Abstract
Purpose
Inflammatory cells can both suppress and stimulate tumor growth, and the influence of inflammatory cells on clinical outcome has been the focus of many studies. The purpose of this study was to evaluate the effectiveness of the neutrophil to lymphocyte ratio (NLR), a measure of the systemic inflammatory response, as an additional discriminative biomarker in epithelial ovarian cancer and to determine whether it predicts survival and recurrence.
Methods
We studied 192 patients with epithelial ovarian cancer, 173 with benign ovarian tumors, 229 with benign gynecologic disease, and 405 healthy controls. Serum CA125 levels and leukocyte counts according to subtypes were recorded prior to treatment in all study subjects. In epithelial ovarian cancer, the diagnostic usefulness of NLR, in combination with CA125, was evaluated. The correlation between NLR and overall and disease-free survival was analyzed using both univariate and multivariate analyses adjusting for the known prognostic factors (age, stage, cell type, and grade).
Results
Preoperative NLR in ovarian cancer subjects (mean 6.02) was significantly higher than that in benign ovarian tumor subjects (mean 2.57), benign gynecologic disease subjects (mean 2.55), and healthy controls (mean 1.98) (P < 0.001). The sensitivity and specificity of NLR in detecting ovarian cancer was 66.1% (95% CI, 59.52–72.68%) and 82.7% (95% CI, 79.02–86.38%), respectively (cutoff value: 2.60). In early stage ovarian cancer, CA125 was not elevated in 19 out of 49 patients. Seven (36.8%) of these 19 patients were NLR positive. On Cox multivariate analysis, NLR positive, stage III/IV, and older age were independent poor prognostic factors, and being NLR positive was the most powerful predictive variable (Hazard Ratio = 8.42 [95% CI: 1.09–64.84], P = 0.041).
Conclusions
Our findings provide evidence for the association between NLR and epithelial ovarian cancer. Preoperative NLR, in combination with CA125, may represent a simple and cost-effective method of identifying ovarian cancers, and an elevated NLR may predict an adverse outcome in ovarian cancer.
Keywords: Neutrophil to lymphocyte ratio (NLR), Leukocyte differential count, Ovarian cancer, Diagnostic marker, Prognostic marker
Introduction
Ovarian cancer is the most lethal gynecological cancer worldwide. Because early stage disease is often asymptomatic, the majority of cases present late [1] when little can be done to cure the disease. However, when discovered in early stage, either due to fortuity or presence of symptoms, prognosis is usually excellent, with 5-year survival exceeding 90% [2]. If screening programs using effective tests performed at regular intervals could identify ovarian cancer in its early stages, a relatively simple operation would be sufficient to treat most ovarian cancer patients.
Currently, clinicians rely on a combination of serum CA125 levels and imaging to diagnose ovarian cancer in patients. However, due to the low sensitivity of CA125 testing and the cost ineffectiveness of imaging studies, the currently used tests are not adequate for early detection [3]. Thus, there is an urgent need for new ovarian cancer biomarkers that could improve sensitivity for the early detection of ovarian cancer. The ideal test would detect ovarian cancer when it is at an early stage, at low volume, and prior to occurrence of any symptoms.
Numerous ovarian cancer serum biomarkers have been identified by both genomic and proteomic approaches and evaluated alone or in combination with CA125 [4–6]. However, any biomarker or combination of biomarkers has not yet been determined to be ideal. Given that no biomarker has been found to surpass CA125 as an diagnostic marker of ovarian cancer in the last 40 years, together with the new technologies available for biomarker discovery, finding new markers that can increase the sensitivity of CA125 by a combination of hematologic, inflammatory, or immunologic markers is warranted [7].
Assessment of the inflammatory response to the tumor may be easier and more cost-effective in clinical practice. Recently the role that the immune system plays in disease cessation or progression has been examined [8], and hematological markers including leukocytes have been proposed as both a diagnostic and prognostic factor in a variety of cancers. The systemic inflammatory response features changes in the relative levels of circulating leukocytes; the well-recognized neutrophilia is accompanied by a relative lymphocytopenia [9]. The neutophil to lymphocyte ratio (NLR) has been suggested as a simple index of systemic inflammatory response in critically ill patients [10].
