Abstract
The discovery of tumor antigens recognized by T lymphocytes has stimulated the development of a variety of cancer treatment protocols aimed at enhancing antitumor-specific T cell responses and tumor rejection. However, immunotherapy-mediated regression of established tumors and clearly positive clinical response to such treatment has not been achieved yet despite the induction of T cells directed against tumor antigens. The failure of the modern immunotherapy protocols can be explained by different tumor escape mechanisms that have been defined in various types of malignancy. The loss or downregulation of MHC class I antigens in tumor cells is one of the best analyzed mechanisms. In this review, we show experimental evidence obtained in our laboratory on human tumors and in a mouse cancer model suggesting that the molecular mechanism responsible for the MHC class I alteration in tumor cells might have a crucial impact on tumor recovery of normal H-2/HLA expression during the natural history of tumor development or after immunotherapy. When the preexisting molecular lesion underlying tumor MHC class I alteration is reversible (regulatory or soft), class I expression can be recovered leading to regression of tumor lesion. In contrast, if the HLA class I alteration is irreversible in nature (structural or hard), the lesion will progress killing the host. This is a new vision of the role of MHC class I alteration in tumors that can explain the failure of immunotherapy in a variety of different clinical protocols.
Keywords: MHC, Cancer, Immunoselection, Hard lesions, Soft lesions, Immunotherapy
Introduction
Growth of tumor cells and cancer progression is associated with the tumor escape from the immune system or its suppression by different mechanisms, including anergy, tolerance and regulatory T cells [1, 2]. Tumor cells produce immunosuppressive factors, such as TGF-β, IL-10, VEGF, gangliosides, 2, 3-indoleamone dioxygenase (IDO) or they also express molecules, such as Fas ligand that inhibit the immune system [3–6]. Deficiency in the dendritic cell function and defects in the signal transduction pathway of CD8+ cytotoxic T cells (CTL) greatly decrease the ability of the immune system to reject tumors [7]. In addition, regulatory T cells play an important role in the suppression of antitumor immunity [8, 9]. Furthermore, various tumor escape mechanisms have been defined over the past years, including loss of tumor-specific antigens, lack of costimulatory signals and/or adhesion molecules, etc. [10]. One of the best documented mechanisms of cancer escape from the immune recognition is total or partial loss of MHC class I molecules in tumor cells [11–13]. Antitumor-specific T lymphocytes are usually found in tumor-bearing host, but they lack the ability to recognize and destroy tumor target cells due to the absence of the restriction element required for efficient T cell cytotoxicity [14]. These findings have been reported both during the natural history of tumor development as well as after different protocols of immunotherapy aimed to boost T cell responses [15].
Among different molecular mechanisms that lead to altered MHC class I expression are (1) loss of heterozygosity (LOH) in human chromosomes 6 or/and 15 in which class I heavy chain or β2-microglobulin genes are located [16, 17]; (2) mutations affecting these genes [18]; (3) coordinated downregulation of HLA A, B or C loci [19]; and (4) downregulation of the antigen processing machinery, including TAP and LMP genes [20]. For further details, see the review by Garrido and Algarra [15].
Frequently normal MHC class I expression on tumor cells can be recovered after treatment with various immunomodulators (i.e. interferons) or pharmacological agents indicating that tumor cells have reversible (soft) defects underlying MHC alterations. However, lack of cytokine-induced restoration of normal MHC expression suggests that a structural-irreversible (hard) defect is responsible for the loss of class I antigens on tumor cell surface. Thus, from the functional point of view MHC alterations can be classified into two main groups: (1) reversible-regulatory or “soft” defects, with a possibility of HLA class I upregulation by IFN-γ; and (2) irreversible-structural or “hard”, when normal HLA I expression cannot be recovered by cytokines. Even though these different molecular mechanisms underlying altered MHC class I expression have been described years ago; only recently, important clinical implications of such findings have been proposed [21, 22].
In this focussed research review, we present the results obtained in our laboratory in a mouse experimental tumor model (the GR9 fibrosarcoma) together with the recent data on HLA expression in metastatic lesion of melanoma patients receiving immunotherapy. We describe that in our experimental model, the primary mouse tumor consists of tumor cells clones with different MHC class I expression patterns, and such heterogeneity has an impact on tumor growth and metastasis capacity. Second, we describe the emergence of additional MHC class I alterations with reversible-soft and irreversible-hard lesions in metastatic colonies generated from a single tumor clone. In other experimental models appearance of antigen-loss tumor variants with reversible or irreversible alterations have been also reported. Finally, we show that cancer immunotherapy led to regression of human metastatic lesions with soft MHC class I alterations due to MHC class I upregulation which can be observed in different clinical immunotherapy protocols.
