Abstract
Introduction
The current systemic anti-metastatic treatment is chemotherapy. Chemotherapy reacts mostly against replicating cells, which makes this therapy not specific. Moreover, resting cancer cells will not be destroyed. A better alternative is an engagement of the host immune system to react against tumor-associated antigens. An efficient immune-stimulating technique is an ablation of the tumor that results in the release of tumor antigens. Our ablation strategy is an innovative alpha-radiation-based technology, diffusing alpha-emitters radiation therapy (DaRT), which efficiently destroys local tumors and provides thereby an antigenic supply for antigen-presenting cells to stimulate T cells.
Methods
Mice bearing weakly immunogenic DA3 adenocarcinoma or highly immunogenic CT26 colon carcinoma were treated by DaRT. Anti-tumor immune responses following tumor destruction were evaluated by (1) the resistance to a tumor challenge; (2) scanning by a CT imaging device for elimination of lung metastases; (3) improved tumor control when combining DaRT with an immunoadjuvant (CpG).
Results
CT26 model: 63–77 % of DaRT-treated mice became resistant to a re-inoculated tumor compared to 29–33 % resistant mice in the control. DA3 model: (1) The growth rate of challenge tumors was the lowest in mice which their primary tumor was treated by DaRT. (2) Most (93 %) mice in the control group developed lung metastases compared to 56 % in the DaRT group. (3) Combining DaRT with CpG resulted in a better control of the primary tumor. Our study offers a technique to eliminate local and distant malignant cells, regardless of their replication status, by stimulating specific anti-tumor immunity through the supply of tumor antigens from the destroyed tumor.
Keywords: Colon cancer, Breast cancer, Radiotherapy, Immune stimulation, Ra-224, Brachytherapy
Introduction
The major risk of treating tumors by radiation is tumor recurrence at the end of the therapy. The challenge of chemotherapy is to avoid appearance of micrometastases and of targeting senescent tumor cells. Therefore, multimodal treatments are urgently needed that induce systemic, specific, and long-lasting anti-tumor responses [1]. Tumor cells express tumor-associated antigens that can be recognized by T cells provided that they are phagocytized and processed by dendritic cells (DCs) [2].
To improve the recruitment of DCs and the tumor antigen uptake by those cells, a massive tumor antigen release is needed. Upon tumor ablation in situ, large amounts of tumor debris are released that could potentially be taken up by the immune system and serve as an antigen source for the induction of anti-tumor immunity [3].
Diffusing alpha-emitters radiation therapy (DaRT) is a novel, alpha-radiation-based, ablation method. In this technique, tumors are destroyed by an insertion of Ra-224-loaded wires that disperse in the tumor alpha particle emitting atoms. DaRT therapy has been tested in our laboratory on both mouse and human experimental tumor models and proven to be effective, specific, and safe [4–10].
Increasing evidence suggests that adaptive immunity contributes to the long-term clinical benefits of anticancer treatments [11]. By destroying the tumor in situ with a Ra-224-loaded wire, tumor cells serve as a source of tumor antigens that can be taken up by DCs and initiate an adaptive immune response.
Low antigen expression and an absence of co-stimulatory signals may be partly responsible for the low immunogenicity of many tumors. It may be possible to overcome this situation by defining a combination of adjuvants and antigens that can activate a high-avidity anti-tumor response [12]. CpG oligodeoxynucleotides (ODNs) are synthetic DNA sequences containing unmethylated cytosine–guanine motifs with potent immune modulatory effects. Via Toll-like receptor 9 agonists of DCs and B cells, CpG ODNs induce cytokines, activate natural killer cells, and elicit vigorous T cell responses that lead to significant anti-tumor effects [13–16].
In this work, we used two models of syngeneic mouse tumors: the DA3 murine breast adenocarcinoma [17] and CT26 mouse colon carcinoma. These tumors differ in their immunogenicity. In contrast to the DA3 model, mice with CT26 mouse colon carcinoma developed a strong anti-tumor immune response [18]. We showed that alpha-radiation therapy suppressed the growth of both breast and colon primary tumors. The destruction of the tumor stimulated anti-tumor immunity and decreased the numbers of distant metastases. The application of DaRT in combination with CpG strongly enhanced the inhibitory effect on primary tumors. These data suggest that the tumor ablation via DaRT augments anti-tumor immune reactions and could be considered as an efficient strategy of anti-tumor immunotherapy.
Methods
Animals
Balb/c male and female mice (20–25 g, 10 weeks old) were obtained from Tel Aviv University (Tel Aviv, Israel) and Harlan (Jerusalem, Israel), and were kept in the animal facility of Tel Aviv University. Experiments were performed in accordance with government and institute guidelines and regulations (Ethical committees permit No. M-10-059 and M-11-104). The survival and general performance of mice were monitored daily.
