,3,4,5,6-hexabromocyclohexane | 50 µM | JAK2 | Calbiochem |
| U0126 | 10 µM | MEK1/2 | Biomol | |
| GW5074 | 10 µM | cRaf | Calbiochem | |
| LY294002 | 25 µM | P13K | LC Labs | |
| Rap | Rapamycin | 20 nM | mTOR | Calbiochem |
| PP2 | 1 mM | Src | Biomol | |
| AG1478 | 0.25 µM | EGF-R | Enzo | |
| GFx | GF109203x | 3 µM | PKCs | LC Labs |
| H1152 | 10 µM | ROCKs | Calbiochem | |
Other cells and reagents
PBMC were obtained from healthy donors by leukapheresis. Lipopolysaccharide (LPS) and phytohemagglutinin (PHA) were purchased from SIGMA. Recombinant human cytokines and antibodies were obtained from the following sources: IL-1β, IL-12 from R&D systems; IL-2 from CHIRON Therapeutics (Emeryville, CA); IL-6 and TNF-α from Invitrogen; IL-8, IL-10, IFN-α and IFN-γ from NCI Biological Resources Branch (Rockville, MD); and GM-CSF from Bayer HealthCare Pharmaceuticals (Wayne, NJ). Neutralizing rat anti-human IL-6 antibody and isotype-matched rat IgG1 were purchased from BD Biosciences (San Jose, CA).
Statistical analysis
The experiments were repeated at least twice to confirm the consistency of data, in which samples were in duplicates or triplicates. The comparisons of data were performed with the Student’s t test and one-way ANOVA using PRISM program version 4.0c (GraphPad). P value of <0.05 was used for statistical significance.
Results
IL-10 production by melanoma cells was enhanced by supernatants from LPS-stimulated culture medium of PBMC
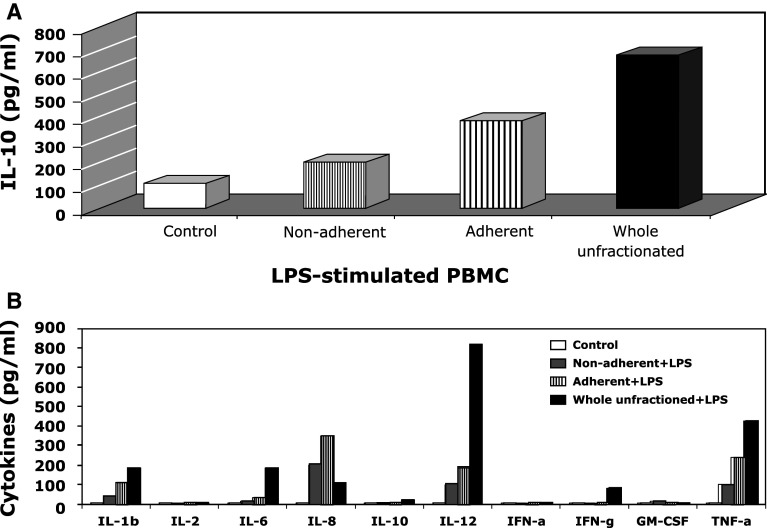
We hypothesized that melanoma cells produce IL-10 in response to cytokines generated by immune cells in the tumor microenvironment, which might explain the decrease or loss of IL-10 production in short-term culture melanoma cell lines deficient of immune cells. To test this hypothesis, we first tested a CM005 cell line since this cell line constitutively and stably produces small amount of IL-10 in culture. PBMC were stimulated with LPS or PHA, and supernatants were collected. The CM005 melanoma cells were cultured with the supernatant obtained from LPS-stimulated or PHA-stimulated PBMC supernatants. IL-10 production from CM005 cells was enhanced when co-cultured with supernatants from LPS-stimulated PBMC and, to a lesser extent, when co-cultured with those from PHA-stimulated PBMC. Therefore, subsequent experiments were conducted with LPS-stimulated supernatants. We further investigated whether it was the non-adherent or adherent cell population of PBMC that produce cytokine(s) to stimulate IL-10 production by CM005 melanoma cells. The CM005 cells were co-cultured with the supernatants obtained from unfractionated PBMC without LPS stimulation (control) or non-adherent, adherent, and whole unfractionated PBMC with LPS stimulation at 10 ng/ml. As shown in Fig. 1a, CM005 cells cultured with the supernatant of LPS-stimulated adherent and unfractionated PBMC produced higher IL-10 protein than supernatant obtained from LPS-stimulated non-adherent PBMC. However, LPS itself did not stimulate IL-10 production by CM005 cells.
Fig. 1.
