Abstract
To investigate the safety and immunological responses of personalized peptide vaccination in combination with oral administration of UFT and UZEL for metastatic colorectal carcinoma (mCRC), fourteen patients were enrolled in the present study. Peptides were determined based on the presence of peptide-specific cytotoxic T lymphocyte precursors and IgG in each patient. A maximum of four peptides were subcutaneously administered weekly with UFT (300 mg/m2 day−1) and UZEL (75 mg/day) for 4 weeks, followed by 1 week of rest. This therapy was well-tolerated although there was a grade-3 skin reaction at the vaccination site in one patient. An increase in peptide-specific interferon-γ production or peptide-specific IgG after the tenth vaccination was observed in nine of ten or eight of ten patients tested, respectively. IgG responses were well correlated with overall survival (P = 0.0215). The safety and immunological responsiveness of the present therapy suggest that this combination would be of clinical benefit for mCRC patients, and further trials are merited.
Keywords: Peptide vaccine, Metastatic colorectal cancer, Personalized treatment, Cytotoxic T lymphocyte, Peptide specific IgG response
Introduction
The field of cancer vaccines is currently in an active state of clinical investigations. Human papilloma virus vaccine has been approved as a prophylactic cancer vaccine [9, 16], while tumor-derived heat shock protein-based cancer vaccine has been approved in Russia, although to date no vaccine have been approved in Japan or the USA [21]. There have been slow but substantial advances in peptide vaccines with regard to both clinical responses and immunological markers [2–4, 15, 22].
We reported that the administration of the standard dose (80 mg/m2 day−1) of 5-fluorouracil derivative (TS-1) for advanced gastric or colorectal carcinoma patients did not impede immunological responses to both the inoculated peptides and tumor cells in advanced gastric or colorectal carcinoma patients under the combined therapy of personalized peptide vaccine and TS-1 [18]. We also reported that a personalized peptide vaccination in combination with estramustine phosphate showed a superior antitumor effect compared to peptide vaccination alone in patients with hormone-refractory prostate cancer [15].
The combined chemotherapy using UFT and UZEL is considered to be one of the standard therapies for colorectal cancers [5, 19]. UFT is an oral anticancer drug consisting of both Tegafur (FT), a prodrug of 5-fluorouracil (5-FU), and uracil, an inhibiter of degradation of 5-FU. UZEL is an oral drug consisting of calcium folinate, which modulates 5-FU. To investigate the safety and immunological responses of personalized peptide vaccination in combination with UFT and UZEL, we conducted a phase I clinical study of this combination therapy for metastatic colorectal cancer patients.
Patients and methods
Patients and eligibility criteria
The study protocol was approved by the Institutional Ethical Review Boards of Kinki University and Kurume University. Complete written informed consent was obtained from all patients at the time of enrolment. All patients were required to have histologically confirmed metastatic colorectal carcinoma (mCRC) unsuitable for surgical resection, and to be HLA-A24 or HLA-A2 positive. All patients but one had failed to respond to the prior chemotherapies, including UFT and UZEL (n = 8) (Table 1). All patients were required to have completed prior chemotherapy at least 4 weeks before trial enrollment, and to have recovered from an adverse event with a toxicity of grade 3 or higher by the Common Terminology Criteria for Adverse Event (CTCAE) scale. All patients were required to have an Eastern Cooperative Oncology Group performance status (PS) of 0–1, to be older than 20 years of age, and to have a life expectancy of at least 3 months. Adequate bone marrow (white blood cell (WBC) count ≥3,000/mm3, hemoglobin ≥10 g/dL and platelet count ≥75,000/mm3), renal function (serum creatinine ≤ 1.4 mg/dL), and liver function (bilirubin ≤ 1.5 mg/dL and transaminase within 1.5× the institution’s upper limit of normal) were required. Patients were excluded, if they had hepatitis B or C virus antigens, or human immunodeficiency virus (HIV), or if they were pregnant.
Table 1.
