Abstract
Background
The human 5T4 (h5T4) oncofoetal antigen is expressed by a wide variety of human carcinomas including colorectal, ovarian, gastric and renal, but rarely on normal tissues. Its restricted expression on tumour tissues as well as its association with tumour progression and bad prognosis has driven the development of a MVA-based vaccine (TroVax) which has been tested in several early phase clinical trials and these studies have led to the start of a phase III trial in renal cell carcinoma patients. We have recently shown that CD8+ T cells recognizing h5T4 can be generated in the absence of CD4+ T cells from peripheral blood lymphocytes of human healthy individuals.
Results
We report the existence and expansion of human CD4+ T cells against h5T4 by stimulation with autologous monocyte-derived dendritic cells infected with a replication defective adenovirus encoding the h5T4 cDNA (Ad-h5T4). The h5T4-specific T-cell responses in normal individuals are enhanced by initial depletion of CD25+ cells (putative T regulatory cells) prior to the in vitro stimulation. We have identified a novel h5T4-derived 15-mer peptide recognized by CD4+ T cells in HLA-DR4 positive healthy individuals. Interestingly, CD4+ T cells spontaneously recognizing a different 5T4 epitope restricted by HLA-DR were identified in tumour-infiltrating lymphocytes isolated from a regressing renal cell carcinoma lung metastasis.
Conclusion
Our data show that CD4+ T cells recognizing h5T4 can be expanded and detected in healthy individuals and a renal cell carcinoma patient. Such h5T4-specific CD4+ T cells boosted or induced by vaccination could act to modulate both cell or antibody mediated anti-tumour responses.
Keywords: 5T4 oncofoetal antigen, Dendritic cells, CD4+ T cells, T regulatory cells, CD25+ cell depletion
Introduction
Following the landmark studies of Boon [1] and Rosenberg [2], the expression of certain antigens on human cancer cells that can be specifically targeted by cellular immunity, is driving the design and clinical testing of antigen-specific cancer immunotherapy [3]. Anti-tumour immune responses appear to be widely mediated by T cells rather than by antibodies [4], and current evidence suggests that both CD8+ CTL and CD4+ T helper cells are required for inducing effective anti-tumour immunity [5]. Therefore, identification of CD8+ and CD4+ epitopes presented by MHC class I and II molecules, respectively, on tumour cells is important for the design of active immunotherapy and/or their immune monitoring. As the tumour-associated antigens (TAA) are often potential autoantigens, it is clear that any induced immunity must be accompanied by minimum deleterious autoimmune reactions.
The human oncofoetal antigen 5T4 (h5T4) is a 72 kDa cell leucine-rich region repeat membrane glycoprotein [6, 7], which is highly expressed in trophoblast but shows relatively limited expression in other normal tissues [8]. In contrast the h5T4 antigen is upregulated in a wide variety of human carcinomas including colorectal, gastric, and ovarian where it is associated with poor clinical outcome [9–13]. Recently, we have also reported that renal cell carcinoma express h5T4 [14]. The restricted expression of h5T4 antigen on tumour tissues as well as its association with tumour progression have led to appropriate preclinical and clinical trials of a MVA based vaccine [15, 16] and an antibody delivered superantigen therapy [17, 18] as well as other preclinical studies using chimeric immune receptors of polyclonal T cell populations [19, 20].
Natural and inducible T regulatory cells (hereafter termed Treg) are subsets of lymphocytes able to suppress immune responses by direct interaction with other immune cell types or through secretion of immune suppressive cytokines and Treg appear crucial in maintaining immune homeostasis, mediating peripheral tolerance and preventing autoimmunity [21–23]. Emerging evidence suggests that these regulatory T cells may also suppress host T-cell activity against TAA [24–26], thereby facilitating tumour escape from immunological control/rejection. Naturally occurring T-regulatory cells are identified by expressing CD4 and high levels of CD25 as well as the nuclear transcription repressor forkhead box P3 (FOXP3) which distinguishes them from activated T cells [27–29].
In this study, we have investigated the existence of CD4+ T cells specific for h5T4 in the circulation of healthy individuals and assessed the impact of depleting T regulatory cells, as defined by CD25 expression, on the generation of h5T4-specific CD4+ T-cell responses in vitro. Healthy donors (HD) required CD25+-cell depletion for in vitro induction of significant h5T4-specific CD4+ T-cell responses. We identified a novel HLA-DR4-restricted h5T4-derived 15-mer peptide. Additionally, a population of CD4+ T cells spontaneously recognizing another HLA-DR restricted h5T4 epitope was also characterized in tumour-infiltrating lymphocytes from a renal cell carcinoma metastasis.
Materials and methods
Peptides
Individual peptides consisting of 32 amino acids, overlapping by 12–15 amino acids, were designed to span the whole sequence of h5T4 and synthesized as previously described [30]. Six peptide pools (PP) were prepared by mixing four individual peptides, except PP6 by mixing three peptides. Sequences of individual peptides and description of PP are shown in Table 1. Potential HLA-DR4 binding peptides derived from h5T4 were predicted using different algorithms. Three peptides were chosen for further investigation and named h5T4106–120 (P1; AGAFEHLPSLRQLDL), h5T4190–204 (P2; SNHFLYLPRDVLAQL), and h5T4222–236 (P3; YVSFRNLTHLESLHL). They were synthesised by Sigma-Genosys Ltd, Haverhill, UK with a purity of ≥95%.
Table 1.
Description of peptides and peptide pools used in this study
| Peptide pool | Peptide code | Sequence |
|---|---|---|
| PP1 | A | SSSSPTSSASSFSSSAPFLASAVSAQPPLPDQ |
| B | PFLASAVSAQPPLPDQCPALCECSEAARTVKC | |
| C | PALCECSEAARTVKCVNRNLTEVPTDLPAYVR | |
| D | VNRNLTEVPTDLPAYVRNLFLTGNQLAVLPAG | |
| PP2 | E | RNLFLTGNQLAVLPAGAFARRPPLAELAALNL |
| F | AFARRPPLAELAALNLSGSRLDEVRAGAFEHL | |
| G | SGSRLDEVRAGAFEHLPSLRQLDLSHNPLADL | |
| H | PSLRQLDLSHNPLADLSPFAFSGSNASVSAPSPLV | |
| PP3 | I | SPFAFSGSNASVSAPSPLVELILNHIVPPEDE |
| J | PLVELILNHIVPPEDERQNRSFEGMVVAALLA | |
| K | RQNRSFEGMVVAALLAGRALQGLRRLELASNH | |
| L | GRALQGLRRLELASNHFLYLPRDVLAQLPSLR | |
| PP4 | M | FLYLPRDVLAQLPSLRHLDLSNNSLVSLTYVS |
| N | HLDLSNNSLVSLTYVSFRNLTHLESLHLEDNA | |
| O | FRNLTHLESLHLEDNALKVLHNGTLAELQGL | |
| P | LKVLHNGTLAELQGLPHIRVFLDNNPWVCDCH | |
| PP5 | Q | HIRVFLDNNPWVCDCHMADMVTWLKETEVVQG |
| R | MADMVTWLKETEVVQGKDRLTCAYPEKMRNRV | |
| S | KDRLTCAYPEKMRNRVLLELNSADLDCDPIL | |
| T | LLELNSADLDCDPILPPSLQTSYVFLGIVLAL | |
| PP6 | V | IGAIFLLVLYLNRKGIKKWMHNIRDACRDHME |
| W | NRKGIKKWMHNIRDACRDHMEGYHYRYEINAD | |
| X | RDACRDHMEGYHYRYEINADPRLTNLSSNSDV |
Each peptide pool (PP) is prepared by mixing four (except PP6 is made of three peptides) of the 32-mer peptides. Each 32-mer peptide is given a code from A to X. The sequence of these peptides is shown and the one-letter code for amino acids is used
