Abstract
Purpose
The programmed death-1 (PD-1)/B7-H1 (also called PD-L1) pathway negatively regulates T cell activation and has been suggested to play an important role in regulating antitumor host immunity. To investigate the clinical significance of B7-H1 expression to the tumor grade and postoperative prognosis of patients with urothelial cancer, we analyzed the relationship between B7-H1 expression and various clinicopathological features and postoperative prognosis.
Experimental design
Sixty-five urothelial cancer cases were examined. B7-H1 expression in tumors and the numbers and phenotypes of tumor-infiltrating lymphocytes were evaluated by immunohistochemistry and flow cytometry.
Results
A substantial expression of B7-H1 was observed in all urothelial cancers investigated. Tumor specimens from patients with higher WHO grade or primary tumor classifications showed significantly higher percentages of tumor-associated B7-H1. Tumor-associated B7-H1 expression was significantly associated with a high frequency of postoperative recurrence and poor survival rate. Furthermore, multivariate analysis indicated that tumor-associated B7-H1 was more significant prognostic factor than WHO grade.
Conclusions
Our results demonstrate that the aberrant expression of B7-H1 in urothelial cancer is associated with aggressive tumors, suggesting a regulatory role of tumor-associated B7-H1 in antitumor immunity. Therefore, the manipulation of tumor-associated B7-H1 may become a beneficial target for immunotherapy in human urothelial cancer.
Keywords: B7-H1/PD-L1, PD-1, Costimulation, Urothelial cancer, T cell, Antitumor immunity
Introduction
Bladder cancers have been characterized as one of the tumor groups in which immunological responses are relatively well preserved [1–3]. A large number of tumor-infiltrating lymphocytes (TILs) are present in bladder cancer, and the secreted cytokines and cell surface molecules in TILs positively control the local antitumor responses. Thus, bladder cancer has been shown to be sensitive to immunotherapy with Bacillus Calmette-Guerin (BCG) [4]. However, it is possible that the tumor cells might be protected from attack by immune cells and might counterattack the immune cells.
Programmed death-1 (PD-1) belongs to the B7-CD28 family of positive and negative costimulating molecules that regulate T cell responses [5–7]. PD-1 is expressed on activated T, B, and myeloid cells, and the ligation of PD-1 inhibits T cell activation and the production of cytokines such as IFN-γ, IL-10, IL-4, and IL-2 [8, 9]. PD-1-deficient mice develop various features of autoimmune diseases [9–11], indicating that PD-1 is involved in the negative regulation of immune responses and peripheral tolerance.
Two ligands, B7-H1/programmed death receptor ligand 1 (PD-L1) [12] and B7-DC/programmed death receptor ligand 2 (PD-L2) [13], have been identified as counter-receptors for PD-1. In contrast to the limited expression of B7-DC on dendritic cells and macrophages, B7-H1 is broadly expressed on non-lymphoid cells as well as lymphoid cells [12–15]. IFN-γ has been shown to be a potent inducer for B7-H1 expression in non-lymphoid tissue cells, including epithelial and endothelial cells and tumor cells [16–20]. Abundant expression of B7-H1 has been observed in tumor cells from lung, ovary, colon, breast, liver, head and neck, kidney, bladder, and skin (melanoma) cancers [18–21].
An initial study demonstrated that tumor-associated B7-H1 cells promoted apoptosis of effector cytotoxic T lymphocytes (CTLs) and escaped the lysis caused by CTLs [18]. In murine syngeneic tumor models, B7-H1 blockade using anti-B7-H1 monoclonal antibody enhanced antitumor immunity and inhibited tumor growth [18, 22, 23]. These findings suggest that the induction of B7-H1 might be one of the immunological escape mechanisms used by tumors.
An association between tumor-associated B7-H1 expression and variable clinicopathological features has been recently reported in lung, esophageal, and renal cell cancers [24–28]. In non-small cell lung cancer cells, a relationship was not seen between the expression of B7-H1 and clinicopathological variables or postoperative survival. However, in the same tissue sections, significantly fewer TILs were identified in B7-H1-positive tumor regions than in B7-H1-negative tumor regions. Moreover, the percentage of TILs expressing PD-1 was significantly lower in B7-H1-positive tumor regions than in B7-H1-negative tumor regions [24]. In esophageal cancer, B7-H1-positive patients had a significantly poorer prognosis than the negative patients. Furthermore, multivariate analysis indicated that the B7-H1 status was an independent prognostic factor and that there was no significant association between B7-H1 expression and TILs [25]. In clear-cell renal cell cancer, patients with B7-H1-positive tumors showed a poorer survival rate than those with B7-H1-negative tumors [26, 27]. A recent study with long-term follow-up suggested that tumor B7-H1 was significantly associated with a risk of rapid cancer progression and accelerated rates of mortality [28].
The above findings prompted us to investigate the B7-H1 status in bladder cancer because the abundant infiltration of TILs in bladder cancer might induce the expression of B7-H1 in tumor cells, which might modulate tumor-host responses at the local tumor site. We investigated B7-H1 expression in urothelial cancer in parallel with an assessment of PD-1 expression in TILs and analyzed the relationship between the expression of these proteins and clinicopathological features.