Tumor clones with different MHC class I expression coexist in primary tumors: biological implications
We used the GR9 fibrosarcoma tumor model in our laboratory to study the biological implications of MHC class I alterations in cancer. The experimental tumor was established using methylcholantrene in Balb/c mice (H-2d) and was adapted to tissue culture without any in vivo passage to avoid immunoselection [23]. The H-2 typing of the different clones revealed the existence of tumor clones with different H-2 class I expression patterns from highly positive tumor cells expressing H-2 K, D and L molecules to completely negative cells (B9 clone). In all instances, the tumor cells could upregulate H-2 class I antigens after IFN treatment. Interestingly, this different MHC class I cell surface expression had an impact on local tumor growth and metastatic capacity. Clones with high class I expression were very immunogenic in local growth but highly metastatic [24]. In contrast, clones with decreased MHC class I expression were growing easily when injected subcutaneously, but showed low metastatic activity (Table 1). Similar heterogeneity in HLA class I expression was observed in a variety of human tumors immunostained with anti-HLA class I antibodies [25]. We favor the idea that the primary tumor is composed of tumor cells with enormous diversity in expression of MHC class I molecules and, therefore, presenting tumor antigens with different intensity. We have gathered experimental evidence strongly supporting this idea, i.e. in GR9 fibrosarcoma tumor the amount of class I antigens directly affect T and NK cell cytotoxicity [26]. Tumor cells with high amount of class I molecules are destroyed more easily and quickly, while the remaining cells with low MHC class I become better targets for NK cells.
Table 1.
MHC class I phenotype and biological behavior of mouse fibrosarcoma tumor clones
| Tumor clone | H-2 class 1 phenotype | IFN response Kd Dd Ld | Local growth | Metastatic capacity | NK sensitivity |
|---|---|---|---|---|---|
| G2 | +++ | +++ | − | +++ | − |
| A7 | +++ | +++ | − | +++ | − |
| BIO | + | +++ | ++ | + | ++ |
| B9 | − | +++ | +++ | +/− | ++ |
Immunoselection leads to emergence of new tumor escape variants with soft and hard lesions
Tumor cell clones with MHC class I soft lesions re-express MHC class I in metastatic colonies produced in T cell-deficient syngeneic athymic mice
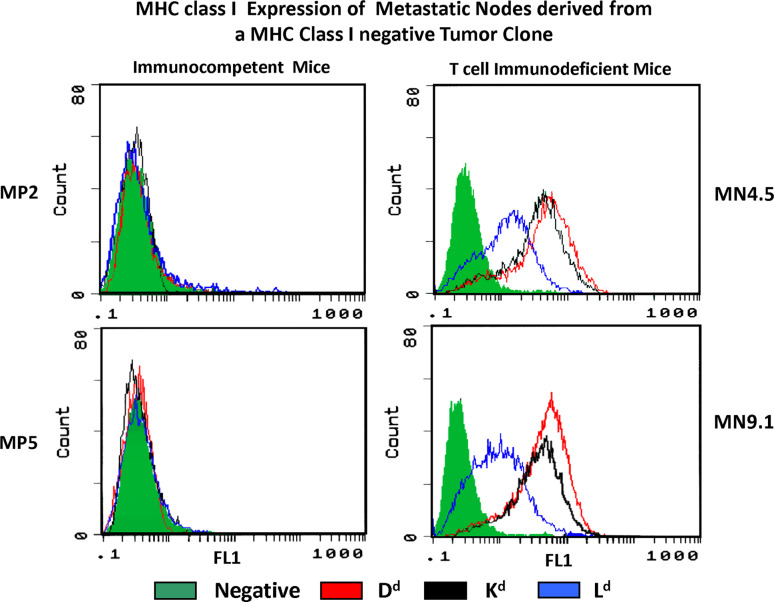
We observed in our mouse fibrosarcoma tumor model that the H-2 negative clone B9 produced H-2 negative metastatic lung colonies when grown in immunocompetent syngeneic Balb/c mice, as described earlier [27]. Interestingly, the same clone produced H-2 positive metastases under basal condition when injected in nude/nude T cell-deficient athymic Balb/c mice [28] (Fig. 1). These results indicate that the immune status of the host is playing a critical role in MHC profile of the tumor. We propose that MHC class I-deficient tumor cells with soft alterations will re-express MHC class when is growing in a T cell-deficient host since there is no immunoselective pressure to escape T cell recognition. Similar findings have been reported in human renal cell carcinomas with immune escape mechanisms independent of T lymphocytes and high levels of HLA expression observed in the majority of the RCC tissue samples [29].
