Abstract
Chronic inflammation typical to various chronic diseases is associated with immunosuppression, mediated primarily by immature myeloid-derived suppressor cells (MDSCs). A variety of factors induce MDSC differentiation arrest, thus manipulating the host’s immune function and suppressing the innate and adaptive immune systems, as reflected by their impaired status associated with down-regulated expression of the CD247 molecule. Such chronic inflammation-induced immunosuppressive features are also found in many tumors, generating tumor micro- and macro-environments that act as critical barriers to effective anti-tumor responses and therapies. This knowledge offers new and novel candidate immune targets for therapeutic interventions, in combination with more conventional approaches as chemotherapy, radiotherapy, and cancer cell targeted therapy. Therapeutic manipulation of chronic inflammation during cancer development is likely to enhance efficacy of treatments such as vaccinations, and adoptive T cell transfer, thus switching the chronic pro-cancer inflammatory environments into an anti-cancer milieu. Based on the functional relevance of immune networking in tumors, it is advantageous to merge monitoring immune biomarkers into the traditional patient’s categorization and treatment regiments, which will provide new prognostic and/or predictive tools to clinical practice. A better identification of environmental and tumor-specific inflammatory mechanisms will allow directing the clinical management of cancer toward a more personalized medicine.
Keywords: Chronic inflammation, Immunosuppression, Cancer, Anti-cancer therapy, Myeloid-derived suppressor cells, CITIM 2013
Suppressive features of chronic inflammation
Inflammation represents a link between intrinsic (oncogenes, tumor suppressors, and genome stability) and extrinsic (immune and stromal components) factors contributing to tumor development. In cancer, immune cells exerting either pro- or anti-tumor activities affect various therapeutic strategies. In the past decade, numerous studies showed that the tumor micro-environment is immunosuppressive, impairing the function of the innate (NK cells) and adaptive (T cells) immune systems, and associated with CD247 down-regulation, which is an indispensable molecule in the structure, expression, and function of the T cell antigen receptor (TCR) and the NK cell activating receptors. In parallel, a similar immunosuppression was observed in various pathologies that differ in their etiology and physiology such as autoimmune disease and infections [1–4], suggesting a common denominator responsible for this phenomenon. We were the first to show that chronic inflammation, which is shared by these diseases, is responsible for the induced immunosuppression. This linkage was discovered based on a pathology-free chronic inflammation mouse model that we established in our laboratory, in which mice were repeatedly exposed to inactivated pathogens or single TLR ligands that induce inflammatory responses. The outcome was surprising since mice that were simply immunized developed an immunosuppression as if displaying one of the above-mentioned pathologies; their T and NK cell ex vivo and in vivo functions were impaired, CD247 was down-regulated, and they were more susceptible to influenza virus infection as compared to control non-inflamed mice. Our cumulative data point out two critical characteristics of the chronic inflammation-induced immunosuppression: a bystander effect and a reversible phenomenon. Under such conditions, all T and NK cells display an impaired function, which is reversed upon their isolation from the generated inflammatory environment or following neutralization of the inflammation/suppressive factors. These results suggested that the observed immunosuppression is due to environmental factors and not to intrinsic defects in the immune cells.
MDSCs and chronic inflammation
In the course of our initial studies using the pathology-free chronic inflammation models, we identified myeloid-derived suppressor cells (MDSCs) as the main cell population being responsible for inducing the observed immunosuppression, which were later described in tumor-bearing mice and patients. The murine MDSCs express both Gr1 and CD11b cell surface markers that could be further segregated into a monocytic (CD11b+Ly6G−Ly6Chigh) and granulocytic (CD11b+Ly6G+Ly6Clow) phenotypes, both accumulating in the bone marrow, spleen, and peripheral blood of inflamed mice, and in tumor settings in the lymph nodes and tumor sites as well. MDSCs are characterized by various suppressive features such as enhanced activities of arginase-1 and iNOS, as reflected by the elevated levels of both intra- and extra-cellular NO− and ROS [5, 6]. They were found to affect ex vivo T cell function; when primary normal T cells are co-incubated with MDSCs isolated from an inflamed environment, they down-regulate CD247 expression and are unable to proliferate in response to TCR-mediated activation signals [3]. A direct T-MDSC contact or close proximity is required for imposing the immunosuppressive effect. In addition, the in vivo depletion of MDSCs in the inflamed mice using monoclonal anti-Gr1 antibodies led to recuperation of the host’s immune status; while adoptively transferred CFSE-labeled OT1 CD8 T cells to inflamed immunized mice showed a decrease in vivo cell proliferative ability, the same cells could successfully proliferate when injected into immunized inflamed mice pre-treated with anti-Gr1 antibodies [7]. Moreover, the inflamed mice were also unable to display NK cell-mediated allogeneic cell clearance, while upon anti-Gr1 treatment NK cell responses were reinforced. Both OT1 CD8 T and NK cell recuperated activities in the anti-Gr1-treated inflamed mice correlate with retrieval of the CD247 expression levels in the host’s cells. These results highlight the major role of MDSCs in chronic inflammation-induced immunosuppression and show that the tumor settings are not required for their induction, recruitment, and suppressive features. Moreover, the harmful effects of the chronic-inflammatory environment on immune-based therapies/modalities are also emphasized as shown by the impaired function of adoptively transferred T cells to inflamed mice and the impaired response to immunization protocols as against influenza virus.
