Abstract
Pericardial effusion (PE) and cardiac tamponade caused by malignant pericarditis are critical conditions in cancer patients, which still lack a recommended protocol for their long-term management. Percutaneous pericardiocentesis and simple drainage are commonly performed as the initial treatment. The aims of this study were to investigate the presence of cytotoxic T lymphocytes (CTLs) in malignant PE and to determine the clinical response to administering autologous tumor-infiltrating lymphocytes (TILs) into the pericardial cavity. Initially, we identified human lymphocyte antigen class-I-restricted and tumor-specific CTLs within the interleukin-2 (IL-2)-activated TILs in PEs from four patients, on the basis of interferon-γ production and lactate dehydrogenase-release assays. Clinically we observed favorable responses to the pericardial transfer of IL-2-activated autologous TILs in four patients: one male with advanced esophageal cancer, one female with recurrent lung cancer and two females with recurrent breast cancer, respectively. Autologous TILs from PEs were expanded in vitro with IL-2, characterized for CD3, CD4 and CD8 markers, checked for contamination and then infused into the patient’s pericardial space through a catheter. This was repeated biweekly. After treatment, there were no signs of recurrence of PE in either case, as determined by radiography, echocardiography and computed tomography. The only adverse effects seen were grade 1 fevers. These results suggested that intrapericardial cellular immunotherapy with autologous TILs could be a safe and effective treatment for controlling malignant pericarditis with associated cardiac tamponade, and that tumor-specific CTLs present in malignant PE might be important for tumor rejection.
Keywords: Immunotherapy, Malignant pericardial effusion, Tumor-infiltrating lymphocytes (TIL)
Introduction
Malignant pericarditis is critically associated with pericardial effusion (PE), which causes cardiac tamponade [1]. This condition is a life-threatening complication of many advanced malignancies, such as lung, breast and esophageal cancers. Untreated malignant pericarditis causes severe complication or death in patients with symptomatic PEs. Effective therapy for malignant pericarditis could significantly improve life expectancy, palliative care and quality of life for these patients. Attempts to administer chemotherapeutic and sclerosing agents intrapericardially [2–4] have been associated with adverse effects, such as high fever, arrhythmia, myelosuppresion and severe nausea, and in most cases the effusions have recurred. Several reports have demonstrated the presence of cytotoxic T lymphocytes (CTLs) at various tumor sites, as well as their clinical efficacy as immunotherapy in advanced cancer patients [5–7]. However, adoptive cellular immunotherapy using tumor-infiltrating lymphocytes (TILs) for malignant PE has not yet been reported. We have shown that the repeated transfer of autologous tumor-stimulated T cells into tumor sites can be effective not only as a local adoptive immunotherapy for the target tumor, but also in spreading the immune response systemically to distant metastatic tumor sites [6, 8]. In this study, we therefore extended the application of local adoptive immunotherapy using autologous interleukin-2 (IL-2)-activated TILs to treat carcinomatous pericarditis arising in advanced cancer.
Materials and methods
Patients, cell lines and autologous tumor cells
Six patients with refractory, advanced or recurrent lung, breast or esophageal cancer, with PE and pericarditis, gave written informed consent before entering the study. The profiles and characteristics of the patients are summarized in Table 1. All subjects had previously received standard radiation or chemotherapy for their primary disease. Other eligibility and exclusion criteria for the patients were similar to those used in our earlier phase I/II study, as approved by the Institutional Review Board of Kurume University Hospital [6]. These patients had not received any immunotherapy before this study. Prior to undergoing pericardial drainage, all patients had complained of weakness, dyspnea and chest discomfort. According to echocardiography, all six patients presented with large PEs (>3 cm) and showed one or more additional criteria for cardiac tamponade. In electrocardiograms (ECGs), all patients showed sinus tachycardia and low voltages in the precordial leads. Electrical alternans was present in two patients (Cases 3 and 4) and large amounts of PE were seen in chest X-rays and/or computed tomography (CT) scans of Cases 1, 2, 5 and 6 (Figs. 3a-1, b-1, 5a-1, a-2, 6a-1). None of the patients manifested cardiac rhythm disturbances.
Table 1.
Patients profile and summary of interleukin-2 (IL-2)-activated tumor-infiltrating lymphocyte (TIL) from patients with carcinomatous pericarditis
| Cases | Sex/age | Origin of primary cancer | Histology | Clinical state of cancer | HLA class-I A locus | Total no. of injected cells | Clinical response in pericardial effusion | Adverse effecta | Period of remission (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M/63 | Esophagus | SCC | Ad. Ca. (stage IV) | 26/- | 12.6×109 | Regression | Grade 1 (fever) | 15 |
| 2 | F/63 | Lung | Adeno. | Recurrence | 24/33 | 2.9×109 | Regression | Grade 1 (fever) | 7 |
| 3 | F/59 | Esophagus | SCC | Recurrence | 26/24 | – | Progression | – | <1 |
| 4 | M/67 | Lung | Adeno. | Recurrence | 24/- | – | Progression | – | <1 |
| 5 | F/53 | Breast | Papi. tubu. adeno. | Recurrence | 26/2 | 1.8×109 | Regression | Grade 1 (fever, tachycardia) | 6 |
| 6 | F/58 | Breast | Papi. tubu. adeno. | Recurrence | 24/2 | 3.2×109 | Regression | Grade 1 (fever) | >11 |
SCC squamous cell carcinoma; Adeno. adenocarcinoma; Papi. tubu. adeno. papillotubular adenocarcinoma; Ad. Ca. advanced cancer staging based on TNM classification
aCommon Toxicity Criteria of National Cancer Institute
Fig. 3.