Patients’ characteristic
| Patient No. | HLA | Gender | Age | Sites of metastases | PS | Previous treatment |
|---|---|---|---|---|---|---|
| 1 | A24 | M | 65 | Peritoneum | 1 | UFT/UZEL |
| 2 | A2/A24 | F | 57 | Lymph nodes | 1 | 5-FU/LV, TS-1 |
| 3 | A24 | F | 73 | Lymph nodes, lung | 1 | UFT/UZEL, CPT-11 |
| 4 | A2/A24 | F | 47 | Lung | 0 | 5-FU/LV, UFT/UZEL |
| 5 | A2 | M | 39 | Lung | 0 | 5-FU/MMC (hepatic artery infusion) |
| 6 | A2/A24 | F | 59 | Lung | 0 | 5-FU/LV, FOLFOX |
| 7 | A24 | M | 37 | Peritoneum | 1 | TS-1/CPT-11, TS-1/CDDP |
| 8 | A24 | F | 52 | Lung | 0 | None |
| 9 | A24 | M | 58 | Lung | 0 | UFT/UZEL, CPT-11 |
| 10 | A2 | M | 54 | Lung | 0 | 5-FU, UFT/UZEL |
| 11 | A2 | F | 69 | Lymph nodes | 1 | UFT/UZEL |
| 12 | A24 | M | 53 | Bone | 1 | UFT/UZEL, radiation |
| 13 | A24 | M | 77 | Lung | 0 | UFT/UZE, 5-FU/CPT-11, TS-1 |
| 14 | A24 | M | 74 | Lung | 0 | UFT/UZEL, radiation |
5-FU 5-fluorouracil, LV leucovorin, MMC mitomycin C, CPT-11 irinotecan, CDDP cisplatin, PS performance status
Clinical protocol
This was an open-label phase I study. Peptide-specific CTL reactivity and peptide-specific IgG antibody were measured using pre-vaccination peripheral blood mononuclear cells (PBMCs) and pre-vaccination plasma. According to the results of the screening, peptides showing higher immune-responses (a maximum of four peptides) were selected for injection as reported previously [15, 18, 22]. The peptides (3 mg/peptide) were subcutaneously injected with incomplete Freund’s adjuvant (IFA) into the thigh once a week for 5 weeks as reported previously [15, 23]. At the same time, patients also received oral administration of UFT (300 mg/m2 day−1) and UZEL (75 mg/day) for 4 weeks, followed by 1 week of rest. We investigated immunological responses to the inoculated peptides after every five vaccinations. After the tenth vaccination, the inoculated peptides were re-selected according to the results of immunological tests. The 1st to 10th, 11th to 20th, and after 20th vaccinations were given weekly, biweekly, and monthly, respectively, while UFT/UZEL was continued to be administered for 4 weeks followed by 1 week of rest during the entire treatment period. Physical examination was performed weekly throughout the entire treatment period. Complete blood count and serum chemistry tests were obtained once per 2 weeks. Clinical responses by means of computed tomography (CT)-scans and tumor markers were evaluated at the end of every 5 weeks. All the vaccinated patients (n = 13) were assessed for toxicity, and immunological and clinical responses. Toxicity and clinical response were assessed according to CTCAE version 3.0 and the Response Evaluation Criteria in Solid Tumors (RECIST), respectively. Survival benefits were analyzed by the Kaplan–Meier method and P values were assessed by a log-rank test.
Peptides and vaccination
Twenty-five peptides for HLA-A24+ patients and 23 peptides for HLA-A2+ patients were prepared under the conditions of Good Manufacturing Practice by the Multiple Peptide System (San Diego, CA, USA) as reported previously [15, 23]. Most of these peptides were encoded by tumor associated antigen genes cloned by means of cDNA-expression techniques in our laboratories, and the remaining peptides were identified by means of reverse-immunology techniques in our laboratories [6, 8, 10, 14, 15, 17, 23, 24]. These peptides have the ability to induce HLA-A24-restricted or HLA-A2-restricted and tumor-specific CTL activity in PBMCs of cancer patients and are expressed frequently on various tumor cell lines [6, 8, 10, 14, 17, 24]. The peptides were dissolved in saline and stored at −80°C. And then the peptides were supplied in vials containing 3 mg/mL sterile solution for injection. Just before use, these solutions were diluted with saline, and mixed with an equal volume of IFA (Montanide ISA-51), and emulsified in a 5-ml sterilized syringe.
Screening of peptide-specific CTL response
Screening of peptide-specific CTL precursors was conducted using 30 mL of peripheral blood obtained from each patient. PBMCs were isolated by means of Ficoll-Conray density gradient centrifugation. Peptide-specific CTL responses in PBMCs were detected using a previously reported culture method [7, 8]. Briefly, PBMCs (1 × 105 cells/well) were incubated with 10 μM of a peptide in 200 μL of culture medium in u-bottom-type 96-well microculture plates (Nunc, Roskilde, Denmark). The culture medium consisted of 45% RPMI-1640 medium, 45% AIM-V medium (Gibco BRL, Walkersville, MA, USA), 10% fetal calf serum, 100 IU/mL of interleukin-2 (IL-2), and 0.1 μM MEM non-essential amino acid solution (Gibco BRL). Half of the medium was removed and replaced with new medium containing the corresponding peptide (20 μM) every 3 days. After incubation for 14 days, these cells were harvested and tested for their ability to produce interferon (IFN)-γ in response to CIR-A2402 or T2 cells that were preloaded with either the corresponding peptide or human immunodeficiency virus (HIV) peptides (RYLRQQLLGI for HLA-A24 and LLFGYPVYV for HLAA2) as a negative control. The level of IFN-γ was determined by enzyme-linked immunosorbent assay (limit of sensitivity: 10 pg/mL). All assays were carried out in quadruplicate and were analyzed by a two-tailed Student’s t-test. A peptide-specific IFN-γ production was estimated as the difference between IFN-γ production in response to target cells with the corresponding peptide and that in response to target cells with an HIV peptide with statistical significance (P ≤ 0.05).
Screening of peptide-specific IgGs
The levels of anti-peptide IgGs were measured using the Luminex system (Austin, TX, USA), as reported previously [11]. In brief, 100 μL of 1/100 diluted plasma was incubated with 25 μL of peptide-coupled color-coded beads for 2 h at room temperature on a plate shaker. After incubation, the mixture was washed with a vacuum manifold apparatus and incubated with 100 μL of biotinylated goat antihuman IgG (γ-chain specific) for 1 hour at room temperature. The plate was then washed, followed by the addition of 100 μL of streptavidin-phycoerythrin (PE) per well, and incubation for an additional 30 min at room temperature on a plate shaker. The bound beads were washed three times followed by the addition of 100 μL of Tween–phosphatebuffered saline into each well. Each sample (50 μL) was then analyzed using the Luminex system.
Cytotoxicity assay
Cytotoxic activity was measured using a standard 6-h 51Cr-release assay, as reported previously [13]. In brief, cryopreserved PBMCs were thawed and cultured in a medium consisting of 100 U/mL of IL-2 alone. After 14 days of culture, the cells were harvested and used as effector cells. SW620 (HLA-A24+ and -A2+ colon cancer cell line), COLO201 (HLA-A2+ colon cancer cell line), COLO320 (HLA-A24+ colon cancer cell line) and phytohemagglutinin (PHA)-stimulated T-cell blasts were used as target cells.
