Abstract
Background
Although regulatory T cells (Tregs) are thought to play an important role in immune suppression, their clinical significance in head and neck squamous cell carcinoma (HNSCC) is unclear. A recent study reported Tregs could be divided into functional subsets based on the expression of CD45RA and Foxp3.
Method
The frequency of circulating Treg subsets was analyzed in patients with HNSCC and compared with the frequency in patients with benign tumors. The association of Treg subsets with the frequency of lymphocyte subsets, status of progression, clinical course, and prognosis were also examined.
Results
The frequency of CD4+Foxp3+ Tregs was comparable between HNSCC patients and age-matched benign tumor patients; however, CD45RA−Foxp3high Tregs were significantly increased in HNSCC patients, in particular those with advanced stage tumors. The high frequency of CD45RA−Foxp3high Tregs correlated with a poor prognosis and the low frequency of CD45RA−Foxp3high Tregs before treatment showed a better clinical outcome, even in patients with advanced stage tumors. CD45RA−Foxp3high Treg numbers were decreased after intensive treatments; however, Treg numbers recovered in the early stages of recurrent cases, even before the clinical manifestation.
Conclusion
CD45RA−Foxp3high Tregs are associated with the clinical course of HNSCC and might be a new target for treatment and an early marker of tumor recurrence in HNSCC patients.
Keywords: Head and neck cancer, Regulatory T cell, Prognosis, Biomarker, Immunotherapy
Introduction
Despite advances in combination treatment modalities involving surgery, radiotherapy, and chemotherapy, the recurrence rate of patients with advanced head and neck squamous cell carcinoma (HNSCC) remains high and the survival rate remains low [1, 2]. The poor prognosis and low responsiveness to treatments are thought to be linked to immune system defects [3–5]. It has been suggested that anti-tumor immune responses are defective in cancer patients based on the accumulation of immunosuppressive cells such as Tregs and myeloid-derived suppressor cells (MDSCs) [6–8]. Therefore, tumors can escape from immunosurveillance [9–11]. Additionally, it has been demonstrated that immune-cell therapy based on the abrogation of immune suppressive effects with molecular targeted drugs has high clinical efficacy against various cancers including HNSCC [12–16]. An enhanced understanding of cancer-induced immunosuppressive mechanisms will increase the development of new strategies for cancer treatment.
Tregs are immunosuppressive cells that have critical roles in the maintenance of immune homeostasis and the control of autoimmunity [17]. However, Tregs may also play a role in the suppression of anti-tumor immunity in patients with cancer.
Conventional CD4+CD25+Foxp3+ Tregs were elevated in the peripheral blood and tumors of patients with various cancers and suppressed the anti-tumor activity of effector cells [18, 19]. However, the clinical significance of Tregs in cancer patients, including HNSCC, remains unclear. Previous studies suggested that the number of CD4+CD25+Foxp3+ Tregs was associated with a poor prognosis in cancer patients [20–22]. In contrast, other studies demonstrated that Tregs did not correlate with a poor clinical outcome [23], and a high density of infiltrating Foxp3+ Tregs was associated with an improved prognosis in patients with various types of cancers, including HNSCC [24–26].
Recently, human CD4+Foxp3+ Tregs were reported to be composed of three phenotypically and functionally distinct subpopulations: CD45RA−Foxp3high cells, CD45RA+Foxp3low cells, and CD45RA−Foxp3low cells [27]. CD45RA−Foxp3high Tregs were immunosuppressive for CD4+CD25− T cells, were increased in the peripheral blood and cancerous tissues of cancer patients, and their frequency in patients correlated with tumor stage and metastases [27, 28].
We hypothesized that CD45RA−Foxp3high Tregs have a negative influence on antitumor immunity and are associated with the clinical outcome of HNSCC patients. Therefore, this study investigated the clinical significance of CD45RA−Foxp3high Tregs in HNSCC patients by monitoring the level of circulating Treg subsets and analyzing their association with the frequency of peripheral lymphocytes, serum cytokines, clinical stage, treatments, recurrence, and prognosis.
Materials and methods
Study subjects
Forty-six patients with HNSCC (36 males and 10 females with a mean age of 63.8 years; range 49–86 years) and 23 age-matched patients with benign tumors (12 males and 11 females with a mean age of 60.7 years; range 39–78 years) were enrolled in this study. The clinical and pathological characteristics are summarized in Table 1. All HNSCC patients were enrolled in the study before receiving standard treatments, including surgery, radiotherapy, and chemotherapy. Nine cases received surgery, 22 were treated with combined chemotherapy and radiotherapy (CCRT), 4 received CCRT and surgery, 3 were treated with combined surgery and radiotherapy, and 7 received radiotherapy alone. One patient did not receive any treatment because he chose palliative care. Cisplatin or cetuximab was used for combined chemotherapy. The initial treatments are summarized in Table 2. All patients were examined once a month and underwent a CT scan or MRI at 1 month and every 3 months after treatment. Patients were excluded if they had tumor recurrence within 1 month after treatment or macroscopically visible residual tumors. Informed consent was obtained from all individual participants included in the study. All procedures performed in the studies involving human participants were approved by the institutional review board and were in accordance with the Helsinki Declaration and its later amendments or comparable ethical standards.
Table 1.
