Abstract
Background
Transplantable B16 melanoma is widely used as a tumor model to investigate tumor immunity. We wished to characterize the leukocyte populations infiltrating B16 melanoma tumors, and the functional properties of tumor-infiltrating dendritic cells (TIDC).
Materials and methods
We used the B16 melanoma cell line expressing ovalbumin protein (OVA) to investigate the phenotype and T cell stimulatory capacity of TIDC.
Results
The majority of leukocytes in B16 melanoma were macrophages, which colocalized with TIDCs, B and T cells to the peripheral area of the tumor. Both myeloid and plasmacytoid DC populations were present within tumors. Most of these DCs appeared immature, but about a third expressed a mature phenotype. TIDCs did not present tumor-derived antigen, as they were unable to induce the proliferation of tumor-specific CD4+ and CD8+ T cells in vitro unless in the presence of specific peptides. Some presentation of tumor-derived antigen could be demonstrated in the tumor-draining lymph node using in vivo proliferation assays. However, while proliferation of CD8+ T cells was reproducibly demonstrated, no proliferation of CD4+ T cells was observed.
Conclusion
In summary, our data suggest that DCs in tumors have limited antigen-presenting function. Inefficient antigen presentation extends to the tumor-draining lymph node, and may affect the generation of antitumor immune responses.
Keywords: Melanoma, Dendritic cells, Tumor immunity
Introduction
Dendritic cells (DCs) are recruited into a wide range of tumors, but still tumors evade immune recognition. Tumors may prevent the induction of an immune response by releasing immunosuppressive factors that induce the development and recruitment of myeloid suppressor cells [10] and regulatory T cells [6]. Furthermore, lack of activation and recruitment of DCs into tumors, as well as compromised function of DCs, have been described as mechanisms by which tumors escape immune recognition. Cytokines and growth factors that can inhibit DCs are secreted by tumors, including IL-10, IL-6 and VEGF [20]. Tumor-infiltrating DCs can often be found in peritumoral areas, and in regressing tumors they express maturation markers and colocalize with T cells [2, 17]. DCs and especially Langerhans cells (LCs), a subset of DCs residing in the epidermis, have been found in human melanoma [17, 24]. LCs showed defective migration and T cell stimulatory function in tumor-bearing mice [9], and human melanoma cells could inhibit differentiation of LCs from precursors [4].
Several reports have demonstrated that DCs infiltrating tumors were compromised in their ability to stimulate T cell responses unless stimulated with maturation factors [5, 22, 26]. However, none of those studies examined the ability of tumor-infiltrating DCs (TIDCs) to present tumor-derived antigen taken up within the tumor. We used the B16.OVA melanoma cell line, which expresses the ovalbumin protein (OVA) as a model tumor antigen [12] to investigate this question. We examined the leukocyte infiltrate in tumors, and the localization, phenotype and functional capabilities of TIDCs from B16.OVA tumors.
Methods
Mice
Breeding pairs of the inbred strains C57BL/6 (CD45.2+) and the congenic strain B6-SJ ptrprca (CD45.1+) were obtained from Jackson Laboratories, Bar Harbour, ME, and from the Animal Resource Centre, Canning Vale, Western Australia, respectively. OT-I mice and OT-II mice express transgenic Vα2 Vβ5.1/5.2 T cell receptors (TCRs) specific for Kb+ ovalbumin (OVA)257–264 and I-Ab+ OVA323–339, respectively [1, 7]. Relevant breeding pairs were provided by Dr. Sarah Hook, School of Pharmacy, Dunedin, NZ, with permission from Prof. Frank Carbone, Melbourne University, Australia. All mice were bred at the animal facility of the Malaghan Institute of Medical Research, and used for experiments at 7–16 weeks of age. All experimental protocols were approved by the Victoria University Animal Ethics Committee and performed according to institutional guidelines.
Media, cell lines and reagents
Culture medium was Iscove’s minimum essential medium (IMDM) supplemented with 5% FBS, 2 mM glutamax, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μM 2-mercapto-ethanol (all Invitrogen, Auckland, NZ). OVA was purchased from Sigma (Castle Hill, NSW, Australia). OVA257–264 (SIINFEKL) and OVA323–339 (ISQAVHAAHAEINEAGR) were purchased from Mimotopes Pty Ltd (Clayton, Australia). The B16.OVA cell line, generated by Drs. Edith Lord and John G. Frelinger, University of Rochester, Rochester, NY [12], was kindly provided by Drs. Roslyn Kemp and Dick Dutton, Trudeau Institute, NY, USA. B16.F1 and B16.F10 melanoma cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA).
