Abstract
Purpose
A wealth of preclinical information, as well as a modest amount of clinical information, indicates that dendritic cell vaccines have therapeutic potential. The aim of this work was to assess the immune response, disease progression, and post-treatment survival of ER/PR double-negative stage II/IIIA breast cancer patients vaccinated with autologous dendritic cells pulsed with autologous tumor lysates.
Methods
Dendritic cell (DC) vaccines were generated from CD14+ precursors pulsed with autologous tumor lysates. DCs were matured with defined factors that induced surface marker and cytokine production. Individuals were immunized intradermally four times. Specific delayed type IV hypersensitivity (DTH) reaction, ex vivo cytokine production, and lymphocyte subsets were determined for the evaluation of the therapeutic efficiency. Overall survival and disease progression rates were analyzed using Kaplan–Meier curves and compared with those of contemporaneous patients who were not administered DC vaccines.
Results
There were no unanticipated or serious adverse effects. DC vaccines elicited Th1 cytokine secretion and increased NK cells, CD8+ IFN-γ+ cells but decreased the percentage of CD3+ T cells and CD3+ HLA-DR+ T cells in the peripheral blood. Approximately 58% (18/31) of patients had a DTH-positive reaction. There was no difference in overall survival between the patients with and without DC vaccine. The 3-year progression-free survival was significantly prolonged: 76.9% versus 31.0% (with vs. without DC vaccine, p < 0.05).
Conclusion
Our findings strongly suggest that tumor lysate-pulsed DCs provide a standardized and widely applicable source of breast cancer antigens that are very effective in evoking anti-breast cancer immune responses.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-011-1192-2) contains supplementary material, which is available to authorized users.
Keywords: Dendritic cells, Breast cancer, Immune response, Immunotherapy
Introduction
The modern era of breast cancer treatment has undergone rapid development due to the efforts of an extremely broad spectrum of basic and clinical scientists whose efforts have redefined our standards for appropriate therapeutic strategies. Estrogen receptor (ER)/progesterone receptor (PR) double-negative breast cancer is associated with a poor prognosis, which is thought to be due to aggressive biology and resistance to currently available endocrine therapies and, in some cases, standard cytotoxic chemotherapies. Clearly, novel approaches that define new targets for the treatment of ER/PR double-negative patients are required. Despite arising from normal host cells, tumor cells can exhibit some degree of immunogenicity, and the immune system has a number of mechanisms by which it acts against tumors. A possible mechanism for targeting micro-metastatic foci or limiting disease is to enhance the immune response of the patient against the tumor cells [1]. By enhancing immunogenicity, a robust and lasting immune response might be attained. Immunotherapy is a therapeutic strategy that manipulates the host’s immune responses against tumor cells [2, 3]. This type of therapy marks a new set of cancer therapies that are directed passively or actively against tumor cells.
Dendritic cells (DCs) are potent antigen-presenting cells that have been under intensive investigation as components of tumor vaccines [4]. Numerous small clinical trials evaluating ex vivo antigen-loaded DCs in patients with a variety of solid and blood tumors have been reported in the literature [5–7]. There is no standardized methodology for preparing vaccines, and many questions remain about the optimal source or type of antigen and maturation state of DCs. Regardless, numerous DC vaccine trials have shown biological activity that deserves further investigation. Previous studies also indicated that a fraction of individuals might derive therapeutic benefits, even though, as expected from phase I/II trials, reports of clinical efficacy are anecdotal. Clinical trials using synthetic peptides as antigens have failed to produce objective clinical responses or improvements in patient survival, probably due to tolerance induction by high-affinity peptides, tumor escape by clones lacking antigen expression, or absence of immunologic danger signals associated with the antigens [8, 9]. In contrast, DCs fused to or loaded with tumor antigens or exposed to tumor lysates have been shown to induce stronger and more extensive immunologic responses against tumors [10].
Notably, none of the clinical trials reported in the literature have evaluated DC vaccines exclusively in ER/PR double-negative stage II/IIIA breast cancer. We initiated a DC vaccine clinical trial for ER-/PR- stage II/IIIA breast cancer to determine treatment feasibility, to gain information with which to build future studies, and to work toward optimizing DC vaccines in breast cancer. This study was designed to investigate the immunologic responses against autologous antigen-pulsed DC vaccines in a heterogeneous group of breast cancer patients treated surgically, medically, and with multimodality approaches. Data are analyzed in the context of host factors that could influence vaccine efficacy and help to define the appropriate application of this strategy.
Patients and methods
Human subjects/patient characteristics
Individuals with histologically confirmed ER-/PR- stage II/IIIA breast cancer who had completed definitive medical, surgical, or multimodality therapy and had stable clinical disease at screening were eligible for the study. Participants were approved under a protocol approved by the ethics committee of Changzhou No. 2 People’s Hospital; informed consent was obtained from all patients. Individuals entered the study anytime from 8 weeks to 3 years after definitive therapy (average, 10 months). The treatment group was heterogeneous with respect to stage, histology, treatment for primary disease, and risk of recurrence. Patient characteristics (31 cases) are summarized in Table 1. The characteristics of the contemporaneous patients (32 cases) who did not receive the DC vaccine are summarized in Table 2.