Results
Patients’ characteristics and vaccinations
Between July 2005 and February 2007, 14 patients with mCRC were enrolled into this trial (Table 1). One patient (patient No. 10) was disqualified for failure to meet the inclusion criteria. The final subject group thus consisted of 13 patients, 7 male and 6 female, with a median age of 57 years (rang 39–77 years). Eleven patients were positive for HLA-A24, and the remaining 3 patients were positive for HLA-A2. For patients having both HLA-A24 and HLA-A2, we used the same peptides as for HLA-A24 patients. All but one (patient No. 8) patient had been previously treated with various chemotherapies, including UFT/UZEL (n = 9). Eight patients had received two different chemotherapy protocols. A total of 224 vaccinations were administered with a median of 17 vaccinations per patient (range 6–36 vaccinations per patient). Vaccinations were terminated when each patient showed a disease progression. All 13 patients received more than 5 vaccinations, and 10 patients received more than 10 vaccinations.
Toxicities
The overall toxicities are shown in Table 2. The most frequent adverse events (AEs) were injection-site reactions (n = 12), anemia (n = 6), lymphopenia (n = 4), elevation of serum transaminase (n = 3), and hyperbilirubinemia (n = 3). With the exception of a grade 3 skin reaction at the vaccination site in one case, all these AEs were grade 1 or 2. In the grade 3 case, the skin ulcer healed after we stopped the administration of the offending peptide. There was no grade 4 toxicity in any case.
Table 2.
Adverse events
| Toxocity | Total | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|
| Anemia | 6 (46.2) | 4 (30.8) | 2 (15.4) | – |
| Neutropenia | 2 (15.4) | 2 (15.4) | – | – |
| Lymphopenia | 4 (30.8) | 2 (15.4) | 2 (15.4) | – |
| Transaminase elevation | 3 (23.1) | 3 (23.1) | – | – |
| Hyperbilirubinemia | 3 (23.1) | 2 (15.4) | 1 (7.7) | – |
| γ-GTP elevation | 1 (7.7) | – | 1 (7.7) | – |
| Colitis | 1 (7.7) | – | 1 (7.7) | – |
| Vaccination site reaction | 12 (92.3) | 11 (84.6) | – | 1 (7.7) |
Numbers and percentages (in parenthesis) of patients who had adverse events were indicated
Immunological responses
Peptide-specific CTL response and IgG level were measured at pre- and post-vaccination (the end of every five vaccinations) (Table 3). We considered CTL response to be augmented if the amount of IFN-γ production induced by the peptide-stimulated post-vaccination samples was 50 pg/mL and also two times higher than that of the pre-vaccination samples. Under these circumstances, augmentation of peptide-specific IFN-γ production by PBMCs after the 5th and 10th vaccinations in response to at least one of the inoculated peptides was observed in 8 of 12 and 9 of 10 patients tested, respectively (Table 3). With regard to the number of peptides eliciting a positive reaction, an augmentation in response to 4, 3, 2, or 1 peptide was observed in 1, 4, 3, or 2 patients, respectively.
Table 3.
Immunological responses and clinical responses
| Patient No. | Peptide | Peptide-specific IgGa | Peptide-specific IFN-γ productionb | Cytotoxicity | Clinical response | Number of vaccination | TTP (day) | OS (day) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post-vaccination | Pre | Post-vaccination | |||||||||
| 5th | 10th | 5th | 10th | |||||||||
| 1 | SART3 109 | 550 | 776 | 446 | 79 | 26 | 169 | SD | 28 | 206 | 376 | |
| Lck 488 | 151 | 243 | 102 | 186 | 118 | 0 | ||||||
| PAP 213 | 164 | 243 | 200 | 25 | 65 | 40 | ||||||
| 2 | Lck 486 | 135 | 257 | 265 | NT | 27 | 0 | PD | 19 | 69 | 659 | |
| Her2/neu 553 | 45 | 117 | 6,676 | NT | 33 | 0 | ||||||
| CEA 425 | 126 | 254 | 242 | NT | 51 | 0 | ||||||
| PTHrp 102 | 60 | 140 | 136 | NT | 114 | 0 | ||||||
| 3 | SART3 109 | 404 | 463 | NT | 0 | 0 | NT | PD | 6 | 35 | 38 | |
| Lck 486 | 44 | 49 | NT | 0 | 0 | NT | ||||||
| MRP3 1293 | 26 | 27 | NT | 0 | 0 | NT | ||||||
| SART1 690 | 9 | 7 | NT | 0 | 0 | NT | ||||||