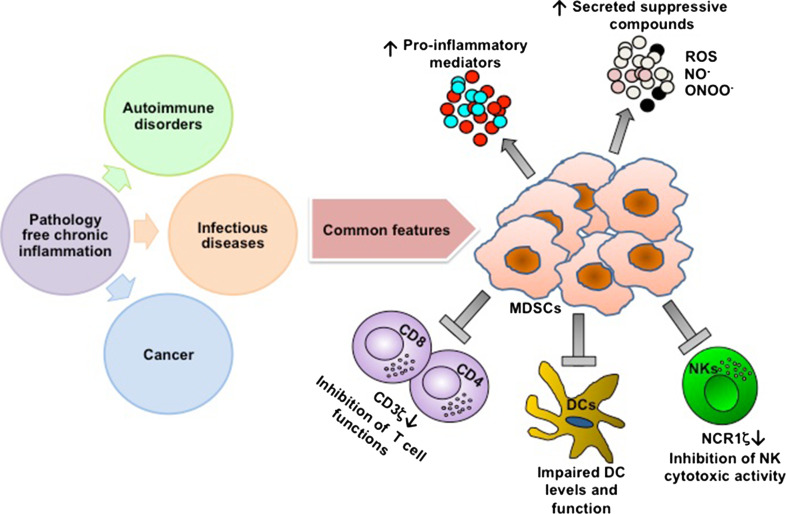
Our studies using the pathology-free chronic inflammation models enabled us to discover that the induced immunosuppression is the normal outcome of the body’s defense system to avoid excessive immune stimulation. While acute inflammation is beneficial to the host’s defense against invading bacteria, viruses, and spontaneous cell transformation, chronic inflammation originating in response to any stimulus that induces a sustained activation of the innate and adaptive immune systems as described for various types of tumors (colorectal, breast, head and neck, melanoma, etc.) results in immunosuppression that starts locally, and at a later stage becomes systemic. Indeed, cumulative data from various laboratories including ours describe MDSC-mediated immunosuppression in various types of tumors in mouse models as well as in humans, and the mechanisms underlying their suppressive activity are well characterized and resemble those detected in MDSCs from a tumor-free chronic-inflammatory environment (Fig. 1).
Fig. 1.
Common features of chronic inflammation-induced immunosuppression. The link between the pathology-free chronic inflammation model we used in the course of our studies and the various pathologies characterized by chronic inflammation as cancer, infectious, and autoimmune diseases is the generation of a suppressive milieu, in which MDSCs play a crucial role in manipulating the micro- and macro-environments. The expansion and activation of MDSCs lead to an increased production of pro-inflammatory mediators and suppressive compounds such as NO− and ROS. The outcome of such a newly generated inflammatory environment is a general suppression of both adaptive and innate immune cells that enables the pathology progression
Regulatory mechanisms controlling MDSC expansion and suppressive features
Since increased levels of MDSCs are observed during chronic inflammation as well as in many types of tumors associated with inflammatory micro- and macro-environments, they serve as key obstacle preventing anti-tumor immune responses and are limiting success of immune-based therapies. A complex milieu of factors associated with inflammation has been identified as regulating MDSC accumulation and suppressive function. These include pro-inflammatory cytokines (TNF-α, IFN-γ, IL-1β, IL-6), chemokines (CXCL5, CCL2), growth factors (GM-CSF, VEGF), and proteins (S100A8/9) that are persistently secreted by normal self-immune cells or modified cells including tumor cells [7–10]. The generated inflammatory milieu regulates different MDSC pathways that control: (1) MDSC differentiation: During chronic inflammation and associated developing tumors, myeloid cell differentiation is distracted from its normal pathway that results in the terminal differentiation of mature macrophages, dendritic cells (DCs) and granulocytes, toward a path that favors the differentiation of “pathological” MDSCs. For example, exogenous PGE2 and diverse COX-2 activators as lipopolysaccharides (LPS), IL-1β, and IFN-γ, all induce monocyte expression of COX-2, blocking their differentiation into CD1a+ DCs and inducing endogenous PGE2, IL-4Rα, IDO1, iNOS, and IL-10, which are typical MDSC-associated suppressive factors [5]. Moreover, our recent studies indicate the role of TNF-α in arresting MDSC differentiation via the induction of S100A8/9 protein and their corresponding receptor for advanced-glycation end products (RAGE) [7], thus, promoting differentiation of MDSCs into mature, non-suppressive DCs and macrophages that enable immune status recovery. (2) MDSC sensitivity to apoptosis: It was demonstrated that chronic-inflammatory and tumor environments increase MDSC levels by conferring resistance to Fas-mediated apoptosis facilitated by FasL+ T cells, as reflected by the low levels of activated caspase 3 and 8 [11, 12]. (3) MDSC-suppressive activity: The above-described inflammatory factors are also responsible for the elevated suppressive activity of MDSCs as reflected by the increased expression and activity of arginase 1 and iNOS, and the production of ROS and NO−. Thus, the use of inhibitors for such pathways should neutralize the immunosuppressive environment and increase immune surveillance.