LPS-stimulated PBMC media increased the production of IL-10 by melanoma cells. a IL-10 levels after co-culture with PBMC-derived media. CM005 melanoma cells were cultured with culture supernatants from unfractionated PBMC without LPS (control) or non-adherent, adherent, and unfractionated PBMC with 10 ng/ml of LPS for 2 days, and then, the supernatants were collected. The IL-10 productions in supernatants were analyzed by the ELISA assay. The average of duplicates in a representative experiment was shown. b Cytokine levels in conditional culture media. The average of duplicates in a representative experiment was shown
To narrow down the cytokines that stimulate IL-10 production by CM005 cells, the levels of key cytokines in individual culture supernatants were measured by commercially available ELISA kits. We assumed that a cytokine present in a greater amount in the supernatant of LPS-stimulated whole unseparated and adherent PBMC as compared with those of non-adherent PBMC would be a candidate mediator. As shown in Fig. 1b, the following cytokines met this criterion: IL-1β, IL-6, IL-12, and TNF-α.
IL-6 is necessary and sufficient for IL-10 production by human metastatic melanoma
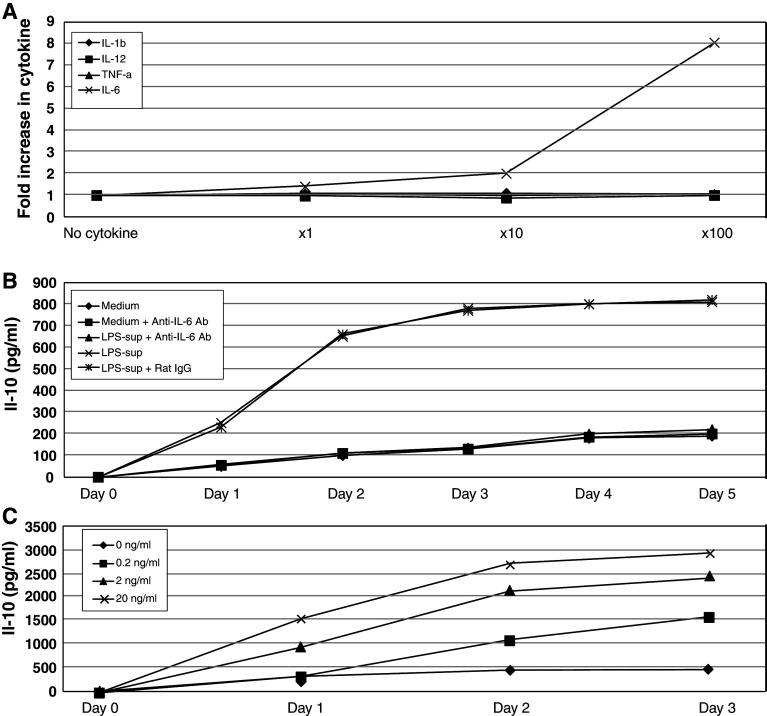
We determined the ability of IL-1β, IL-6, IL-12, and/or TNF-α, at various concentrations, to induce IL-10 production by CM005 cells. Among these cytokines, only IL-6 showed a dose-dependent stimulatory effect on the CM005 cells, while IL-1β, IL-12, and TNF-α had no impact (Fig. 2a). There were no differences in melanoma cell count in individual wells that contained various concentrations of IL-6; therefore, the increase in IL-10 production was not explained by an expansion of melanoma cell numbers. To confirm that IL-6 is a cytokine that stimulates IL-10 production by CM005 cells, the supernatant from LPS-stimulated adherent PBMC was mixed with anti-IL-6 antibody (10 μg/ml) and added to the CM005 cells (1 × 106 cell/ml) in 6-well plate for 3 days. As shown in Fig. 2b, the stimulatory effect on IL-10 production from the supernatant of LPS-stimulated adherent PBMC was almost completely blocked by anti-IL-6 antibody. This finding provided evidence to support our conclusion that IL-6 was the key stimulant for IL-10 production by CM005 cells. We next examined whether the IL-10 production by melanoma cells is proportional to the amount of IL-6 in culture media. CM005 cells were cultured with 0.2, 2, and 20 ng/ml of rhIL-6, and the supernatants were collected daily. As shown in Fig. 2c, IL-10 production in the supernatant of CM005 cells co-cultured with rhIL-6 increased in a dose-dependent fashion. In contrast, healthy donor PBMC did not produce IL-10 in response to rhIL-6 up to 20 ng/ml (data are not shown).
Fig. 2.
