Abstract
Despite the clinical success of CD20-specific antibody rituximab, malignancies of B-cell origin continue to present a major clinical challenge, in part due to an inability of the antibody to activate antibody-dependent cell-mediated cytotoxicity (ADCC) in some patients, and development of resistance in others. Expression of chimeric antigen receptors in effector cells operative in ADCC might allow to bypass insufficient activation via FcγRIII and other resistance mechanisms that limit natural killer (NK)-cell activity. Here we have generated genetically modified NK cells carrying a chimeric antigen receptor that consists of a CD20-specific scFv antibody fragment, via a flexible hinge region connected to the CD3ζ chain as a signaling moiety. As effector cells we employed continuously growing, clinically applicable human NK-92 cells. While activity of the retargeted NK-92 against CD20-negative targets remained unchanged, the gene modified NK cells displayed markedly enhanced cytotoxicity toward NK-sensitive CD20 expressing cells. Importantly, in contrast to parental NK-92, CD20-specific NK cells efficiently lysed CD20 expressing but otherwise NK-resistant established and primary lymphoma and leukemia cells, demonstrating that this strategy can overcome NK-cell resistance and might be suitable for the development of effective cell-based therapeutics for the treatment of B-cell malignancies.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-007-0383-3) contains supplementary material, which is available to authorized users.
Keywords: CD20, scFv antibody, Natural killer cell, Chimeric antigen receptor, Adoptive therapy
Introduction
Introduction of the chimeric anti-CD20 antibody rituximab (MabThera, Rituxan) into clinical practice has considerably changed therapeutic strategies for non-Hodgkin lymphomas (NHL) and other B-cell malignancies [1]. The CD20 antigen is a 33–37 kDa transmembrane phosphoprotein expressed at high levels on most mature B-cells and B-cell lymphomas, but not B-cell progenitors and plasma cells. It harbors four membrane-spanning hydrophobic regions, with N- and C-termini of the protein located in the cytoplasm. So far no natural ligand for CD20 has been described and its physiological role is still poorly defined [2, 3]. It appears to be constitutively organized in lipid rafts, and is thought to be involved in calcium conductance. CD20 is not internalized, down-modulated or shed, further contributing to its attractiveness as a target for monoclonal antibody (Mab) therapy. Rituximab alone or in combination with chemotherapy was shown to be effective against low and high-grade NHL [4–7]. However, despite this success of anti-CD20 antibody therapy, NHL and other malignancies of B-cell origin including chronic lymphocytic leukemia (CLL) and acute lymphoblastic leukemia (ALL) continue to present a major clinical challenge.
While direct effects such as inhibition of proliferation and induction of apoptosis have been observed in vitro [8], studies linking therapeutic efficacy of rituximab with genetic polymorphisms in the FcγRIII suggest antibody-dependent cell-mediated cytotoxicity (ADCC) as a critical mechanism for rituximab action in cancer patients [9, 10]. An inability to activate the cytotoxic activity of natural killer (NK) and other immune effector cells might at least in part explain why only 30–50% of NHL patients respond to initial rituximab treatment [6, 11, 12]. In addition, most patients with CD20-positive NHL will eventually become resistant. Only 40% of patients who had a prior partial or complete response to single-agent rituximab responded to re-treatment after relapse [13]. Expression of chimeric antigen receptors in the effector cells operative in ADCC might allow to bypass insufficient activation via FcγRIII and other resistance mechanisms that limit NK-cell activity. Retargeting of NK cells to cancer cells derived from solid tumors with an ErbB2-specific antigen receptor construct resulted in efficient lysis of otherwise NK-resistant but ErbB2-expressing target cells in vitro, and enhanced tumor localization and anti-tumoral activity in vivo [14, 15]. Likewise, expression of a receptor construct binding to CD19 augmented NK-cell activity and induced specific killing of leukemic cells [16].
Here we have generated genetically modified NK cells carrying a CD20-specific chimeric antigen receptor, and have investigated their cytotoxic activity against established and primary lymphoma and leukemia cells. As effector cells we have employed continuously growing human NK-92 cells. High-intrinsic cytotoxic activity of NK-92 against malignant cells of various origin has been demonstrated in in vitro models and in humanized mouse models in vivo [17–19], and clinical studies investigating safety and efficacy of cellular immunotherapy with unmodified NK-92 cells are ongoing [20]. NK-92 display functional characteristics of activated NK cells. They express typical NK-cell surface receptors, but lack FcγRIII [21]. Their high-endogenous cytotoxic potential is most likely due to the absence of inhibitory NK cell receptors with the exception of KIR2DL4 [20, 22], which through binding to HLA antigens on target cells inhibit NK cell cytolytic activity [23]. Nevertheless, similar to primary NK cells, NK-92 show little or no activity against many cell lines derived from solid tumors such as breast carcinoma [14]. Likewise, in a recent report nearly all B-lineage ALL cells tested proved to be resistant to NK-92 [24], providing a rationale for genetic modification of NK-92 with a chimeric antigen receptor that allows specific recognition of lymphoma and leukemia cells.
To specifically enhance cytotoxic activity of NK-92 against CD20 expressing B-cell malignancies, we have transduced NK-92 cells with a retroviral vector encoding a chimeric antigen receptor that consists of a scFv fragment of the CD20-specific antibody Leu-16 [25], via a flexible hinge region connected to the CD3ζ chain as a signaling moiety. While activity of the gene-modified NK-92 cells against CD20-negative NK-cell targets remained unchanged, NK-92-scFv(Leu-16)-ζ displayed enhanced cytotoxicity toward NK-sensitive CD20 expressing cells. Furthermore, in contrast to parental NK-92, NK-92-scFv(Leu-16)-ζ cells efficiently lysed CD20 expressing but NK-resistant lymphoma and leukemia cells, demonstrating that this strategy can overcome NK-cell resistance and might be suitable for the development of effective cell-based therapeutics for the treatment of B-cell malignancies.
Materials and methods
Cells and culture conditions
Raji Burkitt’s lymphoma cells, DOHH-2 and WSU-NHL follicular lymphoma [26], BV173 and NALM-6 acute B-precursor ALL [24], and erythroleukemic K562 cells were maintained in RPMI-1640 medium (Cambrex, Verviers, Belgium) containing standard supplements (10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin). NIH3T3 fibroblasts and NIH3T3-CD20 cells expressing human CD20 were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Cambrex) containing standard supplements and 2 μg/ml puromycin (NIH3T3-CD20). NK-92 cells and genetically modified NK-92-scFv(Leu-16)-ζ cells were propagated in X-VIVO 10 medium (Cambrex) supplemented with 5% heat-inactivated human serum, 100 U/ml IL-2 (Proleukin, Chiron Corp., Emeryville, CA), and 0.6 mg/ml G418 (NK-92-scFv(Leu-16)-ζ).
Primary CLL cells were obtained with the patients’ informed consent from routine peripheral blood samples. Mononuclear cells were enriched by density gradient centrifugation (Pancoll, PAN Biotech, Aidenbach, Germany), analyzed for expression of CD20 by flow cytometry, and directly used for cytotoxicity experiments. Human B and NK cells were isolated from buffy coats of healthy donors using RosetteSep Human NK Cell and RosetteSep Human B-Cell Enrichment Cocktail (StemCell Technologies, UK) according to the manufacturer’s recommendations. Enriched B-cells were directly used for analysis. Enriched NK cells were cultured for 3 days at a density of 1 × 106 cells/ml in X-VIVO 10 medium supplemented with 5% heat-inactivated human serum and 1,000 U/ml IL-2.
Construction of a chimeric antigen receptor carrying CD20-specific scFv(Leu-16)
cDNA fragments of heavy- (VH) and light-chain variable domains (VL) of CD20-specific Leu-16 antibody were derived by RT-PCR of mRNA from Leu-16 hybridoma cells (kindly provided by BD Biosciences, San Jose, CA, USA) and linked by overlap extension with a GS-linker using the Mouse ScFv Module kit (Amersham Biosciences, Freiburg, Germany) according to the manufacturer’s recommendations. The resulting scFv(Leu-16) PCR product was re-amplified adding SfiI and NotI restriction sites and cloned into pHEN2 [27]. The scFv sequence was verified to correspond to the previously published sequences of Leu-16 VH and VL [25].
For construction of the CD20-specific antigen receptor, the scFv(Leu-16) fragment from the resulting pHEN2-scFv(Leu-16) plasmid was assembled stepwise in frame with an immunoglobulin heavy-chain signal peptide (SP) sequence 5′ of the scFv, and sequences encoding a Myc-tag, the hinge region of CD8α (amino acids 105–165) and CD3ζ chain 3′ of the scFv in plasmid pGEM-1 (Promega, Mannheim, Germany). The complete chimeric receptor sequence was derived from the resulting pGEM-1-scFv(Leu-16)-ζ construct as an SalI, SmaI fragment and cloned into the SalI, HpaI restriction sites of a modified pLXSN retroviral vector [28] yielding pL-scFv(Leu-16)-ζ-SN.