The above-mentioned MDSC pathways that are activated in the course of chronic-inflammatory diseases become critical in inducing immunosuppression that deprives the host from displaying immune responses or reacting toward immune-based therapies. Each of these pathways and the involved molecules could be targeted to manipulate the immune system toward its functional recovery. In the case of cancer, combining a targeted therapy directed against both the tumor and the chronic inflammation-induced immunosuppressive environment, in conjunction with monitoring the hosts’ immune status could provide the optimal conditions for a successful anti-cancer therapy and disease outcomes.
Combating MDSCs
Today, anti-cancer therapies become broad, combating not only the tumor but also the environment, counteracting, or neutralizing tumor supporting inflammation and targeting key regulators of the immune system to allow a cascade of events favoring cancer rejection. Since MDSCs are known to be one of the main mediators orchestrating the suppressive environment, various strategies targeting these cells, both in mouse models and patients were recently established. In parallel to targeting MDSC molecules that regulate their suppressive activity [5], accumulating data suggest biological and chemical agents as potential inhibitors of MDSCs that lead to their elimination, targeting their suppressive activity, and/or stimulating their differentiation. In the next two sections, we will describe MDSC neutralization strategies recently introduced by our and others studies, focusing on the manipulation of the inflammatory environment via TNF-α and by using a variety of drugs.
Targeting MDSCs via TNF-α manipulation
TNF-α was first isolated in 1975, and recognized by its ability to cause rapid hemorrhagic necrosis of cancer cells [13]. However, in the early 90s tumor promoting properties of TNF-α were discovered. In addition, TNF-α has been shown as a harmful master regulator of inflammation that develops in the course of chronic diseases such as rheumatoid arthritis, psoriasis, type II diabetes, and Crohn’s disease [14]. Moreover, studies performed in the last century assessing developing tumors revealed the direct and indirect effects of TNF-α on malignant cells, which lead to increased cell proliferation, survival, and DNA damage. These processes enhance angiogenesis, primary tumor growth, immune evasion, and metastasis [14]. Based on the discoveries of the TNF-α harmful effects, it has been unequivocally validated as a therapeutic target in various immune-mediated inflammatory disorders, which resulted in the development of several therapeutic strategies involving the use of TNF-α neutralizing antibodies and antagonists. Interestingly, the above-described diseases, in addition to their pathological features, are also characterized by chronic inflammation and associated local/systemic immunosuppression that play a critical role in the disease progression and health deterioration. However, until recently, it was unclear whether TNF-α is involved in the manipulation of the host’s immune system toward the generation of a suppressive environment typical of an ongoing chronic inflammation.
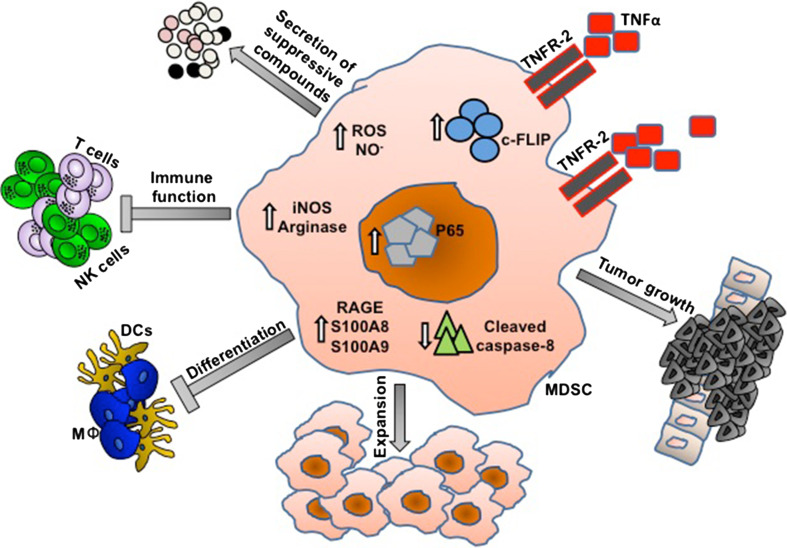
In 2012, Zhao et al. [11] showed that TNF-α promotes the accumulation of MDSCs in a transplanted tumor model. They demonstrated that signaling through TNFR-2 promoted MDSC survival via up-regulating the anti-apoptotic FLICE-inhibitory protein (c-FLIP), which inhibits activation of apoptotic caspase-8, thus is maintaining MDSC accumulation and promoting tumor growth. Our recent study highlights additional mechanisms by which TNF-α manipulates the host immune system toward the generation of a suppressive environment [7]. By using the pathology-free model of chronic inflammation, we showed that TNF-α exhibits a dual function during chronic inflammation: arresting differentiation of MDSCs via the S100A8/9 inflammatory proteins and their corresponding receptor RAGE, and augmenting MDSC-suppressive activity. Moreover, we showed that administration of etanercept (TNF-α-antagonist) during early chronic-inflammatory stages reduced MDSC-suppressive activity and enhanced their maturation into DCs and macrophages. These led to the restoration of in vivo immune functions and a recovery of CD247 expression both in T and NK cells. The results from these two studies provide an insight into the relationship between TNF-α and MDSCs, underscoring TNF-α as an MDSC regulator controlling their expansion, differentiation, and suppressive activities (Fig. 2). In addition, a number of other possible mechanisms for TNF-α-mediated immunosuppressive effects emerged, such as inducing lymphocyte apoptosis, inhibiting TCR signaling and DC function, and activating regulatory T cells (Tregs). However, the results are complicated or controversial due to the indications that both timing and duration of TNF-α expression are important in determining pathogenic versus protective roles.