Tumor cell lines
The DA3 cell line is a dimethylbenzanthracene (DMBA)-induced, undifferentiated breast adenocarcinoma cell line. The cells were grown in Dulbecco’s modified Eagle’s medium (DMEM, Biological industries, Kibbutz Beit Haemek, Israel), supplemented with 10 % fetal calf serum (Biological Industries, Kibbutz Beit Haemek, Israel), l-glutamine (2 mM) (Biological Industries, Kibbutz Beit Haemek, Israel), penicillin (100 U/ml), and streptomycin (100 μg/ml) (Biological industries, Kibbutz Beit Haemek, Israel). The cell line was stored in a humid incubator at a temperature of 37 °C and 5 % CO2.
The CT26 cell line is an N-nitroso-N-methylurethane (NNMU)-induced, undifferentiated colon carcinoma cell line. It was purchased from the ATCC (CRL-2638). The cells were grown in Roswell Park Memorial Institute (RPMI)-1,640 medium (GIBCO, Rehovot, Israel) supplemented with 10 % fetal calf serum (Biological Industries, Kibbutz Beit Haemek, Israel), l-glutamine (2 mM) (Biological Industries, Kibbutz Beit Haemek, Israel), penicillin (100 U/ml), streptomycin (100 μg/ml) (Biological industries, Kibbutz Beit Haemek, Israel), sodium pyruvate (1 mM) (Biological Industries, Kibbutz Beit Haemek, Israel), HEPES buffer 1 M (Biological Industries, Kibbutz Beit Haemek, Israel), and d-glucose (Biological Industries, Kibbutz Beit Haemek, Israel).
CT26 cells infected with the pQC-mCherry retroviral particles were used to evaluate the rate of metastatic spread in the lungs as an expression of activated anti-tumor immunity (kindly provided by Dr. R. Satchi-Fainaro, Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel).
Tumor cell inoculation
Male mice were inoculated intracutaneously into the low lateral side of the back with 5 × 105 CT26 cells in 0.1 ml Hanks’ balanced salt solution (HBSS, Biological Industries, Kibbutz Beit Haemek, Israel). Female mice were injected into either low lateral side of the back or the low left mammary gland with 5 × 105 DA3 cells in 0.1 or 0.05 mL HBSS, respectively.
Tumor volume calculation
Local tumor growth was determined by measuring 3 mutually orthogonal tumor dimensions 2–3 times per week.
Experimental tumor metastasis
Lung metastases developed 10 days after i.v. injection of 2 × 106 mCherry-labeled CT26 cells in 0.2 ml HBSS buffer.
Ra-224-loaded wire (diffusing alpha-emitter radiation therapy wire) preparation
Ra-224 (3.66 days half-life)-loaded wires are prepared using a Th-228 (1.91 years half-life) (Eckert & Zigler, Berlin, Germany) generator as described [4]. The generator is a flat conductive surface partially covered with Th-228 atoms. During the α-disintegration of Th-228, the daughter Ra-224 atom recoils. About 50 % of the Ra-224 atoms recoil out of the generator surface as positive ions and are collected on a thin wire electrostatically. To prevent Ra-224 loss from the wire, the atoms are embedded a few atomic layers below the wire surface through thermal diffusion.
Wire insertion
Wires with the length of 7 mm, either loaded with Ra-224 or inert, were placed near the tip of a 23-gauge needle attached to a 2.5-ml syringe (Picindolor, Rome, Italy) and inserted into the tumor by a plunger placed internally along the syringe axis. Mice of the control group were treated with the inert (non-radioactive) wires.
Inside the tumor, the Ra-224 stays on the wire surface and decays into diffusing atoms that release alpha particles by recoil, which destroy tumor cells.
Treatment protocol
The treatment experiment included 4 experimental groups: (1) Mice with tumors 6–8 mm long (6.5 ± 0.1) (~50–60 mm3 average volume) were treated by a single 7-mm Ra-224-loaded wire. In case tumors did not disappear, they were excised 14 days after wire insertion. (2) Inert control group; tumor-bearing mice treated by an inert wire, whose their tumors were also excised 14 days after wire insertion. (3) Non-treated tumor-bearing mice and (4) mice, whose their tumors were excised when 6–8 mm in diameter.
Challenge assay
Twenty-one days post-wire insertion, mice were re-inoculated with 5 × 105 tumor cells/mouse. Additional control for this assay exclusively was naïve mice that were inoculated with tumor cells for the first time.
Assay scheme: 
Histology
Histological staining of tumors from treated and control tumor-bearing mice was performed as described [6]. Briefly, immediately after removal, tumors were fixed in a 4 % formaldehyde solution (Sigma, Rehevot, Israel) for at least 24 h. The preserved specimens were embedded in paraffin, and sections (5–10 μm) were stained with hematoxylin and eosin (Surgipath, Richmond, Va).