Table 1.
Characteristics of patients and their immunologic response to therapy
| Patient no. | Age (years) | Histology | Stage (TNM) | Additional treatment | DTH (mm) |
|---|---|---|---|---|---|
| 1 | 59 | Ad | IIIA | Neo(C)/Sg/C | 10(+) |
| 2 | 60 | Ad | IIIA | Sg/A (C-XRT) | 7(+) |
| 3 | 49 | Sq | II | Sg/C | (−) |
| 4 | 47 | Ad | IIIA | Neo(C)/Sg/C | 6(+) |
| 5 | 43 | Ad | II | Sg/A (C-XRT) | 8(+) |
| 6 | 44 | Ad | II | Sg/C | (−) |
| 7 | 59 | Sq | II | Sg/C | 7(+) |
| 8 | 41 | Ad | II | Neo(C)/Sg/C | 8(+) |
| 9 | 36 | Ad | IIIA | Neo(C)/Sg/C | (−) |
| 10 | 69 | Sq | II | Sg/C | (−) |
| 11 | 74 | Sq | II | Sg/C | 10(+) |
| 12 | 39 | Ad | II | Sg/C | 7(+) |
| 13 | 61 | Ad/Sq | IIIA | Neo(C)/Sg/C | (−) |
| 14 | 56 | Ad | IIIA | Neo(C)/Sg/C | 7(+) |
| 15 | 40 | Ad | IIIA | Neo(C)/Sg/C | (−) |
| 16 | 56 | Sq | II | Neo(C)/Sg/C | (−) |
| 17 | 49 | Sq | IIIA | Neo(C)/Sg/C | 6(+) |
| 18 | 48 | Ad | II | Neo(C)/Sg/C | 6(+) |
| 19 | 57 | Ad | II | Sg/A (C-XRT) | (−) |
| 20 | 52 | Sq | II | Neo(C)/Sg/C | (−) |
| 21 | 69 | Ad | II | Sg/C | (−) |
| 22 | 56 | Ad | IIIA | Neo(C)/Sg/C | 7(+) |
| 23 | 71 | Ad | IIIA | Neo(C)/Sg/C | 7(+) |
| 24 | 61 | Sq | II | Sg/C | 8(+) |
| 25 | 56 | Ad | II | Sg/C | 7(+) |
| 26 | 53 | Sq | IIIA | Neo(C)/Sg/C | (−) |
| 27 | 56 | Ad/Sq | II | Sg/C | 9(+) |
| 28 | 52 | Ad | II | Sg/C | 8(+) |
| 29 | 57 | Sq | II | Neo(C)/Sg/C | 7(+) |
| 30 | 63 | Sq | II | Neo(C)/Sg/C | (−) |
| 31 | 46 | Sq | IIIA | Neo(C)/Sg/C | (−) |
DTH, delayed type IV hypersensitivity; Ad, adenocarcinoma; Sq, squamous; C, chemotherapy; A, adjuvant; C-XRT, chemotherapy + radiation therapy; Neo(C), neoadjuvant chemotherapy
Table 2.
Characteristics of patients who were not administered DC vaccines
| Patient no. | Age (years) | Histology | Stage (TNM) | Additional treatment |
|---|---|---|---|---|
| 32 | 54 | Ad | II | Sg/C |
| 33 | 52 | Ad | II | Sg/C |
| 34 | 51 | Ad | IIIA | Neo(C)/Sg/C |
| 35 | 37 | Ad | II | Neo(C)/Sg/C |
| 36 | 56 | Sq | IIIA | Sg/A (C-XRT) |
| 37 | 47 | Ad | IIIA | Neo(C)/Sg/C |
| 38 | 58 | Ad | II | Sg/C |
| 39 | 37 | Sq | IIIA | Neo(C)/Sg/C |
| 40 | 45 | Ad | IIIA | Neo(C)/Sg/C |
| 41 | 70 | Ad | II | Sg/C |
| 42 | 72 | Ad | II | Sg/C |
| 43 | 65 | Sq | II | Sg/C |
| 44 | 64 | Sq | IIIA | Neo(C)/Sg/C |
| 45 | 34 | Ad | II | Neo(C)/Sg/C |
| 46 | 42 | Ad | IIIA | Neo(C)/Sg/C |
| 47 | 55 | Ad | II | Sg/C |
| 48 | 34 | Ad/Sq | IIIA | Neo(C)/Sg/C |
| 49 | 58 | Ad | IIIA | Neo(C)/Sg/C |
| 50 | 57 | Ad | II | Sg/C |
| 51 | 59 | Ad | IIIA | Neo(C)/Sg/C |
| 52 | 61 | Sq | II | Sg/C |
| 53 | 53 | Sq | II | Neo(C)/Sg/C |
| 54 | 65 | Sq | II | Neo(C)/Sg/C |
| 55 | 72 | Ad | II | Sg/C |
| 56 | 55 | Ad | II | Neo(C)/Sg/C |
| 57 | 47 | Sq | II | Neo(C)/Sg/C |
| 58 | 53 | Ad | IIIA | Neo(C)/Sg/C |
| 59 | 58 | Ad | II | Sg/A (C-XRT) |
| 60 | 51 | Ad | II | Neo(C)/Sg/C |
| 61 | 68 | Sq | II | Neo(C)/Sg/C |
| 62 | 63 | Sq | IIIA | Neo(C)/Sg/C |
| 63 | 52 | Ad | II | Sg/C |
DTH, delayed type IV hypersensitivity; Ad, adenocarcinoma; Sq, squamous; C, chemotherapy; A, adjuvant; C-XRT, chemotherapy + radiation therapy; Neo(C), neoadjuvant chemotherapy
Trial design
The trial was non-randomized. A measurable immune response to the vaccine was the major end point. Gathering comparative immunological data from both untreated and treated breast cancer patients was the central aim of the study. Individuals were primarily stratified by therapy to assess inhibitory effects of persistent tumor burden and effects of prior chemotherapy and/or radiation on immunologic responses. The small sample size and patient heterogeneity would have precluded meaningful assessment of the therapeutic effects. The ability to incorporate vaccines into a patient’s therapeutic plan with minimal risk and time commitment were paramount. Clinical tolerability was determined by routine safety laboratories and clinical events described by the National Cancer Institute Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events.
