Abstract
Photodynamic therapy (PDT)–generated cancer vaccines have shown promising results in preclinical studies and are being introduced in the clinics. Using an SCCVII mouse squamous cell carcinoma-based whole-cell autologous PDT vaccine model developed in our previous work, we have examined systemic effects in vaccinated mice that could be related to the induction of acute phase response. The upregulation of gene encoding serum amyloid P component (prototypic mouse acute phase reactant) was detected in the liver and to a lesser degree in the tumor of vaccinated mice at 24 h post-PDT vaccine treatment. A strong upregulation of gene for heat shock protein 70 was found in both the liver and tumor of mice at 4 h after their PDT vaccine treatment. Changes in the expression of genes for glucocorticoid-induced leucine zipper and serum- and glucocorticoid-regulated kinase 1 that are highly responsive to glucocorticoid modulation were uncovered in both the tumor and liver of vaccinated mice. A rise in the levels of serum corticosterone was detected in mice at 24 h after PDT vaccine treatment. The results indicate that a sudden appearance of a large number of PDT vaccine cells elicits host responses for securing their optimized clearance, which in addition to producing seminal acute phase reactants includes the engagement of glucocorticoid hormones. It is becoming increasingly clear that a consummate execution of this process of PDT vaccine cell removal is critical for tumor antigen recognition and the attainment of potent antitumor immune response.
Keywords: Photodynamic therapy, Cancer vaccine, Heat shock protein-70, Acute phase response, Glucocorticoid hormones
Introduction
One of the most interesting applications of photodynamic therapy (PDT), a clinically established modality for the eradication of cancers and other lesions [1], is the development of PDT-generated cancer vaccines [2]. In standard application of PDT, tumor-focused illumination using light wavelengths matching absorption characteristics of the photosensitizing drug accumulated in the lesion after previous (usually systemic) administration produces cytotoxic reactive oxidative species directly in targeted lesions [1, 3]. In contrast, for PDT vaccine application, the photosensitizing drug is not administered to the tumor host nor is the tumor illuminated. Instead, the host/patient is injected a vaccine consisting of autologous tumor cells or their lysates previously treated with PDT in vitro [2].
The first report on PDT-generated vaccines was published by Gollnick et al. [4], followed by the findings from our laboratory [5]. In their work, the Gollnick team used lysates of Photofrin PDT-treated mouse tumor cells as a prophylactic vaccine against the challenge with the same tumor. Our work has established autologous therapeutic cancer vaccines based on PDT-treated tumor cells or ex vivo tumor tissue that are effective in attaining clearance of existing tumors [6–8]. While the earlier reports described the development of PDT vaccines relying exclusively on photosensitizer Photofrin [4, 5], subsequent studies revealed that various other photosensitizers, including hematoporphyrin monomethyl ether [9], benzoporphyrin derivative [6], chlorin e6 [7], and hypocrellin SL052 [10], can also be used for generating effective cancer vaccines.
From mounting evidence accumulated in various studies, it is clear that the therapeutic effect of PDT vaccine comes from its capacity to induce a tumor-specific immune response executed by cytotoxic T lymphocytes. The supporting evidence includes the effectiveness against mismatched tumor types, acquisition of resistance against re-challenge with the same tumor, effectiveness against tumors growing distantly from vaccination site, prompted accumulation of dendritic cells and their functional maturation, absence of the vaccine effect in hosts depleted of CD8+ T cells, induction of tumor-specific interferon-γ-secreting T cells, and detection of high numbers of degranulating CD8+ T cells in lesions regressing after vaccination but not in poorly responding lesions [2].
The therapeutic cancer vaccine generated by PDT that was developed in our laboratory belongs to the class of autologous whole-cell vaccines, with the vaccine cell corpses providing material for tumor antigen capture and presentation for attaining immune recognition of the tumor and breakdown of immune tolerance. A key element is the recognition by the host of the presence of cell death patterns on PDT vaccine cells and the engagement of dead cell disposal pathways conducive to the presentation of antigens that were present in the vaccine material [2, 7]. The advantages of whole-cell/polypeptide vaccination strategy over targeting specific epitopes are in securing a greater coverage of potential and diverse tumor antigens (even if most of them remain unknown) with covering also the determinants for engaging tumor-specific helper T cells; this reduces the risk of encountering “tumor escape” by downregulation of antigen expression [11].
The present report shows that PDT vaccine treatment has important systemic effects preceding the development of immune response, as it elicits an acute phase response with the activity of adrenal hormones.
Materials and methods
Tumor model
Squamous cell carcinoma SCCVII, a recognized model for poorly immunogenic head and neck cancer of spontaneous origin [12], was grown subcutaneously in syngeneic C3H/HeN mice. The tumors, implanted subcutaneously in the lower dorsal region, were treated when they reached 5 mm in the largest diameter. The vaccine therapy was carried out as a single peritumoral injection. Its therapeutic outcome was assessed by monitoring changes in tumor size determined by measurement of the lesion’s three orthogonal diameters using a caliper as described in detail elsewhere [8]. The approval for protocol used with mice was received from the Animal Care Committee of the University of British Columbia.
