Abstract
We have generated an anti-Pgp/anti-CD3 diabody which can effectively inhibit the growth of multidrug-resistant human tumors. However, the two chains of the diabody are associated non-covalently and are therefore capable of dissociation. Cysteine residues were introduced into the V-domains to promote disulphide cross-linking of the dimer as secreted by Escherichia coli. Compared with the parent diabody, the ds-Diabody obtained was more stable in human serum at 37°C, without loss of affinity or cytotoxicity activity in vitro. Furthermore, the ds-Diabody showed improved tumor localization and a twofold improved antitumor activity over the parent diabody in nude mice bearing Pgp-overexpressing K562/A02 xenografts. Our data demonstrate that ds-Diabody may be more useful in therapeutic applications than the parent diabody.
Keywords: Drug stability, Bispecific diabody, Diabody, Antibody engineering, P-glycoprotein, Cancer therapy
Introduction
A major issue in the treatment of cancer in terms of a poor response or relapse is the development of multidrug resistance (MDR) by the tumor cells. Pgp, encoded by the MDR1 gene, is a 170 kDa transporter consisting of 1,280 amino acids that is located in the plasma membrane and is responsible for cancer resistance to multiple chemotherapeutic agents [8, 11, 14, 26]. The level of Pgp expression is an adverse prognostic factor for complete remission and survival in malignant diseases [25, 32, 37]. Pgp may therefore act as a potential therapeutic target for cancer intervention.
Immunotherapy with bispecific antibodies (BiAbs) is a very promising approach for targeting to tumors. T cells play a pivotal role acting against tumors by directly eliminating the tumor cells through the formation of cytotoxic T cell-tumor cell synapses [34]. However, the complexity of T cell recognition offers a variety of strategies for tumor cells to evade specific T cell recognition [16, 21, 23, 28, 35]. Anti-CD3/anti-TAA (tumor associated antigen) BiAbs can possess two specificities, one directed at the T cell and the other at the cancer cell. This enables them to serve as mediators between T cells and cancer cells, bypassing the conventional T cell recognition process. There are reports showing that BiAbs can efficiently inhibit tumor growth both in vitro and in vivo [4, 5, 9, 10, 15].
Bispecific diabodies are the smallest BiAbs with about one-third the size of IgG. Bispecific diabodies are dimers, one chain comprising a VH domain from antibody A and a VL domain from antibody B, connected by a short peptide linker, and vice versa. The linker is too short to allow pairing between domains of the same chain, thus driving the pairing between complementary domains on different chains and forming two antigen binding sites that point away from each other [17]. In addition the relatively small size of diabodies (55 kDa) facilitates penetration into solid tumors as compared to larger whole antibodies [40]. Diabodies lack Fc domains thus eliminating the undesirable side-effects they have in immunotherapy. Further, diabodies can be readily produced by secretion from bacteria at a yield of up to 1 g/L, thus possessing a considerable potential for application in a clinical setting [42].
A critical and important factor contributing to the therapeutic effect of recombinant antibodies is stability. The two chains of diabodies are associated non-covalently and are therefore capable of dissociation. By introducing a disulphide bond into the recombinant Fv fragment, between two conserved framework residues, a significant improvement in the antibody stability is achieved. The disulphide bond locks the two peptide chains covalently while retaining full or even improved antigen binding activity [2, 3, 12, 29]. The disulphide bond stabilized Fv fragment or BsAbs may thus have the same, or even higher antitumor activity, compared to its non-stabilized counterpart [1, 29–31].
In our previous study it was suggested that an anti-Pgp/anti-CD3 diabody might be an effective agent in the treatment of MDR tumors [13]. However, the poor stability of the diabody limits the antitumor response and reduces its effectiveness in cancer therapy. The required dose of anti-Pgp/anti-CD3 diabody was relatively high and a rapid tumor relapse occurred only 1 week after therapy. It is therefore possible that improving the stability of the diabody may act to ameliorate these problems. Here we describe a disulphide cross-linking of bispecific diabodies derived from anti-Pgp/anti-CD3 diabody as previously discussed. The disulphide stabilized diabodies produced by Escherichia coli were secreted at high yields in a fully active form without a decrease of affinity. Compared with the parent diabodies, ds-Diabodies were more stable at 37°C in human serum and appeared to have enhanced tumor localizing properties. Immunotherapy with ds-Diabodies in MDR human tumor xenograft mouse models resulted in a greater antitumor effect compared to diabodies in the presence of activated human peripheral blood lymphocytes (PBL).
Materials and methods
Cell lines
Hybridoma cell lines, secreting the anti-CD3 antibody HIT3a (IgG2a, κ) and the anti-Pgp antibody PHMA02 (IgG1, κ), were established at the Institute of Hematology, Chinese Academy of Medical Sciences (Tianjin, China), as described previously [33, 38]. The PHMA02 mAb is directed against the extracellular epitope(s) on Pgp, without interference with the efflux function of the protein [38]. A human chronic myelogenous leukemia cell line K562, its MDR variant K562/A02 [39] established in our laboratory, and human acute T-cell leukemia cell line Jurkat were used in the study. The cells were cultured in RPMI-1640 (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum at 37°C in 5% CO2.
