Abstract
Mast cells may have either antitumor or tumor-promoting potential. Nevertheless, mast cells in tumor microenvironment have been found to promote tumor growth. So far the mechanisms underlying the modulation of mast cell function in tumor microenvironment remains to be fully elucidated. Here, we report that tumor-promoting potential of mast cells could be augmented by molecules released from damaged tumor cells through cooperative stimulation of stem cell factor (SCF) and ligand for Toll-like receptor 4 (TLR4). Co-simulation with SCF and TLR4 ligand inhibited mast cell degranulation, but efficiently induced the production and secretion of VEGF, PDGF, and IL-10. Although TLR4 ligand alone may induce IL-12 expression in mast cells, co-stimulation with SCF and TLR4 ligand induced the expression of IL-10, but not IL-12, in mast cells. The phosphorylation of GSK3β was crucial for the effect of SCF and TLR4 ligand. In addition to inducing phosphorylation of GSK3β at Ser9 through PI3K pathway, SCF and TLR4 ligand cooperated to induce phosphorylation of GSK3β at Tyr216 by simultaneous activation of ERK and p38MAPK pathways. Both phospho-Ser9 and phospho-Tyr216 of GSK3β were required for IL-10 expression induced by SCF/TLR4 ligand, whereas suppressive effect of SCF/TLR4 ligand on mast cell degranulation was related to phospho-Tyr216. Importantly, the effect of SCF and TLR4 ligand on mast cells could be abrogated by inhibiting phosphorylation of GSK3β at Tyr216. These findings disclose the mechanisms underlying the modulation of mast cell function in tumor microenvironment, and suggest that inhibiting GSK3β in mast cells will be beneficial to the treatment of cancer.
Keywords: Mast cells, Tumor, Stem cell factor, Toll-like receptor 4, GSK3β
Introduction
Mast cells are key effector cells in allergic diseases. It has also become apparent that they also influence tumor progression [1, 2]. Although mast cells may have antitumor potential [3, 4], many studies have shown that the number of tumor infiltrating mast cells correlates with increased intra-tumoral microvessel density, enhanced tumor growth, and poor clinical outcome [5–7]. Therefore, it has been suggested that mast cells may serve as a novel therapeutic target for cancer treatment [2, 5, 8]. In this regard, elucidating the mechanisms underlying the modulation of mast cell function in tumor microenvironment will be very important for designing new strategy for tumor therapy.
In addition to promoting angiogenesis [2], mast cells have been found to play a critical role in the suppression of immune reactions [9]. They could produce IL-10 to suppress immune response [10], and are essential for the immune tolerance mediated by regulatory T cells [11]. On the other hand, however, mast cell degranulation is detrimental to tumor [3, 4]. It has also been found that mast cells are a crucial source of IL-12, an important regulator of T cell responses and NK cell function [12]. Therefore, mast cells may have both antitumor potential and tumor-promoting potential. So far the mechanisms underlying the modulation of the function of mast cells in tumor microenvironment have not been fully elucidated. We previously reported that stem cell factor (SCF) could stimulate mast cells to promote tumor growth by remodeling tumor microenvironment [13]. To further investigate the mechanisms underlying the modulation of mast cell function in tumor, in this study, we focused on the modulation of mast cell degranulation and the expressions of IL-10 and IL-12 in mast cells. Our data showed that the tumor-promoting potential of mast cells was augmented by tumor cell-released SCF and ligand for Toll-like receptor 4 (TLR4). SCF and TLR4 ligand cooperated to induce the expression of IL-10 in mast cells and inhibit mast cell degranulation. The underlying mechanism involved the phosphorylation of GSK3β at both Ser9 and Tyr216 induced by SCF and TLR4 ligand. Importantly, the effects of SCF and TLR4 ligand on mast cells could be abrogated by inhibiting phosphorylation at Tyr216 of GSK3β.
Materials and methods
Animal and cell line
BALB/c mice and C57BL/6 mice, 6–8-week-old, were purchased from Center of Medical Experimental Animals of Hubei Province (Wuhan, China) for studies approved by the Animal Care and Use Committee of Tongji Medical College. BALB/c background H22 hepatocarcinoma cell line and C57BL/6 background melanoma B16F1 cell line were purchased from China Center for Type Culture Collection (CCTCC, Wuhan, China) and cultured according to their guidelines.
Reagents and plasmids
Resveratrol (3,4′,5-trihydroxy-trans-stilbene) and LPS (lipopolysaccharide) were purchased from Sigma-Aldrich. SB203580, PD98059, wortmannin, and 6-amino-4-(4-phenoxyphenylethylamino)quinazoline (QNZ) were purchased from Merck4Biosciences (Calbiochem). Murine SCF and IL-3 were purchased from Peprotech (Rocky Hill, NJ). Eukaryotic expression vector psTLR4 [14] carrying the cDNA encoding extracellular domain of murine TLR4 (sTLR4) was constructed by insertion of cDNA into plasmid pcDNA3.1 (Invitrogen, Carlsbad, CA).
Generation of bone marrow-derived mast cells
Bone marrow cells were harvested from femurs of mice and cultured in complete RPMI 1640 medium in presence of 10 ng/ml of IL-3 [13]. The non-adherent cells were passaged every 3 days. 4 weeks later, the generated mast cells (bone marrow-derived mast cells, BMMCs) were used for further experiments. In different batches of BMMCs used for experiment, the percentage of c-Kit+FcεRI+ cells was >93%.
ELISA assay
Cell-free supernatants were harvested after 48-h culture. VEGF, PDGF, IL-10, and IL-12p70 in the supernatants were quantified using ELISA kits (R&D Systems, Minneapolis, MN) as per manufacturer’s instructions. Results from cell culture were normalized between different samples and expressed as pg/ml/2 × 106 cells.