In this study, we investigated the correlation between preoperative differential leukocyte counts (neutrophils, lymphocytes, monocytes, eosinophils, and basophils) and clinical characteristics in epithelial ovarian cancer and were able to determine the effectiveness of the neutrophil to lymphocyte ratio (NLR) as an additional discriminative marker and prognostic predictor for epithelial ovarian cancer.
Materials and methods
Study subjects
This study was carried out in accordance with the ethical standards of the Helsinki Declaration and was approved by the Institute of Review Boards (IRBs) of Yongdong Severance and Severance Hospital (IRB project # 3-2008-0001). Five hundred ninety-four patients who underwent elective surgery at the Department of Obstetrics and Gynecology, Yongdong Severance Hospital and Severance Hospital between January 2003 and June 2006 were retrospectively included in this study. One hundred ninety-two women with primary epithelial ovarian cancer and complete clinical and pathological information documented at the time of surgery were selected. One hundred seventy-three patients had benign ovarian tumors, and 229 patients had benign gynecologic disease. Of the individuals with ovarian tumors, 45 (19.7%) had serous cystadenoma, 40 (7.5%) had mucinous cystadenoma, 68 (29.7%) had mature teratoma, and 20 (9.7%) had hemorrhagic corpus luteal cysts. Of those with gynecological disease, 69 had endometriosis, 39 had adenomyosis, and 121 had leiomyoma. All ovarian cancer patients were surgically staged according to the International Federation of Gynecology and Obstetrics (FIGO) staging system. All stage I/II ovarian cancer patients had pelvic and para-aortic lymph node dissection according to the National Comprehensive Cancer Network (NCCN) clinical practice guidelines. Control groups (n = 405) consisted of health check examinees at Yongdong Severance Hospital and showed no history of cancer or gynecologic disease and no abnormalities in laboratory examinations or gynecologic sonography.
Clinical and laboratory data collection
For all study subjects, leukocyte differential counts and CA125 levels were recorded at primary diagnosis up to 1 week prior to operation. Leukocyte differential counts were analyzed by ADVIA 120/2120 Hematology system (Bayer HealthCare, Diagnostics Division, Tarrytown, NY, USA) and CA125 levels were measured with CA125 II ECLIA (electrochemiluminescence immunoassay) on the Roche/Hitachi Modular Analytics E170 (Roche Diagnostics, Tokyo, Japan). Age, stage, grade, and cell types were recorded for all ovarian cancer patients. The NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count. For the healthy controls, the NLR was calculated from the full blood count performed as part of a health-check examination. For patients who underwent an operation, the NLR was calculated from the full blood count routinely performed the day before operation. Their overall survival and disease-free survival times were recorded. Overall survival was defined as the time from diagnosis to death; disease-free survival was calculated as the time interval between diagnosis and recurrence. Recurrence status was determined using computerized tomography (CT), positron emission tomography (PET), and serum CA125. Non-recurrence was classified as cure with no detectable disease or stable disease with no evidence of progression.
Statistical analysis
Data were summarized with the number of observations, the mean ± SD, and the 95% confidence intervals (CIs) for the mean. Correlation between the tumor marker levels and clinicopathologic variables was analyzed using χ2 test. Comparisons of NLR and serum CA125 levels among the four groups of subjects were analyzed using ANOVA post hoc tests. Receiver operating characteristics (ROC) analysis was used for specificity and sensitivity estimates. The resulting area under the curve (AUC) indicates the average sensitivity of a marker over the entire ROC curve. The diagnostic values of NLR and CA125 were evaluated and the optimal cutoff values of each were determined. A ROC analysis was plotted to investigate the optimal cutoff values that maximized the sum of sensitivity and specificity and the sensitivity and specificity of NLR were compared with those of CA125.
Kaplan–Meier survival analysis was used to determine the univariate relationship of NLR and CA125 with overall and disease-free survival times. The log rank test was utilized to examine the significance of the differences of survival distributions between groups. Subsequently, multivariate analysis with Cox proportional hazards regression was performed to determine which biomarkers predict disease-free and overall survival after having adjusted for the effects of known prognostic factors (age, FIGO-stage, grade, cell type). Generally, for all analyses a P < 0.05 was considered significant. All analyses were performed using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA).