| 4 | SART3 109 | 228 | 1,425 | 13,078 | 30 | 0 | 354 | + | SD (MR) | 31 | 309 | 1125+ |
| Her2/neu 553 | 142 | 147 | 25,684 | 0 | 38 | 0 | ||||||
| CEA 425 | 486 | 822 | 1,205 | 0 | 0 | 1,132 | ||||||
| MRP3 1293 | 386 | 465 | 558 | 0 | 0 | 40 | ||||||
| 5 | UBE2 V 43 | 1927 | 1708 | 4,315 | 1,687 | 2,542 | 2,170 | SD (MR) | 21 | 200 | 1023 | |
| EIF4EBP 51 | 640 | 487 | 451 | 1,206 | 0 | 0 | ||||||
| WHSC2 103 | 490 | 415 | 392 | 528 | 181 | 127 | ||||||
| CypB 129 | 366 | 235 | 223 | 1,293 | 0 | 0 | ||||||
| 6 | SART3 109 | 320 | 202 | NT | 35 | 14 | NT | PD | 8 | 50 | 595 | |
| MRP3 1293 | 36 | 22 | NT | 0 | 799 | NT | ||||||
| EGFR 800 | 23 | 14 | NT | 0 | 25 | NT | ||||||
| PSCA 76 | NT | NT | NT | 0 | 12 | NT | ||||||
| 7 | SART2 93 | 250 | 189 | NT | 0 | 0 | NT | PD | 6 | 37 | 207 | |
| SART3 109 | 720 | 1,907 | NT | 31 | 295 | NT | ||||||
| MRP3 1293 | 941 | 4,779 | NT | 164 | 1,340 | NT | ||||||
| Her2/neu 553 | 748 | 530 | NT | 629 | 129 | NT | ||||||
| 8 | SART3 109 | 572 | 4,779 | 21,235 | 97 | 906 | 174 | + | SD (MR) | 36 | 357 | 1002+ |
| MRP3 1293 | 146 | 124 | 5,592 | 56 | 27 | 168 | ||||||
| Lck 486 | 76 | 64 | 347 | 53 | 44 | 42 | ||||||
| SART2 93 | 43 | 26 | 35 | 0 | 0 | 593 | ||||||
| 9 | SART3 109 | 231 | 222 | 234 | 0 | 3,236 | 2,868 | PD | 11 | 75 | 315 | |
| MRP3 1293 | 40 | 36 | 32 | 14 | 0 | 0 | ||||||
| Her2/neu 553 | 36 | 30 | 31 | 128 | 0 | 6,121 | ||||||
| Lck 488 | 46 | 41 | 42 | 0 | 0 | 2,101 | ||||||
| 11 | SART3 302 | <10 | 768 | 376 | 354 | 0 | 0 | + | PD | 11 | 35 | 450 |
| SART3 309 | <10 | <10 | 25 | 0 | 13,459 | 0 | ||||||
| Lck 246 | <10 | <10 | 154 | 0 | 3,966 | 5,130 | ||||||
| WHSC2 141 | <10 | <10 | <10 | 0 | 349 | 3,843 | ||||||
| 12 | SART3 109 | 448 | 442 | 684 | 73 | 198 | 1,425 | + | SD | 17 | 187 | 725+ |
| Lck 486 | 161 | 179 | 161 | 44 | 76 | 328 | ||||||
| MRP3 1293 | 392 | 610 | 1,177 | 256 | 48 | 0 | ||||||
| PTHrp 102 | 52 | 53 | 49 | 0 | 64 | 827 | ||||||
| 13 | SART2 161 | 2,529 | 1,929 | 2,500 | 27 | 41 | 331 | PD | 15 | 44 | 368 | |
| Lck 486 | 1,486 | 1,397 | 1,409 | 31 | 0 | 0 | ||||||
| MRP3 1293 | 4,463 | 4,011 | 4,240 | 0 | 0 | 0 | ||||||
| PAP 213 | 4,165 | 4,226 | 9,379 | 0 | 0 | 943 | ||||||
| 14 | SART3 109 | 127 | 115 | 127 | 393 | 2,352 | 432 | SD | 17 | 168 | 657+ | |
| Lck 486 | 47 | 38 | 39 | 92 | 0 | 201 | ||||||
| PAP 213 | 155 | 121 | 2,966 | 24 | 0 | 5,936 | ||||||
| PSA 248 | 45 | 36 | 79 | 0 | 0 | 344 | ||||||
NT not tested, TTP time to progression, OS overall survival time, SD stable disease, PD progressive disease, MR minor response
aValue indicates fluorescence intensity units (FIU) of a peptide-specific IgG antibodies level in sera. An increase in FIU was considered to be positive when the absolute value was more than two times higher than that in a prevaccination sample. Positive values are shown in bold
bValue indicates interferon (IFN)-γ production (pg/mL) of peripheral blood mononuclear calls (PBMC) reactive to the vaccinated peptide. An increase in IFN-γ production was considered to be positive when the value was more than two times and 100 pg/mL higher than that in a prevaccination sample
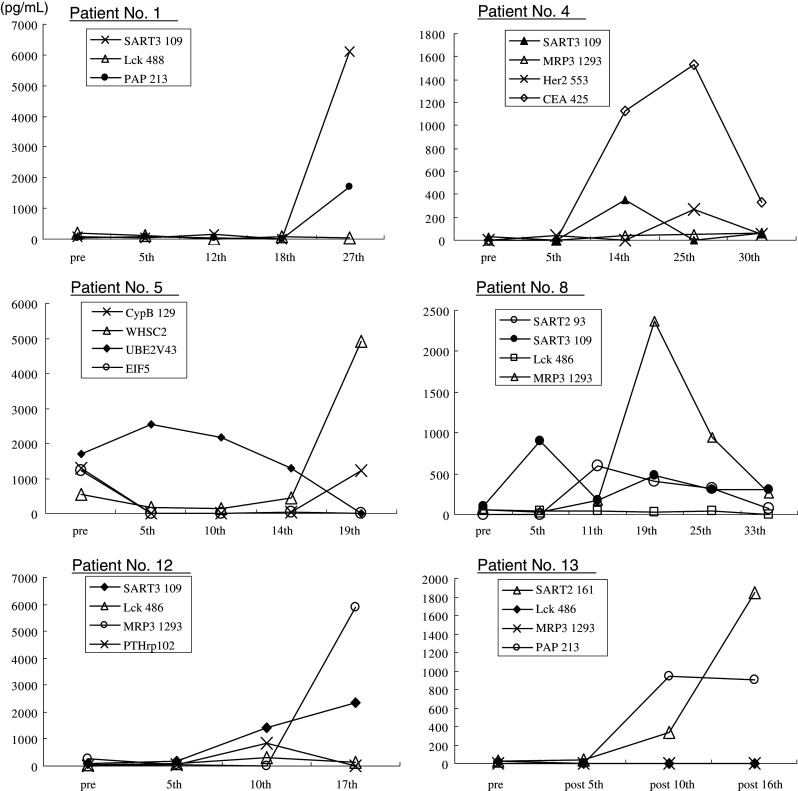
We considered the IgG response to be augmented if the IgG level of post-vaccination samples was more than 10 fluorescence intensity units (FIU) and also two times higher than that of pre-vaccination samples. Under these criteria, augmentation of peptide-specific IgG in plasma after the 5th and 10th vaccinations was observed in 5 of 13 and 8 of 10 patients tested, respectively (Table 3). With regard to the number of peptides eliciting a positive reaction, augmentation in response to 4, 3, 2, or 1 peptide was observed in 0, 4, 1, or 4 patients, respectively. Both peptide-specific CTL response augmentation and peptide-specific IgG increase corresponding to at least one of the inoculated peptides were observed in 8 patients. The results of kinetic studies of IgG and CTL response in patients who received more than 15 vaccinations are shown in Figs. 1 and 2, respectively.