Leukapheresis
The Fresenius COM.TEC blood cell separator (Fresenius, St. Wendel, Germany), which was used for all procedures, is a continuous flow machine that uses centrifugation to separate cells based on specific gravity. Plasma pump flow rates were adjusted to 1.0 mL/min, which is the appropriate interface for isolating mononuclear cells. Two total blood volumes were processed in each procedure, which took approximately 2 h. All leukapheresis procedures required placement of femoral double-lumen hemodialysis catheters for access.
DC Preparation
CD14+ cells were isolated using a commercial magnetic bead separation protocol (Miltenyi Biotec, Auburn, CA). CD14+ cells (with >95% purity) were cultured in GT-T551 medium (106 cells/mL; Takara, Otsu, Shiga, Japan) supplemented with 0.5% human serum albumin, 2 mmol/L l-glutamine, 100 μmol/L non-essential amino acids, 1 mmol/L sodium pyruvate, and 20 mmol/L HEPES buffer. The culture was supplemented with 20 ng/ml GM-CSF and 20 ng/mL interleukin (IL)-4. Additional cytokines were added on days 2 and 5. Tumor antigens (described in the Methods: Antigen Source and Preparation) were added on day 5. On day 5, immature DCs were harvested and resuspended in medium with GM-CSF and IL-4 at 1 × 106 cells/mL. Tumor lysate and DCs were mixed at ratios of 1:3–1:10 (depending on tumor cell yield). On day 6, 10 ng/mL IL-1β, 10 μg/mL PGE2, and 20 ng/mL TNF-α were added. On day 7, antigen-loaded DCs were harvested and suspended in 2 mL of saline solution containing 0.5% human serum albumin for immediate injection.
Microbiologic monitoring
Samples were collected from the culture 24 h before harvest for sterility testing at the Clinical Laboratory of Chanzhou No. 2 People’s Hospital. Sterility was confirmed just prior to delivery. A sample of the final product was also submitted for microbiological testing the day of delivery. It was confirmed that samples were sterile and free of endotoxins by tests described in the Pharmacopoeia of the People’s Republic of China.
Antigen source and preparation
To generate an individual vaccine, autologous tumor tissue was used as a source of tumor-rejection antigens. Tumor tissues (1–2 cm3) obtained during surgery were mechanically minced into portions no larger than 1 mm3 and then placed into a centrifuge tube containing 5 mL of enzyme solution (0.1 mg/mL collagenase, 1 mg/mL DNase, and 1 mg/mL hyaluronidase) for 30 min at 37°C. The patients with a low yield of tumor cells were excluded from the study. The cell suspensions were filtered through 200-mesh stainless steel screens and washed, and the single-cell suspensions were harvested. Subsequently, the tumor cells were heat-shocked for 3 h at 42°C, and then, the cell lysates were prepared by five freeze/thaw cycles (−80°C and 37°C). After verifying by trypan blue staining that no live cells remained, the resulting suspensions were stored at −80°C until use.
Immunization protocol
The protocol, including the route of administration, interval, and dose, was based on the best available information in the literature. Patients received a fixed dose of vaccine at 0, 2, 4, and 6 weeks intradermally near the inguinal lymph nodes in the thigh. The number of DCs injected each time was 1 × 107. Patients were monitored in the outpatient clinic for 2 h following immunization for immediate unanticipated adverse events.
Clinical evaluation
Follow-up by primary care physicians included routine history and physical, chest X-ray, and/or computed tomography scans at regular intervals post-therapy or as directed by signs or symptoms of tumor recurrence.
Immunologic assessment
Serial blood samples were drawn for immunological testing (pre-vaccination and at 1, 6, 8, 12, and 18 weeks post-vaccination) to complete the initial series. Peripheral blood (5 mL) from patients was collected into anticoagulant tubes before and after each vaccination and placed at room temperature for 1–2 h. The serum was then separated by centrifugation at 25°C, removed, and frozen at −80°C. Cytokine levels including those of IL-2, IL-12, IFN-γ, and TNF-α were evaluated by an enzyme-linked immunosorbent assay (ELISA) using commercially available ELISA kits (R&D, Stockholm, Sweden) according to the manufacturer’s instructions.