Characteristics of HNSCC patients
| Squamous cell carcinoma | Benign tumor | ||
|---|---|---|---|
| Total number | 46 | 23 | |
| Mean age (years) | 63.8 (49−86) | 60.7 (39–78) | |
| Gender | |||
| Male | 36 | 12 | |
| Female | 10 | 11 | |
| Tumor site | Tumor site | ||
| Nasopharynx | 1 | Submandibular gland | 1 |
| Oropharynx | 8 | Parotid gland | 10 |
| Maxillary sinus | 9 | Thyroid | 7 |
| Oral cavity | 12 | Larynx | 1 |
| Hypopharynx | 8 | Neck | 3 |
| Larynx | 8 | Paranasal sinus | 1 |
| TNM classification | Pathological diagnosis | ||
| Stage I | 5 | Pleomorphic adenoma | 5 |
| Stage II | 7 | Warthin‘s tumor | 4 |
| Stage III | 10 | Lymphoepithelial cyst | 2 |
| Stage IV | 24 | Adenomatous goiter | 6 |
| T1 | 6 | Papilloma | 1 |
| T2 | 14 | Follicular adenoma | 1 |
| T3 | 7 | Hematoma | 1 |
| T4 | 19 | Lymphangioma | 1 |
| N0 | 25 | Cyst | 1 |
| N1 | 6 | Lipoma | 1 |
| N2 | 14 | ||
| N3 | 1 | ||
TNM classification tumor/node/metastasis classification
Table 2.
Initial treatment for HNSCC patients
| Surgery | 9 | |
| RT | 7 | |
| Surgery, RT | 3 | |
| CCRT | CDDP | 12 |
| Cetuximab | 10 | |
| Surgery, CCRT | CDDP | 3 |
| Cetuximab | 1 | |
RT radiation therapy, CDDP cis-diamminedichloro-platinum (cisplatin), CCRT combined chemotherapy and radiotherapy
Blood samples
Peripheral blood mononuclear cells (PBMCs) were isolated from a heparinized venous blood sample by density gradient centrifugation. The blood was diluted 1:1 with saline before being layered onto Ficoll-Paque PLUS (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). After centrifugation, PBMCs were collected from the plasma–Ficoll interphase and used for flow cytometry and assays.
Antibodies and flow cytometry
PBMCs were stained with the following anti-human antibodies: anti-CD4-Pacific blue, anti-CD3-APC, anti-CD45RA-FITC, anti-HLA-DR-PE, anti-CD33-PE, anti-CD19-PE, anti-CD56-PE, anti-CD14-PerCP, anti-CD15-APC (all BD Bioscience, San Diego, CA, USA), and anti-CD25-APC-Cy7 (BioLegend, San Diego, CA, USA) for surface staining. Anti-CTLA4-PE and anti-Foxp3-eflour660 (eBioscience) were used for intracellular staining. Cell surface and intracellular staining were performed according to the manufacturer’s protocols. Granulocytic MDSCs (G-MDSC) were characterized as HLA-DR-negative, lineage (CD3, CD14, CD19, CD56)-negative, CD15-positive (HLA-DR−Lin−CD15+), and monocytic-MDSC (M-MDSC) were identified as CD14+HLA-DR−/low. Cell phenotypes were evaluated using the FACSCanto II system (BD Biosciences). Data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR, USA).
Cytokine assay
The levels of TGF-β and IL-10 cytokines in the plasma obtained from HNSCC patients were measured with Bio-Plex Pro (Bio-Rad Laboratories, Hercules CA, USA) according to the manufacturer’s protocol. Briefly, samples were exposed to an acidic condition to activate TGF-β. Following activation, the samples were diluted 1:32 in sample diluent. Antibody coupled capture beads were prepared and plated. After washing, the samples and standards were added in duplicate to beads in the wells. The detection antibodies were then added to each well, followed by streptavidin–phycoerythrin solution. The plate was measured with the Bio-Plex System. The absolute concentrations of the samples were determined by construction of a standard curve for each analysis.
Isolation of Treg subsets
Tregs were sorted according to the expression of CD4, CCR4, and CD127 instead of Foxp3 and CD45RA using the FACSAria II cell sorter (BD Biosciences). The fractions of CD4+CD127lowCCR4high cells and CD4+CD127interCCR4low cells corresponded to the fractions of CD45RA−Foxp3high Tregs and CD45RA+Foxp3low Tregs, respectively.
T cell proliferation assay
CD3+ T cells were purified from the whole blood of a healthy volunteer using RosetteSep Human CD3+ T cell Enrichment Cocktail (STEMCELL Technologies). Enriched CD3+ T cells were labeled with CellTrace CFSE (carboxyfluorescein diacetate succinimidyl ester) Cell Proliferation Kit (Molecular Probes, USA) according to the manufacturer’s instructions. CFSE-labeled CD3+ T cells (1 × 105/well) were stimulated with anti-CD3/28-coated beads in the presence of IL-2 (100 JRU/ml), and cultured alone or with CD4+CD127 lowCCR4high cells, or with CD4+CD127interCCR4low cells for 72 h (1:1 ratio). CFSE dilution in CD4+ T cells and CD8+ T cells was analyzed after 72 h by the FACSCanto II system (BD Biosciences).
Statistical analyses
The statistical analyses of the means were evaluated using the parametric unpaired t test or ANOVA, and correlations were assessed using Pearson’s correlation coefficients. The change in Tregs before and after treatment was analyzed using the parametric paired t test, and differences in the overall survival and relapse-free survival were determined using the Log-rank test. All p values were two-sided, and p values <0.05 were considered to be statistically significant.