Patients and methods
Patients and tumor specimens
Sixty-five patients who underwent surgical resection of urothelial cancer at Kumamoto University Medical Hospital (Kumamoto, Japan) between 1996 and 2005 were enrolled in this study. The patients with urothelial cancer included 47 men (72.3%) and 18 women (27.7%), representing 50 bladder cancers, seven renal pelvic cancers, and eight ureteral cancers. Thirty patients underwent transurethral resection of a bladder tumor; 20 patients, radical cystectomy for a bladder tumor; and 15 patients, radical nephroureterectomy for ureter or renal pelvic tumors. Written permission to use human tumor tissues was obtained from the patients prior to surgery.
The surgical specimens were divided into three pieces for immunohistochemical analysis of B7-H1, flow cytometric analysis of PD-1 on TILs, and histopathological analysis. Histopathological analyses were performed by several senior pathologists and reviewed by the chief of clinical pathology in principle, and indicated that all tumors evaluated in this study were transitional cell cancers. The tumors were classified according to the World Health Organization (WHO) grade based on WHO criteria and according to the tumor-node-metastasis (TNM) staging system of the International Union Against Cancer (1997). The median follow-up for all patients was 26 months, with a range of 1–118 months. Nine patients (13.8%) died of urothelial cancer and another 2 (3.1%) died of other causes (pulmonary embolism and sepsis).
Immunohistochemistry
Tumor tissue specimens were immediately embedded in OCT compound (Miles Laboratories, Elkhart, IN) and snap-frozen in liquid nitrogen. Cryostat sections of 4- to 5-μm thickness were fixed in acetone. Immunohistochemical staining was performed using the ABC peroxidase system (Vectastain ABC kit; Vector Laboratories, Burlingame, CA) according to the protocols recommended by the manufacturer, as previously described [29]. Monoclonal antibodies against human B7-H1 (MIH1, mouse IgG1) [30, 31] and CD45 (UCHL1, mouse IgG1, Dako, Kyoto, Japan) as well as control mouse IgG1 were used as the primary antibodies. Reacted sections were visualized with substrate buffer containing diaminobenzidine (DAB; Merck, Darmstat, Germany) and counterstained with hematoxylin. Immunohistochemistry was performed under the guidance of a pathologist and an immunologist.
Evaluation of immunostaining
B7-H1 expression was described as the percentage of tumor cells displaying immunoreactivity in the cytoplasm or on the membrane. Whole areas were surveyed microscopically at 40× magnification to identify focal-staining regions. In cases of multiple areas with focal staining, three randomly selected areas were scored. The percentage of B7-H1+ tumor cells among the total number of tumor cells within focal-staining regions was evaluated. At least 200 tumor cells were scored per 400× field. To evaluate TILs, we quantified the infiltration of CD45+ cells in tumor islet regions. The infiltration ratio of CD45+ cells was evaluated as the CD45+ cell number/total tumor cell number. Cell counts were performed at 400 × in at least three fields. All of the counting was conducted in a blinded fashion; investigators had no knowledge of the outcome of the patients or the results of other analyses.
Isolation of TILs and flow cytometry
TILs from tumor specimens were isolated as previously described [32]. TILs were stained with anti-CD3-PE, anti-CD4-PerCP, anti-CD8-FITC mAb (BD Biosciences), and either biotinylated anti-PD-1 (MIH4) or control mouse IgG, followed by APC-conjugated streptavidin (BD Biosciences). The stained cells were analyzed on the FACSCalibur using CellQuest software (BD Biosciences).
Statistical analysis
All univariate analyses were performed using Statcel software (OMS Publishing Inc, Tokyo, Japan). Multivariate analysis was performed using SPSS 13.0 (SPSS Inc, Chicago, IL, USA). The significance of the differences between the expression of B7-H1 in tumor cells and clinicopathological variables was assessed by Student’s t-test or one-factor ANOVA. The relationship among the expression of B7-H1 in tumor cells, the expression of PD-1 in TILs, and the infiltration ratio of TILs was assessed by Spearman’s correlation coefficient by rank test. Survival times and recurrence-free survival times were calculated from the date of surgery to the date of death or recurrence. The Kaplan-Meier method was used to estimate the probability of survival, and significance was assessed by the log-rank test. Multivariate analysis was done using the Cox regression model in stepwise method to study ten factors (location, manner of surgery, gender, WHO grade, stage classification, T classification, N classification, tumor-associated B7-H1 expression, ratio of TILs, Low B7-H1 group). The level of significance was set at P < 0.05.
Results
B7-H1 expression in tumor cells
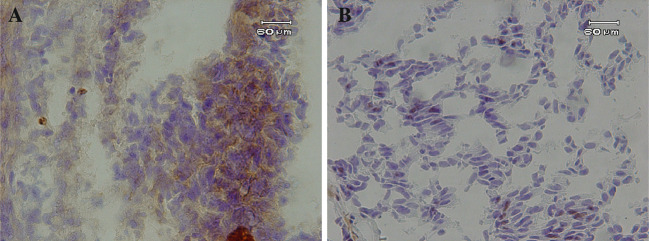
For all 65 surgically resected specimens of urothelial cancer, the expression of B7-H1 was demonstrated on the plasma membrane and in the cytoplasm of cancer cells in a focal pattern (Fig. 1). The mean percentage of B7-H1-positive cells among the cancer cells was 21.1 ± 11.0% (mean ± SD; median, 20.0%; range, 2.1–47.1%).