IL-6 stimulates IL-10 production by CM005 melanoma cells. a Increase in IL-10 in response to exogenously added cytokines. The levels of IL-10 produced by CM005 cells were measured after 2-day incubation with various concentrations (1, 10, and 100 folds of levels in medium of LPS-stimulated adherent PBMC) of individual cytokines (IL-1β, IL-6, IL-12, and TNF-α). Only IL-6 promoted IL-10 production by CM005 cells. b Anti-IL-6 antibody abrogated stimulative effects of LPS-activated PBMC supernatant. c IL-10 production in response to exogenously added IL-6. Exogenously added IL-6 promoted the production of IL-10 by melanoma cells in a dose-dependent manner (P < 0.01)
IL-10 production from freshly isolated tumor specimens
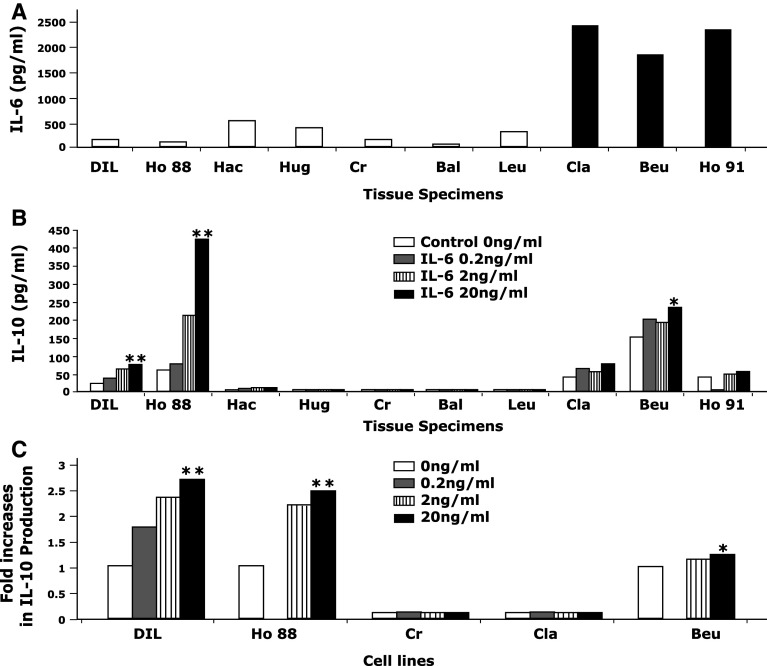
To demonstrate that the above-described phenomenon is not a cell culture artifact, we investigated a series of freshly isolated metastatic melanoma cell suspensions. Freshly isolated melanoma cell suspensions obtained from metastatic tissues were cultured in 10% FBS-enriched RPMI 1640 alone or in combination with rhIL-6 (0.2, 2, and 20 ng/ml) overnight (for 18 h), and the production of IL-10 in supernatants was measured. As shown in Fig. 3a, 3 of 10 melanoma cell suspensions (black bars) constitutively produced a significant amount of IL-6 (>1,000 pg/ml) in 18 h culture (designated as high IL-6 producers). In contrast, 7 melanoma cell suspensions (open bars) produced minimum amount of IL-6 (designated as low IL-6 producers). As shown in Fig. 3b, all of three high IL-6 producers (Cla, Beu, Ho91) constitutively produced IL-10 in 18 h culture. Their IL-10 production slightly increased at the highest dose of exogenously added IL-6 (20 ng/ml). In contrast, only 2 of 7 low IL-6 producers (DIL, Ho88) constitutively produced IL-10 (shown as “control, 0 ng/ml” in Fig. 3b). The production of IL-10 by these two melanoma cell suspensions (DIL, Ho88) increased in a dose-dependent manner when exogenous IL-6 was added (P < 0.01).
Fig. 3.
Relationship of IL-6 and IL-10 productions in metastatic melanoma cells. a IL-6 production by fresh metastatic melanoma cell suspensions. Three fresh metastatic melanoma cell suspensions produced high levels of IL-6 (>1,000 pg/ml, high IL-6 producers, solid bars), while seven suspensions produced small or no quantity of IL-6 (low IL-6 producers, open bars) in 18-h culture. b Increased IL-10 production by fresh metastatic melanoma cell suspensions in response to exogenously added IL-6. Fresh metastatic melanoma cell suspensions were cultured with various concentration of rhIL-6. “Control, 0 ng/ml” indicates constitutive production of IL-10 without IL-6 stimulation. Two of 7 low IL-6 producers (DIL and Ho88) produced IL-10 constitutively and increased IL-10 production in response to exogenously added IL-6 in a dose-dependent manner. The rest of low IL-6 producers had minimum or no constitutive production of IL-10 and did not responded to exogenously added IL-6. All three high IL-6 producers (Cla, Beu, Ho91) constitutively secreted IL-10 with mild increase in IL-10 production in response to high dose of IL-6. *P < 0.05, **P < 0.01. c Maintaining IL-6-induced IL-10 production in long-term cultured metastatic melanoma cell lines. Two cell lines (DIL and Ho88) established from low IL-6 producers maintained their capability of producing IL-10 in response to exogenously added IL-6 in a dose-dependent manner. The cell line, Beu, established from a high IL-6 producer continued to produce the high amount of IL-6 and responded to exogenous IL-6 only at the highest dose (20 ng/ml). In contrast, the two cell lines (Cr and Cla) did not produce IL-10 and did not respond to IL-6. *P < 0.05, **P < 0.01
From these 10 fresh melanoma specimens, five stable melanoma cell lines (more than five passages) were subsequently established. Three cell lines were obtained from low IL-6 producers (DIL, Ho88, and Cr), and two cell lines were established from high IL-6 producers (Cla, Beu). The characterization of these melanoma cell lines is shown in Table 2. Two cell lines (DIL, Ho88) obtained from low IL-6 producing melanoma specimens that initially produced IL-10 continued to produce a small amount of IL-6 and increased their production of IL-10 in response to exogenous IL-6 (Fig. 3c). On the other hand, the cell line (Cr) that was obtained from a melanoma specimen that did not produce IL-10 did not respond to exogenous IL-6 (IL-10 production <4 pg/ml). Two cell lines (Cla, Beu) obtained from high IL-6 producers (black bars in Fig. 3a) maintained their IL-6 production; however, only one cell line (Beu) continued to produce IL-10. The cell line, Beu, continued to produce a significant amount of IL-6 and responded to exogenously added IL-6 only at the highest dose. On the other hand, the cell line, Cla, stopped producing IL-10 and did not respond to exogenously added IL-6, despite the presence of IL-6 receptors (Table 2). In total, 4 of four melanoma cell lines (CM005, DIL, Ho88, Beu) that constitutively produce IL-10 increased IL-10 production in response to exogenously added IL-6. On the other hand, two of two cell lines (Cr, Cla) that do not produce IL-10 did not respond to exogenously added IL-6.