Antibodies
Monoclonal antibodies (mAbs) against CD4 (clone GK1.5), CD11c (clone N418), B cell marker B220/CD45R (clone RA3-6B2), MHC-class II (clone M5/114) and macrophage marker F4/80 (clone BM8) were affinity-purified from hybridoma culture supernatants using protein G-Sepharose (Pharmacia Biotech, Uppsala, Sweden) and conjugated to biotin, allophycocyanin (APC) or fluorescein isothiocyanate (FITC). The following antibodies were purchased from BD-Pharmingen (Auckland, New Zealand): CD45-biotinylated, -FITC or -Phycoerythrin (PE) conjugated (clone 30-F11), CD8α-FITC (clone Ly-2), CD11b-PE (clone M1/70), CD11c-APC (clone HL3), MHC-class II-FITC (clone 2G9), CD86-FITC (clone GL1) and CD40-PE (clone 3/23). The mAb against Langerin was obtained from Dendritics (Lyon, France, clone 929F3). For FACS analysis, cell suspensions were analyzed on a FACS Excalibur using CellQuest (Becton Dickinson, Mountain view, CA). All antibody incubations for FACS analysis were carried out for 10 min on ice.
Isolation and FACS sorting of TIDC
C57BL/6 mice were injected subcutaneously (s.c.) with 105 B16.F1/F10 or B16.OVA tumor cells. After 10–14 days, tumors of size 0.5–1.5 cm2 were excised and digested with 0.5 mg/ml collagenase P (Roche Diagnostics, Mannheim, Germany) for 30 min at 37°C, pressed through cell strainers (Falcon, BD-Pharmingen, Auckland, New Zealand), essentially as described recently for spleen cell suspensions [16]. For pre-enrichment, cells were incubated with anti-CD45-PE (BD-Pharmingen, Auckland, New Zealand) followed by anti-PE-MACS beads (Miltenyi Biotec, Bergisch Gladbach, Germany), and positively sorted with Automacs (Miltenyi), which gave a population containing approximately 20% CD45+ cells. This cell suspension was stained with anti-CD11c-APC and sorted on FACSVantage-SE (BD-Pharmingen, Auckland, New Zealand) for purity routinely above 98%.
Staining of tumor sections
Adult mouse tissue was fixed by cardiac perfusion following CO2 asphyxiation. Briefly, phosphate-buffered saline (PBS) was perfused for 5 min until perfusate was free of blood. The perfusate was then replaced with ice cold 4% paraformaldehyde in PBS (0.15 M, pH 7.4) and perfused for 15 min. Tumors were removed and postfixed in 4% paraformaldehyde for 2–4 h at 4°C. The tissue was then transferred to PBS containing 30% sucrose at 4°C and left overnight. Tumors were stored at −80°C until sectioning. Fixed tumor tissue was mounted in Cryo-M-Bed freezing compound (Bright Instrument Company Ltd, Huntingdon, UK) and 20 μM sections were cut using an HM 500 OM series cryostat microtome (Microm International GmbH, Walldorf, Germany). Frozen sections were mounted directly onto microscope slides and stored at −80°C. Thawed tumor sections were postfixed with acetone for 10 min before staining with antibodies for 30 min at 37°C. As secondary antibody for immunofluorescence stainings, we used Streptavidin-Alexa 555 from Molecular Probes (Invitrogen, Auckland, New Zealand). Stained tumor sections were viewed with an Olympus BX51 immunofluorescence microscope (Olympus, Auckland, New Zealand).
Isolation of T cells
Spleens and lymph nodes were pressed through a cell strainer and red blood cells were lysed with ammonium chloride buffer. Cells were then incubated with anti-CD8 or anti-CD4 mAb conjugated to magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) and positively sorted with Automacs (Miltenyi). Cell purity was routinely over 85%.
In vitro proliferation assays
Graded doses of DCs were incubated with 2 × 105 OVA-specific transgenic T cells for 3 days. Proliferation of T cells was measured by incorporation of 3H-thymidine (activity 1 mCi/ml, Amersham, Aylesbury, UK) during the last 16 h. Data for proliferation assays are expressed as counts per minute (cpm) and represent means of triplicate wells ± SD.
In vivo proliferation assays
T cells were isolated from TCR transgenic OT-I and OT-II mice as described earlier and labelled with 0.2 μM carboxy-fluorescein diacetate, succinimidyl ester (CFSE, Molecular Probes, Invitrogen, New Zealand). CD45.1+ congenic B6-SJ ptrprca mice were injected s.c. with either 105 B16.F1 or B16.OVA melanoma cells, and tumor growth was monitored. On day 10 after tumor cell inoculation, when tumors were around 0.5 cm2, mice received a mix of 2 × 106 CFSE-labelled OT-I T and OT-II cells intraveneously (i.v.). Three days later, lymph nodes were analyzed for T cell proliferation detected by decrease of CFSE fluorescence intensity as described [13]. Injected T cells were identified with CD4-APC (clone GK1.5), CD45.2-PE (clone 104e, eBiosciences, San Diego, CA, USA) and CD8-PerCP-Cy5.5 (clone Ly2, BD-Pharmingen, Auckland, New Zealand). In each of these experiments, three to five mice were used per group and the mean ± SD is shown.