Vaccine treatment
Vaccine generation procedure, referred to as the “post-incubation vaccine protocol” was described in detail in earlier reports [7, 8]. Briefly, SCCVII cells (grown in alpha minimal essential medium supplemented with 10% fetal bovine serum) were treated by PDT by first exposing to photosensitizer chlorin e6 (ce6, Frontier Scientific Inc., Logan, UT, USA) at a concentration of 0.5 μg/ml in serum-free medium for 30 min at 37°C. The cells were next washed and treated with 1 J/cm2 of 665 ± 10 nm light at 15 mW/cm2. After PDT, the cells were kept in serum- and protein-free medium (S8284, Sigma Chemical Co., St. Louis, MO, USA) for 16 h at 37°C. The cells were then collected and concentrated for injecting at 2 × 107 per mouse. Their viability was less than 20% and over 50% were apoptotic. Before injection into mice, they were first treated with X-rays (60 Gy). The same number of SCCVII cells treated with X-rays only was used in the routine control vaccine group. In some experiments, 3,4-methylenedioxy-benzylidine-γ-butyrolactam (KNK437) was added to the medium at 25 μg/ml during the 16-h post-incubation period. This inhibitor of heat shock protein induction was obtained from EMD Bioscience (Mississauga, Ontario, Canada). For blocking the activity of glucocorticoid hormones, either glucocorticoid receptor antagonist mifepristone (M80046, Sigma) or glucocorticoid synthesis inhibitor metyrapone (856525 Sigma Aldrich) was injected i.p. (50 mg/kg and 100 mg/kg, respectively) in selected groups of mice immediately after vaccination.
Gene expression
Expression of the genes of interest was assessed using quantitative real-time RT-PCR described in detail in our previous reports [13]. Briefly, after total RNA isolation from homogenized tumor or liver tissue, 1-μg samples were processed using SuperScript III Platinum Two-Step qRT-PCR kit with SYBR green (Invitrogen Canada Inc., Burlington, Ontario, Canada). Expression of mouse Hsp70, SAP, and GILZ gene analysis including the details of their specific primers was described previously [13–15]. Primer for SGK-1 gene was GAGAAGGATGGGCCTGAACGAT, amplicon size of 171 bp. Housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was served for normalizing the expression of investigated genes.
Corticosterone ELISA
Commercial ELISA kit developed by Kamiya Biomedical Company (Seattle, WA, USA) was used for determining corticosterone levels in mouse serum samples as described in our previous work [15].
Statistical analysis
The difference between means of the data for various treatment groups was analyzed using nonparametric Mann–Whitney test, while tumor growth inhibition results were statistically evaluated using log-rank test. For determining whether the groups were statistically different, the significance threshold was set at 5%.
Results
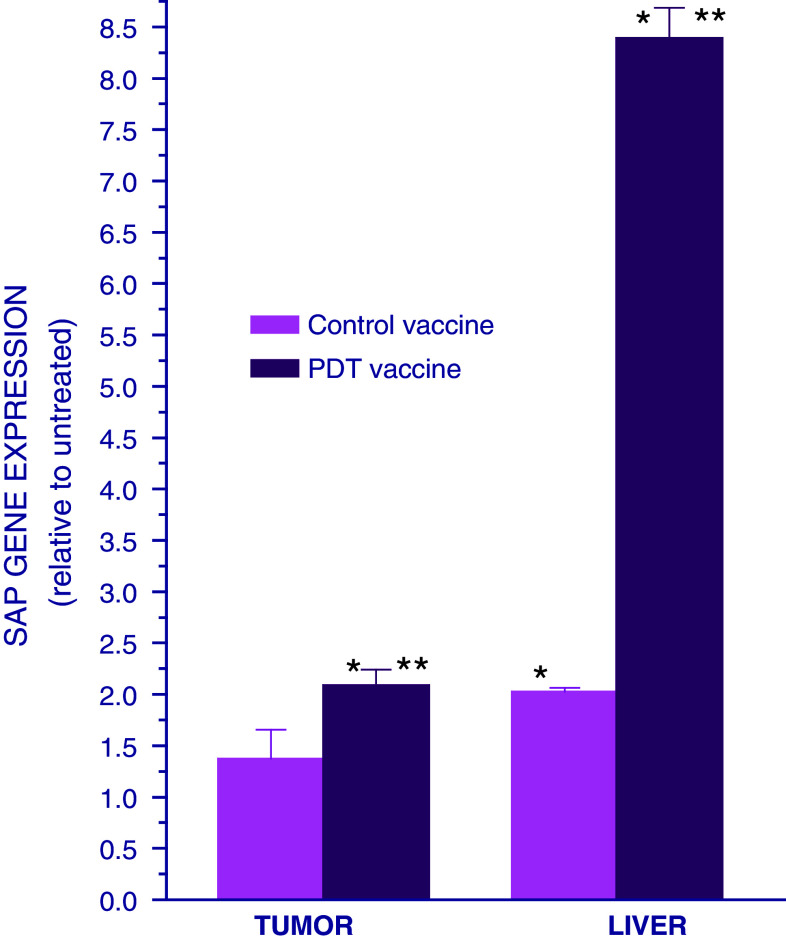
Standard treatment of tumors by PDT, where these lesions are exposed to light in situ after the host was administered a photosensitizing drug, was shown to induce an acute phase response in host mice [13]. To determine whether this type of host response can be instigated by PDT vaccine treatment, the expression of gene encoding serum amyloid P component (SAP, prototypic mouse acute phase reactant) was examined in the livers and tumors of vaccinated mice. The SCCVII tumor-bearing mice were killed at 24 h after they were treated either with PDT vaccine, prepared according to the protocol optimized in our previous work [7, 8], or with control vaccine consisting of SCCVII cells treated only with X-rays. The expression of SAP gene in the tumor and liver of these mice was compared with that found in untreated SCCVII tumor–bearing mice. The results reveal a marked upregulation of SAP gene in the livers of mice treated with PDT vaccine (Fig. 1). A significant, but much smaller liver SAP gene upregulation was found after control vaccine treatment. The treatment with PDT vaccine also caused the upregulation of tumor SAP gene, but the extent was modest, and no significant changes were evident following control vaccine treatment.