Generation and infection of monocyte-derived dendritic cells
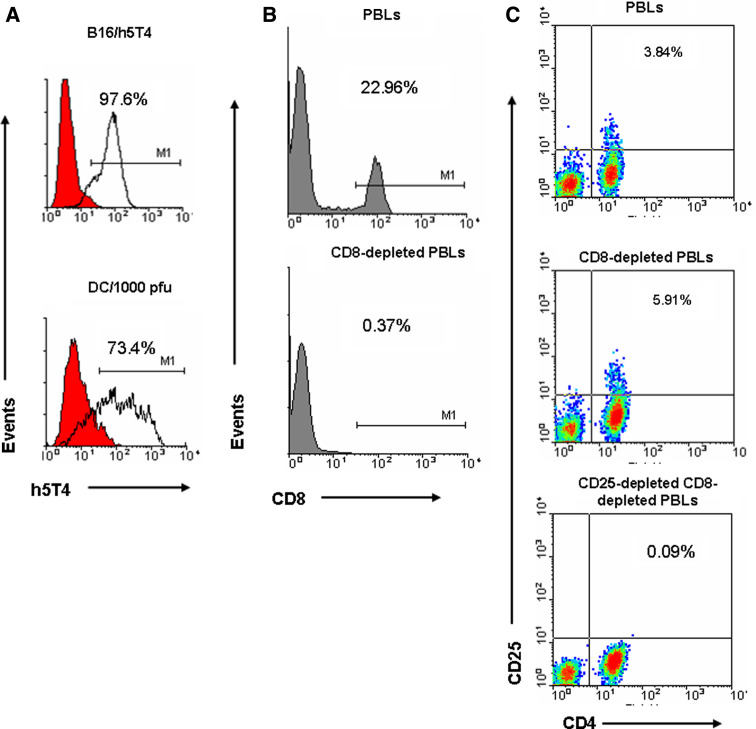
Peripheral blood was collected from HD following informed consent. Peripheral blood mononuclear cells (PBMCs) were isolated from fresh whole blood by Ficoll–Paque density gradient centrifugation. PBMCs (3 × 106/ml) were suspended in X-VIVO 15 medium (Cambrex, UK) supplemented with l-glutamine and penicillin/streptomycin, and 3 ml were added into each well of 6-well plates. Cells were allowed to adhere in a 5% CO2 incubator at 37°C for 90 min. Non-adherent cells were collected as peripheral blood lymphocytes (PBLs) and the adherent cells were washed and cultured in X-VIVO 15 medium supplemented with rhGM-CSF (100 ng/ml) and rhIL-4 (50 ng/ml) (both from R&D, Oxford, UK) for 5–6 days to be used as antigen presenting dendritic cells (DC). DC were infected with adenovirus encoding for human 5T4 (Ad-h5T4) or Ad-GFP, as a control, by co-incubation with 1,000 pfu per cell for 4 h at 37°C. Following the infection period the virus was removed and replaced with fresh complete X-VIVO 15 together with rhGM-CSF and rhIL-4 and culturing was continued for a further 48 hours. Expression of h5T4 on DC was examined by staining with 1 μg/ml mouse anti-human 5T4 monoclonal antibody [31] for 30 min on ice, followed by incubation with FITC-conjugated polyclonal rabbit anti-mouse Ig (Dako, UK) diluted 1:20 in PBS. Usually >70% of DC expressed h5T4 following infection, as shown in Fig. 1a. The mouse melanoma cell line B16 transfected with h5T4 was used as a positive control.
Fig. 1.
Efficiency of DC infection and CD8/CD25 depletion. a B16 cell line expressing h5T4 is stained for h5T4 as a positive control and h5T4 expression on dendritic cells (73.4%) infected with Ad-h5T4. CD4+-enriched subpopulations were assessed by FACS analysis for contamination with residual CD8+ T cells (<0.4%, b) or CD25+ cells (<0.1%, c)
Generation of h5T4-specific CD4+ T cells
Peripheral blood lymphocytes were subjected to CD8+ depletion by attachment of anti-CD8-conjugated microbeads (Miltenyi Biotech, Surrey, UK) and subsequent removal of CD8+-labelled cells using auto MACS™ separator in accordance with the manufacturer’s instructions. After that, negatively selected CD4+-enriched PBLs were either depleted or not of CD25+ cells using anti-CD25-conjugated microbeads (Miltenyi Biotech). Efficiency of CD8 and CD25 depletion was assessed by flow cytometry following direct staining of CD8 or CD25-depleted PBLs with anti-human CD8-FITC or anti-human CD4-FITC and anti-human CD25-PE (Fig. 1b, c). CD4+-enriched PBLs (<0.5% residual CD8+ T cells) or CD25-depleted (<0.1% residual CD25+ cells) were co-incubated with autologous DC (T cells:DC = 10:1) expressing h5T4 (or GFP) in complete RPMI supplemented with 10% human AB serum and 10 ng/ml rhIL-7 (R&D, UK) to generate week one h5T4-specific CD4+ T cells. Subsequent re-stimulations were performed by co-incubation with autologous monocyte-derived DC expressing Ad-h5T4 (or Ad-GFP) in complete RPMI with an additional supplement of 25 IU/ml rhIL-2 (R&D Systems, UK), starting from day 3 of the second stimulation. For generation of peptide-specific CD4+ T cells, day 5 or 6 immature DC were incubated with 0.5 μg/ml LPS (Escherichia coli 026:B6, Sigma, Poole, Dorset, UK) for 48 h at 37°C. Mature DC were then washed three times, resuspended into serum-free RPMI and pulsed with 10 μg/ml of 15-mer individual peptide for 2 h at 37°C. CD4+-enriched PBLs or CD25+-depleted CD4+-enriched PBLs were co-incubated with autologous peptide-pulsed mature DC (T cells:DC = 10:1) for one or two rounds of in vitro stimulation.
Antigen recognition assays
ELISPOT
The protocol for IFN-γ or IL-10 ELISPOT assay (Immunodiagnostic Systems, Boldon, Tyne & Wear, UK) was used with some modifications, as described by the manufacturer (Diaclone Research, Besancon, France). Briefly, Multiscreen-HA 96-well filter plates (Millipore, Etten-Leur, The Netherlands) were coated overnight at 4°C with an anti-IFN-γ capturing antibody in sodium hydrogen carbonate buffer, pH 9.6. PBMCs were pulsed for 2 h at 37°C with 40 μg/ml of peptide pool (10 μg/ml of individual peptide). Unpulsed or peptide-pulsed PBMCs were used as stimulators, and incubated with in vitro-expanded effectors (105/well) overnight. Cells were then removed and plates processed according to the manufacturer’s instructions. Spots were counted with a computer-assisted video-imaging ELISPOT reader (AID ELISPOT, Germany). Specific spots were calculated by subtracting the mean number of spots of the medium only control or vector control from the mean number of spots in experimental wells. h5T4-specific T-cell frequencies were considered to be increased compared to the controls when specific T-cell frequencies were ≥10/105.
IFN-γ intracellular staining
Expanded cells were washed three times and resuspended in RPMI supplemented with 5% human AB serum. Cells were incubated with 10 μg/ml peptide, 1 μg/ml staphylococcal enterotoxin B (Sigma) as a positive control, or mock-stimulated with equivalent concentration of DMSO for 6 h at 37°C in the presence of 1 μl/ml GolgiPlug containing brefeldin A (BD Biosciences, UK). Samples were stained for CD3 and CD4 surface antigens using anti-CD3-PerCP and anti-CD4-FITC (BD Pharmingen, UK) followed by fixation and permeabilisation for 20 min at 4°C using fixation/permeabilisation solution (BD Biosciences). Samples were then washed twice in perm/wash buffer (BD Biosciences), blocked with 1% normal mouse serum for 15 min, and stained for intracellular IFN-γ with 0.2 μg anti-human IFN-γ-PE (BD Pharmingen) or mouse IgG1-PE isotype negative control for 30 min at 4°C. A minimum of 100,000 events for each sample was collected on a FACSCalibur (BD Biosciences, CA, USA) and data were analysed using WinMDI 2.8 software by gating first on the lymphocyte population identified by forward and side scatter and then by analysis of IFN-γ-positive population within the CD3 and CD4 double-positive gate.
Phenotypic identification of T regulatory cells
T regulatory cells were identified by expression of CD4, CD25 and FOXP3 transcription factor using the human regulatory T cell staining kit from eBioscience (San Diego, CA, USA). Cells were first stained for cell surface CD4 and CD25 markers using CD4/25 cocktail (a cocktail of anti-human CD4-FITC and anti-human CD25-APC). Following fixation and permeabilisation, the cells were washed and blocked for non-specific binding sites using normal rat serum. Anti-human FOXP3-PE or rat IgG2a-PE isotype negative control were then added for 30 min before washing twice and flow cytometric analysis using a FACSCalibur flow cytometer.
Antibody blocking assay
To determine whether CD4+ T-cell recognition of P3 15-mer peptide could be blocked by specific antibodies, we measured IFN-γ secretion in an ELISPOT assay in the absence or presence of monoclonal antibodies. These antibodies included W6/32 (anti-HLA-ABC, Serotec, UK), L243 (anti-HLA-DR, BD Pharmingen, UK), and isotype-matched control (mouse IgG2a, Serotec), and were added at 10 μg/ml to P3-expanded CD4+ T cells in the presence of 10 μg/ml of P3.