Fig. 1.

Kinetics of peptide-specific IgG levels in patients who were vaccinated 15 times and more. Peptide-specific IgG levels in the pre- and post-vaccination sera were determined by a Luminex system
Fig. 2.
Kinetics of peptide-specific CTL responses in patients who were vaccinated 15 times and more. Interferon-γ production in response to inoculated peptides was measured as a CTL response in pre- and post-vaccination peripheral blood mononuclear cells by ELISA
The most frequently used peptide was SART3 109-118 (nine patients), which induced CTL and IgG augmentation in seven and three patients, respectively. The second-most frequently used peptide was MRP3 1293-1302 (six patients), which induced CTL and IgG augmentation in three and three patients, respectively. The Lck 486–494 peptide was also used for 6 patients, resulting in CTL and IgG augmentation in three and one patients, respectively.
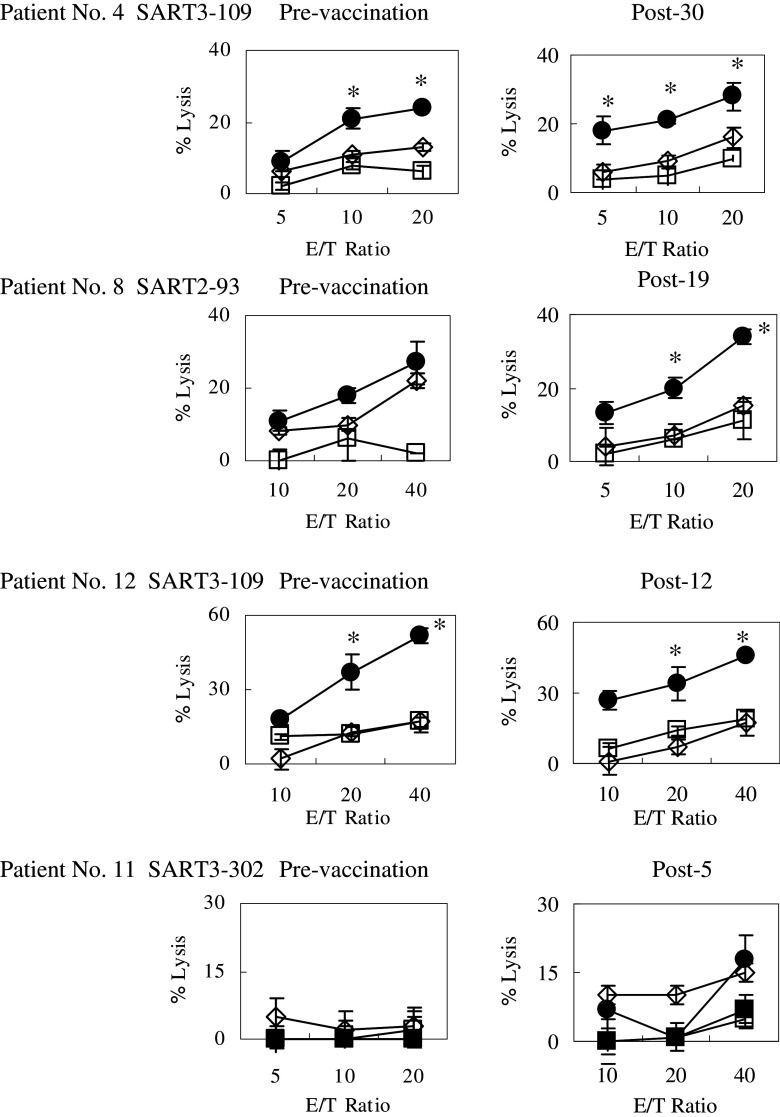
We further examined the cytotoxicity of PBMCs harvested at pre- and post-vaccinations against colon cancer cells by means of a 51Cr release assay (Fig. 3). This assay was conducted simultaneously in pre- and post-vaccination samples in order to avoid bias to the extent possible. Four patients (No. 4, 8, 11, and 12) among the 8 patients whose samples were available for the assay showed HLA-A2 or -A24 restricted cytotoxicity. Namely, PBMCs from patient No. 4 (HLA-A24/A2), who received SART3 109–118 peptide in vivo, were stimulated in vitro with the same peptide for 3 weeks followed by a test of their cytotoxicity against SW620 (HLA-A24+/A2+) and Colo201 (HLA-A2+) tumor cells, and HLA-A24+ phytohemagglutinin (PHA)-stimulated T cells as a negative control. As a result, the post (30th)-vaccination PBMCs showed slightly higher levels of cytotoxicity against SW620 cells as compared to those by the pre-vaccination PBMCs (Fig. 3). None of the PBMCs showed cytotoxicity against either Colo201 or PHA-A24 cells. Similar results were obtained in the post-vaccination PBMCs from patients No. 8 (HLA-A24+) and No. 12 (HLA-A24). PBMCs from patient No. 11 (HLA-A2+), who received SART3 302-110 peptide in vivo, were stimulated in vitro with the same peptide for 3 weeks followed by a test of their cytotoxicity against SW620 (HLA-A24+/A2+), Colo201 (HLA-A2+), and Colo302 (HLA-A24+) tumor cells, and HLA-A2+PHA-stimulated T cells as a negative control. As a result, the post (5th)-vaccination PBMCs showed significantly higher levels of cytotoxicity against both SW620 and Colo201 cells, but not against either Colo302 or PHA-A2 cells (Fig. 3). The pre-vaccination PBMCs failed to show cytotoxicity against any target cells tested.