Fig. 1.
Expression of SAP gene in the tumor and liver of mice treated with PDT vaccine. Mice bearing SCCVII tumors were treated with either PDT vaccine or control vaccine using SCCVII cells exposed only to X-rays (60 Gy). The vaccine was prepared by incubating SCCVII cells with ce6 (0.5 μg/ml) for 30 min followed by exposure to 665 ± 10 nm light (1 J/cm2) and then 16-h post-incubation at 37°C. The cells were then collected, exposed to X-rays (60 Gy), and injected into SCCVII tumor-bearing mice (2 × 107 cells per mouse peritumorally). The mice were killed at 24 h after vaccination and their tumor and liver tissues collected for quantitative RT-PCR-based analysis of the expression of SAP gene. The results are presented as GAPDH-normalized SAP gene expression relative to that in the same tissue of untreated tumor-bearing mice. Each treatment group consisted of 4 mice; bars represent standard deviations. *Statistically significant difference in response (P < 0.05) compared to the unvaccinated control group; **statistically significant difference in response (P < 0.05) compared to control vaccine group
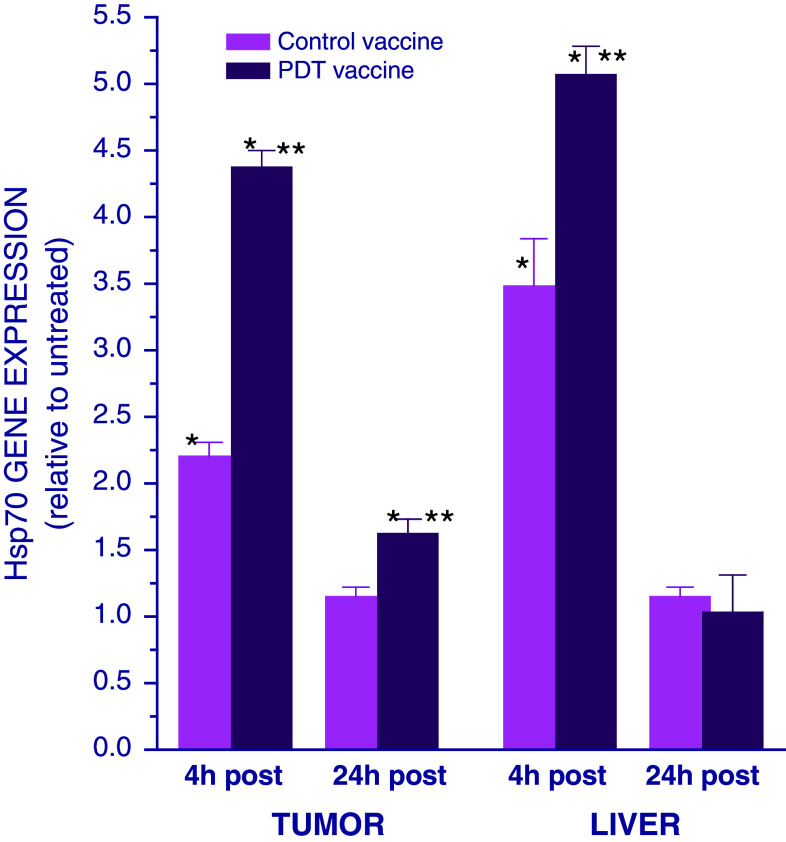
Our recent studies have revealed that proimmune chaperone heat shock protein 70 (Hsp70) can also get expressed as acute phase reactant [14]. The examination of the effect of PDT vaccine on Hsp70 gene expression showed its upregulation in both tumor and liver at 4 h post-treatment (Fig. 2). The upregulation of Hsp70 gene, but to a significantly lower extent, was also evident at the both sites after control vaccine treatment. At 24 h after therapy, the Hsp70 gene expression dropped to pre-treatment levels in the liver, while it remained only slightly elevated in the tumor. No significant effect was seen at this time-point after control vaccine treatment.
Fig. 2.