Periplasmic expression of scFv(Leu-16) in E. coli
CD20-specific scFv(Leu-16), and an ErbB2-specific scFv(FRP5) fragment as a control were subcloned into the bacterial expression vector pSW50 creating in-frame fusions with an N-terminal ompA SP and FLAG epitope, and C-terminal Myc- and polyhistidine tags [29]. The resulting expression constructs were transformed into Escherichia coli XL-1 blue, and single colonies were grown in 500 ml LB medium to an OD600 of 0.8. Then protein expression was induced by addition of 1 mM IPTG at 30°C for 3 h. Cells were harvested by centrifugation, and periplasm was extracted by incubating E. coli cells in 10 ml of buffer containing 50 mM Tris–HCl, pH 8, 20% sucrose, 1 mM EDTA for 1 h on ice. Cell debris was removed by centrifugation. The presence of scFv antibody fragments was confirmed by SDS-PAGE and immunoblot analysis with M2 anti-FLAG antibody followed by horseradish peroxidase (HRP)-conjugated secondary antibody.
Binding of recombinant scFv(Leu-16) to the surface of CD20 expressing cells was investigated by fluorescence activated cell sorter (FACS) analysis. Single cell suspensions (2 × 105) of Raji cells or SKBR3 breast carcinoma cells were incubated for 30 min at 4°C with 20 μl of scFv(Leu-16) or scFv(FRP5) containing periplasmic extracts. Cells were washed twice with phosphate-buffered saline (PBS), treated for another 30 min at 4°C with Myc-tag specific Mab 9E10 [30], washed again and then incubated for 30 min at 4°C with FITC-labeled goat anti-mouse IgG secondary antibody (BD Biosciences, Heidelberg, Germany). Fluorescence of cells was analyzed with a FACScan (BD Biosciences). These data are shown in supplementary Fig. S1.
Production of amphotropic retroviral vector and transduction of NK-92 cells
FLYA-JET packaging cells [31] were transfected with pL-scFv(Leu-16)-ζ-SN by electroporation using the Easyject Optima electroporation system (Equibio, Ashford, UK) with the following parameters: 20 μg of plasmid DNA per 1 × 106 cells in 0.8 ml of DMEM medium in a 0.4 cm cuvette, and ‘standard’ settings according to the manufacturer’s recommendations. Stable transfectants were selected for 1 week in DMEM growth medium containing 2.4 mg/ml G418. For production of amphotropic retroviral vector, FLYA-JET-scFv(Leu-16)-ζ cells were grown over night in NK-92 medium. Culture supernatant was passed through a 0.2 μm filter and incubated with NK-92 cells in the presence of 8 μg/ml polybrene for 5 h at 37°C. Then NK-92 cells were grown over night in fresh X-VIVO 10 medium, before G418 was added to a final concentration of 0.6 mg/ml for selection of NK-92-scFv(Leu-16)-ζ cells.
Analysis of chimeric antigen receptor expression
For RT-PCR, total RNA was isolated from NK-92-scFv(Leu-16)-ζ or parental NK-92 cells using the QIAGEN RNeasy kit (Qiagen, Hilden, Germany). RT-PCR was performed with the QIAGEN OneStep RT-PCR kit using oligonucleotides 5′-pLXSN (5′-CCCTTGAACCTCCTCGTTCGACC-3′) and 3′-pLXSN (5′-GAGCCTGGGGACTTTCCACACCC-3′). For immunoblot analysis, 5 × 106 NK-92-scFv(Leu-16)-ζ or NK-92 cells were harvested by centrifugation, washed with PBS and lysed for 20 min at 4°C in 1 ml of buffer containing 50 mM Tris–HCl, pH 7.5, 1% Triton X-100, 5 mM EGTA, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride. Lysates were cleared by centrifugation, separated by SDS-PAGE and immunoblotted with anti-CD3ζ Mab (BD Biosciences). For FACS analysis, 5 × 105 NK-92-scFv(Leu-16)-ζ or NK-92 cells were incubated for 30 min at 4°C with 1.5 μg of Mab 9E10. Cells were washed twice with PBS and then treated for another 30 min at 4°C with FITC-labeled goat anti-mouse IgG secondary antibody (BD Biosciences). Fluorescence of cells was analyzed with a FACScan.
Enrichment of scFv(Leu-16)-ζ expressing NK-92 cells
NK-92-scFv(Leu-16)-ζ cells expressing high levels of chimeric antigen receptor were enriched by sorting with magnetic beads or FACS. G418 resistant cells were incubated with Mab 9E10 (1.5 μg/5 × 105 cells) and selected using goat anti-mouse IgG MicroBeads and MACS LS+ separation columns (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. For sorting by FACS, cells were incubated with Mab 9E10 (3 μg/1 × 107 cells) followed by FITC-conjugated secondary antibody and separation with a FACSCalibur (BD Biosciences).
Generation of CD20 expressing fibroblasts
cDNA of human CD20 was amplified by RT-PCR from total RNA isolated from Raji cells, using oligonucleotides XhoI-CD20 (5′-AAAACTCGAGGCCACCATGACAACACCCAGAAATTCAG-3′) and CD20-XbaI (5′-TTTTCTAGATTAAGGAGAGCTGTCATTTTCTATTGGT-3′), and cloned into XhoI and XbaI sites of the bicistronic vector pEFIRES-P [32] resulting in plasmid pEFIRES-P-CD20. In this construct, expression of CD20 and the puromycin N-acetyltransferase (pac) resistance gene is driven by the human EF-1α promoter. NIH3T3 fibroblasts were transfected with pEFIRES-P-CD20 DNA by electroporation as described above for FLYA-JET cells. Stable transfectants were selected with puromycin (2 μg/ml) and analyzed by FACS with CD20-specific Mab L27 (BD Biosciences) followed by PE-labeled secondary antibody.
Cytotoxicity assays
Cytotoxicity of NK cells toward B-cell lymphoma, primary CLL and B-cells was analyzed by flow cytometry. About 2 × 106 target cells were incubated for 5 min at room temperature (RT) with the fluorescent dye PKH67-GL (Sigma, Deisenhofen, Germany) according to the manufacturer’s instructions. Incorporation of the dye was stopped by addition of heat-inactivated FBS. Then cells were washed twice with 15 ml of PBS. Target cells (4 × 104 in 100 μl RPMI growth medium/tube) were seeded in triplicates in polypropylene tubes before addition of 100 μl/tube of medium to determine spontaneous lysis, or 100 μl of NK-92 or NK-92-scFv(Leu-16)-ζ cell suspension at various effector to target ratios (E/T). The tubes were incubated for 2 h at 37°C. Cells were centrifuged for 5 min at 500×g, supernatant was removed and 200 μl/tube of a propidium iodide solution (1 μg/ml in PBS) were added. After incubation for 5–10 min at RT, fluorescence was determined using a FACScan. Specific cytotoxicity was calculated using CellQuestPro software (BD Biosciences). Dead target cells were determined as PKH67-GL and PI positive. The number of spontaneously lysed target cells was subtracted.
Cytotoxicity of NK cells toward adherent NIH3T3 fibroblasts was determined in an MTT viability assay. Target cells were seeded over night in 96-well plates at a density of 2 × 104 cells/well. Then NK-92 or NK-92-scFv(Leu-16)-ζ cells were added to triplicate samples at various E/T ratios in a total volume of 200 μl RPMI growth medium/well. After incubation for 3 h at 37°C, non-adherent effector cells and dead target cells were removed by repeated washing with PBS. Then 100 μl of RPMI growth medium and 10 μl of 10 mg/ml MTT (3-(4,5-dimethylthiazole-2-yl)-2,5 diphenyltetrazolium bromide) (Sigma) were added to each well, followed by incubation for another 2 h at 37°C. Cells were lysed by addition of 90 μl of 20% SDS in 50% N, N-dimethyl formamide, pH 5. After solubilization, absorbance at 590 nm was determined in a microplate reader (Dynatech, Denkendorf, Germany) as a measure for the relative number of viable cells.
Microscopy
Microscopic images of cells were taken using a Nikon TMS microscope and a Nikon Coolpix 990 camera (Nikon, Düsseldorf, Germany). To analyze kinetics of cell killing, microscopic images were taken at 1.5-min intervals using an Axiovert 135 microscope (Carl Zeiss, Göttingen, Germany) and a Sony 3CCD color video camera. Cropping of images and adjustment of brightness and contrast was performed with Adobe Photoshop software. The supplementary movie showing kinetics of target cell killing was created with Apple QuickTime Pro software.
In vivo anti-tumor activity
Raji cells (4 × 106) were mixed with 2 × 107 NK-92-scFv(Leu-16)-ζ or parental NK-92 cells (E/T ratio of 5:1) in 0.25 ml of PBS and immediately injected into the flanks of immunodeficient NOD/SCID γ−/−c mice (5–6 animals/group) (Charles River, Sulzfeld, Germany). Tumor growth was followed by caliper measurements, tumor volumes were calculated using the formula: length × (width)2 × 0.5, and data were statistically analyzed. Significance of differences between treatment groups was calculated by double-sided Student’s t-test. Mice were sacrificed when tumors reached 1.5 cm in diameter or when animals appeared to be in distress.