Flow cytometric analysis
The tissues removed from tumor inoculation sites were digested with collagenase and hyaluronidase, and grinded to single cells. For intracellular staining, the cells were first stained with allophycocyanin (APC)-anti-mouse c-Kit and FITC-anti-mouse FcεRIα. Then, the cells were fixed and permeabilized with Fix/Perm solution (eBioscience), and stained with PE-anti-mouse IL-10 or PE-anti-mouse IL-12 antibody or isotype control (eBioscience). For analysis of CD8+ T cells in tissue, the cells were stained with PE-anti-mouse CD8 for flow cytometric analysis. For analysis of mast cells in tissue, the cells were stained with APC-anti-mouse c-Kit and FITC-anti-mouse FcεRIα (eBioscience) for flow cytometric analysis. Parameters were acquired on a FACS Calibur flow cytometer (BD Biosciences) and analyzed with CellQuest software (BD Biosciences).
Tumor growth and treatment experiments
In co-inoculation test, mice were inoculated intramuscularly in the right hind limb with 1 × 105 tumor cells with or without 2 × 105 BMMCs as indicated. In intra-tumor injection test, the mice with palpable tumor were randomly divided into several groups on d7 after inoculation with tumor cells. 5 × 105 BMMCs or equal volume of PBS (50 μl) were carefully injected to tumor. When disodium cromoglycate (DSCG) and LiCl were used for treatment, mice received i.p. injection of either DSCG or LiCl, or equal volume of PBS, once a day within the indicated time frame. Tumors were dissected and weighed at the indicated time point.
When psTLR4 was used in intra-tumor injection test, mice were inoculated intramuscularly in the right hind limb with 1 × 105 tumor cells. At the indicated time point, the mice received the intramuscular (i.m.) injection of 100 μg of psTLR4, or equal volume of saline or equal amount of pcDNA3.1.
Preparation of DTC-Ms and T-sMs
Tumor cells were washed with PBS, and resuspended in PBS to a final concentration of 5 × 107/ml. After four-round frozen-thaw cycles followed by vortexing for 30 s, the cells were removed by centrifugation. The supernatant contained a mixture of molecules from damaged tumor cells (DTC-Ms). The mixture of soluble molecules from tumor (T-sMs) was prepared by digesting tumor peripheral tissue with collagenase and removing debris by centrifugation. The concentration of DTC-Ms and T-sMs was defined by the concentration of protein, which was determined using Coomassie Bradford reagent (Thermo Fisher Scientific Inc., Rockford, IL) according to manufacturer’s instructions.
In vitro migration assay
To determine the existence of functional SCF in DTC-Ms and T-sMs, the medium containing DTC-Ms or T-sMs (10 μg/ml) was set in lower chamber, and 6 × 104 BMMCs were set in upper chamber, in triplicate, of a 24-well transwell apparatus (BD Biosciences, San Jose, CA), and incubated at 37°C for 24 h. BMMCs migrating into the lower chamber were enumerated, and the percentage of migrated cells was calculated. Anti-SCF neutralizing antibody (10 μg/ml) was used to inhibit the activity of SCF.
BMMC degranulation assay
Degranulation of mast cells was determined by β-hexosaminidase release assay [15]. Briefly, BMMCs (5 × 104/well) were incubated for 2 h in triplicate in 96-well plates. To induce degranulation, BMMCs were stimulated with 0.1 mM H2O2 [4]. β-hexosaminidase activity was measured as described previously [15]. β-hexosaminidase release was expressed as the percentage of total cell content.
Reverse transcription-PCR and real-time PCR
After 24-h culture, total RNA was extracted from cells with TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. A reverse transcription-PCR (RT-PCR) procedure was used to determine relative quantities of mRNA. Twenty-eight PCR cycles were used for all of the analyses. The primer sequences were as follows: IL-10, sense 5′-CTCTTACTGACTGGCATGAGG-3′, antisense 5′-CCTTGTAGACACCTTGGTCTTGGAG-3′; IL-12a, sense 5′-GGGACCAAACCAGCAC AT-3′, antisense 5′-AAGGCGTGAAGCAGGATG-3′; β-actin, sense 5′-ATGGGTCAGAAGGACTCCTATG-3′, antisense 5′-ATC TCCTGCTCGAAGTCTAGA G-3′. The quantitative real-time RT-PCR for IL-10 expression was done as described previously [16].
Western blot assay
Western blot assay was done as described previously [13]. Abs against mouse GSK3β, phospho-Ser9-GSK3β, and phospho-Tyr216-GSK3β were purchased from Cell Signaling Technology and BD Biosciences.
Statistics
Results were expressed as mean value ± SD and interpreted by one-way ANOVA. Differences were considered to be statistically significant when P < 0.05.
Results
The function of mast cells is modulated in the established tumor
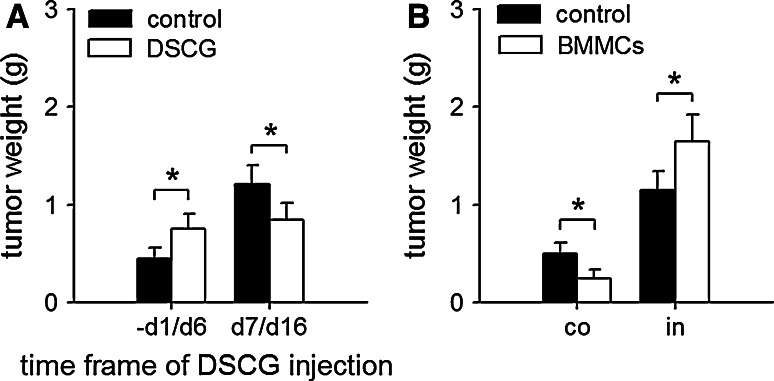
To investigate the effect of tumor microenvironment on the function of mast cells, we first tested the effect of disodium cromoglycate (DSCG), an inhibitor of mast cell function [6, 17], on tumor growth by administration of DSCG within different time frames after tumor inoculation. DSCG promoted tumor growth in early stage of tumor development, but suppressed tumor growth in later stage (Fig. 1a), suggesting that the function of mast cells was modulated in the established tumor. We then tested the effect of mast cells on tumor growth in co-inoculation test and intra-tumor injection test, respectively. The results showed that the BMMCs co-inoculated with tumor cells suppressed the growth of tumor, whereas the BMMCs injected to palpable tumor promoted tumor growth (Fig. 1b), further confirming that the tumor-promoting potential of mast cells was only augmented in the established tumor.