Signal quantification of labeled cancer cells
CRI Maestro™ (Cambridge Research and Instrumentation, USA) fluorescence imaging system was used to measure signal obtained from experimental metastases in mice inoculated i.v. with mCherry-labeled tumor cells. When a mouse was diagnosed as moribund, all organs were taken and analyzed.
Computed tomography imaging of lungs
Mice lungs were scanned, using TomoScope® In vivo CT imaging device (Erlangen, Germany). Images were analyzed using “Amide” and “RadiAnt” softwares.
Adjuvant administration
A day before the wire insertion, mice were injected with 100 μg of CpG (Syntezza, Jerusalem, Israel) in 30 μl PBS (three peri-tumoral injections with 10 μl each) (Tel Aviv University, Tel Aviv, Israel). In addition, a day after the wire insertion, mice were inoculated i.v. with 100 μg CpG in 100 μl PBS. As controls served mice treated by inert wire + PBS, or inert wire + CpG.
Statistical analysis
Statistical significance (p < 0.05) between the experimental groups was determined by two-side Student’s T test or two-way ANOVA without replication or chi-square statistical tests.
Results
Tumor ablation by Ra-224-loaded wires potentiated anti-tumor immunity
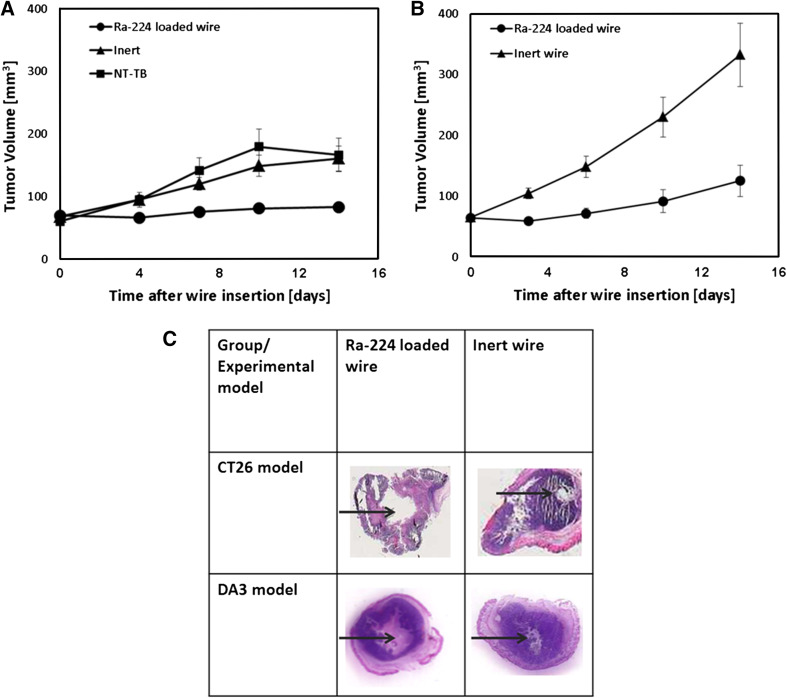
We examined whether Ra-224-loaded wire-based tumor destruction can elicit strong anti-tumor immunity against DA3 and CT26 tumors. In order to test it, DaRT or inert (non-radioactive) wires (one per tumor) were inserted into established tumors (6–8 mm in diameter) and were left there for 14 days. Measurements of tumor volumes revealed that the average tumor volume in the DaRT-treated group was 2–2.6 times smaller than in the control groups in both the DA3 and CT26 models. Histology documented the extent of wire-induced damage inside the tumor tissue (Fig. 1).
Fig. 1.