Results
Generation of natural killer cells carrying a CD20-specific chimeric antigen receptor
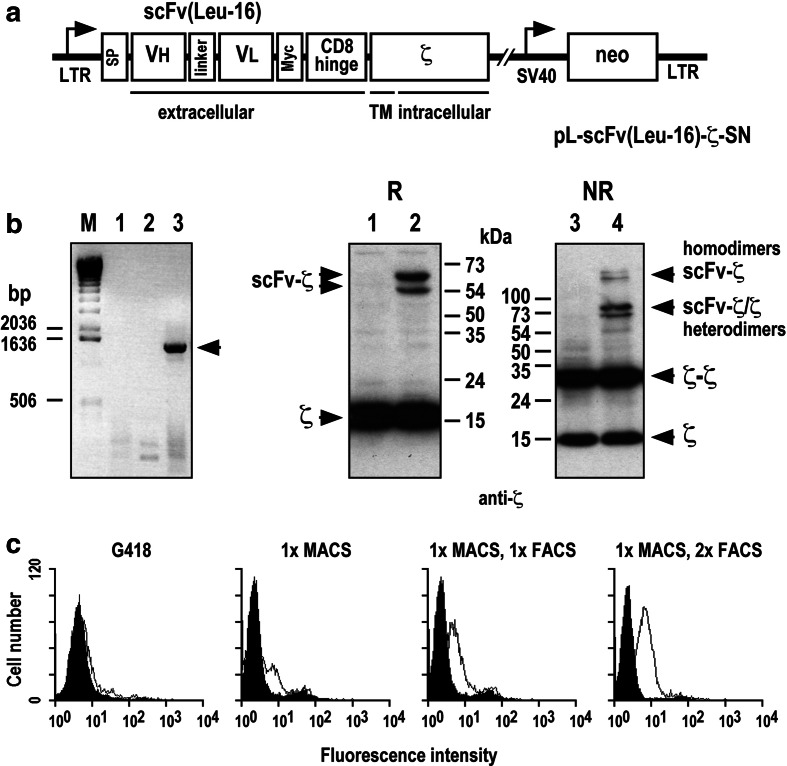
A CD20-specific scFv(Leu-16) antibody fragment was generated by linking cDNA fragments encoding heavy and light chain variable domains of Mab Leu-16 via a synthetic sequence encoding a (G4S)3 linker. Functionality of the antibody fragment was verified by flow cytometry using recombinant protein expressed in E. coli. Similar to CD20-specific Mab, recombinant scFv(Leu-16) from periplasmic extract displayed specific binding to the surface of CD20 expressing Raji cells, while no binding of the molecule to CD20-negative SKBR3 breast carcinoma cells could be detected (data shown in supplementary Fig. S1). A construct for expression of a CD20-specific chimeric antigen receptor in mammalian cells was then constructed by inserting the scFv(Leu-16) fragment in frame between a sequence encoding an N-terminal immunoglobulin heavy-chain SP, and sequences encoding a Myc-tag, the murine CD8α hinge region (amino acids 105–165), and the murine CD3ζ chain in the retroviral expression vector pLXSN [28]. A schematic representation of the resulting pL-scFv(Leu-16)-ζ-SN construct is shown in Fig. 1a.
Fig. 1.
Transduction of NK-92 cells with retroviral vector pL-scFv(Leu-16)-ζ. a Schematic representation of the pL-scFv(Leu-16)-ζ-SN construct. The Moloney murine leukemia virus 5′ long terminal repeat (LTR) controls the expression of the chimeric antigen receptor scFv(Leu-16)-ζ which consists of an N-terminal immunoglobulin heavy-chain leader peptide (SP), the CD20-specific single-chain antibody scFv(Leu-16), a Myc-tag, the hinge region of murine CD8α, and the murine CD3ζ chain. The neomycin-resistance gene for G418 selection of transduced cells is driven by the SV40 early promoter. b Left panel: RT-PCR analysis with primers specific for the chimeric receptor sequence of mRNA from parental NK-92 cells (lane 1), NK-92 cells transduced with empty pLXSN vector (lane 2), and NK-92-scFv(Leu-16)-ζ cells (lane 3). The position of the scFv(Leu-16)-ζ DNA fragment is indicated. Right panel: Immunoblot analysis of scFv(Leu-16)-ζ expression. Lysates of NK-92 (lanes 1 and 3) and transduced NK-92-scFv(Leu-16)-ζ cells (lanes 2 and 4) were separated by SDS-PAGE under non-reducing (NR) or reducing (R) conditions. Immunoblot analysis was performed with a CD3ζ chain specific Mab followed by HRP-conjugated anti-mouse antibody and chemiluminescent detection. The positions of endogenous and chimeric CD3ζ proteins and of molecular weight standards (kDa) are indicated. c Surface expression of chimeric scFv(Leu-16)-ζ antigen receptor. After G418 selection of transduced cells, NK-92-scFv(Leu-16)-ζ cells expressing homogeneous levels of the chimeric antigen receptor on their surface were enriched by sorting with Mab 9E10 and goat anti-mouse IgG-coated magnetic beads, and subsequent sorting by FACS with Mab 9E10 and FITC-conjugated secondary antibody as indicated. After each selection step surface expression of scFv(Leu-16)-ζ was determined by flow cytometry using Mab 9E10 and FITC-labeled goat anti-mouse secondary antibody. NK-92 cells transduced with empty pLXSN served as a control
Amphotropic retroviral vector was produced by stable transfection of FLYA-JET packing cells [31], and used for transduction of human NK-92 NK cells. Following selection of transduced NK-92 cells with G418, expression of scFv(Leu-16)-ζ mRNA was confirmed by RT-PCR (Fig. 1b, left panel). A population of NK-92-scFv(Leu-16)-ζ cells expressing homogeneous levels of the chimeric antigen receptor protein on the cell surface was isolated by sorting with Myc-tag specific Mab 9E10 and immunomagnetic beads, followed by two subsequent rounds of sorting with Mab 9E10 by FACS (Fig. 1c). Expression of the scFv(Leu-16)-ζ receptor was also analyzed by immunoblotting. Cell lysates of parental NK-92 and NK-92-scFv(Leu-16)-ζ cells were separated by SDS-PAGE under reducing or non-reducing conditions, and immunoblotted with a Mab detecting human and murine ζ. The results are shown in Fig. 1b, right panel. Under reducing conditions endogenous ζ chain was detected as a 16 kDa band in lysates of both parental NK-92 (lane 1) and NK-92-scFv(Leu-16)-ζ cells (lane 2). Two additional bands of ∼54 and 65 kDa were only observed in NK-92-scFv(Leu-16)-ζ cells. While the lower band corresponds well with the calculated size of chimeric scFv(Leu-16)-ζ protein (53 kDa), the larger protein species most likely represents the result of complex glycosylation within the hinge region of the chimeric receptor [33]. Under non-reducing conditions a series of additional bands were observed in lysates of NK-92-scFv(Leu-16)-ζ (lane 4), but not of parental NK-92 cells (lane 3), representing scFv(Leu-16)-ζ homodimers and heterodimers of the chimeric receptor with endogenous ζ chain. Monomeric and homodimeric CD3ζ was detected in lysates of both cell populations.
Cytotoxic activity of NK-92-scFv(Leu-16)-ζ cells
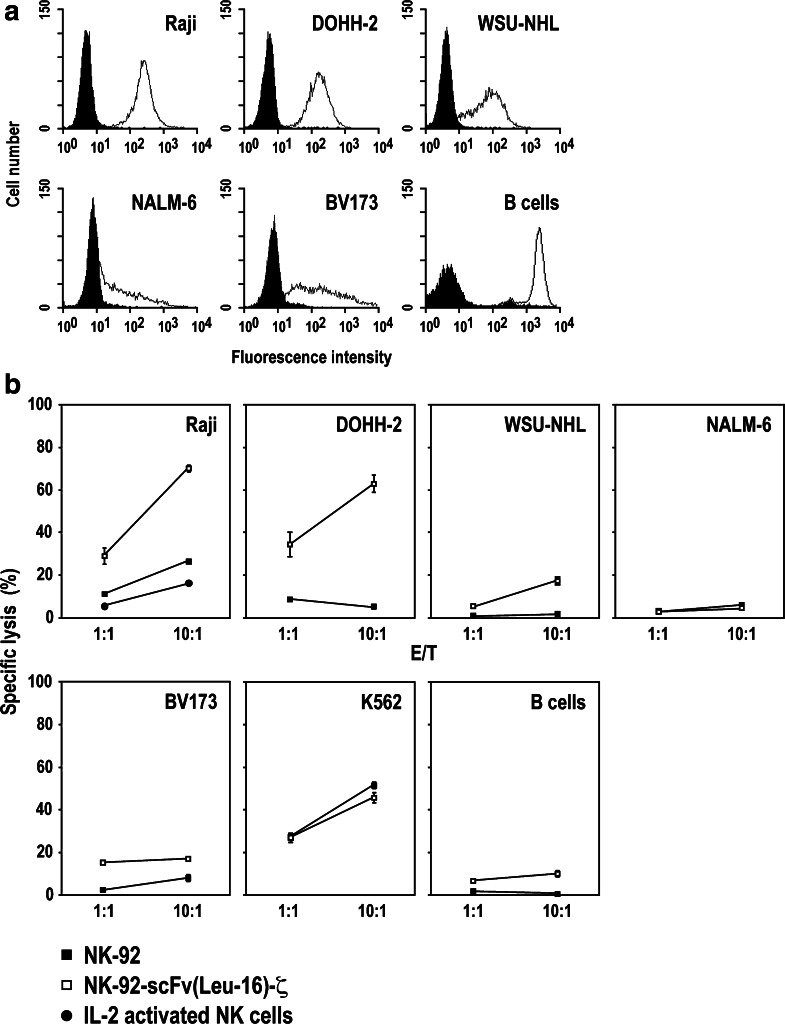
To investigate whether expression of the CD20-specific chimeric receptor can augment cell killing activity of NK-92 and overcome NK-resistance of CD20 expressing targets, we tested a panel of human cancer cell lines expressing different levels of CD20 in cytotoxicity assays with NK-92-scFv(Leu-16)-ζ or unmodified NK-92 cells. In flow cytometric analysis, Raji Burkitt’s lymphoma, and DOHH-2 and WSU-NHL follicular lymphoma cells displayed homogeneous CD20 expression at different levels, whereas BV173 and NALM-6 acute B-precursor ALL cells showed more heterogeneous expression of CD20 (Fig. 2a).