The intracellular staining for IFN-γ production was performed as recently described, with some modifications[11]. In brief, 2 × 106 PBMCs, which were obtained by density gradient centrifugation from 5 mL of the peripheral blood of patients before and 1 week after the immunotherapy, were stimulated with tumor cell lysates at a ratio of 10:1 for 3–4 days. Brefeldin A (Sigma, MO, USA) at a concentration of 10 μg/mL was added during the last 4–5 h. The cells were washed and incubated with PerCP-CD8 (BD, Heidelberg, Germany) for extracellular staining. Then, the cells were washed and fixed with 4% formaldehyde for 15 min, and permeabilization solution (BD, San Jose, CA, USA) was added for 10 min. Finally, the cells were incubated with FITC-IFN-γ (BD, Heidelberg, Germany) for 30 min on ice, washed twice, and analyzed by flow cytometry.
All patients were assessed for in vivo delayed type hypersensitivity (DTH) reactions against tumor cell lysates 1 month after the end of therapy. DTH reaction tests against a mixture containing tumor cell lysate at a density of 0.5 × 106 in 0.1 mL of saline solution and saline solution only as a control were applied intradermally at separate sites on the forearm simultaneously, as described previously. The DTH response was assessed by measuring the diameter of the erythema and induration at the injection site after 48–72 h. If the area exceeded 5 mm, it was scored as positive.
Statistics
Statistical analyses of data were performed using SPSS software version 13.0. The data were presented as mean ± SD. Significance was determined by the analysis of variance. Correlations of events were assessed by linear regression analysis. We measured the overall survival and progression-free survival using a log-rank test and traced survival curves using the Kaplan–Meier method.
Results
DC characteristics
Immature DCs were induced from CD14+ cells with GM-CSF and IL-4 for 5 days. After incubation with the tumor antigens, IL-1β, PGE2, and TNF-α for 2 days, the DCs were mature, had irregular dendrites, and were loosely adherent. The final DCs used for the vaccination were CD14-CD11c+ cells (approximately 90% purity). The viability of mature DCs was greater than 95%. Compared with immature DCs, mature DCs enhanced the expression of surface molecules (HLA-DR, CD80, CD83, CD86) (Fig. 1a). The mature DCs produced large amounts of TNF-α and IL-12 but low amounts of IL-6 and IL-10 (Fig. 1b).
Fig. 1.
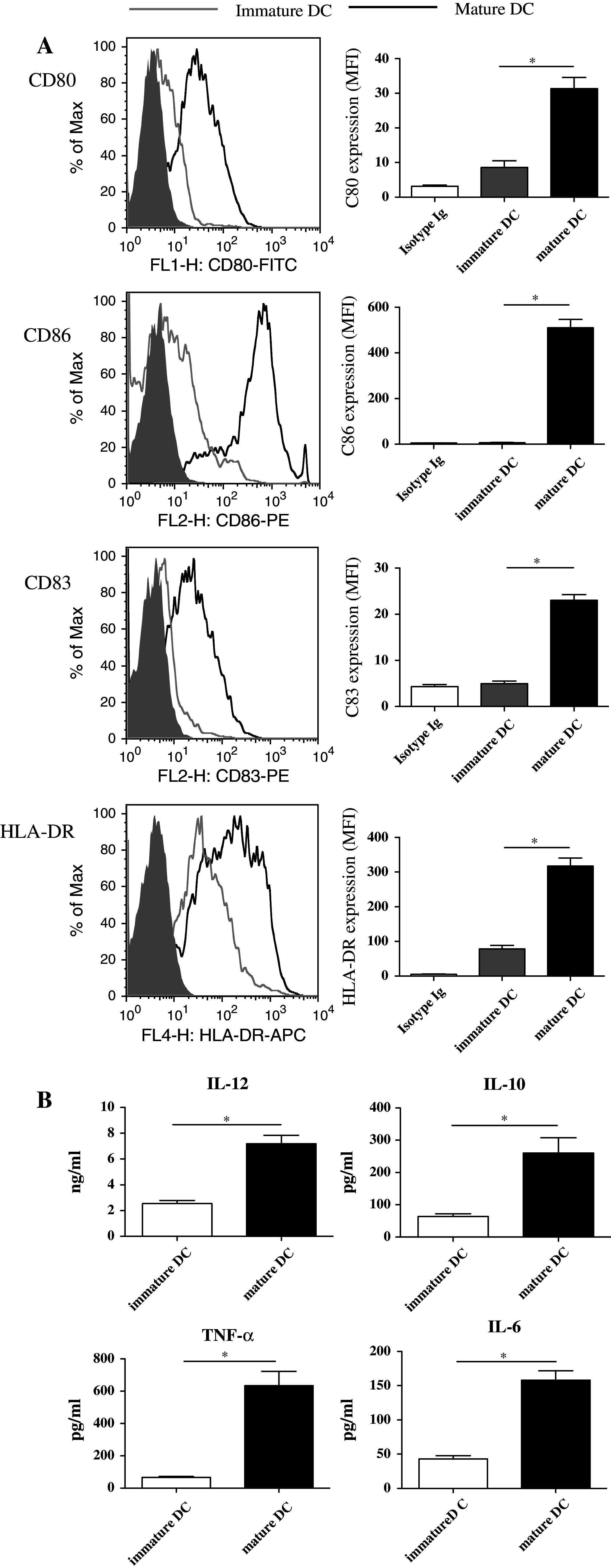
Surface marker expression and cytokine secretion in mature DCs. a PBMCs were incubated for 1.5 h, and the adherent cells were induced by GM-CSF and IL-4 for 5 days (immature DCs) and then stimulated with TNF-α, IL-1β, PGE2, and tumor lysate (mature DCs). The surface marker expression was assessed by flow cytometry. One representative histogram from 3 independent experiments with similar results is shown. b Supernatants from DC culture medium were collected and assayed. (*p < 0.05)
Adverse effects
A self-limited wheal-and-flare skin reaction appeared at the injection site 24–48 h after immunization in 20 of 31 subjects during at least one of the four immunizations. Four individuals noted a more profound reaction with the second vaccine. Four individuals noted minor fatigue in 24 h following the vaccine. Blood from patients that was checked following immunization revealed no abnormalities in hematological parameters or serum chemistries.