Isolation of tumour-infiltrating lymphocytes (TILs) from a lung metastasis of a renal cell carcinoma patient
Tumour material was isolated from a lung lesion by chopping with scalpels. The tumour was then chopped into smaller pieces, and approximately 2 mm3 samples were placed into 6 well plates containing RPMI + 10% AB + 100 IU/ml IL-2. Growing cells were expanded by further culture in RPMI + 10% AB + 100 IU/ml IL-2. The original tumour material was kept in wells containing TILs at all times. More than 95% of TILs were CD4+ T cells as confirmed by flow cytometric analysis.
Results
h5T4-specific CD4+ T-cells are detectable at low frequency in some healthy individuals in the presence of CD25+ T cells
PBLs were depleted of CD8+ T cells to enrich for CD4+ T cells. Efficiency of CD8 depletion is shown in Fig. 1b. CD4+-enriched PBLs undepleted of CD25+ T cells were stimulated with autologous monocyte-derived DC expressing h5T4. A single in vitro stimulation could not induce detectable h5T4-specific CD4+ T-cell responses as tested in IFN-γ ELISPOT versus six pools of peptides covering the sequence of human 5T4 molecules. However, two successive stimulations did generate IFN-γ-secreting CD4+ T cells against h5T4-derived peptides in three of nine healthy donors (HD1, HD3 and HD7, shown in Table 2). No h5T4-specific CD4+ T-cell responses were generated when donor CD4+-enriched PBLs were stimulated twice with autologous Ad-GFP-expressing DC (3 donors tested and frequency always <1/104 against any peptide pool). Presumably the frequency of h5T4-specific CD4+ T cells in the peripheral blood of some HD is too low to be detected without two rounds of stimulation and expansion.
Table 2.
Summary of h5T4-specific CD4+ T-cell responses in nine healthy donors
| Peptide | Pool 1 | Pool 2 | Pool 3 | Pool 4 | Pool 5 | Pool 6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor | CD25+ | CD25− | CD25+ | CD25− | CD25+ | CD25− | CD25+ | CD25− | CD25+ | CD25− | CD25+ | CD25− |
| HD1 | 7 | 7 | 9 | 6 | 5 | 8 | 12 | 202 | 19 | 2 | 5 | 5 |
| HD2 | 0 | 8 | 0 | 5 | 6 | 12 | 0 | 32 | 0 | 6 | 0 | 13 |
| HD3 | 1 | 0 | 40 | 213 | 25 | 51 | 21 | 63 | 10 | 6 | 9 | 0 |
| HD4 | 1 | 21 | 0 | 9 | 0 | 16 | 0 | 17 | 0 | 11 | 0 | 1 |
| HD5 | 0 | 2 | 0 | 4 | 2 | 4 | 1 | 14 | 1 | 4 | 0 | 4 |
| HD6 | 8 | 4 | 0 | 3 | 0 | 13 | 0 | 4 | 0 | 3 | 3 | 28 |
| HD7 | 0 | 0 | 0 | 0 | 49 | 78 | 0 | 0 | 0 | 0 | 31 | 54 |
| HD8 | 0 | 0 | 0 | 0 | 3 | 12 | 0 | 2 | 0 | 0 | 0 | 21 |
| HD9 | 2 | 3 | 0 | 0 | 5 | 2 | 3 | 4 | 0 | 0 | 7 | 0 |
h5T4 specific IFN-γ-producing CD4+ T-cell responses to 6 pools of h5T4 32-mer peptides following 2 rounds of stimulation with autologous DC infected with Ad-h5T4. Specific h5T4-peptide responses were calculated by subtracting the mean number of spots in CD4+ T cells plus PBMC (in the absence of peptides). Specific frequencies are shown per 105 cells. Bold indicates donors where a frequency >1/104 and with an increase in the CD25+-depleted CD4+ T responders compared with undepleted cells
HD Healthy donor, CD25 + undepleted population, CD25 − CD25-depleted population
Higher frequency of h5T4-specific CD4+ T cells can be generated in most healthy individuals in the absence of CD25+ T cells
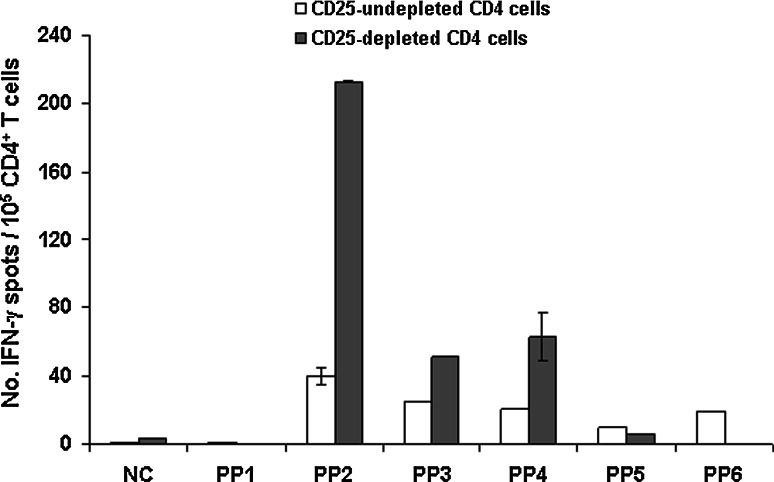
To explore any possible inhibitory effect of T regulatory cells, as defined by CD25 expression, on the h5T4-specific CD4+ T-cell responses, CD4+-enriched PBLs were expanded in the absence of CD25+ T cells. CD4+-enriched PBLs depleted of CD25+ T cells (efficiency of CD25 depletion is shown in Fig. 1c) were cultured with autologous DC expressing h5T4. Specificity to h5T4 was investigated by expanding effector cells with DC infected with Ad-GFP (3 donors frequency always <1/104 against any peptide pool). Interestingly, IFN-γ-secreting h5T4-specific CD4+ T cells were now detected in eight of nine HD, compared to three without CD25+ T-cell depletion. Typically the frequency of IFN-γ-secreting h5T4-specific CD4+ T cells was higher in the CD25+-depleted populations than the undepleted populations (Table 2). For example, in HD3, responses to PP 2, 3, 4 increased 5-, 2- and 3-fold, respectively (Fig. 2), whereas in 5 donors there were de novo responses detected (HD2, 4, 5, 6 and 8). One possible interpretation of this result is that Treg can play a role in inhibiting h5T4-specific CD4+ T-cell responses, and the initial depletion of CD25+ cells enables detection of additional responses in vitro. The differential peptide pool ELISPOT responses of individual donors are likely to reflect the polyclonal repertoire of CD4+ T-cell responses to h5T4 and derived, at least in part, from HLA polymorphism.
Fig. 2.
Depletion of CD25+ cells prior to cell expansion significantly enhances h5T4-specific CD4+ T-cell responses in healthy individuals. CD4+-enriched PBLs undepleted or depleted of CD25+ cells were expanded in vitro for two stimulations using h5T4-expressing DC. The reactivity of expanded effectors against autologous PBMC pulsed with h5T4-derived peptide pools is measured in IFN-γ ELISPOT assays. The data are means of triplicate determinations ±SE. NC is negative control where expanded effectors were incubated with media alone
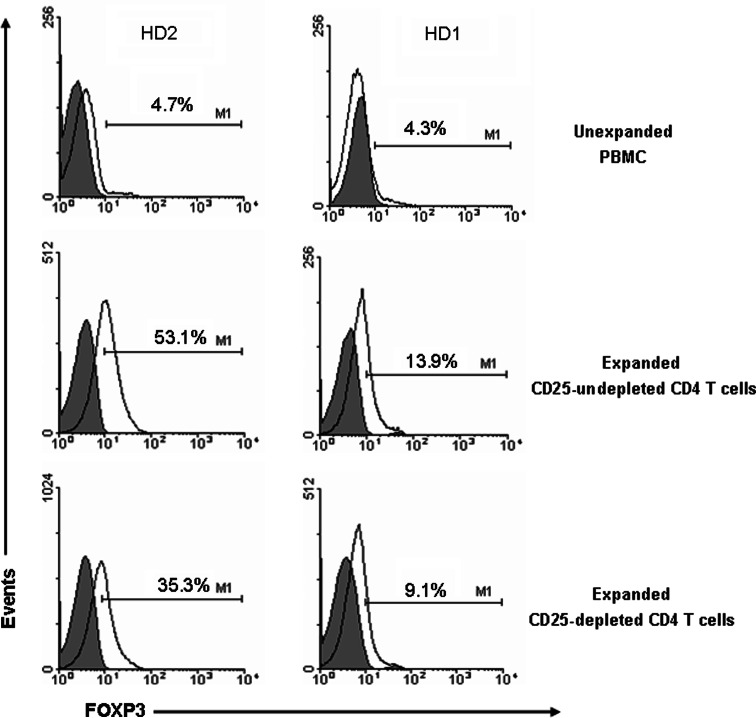
Treg expansion is favoured by the IL-2-based culture conditions used to expand other effector populations [32]. In order to determine the effect of the in vitro culture system on the expansion of Treg and whether the initial depletion of CD25+ cells may reduce Treg percentage (as defined by markers typically expressed by Treg), FOXP3 expression levels were measured in unexpanded or expanded CD4+ T cells depleted or not of CD25+ cells. Indeed, Fig. 3 shows an increased frequency of FOXP3-expressing CD4+-enriched PBLs following two rounds of in vitro stimulation with h5T4-expressing DC. However, the proportion was higher in CD4+ T-cell populations undepleted compared to those previously depleted of CD25+ T cells. These marker studies are consistent with higher h5T4-specific CD4+ T-cell responses detectable when there are lower proportions of Treg natural or induced.