Results
Leukocyte differential counts in study groups
Leukocyte differential counts were analyzed in 192 patients with ovarian cancer, 173 patients with benign ovarian tumor, 229 patients with benign gynecologic disease, and 405 healthy controls according to subtypes (Table 1). The mean ages were 51.8 ± 12.9 years for ovarian cancer patients, 41.1 ± 14.6 years for benign ovarian tumor patients, 41.2 ± 9.9 years for benign gynecologic disease patients, and 44.6 ± 12.5 years for healthy controls. Except for the lymphocytes (Spearman r = −0.10, P = 0.001), the leukocyte differential counts did not correlate with the age of patients but was similar in all age groups. The mean neutrophil and monocyte differential counts (P < 0.001 and 0.001, respectively), in ovarian cancer patients were significantly higher than those of other groups. The mean lymphocyte (P < 0.001), eosinophil (P = 0.003), and basophil (P < 0.001) differential counts in ovarian cancer were significantly lower than those of other groups. Within ovarian cancer patients, neutrophil counts positively correlated with increasing tumor stage (P = 0.029) and tumor grade (P = 0.026), and lymphocyte counts inversely correlated with tumor stage (P = 0.023). Additionally, the difference in monocyte counts between subgroups of benign gynecologic disease were statistically significant (P = 0.042) (Table 2).
Table 1.
Mean counts of leukocyte subsets in study subjects
| Meana ± SD | |||||
|---|---|---|---|---|---|
| Neutrophil | Lymphocyte | Monocyte | Eosinophil | Basophil | |
| Ovarian cancer (n = 192) | 5,776.7 ± 3,328.9 | 1,449.0 ± 669.1 | 343.3 ± 158.3 | 117.2 ± 124.9c | 34.8 ± 31.2b |
| Benign ovarian tumor (n = 173) | 4,430.5 ± 2,508.2b | 1,928.2 ± 579.6b | 283.4 ± 110.9b | 138.1 ± 111.6b,c | 39.3 ± 28.0b,c |
| Benign gynecologic disease (n = 229) | 4,232.5 ± 2,182.2b | 1,864.9 ± 576.4b | 290.4 ± 97.4b | 154.1 ± 133.4b | 36.1 ± 21.2b |
| Healthy (n = 405) | 3,595.7 ± 1,263.9 | 1951.1 ± 588.1b | 271.8 ± 89.5b | 160.4 ± 159.4b | 45.0 ± 22.2c |
| P valued | < 0.001 | < 0.001 | < 0.001 | 0.003 | < 0.001 |
aCells/µl
b, cThere is no statistically significant difference in leukocyte differential counts between the groups with the same symbol
dProbability of a difference between the mean leukocytes differential counts of ovarian cancer and benign ovarian tumor, benign gynecologic disease, or healthy controls, via ANOVA test
Table 2.
Clinicopathologic characteristics associated with leukocyte subsets
| Meana ± SD | |||||
|---|---|---|---|---|---|
| Neutrophil | Lymphocyte | Monocyte | Eosinophil | Basophil | |
| Ovarian cancer (n = 192) | |||||
| Cell type | Pb = 0.616 | P = 0.993 | P = 0.833 | P = 0.889 | P = 0.319 |
| Serous (n = 131) | 5,628.8 ± 3,323.6 | 1,441.6 ± 676.9 | 343.2 ± 164.0 | 114.2 ± 130.1 | 34.6 ± 28.3 |