Results
Characterization of leukocyte infiltrate in melanoma
B16 melanoma cells injected into the subcutaneous tissue form progressively growing tumors within 2–3 weeks. We wished to determine what kind of cells infiltrate B16 melanomas. Mice were injected s.c. with 105 B16 melanoma cells, and after 14 days, when tumors had reached a size of 0.5–1.5 cm2, they were excised and digested with collagenase. The tumor cell suspension was analyzed with markers for subpopulations of CD45+ leukocytes. The majority of the leukocytes was MHC-class II+ and represented CD11c− macrophages expressing myeloid markers such as F4/80 and CD11b. A third of the MHC-class II-positive cells were DCs, since they expressed CD11c, and a small percentage of them were B220 positive, presumably representing plasmacytoid DCs (Fig. 1a). The tumor-infiltrating DCs (TIDCs) were mostly positive for CD11b and MHC-class II, and half of them also expressed F4/80, indicating that they were of myeloid lineage. As expected, F4/80 expression on TIDCs was slightly lower than that on macrophages (data not shown). Further analysis demonstrated that, around 30–40% of TIDCs expressed CD40 and CD86 on the cell surface, indicating that they were phenotypically mature (Fig. 1b).
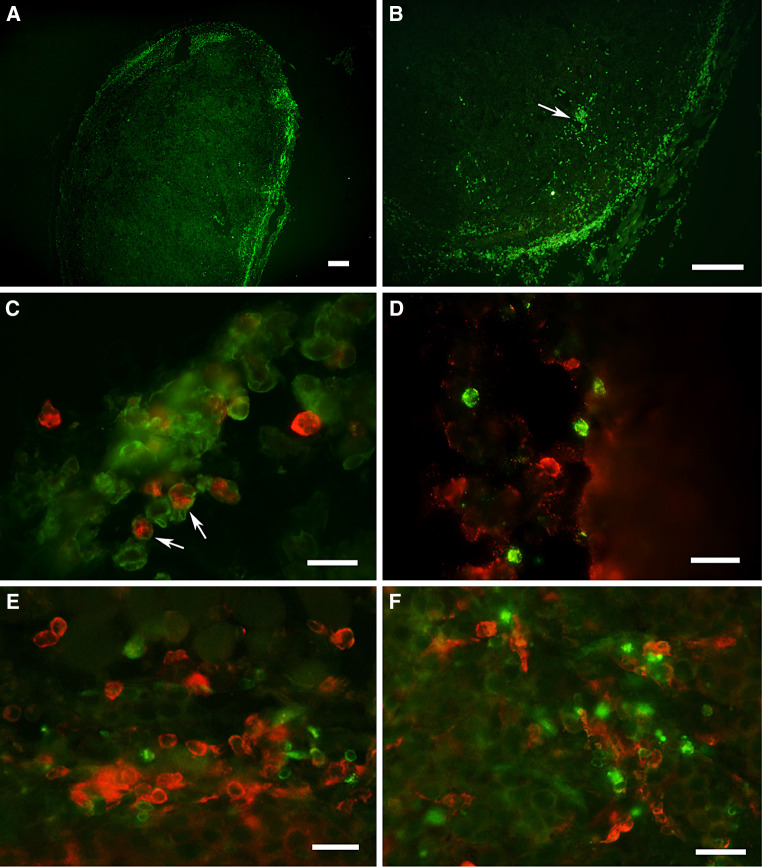
Fig. 1.
Characterization of leukocyte infiltrate in tumors and draining lymph nodes in melanoma-bearing mice. Mice were injected s.c. into the flank with 105 B16.OVA tumor cells. After 10–14 days, tumors had reached a size of 0.5–1.5 cm2 and were excised, digested with collagenase and analyzed by flow cytometry. a CD45+ leukocytes in tumor cell suspensions were gated, and percentages of different cell populations are shown as mean ± SD (summary of five experiments). b Percent expression of lineage and activation markers on CD11c+ TIDCs is shown as mean ± SD (summary of three different experiments). c Tumor-draining and contralateral lymph nodes were excised from mice carrying B16.OVA tumors and compared to control lymph nodes. Cell suspensions were obtained by digestion with collagenase and analyzed by flow cytometry. Total numbers of different cell populations are summarized from four different experiments and are shown as mean ± SD
LCs can infiltrate human melanoma [17, 24]; so we wondered whether LCs are recruited to murine B16 melanoma. In the epidermis overlying the tumors, LCs appeared activated, since they were enlarged and expressed more MHC-class II molecules (data not shown). However, we could hardly find any LCs in the tumor cell suspensions or on tumor sections as judged by staining with the LC-specific marker Langerin (Fig. 1a).
Equal numbers of CD4+ and CD8+ T cells were present in the tumors, and both T cell subsets showed no sign of activation as determined by staining for CD69 (Fig. 1a and data not shown). Around 20% of the tumor-infiltrating leukocytes were B220+ MHC-class II+ B cells.