Fig. 1.
Immunohistostaining with anti-B7-H1 mAb in urothelial cancer sections. Representative cases from WHO grade 3 with high B7-H1 expression (a), and grade 3 with low B7-H1 expression (b) tumors are shown. Bars 60 μm
Association between B7-H1 expression and clinicopathological variables
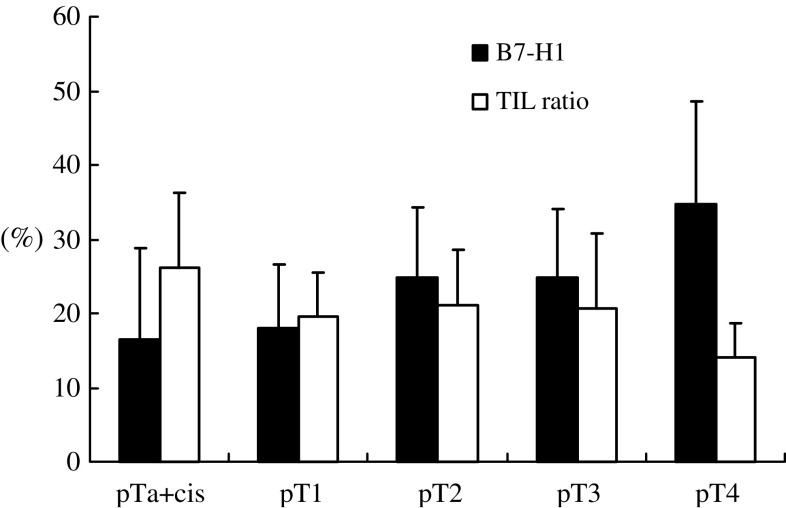
We analyzed the association between the percentage of tumor-associated B7-H1 expression and clinicopathological variables (Table 1). No association was observed between B7-H1 expression and either tumor location or patient gender. When classifying the tumors according to WHO criteria, seven specimens were classified as grades 1 and 2 and 12 specimens were classified as grades 2 and 3. Therefore, we performed the analyses in two ways, one based on single-grade groups and another based on concomitant groups. In the concomitant cases, the specimens were classified into the higher grade group. A significant association between B7-H1 expression and WHO grade was found based on both groupings (P < 0.001). In addition, tumor-associated B7-H1 expression was associated significantly (P = 0.031) with the primary tumor (T) classification (Fig. 2). No significant association was found between B7-H1 expression and either primary node (N) or primary stage classifications.
Table 1.
Correlation between B7-H1 expression on tumor cells and the clinicopathological characteristics of the 65 urothelial carcinomas
| n | B7-H1 expression (%) Mean ± SD | P value | |
|---|---|---|---|
| Location | |||
| Upper | 15 | 24.6 ± 13.2 | 0.161* |
| Lower | 50 | 20 ± 10.2 | |
| Gender | |||
| Male | 47 | 21.1 ± 10.7 | 0.994* |
| Female | 18 | 21.1 ± 12.0 | |
| WHO grade | |||
| G1 | 10 (10) | 8.12 ± 0.05 (8.12 ± 0.05) | <0.001(<0.001)** |
| G2 | 17 (24) | 18.3 ± 0.10 (20.7 ± 0.10 ) | |
| G3 | 19 (31) | 26.1 ± 0.08 (25.6 ± 0.09) | |
| T classifications | |||
| Ta + cis | 18 (13) | 16.4 ± 0.12 | 0.031** |
| T1 | 18 (11) | 18.1 ± 0.08 | |
| T2 | 12 (2) | 24.7 ± 0.09 | |
| T3 | 14 (3) | 24.8 ± 0.09 | |
| T4 | 3 (1) | 34.7 ± 0.13 | |
| N classifications | |||
| N0 | 59 | 20.7 ± 0.11 | 0.376* |
| N1–3 | 6 (1) | 24.9 ± 0.09 | |
| Stage classifications | |||
| 0a + is | 17 (13) | 16.7 ± 0.12 | 0.084*** |
| I | 18 (11) | 18.1 ± 0.08 | |
| II | 11 (2) | 24.1 ± 0.09 | |
| III | 12 (3) | 24.9 ± 0.09 | |
| IV | 7 (1) | 28.1 ± 0.11 | |
WHO grade: pure groups (concomitant groups); T, N, and stage classifications: numbers in brackets are the patients who underwent transurethral resection of bladder tumor
*Student’s paired t test; **One-factor ANOVA; ***Kruskal–Wallis test
Fig. 2.
Association between the primary tumor (T) classification and B7-H1 expression in tumor cells, and the ratio of TILs. Tumor-associated B7-H1 expression is significantly associated with T classification (P = 0.031). The association between TILs and T classification had a tendency toward an inverse association, although the difference was not statistically significant (P = 0.084)
Tumor-associated B7-H1 expression and TILs
We next examined the relationship between tumor-associated B7-H1 expression and the ratio of TILs. The associations between TILs and T classification and the Stage classification tended toward inverse relationships, although the differences were not statistically significant (T, P = 0.084; stage, P = 0.084; Fig. 2).