Fig. 3.
Cytotoxicity against HLA-class I-matched cancer cells. Pre- and post-vaccination peripheral blood mononuclear cells (PBMCs) were cultured with interleukin-2 and the corresponding peptide for 14 days. The cytotoxicity of cultured PBMCs against Colo201 (HLA-A2+ colon carcinoma), SW620 (HLA-A2- and -A24+ colon carcinoma), Colo320 (HLA-A24+ colon carcinoma), and phytohemagglutinin (PHA)-blastoid T cells (HLA-A2+ or HLA-A24+) was measured
Clinical responses
Thirteen patients were assessed for clinical response at the end of 10th vaccination according to the RECIST (Table 3). No patient showed either complete or partial response. Six patients had stable disease (SD), and 7 patients showed progressive disease (PD). The median time of progression free survival (PFS) was 10.7 weeks (range 5.0–51.0 weeks). During the treatment, three of six SD patients showed reduction of tumor size, and we considered these to be cases of minor response (MR). The preceding therapies in these three MR patients were as follows; 5-FU/LU and UFT/UZEL in patient No. 4, 5-FU/MMC in patient No. 5, and none in patient No. 8 (Tables 2, 3).
Immunological responses and clinical benefits
All three patients with MR showed both CTL and IgG responses strongly to at least one of the vaccinated peptide. The other three patients with SD also showed both CTL and IgG responses (n = 2) or a CTL response alone (n = 1). Among the seven patients with PD, three patients showed both CTL and IgG responses, two patients exhibited a CTL response alone, one patient showed an IgG response alone, and the remaining patient showed no response (Table 3).
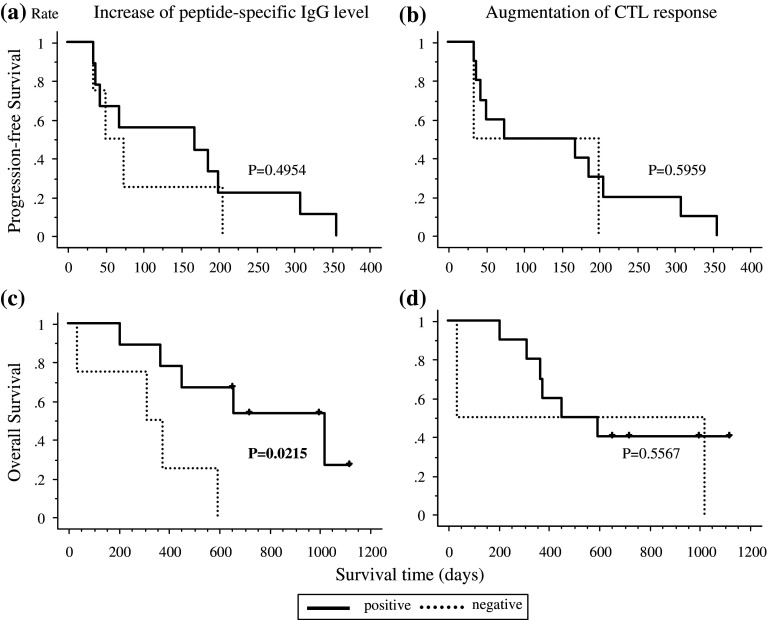
We then investigated whether CTL or IgG response correlated with the PFS and overall survival (OS). OS but PFS in the patients with increased IgG responses to at least one of the inoculated peptides (n = 9) were significantly (P = 0.0215) longer than that in patients with no IgG response (n = 4) (Fig. 4a, c). In contrast, either DFS or OS for the patients with increased CTL responses to at least one of the inoculated peptides (n = 11) was not different with that in the patients with no CTL responses (n = 2) (Fig. 4b, d).
Fig. 4.