Plasmid constructions
The plasmid pLH-T3a-Pgp, encoding anti-CD3 VL and pLH-Pgp-T3a, encoding anti-Pgp VL [13], were each connected by a five-amino-acid linker (G4S) to anti-Pgp VH and anti-CD3 VH, respectively, and were used as templates for the construction of the ds-Diabody. The oligonucleotides 5′-TGGTCCCACAGCCGAACG-3′ and 5′-CATTCCAGGCACTGTCCAG-3′ were used to introduce cysteines at positions Ser-100 of CD3 VL in pLH-T3a-Pgp (pLH-T3a*-Pgp) and Gly-44 of CD3 VH in pLH-Pgp-T3a (pLH-Pgp-T3a*). The expression plasmid was constructed by ligation of the SfiI/NheI and NheI/NotI fragments from pLH-T3a*-Pgp and pLH-Pgp-T3a*, respectively, into the expression vector pCANTAB 5E (Amersham Bioscience, Piscataway, NJ, USA). The nucleotide sequence encoding the ds-Diabody was confirmed by DNA sequencing.
Expression and purification of the anti-Pgp × anti-CD3 ds-Diabody
The soluble expression of the ds-Diabody was as described previously, except that it was propagated at 25°C in a shaker flask for 30 h [41]. Periplasmic lysates from identified mutants were prepared by re-suspending the cell pellet in 25 mM Tris (pH 7.5) containing 20% (w/v) sucrose, 200 mM NaCl, and 1 mM EDTA, followed by incubation at 4°C with gentle shaking for 1 h. After centrifugation at 15,000 rpm for 15 min, the soluble antibodies were purified from the supernatant. The supernatant was loaded onto a cation-exchange chromatographic column (Pharmacia), and the effluent containing the desired protein was then loaded onto an anti-E tag affinity chromatography using a RPAS Purification Module (Amersham Bioscience). SDS-PAGE was carried out in 12% polyacrylamide gels, protein bands were subsequently stained using a colloidal blue stain kit (Novex) to confirm the purity of the ds-Diabody. The parent diabody was expressed and purified as described previously [13].
Flow cytometric analysis
Three cell lines, human acute T-cell leukemia cell line Jurkat, human chronic myelogenous leukemia cell line K562 and its Pgp-overexpressing counterpart K562/A02, were subjected to flow cytometry (FACSCalibur, Becton Dickinson, San Jose, USA). Briefly, 1 × 106 cells in 100 μL phosphate buffered saline (PBS) were first incubated with various antibodies (20 μg/mL) for 1 h at 4°C. After washing 3 times with cold PBS, the cells were incubated with 100 μL of anti-E tag antibody (Amersham Bioscience) at 10 μg/mL for 1 h, followed by incubation with 20 μL of FITC-labeled rabbit anti-mouse antibody (Institute of Hematology) for an additional 30 min. The stained cells were analyzed by flow cytometry (FACSCalibur, Becton Dickinson, San Jose, USA). In a competitive binding assay, 1 × 106 cells in 100 μL PBS were first incubated with ds-Diabody or parent diabody for 1 h at 4°C. After washing 3 times with cold PBS, the cells were incubated with parent mAb IgG (PHMA02 for K562/A02 and HIT3a for Jurkat cells, respectively) for 1 h at 4°C. After washing 3 times with cold PBS, the cells were incubated with an FITC conjugated rabbit anti-mouse IgG (Institute of Hematology) for 30 min. The stained cells were then analyzed by flow cytometry. Control groups only reacted with non-related antibody or parent mAb IgG.
Preparation and stimulation of the effector cells
Human peripheral blood lymphocytes (PBL) were isolated using Ficoll–Hypaque (Institute of Hematology) density-gradient centrifugation from heparinized blood of healthy volunteers and depleted of monocytes by adherence to plastic flasks. For cell activation, the non-adherent cells were cultured in complete RPMI medium (10% FCS) supplemented with 50 IU/mL of IL-2 and 1 μg/mL of HIT3a for 48 h.
In vitro cytotoxicity assay
A non-radioactive cytotoxicity assay (Promega, Madison, WI, USA) was performed to evaluate the efficacy of the diabody in mediating T-cell cytotoxicity. In brief, 2 × 104 K562/A02 target cells were added to effector cells at effector–target cell ratios, ranging from 25:1 to 3:1 in 96-well culture plates. Various diabody dilutions (5–500 ng/mL, in a final volume of 100 μL) were then added. The plates were centrifuged at 250×g for 4 min and incubated for 4 h in a humidified incubator at 37°C in 5% CO2. Control wells were established according to the Manufacturer’s suggestion. The doses for experiments were based on our previous studies or optimized in a pilot experiment. The percentage of cell lysis was calculated by measuring LDH release in supernatants using the standard formula: % cytotoxicity = (experimental − effector spontaneous − target spontaneous)/(target maximum − target spontaneous) × 100.
In vitro stability assays
The diabody prepared as described above was incubated at 37°C for various times in PBS containing 0.2% (w/v) human serum albumin (HSA) at a concentration of 20 μg/mL. The remaining binding activity with Jurkat and K562/A02 cells (1 × 106 cells) was determined by flow cytometry.
In vivo inhibition of growth of MDR human xenografts
Female 6-week-old BALB/C nude mice were inoculated subcutaneously with 2 × 107 of the Pgp-overexpressing K562/A02 cells, into the dorsal thoracic wall, 1 day after the application of total body irradiation (400 rad). Tumors 80–100 mm3 in size developed in all animals 6 days post-tumor inoculation at which point treatment was initiated. Mice were treated by intravenous injections of three different doses (50, 100, or 200 μg/mouse) of ds-Diabody or parent diabody, combined with pre-activated PBL (5 × 106 cells/mouse) in PBS via the tail vein, every week for 3 weeks. Mice received intravenous injections of ds-Diabody, parent diabody, preactivated PBL or PBS, respectively, as control. Tumors were measured with a caliper at 2-day intervals, and the tumor volumes were calculated using the formula: V = (L × W 2)/2, where L represents the longest axis of tumors (in mm) and W represents the axis perpendicular to L (in mm). Mice were killed by cervical dislocation under anesthesia when the tumors reached 3,000 mm3 in size. Tumor volumes were compared between the control and the treatment groups and evaluated by the Student’s test using the SAS system 6.12 software package (SAS Inc., Cary, NC, USA).