Fig. 3.
Increase of FOXP3 expression in the T-cell culture especially in the CD25+-undepleted populations. FOXP3 positive cells are expressed as percentage of CD4 positive cells because it was difficult to delineate CD25high cells in the expanded cell populations. Histograms from two representative experiments out of four performed are shown. Grey filled histograms show cells stained for negative isotype control and M1 region identifies FOXP3 positive population
Predicted HLA-DR4-binding peptides
Prediction of candidate 15-mer epitopes derived from h5T4 that have the potential to bind HLA-DR4 motif was performed using four different algorithms, as shown in Table 3. For example these peptides gave the maximum binding scores using the SYFPEITHI [33] and Leiden [34] algorithms. We noticed responses were generated against PP2, PP3 and PP4 in the majority of donors especially the HLA-DR4 positive subjects (HD3, HD4 and HD5), as shown in Table 2. Therefore, three peptides which gave high binding scores using these prediction algorithms and also their sequences were located within the PP2, PP3 and PP4, as shown in Table 3, were selected as candidate HLA-DR4-restricted peptides for further investigation.
Table 3.
Prediction of HLA-DR4 binding peptides derived from h5T4 using different algorithms
| Peptide code | Position | Sequence | Purity (%) | Score (R) | Score (L) | Score (S) (%) | Score (Re) | 32mer (PP) |
|---|---|---|---|---|---|---|---|---|
| P1 | 106–120 | AGAFEHLPSLRQLDL | 98 | 28 | 50 | 34 | NB | G (PP2) |
| P2 | 190–204 | SNHFLYLPRDVLAQL | 95 | 28 | 50 | 23 | NB | L (PP3) |
| P3 | 222–236 | YVSFRNLTHLESLHL | 95 | 28 | 50 | 51 | 38.7% | N, O (PP4) |
Identification of one HLA-DR4-restricted peptide
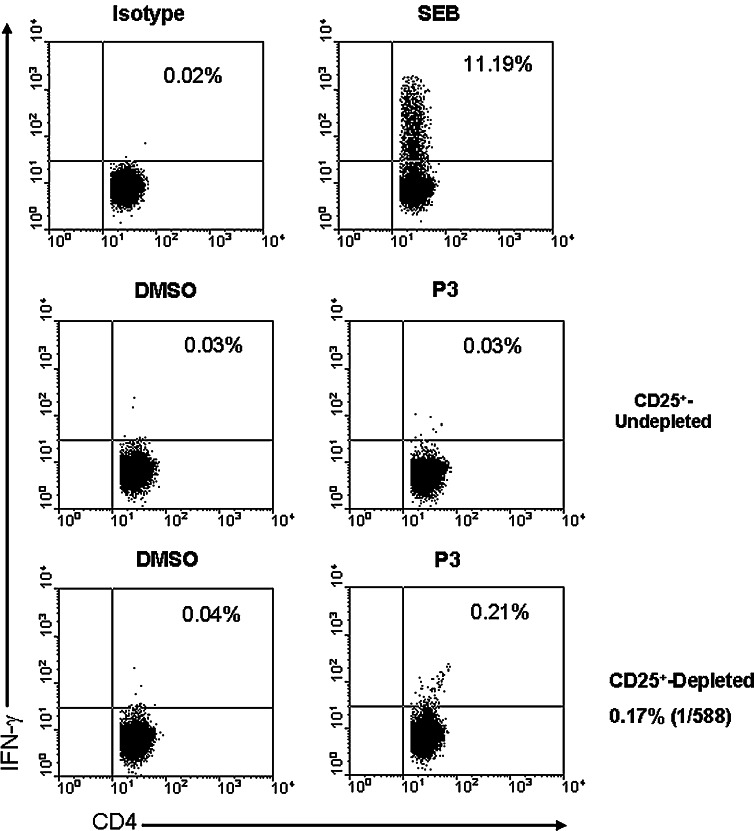
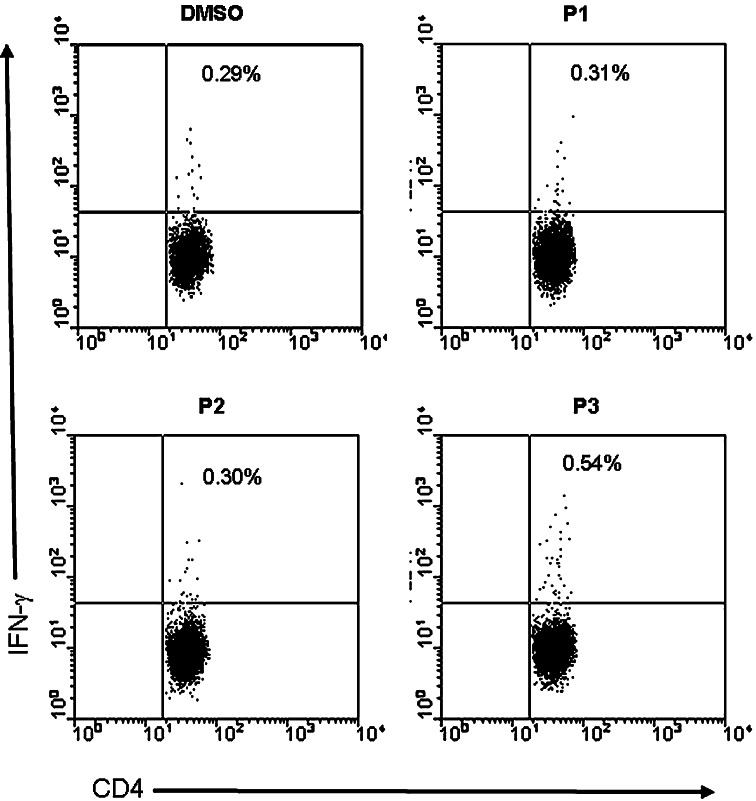
CD4+-enriched PBLs from HLA-DR4 positive HD were stimulated twice with autologous DC expressing h5T4. These effectors were then tested for recognition of candidate 15-mer h5T4 peptides using functional flow cytometry assays detecting peptide-induced intracellular IFN-γ production. As can be seen in Fig. 4, the response against the 15-mer peptides (P3 in this example) was only generated in the CD25+-depleted fraction. Therefore, CD4+-enriched PBLs were depleted of CD25+ cells prior to any polyclonal activation in all subsequent experiments. Figure 5 shows that CD4+ T cells specific for h5T4222–236 15-mer peptide (P3), but not against P1 or P2, were detected in an HLA-DR4 positive donor (one representative plot from two performed is shown) using intracellular IFN-γ flow cytometric assays. The net estimated frequency of P3-specific CD4+ T cells was 0.25% of CD3+CD4+ T cells (Fig. 5).
Fig. 4.