Survival analysis correlated with augmentation of immunological responses. Progression free survival (PFS) and overall survival (OS) of nine patients with increased IgG levels for at least one peptide within ten vaccinations compared to four patients without increased IgG levels are indicated in a and c, respectively. Increased peptide-specific IgG levels correlated with only OS with statistical significance (P = 0.0215). PFS and OS of ten patients with an augmented CTL response for at least one peptide within ten vaccinations compared to two patients without an augmented CTL response are indicated in b and d, respectively. This vaccine induced CTL responses with high frequency but did not show a survival benefit
Discussion
The commonly observed AEs in previously conducted clinical trials of UFT/UZEL for colorectal cancer patients were anemia (47.7%), neutropenia (34.1%), elevation of transaminase (38.6%), elevation of bilirubin (59.1%), and diarrhea (38.6%) [20]. In our previous studies, we also reported grade 1 or 2 inflammatory skin reactions at the injection site as a frequently observed AE of personalized peptide vaccination [15, 18, 22]. These previous results along with the results of this study suggest that vaccination-related AEs were only the injection-site reactions. And the present combination of a personalized peptide vaccination and UFT/UZEL was generally well-tolerated, although one patient experienced a severe AE in the form of an injection-site ulcer. This patient showed strong immune responses to the inoculated peptides and the ulcer healed after vaccination with the offending peptide was stopped.
Cytotoxic anti-cancer drugs generally cause bone marrow suppression in association with anemia, neutropenia and leukopenia. In addition, one might expect that cytotoxic anti-cancer drugs would have the undesired effect of either suppressing or diminishing immune boosting by cancer vaccine. However, TS-1 did not impede immunological responses to both the inoculated peptides and tumor cells in advanced gastric or colorectal carcinoma patients under a personalized peptide vaccination [18]. A personalized peptide vaccination combined with gemcitabine has also been reported to induce peptide-specific immune responses in patients with advanced pancreatic cancer [23]. Dose escalation of the vaccinated peptide in patients with advanced pancreatic cancer who were being treated with a standard dose of gemcitabine resulted in dose-dependent augmentation of the peptide-specific immune responses [23]. This present study also showed increasing IgG levels and CTL responses to the vaccinated peptide in mCRC patients being treated with standard doses of UFT and UZEL. Regardless of the administration of UFT and UZEL, augmentation of CTL responses and IgG responses was observed in 11 of 13 patients and 9 of 13 patients in this study, respectively. Increased cytotoxicity against colon cancer cells was also observed. These results clearly indicate that a personalized peptide vaccine can induce and augment peptide-specific immune responses in mCRC patients being treated with standard doses of UFT and UZEL. All these results suggest that a personalized peptide vaccination approach has an additive potential to increase the clinical benefits for gastrointestinal cancer patients when used in conjunction with standard chemotherapy.
Our kinetic study indicated that the augmentation of IgG responses required ten vaccinations in a half of the mCRC patients with UFT/UZEL. We previously reported that a personalized peptide vaccine alone could generate increasing IgG responses in the majority of patients after the sixth-vaccination in significant correlation with OS [12]. These results suggest that the combination of a personalized peptide vaccination with standard chemotherapy required more vaccinations in order to induce IgG responses than a personalized peptide vaccination alone.
OS was well correlated with increased levels of peptide-specific IgG, whereas there was insignificant correlation between increased levels of CTL responses and PFS or OS. This result is consistent with our previous reports [12, 15]. A similar correlation has also been reported in colorectal carcinoma patients receiving a recombinant CEA vaccine [20]. However, the biological roles of IgGs specific to CTL epitope peptides are presently unknown. Peptide-specific IgGs induced by vaccination may not directly bind to the peptides on HLA class I molecules. CD4+ helper T cells might recognize the inoculated peptides presented on HLA-A24 or -A2 molecules of antigen-presenting cells, resulting in both activation of helper T cells and subsequent promotion of IgG production. It is well known that CD4+ helper T cells are necessary to maintain CD8+ T-cell immunity [1]. If increased levels of peptide-specific IgGs reflect activation levels of CD4+ helper T cells, measurement of peptide-specific IgGs would be worthwhile from the viewpoint of an immunological biomarker to predict clinical benefits of cancer patients under peptide vaccination. Further investigations are needed to clarify this issue.
In conclusion, this study suggests that personalized peptide vaccination combined with UFT/UZEL is well-tolerated, and can induce cellular and humoral immune responses. Furthermore, increased peptide-specific IgGs may be immunological biomarkers predictive of clinical benefits for patients with mCRC.
