Abstract
Immune checkpoints are a series of inhibitory pathways that are crucial for modulating the intensity and duration of immune response. Among these checkpoints, cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) has been shown to be a key regulator of the early activation of naïve and memory T cells. Immune checkpoint blockade is emerging as one of the most promising therapeutic approaches directed toward the activation of the immune response against tumors. The first of these therapies that has been FDA approved is ipilimumab, a fully human monoclonal antibody that blocks CTLA-4. The in cis effects that CTLA-4 blockade has on T cells have been properly described, but there are still questions to be answered regarding the indirect or in trans effects. One of the alternative cellular populations that may play a role in the outcome of CTLA-4 blockade therapy is myeloid-derived suppressor cells (MDSCs), which have recently been associated with clinical outcome in advanced melanoma. In addition to this, MDSCs have been shown to be decreased in number and functional potential after treatment with ipilimumab. A better clarification of what effects CTLA-4 blockade may have on these cellular populations is likely to provide insights on possible predictive biomarkers for CTLA-4 blockade therapy.
Keywords: Ipilimumab, Myeloid-derived suppressor cells, Immune checkpoint blockade, CTLA-4, Immune therapy, 19th Danish Cancer Society Symposium
Immune checkpoints and their role in tumor escape
T cells play an essential role as drivers and effectors of the immune response to defend the host against attack by pathogens. The thin line between an effective immune response and a destructive one is finely balanced by a number of diverse strategies. First of all, central tolerance is generated by thymic selection, a process in which self-reactive T cells are eliminated. Peripheral tolerance intercedes to control the small fraction of these T cells that are not killed in the first step. The mechanisms of peripheral tolerance involve a plethora of co-stimulatory and inhibitory receptors that determine the quality and amplitude of the immune response, against both self and foreign antigens. These receptors are known as immune checkpoints.
Studies aimed at elucidating the different steps required for T cell activation described an innovative paradigm termed the two-signal model. Engagement of the T cell receptor (TCR) is necessary, but independently insufficient. A second co-stimulatory signal, provided by CD28 binding to its ligands (CD80 and CD86), is necessary for achieving a productive activation of T cells, including clonal expansion and acquisition of effector functions [1, 2]. CTLA-4 was subsequently identified as a gene expressed by activated CTLs [3] that shared ligands with CD28 [4]. However, instead of playing a role in activation of T cells, it plays the crucial role of inhibiting T cell activation [reviewed in 5]. The importance of CTLA-4 as a modulator of the T cell response was further confirmed when the phenotype of mice deficient in CTLA-4 was described. These mice succumbed to a lymphoproliferative disease and survived only 3–4 weeks [6, 7].
Cytotoxic T-lymphocyte-associated antigen-4 is a member of the immunoglobulin superfamily and is a type 1 transmembrane glycoprotein. It is mainly expressed on T cells, although recently CTLA-4 expression has been described at considerably lower levels on activated B cells [8], monocytes [9], dendritic cells, [10] and activated granulocytes [11]. On resting T cells, CTLA-4 presents a minimal surface expression pattern and the majority of CTLA-4 in these cells is located in intracellular vesicles [12]. Upon T cell activation, the vesicles are relocalized and CTLA-4 is then expressed on the surface of the activated T cell, where it can exert its modulatory functions [13].
The mechanisms by which engagement of CTLA-4 modulates T cell activation are still under debate, but they can be divided into two principal arms: cell-intrinsic (in cis) or cell-extrinsic (in trans) [reviewed in 14]. The cell-intrinsic mechanisms include three non-excluding alternatives: (1) Downstream signals induced after binding of CTLA-4 to its ligands deliver a negative signal, (2) Competition for CD28 ligands due to CTLA-4’s higher affinity for CD80 and CD86 and (3) CTLA-4 signaling may affect the adhesion and motility of T cells to antigen presenting cells (APC), inhibiting the TCR-mediated stop signal that is required for APC-T cell stability. The proposed in cis mechanisms fail to explain the function of CTLA-4 in Tregs, in which CTLA-4 is constitutively expressed in high levels [15] and CTLA-4 ligation results in activation. This event initiates a straightforward mechanism of in trans suppression by CTLA-4, in which Tregs produce TGF-β upon CTLA-4 engagement, thereby enhancing their suppressive capabilities. Other cell-extrinsic mechanisms include the induction of indoleamine 2,3-dioxygenase (IDO) production in APCs by CTLA-4 expressing T cells, restriction of ligand availability by production of soluble CTLA-4, and CTLA-4 ligand shedding by trans-endocytosis of CD80 and CD86 from APCs into CTLA-4+ T cells [16].