Fig. 1.
A metastatic tumor cell clone with a MHC class I negative phenotype harboring a reversible-soft lesion produce metastases in syngeneic immunocompetent mice without changing the MHC negative profile (MP2, MP5). In contrast, when the same clone is injected in T cell-immunodeficient syngeneic mice, the metastases obtained recover MHC class I expression (MN4.5, MN9.1)
Metastatic colonies develop new altered MHC class I phenotypes when compared with the primary tumor clone: generation of hard MHC class I lesions
MHC class I negative mouse fibrosarcoma tumor clone B9 was isolated from heterogeneous primary tumor and was injected into syngeneic animals to produce metastases. We observed that the obtained colonies where also H-2 class I negative. However, some cells recovered H-2 expression after IFN treatment and others did not (Table 2) [27], especially in cell resistant to cytokine treatment the H-2Ld molecule was not induced. This new heterogenic pattern was not present in the original B9 tumor clone since these cells did respond to IFN treatment and recovered H-2 expression, and was apparently originated in vivo during the metastatic progression. These results indicate that metastases with hard MHC lesions were generated “de novo” during the metastatic development.
Table 2.
Generation of “hard” structural alterations in mouse metastatic colonies
| Tumor | Cell | H-2 class 1 expression | Type of lesion | |||||
|---|---|---|---|---|---|---|---|---|
| Basal | IFN | |||||||
| Kd | Dd | Ld | Kd | Dd | Ld | |||
| Primary tumor | B9 clone | − | − | − | + | + | + | Soft |
| B9 MP2 | − | − | − | + | + | + | Soft | |
| B9 MP3 | − | − | − | + | + | − | Harda | |
| B9 MP5 | − | − | − | + | + | − | Harda | |
| Metastases | B9 MP6 | − | − | − | + | + | − | Harda |
| B9 MP10 | − | − | − | + | + | − | Harda | |
| B9 MP11 | − | − | − | + | + | − | Harda | |
| B9 MP12 | − | − | − | + | + | + | Soft | |
A tumor clone H-2 class I negative (B9) with a soft lesion produced different metastases also H-2 class I negative. Interestingly, now some metastases can recover the Ld gene expression after IFN treatment (soft lesion) while others not (hard lesion); for details, see [27, 28]
aHard lesions affecting the La gene
Immunoselection of antigen-loss tumor variants with soft and hard alterations
In other experimental cancer models, immunoselection of antigen-loss tumor variants has been reported. In some cases, the loss of tumor-specific antigen was due to reversible epigenetic mechanisms. A potent selection of antigen-loss variants of B16 melanoma occurred following inflammatory killing of melanocytes in vivo [30]. The resulting CD8+ T cell response selected B16 variants, which grew aggressively and have lost expression of the tyrosinase and tyrosinase-related protein 2 (TRP-2). In other murine model with B16 melanoma cells [31], cell transfers of activated tumor-associated antigen (TAA) specific T cells promoted the immune selection of the escape variants. Tumors expressing an immunodominant TAA grew in immunocompetent hosts in which >90% of the CD8 T cells were TAA specific. The emergent tumor variants could evade the immune system by a reversible alteration, epigenetic silencing of the TAA gene.
In another studies, irreversible hard alterations are responsible for antigen-loss tumor variants. Adoptively, transferred transgenic T cells specific for P1A into the mice with established P1A-expressing tumors of distinct histological origin caused appearance of tumor escape variants with antigenic drift or antigen loss according to type of tumor [32]. In other study, the mutation of residues within epitopes of HER-2/neu leads to the outgrowth of tumors after immunizing HER-2/neu transgenic mice with therapeutic vaccines expressing fragments of HER-2/neu [33]. Vaccines expressing the kinase domain fragment of HER-2/neu protein were more effective against different tumors than vaccines expressing the extracellular or other intracellular domains indicating that selection of the epitope for immunotherapeutic treatment is very important.