Fig. 2.
TNF-α is a master regulator of MDSCs during chronic inflammation. By signaling through TNFR-2, TNF-α promotes MDSC survival via up-regulation of the anti-apoptotic FLICE-inhibitory protein (c-FLIP), which inhibits activation of apoptotic caspase-8. Moreover, TNF-α also controls MDSC differentiation and activation. The differentiation of MDSCs to non-suppressive myeloid cells such as macrophages (MΦ) and dendritic cells (DCs) is inhibited by TNF-α by up-regulating the expression of both the S100A8/9 pro-inflammatory proteins and their corresponding receptor RAGE. In parallel, TNF-α also controls the ability to produce suppressive compounds such as urea, NO−, and ROS. Taken together, these alterations manifested by TNF-α lead to in vivo inhibition of immune functions
Based on the dual effects of TNF-α, there is still a debate in the clinic as to whether anti-TNF-α treatments in pathologies characterized by chronic inflammation may have beneficial or harmful effects. Many clinical studies analyzing patients suffering from inflammatory bowel disease and rheumatoid arthritis that were treated with anti-TNF-α regiments showed that these patients were more susceptible to opportunistic infections and cancer development [15, 16]. Thus, the observed opposite effects of treatments that neutralize TNF-α in various chronic-inflammatory pathologies, the beneficial versus harmful outcomes remains questionable. Our hypothesis is that TNF-α-mediated inflammatory response, whether acute or chronic, will dictate its beneficial or harmful immunological consequences. During acute inflammation, TNF-α is vital for immediate immune defense against pathogens and clearance of transformed cells, whereas in chronic inflammation, a condition where the host is unable to clear the pathogen or the tumor cells, TNF-α could be harmful due to the promotion of a cell-mediated immunosuppression. Moreover, the balance between the duration and level of TNF-α production could lead to opposite effects on the immune system. Thus, when neutralizing TNF-α, additional parameters such as the presence or absence of a suppressive milieu in the case of MDSCs or M2-macrophages and Tregs should be taken into consideration. Neutralization of TNF-α in time points that are characterized not only by high levels of TNF-α, but also by elevated levels of suppressive cells could lead to more beneficial results, as shown in mouse models of colorectal cancer and ulcerative colitis, as well as in breast, ovarian, and renal cancer patients treated with TNF-α inhibitors.
The knowledge accumulated on TNF-α shows us that this cytokine may have beneficial or harmful effects on disease initiation and progression. Our challenge is not only to find out how to use the beneficial effects of TNF-α under pathological conditions when needed, but also to neutralize it whenever necessary, thus shifting the balance toward better disease outcomes.
Drug warfare against MDSCs
As described above, chronic inflammation-mediated MDSC accumulation in the lymphatic organs, peripheral blood, and tumor sites is one of the main obstacles in cancer therapy [17–19]. It has become clear and indisputable that MDSC-induced immunosuppression contributes to tumor progression and resistance to immune-based therapy [20, 21]. Thus, anti-tumor treatments must be pronged to target the tumor as well as the immunosuppressive environment toward activation of effector immune cells [22–25] (Fig. 3). Cumulative studies including ours demonstrate that MDSC depletion [26], inhibition [27], or induced differentiation [7] result in a moderate or non-suppressive environment that supports the success of effective treatments and enhancing anti-tumor immune responses [19, 28]. Moreover, the fact that the number of MDSCs found in the blood and tumor sites of cancer patients correlate with the disease severity [12, 29] (Sade-Feldman et al. and Kanterman et al. unpublished data) led us to investigate alternative pathways to target in parallel or simultaneously the tumor and MDSCs using the same drug or a combination. This concept was evaluated when testing the effect of various therapies on the tumor and immunosuppressive environment.
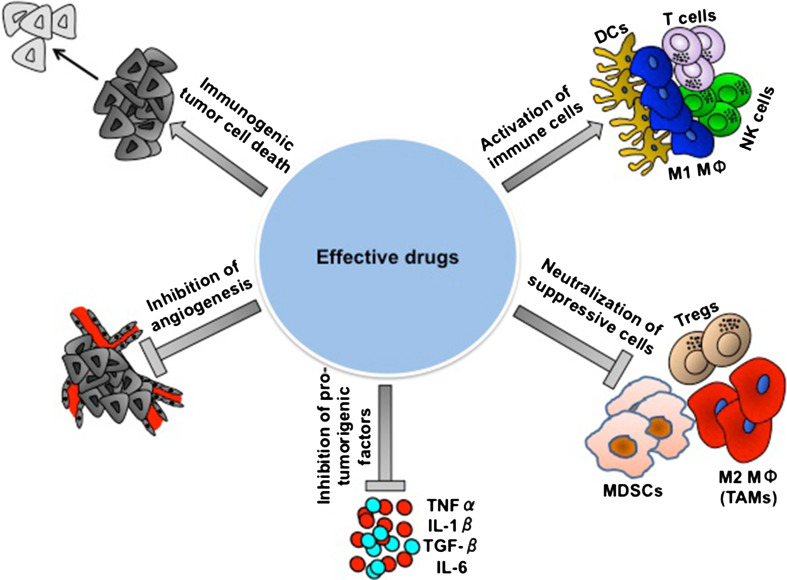
Fig. 3.