Another well-known immune checkpoint molecule is PD-1. In contrast to CTLA-4, PD-1 has been described as responsible for controlling the activation of T cells in the periphery [17]. Suppression mediated by this receptor involves intracellular signaling that directly blocks TCR/CD28-mediated activation. Like CTLA-4, PD-1 is an Ig superfamily transmembrane protein that is marginally expressed in non-activated immune cells of both lymphoid and myeloid lineages. Activation increases its expression levels in T cells after TCR engagement. PD-1 is upregulated on Tregs as well as exhausted T cells present in chronic inflammation sites. As opposed to CTLA-4, PD-1-deficient mice survive well past the first month, showing signs of lupus-like autoimmune disease at 6 months of age [18, 19]. PD-1 has two ligands, PD-L1 and PD-L2. PD-L1 is expressed on various cells of hematopoietic, endothelial and epithelial lineage and can be upregulated via inflammatory signals such as TNF-α and interferons (type I and II) [20]. PD-L2, the second ligand, is restricted to macrophages, dendritic cells and mast cells. Its expression can also be controlled by inflammatory signals, mainly IL-4 and IFN-γ [21].
T cells have proven to have the potential to specifically eliminate tumors provided that they have been properly activated and are present in appropriate numbers. In fact, the presence of circulating tumor-specific T cells in melanoma patients has been extensively described [22], intratumoral CTL infiltration is often associated with favorable clinical outcomes such as decreased disease recurrence and prolonged survival in diverse malignancies [23–25], and adoptive T cell therapy has proven to be a successful approach for the treatment of several malignancies [26]. It is broadly accepted that T cells play a key role in the immune system’s potential to control and eliminate tumors.
Tumors generate a hostile microenvironment for immune cells usurping the same mechanisms that the immune system utilizes to avoid autoimmune responses and immunopathologic sequelae to infectious agents. Among these main mechanisms, tumors can co-opt the immune checkpoints, resulting in a brake being set on possible anti-tumoral immune responses. CTLA-4 is upregulated on circulating and tumor infiltrating T cells [27] and is constitutively expressed in Tregs [15], playing an essential role in their suppressive function [28, 29] that could further impede tumor elimination by the immune system. PD-1 is also upregulated on depleted TILs, and some tumors may also express PD-L1 as an escape mechanism.
In this context, immune checkpoint blockade with monoclonal antibodies is a new type of anticancer therapy that does not target the tumor itself, but has the objective of releasing the brake on the immune system to enable tumor elimination. At the time of writing this review, one antibody against CTLA-4 (ipilimumab (Yervoy®), Bristol-Myers Squibb) was approved by the FDA in 2011 and the EMA in 2012 as the first treatment to show survival benefit in patients with metastatic melanoma [30]. In addition to Ipilimumab, a second CTLA-4 blocking antibody (tremelimumab, Medimmune) is currently undergoing Phase III trials. In the case of PD-1/PD-L1 pathway, there are several candidates that block either the receptor or the ligand with ongoing trials in Phases I-III [reviewed in 31].