In vivo imaging studies
Multidrug resistance human xenografts were established in BALB/C nude mice as described previously. Diabody was labeled with the fluorescent dye Cy5 (Amersham, UK) according to the Manufacturers’ protocol. Retained activity of the labeled antibody was confirmed by flow cytometric analysis. Labeled diabody (100 μg/mouse) or free Cy5 was injected intravenously when tumors were 0.5–1 cm in diameter. The fluorescence emission was recorded at 12, 24, 48 and 72 h post-injection with a whole-body small-animal imaging system (Kodak image station in vivo FX, USA) when animals were anesthetized. Each group consisted of seven animals.
Results
Production of the ds-Diabody
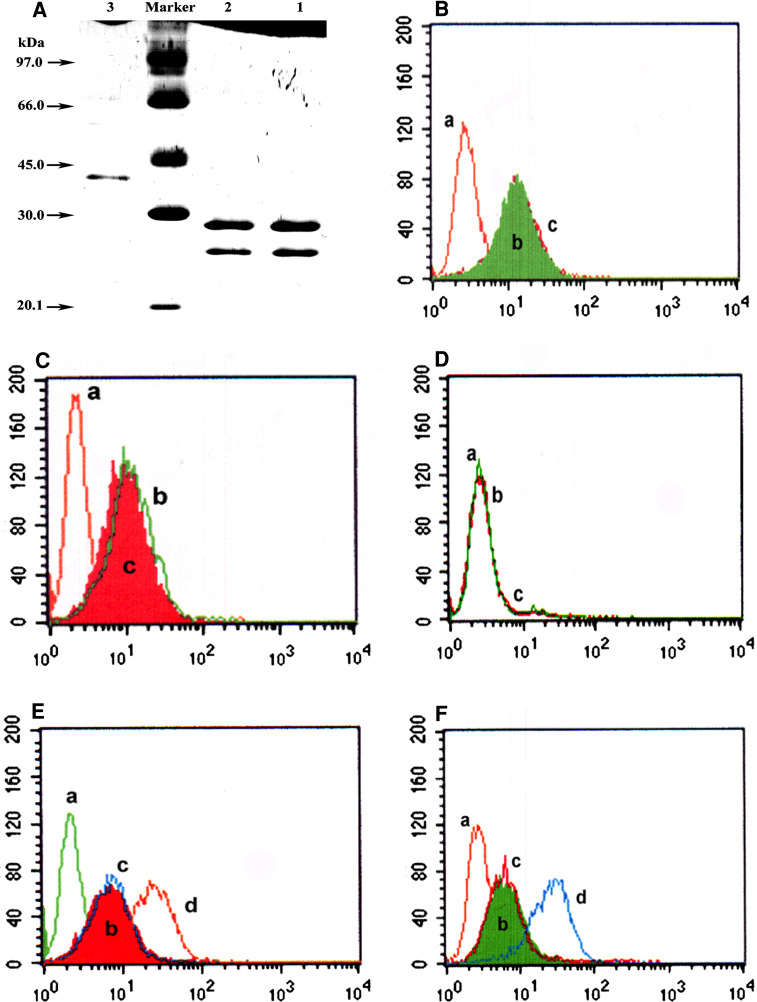
The two hetero-mutated polypeptide chain fragments, pLH-T3a*-Pgp and pLH-Pgp-T3a*, were each separately obtained after secretion from E. coli harboring the appropriate expression plasmid and were localized to the periplasmic space of E. coli, under the guidance of the leader peptide. A disulphide bond was formed between the two hetero-mutated fragments in the periplasmic space. The soluble ds-Diabody was released from the periplasmic space by osmotic shock and purified with a cation-exchange chromatography followed by an anti-E tag affinity chromatography. The final yield of purified ds-Diabody was 0.5–0.8 mg/L. The purified ds-Diabody was analyzed by SDS-PAGE under reducing and non-reducing conditions (Fig. 1A). Under reducing conditions, the ds-Diabody resolved into two protein bands, which is consistent with the calculated molecular weights of 28.3 and 26.1 kDa, for the pLH-T3a*-Pgp and pLH-Pgp-T3a* polypeptide chains, respectively. The ds-Diabody migrated at an apparently lower molecular weight than the expected 54.4 kDa due to the conformational effects of intact inter- and intra-chain disulphide bonds [6].
Fig. 1.