Fig. 1.
Mast cells are converted into tumor-promoting cells in the established tumor. a Effect of DSCG on tumor growth. The mice were inoculated with H22 cells on d0, and received i.p. injection of DSCG (0.6 mg per injection), once a day from −d1 to d6 or from d7 to d16. Tumors (n = 8 in each group) were dissected and weighted on d10 (−d1 to d6 injection) or d17 (d7 to d16 injection) after tumor inoculation. b Effect of BMMCs on tumor growth. BMMCs were co-inoculated with H22 cells (co), or injected into palpable tumor (in). Tumors (n = 8 in each group) were dissected and weighted on d10 (co-inoculation) or d17 (intra-tumor injection) after tumor inoculation. P values, *P < 0.05
TLR4 ligand cooperates with SCF to augment tumor-promoting potential of mast cells
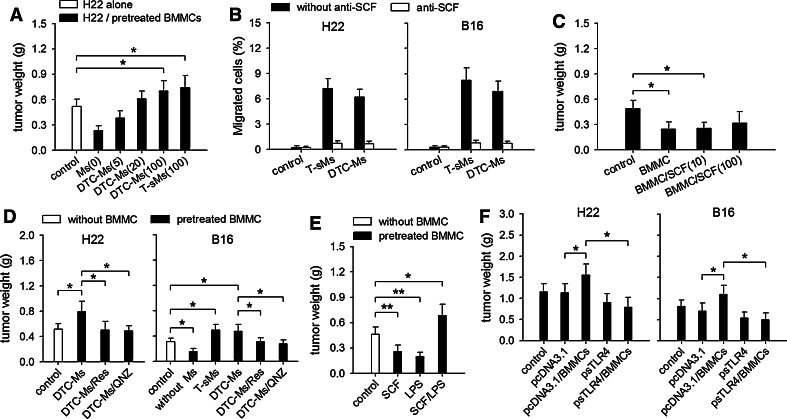
We next investigated whether the function of mast cells could be remodeled by signal molecules in tumor. The soluble molecules in tumor (T-sMs) may contain most of, if not all of, signal molecules which could stimulate mast cells. Since T-sMs also contained the molecules from damaged tumor cells (DTC-Ms), we therefore treated BMMCs with either T-sMs or DTC-Ms in co-inoculation test. The results showed that the BMMCs pretreated with either T-sMs or DTC-Ms could promote tumor growth (Fig. 2a). Our previous study showed that the tumor-promoting effect of mast cells was related to SCF stimulation [13]. Both T-sMs and DTC-Ms indeed contained functional SCF, since the migration of BMMCs induced by DTC-Ms and T-sMs could be inhibited by SCF neutralizing antibody (Fig. 2b). The existence of SCF in DTC-Ms and T-sMs was also confirmed by Western blot (data not shown). However, when we treated BMMCs with SCF alone before they were used in co-inoculation test, BMMCs did not significantly promote tumor growth (Fig. 2c), suggesting that other factor(s) in T-sMs and DTC-Ms might cooperate with SCF to augment tumor-promoting potential of mast cells. Based on same effects of DTC-Ms and T-sMs, we next focused on DTC-Ms to analyze the mechanism underlying the modulation of mast cell function.
Fig. 2.
SCF and TLR4 ligand augment tumor-promoting potential of mast cells. a Effect of pretreated BMMCs on tumor growth. In co-inoculation test, BMMCs were pretreated without [Ms(0)] or with DTC-Ms (5, 20, 100 μg/ml) or T-sMs for 48 h. b Functional SCF in T-sMs and DTC-Ms. The migration of BMMCs in response to T-sMs and DTC-Ms was determined as described in Methods. Anti-SCF neutralizing antibody (10 μg/ml) was used to inhibit the activity of SCF. c In co-inoculation test, H22 cells were inoculated alone, or with untreated BMMCs or the BMMCs pretreated with SCF (10 or 100 ng/ml) for 48 h. d In co-inoculation test, BMMCs were pretreated with DTC-Ms (100 μg/ml) for 48 h in absence or presence of resveratrol (Res, 30 μM) or QNZ (20 nM). Untreated (without Ms) or T-sMs-treated BMMCs were also used for co-inoculation with B16 cells. e In co-inoculation test, H22 cells were inoculated without BMMCs, or with the BMMCs pretreated with SCF (10 ng/ml), LPS (100 ng/ml), or SCF/LPS for 48 h. f sTLR4 abrogates tumor-promoting effect of BMMCs. In intra-tumor injection test, the mice received i.m. injection of psTLR4 or pcDNA3.1 on d4, d5, d6 after tumor inoculation. BMMCs were injected into tumor on d7. P values, *P < 0.05, **P < 0.01. Tumors (n = 8 in each group) were dissected and weighted on d10 (a, c, d, e) or d17 (f) after tumor inoculation
Given that the DTC-Ms contained both TLR2 ligand and TLR4 ligand [14, 18], and that both of these ligands could stimulate mast cells [2, 19], we next investigated whether the effect of DTC-Ms on mast cells involved the cooperation of SCF with TLR2 ligand or TLR4 ligand. To do this, we treated BMMCs with DTC-Ms in presence of QNZ or resveratrol. QNZ, the inhibitor of NF-κB, could inhibit the effect of both TLR2 signaling and TLR4 signaling. Resveratrol inhibits TRIF, and therefore inhibits TLR4 signaling but not TLR2 signaling [20]. QNZ and resveratrol were not toxic to mast cells at the concentration used in the experiments (data not shown). Importantly, each of them significantly suppressed the effect of DTC-Ms on the tumor-promoting potential of BMMCs (Fig. 2d), suggesting that TLR4 ligand might cooperate with SCF to modulate the function of mast cells. To further confirm this, we treated BMMCs with SCF and LPS, a well-known TLR4 ligand. Indeed, BMMCs treated with SCF and LPS promoted tumor growth in co-inoculation test (Fig. 2e). To further evaluate whether TLR4 ligand is required for modulating mast cell function in tumor microenvironment, we expressed soluble TLR4 (sTLR4) in intra-tumor injection test by local transfection of expression vector of sTLR4. The expression of sTLR4 could block TLR4 signaling in local microenvironment [14]. The tumor-promoting effect of BMMCs in intra-tumor injection test was suppressed by local expression of sTLR4 (Fig. 2f), indicating that TLR4 ligand in tumor microenvironment was involved in modulating the function of mast cells.