Mechanisms of action of ipilimumab: searching for predictive and pharmacodynamic biomarkers
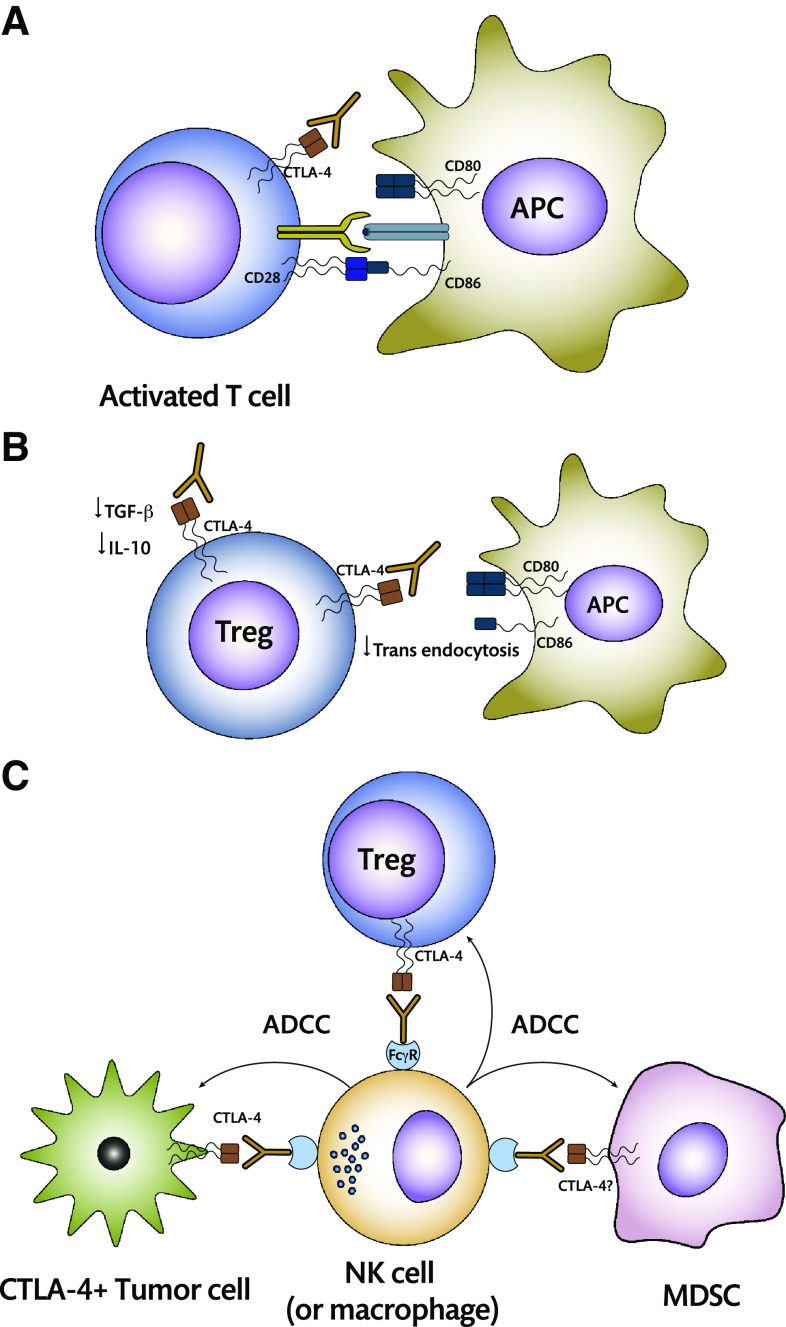
Given the multiple cis and trans pathways by which CTLA-4 exerts its inhibitory function, it is now clear that blocking this receptor can activate T cells via two mechanisms (Fig. 1): Blocking the inhibitory pathways per se of the T cell, allowing normal activation after the two activating signals (TCR/CD28) are delivered, or preventing the in trans inhibition pathways in order to achieve the same effect [32].
Fig. 1.