Fig. 2.
NK-92-scFv(Leu-16)-ζ cells display enhanced cell killing toward target cells expressing high levels of CD20. a Flow cytometric analysis of CD20 surface expression on Raji, DOHH-2, WSU-NHL, NALM-6, BV173, and primary human B-cells with CD20-specific Mab L27 and PE-conjugated secondary antibody. b Raji, DOHH-2, WSU-NHL, NALM-6, and BV173 target cells expressing different levels of CD20, CD20-negative K562 human erythroleukemia cells and primary human B-cells were stained with PKH67-GL and incubated at effector to target ratios of 1:1 or 10:1 with parental NK-92 (filled squares) or NK-92-scFv(Leu-16)-ζ cells (open squares) for 2 h. For comparison, Raji cells were also incubated with IL-2 activated primary human NK cells (filled circles). Cells were stained with propidium iodide, and dead target cells were quantified as double positive cells by flow cytometry. Mean values of triplicate samples are shown. The standard deviation is indicated by error bars
Target cells were stained with green fluorescent PKH67-GL dye, incubated for 2 h with NK cells at E/T ratios of 1:1 or 10:1, and analyzed for dead target cells by flow cytometry after staining with propidium iodide. The data are shown in Fig. 2b. Whereas Raji cells were slightly sensitive to parental NK-92 cells (>20% specific lysis at an E/T of 10:1), there was no significant killing of DOHH-2, WSU-NHL, NALM-6, BV173, and primary human B-cells by NK-92 detectable under the chosen experimental conditions. In contrast, NK-sensitive Raji cells and NK-resistant DOHH-2 expressing CD20 homogeneously on the cell surface at high levels were efficiently killed by NK-92-scFv(Leu-16)-ζ (>60% specific lysis at an E/T of 10:1). Also NK-resistant WSU-NHL cells showed sensitivity to NK-92-scFv(Leu-16)-ζ, albeit to a lower degree correlating with the lower level of CD20 expression. Sensitivity of BV173 cells to NK-92-scFv(Leu-16)-ζ was slightly higher than to parental NK-92, but could not be increased further by increasing the E/T ratio to 20:1 (data not shown). This suggests that only the proportion of target cells with high-CD20 expression was lysed. In the case of NALM-6 cells, NK-resistance was not overcome by expression of the chimeric antigen receptor, correlating with relatively low-CD20 expression. K562 cells that do not express detectable levels of CD20 (data not shown), were lysed equally well by unmodified NK-92 and transduced NK-92-scFv(Leu-16)-ζ cells. These results demonstrate that expression of the chimeric scFv(Leu-16)-ζ protein did not alter the intrinsic cytotoxic activity of NK-92 toward NK-sensitive targets negative for CD20, while it markedly enhanced cell killing activity against cells that homogeneously express high levels of CD20 on their surface, irrespective of whether or not these cells are resistant to parental NK-92.
To investigate the contribution of specific binding of the chimeric receptor to CD20 to the cell killing activity of NK-92-scFv(Leu-16)-ζ, Raji cells were incubated with NK-92-scFv(Leu-16)-ζ cells at an E/T ratio of 10:1 in the presence of CD20-specific Mab rituximab as a competitor. As shown in Fig. 3, rituximab efficiently prevented CD20 binding of the chimeric receptor and reduced specific lysis by NK-92-scFv(Leu-16)-ζ from 71% to a level even below that of parental NK-92 (17% specific lysis), while an irrelevant control Mab did not (70% specific lysis). This demonstrates that enhanced cell killing activity of NK-92-scFv(Leu-16)-ζ is strictly dependent on binding of the chimeric receptor to CD20, and not due to potential general effects that might have been induced by genetic manipulation of the NK-92 cells.
Fig. 3.
Specificity of target cell recognition and cell killing. Raji cells stained with PKH67-GL were incubated with parental NK-92 cells (open bar) or NK-92-scFv(Leu-16)-ζ cells (filled and hatched bars) for 2 h at an effector to target ratio of 10:1 either in the absence of competitor, or in the presence of 15 μg/ml rituximab or an irrelevant control antibody as indicated. Killing of target cells was determined as described in the legend to Fig. 2. Mean values of triplicate samples are shown. The standard deviation is indicated by error bars
CD20 expression is sufficient to render target cells sensitive to NK-92-scFv(Leu-16)-ζ cells
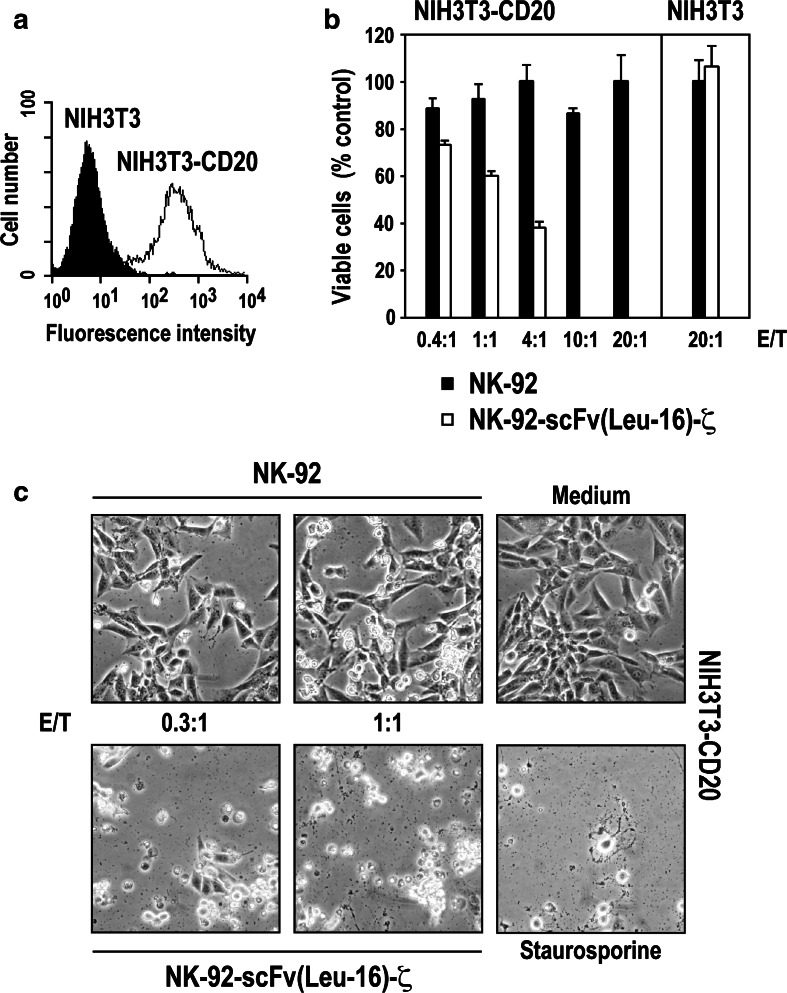
NK-92 cells display no xenogeneic cytotoxic effects against murine cells in SCID mouse models [18, 19]. To analyze whether expression of human CD20 by murine cells alone is sufficient to allow recognition and cytolysis by NK-92-scFv(Leu-16)-ζ, NIH3T3 fibroblasts were stably transfected with a human CD20 cDNA construct. Comparable to Raji cells, flow cytometric analysis revealed high and homogeneous levels of CD20 on the surface of the resulting NIH3T3-CD20 cells (Fig. 4a). Cytotoxic activity of NK cells toward NIH3T3-CD20 and parental NIH3T3 was tested in 3 h MTT cytotoxicity assays. As expected, even at an E/T ratio of 20:1 neither NK-92 nor NK-92-scFv(Leu-16)-ζ were able to kill unmodified NIH3T3 fibroblasts (Fig. 4b). In contrast, while still resistant to parental NK-92, NIH3T3-CD20 cells were highly sensitive to NK-92-scFv(Leu-16)-ζ, resulting in >40% of cell killing at an E/T ratio of 1:1 and complete eradication of target cells at E/T ratios ≥10:1. Similar to Raji cells, the cytotoxic activity of NK-92-scFv(Leu-16)-ζ against NIH3T3-CD20 could be competed with CD20-specific rituximab (data not shown). Likewise, cell killing activity was completely abrogated by pre-incubation of NK-92-scFv(Leu-16)-ζ cells with the serine protease inhibitor DCI (3,4-dichloroisocoumarin) indicating killing via the perforine/granzyme B pathway (data shown in supplementary Fig. S2).
Fig. 4.