Cancer immunotherapy in humans induces additional T cell-mediated immunoselection rejecting tumor cells with MHC class I soft lesions
All the experimental evidence obtained in our cancer model during last several years indicates that cancer progression is associated with emergence of additional MHC class I alterations with reversible-soft and irreversible-hard lesions in metastatic colonies generated from a single tumor clone. We have recently reported that a patient with metastatic melanoma treated with autologous tumor vaccine (M-VAX) plus BCG developed several subcutaneous metastases with distinct HLA class I expression and different ability to grow in response to immunotherapy, i.e. three of the lesions progressed and the three showed considerable regression. All lesions presented a loss of HLA class I haplotype [34]. However, the progressing metastases showed low HLA class I expression and newly additional alterations—LOH in chromosome 15 and HLA B locus downregulation. In contrast, the regressors showed high levels of HLA class I expression with an HLA haplotype loss similar to the progressors. Comparable results indicating a strong association between HLA class I expression and progression/regression of the metastatic lesions were obtained in another melanoma patient treated with IFN-α-2b/autologous tumor vaccine plus BCG [35]. Five lesions were obtained after interferon-α-2b treatment and five after autologous tumor vaccination plus BCG. Eight metastases were regressing after immunotherapy, while two were progressing. The regressing metastases showed high level of HLA class I expression, whereas the two progressing lesions had low HLA levels as measured by real-time PCR and immunohistological techniques.
These data taken together with the results obtained in our mouse experimental model suggest that activation of T lymphocytes after immunotherapy might induce the release of cytokines in the tumor microenvironment and upregulate MHC class I antigen expression in tumor cells with soft lesions (Fig. 2) leading to their rejection, while tumor cells with hard irreversible lesions will progress. Our prediction is that tumor lesions with “soft” and “hard” HLA class I defects coexist during the natural history of tumor development, and after immunotherapy tumor cells with “soft” lesion will be rejected and cells with “hard” lesions will prevail and metastasize.
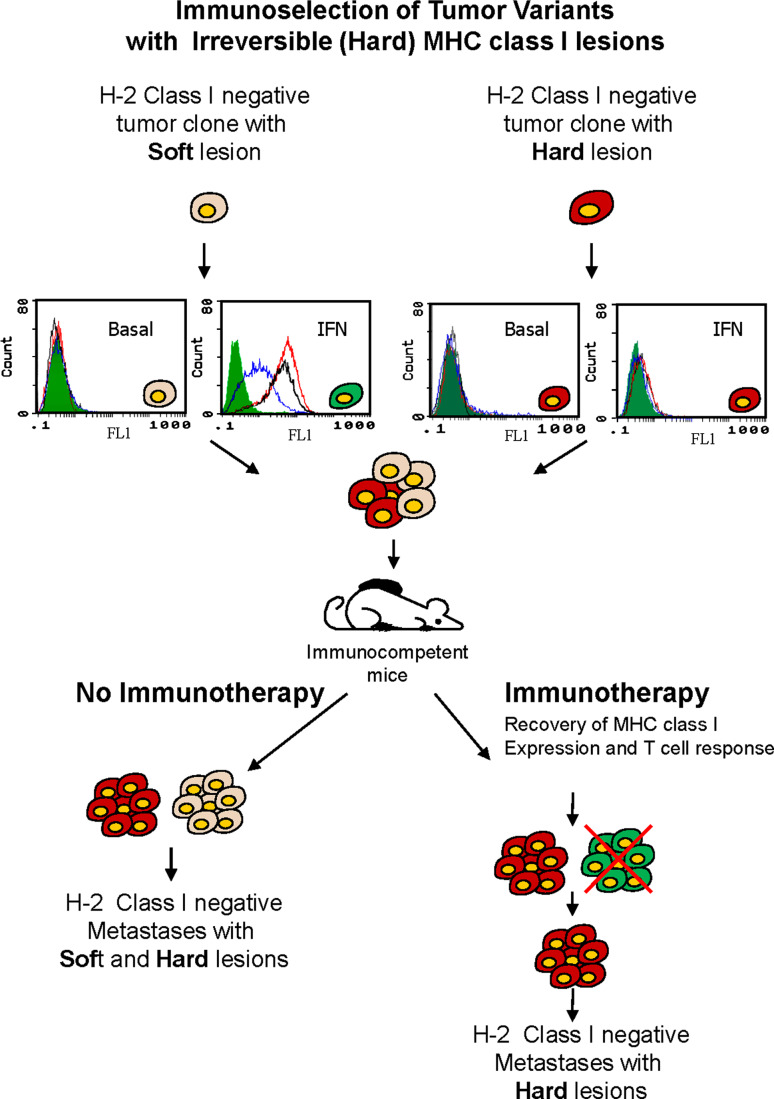
Fig. 2.