Similar to H22 hepatocarcinoma cells, T-sMs from melanoma and DTC-Ms from melanoma B16 cells also contained SCF (Fig. 2b). BMMCs pretreated with T-sMs from melanoma and DTC-Ms from B16 cells could also promote tumor growth in co-inoculation test (Fig. 2d). The effect of DTC-Ms from B16 cells was also suppressed by QNZ and resveratrol. Moreover, the tumor-promoting effect of BMMCs in melanoma was also suppressed by local expression of sTLR4 (Fig. 2f). These data suggested that mast cells could be modulated through same mechanism in different types of tumor.
SCF and TLR4 ligand inhibit degranulation of mast cells but not secretion of growth factors
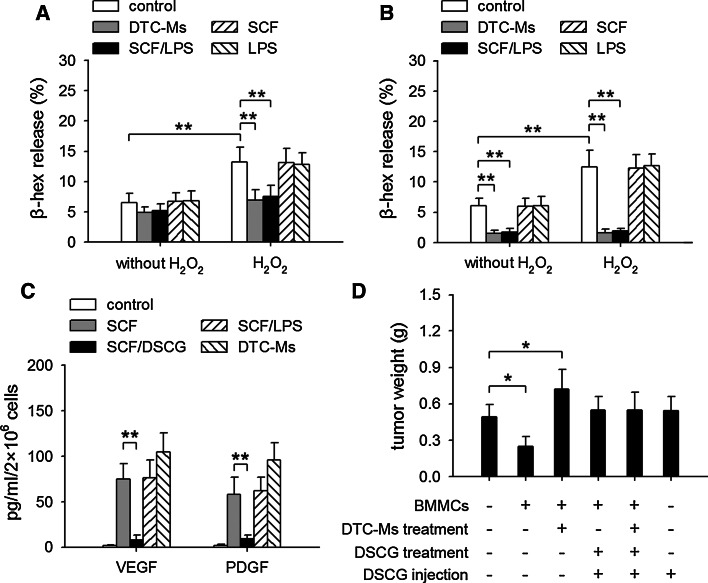
The way that mast cells could be beneficial to tumor is if secretion of cytokines and other molecules from mast cells could occur without degranulation [1, 21]. The antitumor function of mast cells is related to their degranulation induced by H2O2 [4]. Despite the existence of H2O2, however, mast cell degranulation is inhibited in tumor [21, 22]. We therefore investigated whether SCF and TLR4 ligand may suppress H2O2-induced degranulation of mast cells. As shown in Fig. 3a, H2O2-induced degranulation of BMMCs was suppressed by DTC-Ms or SCF/LPS, but not by SCF or LPS alone. Moreover, the treatment of BMMCs with DTC-Ms or SCF/LPS for a longer period of time resulted in a much stronger inhibitory effect (Fig. 3b). Both the viability of mast cells and the intracellular granules of BMMCs were not changed after incubation (data not shown).
Fig. 3.
SCF and TLR4 ligand suppress degranulation of mast cells but not secretion of VEGF and PDGF. a Degranulation of mast cells. BMMCs were unstimulated (without H2O2) or stimulated with 0.1 mM H2O2 for 2 h in absence or presence of DTC-Ms (100 μg/ml), SCF (10 ng/ml), LPS (100 ng/ml), or SCF/LPS. β-hexosaminidase (β-hex) release was measured as described in Methods. b BMMCs were cultured for 6 h in absence or presence of DTC-Ms, SCF, LPS, or SCF/LPS. Then, the cells were stimulated with H2O2 for β-hex release assay. c Production of VEGF and PDGF by mast cells. BMMCs were cultured in absence or presence of SCF, SCF/DSCG(100 μM), SCF/LPS, or DTC-Ms for 48 h. VEGF and PDGF in supernatants were determined by ELISA. d DSCG abrogates the effect of BMMCs on tumor growth. In co-inoculation test, the untreated BMMCs or DTC-Ms-treated BMMCs were directly used, or further treated with DSCG for 2 h. As indicated, the mice received i.p. injection of DSCG (0.6 mg per injection, once a day for 2 days). Tumors (n = 8 in each group) were dissected and weighted on d10 after tumor inoculation. P values, *P < 0.05, **P < 0.01
Although SCF/TLR4 ligand could suppress mast cell degranulation, SCF-induced production of VEGF and PDGF in mast cells was not suppressed by co-stimulation with SCF and TLR4 ligand (Fig. 3c). Differently, DSCG has been known to suppress not only mast cell degranulation but also the secretion of cytokines and growth factors from mast cells. Consistently, DSCG not only abolished the tumor-suppressing effect of untreated BMMCs, but also abrogated the tumor-promoting effect of DTC-Ms-treated BMMCs in co-inoculation test (Fig. 3d). BMMCs treated with DSCG could not influence tumor growth, no matter whether they were treated with DTC-Ms or not. Therefore, DSCG might suppress the function of mast cells, whereas SCF/TLR4 ligand only remodeled the function of mast cells.