Results
Increased frequency of CTLA-4highCD45RA−Foxp3high Tregs and their association with clinical stage in HNSCC patients
A representative staining profile of CD45RA and Foxp3 expressions on peripheral CD4+ T cells from a patient with HNSCC and a patient with a benign tumor before treatment are shown in Fig. 1a. An increase in the proportion of CD45RA−Foxp3high Tregs and a decrease of CD45RA+Foxp3low Tregs were observed in the HNSCC patient compared with the control patient. CD45RA−Foxp3high Tregs showed higher expressions of CTLA-4, CD25, and CCR4 on their surface than CD45RA+Foxp3low Tregs and CD45RA−Foxp3low Tregs (Fig. 1b). Two subsets of Tregs, CD45RA−Foxp3high Tregs and CD45RA+Foxp3low Tregs, were sorted according to the expression of CD4, CCR4, and CD127. We confirmed that the fractions of CD4+CD127lowCCR4high and CD4+CD127interCCR4low cells corresponded to the fraction of CD45RA−Foxp3high Tregs and CD45RA+Foxp3low Tregs, respectively. The suppressive activity of CD4+CD127lowCCR4high and CD4+CD127interCCR4low cells were assessed by co-culture with CD4+ T cells. CD4+CD127lowCCR4high cells induced a significant suppression of CD4+ T cell proliferation compared with the CD4+CD127interCCR4low cells (p < 0.0001) (Fig. 1c, d). Similar results were obtained with CD8+ T cell (data not shown). The percentage of CD4+Foxp3+ Tregs in patients with HNSCC (n = 46) was comparable to patients with benign tumors (n = 23) (Fig. 1e). However, the percentage of CD45RA−Foxp3high Tregs among CD4+ T cells and the ratio of CD45RA−Foxp3high Tregs to CD45RA+Foxp3low Tregs in HNSCC patients were significantly higher than in benign tumor patients (p < 0.05) (Fig. 1f). The percentage of CD45RA+Foxp3low Tregs in HNSCC tended to be lower than that of benign tumor patients, but the difference was not significant (p = 0.09) (Fig. 1g). The ratio of CD45RA−Foxp3high Tregs to CD45RA+Foxp3low Tregs in HNSCC was also significantly higher than in benign tumor patients (Fig. 1h).
Fig. 1.
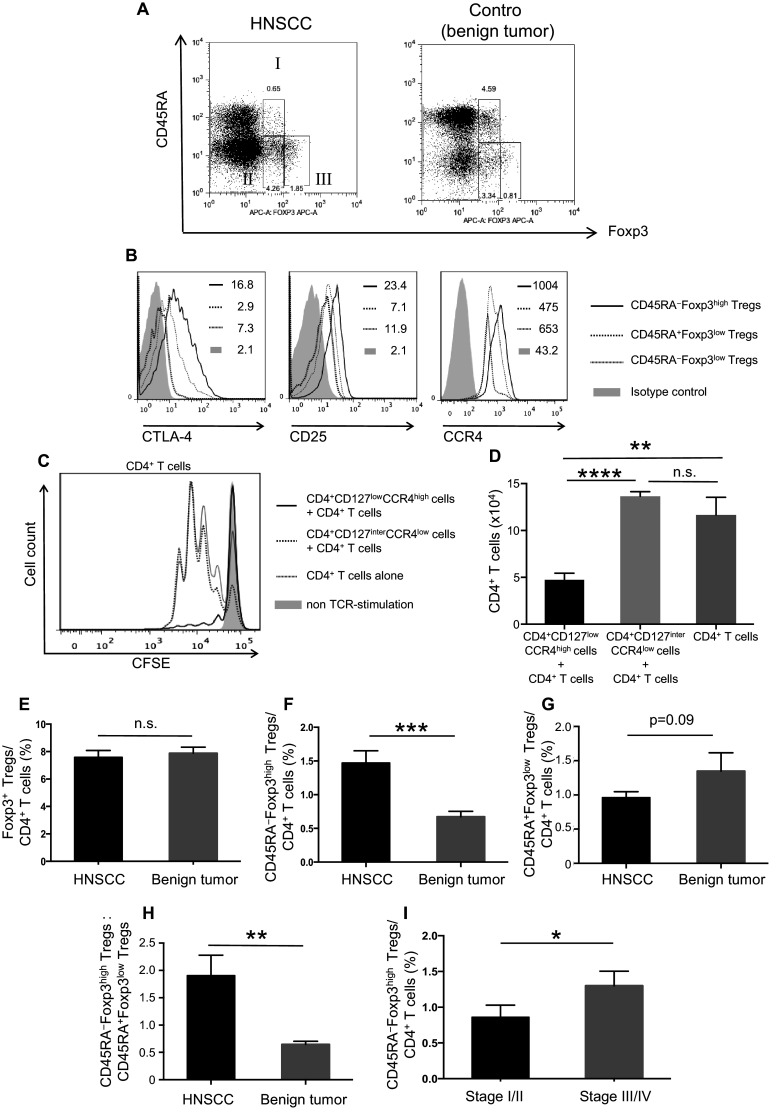
Frequency and characteristics of Treg subsets in patients with HNSCC and in those with benign tumors. The gating strategies for Treg subsets in the PBMCs of patients with HNSCC or benign tumors. I: CD45RA+Foxp3low, II: CD45RA−Foxp3low, and III: CD45RA−Foxp3high (a). The expression of CTLA-4, CD25, and CCR4 on Treg subsets in a representative patient with HNSCC (b). Mean fluorescence intensities (MFI) are indicated in the upper right quadrant. CFSE dilutions of proliferated CD4+ T cells were assessed after 72 h of TCR stimulation with or without indicated Treg subsets at a 1:1 ratio (c), and the number of proliferated CD4+ T cells after the co-culture are shown (d). The percentage of Foxp3+ cells in CD4+ T cells (e), CD45RA−Foxp3high Tregs in CD4+ T cells (F), CD45RA+Foxp3low Tregs in CD4+ T cells (g) and the ratio of CD45RA−Foxp3high Tregs to CD45RA+Foxp3low Tregs (h) in PBMCs of patients with HNSCC (n = 46) and benign tumors (n = 23) before treatment. The percentage of CD45RA−Foxp3high Tregs and the ratio of CD45RA−Foxp3high Tregs to CD45RA+Foxp3low Tregs were increased according to clinical stage (i). Statistical comparisons were performed using the parametric unpaired t test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. n.s. not significant, HNSCC head and neck squamous cell carcinoma, CTLA-4 cytotoxic T-lymphocyte antigen 4, CCR CC chemokine receptor, CFSE carboxyfluorescein diacetate succinimidyl ester, TCR T cell receptor
The percentage of CD45RA−Foxp3high Tregs and the ratio of CD45RA−Foxp3high Tregs to CD45RA+Foxp3low Tregs in patients with advanced stage tumors (cStage III and IV) were significantly higher than in patients with early stage tumors (cStage I and II) (p < 0.05) (Fig. 1I). There was no significant difference in the proportion of peripheral CD45RA−Foxp3high Tregs among patients with different primary cancer sites before treatment (data not shown).