Table 2.
Characteristics of skin melanoma cell lines derived from lymph node metastases
| Melanoma cell lines | Mutation | CD126 (%) | CD130 (%) | sIL-6Ra (pg/ml) | IL-6 (pg/ml) | IL-10 (pg/ml) |
|---|---|---|---|---|---|---|
| CM005 | BRAFV600E | 10.5 | 60.6 | 159.4 | Negative | 130.0 |
| Cr | BRAFV600E | Negative | 75.7 | 546.7 | 100.7 | Negative |
| Ho88 | BRAFV600E | Negative | 38.0 | 188.2 | 132.8 | 1,842.7 |
| DIL | BRAFV600K | Negative | 15.2 | 181.5 | 100.0 | 40.0 |
| Cla | BRAFV600E | Negative | 49.3 | 85.8 | 300.0 | Negative |
| Beu | BRAFV600E | Negative | 24.5 | 29.2 | 2,058.6 | 434.6 |
IL-10 production via IL-6 signaling pathway
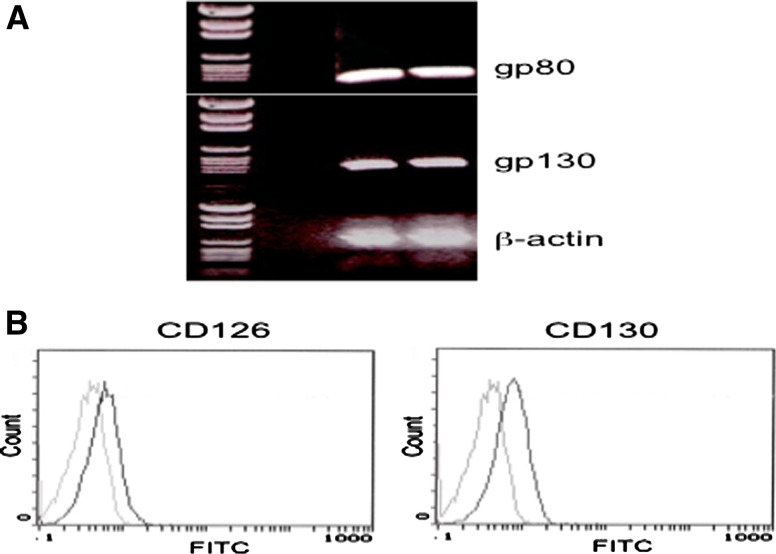
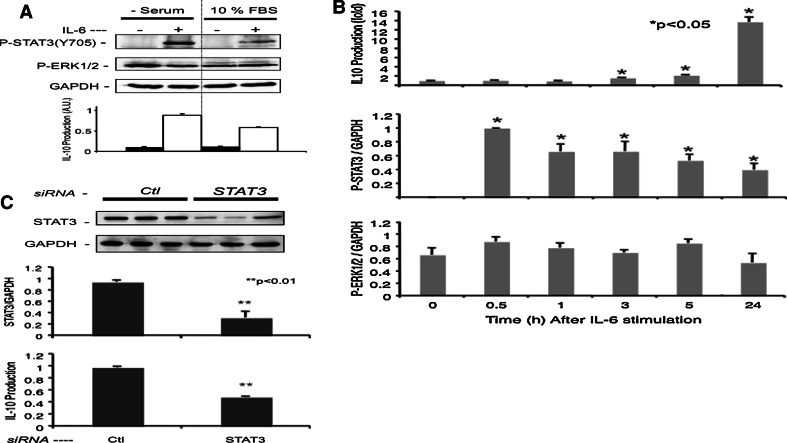
We further investigated the molecular mechanism of IL-6-induced IL-10 production by melanoma cells. Since the CM005 cells stably produce IL-10, increase IL-10 production in response to exogenous IL-6, and do not constitutively produce IL-6, this cell line was chosen for investigations regarding the interaction between IL-6 signaling pathways and IL-10 production. RT–PCR showed that CM005 cells expressed gp80 (CD126) and gp130 (CD130) mRNAs (Fig. 4a). FACS analysis confirmed the expression of CD126 and CD130 proteins on the surface of CM005 cells (Fig. 4b). Figure 5 shows simultaneous assay of IL-6-induced IL-10 production along with phosphorylation of STAT3 and ERK1/2. The CM005 cells were harvested after 16 h in the absence or presence of 10% FBS and then stimulated with 2 ng/ml of rhIL-6 for 24 h. A 24 h stimulus with rhIL-6 increased IL-10 production and STAT3 phosphorylation at Tyr705 (Fig. 5a). On the other hand, ERK1/2 was spontaneously phosphorylated as expected with the basis of the BRAFV600E genotype of this cell line. STAT3 phosphorylation was slightly higher upon serum starvation, compared to the cells cultured in medium containing 10% FBS (Fig. 5a). To avoid unknown effects of serum on the IL-6 signal pathways, subsequent experiments were performed without serum using quiescent cells. As shown in Fig. 5b, STAT3 phosphorylation was peaked at 30 min. IL-10 production started increasing at 3 h after rhIL-6 stimulation and sustained for 24 h. On the other hand, ERK1/2 was unchanged upon IL-6 stimulation (Fig. 5b). As shown in Fig. 5c, the STAT3-specific siRNA knockdown reduced STAT3 expression by 70% in CM005 cells, in parallel with a 50% reduction in IL-10 production, suggesting the role of STAT3 in IL-6-induced IL-10 production.
Fig. 4.
Expression of IL-6 receptors in CM005 cells. a Expression of mRNA for gp80 and gp130. b Expression of CD126 (gp80) and CD130 (gp130) on the surface of CM005 melanoma cells. Thick line: specific antibody, thin line: isotype-matched control antibody
Fig. 5.