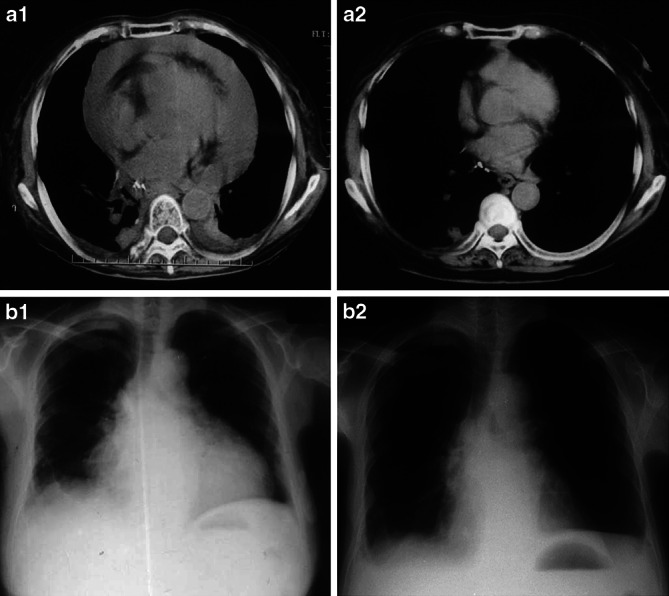
Clinical assessment of adoptive immunotherapy. Chest computed tomography (CT) scans of Case 1 are shown before (a-1) and after (a-2) treatment. X-rays of Case 2 are shown before (b-1) and after (b-2) treatment. In both patients, the regression of pericardial effusion (PE) after treatment was marked
Fig. 5.
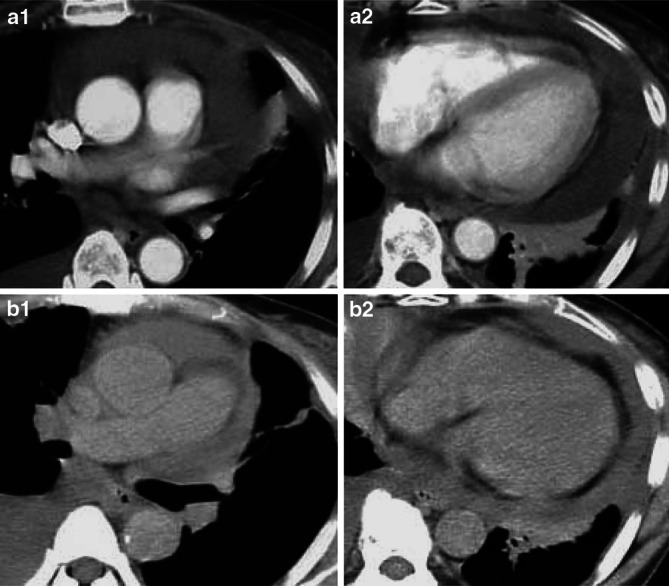
Clinical assessment of adoptive immunotherapy in Case 5. Chest CT scans are shown before (a1, a2) and after (b1, b2) treatment. A reduction of PE after treatment was marked in the patient
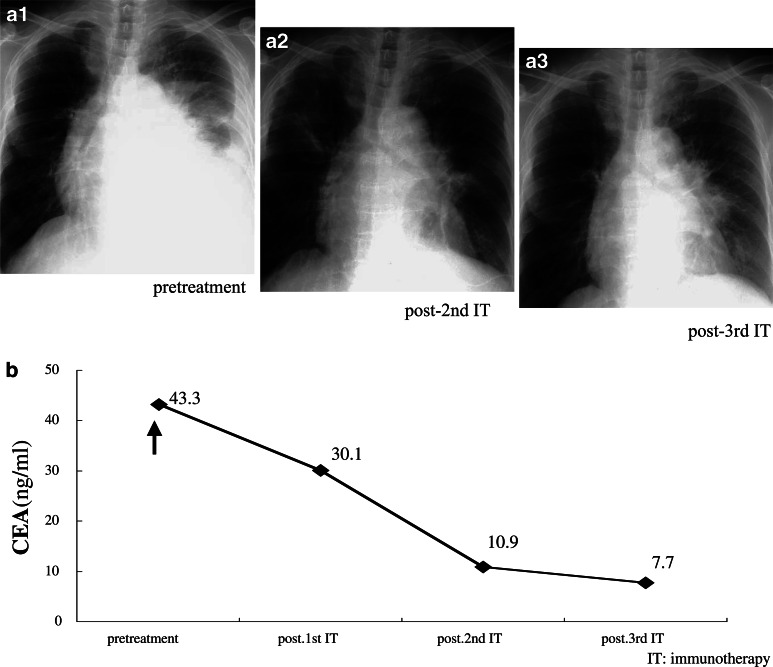
Fig. 6.
Clinical assessment of adoptive immunotherapy in Case 6. X-rays of Case 6 are shown before (a1) and after (a2, a3) treatment. A regression of PE after treatment was marked in the patient. The level of CEA decreased significantly during treatment (b)
Pericardial effusions were collected from all six patients and cytopathology identified neoplastic cells in the pericardial fluid in every case. Cases 1, 2, 5 and 6 passed the eligibility criteria for enrollment into the clinical study and received adoptive immunotherapy with pericardial TILs. The intervals between previous radio- or chemotherapy and the experimental immunotherapy were at least 1 month. But two patients, Cases 3 and 4 with electrical alternans in ECGs were eliminated before treatment because of their poor performance statuses (<40–50% Karnofsky’s score) and inadequate growth of cultured TILs; however, their IL-2-activated TILs were tested for CTL activity in vitro.
Case 1 was a 63-year-old male with advanced invasive cancer of the middle thoracic esophagus, which was inoperable. However, a total dose of local external radiotherapy of 60 Gy had previously had a dramatic effect on the primary tumor, resulting in nearly complete remission (CR), and the symptoms of dysphagia and chest pain due to the primary tumor had almost disappeared. Case 2 was a 63-year-old female with recurrent postoperative carcinomatous peripleuritis, and had failed two cycles of chemotherapy with vindesine and cisplatin. Cases 5 and 6 were 53-year-old and 58-year-old females with recurrent breast cancer postoperatively; both of them showed no expression for hormone receptors of estrogen and/or progesterone, and also no expression for Her2/neu receptor. They failed a series of the standard radiotherapy (50–60 Gy) and chemotherapy. Case 5 had received three cycles with cyclophosphamide, methotrexate and 5FU followed by six cycles of adriamycin and epirubicin hydrochloride. Futhermore, she also failed an additional chemotherapy with capecitabine. Case 6 had failed 11 cycles of docetaxel hydrate combined with doxifluridine and 6 cycles of paclitaxol plus capecitabine.