Gel analysis of ds-Diabody and flow cytometry analysis of the specificity binding of the diabody. A SDS-PAGE of purified ds-Diabody. Lane 1, parent diabody in reducing loading buffer; lane 2, ds-Diabody in reducing loading buffer; lane 3, ds-diabody in non-reducing loading buffer. Binding to K562/A02 (B), Jurkat (C), or K562 cells (D) by non-related antibody (a), ds-Diabody (b), and parent diabody (c). Competitive binding assay for competing diabody. K562/A02 (E) or Jurkat cells (F) were first incubated with ds-Diabody (b), or parent diabody (c) and reacted with parent mAb IgG (PHMA02 was used for K562/A02 and HIT3a for Jurkat cells, respectively), the cells were then incubated with an FITC conjugated anti-mouse IgG. Control groups only reacted with non-related antibody (a), or parent mAb IgG (d)
Antigen binding of the ds-Diabody
The binding specificities of the diabody, determined by flow cytometry analysis, using CD3-positive Jurkat and Pgp-positive K562/A02 cells, are shown in Fig. 1B, C. No binding was detectable on K562 cells, which express neither CD3 nor Pgp (Fig. 1D). To confirm the specificity of the diabody for Pgp and CD3, blocking tests were performed. Flow cytometry analysis showed that the competing diabody could significantly inhibit the binding of parent mAbs (PHMA02 and HIT3a) to K562/A02 or Jurkat cells (Fig. 1E, F). By comparison, the ds-Diabody had an affinity comparable with its parent diabody (Table 1). No significant affinity decreasing was detected.
Table 1.
Binding affinity of the ds-Diabody and parent diabody to their respective target antigens as determined by flow cytometry
| Antibody | K562/A02 cells (nM) | Jurkat cells (nM) |
|---|---|---|
| Parent diabody | 49 ± 6.2 | 16 ± 5.6 |
| ds-Diabody | 48 ± 7.8 | 16 ± 4.8 |
Specific cytotoxic activity against Pgp-positive cell lines
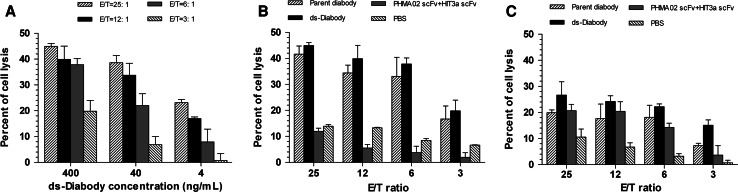
To investigate the ability of the ds-Diabody to induce lyses Pgp-overexpressing tumor cells in the presence of pre-activated human PBL, a non-radioactive cytotoxicity assay was performed. Lysis of Pgp-positive K562/A02 cells in the presence of human PBLs was specifically mediated by the ds-Diabody, in a dose-dependent manner, as shown in Fig. 2a. Enhanced target cell cytotoxicity was observed when the E:T ratio increased (Fig. 2a, b). A mixture of equal amounts of anti-Pgp scFv (PHMA02 scFv) and anti-CD3 scFv (HIT3a scFv) was much less potent than the ds-Diabody in mediating K562/A02 cell lysis (Fig. 2b). Also the ds-Diabody was much less potent in mediating K562 cell lysis, when the cells expressed neither CD3 nor Pgp (Fig. 2c). The scFv mixture showed the same potency towards both K562/A02 and K562 cells (Fig. 2b, c). Importantly, the ds-Diabody was as efficacious as the parent diabody. There was no statistical difference (P = 0.6) between parent diabody and ds-Diabody (400 ng/mL) in mediating the lysis of K562/A02 cells, at various E:T ratios (Fig. 2b). These results indicate that the killing effect is dependent on expression of Pgp on the target cells and that the introduced disulphide bridge does not affect the cytotoxicity against Pgp-positive cells.
Fig. 2.
Cytotoxicity of human PBL by ds-Diabody or parent diabody in a non-radioactive cytotoxicity assay. Cytotoxicity of PBL towards K562/A02 cells mediated by ds-Diabody in different concentrations at various E:T ratios (a). Cytotoxicity of PBL towards K562/A02 cells (b) or K562 cells (c) at different E:T ratios in the presence of PBS, a mixture of the PHMA02 scFv and HIT3a scFv, ds-Diabody, or the parent diabody. The antibody concentration used was 400 ng/mL. Data shown are the mean ± SD of triplicate determinations
In vitro stability of the ds-Diabody
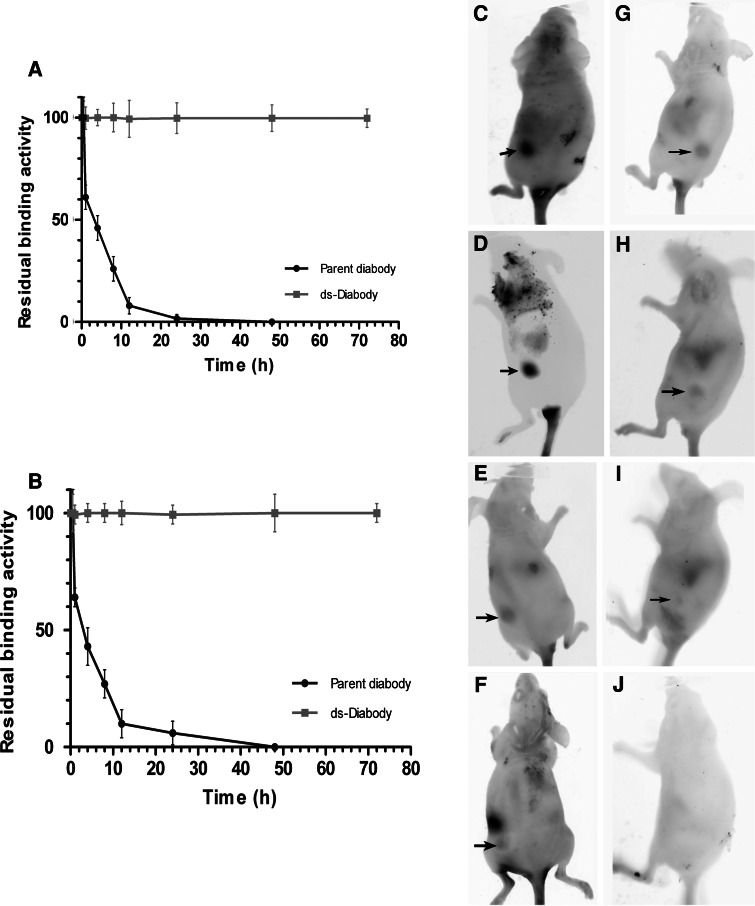
The stability of ds-Diabody was evaluated by flow cytometry after incubation in PBS containing 0.2% (w/v) HSA at 37°C for prolonged periods of time (Fig. 3a, b). After incubation for 4 h, the residual binding activity of the parent diabody dropped to approximately 50% of its initial value, and was undetectable after incubation for 24 h for the Pgp scFv components and for 48 h for the CD3 scFv components, respectively. By contrast, the ds-Diabody retained full antigen binding capacity after 72 h and retained approximately 80% of its antigen binding capacity even after 1 week (data not shown), for each scFv moiety. Taken together, introducing a disulphide bond into CD3 scFv of the diabody resulted in a marked increase of its stability in serum.