Involvement of STAT3 in IL-6-induced production of IL-10. a STAT3 and ERK phosphorylation after stimulation of CM005 melanoma cells with IL-6. The CM005 cells were harvested after 16 h in the absence or presence of 10% FBS and then stimulated with 2 ng/mol of rhIL-6 for 24 h. Phosphorylation of STAT3 (Y705) and subsequent production of IL-10 after IL-6 stimulation were more dominant in CM005 cells cultured in serum-free medium. ERK1/2 was spontaneously phosphorylated without IL-6 stimulation. b Kinetics of IL-6-induced IL-10 production and its association with STAT3 and ERK activation. CM005 cells were stimulated with rhIL-6 (2 ng/ml) for 30 min, 1 h, 3 h, 5 h, and 24 h. The IL-10 levels in supernatant were analyzed by ELISA. Intensities of phosphorylated STAT3 (STAT3Y705) and ERK1/2 bands were compared to those of GAPDH control by the Western blot. *P < 0.05. c Blocking of STAT 3 by siRNA and subsequent decrease in IL-6-induced IL-10 production. After a 2-day knockdown with siRNA fragments of control (Ctl) and STAT3, quiescent CM005 cells were stimulated with 2 ng/ml of IL-6 for 24 h. The STAT3 transcription was decreased by STAT3 siRNA and IL-10 production after IL-6 stimulation was also decreased. **P < 0.01
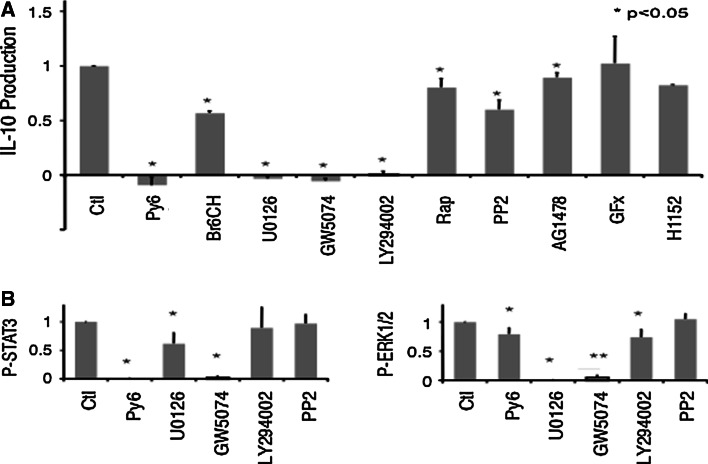
We further investigated signals mediated by IL-6 stimulation of IL-10 production, using various kinase inhibitors listed in Table 1. As shown in Fig. 6a, IL-10 production was completely suppressed by addition of Py6, a pan JAK inhibitor, while Br6CH, a JAK2-specific inhibitor, partially affected the IL-10 production. Tyrosine kinase inhibitors for Src (PP2) and the EGFR (AG1478) had only marginal effects on the IL-10 production. Indeed, Py6 induced inhibition of STAT3 phosphorylation, which is consistent with the inhibition of IL-10 production (Fig. 6b). Furthermore, U0126 and GW5074 completely abrogated IL-10 production by CM005 cells. U0126, a MEK inhibitor, eliminated ERK1/2 phosphorylation (Fig. 6c), with a marginal reduction in STAT3 phosphorylation (Fig. 6b). Thus, suppression of ERK1/2 seems to be sufficient to block IL-6-induced IL-10 production without affecting STAT3 activation. Due to the reduction in STAT3 phosphorylation, an off-target effect of GW5074 (c-RAF inhibitor) is evident. In addition to STAT3 and ERK1/2, LY294002, a PI3K inhibitor, potently blocked IL-10 production. Interestingly, a known immunosuppressant, rapamycin (Rap), showed a minimum effect on the IL-10 production, suggesting that the IL-10 production via PI3K signal is independent of the AKT-mTOR pathway. PKC and ROCK inhibitors (GFx and H1152) had no effect on the IL-6-induced IL-10 production by CM005 cells. These results suggest that the inhibition of just one (STAT3, ERK1/2, or PI3K) signal pathway is sufficient to interfere with IL-6-induced IL-10 production by CM005 cells.