Circulating CD45RA−Foxp3high Tregs negatively correlate with CD3+ cells and positively correlate with HLA-DR−Lin−CD15+ cells and plasma concentrations of TGF-β
The percentage of circulating CD45RA−Foxp3high Tregs and CD3+ cells exhibited a significant, negative correlation (p < 0.05, r = −0.26) (Fig. 2a). Similarly, the ratio of CD45RA−Foxp3high Tregs to CD45RA+Foxp3low Tregs significantly correlated with CD3+ cells (p < 0.01, r = −0.37) (Fig. 2b). Additionally, a significant positive correlation was observed between the percentage of HLA-DR−Lin−CD15+ cells and CD45RA−Foxp3high Tregs (p < 0.05, r = 0.34) (Fig. 2c) and the ratio of CD45RA−Foxp3high Tregs to CD45RA+Foxp3low cells (p < 0.001, r = 0.42) (Fig. 2d). The plasma concentration of TGF-β exhibited a positive correlation with the percentage of CD45RA−Foxp3high Tregs (p < 0.01, r = 0.32) (Fig. 2e) and the ratio of CD45RA−Foxp3high Tregs to CD45RA+Foxp3low Tregs (p < 0.0001, r = 0.49) (Fig. 2f). There was no difference in the plasma concentrations of IL-10 between groups (data not shown).
Fig. 2.
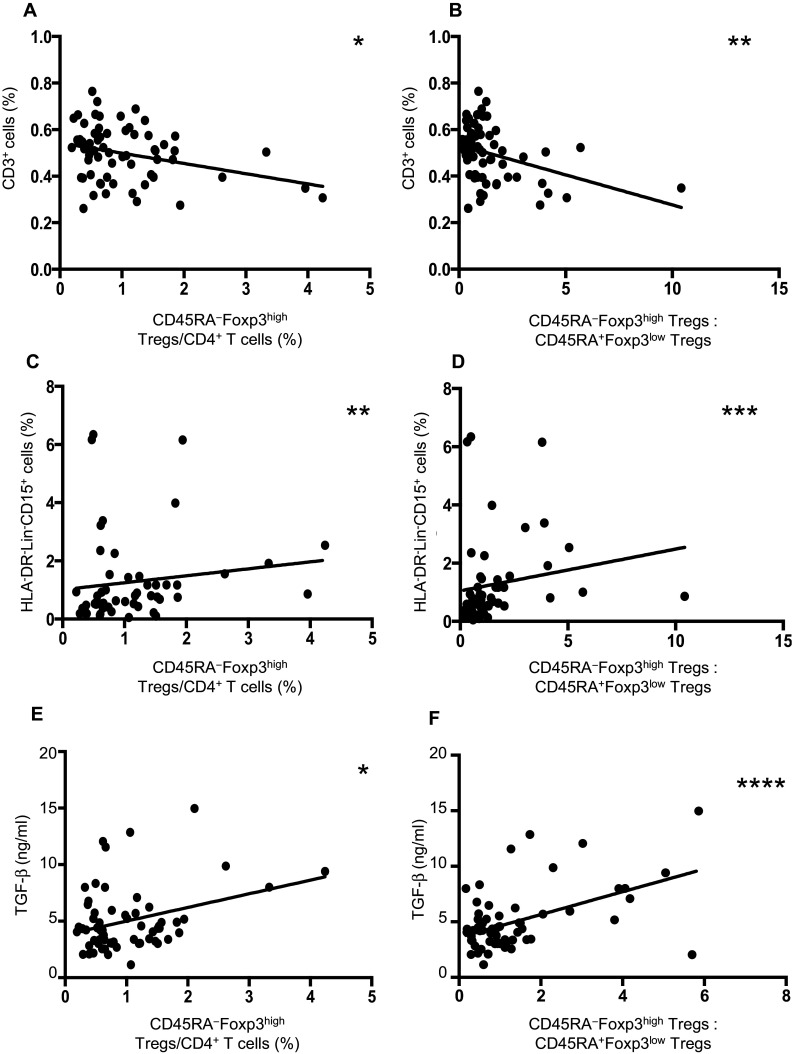
Association of the frequency of Treg subsets with peripheral lymphocytes and serum TGF-β levels in patients with HNSCC. Association of the frequency of CD3+ cells with CD45RA−Foxp3high Tregs (a) and the ratio of CD45RA−Foxp3high Tregs to CD45RA+Foxp3low Tregs (b) before treatment in HNSCC patients. Association of the frequency of HLA-DR−/lowCD14+ cells with CD45RA−Foxp3high cells (c) and the ratio of CD45RA−Foxp3high Tregs to CD45RA+Foxp3low Tregs (d) before treatment in HNSCC patients. Association of the plasma concentrations of TGF-β with CD45RA−Foxp3high Tregs (e) and the ratio of CD45RA−Foxp3high Tregs to CD45RA+Foxp3low Tregs (f) before treatment in HNSCC patients. Statistical analyses were performed using Pearson’s correlation coefficients. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, HLA human leukocyte antigen, Lin lineage
The frequency of CD45RA−Foxp3high Tregs before treatment is associated with tumor recurrence and prognosis of HNSCC patients
The mean value of the percentage of CD45RA−Foxp3high Tregs among all patients including benign tumor patients was 1.05%. Based on this, HNSCC patients at all disease stages (n = 46) were divided into two groups according to the percentage of CD45RA−Foxp3high Tregs among CD4+ T cells before treatment: a high percentage CD45RA−Foxp3high Treg group (>1%) (n = 20) and a low percentage CD45RA−Foxp3high Treg group (<1%) (n = 26). The high percentage CD45RA−Foxp3high Treg group in HNSCC patients had significantly low rates of overall survival and relapse-free survival compared with the low percentage CD45RA−Foxp3high Treg group (p < 0.05, p < 0.01, respectively) (Fig. 3a, b). In HNSCC patients with cStage IV (n = 24), the high percentage CD45RA−Foxp3high Treg group (n = 14) had significantly low rates of overall survival and relapse-free survival compared with the low percentage CD45RA−Foxp3high Treg group (n = 10) (p < 0.05, p < 0.05, respectively) (Fig. 3c, d).