Tumor-specific immune response
We examined whether cytokine profiles in the serum would be altered after DC vaccine treatment. As shown in Fig. 2a and Supplementary Table 1, IL-10, IL-6, IL-17, and TGF-β levels were not significantly changed. Strikingly, IL-2, IL-12, TNF-α, and IFN-γ levels were significantly increased. These data suggest that DC vaccine treatment is capable of promoting secretion of Th1 cytokines, which may lead to anti-tumor effects.
Fig. 2.
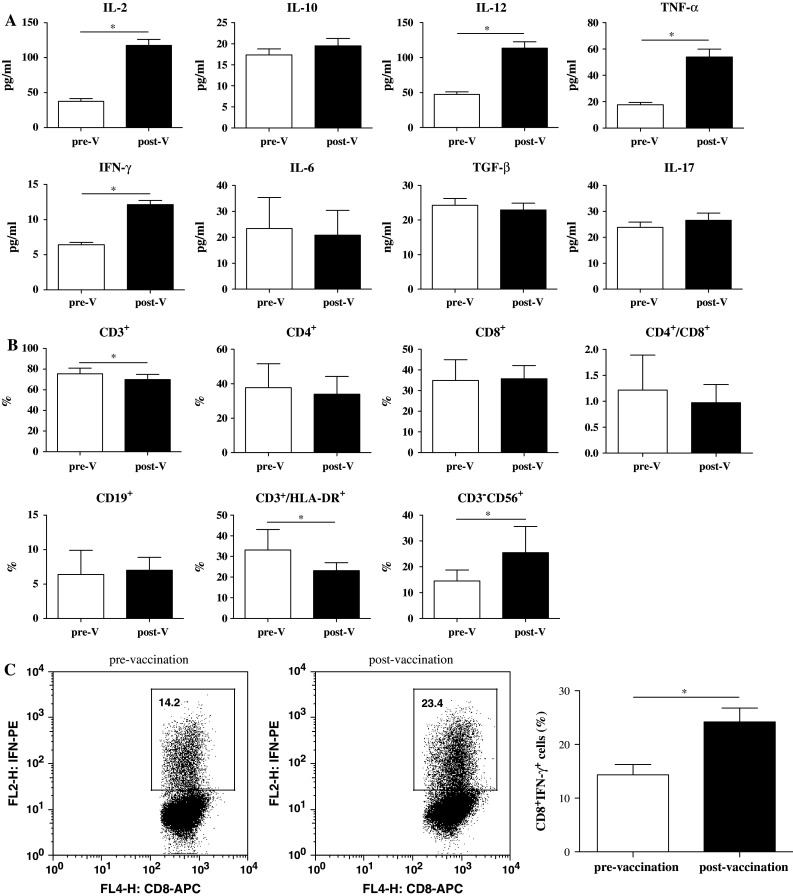
Cytokines and lymphocyte subsets in 31 patients before and after the DC vaccine. a The serums were collected before the vaccination, and 4 weeks after, the DC vaccine was administered. The cytokines were analyzed with ELISA. b Whole blood was collected prior to vaccination and 4 weeks after vaccination. The different subsets were analyzed with flow cytometry. pre-V pre-vaccination, post-V post-vaccination. c Intracellular IFN-γ staining of CD8+ T lymphocytes. The percentage of tumor antigen-specific IFN-γ-producing cells among CD8+ T lymphocytes pre-vaccination versus post-vaccination (1 week after immunotherapy) is indicated. The data were the mean values from these patients, using Student’s t test (*p < 0.05)
We also investigated the lymphocyte subsets in the peripheral blood. As shown in Fig. 2b, CD4+ T, CD8+ T, and B cells were not significantly different. Interestingly, the number of total CD3+ T cells and CD3+/HLA-DR+-activated T cells were decreased, and the number of NK cells increased.