Fig. 6.
Inhibitions of IL-6 pathways to block IL-10 production by CM005 melanoma cells. a Inhibition of IL-6-induced IL-10 productions by various kinase inhibitors. CM005 cells were treated with various kinase inhibitors 30 min prior to rhIL-6 (2 ng/ml) stimulation. After 24 h of incubation, the production of IL-10 in the supernatant was analyzed by ELISA assay. The data were shown as a fraction in comparison with IL-10 production in control (Ctl, no inhibitor). *P < 0.05. b Suppression of STAT3 and ERK1/2 phosphorylation by kinase inhibitors. After treatment with individual kinase inhibitors followed by IL-6 stimulation, CM005 cells were lysed and phosphorylation of STAT3 (left) and ERK1/2 (right) was measured by Western blot. The intensity of bands for phosphorylated STAT and ERK1/2 was compared to that of control (Ctl, no inhibitor). *P < 0.05, **P < 0.01
Discussion
The current study indicates that exogenously added IL-6 directly stimulates the production of IL-10 by melanoma cells if those melanoma cells constitutively produce IL-10 protein. The increase in IL-10 production by exogenously added IL-6 was more significant in melanoma cells that produce relatively small amount of IL-6. The increase in IL-6-mediated IL-10 production was relatively small in melanoma cells that constitutively produce high amount of IL-6. This is probably due to pre-existing stimulation via IL-6 receptors by endogenously produced IL-6. Comparison of data obtained from freshly isolated metastatic melanoma single-cell suspensions and established cell lines from these fresh melanoma cells indicates that the observed phenomena are not an artifact developed during the long-term cell culture. This study also suggests that the autocrine production of IL-6 by melanoma cells could be associated with production of IL-10 although the mechanism of these interactions is complex and remains to be investigated.
IL-6 is a pleiotropic immune modulatory cytokine produced by a variety of immune cells as well as tumor cells. As reported for IL-10 [7, 19], IL-6 level in blood also is elevated in patients affected with malignant tumors including colon cancer [19], renal cell carcinoma [20], hepatocellular carcinoma [21], and melanoma [22]. IL-6 promotes cell growth in advanced stage melanoma, and high IL-6 levels in melanoma patients were associated with large tumor burden [22]. IL-6 binds to the IL-6R (gp80), present either on the cell surface or in solution, and induces dimerization of gp130, resulting in phosphorylation of the associated JAKs such as JAK1, JAK2, or TYK2 [23, 24]. The soluble receptor binds IL-6 with an affinity similar to that of the cognate receptor (0.5–2 nM) [25, 26]. The (sIL-6R/IL-6) complex is capable of activating cells via interaction with membrane-bound gp130. The phosphorylation of IL-6R (gp130) and JAKs permits the binding of STAT3, resulting in the phosphorylation of STAT3. Phospho-STAT3 forms a dimmer and translocates into the nucleus to regulate target gene transcription [27, 28]. In parallel, the phosphorylation of IL-6R (gp130) and JAKs activates MAP kinases, PI3 kinase/Akt and other cellular pathways through the binding of adaptor proteins [29]. Thus, multiple signal pathways are triggered in response to IL-6R/JAK activation.
The importance of IL-6 signaling in mediating tumorigenesis has been examined. However, the association between IL-6 signaling and production of IL-10 has not been well studied. We found that IL-6-induced IL-10 production by melanoma cells was inhibited by siRNA knockdown of STAT3 and a JAK inhibitor. The inhibition of ERK1/2 pathway and PI3K blocks IL-10 production accompanied with minimal effects on STAT3 phosphorylation. Therefore, in addition to STAT3 activation, ERK1/2 and PI3K signals are important in IL-10 production by melanoma cells. However, further study on the regulatory circuit is necessary for dissection of the signal transduction related to IL-6-induced IL-10 production in melanoma cells. As for immune cells such as macrophages, Sp1 has been suggested to play an important role in IL-10 promoter activation. The ERK activation following macrophage FcγR ligation leads to histone H3 phosphorylation across the promoter region of the IL-10 gene and facilitates the binding of Sp1 and STAT3 [30]. Although the data suggest the involvement of signaling pathways similar to that found in our study, the interaction between these signaling pathways and transcription of IL-10 message in cancer cells remains to be investigated.