We observed that tumor-draining lymph nodes were sometimes enlarged; so, we compared cell suspensions from tumor-draining and control lymph nodes. Tumors were injected by subcutaneous injection of 105 B16 melanoma cells into one flank of mice, and 14 days later, the tumor-draining and the contralateral nondraining inguinal lymph nodes were excised. Lymph nodes from nontumor-bearing mice were used as a control. Lymph nodes were digested with collagenase, and cell suspensions were stained with antibodies for CD11c+ DC, Langerin+ LCs, F4/80+ CD11c− macrophages, B220+ B cells, CD4+ T cells and CD8+ T cells. Each leukocyte population tested was found to be equally represented in control and in tumor-draining and nondraining lymph nodes, both in percentage and in absolute cell numbers (Fig. 1c).
Immunofluorescent stainings of tumor sections showed that most of the CD45+ leukocytes were localized in the peripheral area of the tumors, and some were close to blood vessels. The leukocyte infiltrate consisted mainly of MHC-class II+ CD11c− macrophages, which were occasionally found deeper in the tumor tissue. CD11c+ DCs were found in close proximity of CD11c− B220+ B cells, CD4+ and CD8+ T cells and expressed most of their MHC-class II molecules inside the cells indicating a more immature phenotype, though some of it was found on the surface as well. Furthermore, some TIDCs expressed hardly any MHC-class II on tumor sections; however, after isolation from tumors, most of the TIDCs showed surface MHC-class II that was most probably upregulated during the isolation procedure (Fig. 2).
Fig. 2.
Leukocytes are located in the periphery of tumors. Mice were injected s.c. into the flank with 105 B16.OVA tumor cells. Frozen sections from tumors (size 0.5–0.8 cm2) were prepared. Tumor sections were stained with mAb specific for macrophages, DCs, B and T cells, and viewed on a conventional immunofluorescence microscope. a, b Tumor sections stained with anti-CD45 mAb. CD45+ leukocytes are located in the periphery of the tumor. The arrow indicates leukocytes close to blood vessel. Bars in a and b represent 200 μm. c Double staining for CD11c (green fluorescence) and MHC II (red fluorescence). CD11c+ DCs express MHC-class II mostly inside the cells indicating an immature phenotype. MHC-class II-single positive cells could be either macrophages or B cells. d Double staining for CD11c+ DCs (green fluorescence) and B220+ B cells (red fluorescence). e Colocalization of CD4+ T cells (green fluorescence) and CD11c+ DC (red fluorescence) to the same area. f Colocalization of CD8+ T cells (green fluorescence) and CD11c+ DC (red fluorescence). Bars in c–f correspond to 20 μm
Tumor-infiltrating DCs (TIDCs) do not present tumor-derived antigen to T cells in vitro
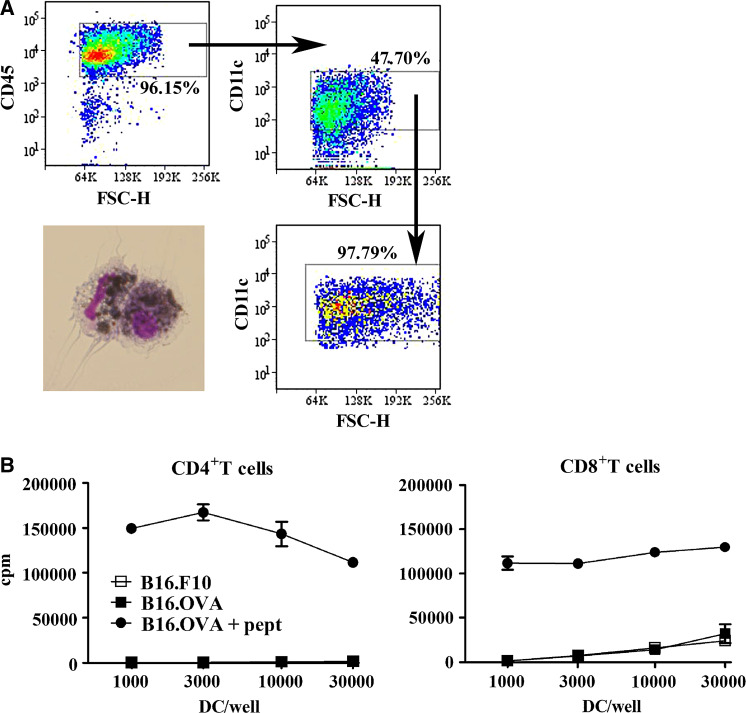
Some of the DCs infiltrating the tumors expressed costimulatory molecules such as CD40 and CD86, and colocalized with T cells. Thus, we asked whether TIDC can present tumor-derived antigen to T cells, either in tumors or in lymph nodes. For these experiments, we used the OVA-expressing B16.OVA tumor [12], and as a negative control the OVA-negative B16.F1 or B16.F10 melanoma cells were used. When tumors had reached a size of 1–1.5 cm2, they were excised and digested with collagenase. CD45+ leukocytes were pre-enriched by magnetic bead sorting, and TIDCs were highly purified by electronic sorting on the basis of CD11c expression. TIDCs had captured antigen from melanoma cells, as shown by melanin uptake in a third of the cells (Fig. 3a). Sorted DCs were cocultured with OVA-specific CD4+ and CD8+T cells, and proliferation of T cells was measured 3 days later. DCs sorted from B16.OVA tumors were unable to stimulate T cell proliferation to a level above that induced by DCs from parental B16 tumors, indicating that TIDCs were unable to present OVA taken up from tumor cell in vivo. The same DCs loaded with OVA peptides induced strong proliferation of both CD4+ and CD8+ T cells in vitro, indicating that they were functional (Fig. 3b). The TLR-4 ligand lipopolysacharide (LPS) added to the proliferation assay to enhance maturation of TIDCs had no effect on the T cell stimulatory capacity of TIDCs (data not shown).