PD-1 expression on TILs
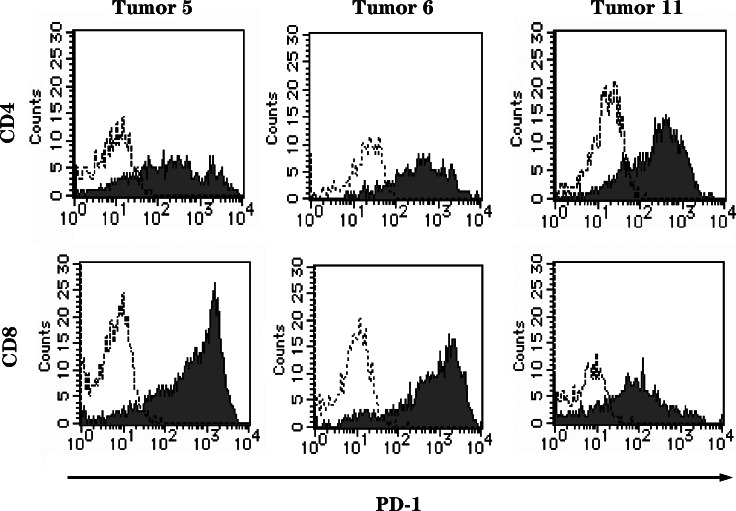
PD-1 expression on TILs was examined in 13 randomly selected cases (Table 2), which included five bladder (three patients underwent transurethral resection; two patients, radical cystectomy), two ureter, and six renal pelvic cancers. The majority of TILs, including both CD4+ and CD8+ T cells, expressed PD-1 at high levels (Fig. 3). The mean percentages of CD4+ and CD8+ TILs expressing PD-1 were 68.0 ± 18.5% and 80.6 ± 14.0%, respectively. In most of the patients, PD-1 expression on CD4+ and CD8+ TILs was highly upregulated compared with expression on peripheral blood lymphocytes (data not shown). PD-1 expression was significantly associated (P = 0.009) between CD4+ and CD8+ TILs, and an inverse association was observed between the CD4/CD8 ratio and PD-1 expression on CD8+ TILs (P = 0.005). These results indicate that a large number of the CD8+ T cells infiltrating into the urothelial cancer expressed PD-1.
Table 2.
The flow cytometric analyses data of TILs, the expression of B7-H1 on tumor cells, the infiltration ratio of CD45+ cell, and the clinicopathological characteristics of 13 patients with urothelial carcinoma
| Tumor specimen | Location | Gender | WHO grade | pStage | pT | pN | B7-H1 expression on tumor cells | Infiltration ratio of CD45+ cell | CD4/CD8 ratio of TILs | PD-1 expression on CD8+ TILs | PD-1 expression on CD4+ TILs | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Renal pelvis | m | G1-2 | 3 | 3 | 0 | 37.8 | 15 | 3.033 | 57.97 | 53.33 | ||
| 2 | Renal pelvis | m | G1-2 | 0 | a | 0 | 38.7 | 14.2 | 0.595 | 83.03 | 53.56 | ||
| 3 | Renal pelvis | f | G2 | 2 | 2 | 0 | 41 | 32 | 0.913 | 89.6 | 63.29 | ||
| 4 | Renal pelvis | m | G2 | 3 | 3 | 0 | 10 | 42.7 | 1.473 | 88 | 49.11 | ||
| 5 | Ureter | f | G2-3 | 1 | 1 | 0 | 10.1 | 10.8 | 0.519 | 95.17 | 77.5 | ||
| 6 | Ureter | f | G3 | 3 | 3 | 0 | 25.7 | 20.4 | 0.417 | 95.13 | 90.73 | ||
| 7 | Bladder | m | G3 | 3 | 3 | 2 | 29.7 | 19.8 | 1.613 | 86.79 | 85.47 | ||
| 8 | Bladder | m | G2-3 | 2 | 2 | 0 | 10.5 | 37.6 | 1.385 | 60.32 | 50.57 | ||
| 9 | Bladder | f | G2 | 1 | 1 | 0 | 12.2 | 31.1 | 2.401 | 81.37 | 87.35 | ||
| 10 | Renal pelvis | m | G1-2 | 3 | 3 | 0 | 21.3 | 34 | 2.2 | 80 | 64.94 | ||
| 11 | Bladder | m | G2-3 | 1 | 1 | 0 | 16.7 | 22.4 | 1.634 | 78.52 | 84.1 | ||
| 12 | Renal pelvis | f | G2 | 1 | 1 | 0 | 33.7 | 21.5 | 0.692 | 96.38 | 89.01 | ||
| 13 | Bladder | m | G2-3 | 1 | 1 | 0 | 32.6 | 23.4 | 4.836 | 56.36 | 35.71 | ||
| Mean ± SD | 24.6 ± 11.7 | 24.9 ± 9.6 | 1.67 ± 1.23 | 80.6 ± 14.0 | 68.0 ± 18.5 | ||||||||
| rs = −0.796 | rs = 0.683 | ||||||||||||
| P = 0.005* | P = 0.009** |
*Spearman’s rank test (correlation between CD4/CD8 ratio of TILs and PD-1 expression on CD8+ TILs)
**Pearson’s correlation coefficient test (correlation between PD-1 expression on CD8+ TILs and CD4+ TILs)
Fig. 3.