It is of note that the CM005 cell has a BRAFV600E point mutation, which is consistent with the spontaneous phosphorylation of ERK1/2 detected in the cells. The BRAF gene mutations, especially substitution for valine by glutamic acid, BRAFV600E, in exon 15, are frequently observed in human melanoma [31]. This mutation causes increased kinase activation and signaling through the MAP kinase pathway [32, 33]. In melanoma cells with BRAFV600E mutation, both MAPK and STAT3 pathways are essential for the production of immunosuppressive factors including IL-10, IL-6, and VEGF [34]. However, BRAF mutation is not considered to be pre-requisite for IL-6-induced production of IL-10 since we confirmed that exogenous IL-6 also induces the production of IL-10 in a malignant melanoma cell line without BRAF mutation (unpublished data). BRAFV600E inhibitors have been under clinical investigation for anti-melanoma treatment, and it is interesting to test whether these antagonists suppress IL-10 production by melanoma cells, which might contribute to the clinical efficacy of BRAF blockades.
In summary, our data suggest that a subset of human melanomas would produce IL-10 in response to microenvironmental IL-6 release. Blockage of the IL-10 produced by melanoma cells potentially increases anti-tumor immunity [35]. Together with the data presented in this paper and published data by others, we propose signals in IL-6/STAT3/IL-10 as potential targets to disrupt the interaction of tumor and tumor-associated immune cells.
Acknowledgments
We thank Dr. Andrew Aplin at Kimmel Cancer Center of Thomas Jefferson University and Dr. Matthew J. McGinniss at Caris Life Sciences for analysis of BRAF and NRAS mutations on melanoma cell lines. We also thank Dr. Henry C. Maguire, Jr. for critical review of this article. This research was supported by the Bonnie Kroll Research Fund, the Marla Brecher Research Fund, and the Eye Melanoma Research Fund at Thomas Jefferson University. M Eto and G. D. Young were supported by a Pennsylvania CURE grant.
Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Colombo MP, Mantovani A. Targeting myelomonocytic cells to revert inflammation-dependent cancer promotion. Cancer Res. 2005;65:9113–9116. doi: 10.1158/0008-5472.CAN-05-2714. [DOI] [PubMed] [Google Scholar]
- 2.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 3.Moore KW, O’Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 4.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy—review of a new approach. Pharmacol Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 5.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 6.Lattime EC, Mastrangelo MJ, Bagasra O, Li W, Berd D. Expression of cytokine mRNA in human melanoma tissues. Cancer Immunol Immunother. 1995;41:151–156. doi: 10.1007/BF01521340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato T, McCue P, Masuoka K, et al. Interleukin 10 production by human melanoma. Clin Cancer Res. 1996;2:1383–1390. [PubMed] [Google Scholar]
- 8.Kim J, Modlin RL, Moy RL, et al. IL-10 production in cutaneous basal and squamous cell carcinomas. A mechanism for evading the local T cell immune response. J Immunol. 1995;155:2240–2247. [PubMed] [Google Scholar]
- 9.Ordemann J, Jacobi CA, Braumann C, Schwenk W, Volk HD, Muller JM. Immunomodulatory changes in patients with colorectal cancer. Int J Colorectal Dis. 2002;17:37–41. doi: 10.1007/s003840100338. [DOI] [PubMed] [Google Scholar]
- 10.Knoefel B, Nuske K, Steiner T, et al. Renal cell carcinomas produce IL-6, IL-10, IL-11, and TGF-beta 1 in primary cultures and modulate T lymphocyte blast transformation. J Interferon Cytokine Res. 1997;17:95–102. doi: 10.1089/jir.1997.17.95. [DOI] [PubMed] [Google Scholar]
- 11.Green AR, Green VL, White MC, Speirs V. Expression of cytokine messenger RNA in normal and neoplastic human breast tissue: identification of interleukin-8 as a potential regulatory factor in breast tumours. Int J Cancer. 1997;72:937–941. doi: 10.1002/(SICI)1097-0215(19970917)72:6<937::AID-IJC3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 12.Dummer W, Becker JC, Schwaaf A, Leverkus M, Moll T, Brocker EB. Elevated serum levels of interleukin-10 in patients with metastatic malignant melanoma. Melanoma Res. 1995;5:67–68. doi: 10.1097/00008390-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Nacinovic-Duletic A, Stifter S, Dvornik S, Skunca Z, Jonjic N. Correlation of serum IL-6, IL-8 and IL-10 levels with clinicopathological features and prognosis in patients with diffuse large B-cell lymphoma. Int J Lab Hematol. 2008;30:230–239. doi: 10.1111/j.1751-553X.2007.00951.x. [DOI] [PubMed] [Google Scholar]