Expression of Hsp70 gene in the tumor and liver of mice treated with PDT vaccine. Mice bearing SCCVII tumors were treated with either PDT vaccine or control vaccine as described for Fig. 1. The mice were killed either 4 or 24 h after vaccination and their tumor and liver tissues collected for quantitative RT-PCR-based analysis of the expression of Hsp70 gene. The results are presented as GAPDH-normalized Hsp70 gene expression relative to that in the same tissue of untreated tumor-bearing mice. Each treatment group consisted of 4 mice; bars represent standard deviations. *Statistically significant difference in response (P < 0.05) compared to the unvaccinated control group; **statistically significant difference in response (P < 0.05) compared to control vaccine group
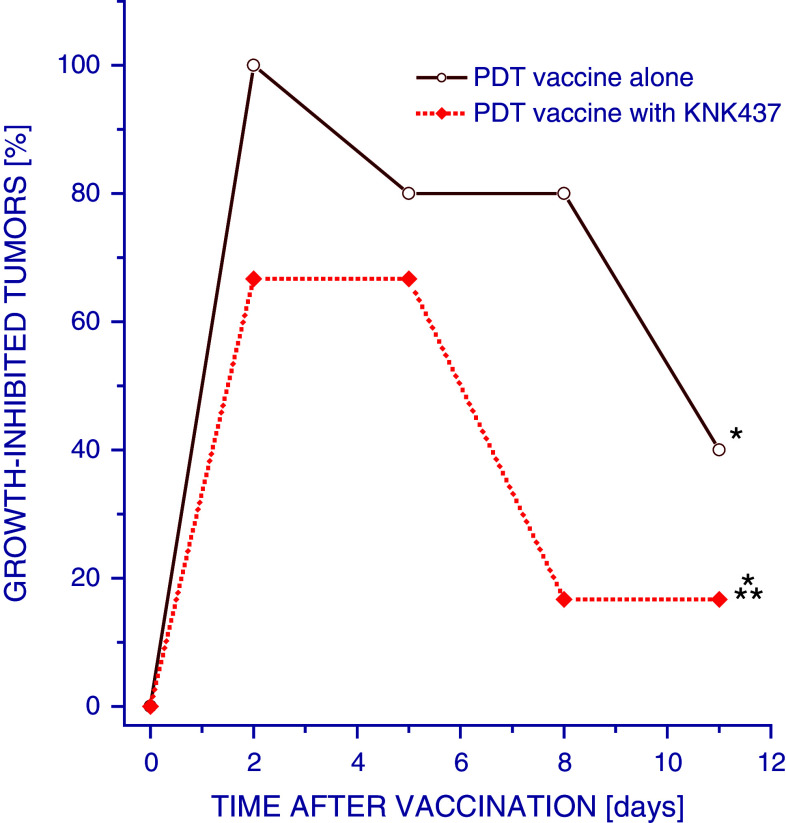
The importance of Hsp70 for the therapeutic impact of PDT vaccines was demonstrated using KNK437, which inhibits selectively the induction of this protein [16, 17]. The preparations of PDT vaccine prepared with KNK437 present during the 16-h post-incubation period were less effective in inhibiting SCCVII tumor growth than PDT vaccines prepared without it (Fig. 3).
Fig. 3.
Effect of inhibition of heat shock protein synthesis induction in PDT vaccine cells on therapy outcome. Mice bearing SCCVII tumors were treated with PDT vaccine or control vaccine as described for Fig. 1. The 16 h post-incubation in this protocol was carried out either in the presence or absence of KNK437 (25 μg/ml). The vaccine response was monitored by tumor size measurement and is shown as percentage of growth-inhibited tumors smaller than the means minus twofold SD of unvaccinated control group. Each treatment group consisted of 6 mice. *Statistically significant difference in response (P < 0.05) compared to the unvaccinated control group; **statistically significant difference in response (P < 0.05) compared to PDT vaccine alone group
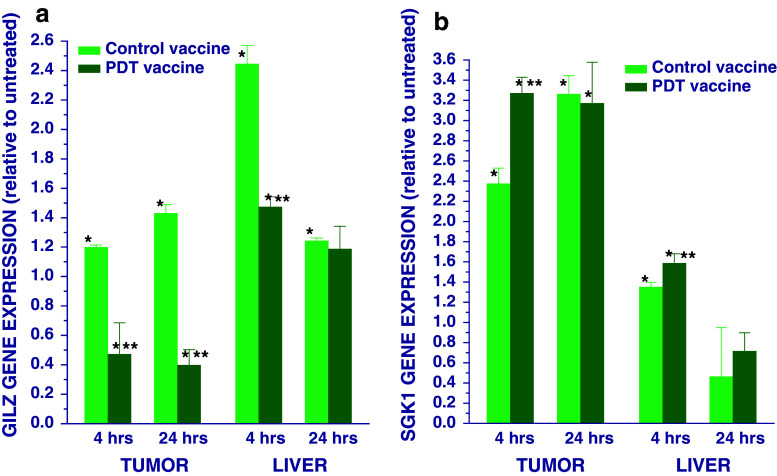
Since adrenal hormones have an important role in PDT-induced acute phase response [13, 15], it was warranted to examine their involvement in the response to PDT vaccine. A reliable indication of such event is changes in the expression of genes particularly susceptible to glucocorticoid regulation. The results for one such gene, glucocorticoid-induced leucine zipper (GILZ) [18], are shown in Fig. 4a. It can be seen that this gene became downregulated in the tumors at both 4 and 24 h after PDT vaccine, while control vaccine had the opposite effect. In the liver, PDT vaccine caused only a modest GILZ gene upregulation at 4 h post-treatment, but the effects seen after control vaccine were much more pronounced. Significant changes in the expression of serum- and glucocorticoid-regulated kinase-1 (SGK1), which is another glucocorticoid-susceptible gene [19], were also detected in the tumor at 4 and 24 h post-PDT vaccine treatment. However, control vaccine exhibited a significantly lower effect on SGK1 gene expression only at the 4-h time-point while its effect at the 24-h time-point was similar to PDT vaccine. In the liver, the changes in SGK1 gene expression were detectable only at 4 h post-treatment and were much less manifested than in the tumor; the effect seen with PDT vaccine was only slightly more pronounced.