Fig. 3.
TIDCs cannot present tumor-derived antigen in vitro. We injected mice with B16.OVA or control B16.F10, excised the tumors after 14 days (size of tumors 0.5–1.5 cm2) and digested them with collagenase. Cell suspensions were pre-enriched for CD45+ cells by magnetic bead sorting and electronically sorted for CD11c+ DC. a The sorting strategy and purity of TIDCs are shown. TIDCs contain melanin that can be seen as black particles inside the sorted DCs. b Graded doses of tumor-derived DC were cocultured with 2 × 105 OVA-specific CD4+ OT-II and CD8+ OT-I T cells for 3 days, and radioactive thymidine was added during the last 16 h. Results are expressed as counts per minute (cpm). Cultures were set up in triplicate; mean ± SD are shown. Results are from one of four experiments that gave similar results
Tumor-derived antigen is presented in tumor-draining lymph nodes
TIDCs were unable to present tumor-derived antigen in vitro despite capture of melanin from tumor cells. We examined whether presentation of tumor-derived antigen occurred in tumor-draining lymph nodes. CD45.1+ mice were injected with either B16.F1 or B16.OVA in the flank. On day 10, after tumor cell injection, when tumors were still small, mice were injected i.v. with OVA-specific CD4+ and CD8+ T cells (CD45.2+) labelled with CFSE. Tumor-draining and contralateral nondraining inguinal lymph nodes were examined for proliferation of T cells 3 days later. T cell proliferation just occurred in the tumor-draining lymph nodes and never in nondraining ones. CD8+ T cells proliferated in mice bearing B16.OVA tumors; however, CD4+ T cell proliferation was never detectable. No proliferation of OVA-specific T cells was observed in mice carrying control B16.F1 tumors (Fig. 4).
Fig. 4.
Tumor-derived antigen is presented to CD8+ T cells in tumor-draining lymph nodes in vivo. CD45.1+ congenic mice were injected subcutaneously into one flank with 105 B16.OVA or control B16.F1 tumor cells. Ten days later, CFSE-labelled OVA-specific CD4+ OT-II and CD8+ OT-I T cells were adoptively transferred into tumor-bearing mice. The tumor-draining and contralateral nondraining lymph nodes were examined for the presence of proliferating T cells 3 days later. a shows complete data for all mice in one experiment are shown. The percent proliferating T cells was determined as shown in b. b Histograms for CFSE-proliferation of CD45.1+ cells from one representative mouse from each group are shown. Each symbol represents one mouse; means are indicated by horizontal bars. Data are from one of three experiments that gave similar results
Discussion
DCs loaded with tumor material are widely used for immunotherapy against cancer. The rationale behind it is that DCs can infiltrate tumors and are involved in immune responses against tumors. However, tumors possess many mechanisms to evade an immune response. In the present study, we demonstrate that murine-transplantable melanoma growing in the subcutis contains a mixture of mature and immature DCs as well as macrophages, T and B cells. DCs isolated from tumors were compromised in their ability to present tumor-derived antigen to T cells in vitro, although they were functional as demonstrated by efficient presentation of peptides. Tumor-derived antigen was presented to CD8+ T cells in tumor-draining lymph nodes but not at distant sites. Antigen-specific CD4+ T cells were unable to proliferate in responses to growing tumors, suggesting a lack of CD4 help to generate cytotoxic antitumor responses. In conclusion, some tumor antigen presentation occurs in the lymphatic tissue draining the tumor, but most probably not in tumors themselves.