Ra-224-loaded wire inhibits both DA3 and CT26 local tumor development. a DA3 tumor-bearing mice were treated with the intratumoral Ra-224-loaded wire (40–50 kBq/wire, n = 52) or with an inert wire (n = 46) or were left untreated. Tumor volumes were recorded for 14 days. P (T test) < 0.05 (except for day 0).b CT26 tumor-bearing mice were treated with the intratumoral Ra-224-loaded wire (40-50 kBq/wire, n = 17) or with an inert wire (n = 17). Tumor volumes were recorded for 14 days. P (T test) <0.05 (except for day 0). c Histological slices of DA3 tumors were taken 14 days, and CT26 tumors 5 days after a single Ra-224-loaded wire (half-life = 3.66 days)
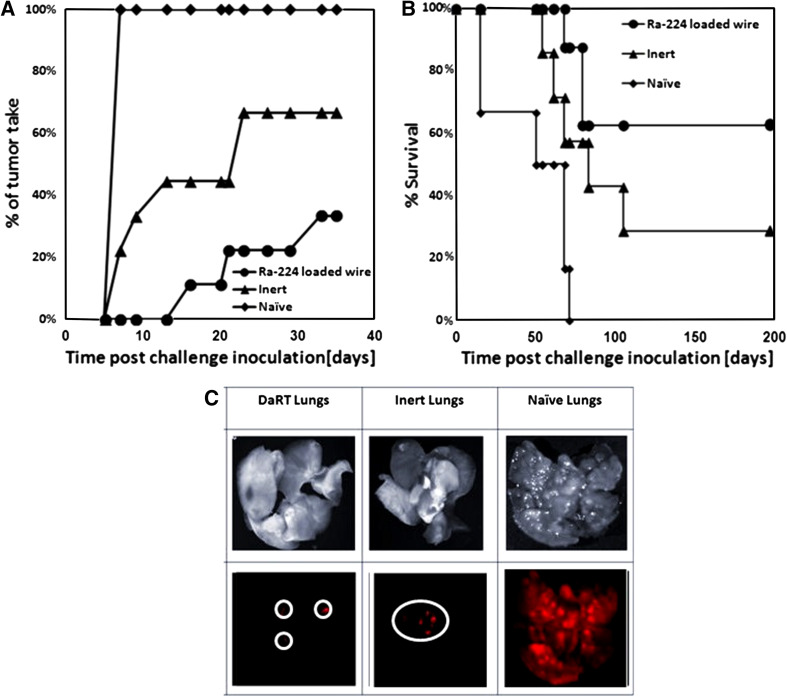
The treated animals were re-inoculated with the same amount of tumor cells as was injected in order to induce a primary tumor. The appearance and growth of secondary tumors were recorded. We found that in the CT26 model, 77 % of mice in the DaRT-treated group did not develop s.c. tumors after a challenge with tumor cells compared to 33 % of mice in the inert-treated group that were resistant to re-inoculation of cells (Fig. 2a).
Fig. 2.
Effect of tumor ablation by Ra-224-loaded wires on the resistance to a subcutaneous or i.v. CT26 tumor rechallenge. a CT26 tumor-bearing mice were treated with either intratumoral Ra-224-loaded wire (40–50 kBq/wire, n = 8–9) or with an inert wire (n = 7–9) followed by the removal of residual tumors 14 days after the treatment. Treated animals were injected again s.c. with 5 × 105 tumor cells. Normal mice were injected s.c. with 5 × 105 CT26 tumor cells at the same time (naïve, n = 9). The percentage of tumor take against time after challenge inoculation is presented, P (T-test) <0.05 (at all points, Ra-224-loaded wire vs. inert or Naive). b Treated animals were injected i.v. with 2 × 106 tumor cells. Naive mice were injected i.v. with 2 × 106 CT26 tumor cells at the same time (naïve, n = 6). Survival of mice is shown as a Kaplan–Meier curve. P (two-way ANOVA without replication) <0.05 (at all points, Ra-224-loaded wire vs. inert or naïve). c Maestro-CRI lung imaging of mice injected with CT-26 mCherry-labeled cells (as described in B): (1) lungs in bright field, (2) fluorescent image of lungs
Next, we tested the anti-tumor immunity in the lungs of animals, which underwent primary tumor ablation by DaRT. Treated animals were injected i.v. with 2 × 106 mCherry-labeled tumor cells, and the survival of the mice was recorded. Survival of DaRT-treated mice was higher than that in the inert-treated group (63 vs. 29 % survival; respectively, Fig. 2b, c). In addition, in the i.v. challenge protocol, as a mouse was diagnosed as moribund, it was killed, and all its organs were harvested and analyzed using a CRI MAESTRO™ Imaging tool. The signal that was measured in the inert group was three times stronger than the signal measured in the DaRT group (data not shown), indicating a higher load of cancer cells in the lungs of inert-treated group compared to the DaRT-treated group. Similar experiments with the less-immunogenic tumor DA3 showed also that the tumors after re-inoculation grew slower in treated animals than in non-treated group (Fig. 3). Moreover, the number of tumor free mice upon the tumor challenge in both DA3 and CT26 model was significantly higher than in control groups.
Fig. 3.