Fig. 4.
Expression of GILZ and SGK1 genes in the tumor and liver of mice treated with PDT vaccine. Mice bearing SCCVII tumors were treated with either PDT vaccine or control vaccine as described for Fig. 1. The mice were killed either 4 or 24 h after vaccination and their tumor and liver tissues collected for quantitative RT-PCR-based analysis of the expression of a GILZ gene and b SGK1 gene. The results are presented as GAPDH-normalized GILZ or SGK1 gene expression relative to that in the same tissue of untreated tumor-bearing mice. Each treatment group consisted of 4 mice; bars represent standard deviations. *Statistically significant difference in response (P < 0.05) compared to the unvaccinated control group; **statistically significant difference in response (P < 0.05) compared to control vaccine group
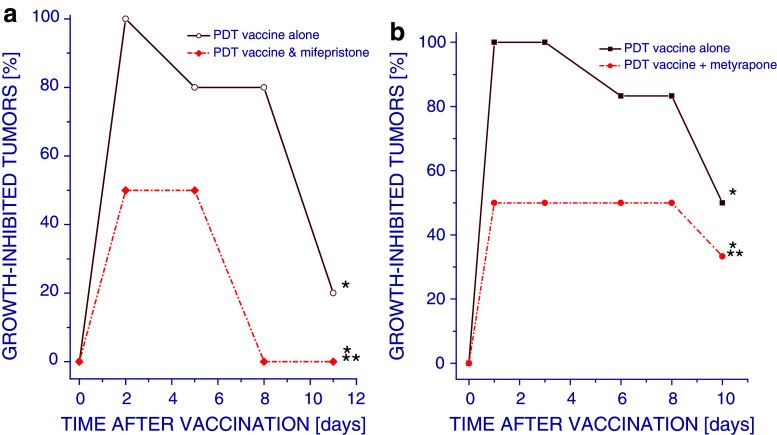
In order to examine the relevance of glucocorticoid activity for the therapeutic potency of PDT vaccines, glucocorticoid receptor antagonist mifepristone was administered to mice immediately after vaccination. The results show that during the first 8 days post-therapy, the percentage of growth-inhibited tumors ranged 80–100% with PDT vaccine alone group, while this inhibitory effect lasted much shorter and never exceeded 50% with PDT plus mifepristone group (Fig. 5a).
Fig. 5.
Effect of glucocorticoid activity inhibition on the therapeutic effect of PDT vaccine. Mice bearing SCCVII tumors were treated with PDT vaccine as described for Fig. 1. At 30 min before vaccination, the mice received intraperitoneal injection of either a mifepristone (50 mg/kg) or b metyrapone (100 mg kg). Tumor responses to vaccine treatment are presented as percentage of growth-inhibited tumors (same as in Fig. 1). Each treatment group consisted of 6 mice. *Statistically significant difference in response (P < 0.05) compared to the unvaccinated control group; **Statistically significant difference in response (P < 0.05) compared to PDT vaccine alone group
Treatment with another glucocorticoid-modulating agent metyrapone (glucocorticoid synthesis inhibitor) also reduced the percentage of growth-inhibited tumors compared to PDT vaccine alone (Fig. 5b). These results demonstrate that the activity of glucocorticoid hormones is important for the therapeutic efficacy of PDT vaccines and have in overall a positive contribution to the therapeutic impact.