Mechanisms of action of CTLA-4 blockade. a Anti-CTLA-4 may be acting directly or in cis on effector T cells by impeding CTLA-4 intracellular signaling that takes place when it binds to its ligand. In addition to this, availability of CD80 and CD86 on the APC surface will be increased, allowing for direct activation after co-stimulatory signals are delivered by CD28 signaling. b In trans mechanisms involve the binding of the antibody to CTLA-4 on the surface of Tregs, decreasing production of inhibitory cytokines, and preventing transendocytosis of CD80 and CD86. c ADCC has been recently suggested as a possible mechanism of action for CTLA-4 blockade. It may involve Treg depletion or directly eliminate CTLA-4+ tumor cells. The possibility that MDSCs may express CTLA-4 on their surface is currently under debate and may provide an additional pathway toward α-CTLA-4-mediated activation
It would therefore be logical that the search for predictive biomarkers should involve analyzing the changes in phenotype of T cell populations in patients during ipilimumab treatment. A large number of studies examining changes in T cell frequencies and phenotype have been performed. None of these have been able to solidly establish any type of predictive biomarkers that correlate with clinical outcomes. These studies have shown increases in absolute lymphocyte counts, activated (HLA-DR+ or CD25+) CD4 and CD8 T cells, increases in central memory (CD4 and CD8) and effector memory (CD8 only), increases in ICOS+ CD4+, early increases in Treg populations, increases in Ki67+ CD4 and CD8 cells, decreases in the frequency of naïve T cells and an increase in the T cell reactivity and humoral response to tumor antigens [33–35]. In summary, patients undergoing treatment respond with an overall activation of their immune system, suggesting possible pharmacodynamic biomarkers although no valid predictive biomarkers have been found. One of the most solid candidates to be considered as a pharmacodynamic biomarker is ICOS expression in CD4 T cells. This costimulatory molecule showed to be essential for antitumoral responses during CTLA-4 blockade in mice [36, 37], and its expression is increased in ipilimumab-treated patients [38].
The possibility that additional cell populations are involved in the response to ipilimumab treatment is presently being explored. Firstly, several independent studies have demonstrated that CTLA-4 is not exclusively expressed on T cells [8–11], and therefore, it is eminently possible that ipilimumab may be directly targeting additional cell populations. In addition to this, an alternative mechanism of action of CTLA-4 blockade through selective elimination of tumor infiltrating Tregs has been recently described in a murine model [39]. This depletion was shown to be dependent on the presence of FcγRIV expressing macrophages, suggesting antibody-dependent cellular cytotoxicity (ADCC) as the responsible mechanism. In humans, the equivalent of FcγRIV is FcγRIIIA, which is expressed on NK cells, macrophages, monocytes and neutrophils, cell populations that may also yield potential biomarkers. Finally, CTLA-4 has recently been detected by immunohistochemistry in fixed tumor samples. In this report, Laurent et al. [40] also demonstrated that CTLA-4 can be expressed on the surface and secreted by patient-derived cutaneous melanoma cell lines and that these CTLA-4 expressing cell lines could be targeted by ipilimumab-mediated ADCC. The cumulative conclusion is that immune monitoring of patients undergoing ipilimumab treatment should not be restricted only to T cells.
Myeloid-derived suppressor cells
Myeloid-derived suppressor cells (MDSCs) are a largely heterogeneous population of cells of myeloid origin that have immune suppressor activity. The term myeloid-derived suppressor cell was first coined in 2007 [41], and since then this cellular population has received increasing focus of attention as one of the main cellular populations responsible for the suppression of the innate and adaptive antitumoral immune response [reviewed in 42].
In most of the studies, MDSCs have been found to be positive for CD33 and CD11b, while expressing very low levels or no HLA-DR. Additionally, there are a wide number of overlapping or mutually excluding phenotypic descriptions. In spite of the diversity of this cellular population, two main subsets can be established according to phenotypic surface markers: Granulocytic MDSCs (GrMDSCs) that express CD15 and monocytic MDSCs (MoMDSCs) that are positive for CD14.
Besides having a distinct phenotype, MDSCs by definition also have suppressor capabilities [42]. GrMDSCs suppress T cells mainly via the production of reactive oxygen species (ROS) (which induces the loss of the TCR ζ chain) or arginase I, (resulting in arginine starvation in T cells). Monocytic MDSCs also suppress T cells via arginine starvation mediated by iNOS (as well as the aforementioned arginase production), in addition to producing suppressive cytokines TGF-β and IL-10. This suppressive mechanism is closely associated with the crosstalk that exists between MDSCs and Tregs: There is a correlation between the frequencies of these cellular populations in patients with several malignancies, and MDSCs have shown, in vitro, to induce the conversion of conventional T cells into Tregs.