Fig. 3.
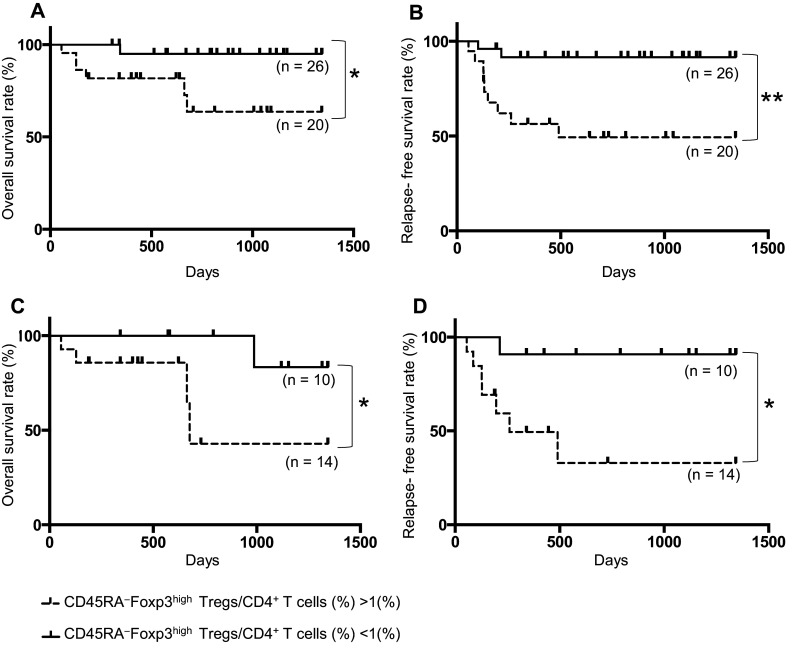
Dependency of survival rate on the frequency of CD45RA−Foxp3high Tregs before treatment in patients with HNSCC. The overall survival rate (a) and the relapse-free survival rate (b) between groups with a high (>1%; n = 20) or low (<1%; n = 26) percentage of CD45RA−Foxp3high Tregs before treatment in all stages of patients with HNSCC who were followed over 2 years after study entry. The overall survival rate (c) and relapse-free survival rate (d) between groups with a high (>1%; n = 14) or low (<1%; n = 10) percentage of CD45RA−Foxp3high Tregs before treatment in cStage IV of patients with HNSCC. Statistical comparisons were performed using the log-rank test *p < 0.05, **p < 0.01
Frequency of CD45RA−Foxp3high Tregs is associated with the clinical course in HNSCC patients
There was no significant difference in the proportion of Foxp3+ cells before and after 1 month of treatment for 23 patients who achieved complete remission (CR) after standard treatments and who were followed for over 2 years after study entry (Fig. 4a); however, the percentage of CD45RA−Foxp3high Tregs was significantly decreased after treatment (p < 0.001) (Fig. 4b). Six patients had tumor recurrence within 12 months after treatment, and showed no significant difference in the percentage of CD4+ Foxp3+ cells before and after 1 month or 3–6 months of treatment (Fig. 4c); however, the percentage of CD45RA−Foxp3high Tregs was significantly decreased to the levels of those with benign tumors at 1 month after treatment (p < 0.05), but was significantly increased after 3–6 months (p < 0.05) (Fig. 4d). In three of these six recurrent cases, an increased percentage of CD45RA−Foxp3high Tregs was confirmed before clinical examination using CT scan or MRI to detect tumor recurrence. In non-recurrent cases (n = 12), the percentage of CD45RA−Foxp3high Tregs was significantly decreased after 1 month of treatment (p < 0.01) and remained low after 3–6 months of treatment (Fig. 4e). The percentage of CD45RA−Foxp3high Tregs in patients who underwent surgery alone (n = 9) was significantly decreased after 1 month of treatment (p < 0.01) (Fig. 4f). In contrast, the percentage of CD45RA−Foxp3high Tregs in patients who underwent both chemotherapy and radiotherapy without surgery (n = 11) was not altered after 1 month of treatment; however, the percentage was significantly decreased after 3–6 months of treatment (n = 6), excluding recurring cases within 3 months after treatment (p < 0.05) (Fig. 4g). Similar results were obtained using the ratio of CD45RA−Foxp3high Tregs to CD45RA+Foxp3low Tregs (data not shown).