IFN-γ-secreting P3-specific CD4+ T cells are generated in the CD25+-depleted subpopulation following polyclonal stimulation with h5T4-expressing DC. Reactivity of the expanded CD4+ T cells (undepleted or depleted of CD25+ cells) from HLA-DR4 positive healthy individual against P3 15-mer was evaluated in intracellular IFN-γ flow cytometric assays. Cells stimulated with 1 μg/ml staphylococcal enterotoxin B (SEB) are shown as a positive control. Cells were incubated with equivalent amount of DMSO (used to dissolve peptide) to subtract any background. No IFN-γ-secreting CD3+CD4+ T cells specific for P3 were detected in the CD25+-undepleted subpopulation, while 0.17% of CD3+CD4+ T cells secreted IFN-γ in response to P3 in the CD25+-depleted subpopulation
Fig. 5.
Response against P3 is generated in HLA-DR4 positive healthy donors following polyclonal stimulation. CD25+-depleted CD4+-enriched PBLs were expanded twice with h5T4-expressing DC. They were then incubated with DMSO (to calculate background), P1, P2, or P3 15-mer peptides for 6 h. IFN-γ secretion from CD3+CD4+ T cells was assessed by flow cytometric analysis. 0.25% of CD3+CD4+ T cells secreted IFN-γ in response to P3 but not to P1 or P2 peptides. One representative experiment of two performed is shown
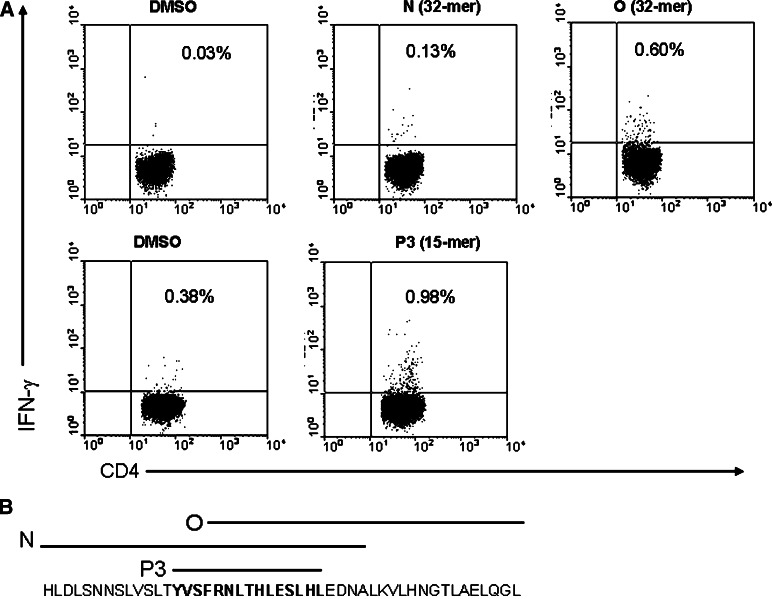
When the individual 32-mer peptides covering the complete sequence of h5T4 were used to restimulate CD25+-depleted CD4+ T cells previously expanded by two stimulations, only peptides N (net 0.1%) and O (net 0.57%) generated specific IFN-γ positive CD4+ cells (Fig. 6a). The other peptides only gave background levels but the same donor showed 0.6% IFN-γ positive CD4 cells when stimulated with peptide P3 (Fig. 6a). Importantly, the whole sequence of P3 peptide is within the N peptide but for the O peptide, 12 amino acids are shared (Fig. 6b). Whereas the 15-mer (P3) or the 32-mer (O) stimulates similar levels of T cells, N peptide does significantly less. This may result from amino acids N-terminal to the tyrosine at position 222 of h5T4, which is the start position of peptide P3, inhibiting presentation. In addition, the O sequence contains 3 further 15-mer sequences which are predicted to bind to DR4, albeit with lower relative strength (26 as predicted by SYFPEITHI), and thus may also contribute to the repertoire of T cells stimulated.
Fig. 6.
Expanded CD4+ T cells from HLA-DR4 positive healthy donor respond to 32-mer peptides (N and O) containing the sequence of P3. CD4+-enriched PBLs were expanded for two stimulations with h5T4-expressing DC. Cells were then incubated for 6 h with DMSO, or any of the 32-mer peptides. IFN-γ secretion was assessed by flow cytometric analysis. Cells responded to N and O 32-mer and P3 15-mer peptides a and the schematic diagram b showing that the sequence of P3 15-mer peptide is located within the sequence of N and O 32-mer peptides
IFN-γ-secreting P3-specific CD4+ T cells are detected in 4 of 9 HLA-DR4 positive HD
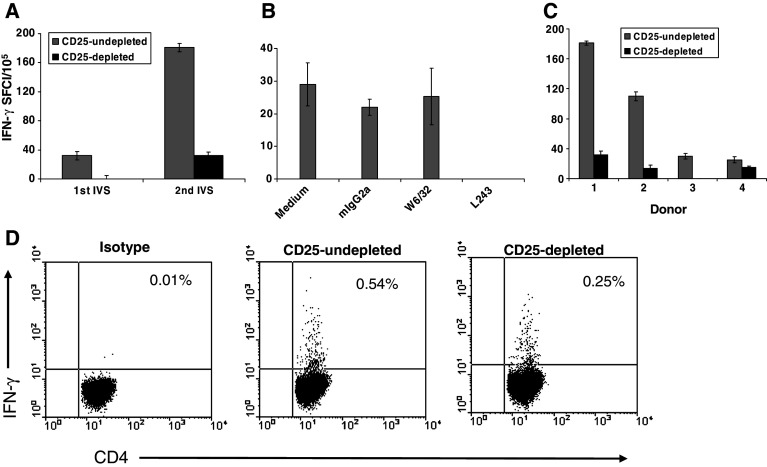
To determine whether the 15-mer P3 can stimulate immune responses, CD4+-enriched PBLs (undepleted or depleted of CD25+ cells) isolated from HLA-DR4 positive HD (or HLA-DR4 negative as controls) were expanded with autologous P3-pulsed DC. ELISPOT assays were used to screen for any IFN-γ secretion in response to P3 or media alone to subtract any background. Following one round of in vitro stimulation, some IFN-γ-secreting CD4+ T cells against P3 were detected in the expanded effectors from CD25+-undepleted population, but their frequency was significantly enhanced after two stimulations (Fig. 7a). The HLA-DR restriction of P3-specific T-cell lines was confirmed using antibody to HLA-DR, but not to HLA-ABC or mIgG2a control (Fig. 7b).
Fig. 7.
Response against P3 15-mer peptide is generated in HLA-DR4 positive healthy donors. CD4+-enriched PBLs (undepleted or depleted of CD25+ cells) from 9 HLA-DR4 positive healthy donors were expanded with mature P3-pulsed DC, and reactivity against P3 15-mer peptide was detected in ELISPOT assays. The data are means of triplicate determinations ±SE. One round of in vitro stimulation generated weak P3-specific CD4+ T-cell responses and two stimulations were required to amplify responses a. Response to P3 peptide was blocked with anti-HLA-DR (L243) but not with anti-HLA-ABC (W6/32) or control mIgG2a b. P3-specific CD4+ T cells were detected in 4 out of 9 healthy donors following two stimulations c. Response against P3 was higher in the CD25+-undepleted subpopulation compared to CD25+-depleted subpopulation c and this was confirmed (0.53 vs 0.24%) by performing intracellular IFN-γ staining for cells expanded from one healthy individual (donor 1, d). SFC is spot forming cell
Unlike polyclonal activation using h5T4-expressing DC, we found that responses to P3 were higher in the CD4+ T cells expanded from CD25+-undepleted population (Fig. 7a). These results were confirmed in four of nine HLA-DR4 positive healthy individuals, as shown in Fig. 7c. Intracellular IFN-γ flow cytometric staining (Fig. 7d) in response to P3 stimulation (6 h) for one of the donors showed the same phenomenon (undepleted = 0.54% versus depleted = 0.25%). The specificity of this peptide was examined in 3 HLA-DR4 negative donors and no IFN-γ secretion was detected in response to P3 in any of them as measured by ELISPOT.
Spontaneous recognition of h5T4 by CD4+ T cells isolated from a regressing renal cell carcinoma lesion
Tumour-infiltrating lymphocytes were isolated from a regressing lung metastasis of a RCC patient. Since most RCC are h5T4 positive [14], we examined the reactivity of TILs against the six different PP derived from h5T4 using ELISPOT assays. There was a prominent (>95%) CD4+ T-cell population with very few (<2%) cells with Treg phenotype (CD4+CD25+FOXP3+) in these TILs. CD4+ T cells secreted IFN-γ in recognition of QRST peptide pool and to a lesser extent to MNOP, as shown in Fig. 8a. Fine epitope mapping against single peptides constituting MNOP and QRST PP confirmed that Q and R single peptides induced significant responses (Fig. 8b). This response was confirmed to be HLA-DR restricted as shown by blocking IFN-γ response to R peptide using antibody to HLA-DR, but not to HLA-ABC or mIgG2a control (Fig. 8c). Interestingly, Q and R peptides overlapped by 16 amino acids (Fig. 8d) and this overlapping sequence (h5T4273–288: MADMVTWLKETEVVQG) may represent another HLA-DR-restricted h5T4-derived epitope, which warrants further investigation. Interestingly, in addition to IFN-γ, these CD4+ T cells secreted IL-10 in response to QRST peptide pool (Fig. 8e) and again such response was against Q and R single peptides, which was blocked with antibody to HLA-DR, but not to HLA-ABC or mIgG2a control (Fig. 8f).