By intracellular cytokine staining, PBMC samples from 31 patients were evaluated for the presence of tumor antigen-reactive CD8+ T cells before and after the last vaccination with tumor lysate antigen-pulsed monocyte-derived DCs. The DC vaccine significantly increased tumor-specific CD8+ T-cell expansion and further differentiation into IFN-γ-producing effector cells (Fig. 2c). These results indicated that vaccinations with DCs recognizing tumor antigens induced cytotoxic T-lymphocyte responses.
DTH reactions against tumor lysates constitute an excellent clinical response predictor because this immune reaction directly correlates with patient survival and lack of disease progression. We found that DTH reactions against tumor lysates ≥5 mm in diameter were detected in 58.1% of vaccinated patients (18 of 31) (Fig. 3). The remaining patients had no reaction (n = 10) or a reaction less than 5 mm (n = 3) (Table 1). Tumor lysate-specific DTH responders (DTH positive) and non-responders (DTH negative) did not differ with respect to age, sex, HLA haplotype, primary tumor or metastasis location, or hematologic condition.
Fig. 3.
DTH responses determined by intradermal injection of tumor antigens 1 week after the immunotherapy. The DTH response was assessed by measuring the diameter of the erythema and induration at the injection site after 48–72 h, and areas larger than 5 mm were scored as positive. Data are representative of the erythema and the induration in the patients compared to controls
Clinical outcomes
Clinical follow-up is available for all individuals for a minimum of 36 months from their primary immunization. As shown in Fig. 4, the 3-year progression-free survival is significantly increased in patients that received the DC vaccine versus patients that did not receive the DC vaccine (76.9% vs. 31.0%; p < 0.05). The overall survival rates were not different between these groups. Three individuals that received the DC vaccine to date have documented disease recurrence or progression, while 9 individuals that did not receive the DC vaccine had disease progression. One individual treated with multimodality therapy developed a lung metastasis 2 months following the initial vaccine and underwent local resection of the metastatic focus. There was no evidence of disease 25 months following the resection.
Fig. 4.
Effect of DC vaccine on progression-free survival (a) and overall survival (b) in breast cancer patients
Discussion
Breast cancer is a heterogeneous disease, and therefore no gold standard therapy exists that is suitable for all tumors of the mammary gland. For many years, tumors of the breast were characterized only by tumor size. However, this subclassification proved to be limiting, for it was unable to define subgroups sharing similar prognostic and therapeutic aspects. Later on, a histological classification system was developed, with the division of breast cancers into subgroups distinguished by the histological appearance of the tumor [12]. For the ER-/PR- expression profile, breast cancer is not amenable to treatment with hormone therapy, and systemic treatment options are currently limited to cytotoxic chemotherapy. ER-/PR- breast tumors show high recurrence and poor survival rates. Novel approaches that define new targets for the treatment of ER-/PR- patients are required.
Over the last decade, the convergence of several lines of research has bolstered interest in the use of dendritic cells (DCs) as the prime platform for immune therapy of breast cancer [13–15]. One of the critical developments in the field is related to gaining a better understanding of the central role of DCs for the initiation of anti-tumor immunity. Ample evidence now indicates that occurrence of an immune response against breast cancer involving DC recruitment and activation within the tumor tissue. Thus, DC-based vaccines are emerging as a crucial strategy to enhance therapeutic alternatives for patients with this disease. Whether the reduction in tumor load is achieved by surgery or by chemo/radiotherapy, an immune response is likely to be more effective against minimal residual disease. Although DC immunotherapy has shown encouraging results, most studies have been performed in the adjuvant setting. The implementation of DC adjuvant therapy for breast cancer is likely to occur in combination with other modalities [16, 17] and will require randomized studies to determine whether immunotherapy provides an additional benefit. Thorough evaluation of its efficacy, in turn, will require the development of standardized protocols. Given the myriad of relevant variables, for example, the type of antigen, antigen loading, type of DCs, and method of delivery, consensus treatment regimes need to be defined. This will ensure that this type of therapy progresses from defined patient study series to successful implementation as a therapeutic alternative for patients with breast cancer. The efficacy of such a vaccine depends on its capacity to overcome tolerance by transforming weak immune responses to ‘self-tumor antigens’ to strongly immunogenic responses, thereby generating vigorous anti-tumor immunity [5, 6, 18]. In this study, we generated tumor lysate antigen-pulsed DCs from peripheral blood monocytes using a panel of cytokines (GM-CSF, IL-4, IL-1β, PGE2, and TNF-α) for 7 days. The processed cells highly expressed surface molecules such as HLA-DR, CD80, CD83, CD86, and secreted cytokines including IL-12, IL-10, TNF-α, and IL-6.