Target-cell killing is mediated via interaction of the chimeric scFv(Leu-16)-ζ antigen receptor with CD20. a Surface expression of human CD20 on NIH3T3 murine fibroblasts stably transfected with a CD20 cDNA construct (NIH3T3-CD20 cells) was determined by flow cytometry using CD20-specific Mab L27 and a secondary PE-labeled antibody. Parental NIH3T3 cells served as a control. b Cytotoxic activity of NK-92 and NK-92-scFv(Leu-16)-ζ toward CD20 expressing NIH3T3-CD20 and CD20-negative NIH3T3 cells at the indicated effector to target ratios was analyzed in a 3 h MTT cytotoxicity assay as described in the methods section. The relative number of viable target cells is expressed in % of untreated controls (set to 100%). Mean values of triplicate samples are shown. The standard deviation is indicated by error bars. c Cytotoxic activity of NK-92 and NK-92-scFv(Leu-16)-ζ toward NIH3T3-CD20 cells was also investigated by microscopical analysis after 16 h. Control cells were incubated in the absence of NK cells, or were treated with 8 μM of the apoptosis-inducing drug staurosporine as indicated. Representative fields are shown
The consequences of NK-92-scFv(Leu-16)-ζ activity on NIH3T3-CD20 cells at low E/T ratios were investigated further. NIH3T3-CD20 cells were grown over night and then incubated for 16 h with NK-92-scFv(Leu-16)-ζ or parental NK-92 cells. NK-cell mediated cytotoxicity was analyzed by light microscopy. The results are shown in Fig. 4c. Comparable to exposure to the apoptosis-inducing reagent staurosporine, at an E/T ratio of 1:1 none of the target cells survived the cytotoxic effects of NK-92-scFv(Leu-16)-ζ, and at an E/T ratio of 0.3:1 only very few viable NIH3T3-CD20 cells could be detected. In contrast, even after prolonged incubation parental NK-92 cells had no effect on the survival of NIH3T3-CD20 fibroblasts. These results demonstrate that expression of CD20 on the surface of target cells is sufficient to mediate recognition and lysis by NK-92-scFv(Leu-16)-ζ. In addition, the high-cell killing activity observed at an E/T ratio below 1:1 suggests that NK-92-scFv(Leu-16)-ζ cells are able to sequentially attack several target cells.
Selectivity and kinetics of target cell killing
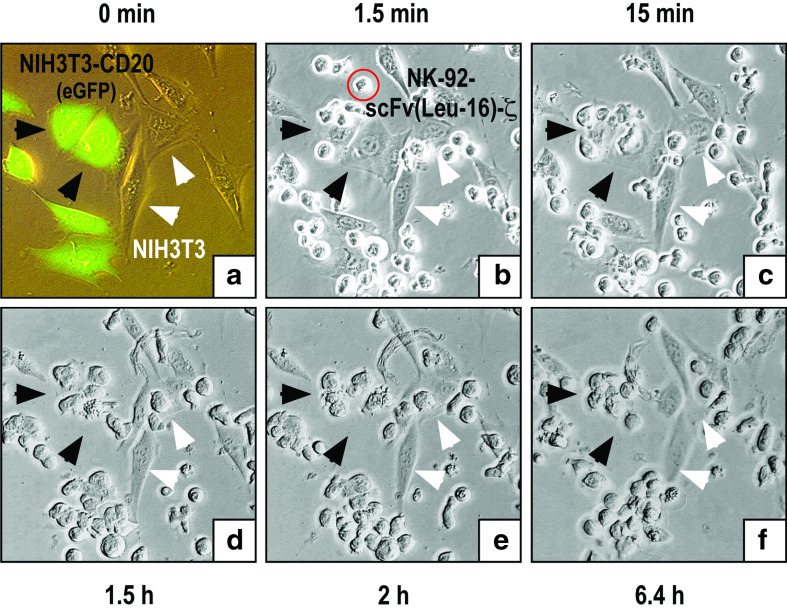
Next we investigated the kinetics of cell killing and the ability of NK-92-scFv(Leu-16)-ζ to selectively eliminate CD20 expressing targets from a mixture of CD20-positive and CD20-negative cells. To distinguish them from parental NIH3T3, NIH3T3-CD20 cells were transduced with a retroviral vector encoding enhanced green fluorescent protein (eGFP), and plated as a 1:1 mixture together with NIH3T3. Then NK-92-scFv(Leu-16)-ζ cells were added at an E/T ratio of 1:1, and microscopic images were taken at 1.5-min intervals for 6.4 h. Figure 5a shows a fluorescence microscopic image of the target cells before addition of NK-92-scFv(Leu-16)-ζ. Microscopic images of the same field taken 1.5 min, 1.5, 2, and 6.4 h after including effector cells in the culture are shown in Fig. 5b–f. The complete image sequence is provided as a QuickTime movie in the supplementary material. Whereas NK-92-scFv(Leu-16)-ζ made contact with both target cell populations immediately after addition, they interacted only with NIH3T3-CD20 cells for a prolonged time resulting in selective target-cell killing within 1.5–2 h. This is indicated by cell contraction, massive membrane blebbing and the appearance of apoptotic bodies visible in Fig. 5d and e. While after a few hours only fragments of the NIH3T3-CD20 cells remained in the mixture, CD20-negative NIH3T3 cells survived long-term co-culture with NK-92-scFv(Leu-16)-ζ unharmed (Fig. 5f).
Fig. 5.
Selectivity and kinetics of target cell killing. NIH3T3-CD20 cells transduced with a retroviral vector encoding enhanced green fluorescent protein (eGFP) were mixed at a 1:1 ratio with parental NIH3T3 cells and grown overnight. Then NK-92-scFv(Leu-16)-ζ cells were added at an effector to target ratio of 1:1, and microscopic images were taken at 1.5-min intervals for 6.4 h. a Fluorescence microscopic image of eGFP- and CD20-positive NIH3T3-CD20(eGFP), and eGFP- and CD20-negative NIH3T3 cells before addition of NK cells. b–f Microscopic images of the same field taken at the indicated time points after addition of NK cells. As examples, some NIH3T3-CD20(eGFP) and NIH3T3 cells are indicated by black and white arrows, respectively. An NK-92-scFv(Leu-16)-ζ cell is indicated by a red circle in b. The complete image sequence is provided as a QuickTime movie in the supplementary material
NK-92-scFv(Leu-16)-ζ inhibit the in vivo growth of CD20 expressing lymphoma cells
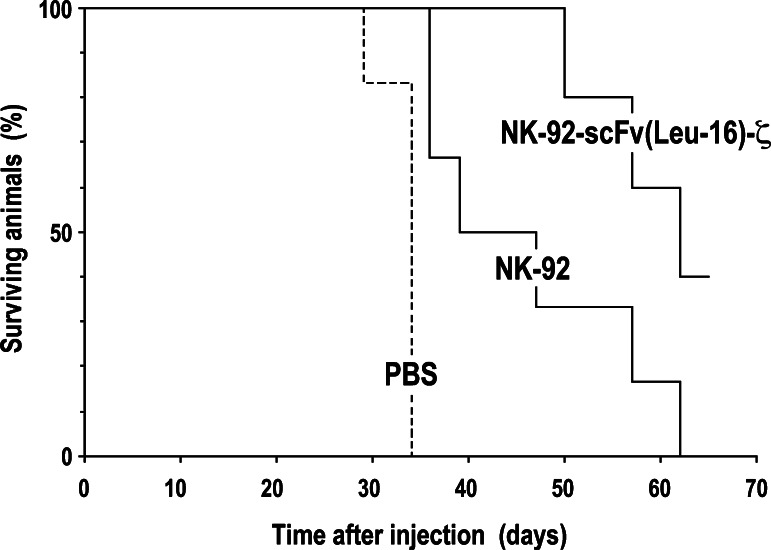
Human Raji Burkitt’s lymphoma cells were used as a model to determine anti-tumoral activity of NK-92-scFv(Leu-16)-ζ cells in vivo. As shown in Fig. 6, subcutaneous injection of Raji cells into NOD/SCID γ−/−c mice led to rapid tumor formation, requiring mice in the untreated control group to be sacrificed latest by day 34 (median survival of 33.2 days). In contrast, simultaneous administration of tumor cells and parental or gene-modified NK-92 cells resulted in marked suppression of tumor growth and extended survival (median survival of 46.2 and 59.8 days, respectively). The difference in outcome after treatment with NK cells in comparison to PBS was statistically significant with P < 0.05 (NK-92 versus control) and P < 0.00001 (NK-92-scFv(Leu-16)-ζ versus control). Thereby retargeted NK-92-scFv(Leu-16)-ζ cells were considerably more effective than unmodified NK-92 (P < 0.05), which is in agreement with their enhanced activity against Raji cells in vitro (Fig. 2). Due to tumor growth at later time points in all animals the experiment was terminated on day 65.
Fig. 6.
In vivo anti-tumor activity of NK-92-scFv(Leu-16)-ζ cells. About 4 × 106 Raji cells were mixed with PBS, or NK-92-scFv(Leu-16)-ζ or parental NK-92 cells at an effector to target ratio of 5:1 as indicated and immediately injected subcutaneously into the flanks of NOD/SCID γ−/−c mice. Tumor growth was followed by caliper measurements. Mice were sacrificed when tumors reached 1.5 cm in diameter or when animals appeared to be in distress
NK-92-scFv(Leu-16)-ζ kill NK-resistant primary CLL cells
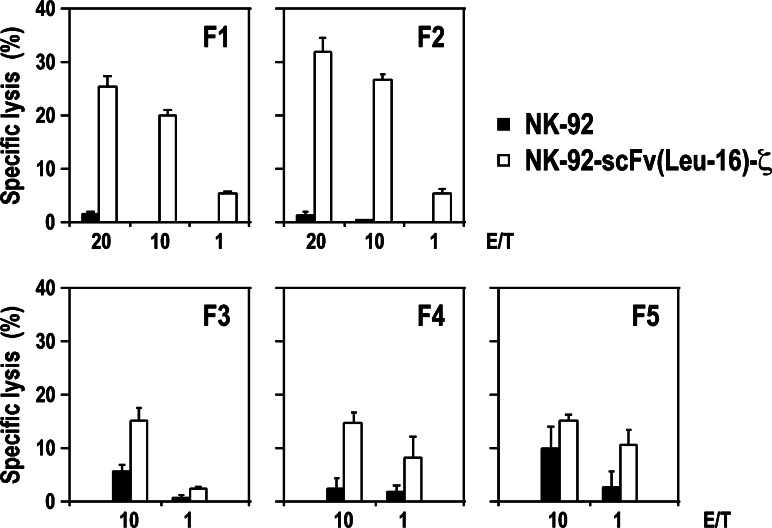
To investigate anti-tumoral activity of NK-92-scFv(Leu-16)-ζ cells against primary B-cell malignancies, mononuclear cells were isolated from blood samples of five previously untreated patients suffering from CLL. CD20 expression on the cell surface was determined by flow cytometry, with relative amounts of CD20-positive cells in these samples ranging from 93.8 to 96.3% and mean fluorescence intensities comparable to that of established lymphoma cells (Table 1). The CLL cells were labeled with PKH67, incubated for 2 h with NK-92-scFv(Leu-16)-ζ or parental NK-92 cells at E/T ratios of 1:1–20:1, and analyzed for dead target cells by flow cytometry after staining with propidium iodide. As shown in Fig. 7, even at high E/T ratios of 20:1 or 10:1 the CLL cells were not or only marginally sensitive to parental NK-92. This NK-resistance was overcome by expression of the chimeric antigen receptor, resulting in markedly enhanced killing of CLL cells from all five patients by NK-92-scFv(Leu-16)-ζ.