A majority of TILs, including both CD4+ and CD8+ T cells, expressed PD-1 at high levels. On CD4+ TILs from tumor specimens 5, 6, and 11, the percentages of PD-1 expression were 77.50, 90.73, and 84.10%, respectively. On CD8+ TILs from tumor specimens 5, 6, and 11, the percentages of PD-1 expression were 95.17, 95.13, and 78.52%, respectively (broken line, stained with control mAb; regular line, stained with antibody against PD-1)
Association between B7-H1 expression and postoperative prognosis
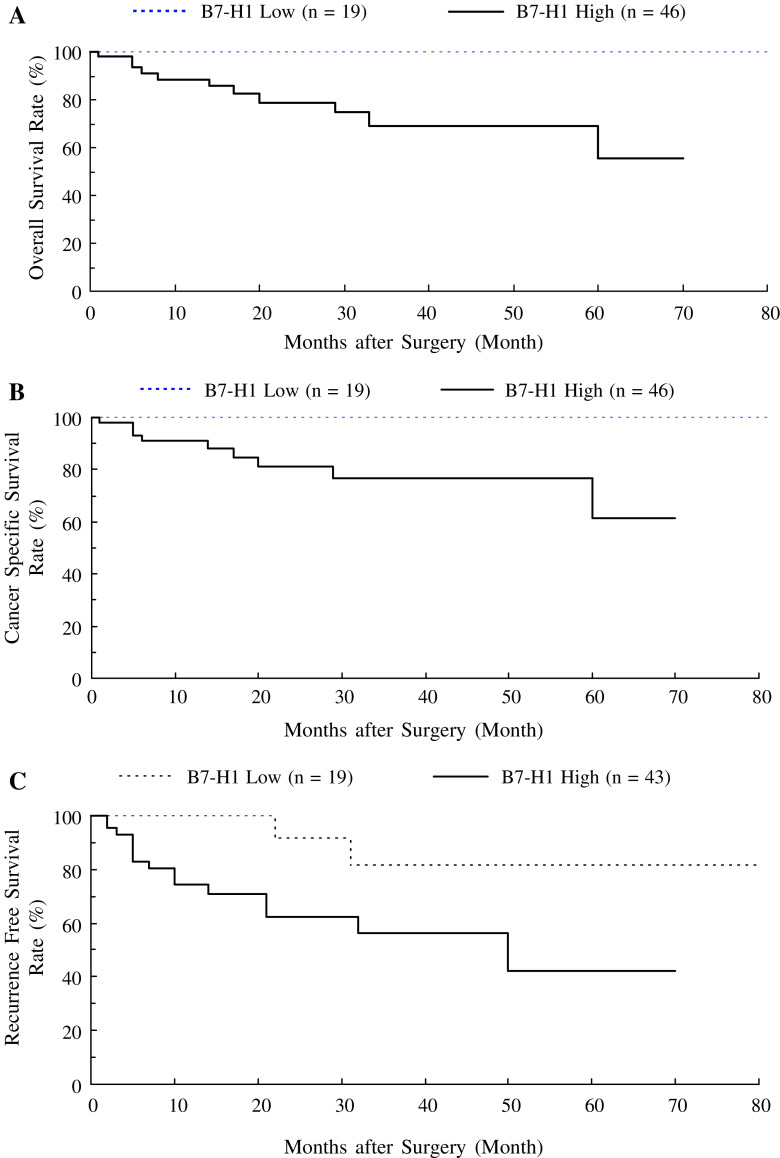
To investigate the association between tumor-associated B7-H1 expression and postoperative prognosis, we divided the 65 patients into two groups based on B7-H1 expression. The cutoff point was set at 12.2%. This value was the mean of the median percentage of B7-H1 expression together with WHO grade 1 (6.8%) and 2 (17.5%) cases. We set this value, because we found the most significant difference of B7-H1 expression between WHO grade 1 and 2 cases. The overall survival was significantly worse in patients with high-B7-H1 tumors (high B7-H1 group) than in those with low-B7-H1 tumors (low B7-H1 group) (5-year survival: 55 vs. 100%, P = 0.021; Fig. 4a). The cause-specific survival was also significantly worse in the high-B7-H1 group than in the low-B7-H1 group (5-year survival: 61 vs. 100%, P = 0.041; Fig. 4b). Among the 62 patients who had no visible rest of tumor after surgery, postoperative recurrence-free survival was significantly worse in the high-B7-H1 group than in the low-B7-H1 group (5-year recurrence-free survival: 42 vs. 81%, P = 0.026; Fig. 4c).
Fig. 4.