| Mucinous (n = 19) | 6,897.0 ± 3,796.5 | 1,506.3 ± 714.7 | 366.2 ± 107.4 | 125.7 ± 121.1 | 46.6 ± 57.5 |
| Endometrioid (n = 12) | 5,338.8 ± 1,902.1 | 1,427.5 ± 497.9 | 348.8 ± 105.4 | 151.6 ± 130.9 | 36.4 ± 26.9 |
| Clear cell (n = 13) | 6,015.2 ± 2,339.8 | 1,416.1 ± 626.8 | 355.2 ± 132.8 | 109.9 ± 108.0 | 32.2 ± 20.5 |
| Others (n = 17) | 5,791.2 ± 4,227.0 | 1,482.3 ± 757.6 | 305.0 ± 211.0 | 111.8 ± 103.0 | 24.4 ± 16.4 |
| Stage | P = 0.029 | P = 0.023 | P = 0.057 | P = 0.868 | P = 0.739 |
| I/II (n = 59) | 5,676.5 ± 3,314.9 | 1,646.6 ± 677.8 | 337.3 ± 126.3 | 114.3 ± 98.7 | 33.3 ± 30.2 |
| III/IV (n = 125) | 6,013.5 ± 3,344.1 | 1,364.4 ± 657.7 | 354.2 ± 169.8 | 119.9 ± 138.7 | 36.0 ± 32.2 |
| Recurrence (n = 8) | 2,817.1 ± 1,463.6 | 1,315.0 ± 519.6 | 217.7 ± 143.4 | 97.6 ± 63.4 | 29.0 ± 23.5 |
| Grade | P = 0.026 | P = 0.675 | P = 0.415 | P = 0.568 | P = 0.530 |
| 1 (n = 31) | 7,269.3 ± 3,941 | 1,373.0 ± 566.3 | 367.45 ± 111.99 | 106.04 ± 83.13 | 29.5 ± 26.5 |
| 2/3 (n = 109) | 5,477.5 ± 2,845.1 | 1,427.3 ± 641.01 | 346.09 ± 165.17 | 117.6 ± 136.01 | 33.4 ± 30.3 |
| Benign ovarian tumor (n = 173) | |||||
| Histologic type | P = 0.384 | P = 0.411 | P = 0.902 | P = 0.894 | P = 0.479 |
| Serous (n = 45) | 4,299.9 ± 3,206.4 | 1,827.6 ± 619.2 | 285.3 ± 150.1 | 141.9 ± 109.7 | 35.3 ± 17.9 |
| Mucinous (n = 40) | 4,484.3 ± 2,226.8 | 1,892.5 ± 529.5 | 295.1 ± 116.0 | 145.6 ± 145.5 | 38.8 ± 22.0 |
| Dermoid (n = 68) | 4,416.5 ± 1,694.4 | 2,040.6 ± 609.1 | 276.2 ± 79.6 | 126.0 ± 91.5 | 44.6 ± 39.9 |
| Corpus luteal cyst (n = 20) | 4,122.7 ± 1,883.4 | 1,931.3 ± 506.6 | 273.3 ± 66.6 | 139.9 ± 76.9 | 36.5 ± 21.7 |
| Benign gynecologic disease (n = 229) | |||||
| Histologic type | P = 0.140 | P = 0.090 | P = 0.042 | P = 0.520 | P = 0.100 |
| Endometriosis (n = 69) | 4,632.8 ± 2,560.1 | 1,957.2 ± 616.9 | 291.2 ± 95.4 | 166.0 ± 135.7 | 39.4 ± 22.0 |
| Adenomyosis (n = 39) | 3,823.9 ± 1,884.8 | 1,704.1 ± 601.0 | 256.0 ± 75.2 | 135.5 ± 128.0 | 30.3 ± 18.1 |
| Myoma (n = 121) | 4,135.8 ± 2,013.2 | 1,864.1 ± 536.5 | 301.0 ± 102.8 | 153.4 ± 134.2 | 36.0 ± 21.4 |
aCells/µl
bProbability of a difference between the mean leukocytes differential counts, via ANOVA test
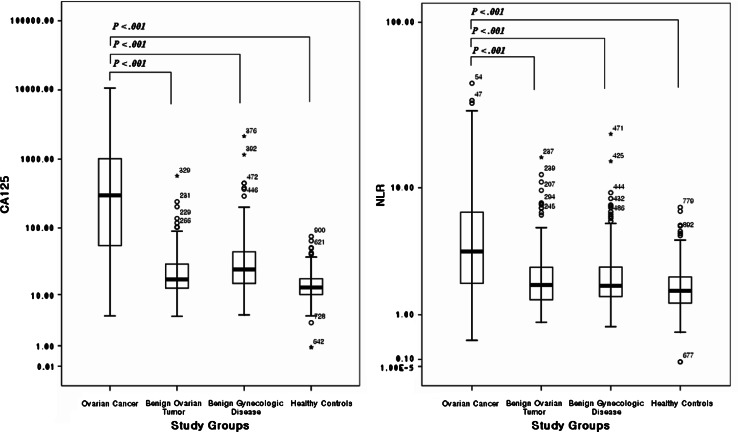
Because the neutrophil and lymphocyte differential counts in ovarian cancer patients were significantly different from those of benign ovarian tumor and benign gynecologic disease patients, we calculated NLR and evaluated the diagnostic and prognostic significance of NLR and correlate it with the tumor stage and grade. We compared the preoperative CA125 level and NLR in all study groups (Fig. 1). The mean values of CA125 and NLR in ovarian cancer patients were significantly higher than those of the other study groups (P < 0.001). NLR did not correlate with the age of study subjects (Spearman r = 0.04, P = 0.21).