The tumor cell lines used in this study have been described previously [8]. Their human lymphocyte antigen (HLA) class-I genotypes and those of their peripheral blood mononuclear cells (PBMCs) were determined by the polymerase chain reaction sequence-specific oligonucleotide probe (PCR-SSOP) method, as reported elsewhere [9]. Where genotyping data were not available, serological HLA class-I typing for PBMCs, determined using HLA Monoclonal Reagent (One Lambda, Canoga Park, CA, USA) was used.
Autologous tumor cells were separated from PEs using the Ficoll-Hypaque method [10, 11] and identified cytologically. These cells were cryopreserved in 90% human AB serum (Blood Center of the Japanese Red Cross) plus 10% dimethylsulfoxide (Sigma Chemical Co., St. Louis, MO, USA) at −178°C in liquid nitrogen for use in the subsequent immunological assays. After cryopreserved fresh tumor cells were thawed and cultured for 2–3 days, they were utilized as target cells in experimental assays.
TIL preparation and expansion
Pericardial effusions were drained from patients through pericardiac catheters and applied to Ficoll-Hypaque solution, in order to separate the TILs from aggregates, as reported previously [10, 11]. Approximately 1×106 effusion-associated TILs per well were expanded over 2 weeks (mean 14.5 days) in a 24-well culture plate at 37°C in 5% CO2. The culture medium used was RPMI-1640 (Gibco Brl, Grand Island, NY, USA) with 10% heat-inactivated human AB serum, 0.1 μM MEM nonessential amino-acid solution (Gibco Brl), 100 U/ml recombinant IL-2 (Chiron B, Amsterdam, The Netherlands), 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml gentamicin and 0.5 μg/ml fungizone. The cultured TILs were checked for contamination by bacteria or by virus before being used for immunotherapy, in accordance with our university’s guideline for cellular therapy. The TIL viability of each culture was >95%, as evaluated by trypan blue dye exclusion. These IL-2-activated TILs were also cryopreserved, as described above, for further experimental assays.
Assays
The surface phenotype of the proliferating TILs was assessed by flow cytometry (FACScan, BD Biosciences, Mountainview, CA, USA) using anti-CD3, anti-CD4 and anti-CD8 monoclonal antibodies (MAbs), as reported previously [12]. The cytotoxic activity of IL-2-activated TILs was assessed in this study by measuring the increase in interferon-γ (IFN-γ) production elicited by autologous tumor cells compared with allogeneic tumor cell lines. Frozen PE-associated tumor cells were thawed and cultured for 2–3 days, before they were used as target cells in these assays. IFN-γ levels in cell-free supernatants were measured using IFN-γ ELISA kits (BioSource International Inc., Camarillo, CA, USA), which had a limit of sensitivity of 5 pg/ml, as reported previously [12]. Cell-mediated cytotoxicity was measured using lactate dehydrogenase (LDH) activity in the CytoTox 96® assay system (Promega Corp., Madison, MI, USA). These cytotoxicity assays used a constant number of target cells (1x105 cell/well) and varying numbers of effector IL-2-activated TILs (at effector-to-target (E/T) ratios of 10:1, 20:1 and 40:1) in triplicate wells. After incubation for 4 h, 100 μl aliquots of supernatants from the effector and target cell mixtures were transferred to 50 μl LDH substrate in the enzymatic assay plates, and the optical absorbance at 490 nm was recorded. The percentage-specific cytotoxicity was calculated using the following formula: % cytotoxicity = 100 × [(experimental − effector spontaneous − target spontaneous) OD/(target maximum − target spontanous) OD]. Four esophageal squamous cell carcinoma (SCC) lines (KE-3, KE-4, TE-9 and TE-10), one head and neck SCC cell line (KUMA-1), five lung adenocarcinoma cell lines (11–18, PC-9, A549, 1–87 and LC1) and one leukemia cell line (K562) were used as target cells. Anti-class-I [W6/32, immunoglobulin (Ig)G2a], anti-class-II (H-DR-1, IgG2a) and anti-CD4 (IgG1) MAbs (Cosmo Bio Co., Ltd, Tokyo, Japan) were used at a concentration of 10 μg/ml to inhibit cytotoxicity, as reported previously [9]. Anti-CD14 MAb (IgG2a) was used as a control antibody. The Student’s two-tailed t test was used for statistical analysis in this study.
Procedure and treatment method
Patients were monitored echocardiographically while percutaneous pericardiocentesis was performed. Briefly, a 6.5 Fr catheter (Unichika Inc., Hyogo, Japan) was inserted percutaneously into the pericardial sac using a sterile subxiphoid approach. The catheter was then connected to a sterile catheter system with a three-way stopcock and left in place to drain. After initial drainage, the catheter was left in place in the pericardial sac until the administration of autologous TILs on day 14. The expanded IL-2-activated TILs were washed three times in PBS, resuspended in 20 ml of 0.9% saline and introduced through the catheter. The pericardial drainage and administration of IL-2-activated TILs were repeated at 2-weekly intervals until no fluid reaccumulated, as assessed by echocardiography. After administering the IL-2-activated TILs the catheter was closed and kept in sterile conditions for next treatment. The clinical response was evaluated at 4-weekly intervals by chest X-ray, echocardiography, CT scan and serum carcinoembryonic antigen (CEA) concentrations. And treatment outcome of malignant PE was evaluated and successful control was defined as follows: (1) reduction or resolution of the effusion for a minimum of 30 days, (2) the absence of symptoms and (3) no requirement for pericardiocentesis within 30 days of initiation of treatment [13]. Survival time was calculated from the date of first instillation of the TILs transfer to the date of death or most recent follow-up. There was no other additional therapy given to these patients during or after this immunotherapy. Adverse effects were evaluated from patient histories and physical examinations, and were graded according to the National Cancer Institute Common Criteria (NCI-CTC).