Primary tumors are composed of heterogeneous population of cells with different MHC class I altered phenotypes. These phenotypes can be produced by a large variety of mechanisms. These defects underlying altered MHC expression can be classified as reversible-regulatory or “soft” (gray) and irreversible-structural-hard (red). Immunotherapy induces the release of different cytokines in the tumor microenvironment upregulating MHC class I expression in tumor cells with soft lesions (green). Now these tumor cells can be recognized and destroyed by antitumor-specific T lymphocytes. Tumor cells with hard lesions (red) that cannot recover MHC expression are immunoselected and kill the host
Concluding remarks
We have recently proposed that the molecular alterations responsible for the altered MHC class I expression can be classified into reversible/regulatory and irreversible/structural defects. These two types of alterations that we named “soft” and “hard” might have profound implications in the T cell-mediated rejection of tumor cells in primary or metastatic lesions and in the outcome of cancer immunotherapy. Here, we present evidence suggesting the existence of these two types of MHC defects both in mouse tumors/metastases, as well as in human metastases. We believe that modification of microenvironment of metastatic lesions in response to various types of immunotherapy might lead to cytokine release and influence the MHC class I expression depending on the nature (reversible or irreversible) of underlying alterations. Cytokine-induced upregulation in soft lesions will lead to regression of metastatic nodule, while resistance to cytokines in tumor cells with hard defects will stimulate progression of metastases. The detailed analysis of the correlation of impaired HLA expression with metastatic progression is important for the improvement of the existing protocols of cancer immunotherapy. Immunoselection and progression of tumor cells with MHC hard lesions requires search for new ways to restore normal MHC expression including gene therapy.
Acknowledgments
The authors would like to thank Dr. Natalia Aptsiauri for helpful discussion. A. García-Lora was supported by Fundación Progreso y Salud and FIS-Research Contract CP03/0111. This study was partially supported by Grants from the Fondo de Investigaciones Sanitarias (FIS, CP03/0111; PI 08/1265), Red Genómica del Cancer RTICCC (RETIC RD 06/020), Consejeria de Salud and Plan Andaluz de Investigación (PAI, Group CTS-143 and projects CTS-695, CTS-3952, CVI-4740) from the Junta de Andalucia, in Spain; by the ENACT project (LSHC-CT-2004-503306) and the Cancer Immunotherapy project (OJ 2004/c158, 18234) from the European Community, and by Kureha Chemical Industry, Tokyo, Japan.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Ninth International Conference on Progress in Vaccination against Cancer (PIVAC 9), held in Sofia, Bulgaria, 8–10 October 2009.
References
- 1.Zou W. Immunosupressive networks in the tumor environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 2.Mapara MY, Sykes M. Tolerance and cancer: mechanisms of tumor evasion and strategies for breaking tolerance. J Clin Oncol. 2004;22:1136–1151. doi: 10.1200/JCO.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 3.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7:1118–1122. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 4.Kawamura K, Bahar R, Natsume W, Sakiyama S, Tagawa M. Secretion of interleukin-10 from murine colon carcinoma cells suppresses systemic antitumor immunity and impairs protective immunity induced against the tumors. Cancer Gene Ther. 2002;9:109–115. doi: 10.1038/sj.cgt.7700418. [DOI] [PubMed] [Google Scholar]
- 5.McKallip R, Li R, Ladisch S. Tumor gangliosides inhibit the tumor-specific immune response. J Immunol. 1999;163:3718–3726. [PubMed] [Google Scholar]