Fig. 1.
CA125 levels and NLR in ovarian cancer, benign ovarian tumor, benign gynecologic disease, and healthy control patients. CA125 levels and NLR in ovarian cancer were significantly higher than those in benign ovarian tumor, benign gynecologic disease, and healthy controls
Diagnostic significance of NLR along with CA125 in ovarian cancer
Statistical tests of NLR and leukocyte differential counts between healthy controls and ovarian cancer patients of different subgroups according to the tumor stage were performed (Table 3). To further compare the utility of NLR and leukocyte differential counts in predicting ovarian cancer, we also analyzed the ROC curve. The areas under curve (AUC) for NLR in all ovarian cancer and early stage ovarian cancer were 0.776 and 0.731, respectively. To categorize patients as NLR positive or negative, an optimal cutoff value that maximized the sum of sensitivity and specificity in the ROC curve was used. In the case of CA125, a fixed cutoff value of 35 U/ml was used for analysis of diagnostic power. In all ovarian cancers, NLR yielded a sensitivity of 66.1% (95% CI, 59.52–72.68%) and a specificity of 82.7% (95% CI, 79.02–86.38%) at a cutoff value of 2.60. At cutoff value of 2.61, NLR yielded a sensitivity of 56.2% (95% CI, 42.31–70.09%) and a corresponding specificity of 84.2% (95% CI, 80.65–87.75%) in early stage ovarian cancer.
Table 3.
Diagnostic sensitivity and specificity of CA125, NLR, and leukocyte subsets in ovarian cancer
| CA125 | NLR | Neutrophil | Lymphocyte | Monocyte | Eosinophil | Basophil | |
|---|---|---|---|---|---|---|---|
| All ovarian cancer (n = 192) | |||||||
| AUC | 0.919 | 0.776 | 0.742 | 0.716 | 0.658 | 0.628 | 0.679 |
| Sensitivity | 0.808 | 0.661 | 0.614 | 0.532 | 0.598 | 0.474 | 0.396 |
| Specificity | 0.965 | 0.827 | 0.819 | 0.823 | 0.671 | 0.778 | 0.909 |
| Cutoffa | 35b | 2.60 | 4,684 | 1,440 | 299 | 65 | 20 |
| Early stage ovarian cancer (n = 49) | |||||||
| AUC | 0.835 | 0.731 | 0.730 | 0.616 | 0.685 | 0.631 | 0.705 |
| Sensitivity | 0.581 | 0.562 | 0.571 | 0.449 | 0.653 | 0.449 | 0.509 |
| Specificity | 0.964 | 0.842 | 0.832 | 0.761 | 0.676 | 0.869 | 0.901 |
| Cutoffa | 35b | 2.61 | 4,748 | 1,535 | 300 | 50 | 20 |
aCutoff value that maximized the sum of sensitivity and specificity in the ROC curve
bFixed cutoff value of 35 U/ml was used for CA125
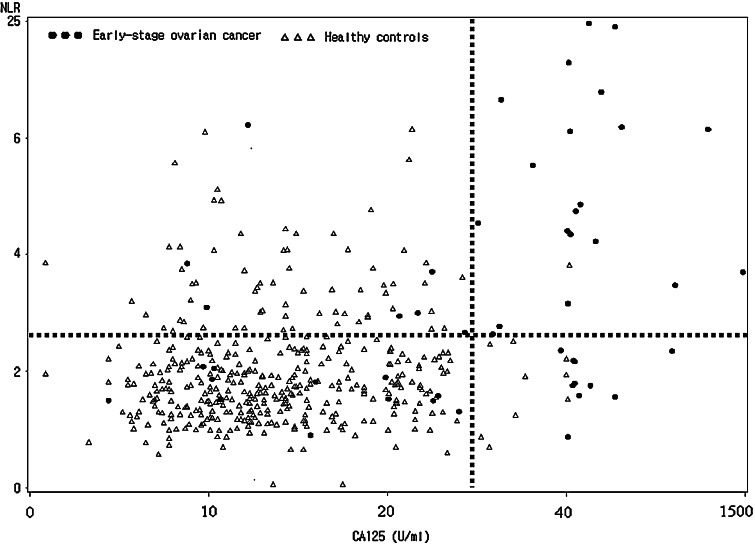
We next assessed the diagnostic significance of NLR along with CA125 for predicting early stage ovarian cancer. In early stage ovarian cancer (n = 49), CA125 was elevated in 30 (61.2%) out of 49 patients, while NLR was elevated in 27 (55.1%) patients. Of the 19 patients who showed a false negative for CA125, 7 patients (36.8%) were NLR positive (Fig. 2).