Results
Laboratory markers
To test whether tumor-specific CTL precursors were present in TILs from malignant PE, we measured IFN-γ production and LDH release by IL-2-activated TILs in the presence of tumor cell lines. After a 2-week culture, the amounts of CD8+ IL-2-activated TILs were 73, 58, 53 and 69% for Cases 1 to 4, respectively. The production of IFN-γ by IL-2-activated TILs from these four patients, in response to autologous tumor cells and various allogeneic tumor cells, were measured (Table 2). All four cases showed a similar pattern of IFN-γ production. The IL-2-activated TILs from Case 1 (HLA class-I-A26/-) and Case 3 (HLA class-I-A26/A24) produced significant levels of IFN-γ in response to autologous tumor cells (325 and 258 pg/ml respectively), and to allogeneic locus-matched tumor cells, KE-4 (356 and 286 pg/ml respectively) and Kuma-1 (199 and 258 pg/ml respectively). By contrast, the IL-2-activated TILs from both these patients produced <70 pg/ml of IFN-γ in response to the 14 other target cells. Moreover, the IL-2-activated TILs from Case 2 (HLA class-I-A24/A33) and Case 4 (HLA class-I-A24/-) produced significant levels of IFN-γ in response to autologous tumor cells (609 and 221 pg/ml respectively) and allogenic, HLA-A-matched 11–18 and PC-9 tumor cells (171 and 621 pg/ml in Case 2, 190 and 209 pg/ml in Case 4). By contrast, activated TILs from Cases 2 and 4 produced only <50 ng/ml of IFN-γ in response to HLA-A locus-unmatched adenocarcinoma cells including A549, 1–87, LC1 and K562 cells (Table 2).
Table 2.
Interferon-γ (INF-γ) production by IL-2-activated TIL in patients with carcinomatous pericarditis
| IFN-γ production (pg/ml) | HLA class-I | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Histology | Stimulator (cell line) | Origin | Case 1 | Case 2 | Case 3 | Case 4 | A | B | Cw |
| Autologous tumor cells | Malignant pericardial effusion | 325 | 609 | 258 | 221 | ||||
| Squamous cell carcinoma | KE-4 | Esophagus | 356 | <5 | 286 | 16 | 2402/2601 | 4001/5102 | 0101/030 |
| KE-3 | Esophagus | 7 | <5 | 29 | <5 | 0206/2402 | 15/53 | 0401/150 | |
| TE-9 | Esophagus | 12 | 20 | <5 | <5 | 3302/- | 1503/- | ||
| TE-10 | Esophagus | 32 | <5 | <5 | 14 | 2/2402 | 51/55 | 0302/010 | |
| Kuma-1 | Head and neck | 199 | 38 | 261 | 10 | 2603/3302 | 1501/44031 | 0303/140 | |
| RERF-LC-A1 | Lung | <5 | <5 | 12 | ND | 2402/- | 5201/- | 1202/- | |
| LC-1 sq | Lung | 7 | <5 | 31 | <5 | 11/2402 | 44031/- | 1203/160 | |
| Adenocarcinoma | A549 | A549 | Lung 21 | 12 | <5 | <5 | 2602/3001 | 1801/44031 | 1203/160 |
| 1–87 | Lung | <5 | <5 | 38 | ND | 0207/1101 | 4601/5401 | 0102/- | |
| 11–18 | Lung | 7 | 171 | <5 | 190 | 0201/2402 | 5201/5401 | 0102/120 | |
| LC-1 | Lung | <5 | <5 | ND | 14 | 3101/3302 | 1511/44031 | 0303/140 | |
| PC-9 | Lung | 67 | 621 | 22 | 209 | 0206/2402 | 0702/5502 | 0303/070 | |
| MKN-28 | Stomach | <5 | <5 | <5 | <5 | 3101/- | 5101/- | 0304/- | |
| COLO-201 | Colon | <5 | 21 | <5 | 40 | 0101/0201 | 0702/0801 | 0701/070 | |
| Panc-1 | Pancreas | <5 | <5 | <5 | <5 | 0201/1101 | 3801/- | 1203/- | |
| Leukemia | K562 | Erythroblast | 25 | 50 | 31 | 20 | |||
IL-2-activated TIL (5x105 cells) were incubated with the stimulators (1x105 cells) for 18 h, and the INF-γ activity in the supernatant was measured using an ELISA kit
ND not determined
Specific cytotoxicity was also measured by LDH-release for Cases 1 and 2, as larger numbers of effector cells were available from these patients (Fig. 1). IL-2-activated TILs from Case 1 (HLA-A26/-) were strongly lytic, in a dose-dependent manner, for autologous tumor cells, HLA-A-26+ KE-4 and KUMA-1 SCC cells. Their lytic activity was significantly lower (<10%) for HLA-A26− esophageal SCC cells (KE-3, TE-9 and TE-10) and K562 cells (Fig. 1, left). IL-2-activated TILs from Case 2 (HLA-A24/A33) showed a significant level of cytotoxicity for autologous tumor cells and HLA-A24+ compared with HLA-A-24− lung adenocarcinoma cell lines and K562 target cells (Fig. 1, right). Furthermore, IL-2-activated TILs from Cases 1, 2, 5 and 6 (at an E/T ratio of 20:1) killed 33.9, 30.0, 23.6 and 25.3% of the autologous tumor cells, respectively. This was specifically inhibited by an anti-CD8 MAb, which decreased the amounts of lysis to 7.1,14.3, 9.2 and 12.5% for Case 1, 2, 5 and 6 respectively, and by an anti-HLA class-I MAb, which decreased the amounts of lysis to 9.5, 11.2, 8.1 and 12.8%, respectively (Fig. 2). By contrast, anti-CD4 and anti-HLA class-II (DR) MAbs had no effect. These results indicated that HLA class-I-restricted CTLs were present in the TILs from the malignant PE in these cases.