Footnotes
T. Hattori and T. Mine equally contributed to this work.
References
- 1.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4 + T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barve M, Bender J, Senzer N, et al. Induction of immune response and clinical efficacy in a phase II trial of IDM-2101, a 10-epitope cytotoxic T-lymphocyte vaccine, in metastatic non-small-cell lung cancer. J Clin Oncol. 2008;27:4418–4425. doi: 10.1200/JCO.2008.16.6462. [DOI] [PubMed] [Google Scholar]
- 3.Becker JC, Wobser M, Hofmeister V et al (2008) Safety, immunologenicity and clinical response of a survivin-based peptide vaccine in therapy-resistant advanced cancer: Results from phase I/II trial. Abstract of Annual Meeting of American Society of Clinical Oncology J Clin Oncol 26:3046, 143pp
- 4.Bolonaki I, Kotsakis A, Papadimitraki E, et al. Vaccination of patients with advanced non-small-cell lung cancer with an optimized cryptic human telomerase reverse transcriptase peptide. J Clin Oncol. 2007;25:2727–2734. doi: 10.1200/JCO.2006.10.3465. [DOI] [PubMed] [Google Scholar]
- 5.Douillard JY, Hoff PM, Skillings JR, et al. Multicenter phase study of uracil/tegafur and oral leucovorin versus fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2002;20:3605–3616. doi: 10.1200/JCO.2002.04.123. [DOI] [PubMed] [Google Scholar]
- 6.Harashima N, Tanaka K, Sasatomi T, et al. Recognition of the Lck tyrosine kinase as a tumor antigen by cytotoxic T lymphocytes of cancer patients with distant metastases. Eur J Immunol. 2001;31:323–332. doi: 10.1002/1521-4141(200102)31:2<323::AID-IMMU323>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Hida N, Maeda Y, Katagiri K, et al. A simple culture protocol to detect peptide-specific cytotoxic T lymphocyte precursors in the circulation. Cancer Immunol Immunother. 2002;51:219–228. doi: 10.1007/s00262-002-0273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito M, Shichijo S, Miyagi Y, et al. Identification of SART3-derived peptides capable of inducing HLA-A2-restricted and tumor specific CTLs in cancer patients with different HLA-A2 subtypes. Int J Cancer. 2000;88:633–639. doi: 10.1002/1097-0215(20001115)88:4<633::AID-IJC18>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 9.Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693–1702. doi: 10.1016/S0140-6736(07)60777-6. [DOI] [PubMed] [Google Scholar]
- 10.Kikuchi M, Nakao M, Inoue Y, et al. Identification of a SART-1-derived peptide capable of inducing HLA-A24-restricted and tumor-specific cytotoxic T lymphocytes. Int J Cancer. 1999;81:459–466. doi: 10.1002/(SICI)1097-0215(19990505)81:3<459::AID-IJC21>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Komatsu N, Shichijo S, Nakagawa M, et al. New multiplexed flow cytometric assay to measure anti-peptide antibody: a novel tool for monitoring immune responses to peptides used for immunization. Scand J Clin Lab Inest. 2004;64:535–545. doi: 10.1080/00365510410007008. [DOI] [PubMed] [Google Scholar]
- 12.Mine T, Sato Y, Noguchi M, et al. Humoral responses to peptides correlate with overall survival in advanced cancer patients vaccinated with peptides based on pre-existing, peptide-specific cellular responses. Clin Cancer Res. 2004;10:929–937. doi: 10.1158/1078-0432.CCR-1117-3. [DOI] [PubMed] [Google Scholar]
- 13.Miyagi Y, Imai N, Sasatomi T, et al. Induction of cellular immune responses to tumor cells and peptides in colorectal cancer patients by vaccination with SART3 peptide. Clin Cancer Res. 2001;12:3950–3962. [PubMed] [Google Scholar]
- 14.Nakao M, Shichijo S, Imaizumi T, et al. Identification of a gene coding for a new squamous cell carcinoma antigen recognized by the CTL. J Immunol. 2000;164:2565–2574. doi: 10.4049/jimmunol.164.5.2565. [DOI] [PubMed] [Google Scholar]
- 15.Noguchi M, Mine T, Yamada A, et al. Combination therapy of personalized peptide vaccination and low-dose estramustine phosphate for metastatic hormone refractory prostate cancer patients: an analysis of prognostic factors in the treatment. Oncol Res. 2007;16:341–349. doi: 10.3727/000000006783980955. [DOI] [PubMed] [Google Scholar]
- 16.Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 17.Sasatomi T, Suefuji Y, Matsunaga K, et al. Expression of tumor rejection antigens in colorectal carcinomas. Cancer. 2002;94:1636–1641. doi: 10.1002/cncr.10421. [DOI] [PubMed] [Google Scholar]
- 18.Sato Y, Fujiwara T, Mine T, et al. Immunological evaluation of personalized peptide vaccination in combination with a 5-fluorouracil derivative (TS-1) for advanced gastric or colorectal carcinoma patients. Cancer Sci. 2007;7:1113–1119. doi: 10.1111/j.1349-7006.2007.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shirao K, Hoff PM, Ohtsu A, et al. Comparison of the efficacy, toxicity, and pharmacokinetics of a uracil/tegafur (UFT) plus oral leucovorin (LV) regimen between Japanese and American patients with advanced colorectal cancer: joint United States and Japan study of UFT/LV. J Clin Oncol. 2004;22:3466–3474. doi: 10.1200/JCO.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Ullenhag GJ, Frödin JE, Jeddi-Tehrani M, et al. Durable carcinoembryonic antigen (CEA)-specific humoral and cellular immune responses in colorectal carcinoma patients vaccinated with recombinant CEA and granulocyte/macrophage colony-stimulating factor. Clin Cancer Res. 2004;15:3273–3328. doi: 10.1158/1078-0432.CCR-03-0706. [DOI] [PubMed] [Google Scholar]
- 21.Wood C, Srivastava P, Bukowski R, et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet. 2008;372:145–154. doi: 10.1016/S0140-6736(08)60697-2. [DOI] [PubMed] [Google Scholar]
- 22.Yajima N, Yamanaka R, Mine T, et al. Immunologic evaluation of personalized peptide vaccination for patients with advanced malignant glioma. Clin Cancer Res. 2005;11:5900–5911. doi: 10.1158/1078-0432.CCR-05-0559. [DOI] [PubMed] [Google Scholar]
- 23.Yanagimoto H, Mine T, Yamamoto K, et al. Immunological evaluation of personalized peptide vaccination with gemcitabine for pancreatic cancer. Cancer Sci. 2007;12:605–611. doi: 10.1111/j.1349-7006.2007.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang D, Nakao M, Shichijo S, et al. Identification of a gene coding for a protein possessing shared tumor epitopes capable of inducing HLA-A24-restricted cytotoxic T lymphocytes in cancer patients. Cancer Res. 1999;59:4056–4063. [PubMed] [Google Scholar]