Overall (a) and cause-specific (b) survival in 65 patients with urothelial cancer in relation to tumor B7-H1 status. The patients with high B7-H1 expression had a poorer prognosis than those with low B7-H1 expression (a P = 0.021 and b P = 0.041). c Recurrence-free survival in 62 patients who had no visible rest of tumor after surgery in relation to tumor B7-H1 status. The patients with high B7-H1 expression had a poorer prognosis than those with low B7-H1 expression (P = 0.026). P values were determined by the log-rank test
To determine the prognostic value of tumor-associated B7-H1 expression, we did multivariate analysis using Cox regression model. WHO grade, stage classification, T classification, N classification, tumor-associated B7-H1 expression, ratio of TILs, and Low B7-H1 group had significant prognostic values. Although most of these seven factors significantly correlated each other, we compared these factors using stepwise progressively Cox regression to clarify which factor influenced the prognosis most strongly. The most significant predictor associated with poor outcomes was T classification. Stage classification correlated with T classification very significantly and was second predictor. We removed T and stage classification from dataset for seeking the best third factor. B7-H1 expression was classified as the significant factor while WHO grade was discarded. This indicated that B7-H1 expression was more significant factor than WHO grade in our samples. Another significant factor ratio of TILs had very small (<0.001) Exp(B), because of large negative B, thus no actual influence to the regression model (Table 3).
Table 3.
The stepwise progressively Cox regression with ten factors
| Before stepwise Cox regression | ||||||
|---|---|---|---|---|---|---|
| −2 Log likelihood 38.617 | ||||||
| χ 2 | df | Significance | ||||
| Location | 1.790 | 1 | 0.181 | |||
| Manner of surgery | 2.426 | 1 | 0.119 | |||
| Gender | 0.541 | 1 | 0.462 | |||
| WHO grade | 6.397 | 1 | 0.011 | |||
| Stage classification | 13.944 | 1 | <0.001 | |||
| T classification | 24.768 | 1 | <0.001 | |||
| N classification | 6.670 | 1 | 0.010 | |||
| B7-H1 expression | 7.507 | 1 | 0.006 | |||
| Ratio of TILs | 6.369 | 1 | 0.012 | |||
| Low B7-H1 groupa | 5.302 | 1 | 0.021 | |||
| Stepwise Cox regression with ten factors | ||||||
|---|---|---|---|---|---|---|
| Coefficients converged after one iteration | ||||||
| −2 Log likelihood 79.909 | ||||||
| χ 2 | df | Significance | ||||
| T classification | 21.784 | 1 | <0.001 | |||
| Variables in the equation | ||||||
|---|---|---|---|---|---|---|
| Variable | B | SE | Wald | df | Significance | Exp(B) |
| T classification | 1.586 | 0.405 | 15.312 | 1 | <0.001 | 4.885 |
| Except T classification, stepwise Cox regression with nine factors | ||||||
|---|---|---|---|---|---|---|
| Coefficients converged after two iterations | ||||||
| −2 Log likelihood 79.909 | ||||||
| χ 2 | df | Significance | ||||
| Stage and location | 22.774 | 2 | <0.001 | |||
| Variables in the equation | ||||||
|---|---|---|---|---|---|---|
| Variable | B | SE | Wald | df | Significance | Exp(B) |
| Stage classification | 1.250 | 0.328 | 14.518 | 1 | <0.001 | 3.489 |
| location | −2.583 | 1.105 | 5.465 | 1 | 0.019 | 0.76 |
| Except T and stage classification, stepwise Cox regression with eight factors | ||||||
|---|---|---|---|---|---|---|
| Coefficients converged after three iterations | ||||||
| −2 Log likelihood 79.909 | ||||||
| χ 2 | df | Significance | ||||
| B7-H1 and ratio of TILs | 15.438 | 2 | <0.001 | |||
| Variables in the equation | ||||||
|---|---|---|---|---|---|---|
| Variable | B | SE | Wald | df | Significance | Exp(B) |
| B7-H1 expression | 6.677 | 2.636 | 6.416 | 1 | 0.011 | 793.827 |
| Ratio of TILs | −18.531 | 7.742 | 5.729 | 1 | 0.017 | <0.001 |
aLow B7-H1 group: patients with tumor-associated B7-H1 less than 12.2%
Discussion
In the present study, we found three important results in human urothelial cancer. First, B7-H1 expression in tumor cells was associated with clinicopathological variables. Second, PD-1 was expressed at high levels on TILs. Third, B7-H1 expression in tumor cells was associated with the postoperative prognosis. Furthermore, it was more significant prognostic factor than WHO grade.
Consistent with previous results in other human tumors [19, 21, 24–28, 33], B7-H1 expression was detected on the cell membrane and in the cytoplasm of urothelial cancer cells. Although the precise mechanism regulating B7-H1 expression in tumor cells is unknown, a previous report showed that B7-H1 was expressed more frequently in freshly isolated cancer tissue specimens than in cultured tumor cell lines [18]. Several cytokines, including interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and interleukin (IL)-2, have been implicated as possible regulators of B7-H1 expression on the surface of several tumor cell lines [18, 19]. T cells and natural killer cells infiltrate tumor lesions and secrete various cytokines, including IFN-γ, TNF-α, and IL-2. In addition, it has been shown that the degree of effector lymphocyte infiltration in urothelial cancer is associated with the WHO grade and T classification [3]. Therefore, the induced B7-H1 in urothelial cancer may be mediated by secreted cytokines from tumor-infiltrating effector lymphocytes. IFN-γ is a potential candidate for the induction of B7-H1.