SCF and TLR4 ligand cooperate to induce the expression of IL-10 in mast cells
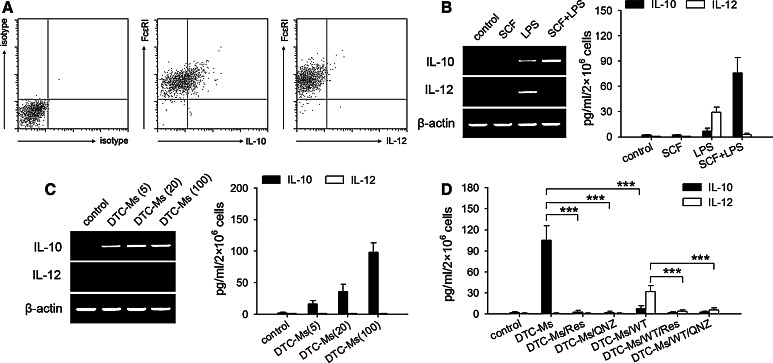
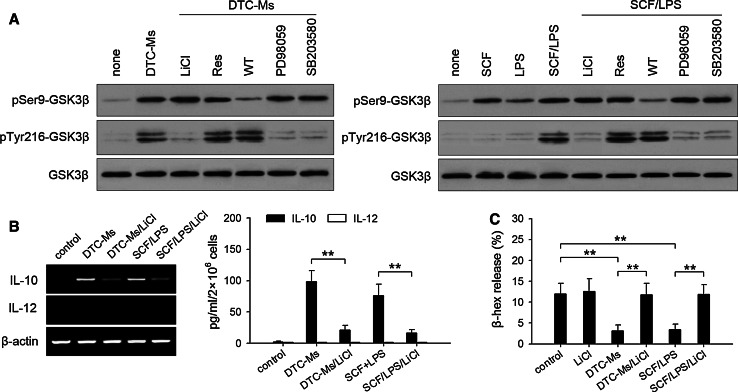
Mast cells have been found to negatively regulate immune response by expressing IL-10 [10]. However, mast cells have also been found to express IL-12 in response to TLR4 signaling [12]. We therefore analyzed the expression of IL-10 and IL-12 in mast cells in tumor. The results showed that the mast cells in tumor expressed IL-10, but not IL-12 (Fig. 4a). To investigate whether the effect of TLR4 ligand on mast cells might be altered by SCF, we stimulated BMMCs with LPS and/or SCF. LPS alone indeed stimulated BMMCs to express IL-12, whereas LPS in combination with SCF stimulated BMMCs to express IL-10 (Fig. 4b). Consistently, DTC-Ms could stimulate BMMCs to express IL-10, but not IL-12 (Fig. 4c). In presence of wortmannin (PI3K inhibitor), IL-12 expression in BMMCs was induced by DTC-Ms, whereas IL-10 expression was significantly suppressed (Fig. 4d). QNZ and resveratrol inhibited IL-10 expression in DTC-Ms-stimulated BMMCs, and also inhibited IL-12 expression in BMMCs stimulated with DTC-Ms in presence of wortmannin (Fig. 4d). These results suggested that TLR4 signaling could stimulate mast cells to express either IL-10 or IL-12, depending on whether PI3K pathway was activated. In line with the results shown in Fig. 3c and d, SCF/TLR4 ligand induced the production of IL-10, whereas DSCG could suppress the release of IL-10 (data not shown).
Fig. 4.
SCF and TLR4 ligand stimulate mast cells to express IL-10. a Flow cytometric analysis of IL-10 and IL-12 expressions in mast cells in tumor as described in Methods. c-Kit+ cells were gated for further analysis. b, c IL-10 and IL-12 expressions in mast cells. BMMCs were cultured in absence or presence of SCF (10 ng/ml), LPS (100 ng/ml), or SCF/LPS (b), or DTC-Ms (μg/ml) (c). IL-10 and IL-12 expressions were determined by RT-PCR and ELISA as described in Methods. d ELISA analysis of IL-10 and IL-12 expressions. BMMCs were cultured in absence or presence of DTC-Ms (100 μg/ml) in different combination with resveratrol (Res, 30 μM), QNZ (20 nM), or wortmannin (WT, 10 nM). P values, ***P < 0.001
Phosphorylation of GSK3β is involved in the effect of SCF/TLR4 ligand on mast cells
PI3K pathway is involved in the regulation of IL-10 and IL-12 expressions. We next wondered whether GSK3β, a downstream molecule of PI3K pathway, was involved in the effect of SCF/TLR4 ligand on mast cell function. It has been known that the activation of PI3K pathway induces the phosphorylation of GSK3β at Ser9 [23–25]. Consistently, DTC-Ms and SCF/LPS induced the phosphorylation of GSK3β at Ser9, which was inhibited by wortmannin (Fig. 5a). Interestingly, DTC-Ms or SCF/LPS also induced the phosphorylation of GSK3β at Tyr216, whereas either SCF or LPS alone could not induce the phosphorylation of Tyr216 (Fig. 5a). The phosphorylation of Tyr216 in response to DTC-Ms or SCF/LPS was not influenced by wortmannin or resveratrol, but inhibited by LiCl (GSK3β inhibitor), SB203580 (p38MAPK inhibitor), and PD98059 (inhibitor of ERK pathway), suggesting that the activated p38MAPK pathway and ERK pathway were required for Tyr216 phosphorylation.
Fig. 5.
Activation of GSK3β and its effect on mast cell function. a BMMCs were untreated or pretreated with LiCl (60 μM), resveratrol (Res, 30 μM), wortmannin (WT, 10 nM), PD98059 (20 μM), SB203580 (20 μM) for 60 min, and then stimulated with DTC-Ms (100 μg/ml), SCF (10 ng/ml), LPS (100 ng/ml), or SCF/LPS for 30 min. Phospho-Ser9-GSK3β, phospho-Tyr216-GSK3β, and GSK3β were detected by Western blot. b Effect of GSK3β on IL-10 expression in mast cells. BMMCs were cultured in absence or presence of DTC-Ms or SCF/LPS with or without LiCl. The expressions of IL-10 and IL-12 were determined by RT-PCR and ELISA as described in Methods. c Effect of GSK3β on mast cell degranulation. BMMCs were cultured in absence or presence of DTC-Ms, SCF/LPS, and/or LiCl for 6 h. Then the cells were stimulated with 0.1 mM H2O2 for the assay of degranulation. P values, **P < 0.01
Using LiCl to inhibit Tyr216 phosphorylation, we next investigated whether phospho-Tyr216 of GSK3β was critical for the effect of SCF/TLR4 ligand on mast cells. LiCl inhibited IL-10 expression in mast cells in response to DTC-Ms and SCF/LPS, but did not promote IL-12 expression (Fig. 5b). Interestingly, LiCl also abrogated the inhibitory effect of DTC-Ms and SCF/LPS on mast cell degranulation (Fig. 5c). Given that wortmannin also suppressed IL-10 expression in mast cells (Fig. 4d), these results indicated that both phospho-Ser9 and phospho-Tyr216 were required for the induction of IL-10 expression in mast cells, whereas phospho-Tyr216 was required for inhibiting mast cell degranulation. Moreover, these results also explained the result in Fig. 2c. QNZ and resveratrol could inhibit DTC-Ms-induced tumor-promoting effect of mast cells, but did not recover their tumor-suppressing effect, since the phosphorylation of GSK3β was not inhibited.