- 14.Guney N, Soydinc HO, Basaran M, et al. Serum levels of interleukin-6 and interleukin-10 in Turkish patients with aggressive non-Hodgkin’s lymphoma. Asian Pac J Cancer Prev. 2009;10:669–674. [PubMed] [Google Scholar]
- 15.Mapara MY, Sykes M. Tolerance and cancer: mechanisms of tumor evasion and strategies for breaking tolerance. J Clin Oncol. 2004;22:1136–1151. doi: 10.1200/JCO.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 16.Berd D, Sato T, Maguire HC, Jr, Kairys J, Mastrangelo MJ. Immunopharmacologic analysis of an autologous, hapten-modified human melanoma vaccine. J Clin Oncol. 2004;22:403–415. doi: 10.1200/JCO.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 17.Berd D, Maguire HC, Jr, Schuchter LM, et al. Autologous hapten-modified melanoma vaccine as postsurgical adjuvant treatment after resection of nodal metastases. J Clin Oncol. 1997;15:2359–2370. doi: 10.1200/JCO.1997.15.6.2359. [DOI] [PubMed] [Google Scholar]
- 18.Berd D, Maguire HC, Jr, Mastrangelo MJ. Induction of cell-mediated immunity to autologous melanoma cells and regression of metastases after treatment with a melanoma cell vaccine preceded by cyclophosphamide. Cancer Res. 1986;46:2572–2577. [PubMed] [Google Scholar]
- 19.Galizia G, Orditura M, Romano C, et al. Prognostic significance of circulating IL-10 and IL-6 serum levels in colon cancer patients undergoing surgery. Clin Immunol. 2002;102:169–178. doi: 10.1006/clim.2001.5163. [DOI] [PubMed] [Google Scholar]
- 20.Negrier S, Perol D, Menetrier-Caux C, et al. Interleukin-6, interleukin-10, and vascular endothelial growth factor in metastatic renal cell carcinoma: prognostic value of interleukin-6–from the Groupe Francais d’Immunotherapie. J Clin Oncol. 2004;22:2371–2378. doi: 10.1200/JCO.2004.06.121. [DOI] [PubMed] [Google Scholar]
- 21.Porta C, De Amici M, Quaglini S, et al. Circulating interleukin-6 as a tumor marker for hepatocellular carcinoma. Ann Oncol. 2008;19:353–358. doi: 10.1093/annonc/mdm448. [DOI] [PubMed] [Google Scholar]
- 22.Mouawad R, Benhammouda A, Rixe O, et al. Endogenous interleukin 6 levels in patients with metastatic malignant melanoma: correlation with tumor burden. Clin Cancer Res. 1996;2:1405–1409. [PubMed] [Google Scholar]
- 23.Taga T, Hibi M, Hirata Y, et al. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 24.Mackiewicz A, Schooltink H, Heinrich PC, Rose-John S. Complex of soluble human IL-6-receptor/IL-6 up-regulates expression of acute-phase proteins. J Immunol. 1992;149:2021–2027. [PubMed] [Google Scholar]
- 25.Oh JW, Revel M, Chebath J. A soluble interleukin 6 receptor isolated from conditioned medium of human breast cancer cells is encoded by a differentially spliced mRNA. Cytokine. 1996;8:401–409. doi: 10.1006/cyto.1996.0055. [DOI] [PubMed] [Google Scholar]
- 26.Muller-Newen G, Kuster A, Hemmann U, et al. Soluble IL-6 receptor potentiates the antagonistic activity of soluble gp130 on IL-6 responses. J Immunol. 1998;161:6347–6355. [PubMed] [Google Scholar]
- 27.Winston LA, Hunter T. JAK2, Ras, and Raf are required for activation of extracellular signal-regulated kinase/mitogen-activated protein kinase by growth hormone. J Biol Chem. 1995;270:30837–30840. doi: 10.1074/jbc.270.52.30837. [DOI] [PubMed] [Google Scholar]
- 28.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 29.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas M, Zhang X, Prasanna V, Mosser DM. ERK activation following macrophage FcgammaR ligation leads to chromatin modifications at the IL-10 locus. J Immunol. 2005;175:469–477. doi: 10.4049/jimmunol.175.1.469. [DOI] [PubMed] [Google Scholar]
- 31.Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. [PubMed] [Google Scholar]
- 32.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–867. doi: 10.1016/S0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 33.Goodall J, Wellbrock C, Dexter TJ, Roberts K, Marais R, Goding CR. The Brn-2 transcription factor links activated BRAF to melanoma proliferation. Mol Cell Biol. 2004;24:2923–2931. doi: 10.1128/MCB.24.7.2923-2931.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terai M, Tamura Y, Alexeev V, et al. Human interleukin 10 receptor 1/IgG1-Fc fusion proteins: immunoadhesins for human IL-10 with therapeutic potential. Cancer Immunol Immunother. 2009;58:1307–1317. doi: 10.1007/s00262-008-0644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]