The interest of our group in MDSCs started in 2010 when we showed that CD14+/HLA-DRlo MoMDSCs were significantly increased in patients with advanced melanoma [43]. These MoMDSCs proved to be suppressive via arginase I production and overexpressed CD80, CD83, and STAT3. We are currently investigating the mechanisms by which these MoMDSCs are generated in the tumor microenvironment of melanoma and have been focusing on the roles of PGE-2 and COX-2 in this process [44]. In addition to this, a recent paper by Weide et al. [45] has confirmed the importance of this population of MoMDSCs, showing that their frequency is inversely related to the frequency of antigen-specific T cells and, most importantly, with survival of melanoma patients.
The GrMDSC subset has one inherent quality that may be hindering its proper study, possibly leading to an underestimation of both the frequency and functionality of this cellular population: Granulocytes are very short-lived and highly sensitive to freezing. In spite of this inconvenience, many studies have described a wide variety of granulocytic MDSCs [42], and in the case of melanoma, possibly assigning them higher suppressive capabilities [46]. We are currently working on several immune monitoring projects that include the collection, processing, and analysis of fresh samples that will allow us to study MDSCs in advanced melanoma patients under treatment.
MDSCs and checkpoint blockade therapies
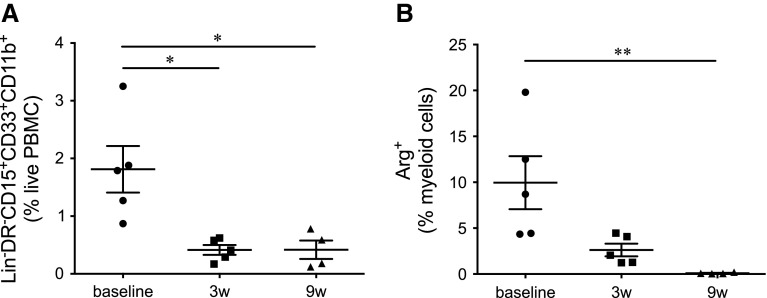
As discussed above, there is a great need for information regarding the possible role that non-T cell populations may be playing during antibody checkpoint blockade. In 2011, we started monitoring the immune system of patients undergoing ipilimumab treatment. The main focus of this study was to look for predictive and pharmacodynamic biomarkers that may aid in the clarification of the mechanisms of action of ipilimumab. We used multicolor flow cytometric analysis of fresh PBMCs purified from patients before, during, and after the treatment. Among the multiple parameters analyzed are the frequencies and some functional markers of two MDSC populations with granulocytic and monocytic phenotype (Lin− HLA-DR−/lo CD15+ CD33+ CD11b+ and CD3− CD19− HLA-DR−/lo CD14+, respectively). We have recently shown that in these patients, the frequency of GrMDSCs decreases significantly 3 weeks after the first dose of ipilimumab is administered [47]. This decrease is maintained during the course of treatment and is accompanied by a significant decrease in the frequencies of ARG1-producing, CD14− myeloid cells (Fig. 2). In the eight patients included in the study, no trend was observed in the MoMDSC population. Along with the frequencies of MDSCs, the frequencies of Tregs and PD1+ T cells were also determined, showing an initial increase, followed by a decrease to lower than baseline levels by the end of ipilimumab treatment. These results provided a first look at responses of MDSCs to CTLA-4 blockade and suggest that GrMDSCs may be useful as a pharmacodynamic biomarker. In spite of the fact that no changes in MoMDSCs were observed, analysis of the PD-L1 expression levels in CD14+ monocytes was also carried out, revealing a profile very similar to that observed in both Tregs and PD1+ T cells. In summary, the combined data obtained from these eight patients and fifteen others that were included in the study after publication suggest that ipilimumab has an effect on both the frequency and suppressive capacity of GrMDSC populations and supports further studies that aim to clarify the possible role that MDSCs are playing in this therapeutic setting. The nature of this effect can be direct or indirect, as with T cells. Given the possibility that myeloid populations express CTLA-4, an in cis effect could imply blocking the possible CTLA-4 related signaling pathways in the myeloid cell. Ipilimumab-mediated ADCC could also be responsible for the depletion of GrMDSCs as it has already been shown for CTLA-4+ melanoma cell lines [40]. In trans effects of ipilimumab on myeloid populations could be related to the higher activation state that is observed for T cells, via mechanisms that have yet to be described.