Fig. 1.
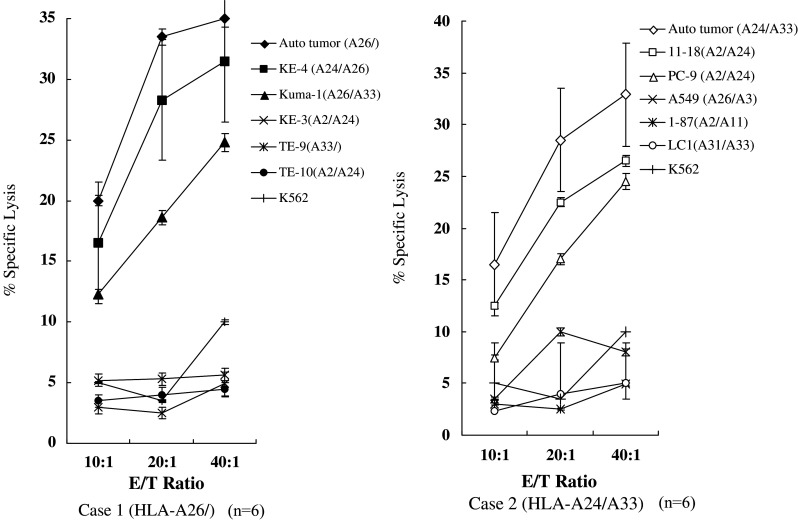
Cytotoxic activity of human lymphocyte antigen (HLA) class-I-restricted interleukin-2 (IL-2)-activated tumor-infiltrating lymphocytes (TILs). IL-2-activated TILs from Case 1 (left) and Case 2 (right) were tested in lactate dehydrogenase (LDH)-release assays at three different effector-to-target (E/T) ratios. Values represent the mean percentage specific lysis from triplicate assay wells
Fig. 2.

Inhibition of the cytotoxic activity of IL-2-activated TILs by monoclonal antibodies (Mabs). IL-2-activated TILs from Case 1 (left) and Case 2 (right) were cultured with autologous tumor cells at a ratio of 20:1, in the presence of the inhibitory MAbs shown. The two-tailed Student’s t test was used for statistical analysis and a P value (*) <0.05 was considered statistically significant
Clinical results
Although all six patients with malignant PE received pericardiocentesis, and Case 1, 2, 5 and 6 received cellular immunotherapy with activated TILs every 2 weeks. The mean volume of fluid drained at the initial pericardiocentesis was 711±309 ml and was hemorrhagic in all six patients. During the procedure, no general or cardiovascular complications were observed, as assessed by clinical examination, radiography, ECG and echocardiography. After undergoing pericardiocentesis, all patients showed rapid improvement in their symptoms. The patient profiles, treatments, adverse effects and clinical responses are summarized in Table 1.
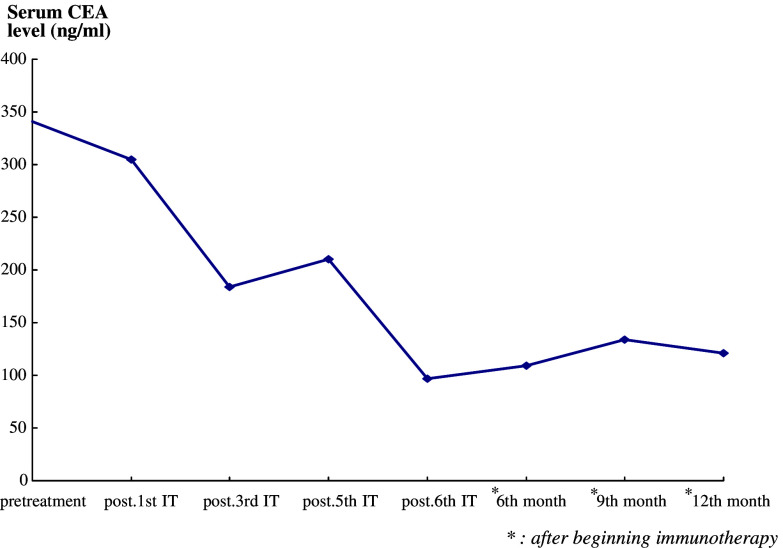
Case 1, who had carcinomatous pericarditis, received six infusions of IL-2-activated TILs into the pericardial cavity, equivalent to a total of 12.6×109 cells. After the third treatment, his quality of life had improved significantly and no PE recurred before the primary esophageal cancer progressed; the patient died 15 months after undergoing the first procedure. Chest CT scans confirmed regression of the PE after the third infusion (Fig. 3a-1, a-2). The serum CEA levels had decreased from 341 to 97 ng/ml after the sixth treatment and that was stabilized within the range between 109 and 134 ng until his last follow-up visit in the 12th month after beginning the IL-2-activated TILs transfer (Fig. 4).
Fig. 4.
Effect of adoptive immunotherapy on serum carcinoembryonic antigen (CEA) levels in Case 1. The level of serum CEA decreased significantly during and after treatment
Case 2 received three intrapericardial transfers of IL-2-activated TILs, equivalent to 2.9×109 lymphocytes in total. Chest X-rays showed a reduction in PE (Fig. 3b-1, b-2). However, she died from respiratory failure, due to the recurrence of lung cancer, 3 months after undergoing the first treatment.
Both Cases 5 and 6 received two and three intrapericardial transfers of IL-2-activated TILs, equivalent to 1.8×109 and 3.2×109 lymphocytes in total, respectively. Chest CT scans in Case 5 showed a reduction in PE (Fig. 5b-1, b-2). However, she died from respiratory failure, due to the progression of multiple lung metastases from breast cancer, 6 months after undergoing the first treatment. And chest X-ray of Case 6 showed a significant reduction in PE and pleural effusion (Fig. 6a-2, a-3). She is still able to live at home, maintain mild household work, and care herself for more than 11 months after undergoing the first treatment, although she complains a mild dyspnea occasionally. The serum CEA levels had decreased from 43.3 to 7.7 ng/ml after the third treatment (Fig. 6b).