The obstacles in obtaining a successful treatment. The important role of the immune system in eliminating cancer is unquestionable. The effective function of the immune system in conjunction with various standard treatments given today to cancer patients is critical, due to the immune system “responsibility” to eliminate cancer cells that fail to be destroyed by the conventional therapies. An effective therapy against cancer should take into consideration not only the tumor itself, but also its unique environment that protects it from complete eradication and should include five important parameters: (a) Elimination of the tumor cells, leading to immunogenic death; (b) Blockade/destruction of the suppressive cells, (c) Activation of effector immune cells, (d) Neutralization of pro-tumorigenic factors, and (e) Inhibition of angiogenesis. These parameters must be routinely monitored prior to and following treatment in order to choose an optimal combinatorial therapy that will lead to a beneficial optimal personalized treatment
It was shown that Sunitinib, a receptor tyrosine kinase inhibitor, significantly decreased MDSC levels in renal cell carcinoma (RCC) patients, by affecting redistribution within the myeloid lineage and tuning toward myeloid DCs [30]. Moreover, the reduced MDSC numbers were correlated both with a decline in Tregs and reversed suppression of effector T cells [31]. A tyrosine kinase inhibitor of VEGFR, Axitinib that was applied to a mouse model of melanoma also reduced both MDSCs and Tregs, and resulted in reinforced tumor infiltrating T cells (TILs) activated by an immune-based vaccine [32].
Recently, various laboratories including ours discovered that conventional cytotoxic chemotherapeutic drugs influence not only the tumor cells, but also have an impact on the tumor micro-environment and the immune system. The in vivo treatment of tumor-bearing mice with 5-fluorouracil (5FU), a pyrimidine analog, led to a major decrease in the number of MDSC in spleens and tumor beds of animals, whereas no significant effect on T cells, NK cells, DCs, or B cells was noted. 5FU showed a stronger efficacy in depleting MDSCs and selectively inducing MDSC-apoptotic cell death in vitro and in vivo. The elimination of MDSCs by 5FU increased IFN-γ production by tumor-specific CD8 T cells infiltrating the tumor, promoting T cell-dependent anti-tumor responses in vivo [33]. Similarly, gemcitabine, a cytidine nucleoside analog, also reduced MDSC numbers and improved the activity of cytotoxic effector CD8 T and NK cells [34]. A later investigation suggested that reduced MDSCs due to gemcitabine-induced apoptosis correlates with decreased tumor progression [35]. Moreover, the combined treatment with gemcitabine and cytokine-based immunotherapy significantly improved the anti-tumor response in mice. However, other studies demonstrated that in some cases, the tumor progressed although MDSC levels were reduced, suggesting that additional mechanisms leading to immune escape and tumor resistance operate. Thus, under such conditions, the timing of applied treatment and the tumor stage are critical for the success of therapy and tumor regression. Recently, it was shown that following 5FU treatment the activity of Nlrp3 inflammasomes was enhanced and resulted in pro-angiogenic effects [36]. Therefore, there is a need to explore the dual effect of such chemotherapeutic agents, and find a combinatorial treatment that could block the parallel harmful effects such as combining anti-angiogenic with direct MDSC-apoptotic drugs.
In contrast, not all cytotoxic drugs are useful in combating MDSCs. Chemotherapeutic agent such as cyclophosphamide (CP), although having the ability to target Tregs [37] and promote immunogenic tumor cell death as shown in ret transgenic murine melanoma model, had no beneficial anti-tumor effects [38]. Assessment of the changes in the immune system and tumor micro- and macro-environments revealed that CP enhanced the generated immunosuppression, increased the accumulation and suppressive features of MDSCs characterized by the elevated levels of NO− and the production of pro-inflammatory cytokines, which together, impaired the activity of cytotoxic T and NK cells [38].