Fig. 2.
Changes in MDSC frequencies and phenotypes during ipilimumab treatment. a Lin− HLA-DR−/lo CD15+ CD33+ CD11b+ frequencies significantly decreased after the first ipilimumab dose and remained low at week 9. b CD3− cells cease ARG1 production at week 9 after treatment. Myeloid cells were gated based on CD3 negativity and FSC/SSC characteristics
Due to the known importance of MoMDSCs in advanced melanoma [43, 45, 48], the lack of changes in the frequency of this population following ipilimumab treatment in our studies was unexpected. Similar results have been observed in the latest study by Speiser et al. [49] in which a larger cohort of patients was analyzed without observing significant changes in MoMDSC frequencies during treatment. The main finding in this study is that patients responding to ipilimumab had significantly lower frequencies of MoMDSCs. In contrast to these results, there is a recent report that has associated 10 mg/kg neoadjuvant ipilimumab treatment with a decrease in MoMDSCs [50]. These are, to our knowledge, the first reports in which MDSCs have been associated with the outcome of checkpoint blockade therapy, warranting further studies with larger patient numbers that could lead to the inclusion of the frequency of this cellular population as a predictive biomarker for treatment outcome. In addition to this, they open the window for combination therapies that include checkpoint blockade and chemotherapeutic approaches that may inhibit expansion or viability of MDSCs such as docetaxel, gemcitabine, or sunitinib [51–53]. The results observed by Meyer et al. [49] are in agreement with those observed by Weide et al. [45], where a strong inverse correlation between MoMDSCs and NY-ESO-1-specific T cells was observed. In this setting, a higher MoMDSC burden may impede maximum ipilimumab-induced activation and expansion of tumor-specific T cells leading toward lower clinical response rates. Ipilimumab has recently been shown to enhance NY-ESO-1-specific responses in a small number of patients [54], and the presence of NY-ESO-1 antibodies combined with CD8-T cell responses has been suggested as a possible predictive marker for ipilimumab in an earlier report [55]. Further studies of this triple correlation between MoMDSCs, tumor-specific T cells, and clinical response rates to ipilimumab could deliver broader insights on the mechanisms of action of ipilimumab and clarify the role of the myeloid compartment in the response to this therapy.
Acknowledgments
The authors would like to thank Raja Choudhury for revising the manuscript. This study was supported by Grants from The Knut and Alice Wallenberg Foundation, The Swedish Cancer Society (12-0598 Cancerfonden), The Stockholm Cancer Society (121103 Cancerföreningen, Radiumhemmets Forskningsfonder), The Swedish Medical Research Council (K2011-66X-15387-07-3VR) and an ALF-Project Grant from Stockholm City Council (20110070 ALF-Medicin-2012).