DA3 tumor in mice upon the tumor ablation by Ra-224-loaded wires followed by subcutaneous administration of tumor cell. DA3 tumor-bearing mice were treated with intratumoral Ra-224-loaded wire (40–50 kBq/wire, n = 18) or with an inert wire (n = 24) followed by the removal of residual tumors at day 14 after the treatment. In some tumor-bearing mice, tumors were excised 14 days after wire insertion (surgery group; n = 26). Non-treated tumor-bearing mice served as a control (NT-TB; n = 7). The control and treated animals were challenged again s.c. with 5 × 105 DA3 tumor cells 21 days after treatment. Naïve–Normal Balb/c mice that were injected only tumor cells at day 21 after the beginning of experiment (n = 25). P (two-way ANOVA without replication) <0.05, P (chi-square) <0.01 (days 10–15), P (chi-square) = 0.06 (days 20–33) (Ra-224-loaded wire vs. controls)
Reduction in incidence of lung metastases in the DA3 tumor model
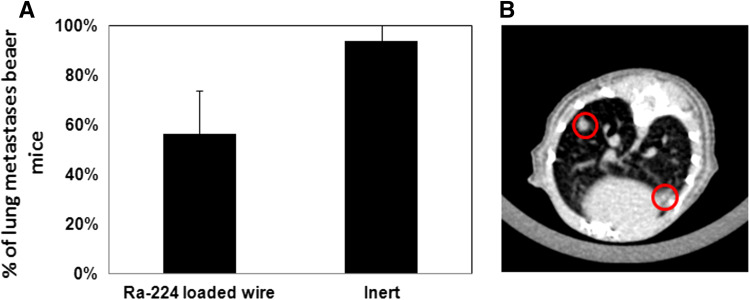
We next studied the effect of treatment of the local tumor with DaRT on distant lung metastases in the DA3 tumor-bearing animals. The lungs of mice treated by a single Ra-224-loaded wire and tumor excision 14 days after wire insertion were investigated at day 50 after the treatment by a CT imaging device. We found that in the DaRT-treated group, 56 % of the mice had lung metastases, whereas in the control group, 93 % of animals developed lung metastases (Fig. 4a).
Fig. 4.
Effect of DaRT treatment on lung metastases. Lung metastases in mice bearing murine breast tumors treated by Ra-224-loaded wires. Treatment was applied to Balb/c mice bearing DA3 tumors (6–8 mm average diameter). a Fifty days post-treatment all mice were CT scanned for lung metastases. Tumor-bearing mice each treated with one Ra-224 wire, carrying activities in the range of 40–50 kBq (Ra-224 wire; n = 16). Inert—tumor-bearing mice treated with an inert wire were used as a control group (n = 16). P value (T test) DaRT vs. Inert <0.05. b Lung metastases in the mouse of control group is shown
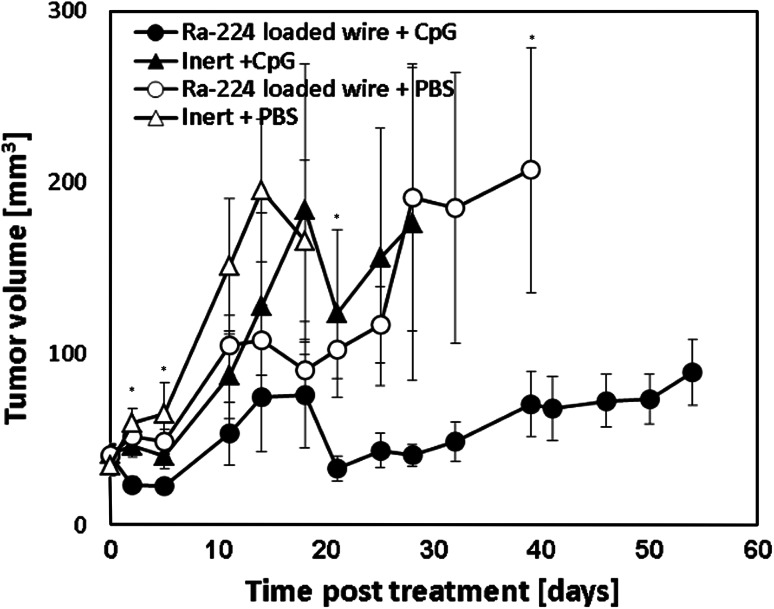
Combined treatments of DaRT and the immunoadjuvant, CpG
In order to augment the anti-tumor immunity triggered by the radioactive ablation, we tested the effect of DaRT and the immunoadjuvant, CpG, on the development of primary tumors. The combined treatment significantly inhibited tumor growth compared to each treatment alone (Fig. 5). The mean survival time of mice treated with Ra-224-loaded wire and CpG was the longest (99 days) compared to animals treated with Ra-224-loaded wire only (83 days), CpG only (88 days), or non-treated mice (64 days).
Fig. 5.