Table 1.
Expression of CD20 by primary CLL cells
| Patient | CD20 positive cells [%] | Mean fluorescence intensity |
|---|---|---|
| F1 | 96.3 | 427.9 |
| F2 | 94.7 | 1,391.2 |
| F3 | 96.3 | 548.6 |
| F4 | 94.7 | 490.6 |
| F5 | 93.8 | 210.1 |
| Rajia | 99.9 | 548.0 |
aRaji cells were included for comparison
Fig. 7.
Cytotoxic activity of NK cells against primary chronic lymphocytic leukemia cells. CLL cells were obtained from routine peripheral blood samples of untreated patients. Mononuclear cells were enriched by density gradient centrifugation, analyzed for expression of CD20 by flow cytometry, and directly used for cytotoxicity experiments. Cytotoxic activity of CD20-specific NK-92-scFv(Leu-16)-ζ and parental NK-92 cells was analyzed as described in the legend of Fig. 2. Mean values of triplicate samples are shown. The standard deviation is indicated by error bars
Discussion
In this study, we have redirected the continuously growing human NK cell line NK-92 to CD20 expressing cancer cells using a chimeric antigen receptor that consists of a CD20-specific scFv fragment linked to the CD3ζ chain of the T-cell receptor complex as an intracellular signaling moiety. This approach was originally developed to bypass MHC-restriction in genetically modified T-cells, and has been investigated in this setting for a number of surface antigens expressed on cancer cells or cells infected by viruses [34–37]. Retargeting of NK cells has been attempted less frequently [14, 16, 38, 39], despite the fact that they constitute a highly potent cytotoxic effector mechanism of the innate immune response [40–42].
Intrinsic cytotoxic activity of unmodified NK-92 cells against a number of established leukemia, lymphoma and melanoma cell lines, and in some cases against primary leukemia and lymphoma cells has previously been demonstrated [17–19]. Normal hematopoetic cells from the bone marrow of healthy donors were not affected by NK-92 [18], providing a basis for the ongoing clinical development of NK-92 as an allogeneic cell therapeutic for adoptive cancer immunotherapy [20]. To extend this approach to target cells derived from solid tumors that display intrinsic resistance to NK cells, we have previously generated an NK-92 variant harboring a chimeric antigen receptor which recognizes the ErbB2/HER2 protooncogene overexpressed on many human malignancies of epithelial origin [14, 15]. The aim of our current study was to investigate whether a similar strategy can be used to successfully attack otherwise NK-resistant malignant cells of hematopoetic origin, and whether expression of a suitable chimeric antigen receptor could further enhance the intrinsic cytotoxic activity of NK-92 against already NK-sensitive cells that carry the chosen target antigen on their surface.
Recently it was shown that T-ALL cells were more susceptible to NK-92 than B-lineage ALL cells [24]. This resistance was at least in part attributed to the lack of MICA/B expression in NK-92 insensitive targets. To overcome resistance and specifically redirect NK-92 to B-cell malignancies, we introduced a CD20-specific chimeric antigen receptor construct into the NK cells. An NK-92-scFv(Leu-16)-ζ cell population with homogeneous surface expression of the chimeric antigen receptor was established by immunomagnetic and fluorescence-activated cell sorting. Retroviral transduction and expression of the CD20-specific receptor did not alter the intrinsic cytotoxic activity of NK-92 against CD20 negative targets, indicated by the very similar activity of NK-92 and NK-92-scFv(Leu-16)-ζ toward K562 cells. In contrast, NK-92-scFv(Leu-16)-ζ cells displayed markedly enhanced cytotoxic activity against NK-sensitive targets that express CD20 on their surface. This enhanced cell killing activity was strictly dependent on binding of the chimeric receptor to CD20, and could be readily inhibited by blocking the target antigen with high concentrations of CD20-specific antibody.
Expression of CD20 on the target cell surface was sufficient to enable recognition and lysis by NK-92-scFv(Leu-16)-ζ. Murine fibroblasts, which like other murine cells are resistant to NK-92 [14], became highly sensitive to the genetically modified variant after stable transfection with a human CD20 cDNA construct. Using these NIH3T3-CD20 cells as a model, we observed high-cell killing activity of NK-92-scFv(Leu-16)-ζ even at an E/T ratio below 1:1. This suggests that during prolonged co-incubation CD20-specific NK-92 in contrast to other NK cell lines are able to sequentially attack multiple target cells [43]. In mixed cultures with CD20 expressing and CD20-negative murine fibroblasts, NK-92-scFv(Leu-16)-ζ cells proved to be highly selective only attacking and eliminating antigen-positive targets. Thereby cell killing followed kinetics in agreement with cell death induced via the perforin/granzyme B pathway [44], with clear signs of apoptosis appearing between 1.5 and 2 h after initial contact with NK cells. Furthermore, target cell killing could be completely inhibited by pre-incubation of NK-92-scFv(Leu-16)-ζ with a serine protease inhibitor, indicating that similar to parental NK-92 cells their cytotoxic activity is dependent on granzyme B [45].
NK-resistant follicular lymphoma and B-precursor ALL cells were not affected by co-culture with parental NK-92, but depending on the level and homogeneity of CD20 expression, were efficiently lysed by NK-92-scFv(Leu-16)-ζ. More importantly, also primary CLL cells, which displayed little or no sensitivity to NK-92-mediated lysis, showed markedly enhanced sensitivity to NK-92-scFv(Leu-16)-ζ. These results demonstrate that expression of the CD20-specific antigen receptor in NK cells can overcome the resistance mechanisms operative in a variety of malignancies of B-cell origin. Similar results were recently reported for donor-derived primary NK cells targeted to CD19 [16]. In this case high-cell killing activity was dependent on a costimulatory 4-1BB domain included in the chimeric antigen receptor in addition to CD3ζ. NK-92 cells do not express endogenous 4-1BB (data not shown), and CD20-specific NK-92-scFv(Leu-16)-ζ cells carry a more basic receptor construct. Nevertheless, they displayed high and specific cytotoxicity, possibly due to the high-intrinsic activation state of NK-92. Enhanced anti-tumoral activity of NK-92-scFv(Leu-16)-ζ cells was also observed when these cells were implanted simultaneously with human Raji lymphoma cells into NOD/SCID γ−/−c mice. While treatment with parental NK-92 cells already delayed tumor growth significantly, survival of animals was extended considerably longer by NK-92-scFv(Leu-16)-ζ. This indicates that the gene-modified effector cells retained specificity and cytolytic activity in vivo even though no exogenous IL-2 was included in the treatment schedule.
In patients with malignant disorders NK cell function can be impaired, resulting in a reduced proliferative response and reduced cytotoxic activity [46]. Consequently, it might not be possible to generate suitable autologous effector cells in all cases where this is desired, and clinically applicable human cell lines such as NK-92 could provide a valuable alternative. Furthermore, in contrast to retroviral transduction of primary cells, individual clones of genetically modified NK-92 with molecularly defined retroviral insertion sites can be generated, limiting the risk of insertional mutagenesis and oncogenic activation [47]. Remaining safety concerns could be relieved by inclusion of suicide gene constructs [48, 49], or irradiation of the cells before in vivo application to block proliferation. This has previously been shown not to affect cell killing activity of unmodified and retrovirally transduced NK-92 cells [14, 20].