Thus, despite of the destructive effects of the chemotherapy on the tumor and MDSCs, there are still evidences for tumor prosperity at later stages, suggesting a crucial role of the residual peripheral and/or tumor resident MDSCs. In such cases, there is a need for a complete depletion or inactivation of the suppressive cell population. It was shown that the administration of anti-Gr1 antibodies enhanced the anti-tumor activity against lung [26] and colorectal cancer (Sade-Feldman et al. manuscript in preparation), and together with COX-2 inhibitor, prevented the spread of brain metastasis in breast cancer-bearing mice [39]. In contrast, a recent study demonstrated that the use of anti-Gr1 is not suitable for the depletion of MDSCs from all organs. Although MDSCs were completely depleted from the peripheral blood and spleen, their levels in the liver remained untargeted and immunosuppressive [40]. Thus, the additional neutralization of the MDSC-suppressive activity could complete the mission. Suitable inhibitors could be those that directly target a specific MDSC-suppressive pathway or alternatively eliminating several MDSC-immunosuppressive machineries. Specific drugs such as the COX-2 inhibitor, celecoxib [41], which blocks the production of PGE2, lead to a decrease in NO− and ROS production and a restoration of effector immunity. Additionally, inhibitors of phosphodiesterase-5 (PDE5) such as sildenafil could be used to target and reduce the expression of arginase 1 and iNOS [17, 42]. Non-specific inhibitors that block several pathways regulating MDSC activity could be more beneficial in inducing the final outcome of the immunosuppressive disease. For example, a derivate of Icariin (ICA), an ingredient of HerbaEpimedii that is used in alternative medicine treatments [43], could be suitable for such a goal. It was shown that ICA serves as a component that is able to block tumor growth, together with reducing MDSC numbers, accompanied by reinforced activity of CD8 T cells measured by the production of IFN-γ. Targeting MDSCs is achieved not only by decreasing their levels, but also by reducing their immunosuppressive activity, measured by decreased amounts of NO− and ROS as well as the low expression levels of S100A8/9 proteins within the cells [44]. Another study showed that advanced melanoma patients treated with vemurafenib displayed reduced MDSC-immunosuppressive activity, suggesting a dual role targeting not only tumor progression, but also up-regulating anti-tumor immunity by reducing MDSC levels and suppressive efficiency [45]. However, there are components that could worsen the disease stage: for example, administration of low-dose CP resulted not only in MDSC retention, but also in elevated suppressive activity measured by NO− and ROS production as well as the inhibition of T cell proliferation in melanoma mouse model [38]. These led to therapy failure, even though the cytotoxic effect of the CP chemotherapy on the tumor is well established. Supporting data show that MDSC-increasing levels were also detected in breast cancer patients treated with combined CP and doxorubicin therapy [46], which correlated with the spread of metastasis. Importantly, in the latest study, a standard high CP dose was used, canceling the importance of the administrated drug dosage and highlights the need for alternative treatment in case of the presence of the immunosuppression.
Altering MDSC differentiation program is an alternative strategy to decrease this immature immunosuppressive population. The most popular way to activate the differentiation pathway today is by all-trans retinoic acid (ATRA), which was found to reduce MDSCs in tumor models [47], turning the myeloid distribution toward DCs, macrophages, and granulocytes. Moreover, it was shown that a combined treatment between ATRA and vaccines improved the anti-tumor effects and yielded the desired results, lowering MDSCs in metastatic RCC patients. It was also recently demonstrated that anti-mitotic chemotherapeutic agent such as paclitaxel in very low and not cytotoxic concentrations directly affect MDSCs, stimulating their differentiation toward DCs [48]. Similarly, another member of the taxane family, docetaxel, was found not only to abrogate tumor progression, but also to decrease MDSCs [49], polarizing them to M1-like phenotype that supports anti-tumor immunity, accompanied with a restoration of Th1 activity [50]. Additionally, there are evidences for natural components that lead to the same results. β-glucans, cell-wall components of numerous micro-organisms, can activate T cells via dectin-1 sign, elicit specific MDSC differentiation via the NF-kB pathway and abrogate the generated immunosuppression. This component could be added to some of the above-described beneficial drugs such as ATRA, docetaxel, or ICA. As mentioned in the previous section, we have recently shown the unique role of TNF-α in mediating MDSC-associated immunosuppression primarily by inducing their differentiation arrest via the S100A8/9 proteins and increasing their immunosuppressive features. By using the FDA-approved TNF-α inhibitor, etanercept, such MDSC “harmful” characteristics could be successfully neutralized. The outcome being stimulation of myeloid cell differentiation and maturation to DCs and macrophages, and reinforced in vivo activity of effector T and NK cells [7].
Conclusion
Taken together, based on the cumulative data, it is undoubtedly clear that the immunosuppression generated under chronic pathological conditions as in cancer possesses is a serious obstacle in the therapeutic area that includes stimulation of anti-tumor immune response and immune-based therapies. There are already convincing evidence showing that the selection of an appropriate and effective therapy must take into consideration not only the tumor-linked parameters or its type and stage, but also the host’s immune status; thus, the therapy must dually attack the tumor cells as well as the generated immunosuppressive environment, aiming at eradicating the suppressive mediators and ensuring the execution of effector anti-tumor immune responses [21, 25]. Components that were identified as displaying a dual function: (1) directing cytotoxic effects on tumor cells and (2) regulating the immunosuppressive mediators as MDSCs could be potentially combined with immunotherapies as complementary and supporting treatment that will magnify the treatment effectiveness. Therefore, prior to a given therapy, it is mandatory that the cancer-bearing host’s immune status should be evaluated by specific biomarkers as CD247 and MDSCs. If immunosuppression is evident, it must be taken into consideration when designing chemo-, biological-, and immune-based treatments. Key parameters such as (1) the dosages of the given treatments, (2) the disease severity, and (3) the host’s immune status and associated immunosuppressive stage must be taken into consideration when designing anti-cancer therapy. These must be routinely monitored prior to and following treatment in order to maintain an optimal combinatorial therapy that will lead to a beneficial personalized treatment.