Effect of combined treatment on tumor development. Mice bearing breast DA3 tumors were treated with Ra-224-loaded wire (39.6–52.7 kBq/wire) combined with two injection of 100 µg CpG (Ra-224-loaded wire + CpG) (n = 11), with Ra-224-loaded wire only (Ra-224-loaded wire + PBS) (n = 9), with CpG alone (inert + CpG; n = 10) or left untreated (inert + PBS; n = 6). *P (T test) < 0.05 (on days: 2, 5, 21, and 39), P (two-way ANOVA w/o replication) <0.05 (Ra-224 load wires + CpG vs. controls, at all time points)
Discussion
In most cases, the main problem of cancer is not the primary tumor, which can be removed, but the metastases in distant organs. Tumor immunotherapy represents a promising approach. Local irradiation, especially alpha based, may induce systemic anti-tumor immune responses. Therefore, a combination of radiotherapy and immunotherapy may serve as a valuable strategy [19]. In this work, we investigated two tumor models: the murine DA3 breast adenocarcinoma and mouse CT26 colon carcinoma. These two tumors differ in their immunogenicity. DA3 cells that exhibit low immunogenicity [20] were injected to females as the relevant choice. The highly immunogenic CT26 tumor cell line [21], which was used in our laboratory for many years, was injected to male mice [22, 23], as in many other studies that used CT26.
Here we demonstrated that the treatment with a single Ra-224-loaded wire inhibited the tumor growth in both DA3 and CT-26 models. Interestingly, both low (DA3)- and high (CT26)-immunogenic tumors are sensitive to the local DaRT treatment. However, the destructive effect is stronger in the case of CT-26 tumors. This could be explained by a higher sensitivity of CT-26 cells to alpha radiation and by a higher immunogenicity of these tumor cells. In addition, according to our findings, not all the tumor masses were destroyed by the alpha particles; however, even sublethal doses of radiation can generate potent immune responses. Irradiated dying cancer cells release the nuclear protein HMGB1 that provides a “danger” signal to DCs [24].
Besides the local tumor destruction, the DaRT treatment was found to induce also anti-tumor immunity. Upon the ablation of DA3 and CT-26 primary tumors, mice were re-inoculated with the same tumor cells (challenge assay). In both models, in the DaRT-treated group, secondary tumors were the smallest or have not developed at all. Here again we observed a better effect in the CT-26 tumor model. It is important to point out that the CT26 tumor is immunogenic, and its growth in the mouse was sufficient to induce an anti-tumor immune response [22, 23], an effect which might be elevated by the minimal tissue damage caused by the inert wire. However, when treating local CT26 tumors by DaRT, 63–77 % of mice were resistant to a tumor re-challenge both i.v. and s.c., respectively, while only 29–33 % of the mice in the inert-treated group became resistant. Based on our findings and due to the low immunogenicity of the DA3 model, we decided to focus on this challenging model. First, we examined lung metastases at day 50 after the DaRT treatment and found a significant decrease in the number of lung metastases compared to control group. It is conceivable that irradiated tumors contained less cancer cells that can induce lung metastases than non-irradiated tumors. In addition, this inhibition of metastases formation could be due the “bystander” effect of irradiation that was reported to stimulate T cell-mediated anti-tumor reactions [25].
Since FACS analyses that were preformed 3 days post-ablation revealed that CpG elevated DCs in the tumor draining lymph nodes (data not shown), we combined DaRT treatment with the application of the immunostimulatory agent CpG [26]. This combined therapy was demonstrated to induce a significant inhibition of the tumor progression. However, the life expectancy was not significantly prolonged. This may indicate that in the DA3 model, suppressor immune cells such as regulatory T cells or myeloid derived suppressor cells may play a significant role [27, 28], and it could be that combining DaRT ablative technique with immune suppressor cells inhibitors would affect survival more significantly.
Alpha particles destroy tumor cells effectively due to DNA double-strand breaks [29]. However, there are many tumor ablation methods that can destroy a tumor [30]. The DaRT ablation method has two important advantages as compared to other ablative approaches. First, since alpha particles release during 10 days, it gives more time for the activation of immune system. Second, the alpha particles target the cancer cells without harming healthy cells [7], thus avoiding the development of auto-immune reactions.
In summary, our results indicate that alpha-radiation-based therapy inhibits the growth of both breast and colon primary tumors. The destruction of the tumor stimulated anti-tumor immunity and inhibited the formation of metastases in distant organs. The combination of DaRT and CpG augmented the anti-tumor effect.
The DaRT vaccination form serves as a novel and powerful strategy for “personalized vaccination,” since the tumor is the antigen supplier and the DaRT therapy induces the release of those antigens to stimulate the immune system. Therefore, DaRT technology could be considered as an optimal candidate for the combination with different immunotherapeutic strategies to better target cancer cells both locally and systemically [31, 32].
Acknowledgments
We thank Dr. Gideon Halpern for assistance with the statistical analysis. This work was supported in part by The Roberts-Guthman Chair in Immunopharmacology and The German-Israeli Foundation. This work was performed in partial fulfillment of the requirements toward a PhD degree of Hila Confino, Sackler Faculty of Medicine, Tel Aviv University.