Clinical responses are the final goal of cancer immunotherapy. Nevertheless, short-term response criteria, which are conventionally based on the shrinkage of the tumor mass of solid tumors, are not always applicable to DC vaccines because they rely on an indirect effect: inducing host immunity [19]. Alternative evaluations of immunotherapy are required. Recently, studies by other researchers and our group indicated that there was a relationship between clinical response and measurable immune response [20–23]. The immune responses could be evaluated by different ways, such as a lymphocyte proliferation assay, intracellular staining for IFN-γ or tetramers, a skin DTH test, and an ELISA for Th1/Th2 cytokines [24, 25]. Here, we used an ELISA to detect cytokines, flow cytometry to evaluate lymphocyte subsets, and a skin DTH test. There was some evidence that immune responses in cancer patients are biased toward a Th2-type response, which is reflected by decreased Th1 cytokine secretion and impaired Th1-mediated anti-tumor activity [26]. The Th1/Th2 cytokine levels play an important role in tumor progression [27]. In this study, it was shown with ELISA that the post-vaccination levels of Th1 cytokines rose significantly compared to pre-vaccination levels (p < 0.05). In the intracellular IFN-γ staining test, the percentage of IFN-γ+ CD8+ T cells was upregulated after the patients were given the DC vaccines. Furthermore, eighteen patients demonstrated a positive response against autologous tumor antigens after immunotherapy with the skin DTH test, and the diameter of induration in three patients was greater than 15 mm. DTH is a typical cell-mediated immune response, involving both CD4+ and CD8+ T cells and Th1 cytokines such as IL-2 and IFN-γ. The prognosis of patients may be associated with the degree of DTH reaction [28]. These results suggested that autologous tumor cell-pulsed DCs were able to elicit antigen-specific Th1 immune responses in vivo.
The current work is a follow-up study that confirms the effectiveness of DC-based immunotherapy in treatment stage II/IIIA breast cancer patients. The study data demonstrated a correlation between the positive immune responses induced by DC vaccination and improved long-term progress-free patient survival in late-stage breast cancer patients. However, we did not find the difference between the patients with and without DC vaccine in overall survival.
The specificity of the immune response induced by our therapy can also be indirectly deduced by the analysis of patient survival. Indeed, the post-vaccination median survival of the stage II/IIIA patients in our study was longer than the overall median survival observed in non-vaccinated patients. DTH-positive patients with minimal tumor mass showed the best clinical performance, supporting other findings of studies that combined tumor resection and adjuvant immunotherapy. These results suggest that the specific immune responses induced by DC vaccines impact the general condition of the patients and their quality of life by controlling tumor metastatic dissemination rather than by the destruction of the established tumor.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (30901304), the Natural Science Foundation of Jiangsu Province (BK2011244), and the Key Project of Changzhou Health Bureau (ZD200902, ZD200907, ZD201001).
Footnotes
Chun-Jian Qi and Yong-Ling Ning contributed equally to this work.
References
- 1.Schietinger A, Philip M, Schreiber H. Specificity in cancer immunotherapy. Semin Immunol. 2008;20(5):276–285. doi: 10.1016/j.smim.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Copier J, Bodman-Smith M, Dalgleish A. Current status and future applications of cellular therapies for cancer. Immunotherapy. 2011;3(4):507–516. doi: 10.2217/imt.11.18. [DOI] [PubMed] [Google Scholar]
- 3.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12(6):509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29(3):372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Lopez MN, Pereda C, Segal G, Munoz L, Aguilera R, Gonzalez FE, Escobar A, Ginesta A, Reyes D, Gonzalez R, Mendoza-Naranjo A, Larrondo M, Compan A, Ferrada C, Salazar-Onfray F. Prolonged survival of dendritic cell-vaccinated melanoma patients correlates with tumor-specific delayed type IV hypersensitivity response and reduction of tumor growth factor beta-expressing T cells. J Clin Oncol. 2009;27(6):945–952. doi: 10.1200/JCO.2008.18.0794. [DOI] [PubMed] [Google Scholar]
- 6.Hirschowitz EA, Foody T, Kryscio R, Dickson L, Sturgill J, Yannelli J. Autologous dendritic cell vaccines for non-small-cell lung cancer. J Clin Oncol. 2004;22(14):2808–2815. doi: 10.1200/JCO.2004.01.074. [DOI] [PubMed] [Google Scholar]
- 7.Yi Q, Szmania S, Freeman J, Qian J, Rosen NA, Viswamitra S, Cottler-Fox M, Barlogie B, Tricot G, van Rhee F. Optimizing dendritic cell-based immunotherapy in multiple myeloma: intranodal injections of idiotype-pulsed CD40 ligand-matured vaccines led to induction of type-1 and cytotoxic T-cell immune responses in patients. Br J Haematol. 2010;150(5):554–564. doi: 10.1111/j.1365-2141.2010.08286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridgway D. The first 1000 dendritic cell vaccinees. Cancer Invest. 2003;21(6):873–886. doi: 10.1081/CNV-120025091. [DOI] [PubMed] [Google Scholar]