Phosphorylation of GSK3β at Tyr216 is crucial in augmenting the tumor-promoting potential of mast cells
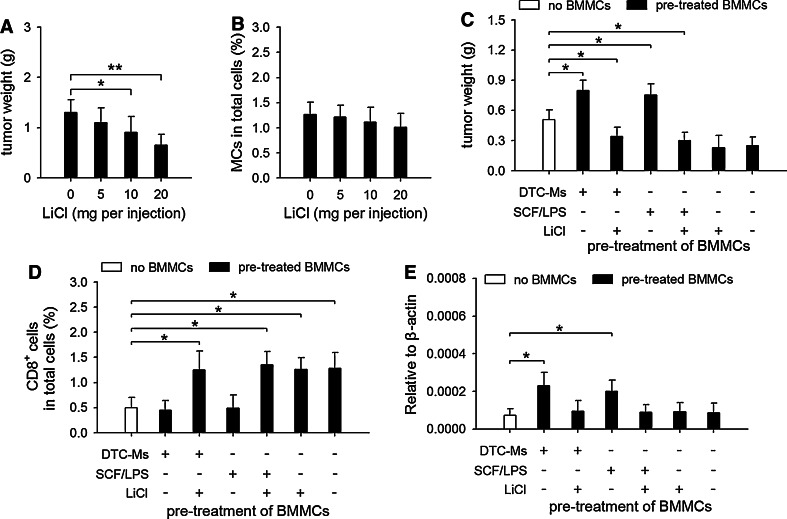
The above results suggested that inhibiting phosphorylation of GSK3β at Tyr216 may suppress the tumor-promoting potential of mast cells. Indeed, LiCl could suppress tumor growth in vivo (Fig. 6a), although it did not significantly influence tumor cell proliferation in vitro (data not shown). Moreover, the accumulation of mast cells in tumor was not significantly influenced (Fig. 6b), suggesting that LiCl might mainly inhibit their function. We then treated BMMCs with DTC-Ms and SCF/LPS in presence of LiCl, and tested the effect of BMMCs on tumor growth in co-inoculation test. The results showed that LiCl abrogated the tumor-promoting effect of BMMCs pretreated with DTC-Ms or SCF/LPS (Fig. 6c). Due to the difficulty of the quantification of mast cell degranulation in tissue, we detected CD8+ T cells in local tissue, since degranulation of mast cells could increase the recruitment of CD8+ T cells [26]. In co-inoculation test, the BMMCs pretreated with DTC-Ms or SCF/LPS could not increase the recruitment of CD8+ T cells in local tissue (Fig. 6d), but increased IL-10 expression in local tissue (Fig. 6e). The effect of DTC-Ms and SCF/LPS on mast cells was abrogated by LiCl. Taken together, these results indicate that the phosphorylation of GSK3β at Tyr216 is indeed critical in augmenting tumor-promoting potential of mast cells.
Fig. 6.
Inhibiting phosphorylation of GSK3β at Tyr216 interferes with the effect of SCF/TLR4 ligand on mast cells. a, b Effect of LiCl on tumor growth in vivo. The mice inoculated with H22 cells received i.p. injection of LiCl. Tumors (n = 8 in each group) were dissected and weighted on d15 after tumor inoculation (a). Single cell suspension was prepared from the tumor tissues. The cells were analyzed by flow cytometry. The percentage of mast cells (MCs) in total cells was calculated (b). c The effect of LiCl on mast cells. In co-inoculation test, tumor cells were inoculated alone (no BMMCs), or co-inoculated with the BMMCs pretreated for 48 h in absence or presence of DTC-Ms (100 μg/ml), SCF (10 ng/ml)/LPS (100 ng/ml), and/or LiCl (60 μM). Tumors (n = 8 in each group) were dissected and weighted on d17 (a, b) or d10 (c) after tumor inoculation. d, e Using same protocol as that in c, tumor cells were inoculated alone (no BMMCs), or co-inoculated with pretreated BMMCs. On d3 after inoculation, single cell suspension was prepared from the tissues at inoculation sites (n = 8 in each group). The cells were analyzed by flow cytometry. The percentage of CD8+ T cells in total cells was calculated (d). IL-10 expression in the tissues at inoculation sites (n = 8 in each group) was detected by real-time RT-PCR 24 h after inoculation (e). P values, *P < 0.05
Discussion
In tumor microenvironment, SCF is released from living tumor cells [13] and the damaged tumor cells. TLR4 ligand could be released from the damaged tumor cells [14, 18], and produced by bacteria and fungi existent in tumor [27]. SCF activates multiple signaling pathways in mast cells, including PI3K and ERK pathways [28–31]. TLR4 signaling also activates several signaling pathways, including MyD88, TRIF, PI3K, and p38MAPK pathways [23, 32, 33]. Our data in this study showed that SCF and TLR4 ligand cooperated to augment tumor-promoting potential of mast cells by activating these signaling pathways. Importantly, here we found that SCF/TLR4 ligand induced the phosphorylation of GSK3β at both Ser9 and Tyr216 in mast cells, which was critical for the tumor-promoting potential of mast cells. It has been known that the activation of PI3K pathway induces the phosphorylation of GSK3β at Ser9 [23–25]. The mechanism underlying the phosphorylation of GSK3β at Tyr216 has not been identified to date. Here, we found that the phosphorylation of GSK3β at Tyr216 in mast cells could result from the simultaneous activation of ERK and p38MAPK pathways. Thus, the cooperative stimulation of SCF and TLR4 ligand is required for distinct phosphorylation pattern of GSK3β in mast cells.