No echocardiographic evidence of PE was detected in Cases 1, 2 and 6 at their last follow-up visits, except that of Case 5 with only a small volume nonsymptomatic PE in the CT scans (Fig. 5b-1, b-2). During intrapericardial cell transfer, no acute clinical or ECG alterations were observed in these patients and they did not complain of chest pain or circulatory symptoms. Four patients (Cases 1, 2, 5 and 6) developed a transient febrile reaction (<38°C) and a grade 1 tachycardia (Case 5) within 72 h of treatment, but no other adverse effects were seen (Table 1). By contrast, the two patients (Cases 3 and 4) who underwent percutaneous pericardiocentesi, but did not receive any immunotherapy with TILs, died on days 24 and 27 after undergoing simple pericardial drainage. These results suggest that intrapericardial administration of autologous IL-2-activated TILs, following the drainage of PEs, could successfully control malignant PE in carcinomatous pericarditis with few side effects.
Discussion
Pericardial effusion and cardiac tamponade, due to malignant pericarditis, are common complications in patients with advanced cancer. Standard management remains controversial [2, 4, 14]. Pericardial drainage followed by the topical infusion of sclerosing or cytotoxic agents is reportedly effective and might even be lifesaving, but these procedures alone are inadequate as there is a high rate of recurrence of PE and the associated symptoms. Cytotoxic treatment is also usually accompanied by adverse reactions [2, 4, 15, 16]. Consequently, the development of a new therapeutic approach, such as immunotherapy, could provide a more effective treatment for malignant PE. Adoptive immunotherapy has been used in a number of clinical trials in cancer patients [17–20]. TILs can be isolated and expanded in vitro with IL-2 from a variety of human cancers [21], and some TILs lyse not only autologous fresh tumor cells, but also allogeneic targets in a class-I MHC-restricted manner in cytolytic assays. TIL with such specific lytic activity have been isolated from human malignant melanoma [22, 23], lung carcinoma [24], prostate carcinoma [25] and esophageal, colon, breast tumors [26, 27, 28]. However, to our knowledge, the use of TILs from PE, specific for autologous tumor cells, as a cellular immunotherapy for malignant pericarditis, has not been reported previously.
In this study, we demonstrated the presence of HLA class-I-restricted tumor-specific CTLs in IL-2-activated TILs from malignant PEs in each of four tested patients (Cases 1 to 4) with carcinomatous pericarditis. Furthermore, we reported for the first time the usefulness of pericardiocentesis combined with the intrapericardial transfer of IL-2-activated TILs in the treatment of malignant PEs in four patients with advanced cancer. And our data suggest that local immune responses might be developed within malignant PEs following subsequent IL-2-activated TIL transfer [21].
In four tested cases, IL-2-activated TILs produced high levels of IFN-γ in response to autologous or HLA-A-matched tumor cells. However, they simultaneously produced <70 μg/ml of IFN-γ nonspecifically, in response to non-HLA-A-matched tumor cells. This might indicate the presence of IL-2-activated natural killer (NK) cells and T cells with non-HLA class-I-restricted lymphokine-activated killer (LAK) cell activity in these IL-2-activated TILs simultaneously. We previously reported that the LAK activity in IL-2-activated TILs decreased in mixed lymphocyte-tumor cell cultures after 5–7 weeks [10, 12]. The CTLs from Case 1 (HLA-A26/-) lysed esophageal SCC cells expressing the HLA-A26 alleles, but not HLA-A26− SCC or K562 cells. The CTLs from Case 2 (HLA-A24/A33) lysed lung adenocarcinoma cells expressing the HLA-A24 alleles, but not HLA-A33+ lung adenocarcinoma cell (LC1) or K562. These cytotoxic effects were inhibited by anti-class-I and anti-CD8 MAbs. These results suggested that after 2 weeks of culture with IL-2, the activated TILs contained LAK/NK cells, as well as tumor-specific CTLs (precursor) restricted by HLA class-I-A26 and I-A24. Cytotoxic T cells that are HLA-restricted and specific for autologous tumor cells are believed to be more effective in adoptive immunotherapy [29–31].
Injecting autologous IL-2-activated TILs into the pericardium theoretically introduces a high concentration of therapeutic T cells directly into the site of PE and avoids the systemic loss that can occur after intravenous transfusion. Direct interactions between the effector and tumor cells are considered imperative for effective tumor cell lysis in vivo as well as in vitro [32–34]. Our results showed that transferring IL-2-activated TILs directly into the pericardium prevented the recurrence of PE in four cases for a prolonged period, until the three patients (Cases 1, 2 and 5) died as a result of the progression of primary or metastatic tumors, and one patient (Case 6) still alive without reaccumulation of PE. However, the mechanism involving how this immunotherapy prevented reaccumulation of fluid, and tamponade was still unknown; further study will be warrant including whether the inflammation and obliteration occurred in the pericardial space after intrapericardial TIL instillation. We previously observed that the repeated transfer of autologous tumor-stimulated T cells into the site of a tumor could effectively treat the target tumor, but could also influence the systemic immune response and thereby affect distant metastatic tumor sites [6, 8]. It is notable that Case 1 survived for 15 months and Case 6 is still alive and well for more than 11 months after treatment without developing other distant metastases, and we speculate that, in these cases, local therapy might have also had immunological effects systemically. Further investigations of the migration and expansion of T cells injected into the pericardium and the circulation will be necessary to substantiate this theory [35, 36].