Conflict of interest
Rolf Kiessling and Johan Hansson have received fees for lectures and other educational activities from Bristol-Myers Squibb. The remaining authors have no conflict of interests.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the 19th Danish Cancer Society Symposium in Copenhagen, Denmark, 23rd–25th September 2013, on the topic “Immunotherapy of Cancer—Present Status and Future Promise”. It is a part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.June CH, Ledbetter JA, Gillespie MM, Lindsten T, Thompson CB. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol Cell Biol. 1987;7(12):4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson CB, Lindsten T, Ledbetter JA, Kunkel SL, Young HA, Emerson SG, Leiden JM, June CH. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci USA. 1989;86(4):1333–1337. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, Golstein P. A new member of the immunoglobulin superfamily–CTLA-4. Nature. 1987;328(6127):267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 4.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174(3):561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65–97. doi: 10.1146/annurev.immunol.24.021605.090535. [DOI] [PubMed] [Google Scholar]
- 6.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270(5238):985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 7.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 8.Pioli C, Gatta L, Ubaldi V, Doria G. Inhibition of IgG1 and IgE production by stimulation of the B cell CTLA-4 receptor. J Immunol. 2000;165(10):5530–5536. doi: 10.4049/jimmunol.165.10.5530. [DOI] [PubMed] [Google Scholar]
- 9.Wang XB, Giscombe R, Yan Z, Heiden T, Xu D, Lefvert AK. Expression of CTLA-4 by human monocytes. Scand J Immunol. 2002;55(1):53–60. doi: 10.1046/j.0300-9475.2001.01019.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang XB, Fan ZZ, Anton D, Vollenhoven AV, Ni ZH, Chen XF, Lefvert AK. CTLA4 is expressed on mature dendritic cells derived from human monocytes and influences their maturation and antigen presentation. BMC Immunol. 2011;12:21. doi: 10.1186/1471-2172-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pistillo MP, Tazzari PL, Palmisano GL, Pierri I, Bolognesi A, Ferlito F, Capanni P, Polito L, Ratta M, Pileri S, Piccioli M, Basso G, Rissotto L, Conte R, Gobbi M, Stirpe F, Ferrara GB. CTLA-4 is not restricted to the lymphoid cell lineage and can function as a target molecule for apoptosis induction of leukemic cells. Blood. 2003;101(1):202–209. doi: 10.1182/blood-2002-06-1668. [DOI] [PubMed] [Google Scholar]
- 12.Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4(6):535–543. doi: 10.1016/S1074-7613(00)80480-X. [DOI] [PubMed] [Google Scholar]
- 13.Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16(1):23–35. doi: 10.1016/S1074-7613(01)00259-X. [DOI] [PubMed] [Google Scholar]
- 14.Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11(12):852–863. doi: 10.1038/nri3108. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192(2):303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151. doi: 10.1016/S1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 20.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 22.Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, Johnson D, Swetter S, Thompson J, Greenberg PD, Roederer M, Davis MM. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5(6):677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 23.Clark WH, Jr, Elder DE, Guerry Dt, Braitman LE, Trock BJ, Schultz D, Synnestvedt M, Halpern AC. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81(24):1893–1904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 24.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25(18):2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 26.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3(9):666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke B, Romero P, Rufer N, Speiser DE. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121(6):2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192(2):295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 30.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Callahan MK, Wolchok JD. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J Leukoc Biol. 2013;94(1):41–53. doi: 10.1189/jlb.1212631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blank CU (2014) The perspective of immunotherapy: new molecules and new mechanisms of action in immune modulation. Curr Opin Oncol 26(2):204–214 [DOI] [PubMed]
- 33.Kavanagh B, O’Brien S, Lee D, Hou Y, Weinberg V, Rini B, Allison JP, Small EJ, Fong L. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112(4):1175–1183. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W, Yu D, Sarnaik AA, Yu B, Hall M, Morelli D, Zhang Y, Zhao X, Weber JS. Biomarkers on melanoma patient T Cells associated with ipilimumab treatment. J Transl Med. 2012;10(1):146. doi: 10.1186/1479-5876-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber JS, Hamid O, Chasalow SD, Wu DY, Parker SM, Galbraith S, Gnjatic S, Berman D. Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma. J Immunother. 2012;35(1):89–97. doi: 10.1097/CJI.0b013e31823aa41c. [DOI] [PubMed] [Google Scholar]