Fig. 3.
The in vitro and in vivo stability of the ds-Diabody in comparison with the parent diabody. Serum stability of the anti-Pgp-scFv component of the ds-Diabody and the parent diabody (a). Stability of the anti-CD3-scFv component of the ds-Diabody and the parent diabody (b). All data are normalized to time-point t 0 = 100%. Data shown are the mean percentage of residual binding ± SD of three independent experiments. Comparison of tumor localization between the ds-Diabody and parent diabody. Tumor localization by the ds-Diabody (c–f). Tumor localization by the parent diabody (g–j). The images were taken 2 (c, g), 12 (d, h), 24 (e, i), and 72 h (f, j) post-intravenous injection
In vivo stability of the ds-Diabody
In vivo near-infrared fluorescent (Cy5) imaging was performed to assess the stability and the effect of the di-Diabody on tumor localization, using Pgp-overexpressing xenografts established in BALB/C nude mice. All mice with xenografts showed apparent fluorescent spots in the tumor tissue after 2 h (Fig. 3c, d). The fluorescence of the tumor xenografts injected with labeled ds-Diabody was more intense than the labeled parent diabody, at each time point (Fig. 3c–j). After 24 h, staining by the parent diabody was poor, whereas the ds-Diabody showed clear staining (Fig. 3e, i). Intense tumor staining could be detected for the ds-Diabody, while no fluorescence for parent diabody was apparent at 72 h post-injection (Fig. 3f, j). The dye alone did not show any staining within tumors (data not shown).
Experimental immunotherapy in xenografted nude mice
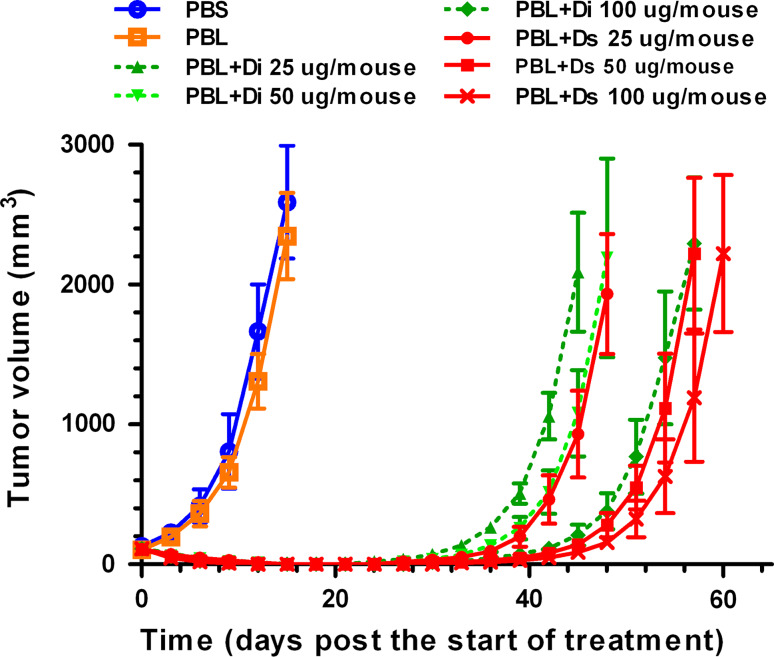
To determine whether the disulphide stabilized diabody had greater antitumor activity than the parent diabody, we established a nude mice model bearing the Pgp-overexpressing K562/A02 xenografts. The mice were treated with the ds-Diabody, parent diabody, pre-activated PBL alone, or a combination of the ds-Diabody or parent diabody, with the pre-activated PBL. Three doses (25, 50, and 100 μg/mouse) of diabody were chosen to assess the ability to cause regressions of established xenografts in nude mice. The antitumor activity of the ds-Diabody and the parent diabody is shown in Fig. 4. Control animals received only diluent, PBL, three doses of ds-Diabody or parent diabody (data not shown) developed large tumors and were sacrificed on day 15, when the tumors had grown to approximately 3,000 mm3 in size. Compared with the mice in the control groups, the mice treated with ds-Diabody or the parent diabody combined with PBL showed significant dose-related tumor regressions. The antitumor activity of the ds-Diabody was better than that of the parent diabody when equivalent doses of antibody and PBL were compared. When the dose for the ds-Diabody was decreased twofold, the antitumor activity for both the stabilized and un-stabilized diabodies were similar (Fig. 4), indicating that the antitumor activity of the disulphide stabilized diabody was approximately twofold greater than its non-stabilized counterpart.