Fig. 4.
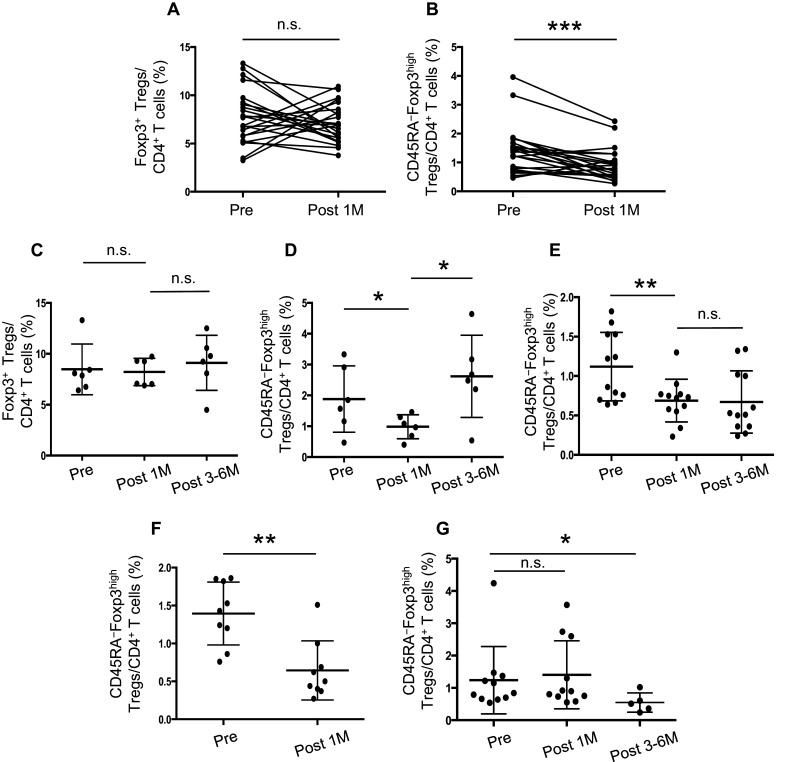
Frequency of Treg subsets in the clinical course of patients with HNSCC. The percentage of CD4+Foxp3+ cells (a) and CD45RA−Foxp3high Tregs (b) in PBMCs of patients with HNSCC (n = 23) before and after 1 month of standard treatments. The percentage of CD4+Foxp3+ cells (c), CD45RA−Foxp3high Tregs in recurrent cases (d) (n = 6), and CD45RA−Foxp3high Tregs in non-recurrent cases (e) (n = 12) of patients with HNSCC before treatment and after 1 month and 3–6 months of standard treatments. The percentage of CD45RA−Foxp3high Tregs in patients who underwent surgery (f) and chemoradiotherapy (g) before and after treatment. Statistical comparisons were performed using the paired t test. *p < 0.05, **p < 0.01, ***p < 0.001. n.s. not significant, M month
Discussion
In this study, we examined the frequency of Treg subsets, based on the functional classification of Tregs using CD45RA and Foxp3 expression, in PBMCs obtained from patients with HNSCC, and analyzed their association with clinical course, clinical stage, recurrence, and prognosis.
Although the frequencies of CD4+Foxp3+ Tregs were comparable between patients with HNSCC and patients with benign tumors, significantly higher numbers of CD45RA−Foxp3high Tregs and lower numbers of CD45RA+Foxp3low Tregs were observed in HNSCC patients compared with patients with benign tumors. Furthermore, the ratio of CD45RA−Foxp3high Tregs to CD45RA+Foxp3low Tregs was significantly increased in HNSCC patients.
The expression levels of CD25 and CTLA-4, which is an immune checkpoint molecule and a key negative regulator of T cell activation [29–31], on the surface of CD45RA−Foxp3high Tregs were higher than for CD45RA+Foxp3low Tregs. Additionally, the fraction of CD4+CD127lowCCR4high cells that corresponded to CD45RA−Foxp3high Tregs had a stronger suppressive activity to T cells than the CD4+CD127interCCR4low cells, which corresponded to CD45RA+Foxp3low Tregs. These results suggested that CD45RA−Foxp3high Tregs are a subpopulation with potent immunosuppressive activity. In this study, the number of CD45RA−Foxp3high Tregs and the ratio of CD45RA−Foxp3high Tregs to CD45RA+Foxp3low Tregs were negatively correlated with CD3+ cells. The frequency of CD45RA−Foxp3high Tregs was also significantly associated with the serum level of TGF-β. Therefore, an increased frequency of CD45RA−Foxp3high Tregs might be associated with the suppression of immune activity and the viability of effector T cells in tumor sites and the circulation, and might contribute to the expansion of tumors.