Numerous clinical trails of adoptive cellular immunotherapy for various cancer sites have been performed worldwide using LAK, NK or dendritic cells derived from peripheral blood lymphocytes or cancer TILs. We believe that immunotherapy using HLA class-I-restricted tumor-specific CTLs will be preferable to nonspecific LAK/NK cells, as the adverse effects due to cytokines are likely to be more transient and therefore more tolerable [36]. In this study, both the characterization of the lymphocytes and the clinical responses lead us to believe that tumor-specific CTLs might be important effector cells in malignant PE. Our results suggest that intrapericardial cellular immunotherapy might be an effective novel treatment for PE or cardiac tamponade arising from malignant pericarditis and warrant further clinical investigation in a larger-scale study, although this developmental therapeutic technique currently only can be applied at some academy institute with sophisticated immunotherapy programs.
In summary, we demonstrated the existence of HLA class-I-restricted tumor-specific CTLs in IL-2-activated TILs from malignant PE in patients with carcinomatous pericarditis. Furthermore, we confirmed a safe treatment regimen that resulted in good control of malignant PE.
References
- 1.Press OW, Livingston R. Management of malignant pericardial effusion and tamponade. JAMA. 1987;257:1088–1092. doi: 10.1001/jama.257.8.1088. [DOI] [PubMed] [Google Scholar]
- 2.Vaitkus PT, Herrmann HC, LeWinter MM. Treatment of malignant pericardial effusion. JAMA. 1994;272:59–64. doi: 10.1001/jama.272.1.59. [DOI] [PubMed] [Google Scholar]
- 3.Maher EA, Shepherd FA, Todd TJ. Pericardial sclerosis as the primary management of malignant pericardial effusion and cardiac tamponade. J Thorac Cardiovasc Surg. 1996;112:637–643. doi: 10.1016/S0022-5223(96)70046-6. [DOI] [PubMed] [Google Scholar]
- 4.Girardi LN, Ginsberg RJ, Burt ME. Pericardiocentesis and intrapericardial sclerosis: effective therapy for malignant pericardial effusions. Ann Thorac Surg. 1997;64:1422–1427. doi: 10.1016/S0003-4975(97)00992-2. [DOI] [PubMed] [Google Scholar]
- 5.Nakazato H, Koike A, Saji S, Ogawa N, Sakamoto J. Efficacy of immunochemotherapy as adjuvant treatment after curative resection of gastric cancer. Study Group of Immunochemotherapy with PSK for Gastric Cancer. Lancet. 1994;343:1122–1126. doi: 10.1016/S0140-6736(94)90233-X. [DOI] [PubMed] [Google Scholar]
- 6.Toh U, Yamana H, Sueyoshi S, Tanaka T, Niiya F, Katagiri K, Fujita H, Shirozou K, Itoh K. Locoregional cellular immunotherapy for patients with advanced esophageal cancer. Clin Cancer Res. 2000;6:4663–4673. [PubMed] [Google Scholar]
- 7.Ueda Y, Yamagishi H, Tanioka Y, Fujiwara H, Fujii N, Itoh T. Clinical application of adoptive immunotherapy and IL-2 for treatment of advanced digestive tract cancer. Hepato-gastroenterology. 1999;46:1274–1279. [PubMed] [Google Scholar]
- 8.Toh U, Sudo T, Kido K, Matono S, Sasahara H, Mine T, Tanaka T, Sueyoshi S, Fujita H, Shirouzu K, Yamana H. Locoregional adoptive immunotherapy resulted in regression in distant metastases of a recurrent esophageal cancer. Int J Clin Oncol. 2002;7:372–375. doi: 10.1007/s101470200058. [DOI] [PubMed] [Google Scholar]
- 9.Itoh K, Platsoucas CD, Balch CM. Autologous tumor-specific cytotoxic T lymphocytes in the infiltrate of human metastatic melanomas. Activation by interleukin 2 and autologous tumor cells, and involvement of the T cell receptor. J Exp Med. 1988;168:1419–1441. doi: 10.1084/jem.168.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toh U, Yamana H, Nakao M, Imai Y, Takasu H, Fujita H, Shirouzu K, Itoh K. HLA class I-restricted and tumor-specific cytotoxic T lymphocytes from metastatic lymph nodes of esophageal cancers. Cell Immunol. 1997;177:137–143. doi: 10.1006/cimm.1997.1105. [DOI] [PubMed] [Google Scholar]
- 11.Chen YM, Yang WK, Ting CC, Tsai WY, Yang DM, Whang-Peng J, Perng RP. Cross regulation by IL-10 and IL-2/IL-12 of the helper T cells and the cytolytic activity of lymphocytes from malignant effusions of lung cancer patients. Chest. 1997;112:960–966. doi: 10.1378/chest.112.4.960. [DOI] [PubMed] [Google Scholar]
- 12.Nakao M, Yamana H, Imai Y, Toh Y, Toh U, Kimura A, Yamana H, Kakegawa T, Itoh K. HLA A2601-restricted CTLs recognize a peptide antigen expressed on squamous cell carcinoma. Cancer Res. 1995;55:4248–4252. [PubMed] [Google Scholar]
- 13.Smith FE, Lane M, Hudgins PT. Conservative management of malignant pericardial effusion. Cancer. 1974;33(1):47–57. doi: 10.1002/1097-0142(197401)33:1<47::AID-CNCR2820330110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Cullinane CA, Paz IB, Smith D, Carter N, Grannis FW., Jr Prognostic factors in the surgical management of pericardial effusion in the patient with concurrent malignancy. Chest. 2004;125:1328–1334. doi: 10.1378/chest.125.4.1328. [DOI] [PubMed] [Google Scholar]
- 15.Morita T, Takiguchi Y, Tabeta H, Watanabe R, Kimura H, Kuriyama T. Controlling malignant pericardial effusion by intrapericardial carboplatin administration in patients with primary non-small-cell lung cancer. Br J Cancer. 2000;83:858–862. doi: 10.1054/bjoc.2000.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinoni A, Cipolla CM, Cardinale D, Civelli M, Lamanitia G, Colleoni M, Fiorentini C. Long-term results of intrapericardial chemotherapeutic treatment of malignant pericardial effusions with thiotepa. Chest. 2004;126:1412–1416. doi: 10.1378/chest.126.5.1412. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg SA, Lotze MT. Cancer immunotherapy using interleukin-2 and interleukin-2-activated lymphocytes. Annu Rev Immunol. 1986;4:681–709. doi: 10.1146/annurev.iy.04.040186.003341. [DOI] [PubMed] [Google Scholar]