Acknowledgments
We gratefully acknowledge the support of the Society of Research Associates of the Lautenberg Center, the Concern Foundation of Los Angeles, and the Harold B. Abramson Chair in Immunology. This study was supported by the Israel Science foundation (ISF), the Israeli Ministry of Health, the Joint German-Israeli Research Program (DKFZ-MOST), the Israel Cancer Research Fund (ICRF), and the United States-Israel Binational Science Foundation (BSF) and by the Joseph and Matilda Melnick Funds. We thank Dr. Lynn Wang for reading and commenting on the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Moshe Sade-Feldman and Julia Kanterman contributed equally to this review.
References
- 1.Baniyash M. TCR zeta-chain downregulation: curtailing an excessive inflammatory immune response. Nat Rev Immunol. 2004;4(9):675–687. doi: 10.1038/nri1434. [DOI] [PubMed] [Google Scholar]
- 2.Ciszak L, Pawlak E, Kosmaczewska A, Potoczek S, Frydecka I. Alterations in the expression of signal-transducing CD3 zeta chain in T cells from patients with chronic inflammatory/autoimmune diseases. Arch Immunol Ther Exp (Warsz) 2007;55(6):373–386. doi: 10.1007/s00005-007-0042-6. [DOI] [PubMed] [Google Scholar]
- 3.Vaknin I, Blinder L, Wang L, Gazit R, Shapira E, Genina O, Pines M, Pikarsky E, Baniyash M. A common pathway mediated through Toll-like receptors leads to T- and natural killer-cell immunosuppression. Blood. 2008;111(3):1437–1447. doi: 10.1182/blood-2007-07-100404. [DOI] [PubMed] [Google Scholar]
- 4.Bronstein-Sitton N, Cohen-Daniel L, Vaknin I, Ezernitchi AV, Leshem B, Halabi A, Houri-Hadad Y, Greenbaum E, Zakay-Rones Z, Shapira L, Baniyash M. Sustained exposure to bacterial antigen induces interferon-gamma-dependent T cell receptor zeta down-regulation and impaired T cell function. Nat Immunol. 2003;4(10):957–964. doi: 10.1038/ni975. [DOI] [PubMed] [Google Scholar]
- 5.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunt SK, Clements VK, Hanson EM, Sinha P, Ostrand-Rosenberg S. Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J Leukoc Biol. 2009;85(6):996–1004. doi: 10.1189/jlb.0708446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sade-Feldman M, Kanterman J, Ish-Shalom E, Elnekave M, Horwitz E, Baniyash M. Tumor necrosis factor-alpha blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity. 2013;38(3):541–554. doi: 10.1016/j.immuni.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176(1):284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 9.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64(17):6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13(1):23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao X, Rong L, Li X, Liu X, Deng J, Wu H, Xu X, Erben U, Wu P, Syrbe U, Sieper J, Qin Z. TNF signaling drives myeloid-derived suppressor cell accumulation. J Clin Investig. 2012;122(11):4094–4104. doi: 10.1172/JCI64115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava MK, Andersson A, Zhu L, Harris-White M, Lee JM, Dubinett S, Sharma S. Myeloid suppressor cells and immune modulation in lung cancer. Immunotherapy. 2012;4(3):291–304. doi: 10.2217/imt.11.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA. 1975;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9(5):361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 15.Thompson AE, Rieder SW, Pope JE. Tumor necrosis factor therapy and the risk of serious infection and malignancy in patients with early rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum. 2011;63(6):1479–1485. doi: 10.1002/art.30310. [DOI] [PubMed] [Google Scholar]
- 16.Kopylov U, Ben-Horin S, Zmora O, Eliakim R, Katz LH. Anti-tumor necrosis factor and postoperative complications in Crohn’s disease: systematic review and meta-analysis. Inflamm Bowel Dis. 2012;18(12):2404–2413. doi: 10.1002/ibd.22954. [DOI] [PubMed] [Google Scholar]
- 17.Meyer C, Sevko A, Ramacher M, Bazhin AV, Falk CS, Osen W, Borrello I, Kato M, Schadendorf D, Baniyash M, Umansky V. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci USA. 2011;108(41):17111–17116. doi: 10.1073/pnas.1108121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;13(18 Pt 1):5243–5248. doi: 10.1158/1078-0432.CCR-07-0182. [DOI] [PubMed] [Google Scholar]
- 19.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 20.Martin F, Apetoh L, Ghiringhelli F. Role of myeloid-derived suppressor cells in tumor immunotherapy. Immunotherapy. 2012;4(1):43–57. doi: 10.2217/imt.11.154. [DOI] [PubMed] [Google Scholar]
- 21.Kanterman J, Sade-Feldman M, Baniyash M. New insights into chronic inflammation-induced immunosuppression. Semin Cancer Biol. 2012;22(4):307–318. doi: 10.1016/j.semcancer.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Fujimura T, Mahnke K, Enk AH. Myeloid derived suppressor cells and their role in tolerance induction in cancer. J Dermatol Sci. 2010;59(1):1–6. doi: 10.1016/j.jdermsci.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Apetoh L, Vegran F, Ladoire S, Ghiringhelli F. Restoration of antitumor immunity through selective inhibition of myeloid derived suppressor cells by anticancer therapies. Curr Mol Med. 2011;11(5):365–372. doi: 10.2174/156652411795976574. [DOI] [PubMed] [Google Scholar]