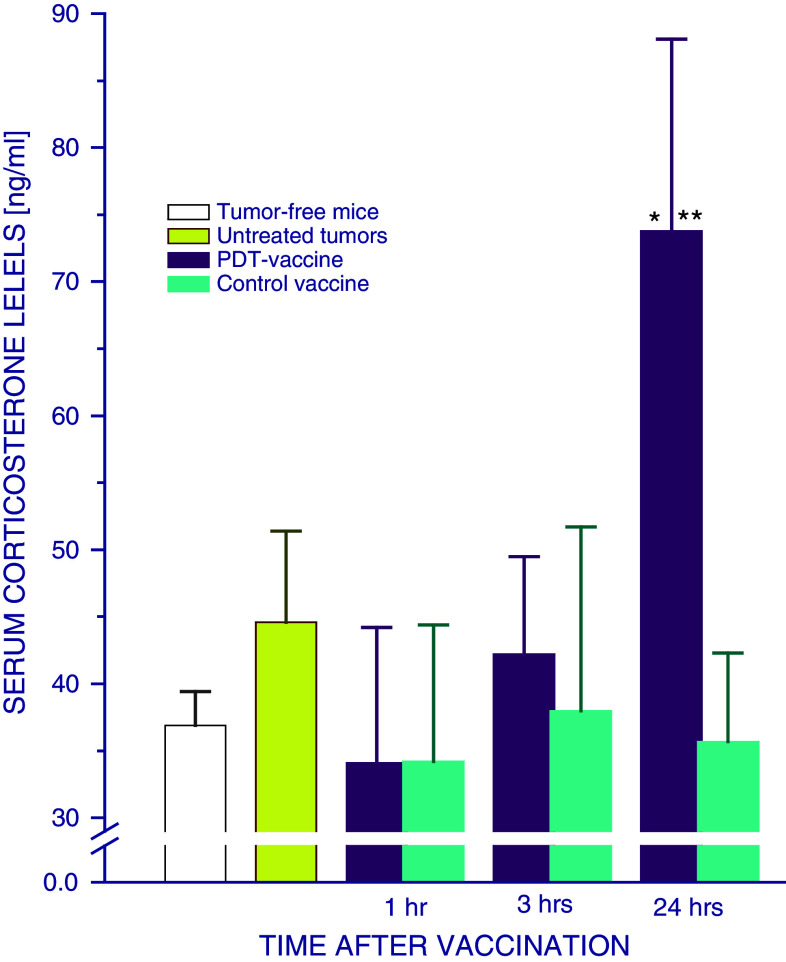
Evidence consistent with raised activity of glucocorticoid hormones after PDT vaccine treatment was obtained by examining the levels of serum corticosterone, which is a primary glucocorticoid in mice. A significant rise in serum corticosterone was found in SCCVII tumor-bearing mice 24 h after they received PDT vaccine treatment (Fig. 6). In contrast, no significant changes in serum corticosterone were detected in mice treated with control vaccine.
Fig. 6.
Serum corticosterone levels in mice treated by PDT vaccine. Mice bearing SCCVII tumors were treated with either PDT vaccine or control vaccine as described for Fig. 1. Their blood was collected at indicated post-vaccination time intervals for the ELISA-based determination of serum corticosterone. Each treatment group consisted of 4 mice; bars represent standard deviations. *Statistically significant difference in response (P < 0.05) compared to the unvaccinated control group; **statistically significant difference in response (P < 0.05) compared to PDT vaccine alone group
Discussion
It is clearly established that the therapeutic effect of PDT-generated cancer vaccines is based on inducing a strong adaptive immune response against the treated malignancy [2]. This evidently systemic response [7] is, as demonstrated in the present study, preceded by other systemic host defense activity. Our recent work has shown that the treatment for solid tumors by PDT induces a strong acute phase response including its hormonal component manifested by the activity of adrenal hormones [13, 15]. This prompted us to examine in the present work whether PDT vaccine treatment, where 2 × 107 in vitro PDT-treated tumor cells are injected in the host, elicits any comparable host activity. The hallmark of acute phase response, an effector process mobilizing resources from the entire organism for the execution of a host-protecting response, is the production of acute phase reactants at sites distant to the local insult with liver representing a major participating organ [20, 21]. This work provides evidence of the activation of genes of two important acute phase reactants, SAP and Hsp70, in the liver of mice that received tumor-localized injection of PDT vaccine.
The upregulation of gene encoding SAP, which is a prototypic acute phase reactant of mice [22], was demonstrated in PDT vaccine-treated mice at 24 h post-treatment that was establish previously as the peak time interval for the upregulation of this gene after standard PDT treatment [13]. The increased activity of SAP gene was detected in both the liver and tumor of vaccinated mice, but the effect was much more pronounced in the liver.
We have demonstrated recently that Hsp70, known not only for its protein chaperoning function but also as signal transduction pathways participant and important regulator of inflammatory and immune responses [23], functions as one of the key acute phase reactants produced in the liver and spleen of mice bearing PDT-treated tumors [14]. The upregulation of Hsp70 gene peaking at the 4-h time-point (similarly as with PDT) was evident in both the liver and tumor of PDT vaccine–treated mice (Fig. 2). The importance of this protein for the therapeutic impact of PDT vaccines, which was already implicated in our earlier work [6], is clearly demonstrated by the loss of their efficacy when Hsp70 induction in vaccine cells was blocked by KNK437 (Fig. 3). The expression of Hsp70 on the surface of PDT vaccine cells appears critical for their disposal orchestrating immune recognition of tumor antigens and leading to an efficient antitumor response. Since Hsp70 released into circulation has tendency to accumulate in PDT-treated tumors and be retained on the surface of cancer cells [14], it could be expected that Hsp70 produced in the liver of PDT vaccine–treated mice will also be attracted to the vaccine cells. Pentraxin SAP also functions as one of the mediators facilitating disposal of dead cells including those sustaining the insult from PDT treatment [24]. Thus, both SAP and Hsp70, produced as acute phase reactants during the acute phase response induced in PDT vaccine–treated mice, are probably mobilized to facilitate the process of vaccine cell disposal and will influence the development of antitumor immunity.