The underlying mechanism of the increased frequency of circulating CD45RA−Foxp3high Tregs in HNSCC patients remains unclear. However, the expression level of chemokine receptor CCR4 on the surface of CD45RA−Foxp3high Tregs was higher compared with CD45RA+Foxp3low Tregs. The CCR4 ligand, CCL22, is produced by tumor-infiltrating macrophages and tumor cells [32, 33]. Therefore, CD45RA−Foxp3high Tregs might be recruited to the tumor site by such tumor-resident cells. Additionally, MDSC were associated with the accumulation of Tregs at tumor sites [34]. In this study, HLA-DR−Lin−CD15+ cells, granulocytic MDSC, had a significant positive correlation with the percentage of CD45RA−Foxp3high Tregs. The recruitment of Tregs, or the de novo conversion of Foxp3− T cells into Tregs, might be induced in the tumor microenvironment by the presence of abundant Treg-inducing factors such as TGF-β, IL-10, and indoleamine 2,3-dioxygenase [35–38]. Abundant Tregs expressing immunosuppressive molecules and with potent immunosuppressive activity in the tumor site were reported [39]. The presence of these factors in the tumor microenvironment may play an important role in the accumulation and activation of Tregs in cancer patients.
In this study, there was no significant difference in the frequency of CD4+Foxp3+ Tregs in HNSCC patients before and after standard treatment. However, CD45RA+Foxp3low Tregs and the ratio of CD45RA−Foxp3high Tregs to CD45RA+Foxp3low Tregs were significantly decreased after treatment.
The high frequency of CD45RA−Foxp3high Tregs before treatment correlated with a significantly poor prognosis in HNSCC patients, whereas a low frequency of CD45RA−Foxp3high Tregs was associated with a better prognosis, even in patients with advanced stage tumors. The percentage of CD45RA−Foxp3high Tregs in patients who underwent surgery alone was significantly decreased because of the lack of suitable conditions for maintaining CD45RA−Foxp3high Treg expansion. However, the percentage of patients who underwent chemoradiotherapy slightly declined over time. Chemoradiotherapy itself might induce Tregs, or it might take longer to eliminate tumor tissues after chemoradiotherapy; thus, existing tumor tissues, regardless of viability, might be sufficient for the induction of Tregs. Recently, it was reported that naïve T cells could not differentiate to effector T cells even in the presence of presented antigens because of the long-lasting suppressive effect of Tregs [40, 41]. Because CD45RA−Foxp3high Tregs might maintain anergy in effector T cells for some time after treatment, the function of suppressed effector T cells may not recover even if the frequency of CD45RA−Foxp3high Tregs is decreased, as in those who achieved CR. This might explain why a high percentage of CD45RA−Foxp3high Tregs before treatment is associated with a poor prognosis. Therefore, patients with an increased frequency of CD45RA−Foxp3high Tregs after standard treatments may require further intensive treatment or immunomodulating therapy.
In this study, six patients suffered from tumor recurrence among 23 patients followed up for 24 months from study entry. In these recurrent cases, the percentage of CD45RA−Foxp3high Tregs was decreased after 1 month of treatment and then significantly increased again after 3–6 months; however, in non-recurrent cases, the percentage of CD45RA−Foxp3high Tregs remained low. Furthermore, in three cases, an increased frequency of CD45RA−Foxp3high Tregs was observed even before the detection of recurrence by clinical examination. These results suggested that the percentage of CD45RA−Foxp3high Tregs is closely associated with the clinical course and tumor status of HNSCC patients compared with CD4+Foxp3+ Tregs, and that the re-elevation of the frequency of circulating CD45RA−Foxp3high Tregs after treatment may reflect tumor recurrence. Thus, the frequency of circulating CD45RA−Foxp3high Tregs might be a useful marker of therapeutic effects and tumor recurrence. Moreover, the CD45RA−Foxp3high Tregs had a stronger suppressive activity to T cells compared with CD45RA+Foxp3low Tregs. Therefore, a targeted therapy that depletes CD45RA−Foxp3high Tregs might inhibit tumor progression and recurrence in combination with standard therapy and contribute to improving the prognosis of HNSCC patients.
In conclusion, our data demonstrated that increased frequencies of circulating CD45RA−Foxp3high Tregs were significantly associated with decreased frequencies of CD3+ T cells, tumor recurrence, and a poor prognosis in patients with HNSCC. These results suggest that CD45RA−Foxp3high Tregs are involved in the suppression of T cell-mediated anti-tumor activity and might be a useful marker for therapeutic efficacy and tumor recurrence as well as a new target for treatments to improve the prognosis of patients with HNSCC.
Acknowledgements
This study was funded by a Grants-in-Aid for Scientific Research from MEXT KAKENHI (Ministry of Education, Culture, Sports, Science and Technology) Grant number 15K10799.
Abbreviations
- CDDP
Cisplatin
- CCRT
Combined chemotherapy and radiotherapy
- CFSE
Carboxyfluorescein diacetate succinimidyl ester
- CR
Complete remission
- CTLA-4
Cytotoxic T-lymphocyte-associated protein 4
- HNSCC
Head and neck squamous cell carcinoma
- MDSCs
Myeloid derived suppressor cells
- PBMCs
Peripheral blood mononuclear cells
- RT
Radiation therapy
- Tregs
Regulatory T cells
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Fumie Ihara and Daiju Sakurai contributed equally to this study.