- 19.Topalian SL, Solomon D, Avis FP, Simpson AE, Freeksen DL, Lineham WM, Lotze MT, Robertson CN, Seipp CA, Simon P, Simpson CG, Rosenberg SA. Immunotherapy of patients with advanced cancer using tumor-infiltrating lymphocytes and recombinant interleukin-2: a pilot study. J Clin Oncol. 1988;6:839–853. doi: 10.1200/JCO.1988.6.5.839. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Lineham WM, Seipp CA, White DE. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210:474–484. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yannelli JR, Hyatt C, McConnell S, Hines K, Jacknin L, Parker L, Sanders M, Rosenberg SA. Growth of tumor-infiltrating lymphocytes from human solid cancers: summary of a 5-year experience. Int J Cancer. 1996;65:413–421. doi: 10.1002/(SICI)1097-0215(19960208)65:4<413::AID-IJC3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Topalian SL, Solomon D, Rosenberg SA. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol. 1989;142:3714–3725. [PubMed] [Google Scholar]
- 23.Dreno B, Nguyen JM, Khammari A, Pandolfino MC, Tessier MH, Bercegeay S, Cassidanius A, Lemarre P, Billaudel S, Labarriere N, Jotereau F. Randomized trial of adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol Immunother. 2002;51(10):539–546. doi: 10.1007/s00262-002-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortegel JW, Staren ED, Faber LP, Warren WH, Braun DP. Modulation of tumor-infiltrating lymphocyte cytolytic activity against human non-small cell lung cancer. Lung Cancer. 2002;36(1):17–25. doi: 10.1016/S0169-5002(01)00472-X. [DOI] [PubMed] [Google Scholar]
- 25.Bronte V, Kasic T, Gri G, Gallana K, Borsellino G, Marigo I, Battistini L, Iafrate M, Prayer-Galetti T, Pagano F, Viola A. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201(8):1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toh U, Yamana H, Nakao M, Imai Y, Seki N, Takasu H, Kaneshige T, Fujita H, Shirouzu K, Itoh K. HLA class I-restricted and tumor-specific cytotoxic T lymphocytes from metastatic lymph nodes of esophageal cancers. Cell Immunol. 1997;177(2):137–143. doi: 10.1006/cimm.1997.1105. [DOI] [PubMed] [Google Scholar]
- 27.Ridolfi R, Flamini E, Riccobon A, De Paola F, Maltoni R, Gardini A, Ridolfi L, Medri L, Poletti G, Amadori D. Adjuvant adoptive immunotherapy with tumour-infiltrating lymphocytes and modulated doses of interleukin-2 in 22 patients with melanoma, colorectal and renal cancer, after radical metastasectomy, and in 12 advanced patients. Cancer Immunol Immunother. 1998;51(10):539–546. doi: 10.1007/s002620050477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kass R, Bellone S, Palmieri M, Cane S, Bignotti E, Henry-Tillman R, Hutchins L, Cannon MJ, Klimberg S, Santin AD. Restoration of tumor-specific HLA class I restricted cytotoxicity in tumor infiltrating lymphocytes of advanced breast cancer patients by in vitro stimulation with tumor antigen-pulsed autologous dendritic cells. Breast Cancer Res Treat. 2003;80(3):275–285. doi: 10.1023/A:1024938215782. [DOI] [PubMed] [Google Scholar]
- 29.Aruga A, Shu S, Chang AE. Tumor-specific granulocyte/macrophage colony-stimulating factor and interferon γ secretion is associated with in vivo therapeutic efficacy of activated tumor-draining lymph node cells. Cancer Immunol Immunother. 1995;41:317–324. doi: 10.1007/BF01517220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt-Wolf GD, Negrin RS, Schmidt-Wolf IG. Activated T cells and cytokine-induced CD3+CD56+ killer cells. Ann Hematol. 1997;74:51–56. doi: 10.1007/s002770050257. [DOI] [PubMed] [Google Scholar]
- 31.Kjaergaard J, Hokland M, Nannmark U, Hokland P, Basse P. Infiltration patterns of short- and long-term cultured A-NK and T-LAK cells following adoptive immunotherapy. Scand J Immunol. 1998;47:532–540. doi: 10.1046/j.1365-3083.1998.00339.x. [DOI] [PubMed] [Google Scholar]
- 32.Basse P, Herberman RB, Nannmark U, Johansson BR, Hokland M, Wasserman K, Goldfarb RH. Accumulation of adoptively transferred adherent, lymphokine-activated killer cells in murine metastases. J Exp Med. 1991;174:479–488. doi: 10.1084/jem.174.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basse PH, Nannmark U, Johansson BR, Herberman RB, Goldfarb RH. Establishment of cell-to-cell contact by adoptively transferred adherent lymphokine-activated killer cells with metastatic murine melanoma cells. J Natl Cancer Inst. 1991;83:944–950. doi: 10.1093/jnci/83.13.944. [DOI] [PubMed] [Google Scholar]
- 34.Zoll B, Lefterova P, Csipai M, Finke S, Trojaneck B, Ebert O, Micka B, Roigk K, Fehlinger M, Schmidt-Wolf GD, Huhn D, Schmidt-Wolf IG. Generation of cytokine-induced killer cells using exogenous interleukin-2, -7 or -12. Cancer Immunol Immunother. 1998;47:221–226. doi: 10.1007/s002620050524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burger UL, Chang MP, Goedegebuure PS, Eberlein TJ, Adams-Hodgins S. Recruitment of host CD8+ T cells by tumor-infiltrating lymphocytes and recombinant interleukin-2 during adoptive immunotherapy of cancer. Surgery. 1995;117:325–333. doi: 10.1016/S0039-6060(05)80209-0. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]