- 9.Schadendorf D, Ugurel S, Schuler-Thurner B, Nestle FO, Enk A, Brocker EB, Grabbe S, Rittgen W, Edler L, Sucker A, Zimpfer-Rechner C, Berger T, Kamarashev J, Burg G, Jonuleit H, Tuttenberg A, Becker JC, Keikavoussi P, Kampgen E, Schuler G. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol. 2006;17(4):563–570. doi: 10.1093/annonc/mdj138. [DOI] [PubMed] [Google Scholar]
- 10.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4(3):328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 11.Qi C, Cai Y, Gunn L, Ding C, Li B, Kloecker G, Qian K, Vasilakos J, Saijo S, Iwakura Y, Yannelli JR, Yan J. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived beta-glucans. Blood. 2011;117(25):6825–6836. doi: 10.1182/blood-2011-02-339812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Ruijter TC, Veeck J, de Hoon JP, van Engeland M, Tjan-Heijnen VC. Characteristics of triple-negative breast cancer. J Cancer Res Clin Oncol. 2011;137(2):183–192. doi: 10.1007/s00432-010-0957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams P, Bouchentouf M, Rafei M, Romieu-Mourez R, Hsieh J, Boivin MN, Yuan S, Forner KA, Birman E, Galipeau J. A dendritic cell population generated by a fusion of GM-CSF and IL-21 induces tumor-antigen-specific immunity. J Immunol. 2010;185(12):7358–7366. doi: 10.4049/jimmunol.1002201. [DOI] [PubMed] [Google Scholar]
- 14.Gong J, Zhang Y, Durfee J, Weng D, Liu C, Koido S, Song B, Apostolopoulos V, Calderwood SK. A heat shock protein 70-based vaccine with enhanced immunogenicity for clinical use. J Immunol. 2010;184(1):488–496. doi: 10.4049/jimmunol.0902255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Svane IM, Pedersen AE, Johansen JS, Johnsen HE, Nielsen D, Kamby C, Ottesen S, Balslev E, Gaarsdal E, Nikolajsen K, Claesson MH. Vaccination with p53 peptide-pulsed dendritic cells is associated with disease stabilization in patients with p53 expressing advanced breast cancer; monitoring of serum YKL-40 and IL-6 as response biomarkers. Cancer Immunol Immunother. 2007;56(9):1485–1499. doi: 10.1007/s00262-007-0293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu B, Kusmartsev S, Cheng F, Paolini M, Nefedova Y, Sotomayor E, Gabrilovich D. Effective combination of chemotherapy and dendritic cell administration for the treatment of advanced-stage experimental breast cancer. Clin Cancer Res. 2003;9(1):285–294. [PubMed] [Google Scholar]
- 17.Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 2005;65(18):8059–8064. doi: 10.1158/0008-5472.CAN-05-1797. [DOI] [PubMed] [Google Scholar]
- 18.Turnis ME, Rooney CM. Enhancement of dendritic cells as vaccines for cancer. Immunotherapy. 2010;2(6):847–862. doi: 10.2217/imt.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoos A, Parmiani G, Hege K, Sznol M, Loibner H, Eggermont A, Urba W, Blumenstein B, Sacks N, Keilholz U, Nichol G. A clinical development paradigm for cancer vaccines and related biologics. J Immunother. 2007;30(1):1–15. doi: 10.1097/01.cji.0000211341.88835.ae. [DOI] [PubMed] [Google Scholar]
- 20.Hirschowitz EA, Foody T, Hidalgo GE, Yannelli JR. Immunization of NSCLC patients with antigen-pulsed immature autologous dendritic cells. Lung Cancer. 2007;57(3):365–372. doi: 10.1016/j.lungcan.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N, Pineiro L, Steinman R, Fay J. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61(17):6451–6458. [PubMed] [Google Scholar]
- 22.Wheeler CJ, Black KL, Liu G, Mazer M, Zhang XX, Pepkowitz S, Goldfinger D, Ng H, Irvin D, Yu JS. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 2008;68(14):5955–5964. doi: 10.1158/0008-5472.CAN-07-5973. [DOI] [PubMed] [Google Scholar]
- 23.Qi CJ, Zheng L, Ma HB, Fei M, Qian KQ, Shen BR, Wu CP, Vihinen M, Zhang XG. A novel mutation in CD40 and its functional characterization. Hum Mutat. 2009;30(6):985–994. doi: 10.1002/humu.20967. [DOI] [PubMed] [Google Scholar]
- 24.Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Annu Rev Immunol. 2000;18:245–273. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- 25.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15(2):138–147. doi: 10.1016/S0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 26.Sheu BC, Lin RH, Lien HC, Ho HN, Hsu SM, Huang SC. Predominant Th2/Tc2 polarity of tumor-infiltrating lymphocytes in human cervical cancer. J Immunol. 2001;167(5):2972–2978. doi: 10.4049/jimmunol.167.5.2972. [DOI] [PubMed] [Google Scholar]
- 27.Elsasser-Beile U, Gierschner D, Jantscheff P, Schultze-Seemann W, Katzenwadel A, Wetterauer U. Different basal expression of type T1 and T2 cytokines in peripheral lymphocytes of patients with adenocarcinomas and benign hyperplasia of the prostate. Anticancer Res. 2003;23(5A):4027–4031. [PubMed] [Google Scholar]
- 28.Nakai N, Katoh N, Germeraad WT, Kishida T, Ueda E, Takenaka H, Mazda O, Kishimoto S. Immunohistological analysis of peptide-induced delayed-type hypersensitivity in advanced melanoma patients treated with melanoma antigen-pulsed mature monocyte-derived dendritic cell vaccination. J Dermatol Sci. 2009;53(1):40–47. doi: 10.1016/j.jdermsci.2008.07.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