We observed that PD-1 was expressed at high levels on major populations of both CD4+ and CD8+ TILs. Thus, it is possible that the induced B7-H1 on urothelial cancer cells interacts with PD-1 on TILs and that these interactions regulate the effector function of TILs, resulting in tumor progression.
The above assumption is strongly supported by the results of our clinicopathological analyses. We observed a significant association between B7-H1 expression and both T classification and WHO grade. In addition, the T4 group expressing a high percentage of tumor B7-H1 showed the lowest TIL ratio. As both WHO grade and T classification are recognized as prognostic factors in human urothelial cancer [1–3], B7-H1 expression may be associated with prognosis. However, tumor-associated B7-H1 expression had a significant prognostic value. The prognostic value was more significant than that of WHO grade. This result may indicate an importance of tumor-associated B7-H1 expression as prognostic factors in human urothelial cancer. Nevertheless, B7-H1 expression associating with prognosis may be an epiphenomenon. For example, tumors that have a specific molecular defect that makes them more aggressive may incidentally secondarily express higher levels of various genes and/or proteins, including B7-H1.
We found a significant association between B7-H1 expression and postoperative recurrence-free survival, suggesting that the degree of B7-H1 expression may be a crucial determinant of tumor invasiveness not only for primary tumors but also for recurrent tumors. However, in subgroup analysis on several manners of surgery, we could not found any association between B7-H1 expression and each survival. Probably, this result was caused by few numbers of patients.
Urothelial tumors have been characterized as immunoresponsive tumors that contain large amounts of TILs and are sensitive to local instillations of BCG [3, 4]. Thus, tumor-specific immunotherapy has been suggested as a potentially useful strategy against bladder cancer. Dendritic cells transfected with bladder cancer-derived mRNA or pulsed with MAGE-3 peptide were able to induce CTLs against autologous tumor cells in vitro. [34, 35]. However, in advanced bladder cancers, immunotherapy using these pulsed dendritic cells showed only partial antitumor responses, and no effect was observed in a case of local recurrence [35]. In a recent clinical melanoma trial, vaccination with tumor-associated peptides combined with CTLA-4 blockade showed better antitumor responses than vaccination with tumor-associated peptide alone [36]. Similar to B7-H1, CTLA-4 is a negative regulator of T cell activation. These results suggest that adverse factors capable of reducing antitumor immune responses may exist in tumor-bearing hosts. Tumor-associated B7-H1 might be one of these regulatory factors.
Renal cell cancer (RCC) is also regarded as an immunogenic tumor like urothelial cancer. High levels of infiltrating T cells are frequently observed within RCC tumors but are paradoxically associated with diminished cancer-specific patient survival [37]. Survival among RCC patients is commonly predicted based on TNM stage, nuclear grade, and performance status [38]. However, these predictors show the same paradoxical association [39]. In a recent long-term follow-up study, RCC patients with high tumor B7-H1 expression showed a significantly increased risk of death, which was independent of current clinical predictors [28]. Furthermore, this risk was associated with metastatic progression of localized RCC.
In conclusion, we have demonstrated the remarkable expression of B7-H1 in urothelial cancer. The B7-H1 expression in tumor cells was well associated with WHO grade, T classification, recurrence rate, and recurrence-free survival. These results suggest that the expression of B7-H1 in urothelial cancer may contribute to the prognosis as well as local progression of tumors. The assessment of tumor-associated B7-H1 in specimens may provide a rationale for intensive treatment of high-risk cases. Furthermore, the manipulation of tumor-associated B7-H1 or PD-1 signaling in TILs may provide an improved outcome in the treatment of urothelial cancer.