The degranulation of mast cells could be induced by H2O2 in local microenvironment, which is involved in the antitumor function of mast cells [4]. H2O2 could be produced by phagocytes in inflammation site. Inflammation is a fundamental character of tumor microenvironment [34, 35]. Despite the existence of H2O2, however, mast cell degranulation is usually inhibited in tumor [21, 22]. The underlying mechanism has not been elucidated to date. Our data in this study showed that degranulation of mast cells could be inhibited by SCF and TLR4 ligand. The phosphorylation of GSK3β was required for this inhibitory effect. The degranulation of mast cells was not influenced by phosphorylation of GSK3β at Ser9, since SCF alone induced phosphorylation of GSK3β at Ser9, but did not influence degranulation of mast cells. TLR4 ligand synergized with SCF to inhibit degranulation of mast cells by inducing phosphorylation of GSK3β at Tyr216. After recruited to tumor, mast cells could be stimulated by SCF to express cytokines and growth factors. These factors may promote tumor growth through different mechanisms [36–38]. However, the mast cells expressing these factors may not be certainly beneficial to tumor unless the degranulation of mast cells is inhibited [1, 21]. In this respect, TLR4 ligand plays an important role in the augmentation of tumor-promoting potential of mast cells by synergizing with SCF to inhibit mast cell degranulation.
TLR4 signaling-activated NF-κB could induce IL-12 expression in mast cells. Despite the existence of TLR4 ligand, however, the mast cells in tumor only expressed IL-10. Our data showed that the response of mast cells to TLR4 ligand was altered by SCF. The underlying mechanism involved the phosphorylation of GSK3β induced by co-stimulation of TLR4 ligand and SCF. Phosphorylated GSK3β suppressed NF-κB-induced IL-12 expression, but cooperated with NF-κB to induce IL-10 expression in mast cells. Phospho-Ser9 was required for GSK3β to inhibit IL-12 expression, whereas both phospho-Ser9 and phospho-Tyr216 were required for GSK3β to up-regulate IL-10 expression in mast cells. Therefore, tumor cells could induce IL-10 expression, but not IL-12 expression, in mast cells by releasing SCF and TLR4 ligand. We previously reported that mast cells in tumor could suppress the function of T cells and NK cells [13], which could now be explained by the production of IL-10 by mast cells in response to SCF/TLR4 ligand.
The effect of SCF/TLR4 ligand on mast cells was different from that of DSCG. DSCG is a stabilizer which acts on cellular membrane and inhibits the release of preformed and newly synthesized mediators from mast cells [39]. DSCG may completely inhibit the function of mast cells by inhibiting both degranulation and the secretion of cytokine and growth factors. Therefore, DSCG may promote tumor growth in early stage of tumor development by suppressing mast cell degranulation, but suppress tumor growth in later stage by inhibiting the secretion of mast cells. Different from DSCG, SCF/TLR4 ligand augmented the tumor-promoting potential of mast cells by inhibiting degranulation of mast cells and simultaneously inducing production of cytokine and growth factors such as IL-10, VEGF and PDGF.
In summary, the findings in this report disclose the mechanisms underlying the modulation of mast cell function in tumor microenvironment. Given that the cooperative stimulation of SCF and TLR4 ligand was required for tumor-promoting potential of mast cells, and that both SCF and TLR4 ligand could be released from damaged tumor cells, the damage of tumor cells by different elements may indirectly promote tumor growth by modulating the function of mast cells.
Acknowledgments
This work was supported by National Science Foundation of China (No. 30830095, 30771974, 30772589), Science Foundation of Ministry of Education of China (No. 20070487004), and National Development Program (973) For Key Basic Research of China (No. 2009CB521806).
Abbreviations
- DTC-Ms
Molecules from damaged tumor cells
- T-sMs
Soluble molecules from tumor
- SCF
Stem cell factor
- BMMC
Bone marrow-derived mast cell
- sTLR4
Soluble form of TLR4
References
- 1.Conti P, Castellani ML, Kempuraj D, Salini V, Vecchiet J, Tetè S, et al. Role of mast cells in tumor growth. Ann Clin Lab Sci. 2007;37:315–322. [PubMed] [Google Scholar]
- 2.Maltby S, Khazaie K, McNagny KM. Mast cells in tumor growth: angiogenesis, tissue remodelling and immune-modulation. Biochim Biophys Acta. 2009;1796:19–26. doi: 10.1016/j.bbcan.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinnamon MJ, Carter KJ, Sims LP, Lafleur B, Fingleton B, Matrisian LM. A protective role of mast cells in intestinal tumorigenesis. Carcinogenesis. 2008;29:880–886. doi: 10.1093/carcin/bgn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henderson WR, Chi EY, Jong EC, Klebanoff SJ. Mast cell-mediated tumor-cell cytotoxicity. Role of the peroxidase system. J Exp Med. 1981;153:520–533. doi: 10.1084/jem.153.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kormelink TG, Abudukelimu A, Redegeld FA. Mast cells as target in cancer therapy. Curr Pharm Des. 2009;15:1868–1878. doi: 10.2174/138161209788453284. [DOI] [PubMed] [Google Scholar]
- 6.Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 7.Ribatti D, Ennas MG, Vacca A, Ferreli F, Nico B, Orru S, Sirigu P. Tumor vascularity and tryptase-positive mast cells correlate with a poor prognosis in melanoma. Eur J Clin Invest. 2003;33:420–425. doi: 10.1046/j.1365-2362.2003.01152.x. [DOI] [PubMed] [Google Scholar]
- 8.Ribatti D, Crivellato E. The controversial role of mast cells in tumor growth. Int Rev Cell Mol Biol. 2009;275:89–131. doi: 10.1016/S1937-6448(09)75004-X. [DOI] [PubMed] [Google Scholar]