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- DaRT
Diffusing alpha-emitters radiation therapy
- DCs
Dendritic cells
- DMBA
Dimethylbenzanthracene
- DMEM
Dulbecco’s modified Eagle’s medium
- HBSS
Hanks’ balanced salt solution
- NNMU
N-Nitroso-N-methylurethane
- ODNs
Oligodeoxynucleotides
- RPMI
Roswell Park Memorial Institute
References
- 1.Weiss EM, Frey B, Rodel F, Herrmann M, Schlucker E, Voll RE, Fietkau R, Gaipl US. Ex vivo– and in vivo–induced dead tumor cells as modulators of antitumor responses. Ann N.Y. Acad Sci. 2010;1209:109–117. doi: 10.1111/j.1749-6632.2010.05743.x. [DOI] [PubMed] [Google Scholar]
- 2.Palucka K, Ueno H, Fay J, Banchereau J. Dendritic cells and immunity against cancer. J Intern Med. 2011;269:64–73. doi: 10.1111/j.1365-2796.2010.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Den Brok MH, Sutmuller RP, van der Voort R, Bennink EJ, Figdor CG, Ruers TJ, Adema GJ. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024–4029. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- 4.Arazi L, Cooks T, Schmidt M, Keisari Y, Kelson I. Treatment of solid tumours by interstitial release of recoiling short-lived alpha emitters. Phys Med Biol. 2007;52:5025–5042. doi: 10.1088/0031-9155/52/16/021. [DOI] [PubMed] [Google Scholar]
- 5.Cooks T, Arazi L, Schmidt M, Marshak G, Kelson I, Keisari Y. Growth retardation and destruction of experimental Squamous cell carcinoma by interstitial radioactive wires releasing diffusing alpha-emitting atoms. Int J Cancer. 2008;122:1657–1664. doi: 10.1002/ijc.23268. [DOI] [PubMed] [Google Scholar]
- 6.Cooks T, Arazi L, Efrati M, Schmidt M, Marshak G, Kelson I, Keisari Y. Interstitial wires releasing diffusing alpha-emitters combined with chemotherapy improved local tumor control and survival in squamous cell carcinoma bearing mice. Cancer. 2009;115:1791–1801. doi: 10.1002/cncr.24191. [DOI] [PubMed] [Google Scholar]
- 7.Cooks T, Schmidt M, Bittan H, Lazarov E, Arazi L, Kelson I, Keisari Y. Local control of lung derived tumors by diffusing alpha-emitting atoms released from intratumoral wires loaded with Radium-224. Int J Radiat Oncol Biol Phys. 2009;74:966–973. doi: 10.1016/j.ijrobp.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 8.Arazi L, Cooks T, Schmidt M, Keisari Y, Kelson I. The treatment of solid tumors by alpha emitters released from 224Ra-loaded sources: internal dosimetry analysis. Phys Med Biol. 2010;55:1203–1218. doi: 10.1088/0031-9155/55/4/020. [DOI] [PubMed] [Google Scholar]
- 9.Horev-Drori G, Cooks T, Bittan H, Lazarov E, Schmidt M, Arazi L, Efrati M, Kelson I, Keisari Y. Local control of experimental malignant pancreatic tumors by treatment with a combination of chemotherapy and intratumoral 224Radium-loaded wires releasing alpha-emitting atoms. Transl Res. 2012;159:32–41. doi: 10.1016/j.trsl.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Cooks T, Tal M, Raab S, Efrati M, Reitkopf S, Lazarov E, Etzyoni R, Schmidt M, Arazi L, Kelson I, Keisari Y. Intratumoral 224Ra-loaded wires spread alpha-emitters inside solid human tumors in athymic mice achieving tumor control. Anticancer Res. 2012;32:5315–5321. [PubMed] [Google Scholar]
- 11.Coulie PG, Van den Eynde BJ, Van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14:135–146. doi: 10.1038/nrc3670. [DOI] [PubMed] [Google Scholar]
- 12.Aranda F, Llopiz D, Díaz-Valdés N, Riezu-Boj JI, Bezunartea J, Ruiz M, Martínez M, Durantez M, Mansilla C, Prieto J, Lasarte JJ, Borrás-Cuesta F, Sarobe P. Adjuvant combination and antigen targeting as a strategy to induce polyfunctional and high-avidity T-cell responses against poorly immunogenic tumors. Cancer Res. 2011;71:3214–3224. doi: 10.1158/0008-5472.CAN-10-3259. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein M, Varghese B, Brody J, Rajapaksa R, Kohrt H, Czerwinski D, Levy S, Levy RA. CpG-loaded tumor cell vaccine induces antitumor CD4 T cells that are effective in adoptive therapy for large and established tumors. Blood. 2011;117:118–127. doi: 10.1182/blood-2010-06-288456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nierkens S, den Brok MH, Garcia Z, Togher S, Wagenaars J, Wassink M, Boon L, Ruers TJ, Figdor CG, Schoenberger SP, Adema GJ, Janssen EM. Immune adjuvant efficacy of CpG oligonucleotide in cancer treatment is founded specifically upon TLR9 function in plasmacytoid dendritic cells. Cancer Res. 2011;71:6428–6437. doi: 10.1158/0008-5472.CAN-11-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nierkens S, den Brok MH, Sutmuller RP, Grauer OM, Bennink E, Morgan ME, Figdor CG, Ruers TJ, Adema GJ. In vivo colocalization of antigen and CpG within dendritic cells is associated with the efficacy of cancer immunotherapy. Cancer Res. 2008;68:5390–5396. doi: 10.1158/0008-5472.CAN-07-6023. [DOI] [PubMed] [Google Scholar]