Tumor-infiltrating DCs are inefficient in presenting tumor antigens
To generate immune responses against cancer, tumor-derived antigen must be presented by antigen-presenting cells. We were interested in whether DC-infiltrating murine B16 melanoma would be able to capture and present tumor antigen. The leukocyte infiltrate in B16 tumors consisted mainly of macrophages, and they resided predominately in the periphery of the tumors; however, occasionally, we found macrophages infiltrating deeper into the tissue of the tumors. CD11c+ TIDCs were restricted to the same peripheral area and colocalized with B and T cells. TIDC in the tumor tissue expressed low amounts or intracellular MHC-class II indicating a more immature phenotype; however, some of them showed extracellular expression. We occasionally found CD86-positive TIDCs in the tumor tissue. After isolation from the tumors, most TIDCs showed MHC-class II molecules on the surface, indicating an upregulation during the isolation procedure. Findings about the maturation state of TIDCs in melanoma are somehow contradictory. One study about murine B16.F0 melanoma demonstrated that part of the TIDC isolated displayed a mature phenotype [26]. In contrast, in diverse spontaneous and transplantable murine B16 melanoma models not part but all DC infiltrating tumor tissue expressed a mature phenotype [22]. Analysis of B16.OVA melanoma cell suspensions in our study confirmed that most of the TIDCs expressed MHC-class II and myeloid markers, and that a third of the TIDCs coexpressed CD40 and CD86. Highly purified TIDCs showed incorporation of tumor material as judged by capture of melanin; however, they could not stimulate proliferation of antigen-specific CD4 and CD8+ T cells in vitro. When TIDCs were loaded with OVA-peptides in vitro, they induced efficient proliferation of both T cell subsets, indicating that they were functional and able to interact with T cells. Recently, a report demonstrated that TIDCs were capable of stimulating proliferation of T cells after loading with a protein antigen and could inhibit tumor growth, though the ability of TIDC to present tumor-derived antigen was not formally tested in the study [22]. Moreover, TIDCs isolated from colon carcinoma were able to stimulate cytotoxic T cells, suggesting some presentation of tumor antigens; however, activation of those TIDCs with TLR ligands and antibodies against IL-10 was required for efficient tumor immunity [26]. Transfection of colon carcinoma tumor cells with granulocyte/macrophages colony-stimulating factor (GM-CSF) induced a mature phenotype in all tumor-infiltrating DCs, which contained apoptotic bodies and were able to stimulate interferon (IFN)-γ production in cytotoxic T cells [5]. A recent report confirmed that TIDCs might have some antigen-presenting ability, since melanoma-infiltrating DCs were able to protect naïve mice against a lethal challenge with tumor cells [21]. One critical factor for antigen presentation by TIDC might be their maturational status. In our study, the addition of the TLR-4 ligand LPS could not overcome the inability of TIDCs to present tumor antigen. Thus, it seems unlikely that tumor-infiltrating DCs possess the ability to present tumor-derived antigen in situ. However, external factors such as inflammatory stimuli and growth factors might help TIDCs to regain their function.
Anti-tumor responses are detectable in tumor-draining lymph nodes
Development of cytotoxic activity against tumor cells is a prerequisite for the development of tumor immunity. Lymph nodes draining progressively growing tumors were sometimes enlarged, but analysis of their cellular composition revealed no changes in numbers or percentages of T cells, B cells or DC populations. By using the model tumor antigen OVA, we detected proliferation of CD8+ T cells in the tumor-draining lymph nodes but never in distant lymphatic tissues. In contrast to CD8+ T cells, adoptively transferred CD4+ T cells did not proliferate at all. Naïve OVA-specific CD8+ T cells transferred into mice, before tumor cell injection, did not inhibit tumor growth, and their numbers in blood remained unchanged indicating no extensive T cell expansion when tumors were developing (data not shown), suggesting that the proliferation observed in draining lymph nodes was ineffectual. Other studies have reported that tumor antigens are presented exclusively in the tumor-draining lymph nodes to CD4+ and CD8+ T cells. The level of proliferation depends on the availability of tumor antigen so that the size and level of expression determines the extent of CD8+ T cells proliferation [15, 23]. Helper CD4+ T cells are required for efficient and long-lasting immune responses against tumors, since they help CD8+ T cells to develop into effective cytotoxic T cells. Cotransfer of tumor-specific CD4+ T cells increases the proliferation of CD8+ T cells and maintains the cytotoxic T cell responses [14, 19]. Impaired proliferation of CD4+ T cells might cause inefficient activation of CD8+ T cells leading to progressive tumor growth as described by other authors [14]. The most likely cell type presenting tumor antigen to T cells in the lymphatic tissue are the DCs. When we isolated DCs from tumor-draining lymph nodes, they stimulated T cell proliferation in vitro; however, results between different experiments were variable possibly due to low expression of the tumor model antigen and the low numbers of DCs presenting the antigen in the lymph node (data not shown). The B16 melanoma cell line used in our study expressed low to undetectable levels of MHC-class I and II molecules (data not shown), suggesting that direct antigen presentation by tumor cells was unlikely. Several reports have clearly demonstrated that bone marrow-derived lymph node DCs cross-present tumor antigen to CD8+ T cells in vitro and in vivo [8, 25]. A recent study using B16 melanoma-expressing OVA showed the same inability of CD4+ T cells to proliferate in tumor-draining lymph nodes. The cells presenting the tumor model antigen were identified to be bone-marrow-derived cells. The inhibition of CD4+ T cell responses could neither be overcome by inducing cell death in tumor cells to increase release of tumor antigen nor by injection of DC maturation stimuli intratumorally. As a consequence of lacking CD4-help, antigen-specific cytotoxicity could not be measured in tumor-bearing mice and CD8+ T cell anergy developed [21].