A major event of acute phase response is the activation of hypothalamic–pituitary–adrenal axis resulting in the release of adrenal hormones [25]. Glucocorticoids produced by adrenal gland were shown to have an important impact on the development of acute phase response in mice bearing PDT-treated tumors [15]. The present study demonstrates that the same hormones are mobilized after PDT vaccine treatment and contribute to the therapeutic outcome. Our results uncover changes in the expression of glucocorticoid highly responsive genes GILZ and SGK1 in the tumors and livers of vaccinated mice. By suppressing inflammatory and both innate and adaptive immune reactions through inhibitory effects on transcription factors NF-κB and AP-1 as well as other elements of pro-inflammatory and immune signal transduction pathways, GILZ is established as a pivotal mediator of glucocorticoid-induced responses [18]. Importantly, GILZ gene expression in tumors becomes downregulated after PDT vaccine treatment (in contrast to the effect of control whole-cell vaccine), thus creating a supportive environment for the development of antitumor immune responses. Significant PDT vaccine-induced changes, particularly in the early phase of response in tumor tissue, were found in the expression of gene encoding SGK1. This regulatory kinase transduces signals from glucocorticoid hormone and growth factor pathways to downstream effectors ensuring appropriate timing/context of physiological responses and thus controlling a wide range of biological processes including cell proliferation, cell survival, and (by influencing sodium (Na+) homeostasis) the function/activity of dendritic cells [19, 26].
Although glucocorticoids are primarily known as powerful suppressors of the immune response, they act by both suppressing and stimulating a large number of inflammatory and immune mediators [27]. Given that glucocorticoid activity contributes positively to its therapeutic impact (Fig. 5), PDT vaccine treatment is evidently not associated with substantial immunosuppressive activity of these hormones. One such positive contribution is manifestly the downregulation of genes that dampen immune response such as GILZ. Other way for glucocorticoids to bolster the antitumor effect of PDT vaccines is their engagement in phagocytic engulfment and processing of vaccine cells. Glucocorticoids promote the clearance of dead cells by enhancing opsonization and the activity of scavenger systems and by stimulating macrophage phagocytic ability and antigen uptake [27, 28]. Therefore, faced with a sudden appearance of a large number of (mostly dying) PDT vaccine cells, the host will launch responses to secure the most effective clearance of these cells that in addition to the production of supporting acute phase reactants include the engagement of glucocorticoid hormones.
The exceptional potential for therapeutic efficacy of whole-cell cancer vaccines generated by PDT is becoming recognized owing to their manifested advantages over other polyvalent vaccination strategies [2, 29]. The present study suggests that the potency of PDT vaccines could be related in part to their capacity to elicit acute phase response that ensures the production of seminal acute phase reactants and engagement of glucocorticoid hormones. The latter represents one of the most powerful endogenous regulatory systems for the control of immune and inflammatory responses [25]. To our knowledge, this is the first finding of the proficiency of a cancer vaccine for inducing an acute phase response, and it remains to be seen whether PDT vaccines are in this respect unique among cancer vaccines.
Acknowledgments
Technical assistance in this study was ably provided by Jingahi Sun and Wei Zhang.
Conflict of interest
The authors declare to have no conflict of interest in any form with respect to this article.
References
- 1.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. Photodynamic therapy of cancer; an update. CA Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korbelik M. Cancer vaccines generated by photodynamic therapy. Photochem Photobiol Sci. 2011;10:664–669. doi: 10.1039/c0pp00343c. [DOI] [PubMed] [Google Scholar]
- 3.Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem Photobiol. 1992;55:145–157. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- 4.Gollnick SO, Vaughan LA, Henderson BW. Generation of effective anti-tumor vaccines using photodynamic therapy. Cancer Res. 2002;62:1604–1608. [PubMed] [Google Scholar]
- 5.Korbelik M, Cecic I. Mechanism of tumor destruction by photodynamic therapy. In: Nalwa HS, editor. Handbook of photochemistry and photobiology. Stevenson Ranch: American Scientific Publishers; 2003. pp. 39–77. [Google Scholar]