Fig. 8.
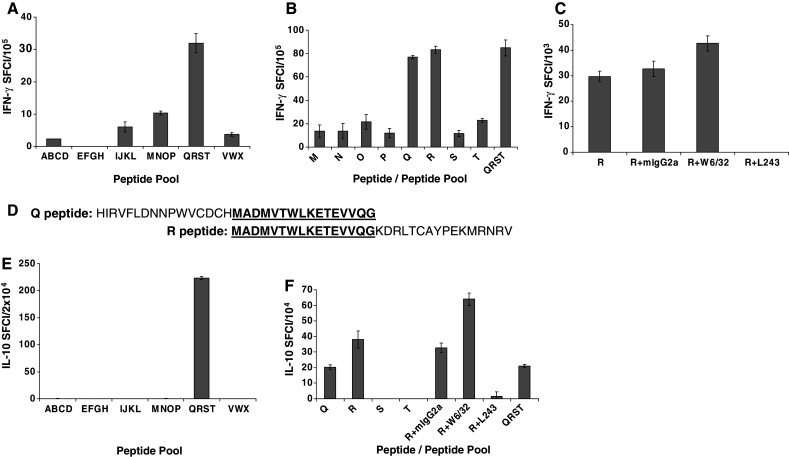
Response to h5T4-derived peptides in TILs isolated from a regressing lung metastasis of a RCC patient. TILs were incubated overnight with the peptide pools of h5T4 and their reactivity was measured using ELISPOT assays. The data are means of triplicate determinations ±SE. TILs secreted IFN-γ in response to peptide pool 5 (QRST), and weaker response to peptide pool 4 (MNOP) a. By performing fine epitope mapping, the IFN-γ secretion was in response to Q or R 32-mer peptides b. IFN-γ response to R peptide was blocked with anti-HLA-DR (L243) but not with anti-HLA-ABC (W6/32) or control mIgG2a c. Q and R peptides overlap by 16 amino acids d. TILs secreted IL-10 e in addition to IFN-γ in response to the same peptide pool (QRST), and this response was blocked with anti-HLA-DR (f)
Discussion
Over the past decades, several TAA have been characterized as targets for cancer immunotherapy, and many efforts have been made to identify MHC class I and II-restricted epitopes. It is now apparent that both CD8+ CTL and CD4+ T helper cells are essential for generating effective anti-tumour immune responses. Indeed, several cancer immunotherapy trials may have failed because of the focus on inducing CD8+ CTL responses without taking into consideration the importance of CD4+ T helper cells in generating and maintaining effective anti-tumour immune responses [35]. In considering cancer vaccine approaches, it is important to investigate the recognition of any TAA by both CD8+ and CD4+ T cells. The h5T4 oncofoetal antigen is the target of different immunotherapeutic approaches for cancer including as a vaccine [15, 16]. We have previously shown that CD4+-depleted CD8+ T cells can recognize HLA class I restricted h5T4 antigens when stimulated by autologous DC infected with Ad-h5T4 [30]. In this study, we show h5T4 recognition by CD4+ T cells from normal donors and that the frequency of response is enhanced with the initial depletion of the CD25+ (putative Treg) cells prior to antigen polyclonal stimulation. These observations might imply that the availability of the h5T4 recognition of both CD4+ and CD8+ T cells are optimally accessed in the absence of T regulatory activity.
The frequency of h5T4-specific CD4+ T cells generated by two stimulations with Ad-h5T4-transfected DC of CD8+-depleted CD4+ T cells was generally low. While the frequency of IFN-γ-secreting h5T4-specific CD4+ T cells is, in general, higher in the CD25+-depleted populations than in the non-depleted populations, there are occasionally some exceptions. For example, in HD1, the response to peptide pool five in the CD25+-depleted population (2/105) is lower than in the non-depleted population (19/105) (Table 2). The higher frequency in the CD25+-depleted populations may reflect the removal of T regulatory cells because the vast majority of Treg cells resides in the CD25 high region and these express FOXP3 but also some CD25 intermediate cells express FOXP3. Therefore, we depleted CD25+ cells (CD25 high and CD25 intermediate cells) prior to T-cell expansion to remove any potential contamination of CD4+ T cells with low levels of CD4+CD25+ Treg cells, which can be expanded during the in vitro stimulation process. Similar results have been obtained by Mesel-Lemoine et al. [36] where CD25+ Treg-cell depletion prior to the activation/expansion of T cells in the presence of IL-2 preserved the functionality of T cells by preventing any possible imbalance between T-effector cells and Treg.
Although, Foxp3 expression can be considered as a definitive marker for Treg in mouse because it is not inducible following T-cell activation [37], in human Treg FOXP3 expression is inducible [38]. Therefore, in stimulated CD25+-depleted or undepleted lymphocyte populations, FOXP3+ cells could be generated by the conversion of CD4+CD25− T cells, and/or the expansion of previously existing natural Treg, respectively. We found that the final frequency of CD4+FOXP3+ cells in stimulated lymphocyte cultures is reduced in CD25+-depleted compared to undepleted populations and this correlated with increased detection of h5T4-specific CD4+ T-cell responses. Studies of the association of FOXP3 induction with suppressive activity have provided conflicting results. For example, induction of FOXP3 expression conferred a regulatory activity in stimulated human CD4+CD25− T cells [39], and TGF-beta induced a regulatory activity in CD4+CD25− T cells through the induction of FOXP3 expression [40], while other reports showed that the induction of FOXP3 did not correlate with Treg characteristics (anergy and suppressive activity) [41–44]. Since we did not perform functional assays for the expanded effectors, the induction of lower FOXP3 expression in the CD25+-depleted populations, by itself, might not explain the higher h5T4-specific CD4+ T-cell responses and there might be other factors contributing towards such stronger response.
Depletion of CD25+ cells prior to initial expansion using DC genetically modified to express prostate-specific antigen leads to generation of T cells reactive to both dominant and subdominant epitopes [45]. In our study, depletion of CD25+ cells prior to polyclonal expansion using h5T4-expressing DC significantly enhanced the h5T4-specific CD4+ T-cell responses, while responses to 15-mer peptide (P3) were higher in the presence of CD25+ cells, following expansion with mature peptide-pulsed DC. One possible explanation for this difference is feeding DC with whole protein requires processing of the protein before class II epitopes are presented, and the peptides generated resemble the natural situation. On the other hand, loading a 15-mer peptide allows for direct binding on the outside of the DC in empty class II molecules which is not likely to be a natural situation. Since it has been suggested that Treg are educated to induce tolerising responses to “self”, it may not be surprising that Treg activity is maximal following more natural processing. A second possibility is the specific DR4 peptide may not generate any Treg activity whereas the CD25 depletion may reduce non-specifically the T-cell precursor frequency. By contrast in the polyclonal stimulation, the potentially entire h5T4 repertoire can be activated as well as viral-related responses and these include populations capable of immune modulation (specific and/or non-specific Treg which limit the detectable h5T4-specific IFN-γ responses). A third possibility is the difference in phenotype and/or functional activity of LPS-matured DC versus transfected DC. There are some published studies supporting the third possibility that different DC types might have different effects on controlling Treg activity and inducing antigen-specific responses. By comparing different types of APC, Fehervari and Sakaguchi showed that LPS-matured DC are the most effective APC at overcoming the suppressive activity of Treg while maintaining stable expression of Foxp3 [46]. We used LPS to mature DC before pulsing with the P3 15-mer and these DC might be able to overcome tolerance to P3 peptide in the presence of Treg activity. In an in vivo mouse model for comparing hemagglutinin-encoding recombinant viruses versus hemagglutinin-pulsed DC to reverse hemagglutinin-specific CD8 tolerance, it has been reported that viral vaccines could break tolerance in the presence of CD4+CD25+ Treg, while DC-based vaccine required Treg depletion to achieve that [47]. In another study, two different types of genetically modified DC were compared for inducing antigen-specific responses; DC transfected with the full length of the human prostate-specific antigen with a signal peptide sequence (named secreted vaccine, sVan) or DC transfected with antigen lacking the signal peptide sequence (antigen retained in the cytosol, named truncated vaccine, tVan) [45]. Differences between these DC in inducing antigen-specific responses were reported. tVan induced response to dominant and subdominant epitopes while sVan induced responses to only dominant epitopes and depletion of CD25+ T cells prior to initial expansion was required to reveal responses to subdominant epitopes [45]. Therefore, it is clear that different types of DC can induce different responses and CD25+ T-cell depletion can be necessary in some cases to reveal responses to certain epitopes. It was postulated that CD4+CD25+ Treg selectively suppress weakly activated T cells, but not T cells strongly activated by stimuli such as pathogenic microbes, to undergo clonal expansion. Supporting this notion, it has been shown in the literature that T cells receiving strong TCR or costimulatory signal are resistant to Treg [48]. Given that optimally activated DC (such as LPS-matured DC) upregulate a panel of costimulators, which can, in turn, render T cells resistant to Treg-mediated suppression, it is possible that depletion of CD25+ cells may not enhance the responses of h5T4-specific T cells that are stimulated by LPS-matured DC. The higher frequency of h5T4-specific CD4+ T cells in undepleted population compared to CD25+-depleted cells following stimulation with peptide-pulsed DC might be explained by the removal of some effector cells as activated T cells are known to express CD25 marker. The in vitro polyclonal stimulation of CD4+ T-cell responses to h5T4 is clearly potentiated by CD25 depletion in vitro and this observation prompts speculation about exploitation in cancer vaccination in vivo.