- 24.Kodumudi KN, Weber A, Sarnaik AA, Pilon-Thomas S. Blockade of myeloid-derived suppressor cells after induction of lymphopenia improves adoptive T cell therapy in a murine model of melanoma. J Immunol. 2012;189(11):5147–5154. doi: 10.4049/jimmunol.1200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Investig. 2008;118(6):1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srivastava MK, Zhu L, Harris-White M, Kar UK, Huang M, Johnson MF, Lee JM, Elashoff D, Strieter R, Dubinett S, Sharma S. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One. 2012;7(7):e40677. doi: 10.1371/journal.pone.0040677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Najjar YG, Finke JH. Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front Oncol. 2013;3:49. doi: 10.3389/fonc.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40(11):2969–2975. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Chang EW, Wong SC, Ong SM, Chong DQ, Ling KL. Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. J Immunol. 2013;190(2):794–804. doi: 10.4049/jimmunol.1202088. [DOI] [PubMed] [Google Scholar]
- 30.van Cruijsen H, van der Veldt AA, Vroling L, Oosterhoff D, Broxterman HJ, Scheper RJ, Giaccone G, Haanen JB, van den Eertwegh AJ, Boven E, Hoekman K, de Gruijl TD. Sunitinib-induced myeloid lineage redistribution in renal cell cancer patients: CD1c+ dendritic cell frequency predicts progression-free survival. Clin Cancer Res. 2008;14(18):5884–5892. doi: 10.1158/1078-0432.CCR-08-0656. [DOI] [PubMed] [Google Scholar]
- 31.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R, Finke JH. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15(6):2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 32.Bose A, Lowe DB, Rao A, Storkus WJ. Combined vaccine + axitinib therapy yields superior antitumor efficacy in a murine melanoma model. Melanoma Res. 2012;22(3):236–243. doi: 10.1097/CMR.0b013e3283538293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70(8):3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11(18):6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 35.Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. 2009;9(7–8):900–909. doi: 10.1016/j.intimp.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, Simon B, Ryffel B, Connat JL, Kanellopoulos J, Martin F, Rebe C, Apetoh L, Ghiringhelli F. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19(1):57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 37.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105(7):2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 38.Sevko A, Sade-Feldman M, Kanterman J, Michels T, Falk CS, Umansky L, Ramacher M, Kato M, Schadendorf D, Baniyash M, Umansky V. Cyclophosphamide promotes chronic inflammation-dependent immunosuppression and prevents antitumor response in melanoma. J Investig Dermatol. 2013;133(6):1610–1619. doi: 10.1038/jid.2012.444. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Kosaka A, Ikeura M, Kohanbash G, Fellows-Mayle W, Snyder LA, Okada H. Premetastatic soil and prevention of breast cancer brain metastasis. Neuro Oncol. 2013;15(7):891–903. doi: 10.1093/neuonc/not031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma C, Kapanadze T, Gamrekelashvili J, Manns MP, Korangy F, Greten TF. Anti-Gr-1 antibody depletion fails to eliminate hepatic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;92(6):1199–1206. doi: 10.1189/jlb.0212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Aerts JG, Hegmans JP. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer. 2010;10:464. doi: 10.1186/1471-2407-10-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203(12):2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou J, Wu J, Chen X, Fortenbery N, Eksioglu E, Kodumudi KN, Pk EB, Dong J, Djeu JY, Wei S. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int Immunopharmacol. 2011;11(7):890–898. doi: 10.1016/j.intimp.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen SR, Xu XZ, Wang YH, Chen JW, Xu SW, Gu LQ, Liu PQ. Icariin derivative inhibits inflammation through suppression of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways. Biol Pharm Bull. 2010;33(8):1307–1313. doi: 10.1248/bpb.33.1307. [DOI] [PubMed] [Google Scholar]
- 45.Schilling B, Sucker A, Griewank K, Zhao F, Weide B, Gorgens A, Giebel B, Schadendorf D, Paschen A. Vemurafenib reverses immunosuppression by myeloid derived suppressor cells. Int J Cancer. 2013;133:1653–1664. doi: 10.1002/ijc.28168. [DOI] [PubMed] [Google Scholar]
- 46.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58(1):49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66(18):9299–9307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michels T, Shurin GV, Naiditch H, Sevko A, Umansky V, Shurin MR. Paclitaxel promotes differentiation of myeloid-derived suppressor cells into dendritic cells in vitro in a TLR4-independent manner. J Immunotoxicol. 2012;9(3):292–300. doi: 10.3109/1547691X.2011.642418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16(18):4583–4594. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Djeu J, Wei S. Chemoimmunomodulation of MDSCs as a novel strategy for cancer therapy. Oncoimmunology. 2012;1(1):121–122. doi: 10.4161/onci.1.1.18074. [DOI] [PMC free article] [PubMed] [Google Scholar]