Isolation and expansion of primary NK and T-cells is often labor intensive and may yield variable results. A cytotoxic cell line such as NK-92 is very attractive, since it offers the possibility to generate a highly selective and generally applicable effector cell population with unlimited expansion potential, as demonstrated here for an NK-92 derivative that specifically recognizes and efficiently lyses malignant cells of B-cell origin. Moreover, such an ‘allogeneic’ cell therapeutic could also circumvent the problems of defective effector cells encountered in some instances in malignancies, justifying further development of retargeted NK-92 variants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1 Recombinant scFv(Leu-16) binds to CD20 expressing lymphoma cells. a For periplasmic expression of CD20-specific scFv(Leu-16) under control of the IPTGinducible tac promoter, cDNA fragments encoding heavy (VH) and light chain variable domains (VL) of monoclonal antibody Leu-16 were connected by a flexible linker sequence and fused to the ompA signal peptide (SP) in the bacterial expression vector pSW50. Synthetic FLAG- and Myc-tags are included at N- and C-termini of the gene product. b The presence of ErbB2-specific control protein scFv(FRP5) (lane 1) and CD20-specific scFv(Leu-16) (lane 2) in periplasmic extracts was confirmed by SDS-PAGE and immunoblot analysis with FLAG-tag specific Mab M2. c Binding of recombinant scFv molecules to CD20 expressing but ErbB2-negative Raji lymphoma cells (upper panel), and ErbB2 expressing but CD20-negative SKBR3 breast carcinoma cells was analyzed by flow cytometry with Myc-tag specific Mab 9E10 and FITC-conjugated secondary antibody. CD20-specific Mab L27 served as a control. (PDF 59 kb)
Supplementary Fig. S2 Granzyme B activity is required for target cell killing by NK-92-scFv(Leu-16)-ζ cells. NK-92-scFv(Leu-16)-ζ cells were incubated with 100 μM of the serine protease inhibitor DCI (3,4-dichloroisocoumarin) (Roche, Mannheim, Germany) in X-VIVO 10 medium for 1 h at 37°C, before their cytotoxic activity towards CD20 expressing NIH3T3-CD20 cells was analyzed in a 3 h MTT cytotoxicity assay as described in the methods section (E/T ratio of 10:1). Untreated NK-92 and NK-92-scFv(Leu-16)-ζ cells, and NK-92-scFv(Leu-16)-ζ cells treated with DMSO served as controls. The relative number of viable target cells is expressed in % of NIH3T3-CD20 grown in the absence of NK cells (set to 100 %). Mean values of triplicate samples are shown. The standard deviation is indicated by error bars. At the concentration applied DCI was not toxic to NK-92 cells as evaluated by propidium iodide staining (data not shown). (PDF 8.85 kb)
Supplementary movie Selectivity and kinetics of target cell killing. NIH3T3-CD20 cells transduced with a retroviral vector encoding enhanced green fluorescent protein (eGFP) were mixed at a 1:1 ratio with parental NIH3T3 cells and grown overnight. Then NK-92-scFv(Leu-16)-ζ cells were added at an effector to target ratio of 1:1, microscopic images of a single field were taken at 1.5 min intervals for 6.4 h, and assembled into a QuickTime movie at 10 frames per second. At the beginning of the movie, a fluorescence microscopic image of eGFP- and CD20-positive NIH3T3-CD20(eGFP), and eGFP- and CD20-negative NIH3T3 cells before addition of NK cells is shown. Exemplary NIH3T3-CD20(eGFP) cells are indicated by white circles, exemplary parental NIH3T3 cells by black arrows. Selected images from this experiment are also shown in Fig. 5. (MOV 2.33 mb)
Acknowledgments
We thank Dr. Barbara Schnierle for providing pEFIRES-P vector, Dr. Annette Romanski for BV173 and NALM-6 cells, Dr. Byoung S. Kwon for anti-4-1BB antibody BBK-1, Daniela Bott for isolation of primary B and NK cells, Dr. Brigitte Rüster for help with microscopical analysis, Dipl. Ing. Nicola Krzossok for help with NK-92 cytotoxicity assays, Dr. Boris Brill, Sabrina Lehmen and Christiane Peter for help with animal experiments, and Dr. Markus Biburger for helpful suggestions. This work was supported in part by research grant 102386/10-2244 from Deutsche Krebshilfe. G. Maki was supported by grant CLL-63119, Section of Hematology, Rush University Medical Center.
References
- 1.Maloney DG. Immunotherapy for non-Hodgkin’s lymphoma: monoclonal antibodies and vaccines. J Clin Oncol. 2005;23:6421–6428. doi: 10.1200/JCO.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Cartron G, Watier H, Golay J, Solal-Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood. 2004;104:2635–2642. doi: 10.1182/blood-2004-03-1110. [DOI] [PubMed] [Google Scholar]
- 3.Schilling V. Immunotherapy with anti-CD20 compounds. Semin Cancer Biol. 2003;13:211–222. doi: 10.1016/S1044-579X(03)00018-X. [DOI] [PubMed] [Google Scholar]
- 4.Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo-Lopez A, Levy R. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84:2457–2466. [PubMed] [Google Scholar]
- 5.Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, Janakiraman N, Foon KA, Liles TM, Dallaire BK, Wey K, Royston I, Davis T, Levy R. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:2188–2195. [PubMed] [Google Scholar]
- 6.McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F, Jain V, Ho AD, Lister J, Wey K, Shen D, Dallaire BK. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 7.Vose JM, Link BK, Grossbard ML, Czuczman M, Grillo-Lopez A, Gilman P, Lowe A, Kunkel LA, Fisher RI. Phase II study of rituximab in combination with chop chemotherapy in patients with previously untreated, aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2001;19:389–397. doi: 10.1200/JCO.2001.19.2.389. [DOI] [PubMed] [Google Scholar]
- 8.Jazirehi AR, Bonavida B. Cellular and molecular signal transduction pathways modulated by rituximab (rituxan, anti-CD20 mAb) in non-Hodgkin’s lymphoma: implications in chemosensitization and therapeutic intervention. Oncogene. 2005;24:2121–2143. doi: 10.1038/sj.onc.1208349. [DOI] [PubMed] [Google Scholar]
- 9.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.V99.3.754. [DOI] [PubMed] [Google Scholar]
- 10.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Hainsworth JD, Burris HA, III, Morrissey LH, Litchy S, Scullin DC, Jr, Bearden JD, III, Richards P, Greco FA. Rituximab monoclonal antibody as initial systemic therapy for patients with low-grade non-Hodgkin lymphoma. Blood. 2000;95:3052–3056. [PubMed] [Google Scholar]
- 12.Colombat P, Salles G, Brousse N, Eftekhari P, Soubeyran P, Delwail V, Deconinck E, Haioun C, Foussard C, Sebban C, Stamatoullas A, Milpied N, Boue F, Taillan B, Lederlin P, Najman A, Thieblemont C, Montestruc F, Mathieu-Boue A, Benzohra A, Solal-Celigny P. Rituximab (anti-CD20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: clinical and molecular evaluation. Blood. 2001;97:101–106. doi: 10.1182/blood.V97.1.101. [DOI] [PubMed] [Google Scholar]
- 13.Davis TA, Grillo-Lopez AJ, White CA, McLaughlin P, Czuczman MS, Link BK, Maloney DG, Weaver RL, Rosenberg J, Levy R. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: safety and efficacy of re-treatment. J Clin Oncol. 2000;18:3135–3143. doi: 10.1200/JCO.2000.18.17.3135. [DOI] [PubMed] [Google Scholar]
- 14.Uherek C, Tonn T, Uherek B, Becker S, Schnierle B, Klingemann HG, Wels W. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood. 2002;100:1265–1273. [PubMed] [Google Scholar]
- 15.Daldrup-Link HE, Meier R, Rudelius M, Piontek G, Piert M, Metz S, Settles M, Uherek C, Wels W, Schlegel J, Rummeny EJ. In vivo tracking of genetically engineered, anti-HER2/neu directed natural killer cells to HER2/neu positive mammary tumors with magnetic resonance imaging. Eur Radiol. 2005;15:4–13. doi: 10.1007/s00330-004-2526-7. [DOI] [PubMed] [Google Scholar]
- 16.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klingemann HG, Wong E, Maki G. A cytotoxic NK-cell line (NK-92) for ex vivo purging of leukemia from blood. Biol Blood Marrow Transplant. 1996;2:68–75. [PubMed] [Google Scholar]
- 18.Yan Y, Steinherz P, Klingemann HG, Dennig D, Childs BH, McGuirk J, O’Reilly RJ. Antileukemia activity of a natural killer cell line against human leukemias. Clin Cancer Res. 1998;4:2859–2868. [PubMed] [Google Scholar]
- 19.Tam YK, Miyagawa B, Ho VC, Klingemann HG. Immunotherapy of malignant melanoma in a SCID mouse model using the highly cytotoxic natural killer cell line NK-92. J Hematother. 1999;8:281–290. doi: 10.1089/106161299320316. [DOI] [PubMed] [Google Scholar]
- 20.Tonn T, Becker S, Esser R, Schwabe D, Seifried E. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J Hematother Stem Cell Res. 2001;10:535–544. doi: 10.1089/15258160152509145. [DOI] [PubMed] [Google Scholar]
- 21.Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–658. [PubMed] [Google Scholar]