It is now well established that CD4+ CD25+ T regulatory cells can suppress auto- and anti-tumour immunities [49]. The T cell receptor (TCR) repertoires of Treg and CD4+CD25− are similarly diverse, but Treg seems to have substantially more efficient interactions with MHC class II bound self-peptides from the periphery than CD4+CD25− T cells [50]. Given that many TAA recognized by T cells are self-antigens, Treg cells engaged in the maintenance of self-tolerance are more likely to simultaneously inhibit anti-tumour immune responses, and their removal might improve these responses. Autoreactive CD4+ T cells were detected in healthy individuals, and the removal of Treg enabled a rapid expansion of these autoreactive cells [51]. Similarly, Treg were found to control the induction of NY-ESO-1 specific CD4+ T-cell responses in cancer patients [52]. In case of the h5T4, it is possible that the T regulatory activity is generated to control any potential autoimmunity against this oncofoetal antigen. In a recent study, it has been reported that Treg cells inhibit the h5T4-specific immune responses in colorectal cancer patients [53]. More recently, we have shown potentiation of h5T4-specific proliferative responses following adoptive transfer of Treg-depleted autologous T cells in one of six patients with renal cell carcinoma, and this increase was associated with a nadir of Treg frequency [54].
In summary, the present study shows that CD4+ T-cell precursors with specificity for h5T4 are present and can be expanded in most healthy individuals and the initial depletion of CD25+ cells prior to cell activation and/or expansion is a useful approach to prevent putative Treg activity and generate stronger h5T4-specific T-cell responses in vitro. Using this approach, a new h5T4-derived MHC class II restricted epitope was identified. In addition, h5T4 specific CD4+ T cells were detected in a regressing tumour lesion. Interestingly, these cells produced both IFN-γ and IL-10, which is a characteristic of Treg specific for human papillomavirus identified in cervical carcinoma TILs [55]. Interestingly, disease control in TroVax immunized colorectal cancer patients correlates with induction of high titre anti-h5T4 antibody responses [15] and possibly 5T4-specific CD4+ T-cell responses may be important in this regard. A more systematic clonal analysis of such h5T4-specific CD4+ T cells to assess their biological relevance must now be pursued including in cancer patients unvaccinated or vaccinated with TroVax.
Acknowledgments
This work was funded by Cancer Research UK. We are very grateful to all the volunteers who donated blood to this study. We are also grateful to Dr Alaaeldin Shablak for providing the tumour sample from which TILs were isolated.
Abbreviations
- Ad
Adenovirus
- CTL
Cytotoxic T lymphocyte
- DC
Dendritic cells
- DMSO
Dimethyl sulfoxide
- ELISA
Enzyme linked immunosorbent assay
- ELISPOT
Enzyme linked immunospot
- GFP
Green fluorescent protein
- GM-CSF
Granulocyte-macrophage colony stimulating factor
- h5T4
Human 5T4
- HD
Healthy donor
- IFN-γ
Interferon gamma
- LPS
Lipopolysaccharide
- MHC
Major histocompatibility complex
- MVA
Modified vaccinia Ankara
- PBL
Peripheral blood lymphocytes
- PBMC
Peripheral blood mononuclear cells
- Pfu
Plaque forming unit
- PP
Peptide pool
- rhIL
Recombinant human interleukin
- SEB
Staphylococcal enterotoxin B
- TAA
Tumour-associated antigen
- TCR
T cell receptor
- TILs
Tumour-infiltrating lymphocytes
- Treg
T regulatory cells
Footnotes
This work was supported by Cancer Research UK.
References
- 1.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 2.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Sakaguchi K, Appella E, Yannelli JR, Adema GJ, Miki T, Rosenberg SA. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci USA. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultze JL, Vonderheide RH. From cancer genomics to cancer immunotherapy: toward second-generation tumor antigens. Trends Immunol. 2001;22:516–523. doi: 10.1016/S1471-4906(01)02015-4. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg PD. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol. 1991;49:281–355. doi: 10.1016/S0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- 5.Chikamatsu K, Albers A, Stanson J, Kwok WW, Appella E, Whiteside TL, DeLeo AB. P53(110–124)-specific human CD4+ T-helper cells enhance in vitro generation and antitumor function of tumor-reactive CD8+ T cells. Cancer Res. 2003;63:3675–3681. [PubMed] [Google Scholar]
- 6.Hole N, Stern PL. Isolation and characterization of 5T4, a tumour-associated antigen. Int J Cancer. 1990;45:179–184. doi: 10.1002/ijc.2910450132. [DOI] [PubMed] [Google Scholar]
- 7.Myers KA, Rahi-Saund V, Davison MD, Young JA, Cheater AJ, Stern PL. Isolation of a cDNA encoding 5T4 oncofetal trophoblast glycoprotein. An antigen associated with metastasis contains leucine-rich repeats. J Biol Chem. 1994;269:9319–9324. [PubMed] [Google Scholar]
- 8.Southall PJ, Boxer GM, Bagshawe KD, Hole N, Bromley M, Stern PL. Immunohistological distribution of 5T4 antigen in normal and malignant tissues. Br J Cancer. 1990;61:89–95. doi: 10.1038/bjc.1990.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulder WM, Stern PL, Stukart MJ, de Windt E, Butzelaar RM, Meijer S, Ader HJ, Claessen AM, Vermorken JB, Meijer CJ, Wagstaff J, Scheper RJ, Bloemena E. Low intercellular adhesion molecule 1 and high 5T4 expression on tumor cells correlate with reduced disease-free survival in colorectal carcinoma patients. Clin Cancer Res. 1997;3:1923–1930. [PubMed] [Google Scholar]
- 10.Starzynska T, Marsh PJ, Schofield PF, Roberts SA, Myers KA, Stern PL. Prognostic significance of 5T4 oncofetal antigen expression in colorectal carcinoma. Br J Cancer. 1994;69:899–902. doi: 10.1038/bjc.1994.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starzynska T, Rahi V, Stern PL. The expression of 5T4 antigen in colorectal and gastric carcinoma. Br J Cancer. 1992;66:867–869. doi: 10.1038/bjc.1992.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starzynska T, Wiechowska-Kozlowska A, Marlicz K, Bromley M, Roberts SA, Lawniczak M, Kolodziej B, Zyluk A, Stern PL. 5T4 oncofetal antigen in gastric carcinoma and its clinical significance. Eur J Gastroenterol Hepatol. 1998;10:479–484. doi: 10.1097/00042737-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Wrigley E, McGown AT, Rennison J, Swindell R, Crowther D, Starzynska T, Stern PL. 5T4 oncofetal antigen expression in ovarian carcinoma. Int J Gynecol Cancer. 1995;5:269–274. doi: 10.1046/j.1525-1438.1995.05040269.x. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths RW, Gilham DE, Dangoor A, Ramani V, Clarke NW, Stern PL, Hawkins RE. Expression of the 5T4 oncofoetal antigen in renal cell carcinoma: a potential target for T-cell-based immunotherapy. Br J Cancer. 2005;93:670–677. doi: 10.1038/sj.bjc.6602776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrop R, Connolly N, Redchenko I, Valle J, Saunders M, Ryan MG, Myers KA, Drury N, Kingsman SM, Hawkins RE, Carroll MW. Vaccination of colorectal cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: a phase I/II trial. Clin Cancer Res. 2006;12:3416–3424. doi: 10.1158/1078-0432.CCR-05-2732. [DOI] [PubMed] [Google Scholar]