Fig. 4.
Antitumor effect of ds-Diabody and parent diabody in nude mice bearing Pgp-overexpressing K562/A02 tumors. Animals were injected with 2 × 107 K562/A02 cells and treated intravenously on days 0, 7, and 14. Control groups received PBS or PBL only. The size of the xenografts was measured every other day. Error bars indicate SD (n = 5)
Discussion
In this study, we have constructed a recombinant anti-Pgp/anti-CD3 diabody, in which the heterodimer is stabilized by a disulphide bond. Compared with other approaches to generate bivalent dsFv, our approach is simple, resulting in a soluble ds-Diabody with high yield in E. coli. The ds-Diabody had the same antigen binding specificity and cytotoxic activity as the parent diabody. Furthermore, the disulphide cross-linking of the Fv fragments led to improved serum stability. The parent diabody begins to be inactivated at 1 h post incubation in 37°C PBS with a t 1/2 of ~4 h (Fig. 3a, b). By contrast, the ds-Diabody retains 80% activity even after 1 week. Thus, compared with the parent diabody, the more stable ds-Diabody may penetrate deeper into a tumor before losing activity. In addition, according to the “binding site barrier” theory, tumor cells can form a barrier preventing an antibody from reaching the center of the tumor mass [22]. Thus, the number of freely diffusing antibodies that reach deeper regions of the tumor will be diminished, whereas the more stable ds-Diabody should not be subject to this effect. This is confirmed by the data shown in Fig. 3c–j, the ds-Diabody persisting in the tumor longer than the parent diabody. Taken together, the more active ds-Diabody penetrates into the tumor and stays longer in the tumor, resulting in a twofold greater antitumor activity compared to the non-stabilized counterpart. Put another way, to achieve the same efficacy, the dose of diabody can be reduced by half.
In recent years, it has become evident that acute myeloid leukemia (AML) is a disease originating from CD34+CD38− hematopoietic stem cells [19]. Evidence for the existence of a subpopulation called “leukemic stem cells” (LSCs) in AML has come from studies using the non-obese diabetic-severe combined immunodeficient (NOD/SCID) mouse model, showing that cells with leukemic engraftment and self-renewal potential are in the CD34+CD38− subpopulation [20]. Conventional chemotherapies kill differentiated or differentiating tumor cells, whilst being ineffective against LSCs. Moreover, it has been reported that Pgp expression is significantly higher in LSCs compared to other AML cell subpopulations [7]. Thus, LSCs can survive chemotherapy and can re-grow. Chronic myeloid leukemia (CML) is also a disease originating from LSCs [18].
Treatment with imatinib has greatly improved the outcome of CML but patients often relapse. It has been suggested that CML LSCs survive the treatment with imatinib, while evidence supports the hypothesis that Pgp plays a role in resistance against the drug. Elsewhere treatment with imatinib has been shown to cause selected expression of Pgp and other ABC transporters [36]. Taken together, this suggests that Pgp could be a promising target for overcoming myeloid leukemia. However, ABCB1 inhibitors have not been able to achieve complete remission. One of the reasons may be that although the expression of ABCB1 could render LSCs resistant to drugs, there may be other factors involved in resistance.
There are other mechanisms involved in the ability of LSCs to evade chemotoxicity, such as the simultaneous expression of other ABC transporters, damage of the DNA-repair capacity, reluctance to enter apoptosis etc. Notably, in one study it was shown that although Pgp is over-expressed in LSCs, the efflux function is reduced, compared with residual normal progenitors [27]. Regarding the anti-Pgp/anti-CD3 diabody, there is a recognition of the antigen epitope of Pgp that is independent of the efflux function. In spite of the fact that Pgp-mediated drug extrusion is reduced in LSCs, the anti-Pgp/anti-CD3 diabody can still direct T cells to Pgp-over-expressing LSCs and may thus achieve complete remission. Interestingly, it has been reported that MDR1 (+) cells can be considered to be melanoma stem cells [24]. In this regard Pgp may be a unique target for anti-melanoma therapies in the future. Taken together, the anti-Pgp/anti-CD3 diabody could target tumor stem cells and may be a very promising antibody for tumor immunotherapy.
In summary, the results of this study indicate that a disulphide linked diabody has improved serum stability, improved antitumor activity, enhanced tumor targeting properties and persists longer in MDR tumors. The use of a ds-Diabody approach may therefore provide a more potent treatment of human tumors.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant number: 30400558) and from the Natural Science Foundation of Tianjin (Grant number: 08ZCKFSH04100, 05YFJZJC01200).
Contributor Information
Yingdai Gao, Email: gaoyingdai@hotmail.com.
Dongsheng Xiong, Email: dsxiong1961@yahoo.com.cn.