References
- 1.Howell GM, Grandis JR. Molecular mediators of metastasis in head and neck squamous cell carcinoma. Head Neck. 2005;27:710–717. doi: 10.1002/hed.20222. [DOI] [PubMed] [Google Scholar]
- 2.Weed DT, Vella JL, Reis IM, et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:39–48. doi: 10.1158/1078-0432.CCR-14-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albers AE, Strauss L, Liao T, Hoffmann TK, Kaufmann AM. T cell-tumor interaction directs the development of immunotherapies in head and neck cancer. Clin Dev Immunol. 2010;2010:236378. doi: 10.1155/2010/236378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duray A, Demoulin S, Hubert P, Delvenne P, Saussez S. Immune suppression in head and neck cancers: a review. Clin Dev Immunol. 2010;2010:701657. doi: 10.1155/2010/701657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freiser ME, Serafini P, Weed DT. The immune system and head and neck squamous cell carcinoma: from carcinogenesis to new therapeutic opportunities. Immunol Res. 2013;57:52–69. doi: 10.1007/s12026-013-8462-3. [DOI] [PubMed] [Google Scholar]
- 6.Horinaka A, Sakurai D, Ihara F, Makita Y, Kunii N, Motohashi S, Nakayama T, Okamoto Y. Invariant NKT cells are resistant to circulating CD15+ myeloid-derived suppressor cells in patients with head and neck cancer. Cancer Sci. 2015;107:207–216. doi: 10.1111/cas.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker KH, Beury DW, Ostrand-Rosenberg S. Myeloid-derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. Adv Cancer Res. 2015;128:95–139. doi: 10.1016/bs.acr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol. 2016;28:401–409. doi: 10.1093/intimm/dxw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnet M. Cancer; a biological approach. I. The processes of control. Br Med J. 1957;1:779–786. doi: 10.1136/bmj.1.5022.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 11.Fujiwara J, Sowa Y, Horinaka M, Koyama M, Wakada M, Miki T, Sakai T. The anti-obesity drug orlistat promotes sensitivity to TRAIL by two different pathways in hormone-refractory prostate cancer cells. Int J Oncol. 2016;48:854. doi: 10.3892/ijo.2015.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 16.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wing JB, Sakaguchi S. Foxp3(+) T(reg) cells in humoral immunity. Int Immunol. 2014;26:61–69. doi: 10.1093/intimm/dxt060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105–115. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alizadeh D, Larmonier N. Chemotherapeutic targeting of cancer-induced immunosuppressive cells. Cancer Res. 2014;74:2663–2668. doi: 10.1158/0008-5472.CAN-14-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okita R, Saeki T, Takashima S, Yamaguchi Y, Toge T. CD4+ CD25+ regulatory T cells in the peripheral blood of patients with breast cancer and non-small cell lung cancer. Oncol Rep. 2005;14:1269–1273. [PubMed] [Google Scholar]
- 21.Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, Omata H, Fujii H. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother. 2006;55:1064–1071. doi: 10.1007/s00262-005-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alhamarneh O, Agada F, Madden L, Stafford N, Greenman J. Serum IL10 and circulating CD4(+) CD25(high) regulatory T cell numbers as predictors of clinical outcome and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2011;33:415–423. doi: 10.1002/hed.21464. [DOI] [PubMed] [Google Scholar]
- 24.Badoual C, Hans S, Rodriguez J, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 25.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 26.Alvaro T, Lejeune M, Salvado MT, et al. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11:1467–1473. doi: 10.1158/1078-0432.CCR-04-1869. [DOI] [PubMed] [Google Scholar]
- 27.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Lin YC, Mahalingam J, Chiang JM, et al. Activated but not resting regulatory T cells accumulated in tumor microenvironment and correlated with tumor progression in patients with colorectal cancer. Inter J Cancer. 2013;132:1341–1350. doi: 10.1002/ijc.27784. [DOI] [PubMed] [Google Scholar]
- 29.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi T, Kishi A, Osaki M, Morikawa H, Prieto-Martin P, Wing K, Saito T, Sakaguchi S. Construction of self-recognizing regulatory T cells from conventional T cells by controlling CTLA-4 and IL-2 expression. Proc Natl Acad Sci USA. 2013;110:E2116–E2125. doi: 10.1073/pnas.1307185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci USA. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jafarzadeh A, Fooladseresht H, Minaee K, Bazrafshani MR, Khosravimashizi A, Nemati M, Mohammadizadeh M, Mohammadi MM, Ghaderi A. Higher circulating levels of chemokine CCL22 in patients with breast cancer: evaluation of the influences of tumor stage and chemokine gene polymorphism. Tumour Biol. 2015;36:1163–1171. doi: 10.1007/s13277-014-2739-6. [DOI] [PubMed] [Google Scholar]
- 33.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 34.Fujimura T, Kambayashi Y, Aiba S. Crosstalk between regulatory T cells (Tregs) and myeloid derived suppressor cells (MDSCs) during melanoma growth. Oncoimmunology. 2012;1:1433–1434. doi: 10.4161/onci.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou G, Levitsky HI. Natural regulatory T cells and de novo-induced regulatory T cells contribute independently to tumor-specific tolerance. J Immunology. 2007;178:2155–2162. doi: 10.4049/jimmunol.178.4.2155. [DOI] [PubMed] [Google Scholar]
- 36.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+ CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu VC, Wong LY, Jang T, et al. Tumor evasion of the immune system by converting CD4+ CD25− T cells into CD4+ CD25+ T regulatory cells: role of tumor-derived TGF-beta. J Immunology. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 38.Oleinika K, Nibbs RJ, Graham GJ, Fraser AR. Suppression, subversion and escape: the role of regulatory T cells in cancer progression. Clin Exp Immunol. 2013;171:36–45. doi: 10.1111/j.1365-2249.2012.04657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jie HB, Gildener-Leapman N, Li J, Srivastava RM, Gibson SP, Whiteside TL, Ferris RL. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer. 2013;109:2629–2635. doi: 10.1038/bjc.2013.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maeda Y, Nishikawa H, Sugiyama D, et al. Detection of self-reactive CD8(+) T cells with an anergic phenotype in healthy individuals. Science. 2014;346:1536–1540. doi: 10.1126/science.aaa1292. [DOI] [PubMed] [Google Scholar]
- 41.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]