- 6.Munn DH, Mellor AL. Indoleamine 2, 3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaput N, Confori R, Viaud S, Spatz A, Zitvogel L. The Janus face of dendritic cells in cancer. Oncogene. 2008;27:5920–5931. doi: 10.1038/onc.2008.270. [DOI] [PubMed] [Google Scholar]
- 8.Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC. Regulatory T cells in cancer. Adv Cancer Res. 2010;107:57–117. doi: 10.1016/S0065-230X(10)07003-X. [DOI] [PubMed] [Google Scholar]
- 9.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 10.Drake CG, Jaffee E, Pardoll DM. Mechanism of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 11.Garrido F, Cabrera T, Concha A, Glew S, Ruiz-Cabello F, Stern PL. Natural history of HLA expression during tumour development. Immunol Today. 1993;14:491–499. doi: 10.1016/0167-5699(93)90264-L. [DOI] [PubMed] [Google Scholar]
- 12.Garrido F, Ruiz-Cabello F, Cabrera T, Pérez-Villar JJ, López-Botet M, Duggan-Keen M, Stern PL. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today. 1997;18:89–95. doi: 10.1016/S0167-5699(96)10075-X. [DOI] [PubMed] [Google Scholar]
- 13.Marincola FM, Jafee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/S0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 14.Serrano A, Tanzarella S, Lionello I, Mendez R, Traversari C, Ruiz-Cabello F, Garrido F. Expression of HLA class I antigens and restoration of antigen-specific CTL response in melanoma cells following 5-aza-2′-deoxycytidine treatment. Int J Cancer. 2001;94:243–251. doi: 10.1002/ijc.1452. [DOI] [PubMed] [Google Scholar]
- 15.Garrido F, Algarra I. MHC antigens and tumor escape from immune surveillance. Adv Cancer Res. 2001;83:117–158. doi: 10.1016/S0065-230X(01)83005-0. [DOI] [PubMed] [Google Scholar]
- 16.Maleno I, López-Nevot MA, Cabrera T, Salinero J, Garrido F. Multiple mechanisms generate HLA class I altered phenotypes in laryngeal carcinomas: high frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21. Cancer Immunol Immunother. 2002;51:389–396. doi: 10.1007/s00262-002-0296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez T, Mendez R, Roberts CH, Ruiz-Cabello F, Dodi IA, Lopez Nevot MA, Paco L, Maleno I, Marsh SG, Pawelec G, et al. High frequency of homozygosity of the HLA region in melanoma cell lines reveals a pattern compatible with extensive loss of heterozygosity. Cancer Immunol Immunother. 2005;54:141–148. doi: 10.1007/s00262-004-0561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pérez B, Benitez R, Fernandez MA, Oliva MR, Soto JL, Serrano S, López Nevot MA, Garrido F. A new beta 2 microglobulin mutation found in a melanoma tumor cell line. Tissue Antigens. 1999;53:569–572. doi: 10.1034/j.1399-0039.1999.530607.x. [DOI] [PubMed] [Google Scholar]
- 19.Mendez R, Rodriguez T, Del Campo A, Monge E, Maleno I, Aptsiauri N, Jimenez P, Pedrinaci S, Pawelec G, Ruiz-Cabello F, et al. Characterization of HLA class I altered phenotypes in a panel of human melanoma cell lines. Cancer Immunol Immunother. 2008;57:719–729. doi: 10.1007/s00262-007-0411-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seliger B, Atkins D, Bock M, Ritz U, Ferrone S, Huber C, Störkel S. Characterization of human lymphocyte antigen-processing machinery defects in renal cell carcinoma lesions with special emphasis on transporter-associated with antigen-processing down-regulation. Clin Cancer Res. 2003;9:1721–1727. [PubMed] [Google Scholar]
- 21.Aptsiauri N, Carretero R, García-Lora A, Real LM, Cabrera T, Garrido F. Regressing and progressing metastatic lesions: resistance to immunotherapy is predetermined by irreversible HLA class I antigen alterations. Cancer Immunol Immunother. 2008;57:1727–1733. doi: 10.1007/s00262-008-0532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrido F, Cabrera T, Apsiauri N. Hard and soft lesions underlaying the HLA class I alterations in cancer cells: implications for immunotherapy. Int J Cancer. 2010;127:249–256. doi: 10.1002/ijc.25270. [DOI] [PubMed] [Google Scholar]