- 22.Burshtyn DN, Scharenberg AM, Wagtmann N, Rajagopalan S, Berrada K, Yi T, Kinet JP, Long EO. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitor receptor. Immunity. 1996;4:77–85. doi: 10.1016/S1074-7613(00)80300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 24.Romanski A, Bug G, Becker S, Kampfmann M, Seifried E, Hoelzer D, Ottmann OG, Tonn T. Mechanisms of resistance to natural killer cell-mediated cytotoxicity in acute lymphoblastic leukemia. Exp Hematol. 2005;33:344–352. doi: 10.1016/j.exphem.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Wu AM, Tan GJ, Sherman MA, Clarke P, Olafsen T, Forman SJ, Raubitschek AA. Multimerization of a chimeric anti-CD20 single-chain Fv-Fc fusion protein is mediated through variable domain exchange. Protein Eng. 2001;14:1025–1033. doi: 10.1093/protein/14.12.1025. [DOI] [PubMed] [Google Scholar]
- 26.Chow KU, Sommerlad WD, Boehrer S, Schneider B, Seipelt G, Rummel MJ, Hoelzer D, Mitrou PS, Weidmann E. Anti-CD20 antibody (IDEC-C2B8, rituximab) enhances efficacy of cytotoxic drugs on neoplastic lymphocytes in vitro: role of cytokines, complement, and caspases. Haematologica. 2002;87:33–43. [PubMed] [Google Scholar]
- 27.Hoogenboom HR, Griffiths AD, Johnson KS, Chiswell DJ, Hudson P, Winter G. Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains. Nucleic Acids Res. 1991;19:4133–4137. doi: 10.1093/nar/19.15.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altenschmidt U, Kahl R, Moritz D, Schnierle BS, Gerstmayer B, Wels W, Groner B. Cytolysis of tumor cells expressing the Neu/erbB-2, erbB-3, and erbB-4 receptors by genetically targeted naive T lymphocytes. Clin Cancer Res. 1996;2:1001–1008. [PubMed] [Google Scholar]
- 29.Rohrbach F, Gerstmayer B, Biburger M, Wels W. Construction and characterization of bispecific costimulatory molecules containing a minimized CD86 (B7-2) domain and single chain antibody fragments for tumor targeting. Clin Cancer Res. 2000;6:4314–4322. [PubMed] [Google Scholar]
- 30.Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerstmayer B, Groner B, Wels W, Schnierle BS. Stable expression of the ecotropic retrovirus receptor in amphotropic packaging cells facilitates the transfer of recombinant vectors and enhances the yield of retroviral particles. J Virol Methods. 1999;81:71–75. doi: 10.1016/S0166-0934(99)00053-1. [DOI] [PubMed] [Google Scholar]
- 32.Hobbs S, Jitrapakdee S, Wallace JC. Development of a bicistronic vector driven by the human polypeptide chain elongation factor 1alpha promoter for creation of stable mammalian cell lines that express very high levels of recombinant proteins. Biochem Biophys Res Commun. 1998;252:368–372. doi: 10.1006/bbrc.1998.9646. [DOI] [PubMed] [Google Scholar]
- 33.Moritz D, Wels W, Mattern J, Groner B. Cytotoxic T lymphocytes with a grafted recognition specificity for ERBB2-expressing tumor cells. Proc Natl Acad Sci USA. 1994;91:4318–4322. doi: 10.1073/pnas.91.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bitton N, Debre P, Eshhar Z, Gorochov G. T-bodies as antiviral agents. Curr Top Microbiol Immunol. 2001;260:271–300. doi: 10.1007/978-3-662-05783-4_14. [DOI] [PubMed] [Google Scholar]
- 35.Uherek C, Groner B, Wels W. Chimeric antigen receptors for the retargeting of cytotoxic effector cells. J Hematother Stem Cell Res. 2001;10:523–534. doi: 10.1089/15258160152509136. [DOI] [PubMed] [Google Scholar]
- 36.Abken H, Hombach A, Heuser C, Kronfeld K, Seliger B. Tuning tumor-specific T-cell activation: a matter of costimulation? Trends Immunol. 2002;23:240–245. doi: 10.1016/S1471-4906(02)02180-4. [DOI] [PubMed] [Google Scholar]
- 37.Kershaw MH, Teng MW, Smyth MJ, Darcy PK. Supernatural T cells: genetic modification of T cells for cancer therapy. Nat Rev Immunol. 2005;5:928–940. doi: 10.1038/nri1729. [DOI] [PubMed] [Google Scholar]
- 38.Bach N, Waks T, Eshhar Z. Specific lysis of tumor cells by an NK-like cell line transfected with chimeric receptor genes. Tumor Target. 1995;1:203–209. [Google Scholar]
- 39.Tran AC, Zhang D, Byrn R, Roberts MR. Chimeric zeta-receptors direct human natural killer (NK) effector function to permit killing of NK-resistant tumor cells and HIV-infected T lymphocytes. J Immunol. 1995;155:1000–1009. [PubMed] [Google Scholar]
- 40.Whiteside TL, Vujanovic NL, Herberman RB. Natural killer cells and tumor therapy. Curr Top Microbiol Immunol. 1998;230:221–244. doi: 10.1007/978-3-642-46859-9_13. [DOI] [PubMed] [Google Scholar]
- 41.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 42.Farag SS, Fehniger TA, Ruggeri L, Velardi A, Caligiuri MA. Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect. Blood. 2002;100:1935–1947. doi: 10.1182/blood-2002-02-0350. [DOI] [PubMed] [Google Scholar]
- 43.Mahle NH, Radcliff G, Sevilla CL, Kornbluth J, Callewaert DM. Kinetics of cellular cytotoxicity mediated by a cloned human natural killer cell line. Immunobiology. 1989;179:230–243. doi: 10.1016/S0171-2985(89)80019-1. [DOI] [PubMed] [Google Scholar]
- 44.Dälken B, Giesübel U, Knauer SK, Wels WS. Targeted induction of apoptosis by chimeric granzyme B fusion proteins carrying antibody and growth factor domains for cell recognition. Cell Death Differ. 2006;13:576–585. doi: 10.1038/sj.cdd.4401773. [DOI] [PubMed] [Google Scholar]
- 45.Mahrus S, Craik CS. Selective chemical functional probes of granzymes A and B reveal granzyme B is a major effector of natural killer cell-mediated lysis of target cells. Chem Biol. 2005;12:567–577. doi: 10.1016/j.chembiol.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Sedlmayr P, Rabinowich H, Elder EM, Ernstoff MS, Kirkwood JM, Herberman RB, Whiteside TL. Depressed ability of patients with melanoma or renal cell carcinoma to generate adherent lymphokine-activated killer cells. J Immunother. 1991;10:336–346. doi: 10.1097/00002371-199110000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Baum C, Dullmann J, Li Z, Fehse B, Meyer J, Williams DA, von Kalle C. Side effects of retroviral gene transfer into hematopoietic stem cells. Blood. 2003;101:2099–2114. doi: 10.1182/blood-2002-07-2314. [DOI] [PubMed] [Google Scholar]
- 48.Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, Ponzoni M, Rossini S, Mavilio F, Traversari C, Bordignon C. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 49.Junker K, Koehl U, Zimmerman S, Stein S, Schwabe D, Klingebiel T, Grez M. Kinetics of cell death in T lymphocytes genetically modified with two novel suicide fusion genes. Gene Ther. 2003;10:1189–1197. doi: 10.1038/sj.gt.3301977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1 Recombinant scFv(Leu-16) binds to CD20 expressing lymphoma cells. a For periplasmic expression of CD20-specific scFv(Leu-16) under control of the IPTGinducible tac promoter, cDNA fragments encoding heavy (VH) and light chain variable domains (VL) of monoclonal antibody Leu-16 were connected by a flexible linker sequence and fused to the ompA signal peptide (SP) in the bacterial expression vector pSW50. Synthetic FLAG- and Myc-tags are included at N- and C-termini of the gene product. b The presence of ErbB2-specific control protein scFv(FRP5) (lane 1) and CD20-specific scFv(Leu-16) (lane 2) in periplasmic extracts was confirmed by SDS-PAGE and immunoblot analysis with FLAG-tag specific Mab M2. c Binding of recombinant scFv molecules to CD20 expressing but ErbB2-negative Raji lymphoma cells (upper panel), and ErbB2 expressing but CD20-negative SKBR3 breast carcinoma cells was analyzed by flow cytometry with Myc-tag specific Mab 9E10 and FITC-conjugated secondary antibody. CD20-specific Mab L27 served as a control. (PDF 59 kb)
Supplementary Fig. S2 Granzyme B activity is required for target cell killing by NK-92-scFv(Leu-16)-ζ cells. NK-92-scFv(Leu-16)-ζ cells were incubated with 100 μM of the serine protease inhibitor DCI (3,4-dichloroisocoumarin) (Roche, Mannheim, Germany) in X-VIVO 10 medium for 1 h at 37°C, before their cytotoxic activity towards CD20 expressing NIH3T3-CD20 cells was analyzed in a 3 h MTT cytotoxicity assay as described in the methods section (E/T ratio of 10:1). Untreated NK-92 and NK-92-scFv(Leu-16)-ζ cells, and NK-92-scFv(Leu-16)-ζ cells treated with DMSO served as controls. The relative number of viable target cells is expressed in % of NIH3T3-CD20 grown in the absence of NK cells (set to 100 %). Mean values of triplicate samples are shown. The standard deviation is indicated by error bars. At the concentration applied DCI was not toxic to NK-92 cells as evaluated by propidium iodide staining (data not shown). (PDF 8.85 kb)
Supplementary movie Selectivity and kinetics of target cell killing. NIH3T3-CD20 cells transduced with a retroviral vector encoding enhanced green fluorescent protein (eGFP) were mixed at a 1:1 ratio with parental NIH3T3 cells and grown overnight. Then NK-92-scFv(Leu-16)-ζ cells were added at an effector to target ratio of 1:1, microscopic images of a single field were taken at 1.5 min intervals for 6.4 h, and assembled into a QuickTime movie at 10 frames per second. At the beginning of the movie, a fluorescence microscopic image of eGFP- and CD20-positive NIH3T3-CD20(eGFP), and eGFP- and CD20-negative NIH3T3 cells before addition of NK cells is shown. Exemplary NIH3T3-CD20(eGFP) cells are indicated by white circles, exemplary parental NIH3T3 cells by black arrows. Selected images from this experiment are also shown in Fig. 5. (MOV 2.33 mb)