The environment in tumors is very hostile for the induction of effective immune responses. Immunosuppressive cytokines, such as IL-10 or gangliosides, are secreted by melanoma and inhibit maturation and induce apoptosis in DCs and LCs [3, 20]. Accumulation of immature DCs [20] and myeloid-derived suppressor cells [18] suppresses CD8+ T cell responses and induces anergy. Another tumor-independent aspect one should be aware of is the differential efficiency of DCs to present cell-associated antigen. The tumor model antigen in our system is expressed in the cytoplasm of the melanoma cells. It has been reported that, compared to soluble OVA, cell-associated OVA is much more efficiently cross-presented on the MHC I of host APC, while presentation on MHC II differs less profoundly. This may explain why in tumour-bearing mice CD8+ T cells frequently become activated, while CD4+ T cells fail to respond [11].
In conclusion, DCs but not LCs infiltrated transplantable melanomas, colocalized with T cells at the periphery of tumors, and in some cases showed a mature phenotype. Nonetheless, DCs isolated from tumors were unable to stimulate antigen-specific T cell proliferation in vitro. Tumor-specific CD8+ T cells, but not CD4+ T cells, were able to proliferate in lymph nodes draining the tumor site, demonstrating that tumor antigen was presented, although inefficiently, in the draining lymph node. The lack of CD4 help is likely to contribute to the inability of CD8+ T cells to develop into long-lived cytotoxic effector cells in tumor-bearing mice, and to their inability to control tumor growth.
Acknowledgments
We thank Drs E. Lord, J.G. Frelinger and F. Carbone for generously providing cell lines and mouse strains used in this study, the staff of the Malaghan Experimental Research Facility for animal husbandry and care, and the staff of the Malaghan Institute for useful suggestions and discussion. This work was supported by research grants from the Health Research Council and Cancer Society of NZ, the Wellington Medical Research Foundation, and the Genesis Oncology Trust. PS was supported by the Erwin Schroedinger Auslandsstipendium from the Austrian Science Fund (FWF-J2479).
Abbreviations
- DCs
Dendritic cells
- TIDC
Tumor-infiltrating DC
- LCs
Langerhans cells
- OVA
Ovalbumin
- TCR
T cell receptor
- mAb
Monoclonal antibody
- APC
Allophycocyanin
- FITC
fluorescein isothiocyanate
- PE
Phycoerythrin
- PBS
Phosphate-buffered saline
- s.c.
Subcutaneously
- cpm
Counts per minute
- CFSE
Carboxy-fluorescein diacetate succinimidyl ester
- i.v.
Intraveneously
- PerCP
Peridinin chlorophyll A protein
- GM-CSF
Granulocyte/macrophages colony-stimulating factor
- LPS
Lipopolysacharide
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s00262-008-0538-x
References
- 1.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 2.Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA, Banchereau J. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190:1417–1426. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennaceur K, Popa I, Portoukalian J, Berthier-Vergnes O, Peguet-Navarro J. Melanoma-derived gangliosides impair migratory and antigen-presenting function of human epidermal Langerhans cells and induce their apoptosis. Int Immunol. 2006;18:879–886. doi: 10.1093/intimm/dxl024. [DOI] [PubMed] [Google Scholar]
- 4.Berthier-Vergnes O, Gaucherand M, Peguet-Navarro J, Plouet J, Pageaux JF, Schmitt D, Staquet MJ. Human melanoma cells inhibit the earliest differentiation steps of human Langerhans cell precursors but failed to affect the functional maturation of epidermal Langerhans cells. Br J Cancer. 2001;85:1944–1951. doi: 10.1054/bjoc.2001.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiodoni C, Paglia P, Stoppacciaro A, Rodolfo M, Parenza M, Colombo MP. Dendritic cells infiltrating tumors cotransduced with granulocyte/macrophage colony-stimulating factor (GM-CSF) and CD40 ligand genes take up and present endogenous tumor-associated antigens, and prime naive mice for a cytotoxic T lymphocyte response. J Exp Med. 1999;190:125–133. doi: 10.1084/jem.190.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 7.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 8.Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 9.Ishida T, Oyama T, Carbone DP, Gabrilovich DI. Defective function of Langerhans cells in tumor-bearing animals is the result of defective maturation from hemopoietic progenitors. J Immunol. 1998;161:4842–51. [PubMed] [Google Scholar]