- 36.Fu T, He Q, Sharma P. The ICOS/ICOSL pathway is required for optimal antitumor responses mediated by anti-CTLA-4 therapy. Cancer Res. 2011;71(16):5445–5454. doi: 10.1158/0008-5472.CAN-11-1138. [DOI] [PubMed] [Google Scholar]
- 37.Fan X, Quezada SA, Sepulveda MA, Sharma P, Allison JP. Engagement of the ICOS pathway markedly enhances efficacy of CTLA-4 blockade in cancer immunotherapy. J Exp Med. 2014;211(4):715–725. doi: 10.1084/jem.20130590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng Tang D, Shen Y, Sun J, Wen S, Wolchok JD, Yuan J, Allison JP, Sharma P. Increased frequency of ICOS+ CD4 T cells as a pharmacodynamic biomarker for anti-CTLA-4 therapy. Cancer Immunol Res. 2013;1(4):229–234. doi: 10.1158/2326-6066.CIR-13-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, Peggs KS, Ravetch JV, Allison JP, Quezada SA. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laurent S, Queirolo P, Boero S, Salvi S, Piccioli P, Boccardo S, Minghelli S, Morabito A, Fontana V, Pietra G, Carrega P, Ferrari N, Tosetti F, Chang LJ, Mingari MC, Ferlazzo G, Poggi A, Pistillo MP. The engagement of CTLA-4 on primary melanoma cell lines induces antibody-dependent cellular cytotoxicity and TNF-alpha production. J Transl Med. 2013;11:108. doi: 10.1186/1479-5876-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67(1):425. doi: 10.1158/0008-5472.CAN-06-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poschke I, Kiessling R. On the armament and appearances of human myeloid-derived suppressor cells. Clin Immunol. 2012;144(3):250–268. doi: 10.1016/j.clim.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+ HLA-DR−/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70(11):4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 44.Mao Y, Poschke I, Wennerberg E, Pico de Coana Y, Egyhazi Brage S, Schultz I, Hansson J, Masucci G, Lundqvist A, Kiessling R. Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res. 2013;73(13):3877–3887. doi: 10.1158/0008-5472.CAN-12-4115. [DOI] [PubMed] [Google Scholar]
- 45.Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM, Maio M, Sucker A, Schilling B, Schadendorf D, Buttner P, Garbe C, Pawelec G. Myeloid-derived suppressor cells predict survival of advanced melanoma patients: comparison with regulatory T cells and NY-ESO-1- or Melan-A-specific T cells. Clin Cancer Res. 2013;20(6):1601–1609. doi: 10.1158/1078-0432.CCR-13-2508. [DOI] [PubMed] [Google Scholar]
- 46.Schilling B, Sucker A, Griewank K, Zhao F, Weide B, Gorgens A, Giebel B, Schadendorf D, Paschen A. Vemurafenib reverses immunosuppression by myeloid derived suppressor cells. Int J Cancer. 2013;133(7):1653–1663. doi: 10.1002/ijc.28168. [DOI] [PubMed] [Google Scholar]
- 47.Pico de Coaña Y, Gentilcore G, Mao Y, Nyström M, Hansson J, Masucci GV, Kiessling R. Ipilimumab treatment results in an early decrease in the frequency of circulating granulocytic myeloid-derived suppressor cells as well as their arginase1 production. Cancer Immunol Res. 2013;1(3):158–162. doi: 10.1158/2326-6066.CIR-13-0016. [DOI] [PubMed] [Google Scholar]
- 48.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25(18):2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 49.Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, Michielin O, Romano E, Speiser DE. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother. 2014;63(3):247–257. doi: 10.1007/s00262-013-1508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tarhini AA, Edington H, Butterfield LH, Lin Y, Shuai Y, Tawbi H, Sander C, Yin Y, Holtzman M, Johnson J, Rao UN, Kirkwood JM. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS ONE. 2014;9(2):e87705. doi: 10.1371/journal.pone.0087705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11(18):6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 52.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16(18):4583–4594. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R, Finke JH. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15(6):2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 54.Kitano S, Tsuji T, Liu C, Hirschhorn-Cymerman D, Kyi C, Mu Z, Allison JP, Gnjatic S, Yuan JD, Wolchok JD. Enhancement of tumor-reactive cytotoxic CD4 T cell responses after ipilimumab treatment in four advanced melanoma patients. Cancer Immunol Res. 2013;1:235–244. doi: 10.1158/2326-6066.CIR-13-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, Xu Y, Pogoriler E, Terzulli SL, Kuk D, Panageas KS, Ritter G, Sznol M, Halaban R, Jungbluth AA, Allison JP, Old LJ, Wolchok JD, Gnjatic S. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci USA. 2011;108(40):16723–16728. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]