- 16.Mulryan K, Ryan MG, Myers KA, Shaw D, Wang W, Kingsman SM, Stern PL, Carroll MW. Attenuated recombinant vaccinia virus expressing oncofetal antigen (tumor-associated antigen) 5T4 induces active therapy of established tumors. Mol Cancer Ther. 2002;1:1129–1137. [PubMed] [Google Scholar]
- 17.Forsberg G, Ohlsson L, Brodin T, Bjork P, Lando PA, Shaw D, Stern PL, Dohlsten M. Therapy of human non-small-cell lung carcinoma using antibody targeting of a modified superantigen. Br J Cancer. 2001;85:129–136. doi: 10.1054/bjoc.2001.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw DM, Connolly NB, Patel PM, Kilany S, Hedlund G, Nordle O, Forsberg G, Zweit J, Stern PL, Hawkins RE. A phase II study of a 5T4 oncofoetal antigen tumour-targeted superantigen (ABR-214936) therapy in patients with advanced renal cell carcinoma. Br J Cancer. 2007;96:567–574. doi: 10.1038/sj.bjc.6603567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dermime S, Gilham DE, Shaw DM, Davidson EJ, Meziane el K, Armstrong A, Hawkins RE, Stern PL. Vaccine and antibody-directed T cell tumour immunotherapy. Biochim Biophys Acta. 2004;1704:11–35. doi: 10.1016/j.bbcan.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Guest RD, Hawkins RE, Kirillova N, Cheadle EJ, Arnold J, O’Neill A, Irlam J, Chester KA, Kemshead JT, Shaw DM, Embleton MJ, Stern PL, Gilham DE. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J Immunother. 2005;28:203–211. doi: 10.1097/01.cji.0000161397.96582.59. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065X.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 22.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi T, Sakaguchi S. The role of regulatory T cells in controlling immunologic self-tolerance. Int Rev Cytol. 2003;225:1–32. doi: 10.1016/S0074-7696(05)25001-5. [DOI] [PubMed] [Google Scholar]
- 24.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 25.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- 26.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 29.Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S, Sakaguchi S. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 30.Smyth LJ, Elkord E, Taher TE, Jiang HR, Burt DJ, Clayton A, van Veelen PA, de Ru A, Ossendorp F, Melief CJ, Drijfhout JW, Dermime S, Hawkins RE, Stern PL. CD8 T-cell recognition of human 5T4 oncofetal antigen. Int J Cancer. 2006;119:1638–1647. doi: 10.1002/ijc.22018. [DOI] [PubMed] [Google Scholar]
- 31.Hole N, Stern PL. A 72 kD trophoblast glycoprotein defined by a monoclonal antibody. Br J Cancer. 1988;57:239–246. doi: 10.1038/bjc.1988.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antony PA, Restifo NP. CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin-2. J Immunother. 2005;28:120–128. doi: 10.1097/01.cji.0000155049.26787.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 34.D’Amaro J, Houbiers JG, Drijfhout JW, Brandt RM, Schipper R, Bavinck JN, Melief CJ, Kast WM. A computer program for predicting possible cytotoxic T lymphocyte epitopes based on HLA class I peptide-binding motifs. Hum Immunol. 1995;43:13–18. doi: 10.1016/0198-8859(94)00153-H. [DOI] [PubMed] [Google Scholar]
- 35.Ito D, Albers A, Zhao YX, Visus C, Appella E, Whiteside TL, DeLeo AB. The wild-type sequence (wt) p53(25–35) peptide induces HLA-DR7 and HLA-DR11-restricted CD4+ Th cells capable of enhancing the ex vivo expansion and function of anti-wt p53(264–272) peptide CD8+ T cells. J Immunol. 2006;177:6795–6803. doi: 10.4049/jimmunol.177.10.6795. [DOI] [PubMed] [Google Scholar]
- 36.Mesel-Lemoine M, Cherai M, Le Gouvello S, Guillot M, Leclercq V, Klatzmann D, Thomas-Vaslin V, Lemoine FM. Initial depletion of regulatory T cells: the missing solution to preserve the immune functions of T lymphocytes designed for cell therapy. Blood. 2006;107:381–388. doi: 10.1182/blood-2005-07-2658. [DOI] [PubMed] [Google Scholar]
- 37.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 38.Knutson KL, Disis ML, Salazar LG. CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunol Immunother. 2007;56:271–285. doi: 10.1007/s00262-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 41.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 42.Morgan ME, van Bilsen JH, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC, Elferink BG, van der Zanden L, de Vries RR, Huizinga TW, Ottenhoff TH, Toes RE. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 43.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3- T cells by T cell receptor stimulation is TGF{beta}-dependent but does not confer a regulatory phenotype. Blood. 2007;110(8):2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 45.Mincheff M, Zoubak S, Altankova I, Tchakarov S, Pogribnyy P, Makogonenko Y, Botev C, Meryman HT. Depletion of CD25+ cells from human T-cell enriched fraction eliminates immunodominance during priming with dendritic cells genetically modified to express a secreted protein. Cancer Gene Ther. 2005;12:185–197. doi: 10.1038/sj.cgt.7700778. [DOI] [PubMed] [Google Scholar]
- 46.Fehervari Z, Sakaguchi S. Control of Foxp3+ CD25+CD4+ regulatory cell activation and function by dendritic cells. Int Immunol. 2004;16:1769–1780. doi: 10.1093/intimm/dxh178. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508–515. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 48.Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin Immunol. 2004;16:89–98. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Chattopadhyay S, Mehrotra S, Chhabra A, Hegde U, Mukherji B, Chakraborty NG. Effect of CD4+CD25+ and CD4+CD25- T regulatory cells on the generation of cytolytic T cell response to a self but human tumor-associated epitope in vitro. J Immunol. 2006;176:984–990. doi: 10.4049/jimmunol.176.2.984. [DOI] [PubMed] [Google Scholar]
- 50.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 51.Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. J Immunol. 2004;172:5967–5972. doi: 10.4049/jimmunol.172.10.5967. [DOI] [PubMed] [Google Scholar]
- 52.Nishikawa H, Jager E, Ritter G, Old LJ, Gnjatic S. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood. 2005;106:1008–1011. doi: 10.1182/blood-2005-02-0607. [DOI] [PubMed] [Google Scholar]
- 53.Clarke SL, Betts GJ, Plant A, Wright KL, El-Shanawany TM, Harrop R, Torkington J, Rees BI, Williams GT, Gallimore AM, Godkin AJ. CD4CD25FOXP3 regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS ONE. 2006;1:e129. doi: 10.1371/journal.pone.0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thistlethwaite FC, Elkord E, Griffiths RW, Burt DJ, Shablak AM, Campbell JD, Gilham DE, Austin EB, Stern PL, Hawkins RE (2007) Adoptive transfer of T(reg) depleted autologous T cells in advanced renal cell carcinoma. Cancer Immunol Immunother. doi:10.1007/s00262-007-0400-6 [DOI] [PMC free article] [PubMed]
- 55.van der Burg SH, Piersma SJ, de Jong A, van der Hulst JM, Kwappenberg KM, van den Hende M, Welters MJ, Van Rood JJ, Fleuren GJ, Melief CJ, Kenter GG, Offringa R. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc Natl Acad Sci USA. 2007;104:12087–12092. doi: 10.1073/pnas.0704672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh H, Raghava GP. ProPred: prediction of HLA-DR binding sites. Bioinformatics. 2001;17:1236–1237. doi: 10.1093/bioinformatics/17.12.1236. [DOI] [PubMed] [Google Scholar]
- 57.Reche PA, Glutting JP, Zhang H, Reinherz EL. Enhancement to the RANKPEP resource for the prediction of peptide binding to MHC molecules using profiles. Immunogenetics. 2004;56:405–419. doi: 10.1007/s00251-004-0709-7. [DOI] [PubMed] [Google Scholar]