- 10.Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002;51:293–298. doi: 10.1007/s00262-002-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Davey GM, Sutherland RM, Kurts C, Lew AM, Hirst C, Carbone FR, Heath WR. Cell-associated ovalbumin is cross-presented much more efficiently than soluble ovalbumin in vivo. J Immunol. 2001;166:6099–6103. doi: 10.4049/jimmunol.166.10.6099. [DOI] [PubMed] [Google Scholar]
- 12.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 13.Lyons AB. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Methods. 2000;243:147–154. doi: 10.1016/S0022-1759(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 14.Marzo AL, Kinnear BF, Lake RA, Frelinger JJ, Collins EJ, Robinson BW, Scott B. Tumor-specific CD4+ T cells have a major “post-licensing” role in CTL mediated anti-tumor immunity. J Immunol. 2000;165:6047–6055. doi: 10.4049/jimmunol.165.11.6047. [DOI] [PubMed] [Google Scholar]
- 15.Marzo AL, Lake RA, Lo D, Sherman L, McWilliam A, Nelson D, Robinson BW, Scott B. Tumor antigens are constitutively presented in the draining lymph nodes. J Immunol. 1999;162:5838–5845. [PubMed] [Google Scholar]
- 16.McLellan AD, Kapp M, Eggert A, Linden C, Bommhardt U, Brocker EB, Kammerer U, Kampgen E. Anatomic location and T-cell stimulatory functions of mouse dendritic cell subsets defined by CD4 and CD8 expression. Blood. 2002;99:2084–2093. doi: 10.1182/blood.V99.6.2084. [DOI] [PubMed] [Google Scholar]
- 17.Movassagh M, Spatz A, Davoust J, Lebecque S, Romero P, Pittet M, Rimoldi D, Lienard D, Gugerli O, Ferradini L, Robert C, Avril MF, Zitvogel L, Angevin E. Selective accumulation of mature DC-Lamp+ dendritic cells in tumor sites is associated with efficient T-cell-mediated antitumor response and control of metastatic dissemination in melanoma. Cancer Res. 2004;64:2192–2198. doi: 10.1158/0008-5472.CAN-03-2969. [DOI] [PubMed] [Google Scholar]
- 18.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ossendorp F, Toes RE, Offringa R, van der Burg SH, Melief CJ. Importance of CD4(+) T helper cell responses in tumor immunity. Immunol Lett. 2000;74:75–79. doi: 10.1016/S0165-2478(00)00252-2. [DOI] [PubMed] [Google Scholar]
- 20.Pinzon-Charry A, Maxwell T, Lopez JA. Dendritic cell dysfunction in cancer: a mechanism for immunosuppression. Immunol Cell Biol. 2005;83:451–461. doi: 10.1111/j.1440-1711.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 21.Preynat-Seauve O, Contassot E, Schuler P, Piguet V, French LE, Huard B. Extralymphatic tumors prepare draining lymph nodes to invasion via a T-cell cross-tolerance process. Cancer Res. 2007;67:5009–5016. doi: 10.1158/0008-5472.CAN-06-4494. [DOI] [PubMed] [Google Scholar]
- 22.Preynat-Seauve O, Schuler P, Contassot E, Beermann F, Huard B, French LE. Tumor-infiltrating dendritic cells are potent antigen-presenting cells able to activate T cells and mediate tumor rejection. J Immunol. 2006;176:61–67. doi: 10.4049/jimmunol.176.1.61. [DOI] [PubMed] [Google Scholar]
- 23.Robinson BW, Lake RA, Nelson DJ, Scott BA, Marzo AL. Cross-presentation of tumour antigens: evaluation of threshold, duration, distribution and regulation. Immunol Cell Biol. 1999;77:552–558. doi: 10.1046/j.1440-1711.1999.00876.x. [DOI] [PubMed] [Google Scholar]
- 24.Tefany FJ, Barnetson RS, Halliday GM, McCarthy SW, McCarthy WH. Immunocytochemical analysis of the cellular infiltrate in primary regressing and non-regressing malignant melanoma. J Invest Dermatol. 1991;97:197–202. doi: 10.1111/1523-1747.ep12479662. [DOI] [PubMed] [Google Scholar]
- 25.van Mierlo GJ, Boonman ZF, Dumortier HM, den Boer AT, Fransen MF, Nouta J, van der Voort EI, Offringa R, Toes RE, Melief CJ. Activation of dendritic cells that cross-present tumor-derived antigen licenses CD8+ CTL to cause tumor eradication. J Immunol. 2004;173:6753–6759. doi: 10.4049/jimmunol.173.11.6753. [DOI] [PubMed] [Google Scholar]
- 26.Vicari AP, Chiodoni C, Vaure C, Ait-Yahia S, Dercamp C, Matsos F, Reynard O, Taverne C, Merle P, Colombo MP, O’Garra A, Trinchieri G, Caux C. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J Exp Med. 2002;196:541–549. doi: 10.1084/jem.20020732. [DOI] [PMC free article] [PubMed] [Google Scholar]