- 23.Pérez M, Garrido A, Algarra I, Caballero A, Delgado C, Collado MD, Fernandez-Cruz E, Garrido F. H-2 antigens and tumour-associated transplantation antigens in clones derived from a methylcholanthrene-induced BALB/c tumour: their influence on the generation in vitro and in vivo of the specific anti-tumour immune response. Exp Clin Immunogenet. 1989;6:204–218. [PubMed] [Google Scholar]
- 24.Pérez M, Algarra I, Ljunggren HG, Caballero A, Mialdea MJ, Gaforio JJ, Klein G, Kärre K, Garrido F. A weakly tumorigenic phenotype with high MHC class-I expression is associated with high metastatic potential after surgical removal of the primary murine fibrosarcoma. Int J Cancer. 1990;46:258–261. doi: 10.1002/ijc.2910460219. [DOI] [PubMed] [Google Scholar]
- 25.Cabrera T, Maleno I, Collado A, López Nevot MA, Tait BD, Garrido F. Analysis of HLA class I alterations in tumors: choosing a strategy based on known patterns of underlying molecular mechanisms. Tissue Antigens. 2007;69:264–268. doi: 10.1111/j.1399-0039.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 26.Algarra I, Öhlen C, Pérez M, Ljunggren HG, Klein G, Garrido F, Kärre K. NK sensitivity and lung clearance of MHC-class-I-deficient cells within a heterogeneous fibrosarcoma. Int J Cancer. 1989;44:675–680. doi: 10.1002/ijc.2910440420. [DOI] [PubMed] [Google Scholar]
- 27.García-Lora A, Algarra I, Gaforio JJ, Ruiz-Cabello F, Garrido F. Immunoselection by T lymphocytes generates repeated MHC class I-deficient metastatic tumor variants. Int J Cancer. 2001;91:109–119. doi: 10.1002/1097-0215(20010101)91:1<109::AID-IJC1017>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 28.García-Lora A, Martinez M, Algarra I, Gaforio JJ, Garrido F. MHC class I-deficient metastatic tumor variants immunoselected by T lymphocytes originate from the coordinated downregulation of APM components. Int J Cancer. 2003;106:521–527. doi: 10.1002/ijc.11241. [DOI] [PubMed] [Google Scholar]
- 29.Sáenz-López P, Gouttefangeas C, Hennenlotter J, Concha A, Maleno I, Ruiz-Cabello F, Cózar JM, Tallada M, Stenzl A, Rammensee HG, Garrido F, Cabrera T. Higher HLA class I expression in renal cell carcinoma than in autologous normal tissue. Tissue Antigens. 2010;75:110–118. doi: 10.1111/j.1399-0039.2009.01409.x. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Perez L, Kottke T, Diaz RM, Ahmed A, Thompson J, Chong H, Melcher A, Holmen S, Daniels G, Vile RG. Potent selection of antigen loss variants of B16 melanoma following inflammatory killing of melanocytes in vivo. Cancer Res. 2005;65:2009–2017. doi: 10.1158/0008-5472.CAN-04-3216. [DOI] [PubMed] [Google Scholar]
- 31.Goldberger O, Volovitz I, Machlenkin A, Vadai E, Tzehoval E, Eisenbach L. Exuberated numbers of tumor-specific T cells result in tumor escape. Cancer Res. 2008;68:3450–3457. doi: 10.1158/0008-5472.CAN-07-5006. [DOI] [PubMed] [Google Scholar]
- 32.Bai XF, Liu JQ, Joshi PS, Wang L, Yin L, Labanowska J, Heerema N, Zheng P, Liu Y. Different lineages of P1A-expressing cancer cells use divergent modes of immune evasion for T-cell adoptive therapy. Cancer Res. 2006;66:8241–8249. doi: 10.1158/0008-5472.CAN-06-0279. [DOI] [PubMed] [Google Scholar]
- 33.Singh R, Paterson Y. Immunoediting sculpts tumor epitopes during immunotherapy. Cancer Res. 2007;67:1887–1892. doi: 10.1158/0008-5472.CAN-06-3960. [DOI] [PubMed] [Google Scholar]
- 34.Cabrera T, Lara E, Romero JM, Maleno I, Real LM, Ruiz-Cabello F, Valero P, Camacho FM, Garrido F. HLA class I expression in metastatic melanoma correlates with tumor development during autologous vaccination. Cancer Immunol Immunother. 2007;56:709–717. doi: 10.1007/s00262-006-0226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carretero R, Romero JM, Ruiz-Cabello F, Maleno I, Rodriguez F, Camacho FM, Real LM, Garrido F, Cabrera T. Analysis of HLA class I expression in progressing and regressing metastatic melanoma lesions after immunotherapy. Immunogenetics. 2008;60:439–447. doi: 10.1007/s00251-008-0303-5. [DOI] [PubMed] [Google Scholar]