- 16.den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Toonen LW, Figdor CG, Ruers TJ, Adema GJ. Synergy between in situ cryoablation and TLR9 stimulation results in a highly effective in vivo dendritic cell vaccine. Cancer Res. 2006;66:7285–7292. doi: 10.1158/0008-5472.CAN-06-0206. [DOI] [PubMed] [Google Scholar]
- 17.Yu B, Kusmartsev S, Cheng F, Paolini M, Nefedova Y, Sotomayor E, Gabrilovich D. Effective combination of chemotherapy and dendritic cell administration for the treatment of advanced-stage experimental breast cancer. Clin Cancer Res. 2003;9:285–294. [PubMed] [Google Scholar]
- 18.Robinson M, et al. Novel immunocompetent murine tumor model for evaluation of conditionally replication-competent (oncolytic) murine adenoviral vectors. J Virol. 2009;83:3450–3462. doi: 10.1128/JVI.02561-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindner M, Schirrmacher V. Tumour cell-dendritic cell fusion for cancer immunotherapy: comparison of therapeutic efficiency of polyethylen-glycol versus electro-fusion protocols. Eur J Clin Invest. 2002;32:207–217. doi: 10.1046/j.1365-2362.2002.00968.x. [DOI] [PubMed] [Google Scholar]
- 21.Robinson M, Li B, Ge Y, Ko D, Yendluri S, Harding T, VanRoey M, Spindler KR, Jooss K. Novel immunocompetent murine tumor model for evaluation of conditionally replication –competent (oncolytic) murine adenoviral vectors. J Virol. 2009;83:3450–3462. doi: 10.1128/JVI.02561-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plotnikov A, Fishman D, Tichler T, Korenstein R, Keisari Y. Low electric field enhanced chemotherapy can cure mice with CT-26 colon carcinoma and induce anti-tumour immunity. Clin Exp Immunol. 2004;138:410–416. doi: 10.1111/j.1365-2249.2004.02636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plotnikov A, Tichler T, Korenstein R, Keisari Y. Involvement of the immune response in the cure of metastatic murine CT-26 colon carcinoma by low electric field-enhanced chemotherapy. Int J Cancer. 2005;117:816–824. doi: 10.1002/ijc.21261. [DOI] [PubMed] [Google Scholar]
- 24.Hodge JW, Guha C, Neefjes J, James L, Gulley Synergizing radiation therapy and immunotherapy for curing incurable cancers: opportunities and challenges. Oncology. 2008;22:1064–1084. [PMC free article] [PubMed] [Google Scholar]
- 25.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Jahrsdörfer B, Weiner GJ. CpG oligodeoxynucleotides as immunotherapy in cancer. Update Cancer Ther. 2008;3:27–32. doi: 10.1016/j.uct.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakuishi K, Ngiow SF, Sullivan JM, Teng MW, Kuchroo VK, Smyth MJ, Anderson AC. TIM3 + FOXP3 + regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology. 2013;2:1–13. doi: 10.4161/onci.23849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youn JI, Nagaraj S, Gabrilovich MI. Subset of myeolid: derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stap J, Krawczyk PM, Van Oven CH, Barendsen GW, Essers J, Kanaar R, Aten JA. Induction of linear tracks of DNA double-strand breaks by alpha-particle irradiation of cells. Nat Methods. 2008;5:261–266. doi: 10.1038/nmeth.f.206. [DOI] [PubMed] [Google Scholar]
- 30.Keisari Y. Tumor ablation: effects on systemic and local anti-tumor immunity and on other tumor-microenvironment interactions. Dordrecht: Springer; 2013. [Google Scholar]
- 31.Gaipl US, Multhoff G, Scheithauer H, Lauber K, Hehlgans S, Frey B, Rödel F. Kill and spread the word: stimulation of antitumor immune responses in the context of radiotherapy. Immunotherapy. 2014;6:597–610. doi: 10.2217/imt.14.38. [DOI] [PubMed] [Google Scholar]
- 32.Frey B, Rubner Y, Kulzer L, Werthmöller N, Weiss EM, Fietkau R, Gaipl US. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother. 2014;63:29–36. doi: 10.1007/s00262-013-1474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]