References
- 1.Benhar I, Pastan I. Characterization of B1(Fv)PE38 and B1(dsFv)PE38: single-chain and disulfide-stabilized Fv immunotoxins with increased activity that cause complete remissions of established human carcinoma xenografts in nude mice. Clin Cancer Res. 1995;1:1023–1029. [PubMed] [Google Scholar]
- 2.Bera TK, Onda M, Brinkmann U, Pastan I. A bivalent disulfide-stabilized Fv with improved antigen binding to erbB2. J Mol Biol. 1998;281:475–483. doi: 10.1006/jmbi.1998.1948. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann U, Reiter Y, Jung SH, Lee B, Pastan I. A recombinant immunotoxin containing a disulfide-stabilized Fv fragment. Proc Natl Acad Sci USA. 1993;90:7538–7542. doi: 10.1073/pnas.90.16.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buhler P, Wolf P, Gierschner D, Schaber I, Katzenwadel A, Schultze-Seemann W, Wetterauer U, Tacke M, Swamy M, Schamel WW, Elsasser-Beile U. A bispecific diabody directed against prostate-specific membrane antigen and CD3 induces T-cell mediated lysis of prostate cancer cells. Cancer Immunol Immunother. 2008;57:43–52. doi: 10.1007/s00262-007-0348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cochlovius B, Kipriyanov SM, Stassar MJJG, Christ O, Schuhmacher J, Strau G, Moldenhauer G, Little M. Treatment of human B Cell lymphoma xenografts with a CD3 CD19 diabody and T Cells. J Immunol. 2000;165:888–895. doi: 10.4049/jimmunol.165.2.888. [DOI] [PubMed] [Google Scholar]
- 6.Creighton TE. Disulfide bond formation in proteins. Methods Enzymol. 1984;107:305–329. doi: 10.1016/0076-6879(84)07021-X. [DOI] [PubMed] [Google Scholar]
- 7.de Figueiredo-Pontes LL, Pintao MC, Oliveira LC, Dalmazzo LF, Jacomo RH, Garcia AB, Falcao RP, Rego EM. Determination of P-glycoprotein, MDR-related protein 1, breast cancer resistance protein, and lung-resistance protein expression in leukemic stem cells of acute myeloid leukemia. Cytometry B Clin Cytom. 2008;74:163–168. doi: 10.1002/cyto.b.20403. [DOI] [PubMed] [Google Scholar]
- 8.Deuchars KL, Ling V. P-glycoprotein and multidrug resistance in cancer chemotherapy. Semin Oncol. 1989;16:156–165. [PubMed] [Google Scholar]
- 9.Dreier T, Baeuerle PA, Fichtner I, Grun M, Schlereth B, Lorenczewski G, Kufer P, Lutterbuse R, Riethmuller G, Gjorstrup P, Bargou RC. T cell costimulus-independent and very efficacious inhibition of tumor growth in mice bearing subcutaneous or leukemic human B cell lymphoma xenografts by a CD19-/CD3- bispecific single-chain antibody construct. J Immunol. 2003;170:4397–4402. doi: 10.4049/jimmunol.170.8.4397. [DOI] [PubMed] [Google Scholar]
- 10.Dreier T, Lorenczewski G, Brandl C, Hoffmann P, Syring U, Hanakam F, Kufer P, Riethmuller G, Bargou R, Baeuerle PA. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer. 2002;100:690–697. doi: 10.1002/ijc.10557. [DOI] [PubMed] [Google Scholar]
- 11.Endicott JA, Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- 12.FitzGerald K, Holliger P, Winter G. Improved tumour targeting by disulphide stabilized diabodies expressed in Pichia pastoris. Protein Eng. 1997;10:1221–1225. doi: 10.1093/protein/10.10.1221. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y, Xiong D, Yang M, Liu H, Peng H, Shao X, Xu Y, Xu C, Fan D, Qin L, Yang C, Zhu Z. Efficient inhibition of multidrug-resistant human tumors with a recombinant bispecific anti-P-glycoprotein x anti-CD3 diabody. Leukemia. 2004;18:513–520. doi: 10.1038/sj.leu.2403267. [DOI] [PubMed] [Google Scholar]
- 14.Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi H, Asano R, Tsumoto K, Katayose Y, Suzuki M, Unno M, Kodama H, Takemura S, Yoshida H, Makabe K, Imai K, Matsuno S, Kumagai I, Kudo T. A highly effective and stable bispecific diabody for cancer immunotherapy: cure of xenografted tumors by bispecific diabody and T-LAK cells. Cancer Immunol Immunother. 2004;53:497–509. doi: 10.1007/s00262-003-0465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hicklin DJ, Marincola FM, Ferrone S. HLA class I antigen downregulation in human cancers: T-cell immunotherapy revives an old story. Mol Med Today. 1999;5:178–186. doi: 10.1016/S1357-4310(99)01451-3. [DOI] [PubMed] [Google Scholar]
- 17.Holliger P, Prospero T, Winter G. “Diabodies”: small bivalent and bispecific antibody fragments. Proc Natl Acad Sci USA. 1993;90:6444–6448. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holyoake T, Jiang X, Eaves C, Eaves A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood. 1999;94:2056–2064. [PubMed] [Google Scholar]
- 19.Huntly BJ, Gilliland DG. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer. 2005;5:311–321. doi: 10.1038/nrc1592. [DOI] [PubMed] [Google Scholar]
- 20.Huntly BJ, Gilliland DG. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer. 2005;5:311–321. doi: 10.1038/nrc1592. [DOI] [PubMed] [Google Scholar]
- 21.Johnsen AK, Templeton DJ, Sy M, Harding CV. Deficiency of transporter for antigen presentation (TAP) in tumor cells allows evasion of immune surveillance and increases tumorigenesis. J Immunol. 1999;163:4224–4231. [PubMed] [Google Scholar]