- 9.Ullrich SE, Nghiem DX, Khaskina P. Suppression of an established immune response by UVA–a critical role for mast cells. Photochem Photobiol. 2007;83:1095–1100. doi: 10.1111/j.1751-1097.2007.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 11.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 12.Nakano N, Nishiyama C, Kanada S, Niwa Y, Shimokawa N, Ushio H, Nishiyama M, Okumura K, Ogawa H. Involvement of mast cells in IL-12/23 p40 production is essential for survival from polymicrobial infections. Blood. 2007;109:4846–4855. doi: 10.1182/blood-2006-09-045641. [DOI] [PubMed] [Google Scholar]
- 13.Huang B, Lei Z, Zhang GM, Li D, Song C, Li B, Liu Y, Yuan Y, Unkeless J, Xiong H, Feng ZH. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008;112:1269–1279. doi: 10.1182/blood-2008-03-147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu YY, Sun LC, Wei JJ, Li D, Yuan Y, Yan B, et al. Tumor cell-Released TLR4 ligands stimulate Gr-1+CD11b+F4/80+ cells to induce apoptosis of activated T cells. J Immunol. 2010;185:2773–2782. doi: 10.4049/jimmunol.1000772. [DOI] [PubMed] [Google Scholar]
- 15.Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, et al. Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–1011. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- 16.Geng H, Zhang GM, Xiao H, Yuan Y, Li D, Zhang H, Qiu H, He YF, Feng ZH. HSP70 vaccine in combination with gene therapy with plasmid DNA encoding sPD-1 overcomes immune resistance and suppresses the progression of pulmonary metastatic melanoma. Int J Cancer. 2006;118:2657–2664. doi: 10.1002/ijc.21795. [DOI] [PubMed] [Google Scholar]
- 17.Walsh SK, Kane KA, Wainwright CL. Mast cell degranulation–a mechanism for the anti-arrhythmic effect of endothelin-1? Br J Pharmacol. 2009;157:716–723. doi: 10.1111/j.1476-5381.2009.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 19.Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. FcepsilonR1 and toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood. 2006;107:610–618. doi: 10.1182/blood-2005-06-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Youn HS, Lee JY, Fitzgerald KA, Young HA, Akira S, Hwang DH. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: molecular targets are TBK1 and RIP1 in TRIF complex. J Immunol. 2005;175:3339–3346. doi: 10.4049/jimmunol.175.5.3339. [DOI] [PubMed] [Google Scholar]
- 21.Theoharides TC, Conti P. Mast cells: the Jekyll and Hyde of tumor growth. Trends Immunol. 2004;25:235–241. doi: 10.1016/j.it.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 23.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3 beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/S0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 25.Miyashita K, Kawakami K, Nakada M, Mai W, Shakoori A, Fujisawa H, Hayashi Y, Hamada J, Minamoto T. Potential therapeutic effect of glycogen synthase kinase 3 beta inhibition against human glioblastoma. Clin Cancer Res. 2009;15:887–897. doi: 10.1158/1078-0432.CCR-08-0760. [DOI] [PubMed] [Google Scholar]
- 26.Ott VL, Cambier JC, Kappler J, Marrack P, Swanson BJ. Mast cell-dependent migration of effector CD8+ T cells through production of leukotriene B4. Nat Immunol. 2003;4:974–981. doi: 10.1038/ni971. [DOI] [PubMed] [Google Scholar]
- 27.Szczepanski MJ, Czystowska M, Szajnik M, Harasymczuk M, Boyiadzis M, Kruk-Zagajewska A, Szyfter W, Zeromski J, Whiteside TL. Triggering of Toll-like receptor 4 expressed on human head and neck squamous cell carcinoma promotes tumor development and protects the tumor from immune attack. Cancer Res. 2009;69:3105–3113. doi: 10.1158/0008-5472.CAN-08-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yasuda A, Sawai H, Takahashi H, Ochi N, Matsuo Y, Funahashi H, Sato M, Okada Y, Takeyama H, Manabe T. Stem cell factor/c-kit receptor signaling enhances the proliferation and invasion of colorectal cancer cells through the PI3K/Akt pathway. Dig Dis Sci. 2007;52:2292–2300. doi: 10.1007/s10620-007-9759-7. [DOI] [PubMed] [Google Scholar]
- 29.Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol. 2009;124:639–646. doi: 10.1016/j.jaci.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim MS, Rådinger M, Gilfillan AM. The multiple roles of phosphoinositide 3-kinase in mast cell biology. Trends Immunol. 2008;29:493–501. doi: 10.1016/j.it.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalesnikoff J, Rios EJ, Chen CC, Nakae S, Zabel BA, Butcher EC, Tsai M, Tam SY, Galli SJ. RabGEF1 regulates stem cell factor/c-Kit-mediated signaling events and biological responses in mast cells. Proc Natl Acad Sci USA. 2006;103:2659–2664. doi: 10.1073/pnas.0511191103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J Immunol. 2001;166:3837–3845. doi: 10.4049/jimmunol.166.6.3837. [DOI] [PubMed] [Google Scholar]
- 33.Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96:1039–1046. [PubMed] [Google Scholar]
- 34.Lawrence T. Inflammation and cancer: a failure of resolution? Trends Pharmacol Sci. 2007;28:162–165. doi: 10.1016/j.tips.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin WW, Karin MA. Cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, Mayer L, Unkeless JC, Xiong H. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 38.Huang B, Lei Z, Zhao J, Gong W, Liu J, Chen Z, Liu Y, Li D, Yuan Y, Zhang GM, Feng ZH. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007;252:86–92. doi: 10.1016/j.canlet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Shin HY, Kim JS, An NH, Park RK, Kim HM. Effect of disodium cromoglycate on mast cell-mediated immediate-type allergic reactions. Life Sci. 2004;74:2877–2887. doi: 10.1016/j.lfs.2003.10.026. [DOI] [PubMed] [Google Scholar]