References
- 1.Tsujihashi H, Matsuda H, Uejima S, Akiyama T, Kurita T. Immunocompetence of tissue infiltrating lymphocytes in bladder tumors. J Urol. 1988;140:890–894. doi: 10.1016/s0022-5347(17)41851-9. [DOI] [PubMed] [Google Scholar]
- 2.Tsujihashi H, Matsuda H, Uejima S, Akiyama T, Kurita T. Immunoresponse of tissue infiltrating lymphocytes in bladder tumors. J Urol. 1989;141:1467–1470. doi: 10.1016/s0022-5347(17)41348-6. [DOI] [PubMed] [Google Scholar]
- 3.Lipponen PK, Eskelinen MJ, Jauhiainen K, Harju E, Terho R. Tumor-infiltrating lymphocytes as an independent prognostic factor in transitional cell bladder cancer. Eur J Cancer. 1993;29A:69–75. doi: 10.1016/0959-8049(93)90579-5. [DOI] [PubMed] [Google Scholar]
- 4.Patard JJ, Saint F, Velotti F, Abbou CC, Chopin DK. Immune response following intravesical Bacillus Calmette-Guerin instillations in superficial bladder cancer: a review. Urol Res. 1998;26:155–159. doi: 10.1007/s002400050039. [DOI] [PubMed] [Google Scholar]
- 5.Dong H, Chen L. B7-H1 pathway and its role in the evasion of tumor immunity. J Mol Med. 2003;81:281–287. doi: 10.1007/s00109-003-0430-2. [DOI] [PubMed] [Google Scholar]
- 6.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307–314. doi: 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okazaki T, Iwai Y, Honjo T. New regulatory co-receptors: inducible co-stimulator and PD-1. Curr Opin Immunol. 2002;14:779–782. doi: 10.1016/S0952-7915(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 8.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura H, Nose M, Hirai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/S1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10:1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsunori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 12.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 13.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 14.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamura H, Dong H, Zhu G, Sica GL, Flies DB, Tamada K, Chen L. B7-H1 costimulation preferentially enhances CD28-independent T-help cell function. Blood. 2001;97:1809–1816. doi: 10.1182/blood.V97.6.1809. [DOI] [PubMed] [Google Scholar]
- 16.Youngnak-Piboonratanakit P, Tsushima F, Otsuki N, Igarashi H, Machida U, Iwai H, Takahashi Y, Omura K, Yokozeki H, Azuma M. The expression of B7-H1 on keratinocytes in chronic inflammatory mucocutaneous disease and its regulatory role. Immunol Lett. 2004;94:215–222. doi: 10.1016/j.imlet.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.CAN-03-3259. [DOI] [PubMed] [Google Scholar]
- 18.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 19.Wintterle S, Screiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, Weller M, Wiendl H. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462–7467. [PubMed] [Google Scholar]
- 20.Tsuda M, Matsumoto K, Inoue H, Matsumura M, Nakano T, Mori A, Azuma M, Nakanishi Y. Expression of B7-H1 and B7-DC on the airway epithelium is enhanced by double-stranded RNA. Biochem Biophys Res Commun. 2005;330:263–270. doi: 10.1016/j.bbrc.2005.02.161. [DOI] [PubMed] [Google Scholar]
- 21.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 22.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggern R, Hemminiki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 23.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 25.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeba N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, Azuma M, Nakajima Y. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 26.Thompson RH, Webster WS, Cheville JC, Lohse CM, Dong H, Leibovich BC, Kuntz SM, Sengupta S, Kwon ED, Blute ML. B7-H1 glycoprotein blockade: a novel strategy to enhance immunotherapy in patients with renal cell carcinoma. Urology. 2005;66:10–14. doi: 10.1016/j.urology.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, Zincke H, Blute ML, Strome SE, Leibovich BC, Kwon ED. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, Blute ML, Sebo TJ, Cheville JC, Kwon ED. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 29.Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, Collins M, Honjo T, Freeman GJ, Carreno BM. PD-1: PD-L inhibitory pathway affects both CD4 (+) and CD8 (+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Youngnak P, Kozono Y, Kozono H, Iwai H, Otsuki N, Jin H, Omura K, Yagita H, Pardoll DM, Chen L, Azuma M. Differential binding properties of B7-H1 and B7-DC to programmed death-1. Biochem Biophys Res Commun. 2003;307:672–677. doi: 10.1016/S0006-291X(03)01257-9. [DOI] [PubMed] [Google Scholar]
- 31.Tsushima F, Tanaka K, Otsuki N, Youngnak P, Iwai H, Omura K, Azuma M. Predominant expression of B7-H1 and its immunoregulatory roles in oral squamous cell carcinoma. Oral Oncol. 2006;42:268–274. doi: 10.1016/j.oraloncology.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Mehal WZ, Azzaroli F, Crispe IN. Antigen presentation by liver cells controls intrahepatic T cell trapping, whereas bone marrow-derived cells preferentially promote intrahepatic T cell apoptosis. J Immunol. 2001;167:667–673. doi: 10.4049/jimmunol.167.2.667. [DOI] [PubMed] [Google Scholar]
- 33.Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, Kasperbauer JL, Ballman KV, Chen L. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 34.Schmitt WE, Stassar MJ, Schmitt W, Little M, Cochlovius B. In vitro induction of a bladder cancer-specific T-cell response by mRNA-transfected dendritic cells. J Cancer Res Clin Oncol. 2001;127:203–206. doi: 10.1007/s004320000201. [DOI] [PubMed] [Google Scholar]
- 35.Nishiyama T, Tachibana M, Horiguchi Y, Nakamura K, Ikebe Y, Takesako K, Murai M. Immunotherapy of bladder cancer using autologous dendritic cells pulsed with human lymphocyte antigen-A24-specific MAGE-3 peptide. Clin Cancer Res. 2001;7:23–31. [PubMed] [Google Scholar]
- 36.Hsu FJ, Komarovskaya M. CTLA4 blockade maximizes antitumor T-cell activation by dendritic cells presenting idiotype protein or opsonized anti-CD20 antibody-coated lymphoma cells. J Immunother. 2002;25:455–268. doi: 10.1097/00002371-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–5136. [PubMed] [Google Scholar]
- 38.Zisman A, Pantuck AJ, Dorey F, Said JW, Shvarts O, Quintana D, Gitlitz BJ, deKernion JB, Figlin RA, Belldegrun AS. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol. 2001;19:1649–1657. doi: 10.1200/JCO.2001.19.6.1649. [DOI] [PubMed] [Google Scholar]
- 39.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]