- 6.Korbelik M, Sun J. Photodynamic therapy-generated vaccine for cancer therapy. Cancer Immunol Immunother. 2006;55:900–909. doi: 10.1007/s00262-005-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korbelik M, Stott B, Sun J. Photodynamic therapy-generated vaccines: relevance of tumour cell death expression. Br J Cancer. 2007;97:1381–1387. doi: 10.1038/sj.bjc.6604059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korbelik M. Photodynamic therapy-generated vaccines. Methods Mol Biol. 2010;635:147–153. doi: 10.1007/978-1-60761-697-9_11. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Ma W, Li Y. Generation of effective vaccines against liver cancer by using photodynamic therapy. Lasers Med Sci. 2009;24:549–552. doi: 10.1007/s10103-008-0609-4. [DOI] [PubMed] [Google Scholar]
- 10.Korbelik M, Merchant S, Huang N. Exploitation of immune response-eliciting properties of hypocrellin photosensitizer SL052-based photodynamic therapy for eradication of malignant tumors. Photochem Photobiol. 2009;85:1418–1424. doi: 10.1111/j.1751-1097.2009.00610.x. [DOI] [PubMed] [Google Scholar]
- 11.Kaluza KM, Thompson JM, Kottke TJ, Flynn Gilmer HC, Knutson DL, Vile RG (2011) Adoptive T cell therapy promotes the emergence of genomically altered tumor escape variants. Int J Cancer (Epub Sep 20). doi:10.10002/ijc.26447 [DOI] [PMC free article] [PubMed]
- 12.Khurana D, Martin EA, Kasperbauer JL, O’Malley BW, Jr, Salomao DR, Chen L, Strome SE. Characterization of a spontaneously arising murine squamous cell carcinoma (SCC VII) as a prerequisite for head and neck cancer immunotherapy. Head Neck. 2001;23:899–906. doi: 10.1002/hed.1130. [DOI] [PubMed] [Google Scholar]
- 13.Korbelik M, Cecic I, Merchant S, Sun J. Acute phase response induction by cancer treatment with photodynamic therapy. Int J Cancer. 2008;122:1411–1417. doi: 10.1002/ijc.23248. [DOI] [PubMed] [Google Scholar]
- 14.Merchant S, Korbelik M. Heat shock protein 70 is acute phase reactant: response elicited by tumor treatment with photodynamic therapy. Cell Stress Chaperones. 2011;16:153–162. doi: 10.1007/s12192-010-0227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merchant S, Huang N, Korbelik M. Expression of complement and pentraxin proteins in acute phase response elicited by tumor photodynamic therapy. Int Immunopharmacol. 2010;10:1595–1601. doi: 10.1016/j.intimp.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Yokota S, Kitahara M, Nagata K. Benzylidene lactam compound, KNK437, a novel inhibitor of acquisition of thermotolerance and heat shock protein induction in human colon carcinoma cells. Cancer Res. 2000;60:2942–2948. [PubMed] [Google Scholar]
- 17.Koishi M, Yokota S, Mae T, Nishimura Y, Kanamori S, Horii N, Shibuya K, Sasai K, Hiraoka M. The effects of KNK437, a novel inhibitor of heat shock protein synthesis, on the acquisition of thermotolerance in a murine transplantable tumor in vivo. Clin Cancer Res. 2001;7:215–219. [PubMed] [Google Scholar]
- 18.Beaulieu E, Morand EF. Role of GILZ in immune regulation, glucocorticoid actions and rheumatoid arthritis. Nat Rev Rheumatol. 2011;7:340–348. doi: 10.1038/nrrheum.2011.59. [DOI] [PubMed] [Google Scholar]
- 19.Thomas SV, Kathpalia PP, Rajagopal M, Carlton C, Zhang J, Eaton DC, Helms MN, Pao AC. Epithelial sodium channel regulation by cell surface-associated serum- and glucocorticoid-regulated kinase 1. J Biol Chem. 2011;286:32074–32085. doi: 10.1074/jbc.M111.278283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 21.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 22.Le PT, Muller T, Mortensen RF. Acute phase reactants of mice. I. Isolation of serum amyloid P-component (SAP) and its induction by a monokine. J Immunol. 1982;129:665–672. [PubMed] [Google Scholar]
- 23.Gehrmann M, Radons J, Molls M, Multhoff G. The therapeutic implications of clinically applied modifiers of heat shock protein 70 (Hsp70) expression by tumor cells. Cell Stress Chaperones. 2008;13:1–10. doi: 10.1007/s12192-007-0006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merchant S, Sun J, Korbelik M. Dying cells program their expedient disposal: serum amyloid P component upregulation in vivo and in vitro induced by photodynamic therapy of cancer. Photochem Photobiol Sci. 2007;6:1284–1289. doi: 10.1039/b709439f. [DOI] [PubMed] [Google Scholar]
- 25.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 26.Rotte A, Pasham V, Eichenmüller M, Yang W, Bhandaru M, Lang F. Influence of dexamethasone on Na+/H+ exchanger activity in dendritic cells. Cell Physiol Biochem. 2011;28:305–314. doi: 10.1159/000331746. [DOI] [PubMed] [Google Scholar]
- 27.Franchimont D. Overview of the actions of glucocorticoids on the immune response. A good model to characterize new pathways of immunosuppression for new treatment strategies. Ann N Y Acad Sci. 2004;1024:124–137. doi: 10.1196/annals.1321.009. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Cousin JM, Hughes J, Van Damme J, Seckl JR, Haslett C, Dransfield I, Savill J, Rossi AG. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J Immunol. 1999;162:3639–3646. [PubMed] [Google Scholar]
- 29.Gollnick SO, Brackett CM. Enhancement of anti-tumor immunity by photodynamic therapy. Immunol Res. 2010;46:216–226. doi: 10.1007/s12026-009-8119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]