- 22.Juweid M, Neumann R, Paik C, Perez-Bacete MJ, Sato J, van Osdol W, Weinstein JN. Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res. 1992;52:5144–5153. [PubMed] [Google Scholar]
- 23.Kageshita T, Hirai S, Ono T, Hicklin DJ, Ferrone S. Down-regulation of HLA class I antigen-processing molecules in malignant melanoma: association with disease progression. Am J Pathol. 1999;154:745–754. doi: 10.1016/S0002-9440(10)65321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keshet GI, Goldstein I, Itzhaki O, Cesarkas K, Shenhav L, Yakirevitch A, Treves AJ, Schachter J, Amariglio N, Rechavi G. MDR1 expression identifies human melanoma stem cells. Biochem Biophys Res Commun. 2008;368:930–936. doi: 10.1016/j.bbrc.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 25.Ling V. Multidrug resistance: molecular mechanisms and clinical relevance. Cancer Chemother Pharmacol. 1997;40(suppl):S3–S8. doi: 10.1007/s002800051053. [DOI] [PubMed] [Google Scholar]
- 26.Moscow JA, Schneider E, Ivy SP, Cowan KH. Multidrug resistance. Cancer Chemother Biol Response Modif. 1997;17:139–177. [PubMed] [Google Scholar]
- 27.Raaijmakers MH, de Grouw EP, van der Reijden BA, de Witte TJ, Jansen JH, Raymakers RA. ABCB1 modulation does not circumvent drug extrusion from primitive leukemic progenitor cells and may preferentially target residual normal cells in acute myelogenous leukemia. Clin Cancer Res. 2006;12:3452–3458. doi: 10.1158/1078-0432.CCR-05-1945. [DOI] [PubMed] [Google Scholar]
- 28.Rees RC, Mian S. Selective MHC expression in tumours modulates adaptive and innate antitumour responses. Cancer Immunol Immunother. 1999;48:374–381. doi: 10.1007/s002620050589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reiter Y, Brinkmann U, Jung SH, Lee B, Kasprzyk PG, King CR, Pastan I. Improved binding and antitumor activity of a recombinant anti-erbB2 immunotoxin by disulfide stabilization of the Fv fragment. J Biol Chem. 1994;269:18327–18331. [PubMed] [Google Scholar]
- 30.Reiter Y, Kreitman RJ, Brinkmann U, Pastan I. Cytotoxic and antitumor activity of a recombinant immunotoxin composed of disulfide-stabilized anti-Tac Fv fragment and truncated Pseudomonas exotoxin. Int J Cancer. 1994;58:142–149. doi: 10.1002/ijc.2910580123. [DOI] [PubMed] [Google Scholar]
- 31.Reiter Y, Pai LH, Brinkmann U, Wang QC, Pastan I. Antitumor activity and pharmacokinetics in mice of a recombinant immunotoxin containing a disulfide-stabilized Fv fragment. Cancer Res. 1994;54:2714–2718. [PubMed] [Google Scholar]
- 32.Schaich M, Soucek S, Thiede C, Ehninger G, Illmer T. MDR1 and MRP1 gene expression are independent predictors for treatment outcome in adult acute myeloid leukaemia. Br J Haematol. 2005;128:324–332. doi: 10.1111/j.1365-2141.2004.05319.x. [DOI] [PubMed] [Google Scholar]
- 33.Shen DC, Yang XF. A high affinity CD3 monoclonal antibody HIT3a. Acta Academiae Medicinae Sinicae. 1993;15:157–162. [PubMed] [Google Scholar]
- 34.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/S1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 35.Stopeck AT, Gessner A, Miller TP, Hersh EM, Johnson CS, Cui H, Frutiger Y, Grogan TM. Loss of B7.2 (CD86) and intracellular adhesion molecule 1 (CD54) expression is associated with decreased tumor-infiltrating T lymphocytes in diffuse B-cell large-cell lymphoma. Clin Cancer Res. 2000;6:3904–3909. [PubMed] [Google Scholar]
- 36.Stromskaya TP, Rybalkina EY, Kruglov SS, Zabotina TN, Mechetner EB, Turkina AG, Stavrovskaya AA. Role of P-glycoprotein in evolution of populations of chronic myeloid leukemia cells treated with imatinib. Biochemistry (Mosc) 2008;73:29–37. doi: 10.1134/s0006297908010045. [DOI] [PubMed] [Google Scholar]
- 37.Tsukamoto F, Shiba E, Taguchi T, Sugimoto T, Watanabe T, Kim SJ, Tanji Y, Kimoto Y, Izukura M, Takai SI. Immunohistochemical Detection of P-glycoprotein in Breast Cancer and Its Significance as a Prognostic Factor. Breast Cancer. 1997;4:259–263. doi: 10.1007/BF02966518. [DOI] [PubMed] [Google Scholar]
- 38.Xiong DS, Yang CZ. Generation and characterization of monoclonal antibodies against P-glycoprotein (Pgp) Clin J Hematol. 1999;20:326–327. [Google Scholar]
- 39.Yang CZ, Luan FJ, Xiong DS, Liu BR, Xu YF, Gu KS. Multidrug resistance in leukemic cell line K562/A02 induced by doxorubicin. Zhongguo Yao Li Xue Bao. 1995;16:333–337. [PubMed] [Google Scholar]
- 40.Yokota T, Milenic DE, Whitlow M, Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992;52:3402–3408. [PubMed] [Google Scholar]
- 41.Zhu Z, Rockwell P, Lu D, Kotanides H, Pytowski B, Hicklin DJ, Bohlen P, Witte L. Inhibition of vascular endothelial growth factor-induced receptor activation with anti-kinase insert domain-containing receptor single-chain antibodies from a phage display library. Cancer Res. 1998;58:3209–3214. [PubMed] [Google Scholar]
- 42.Zhu Z, Zapata G, Shalaby R, Snedecor B, Chen H, Carter P. High level secretion of a humanized bispecific diabody from Escherichia coli . Biotechnology (NY) 1996;14:192–196. doi: 10.1038/nbt0296-192. [DOI] [PubMed] [Google Scholar]