Fig. 2.
Composite analysis of NLR (y-axis) and CA125 (x-axis) levels in patients with early stage ovarian cancer (filled circle) and healthy controls (open diamond). The dotted lines indicate the optimal cutoff value (2.61) of NLR that maximize the sum of sensitivity and specificity and cutoff value of CA125 (35 U/ml)
Prognostic significance of NLR in ovarian cancer
Clinicopathologic and outcome information and marker values for CA125 and NLR were available for 192 ovarian cancer patients who were monitored for survival and recurrence. The mean follow up time was 20.9 months. Twenty patients (10.4%) died within this period, 48 (25.0%) survived but suffered recurrence, and 124 (64.6%) showed no evidence of disease after treatment. In the recurrent patients group, the mean time to recurrence after initial treatment was 9.7 months.
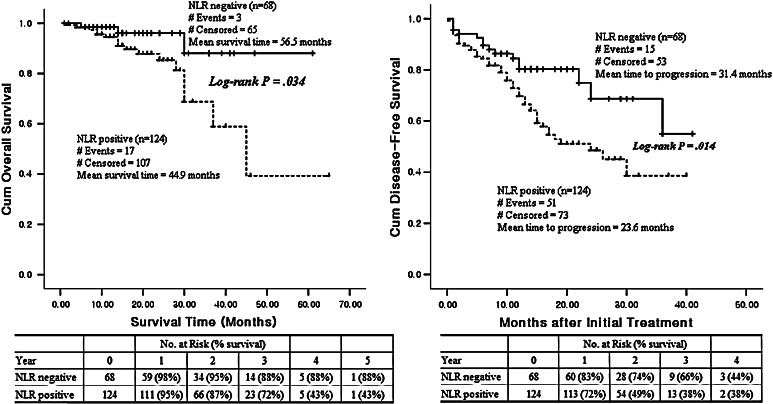
Kaplan–Meier estimates of survival for patients with different NLR levels are shown in Fig. 3. The overall survival and disease-free survival rates for NLR positive patients (>2.60) were significantly lower than the survival rates of NLR negative patients (P = 0.034, 0.014, respectively). A Cox univariate proportional hazards analysis showed that patients with a positive NLR (P = 0.004), stage III/IV (P = 0.041), older age (P = 0.001), and low lymphocyte (P = 0.008) and eosinophil counts (P = 0.040) were of prognostic significance for poor overall survival (Table 4). On multivariate analysis, a positive NLR, stage III/IV, and older age were independent poor prognostic factors, and a positive NLR was the most powerful predictive variable in both the univariate (Hazard Ratio = 6.05 [95% CI: 1.77–20.71], P = 0.004) and multivariate analyses (Hazard Ratio = 8.42 [95% CI: 1.09–64.84], P = 0.041).
Fig. 3.
Kaplan–Meier overall survival (P = 0.034) and disease-free survival curves (P = 0.014) for patients with ovarian cancer. NLR positive (>2.60), n = 124, dotted line; NLR negative (≤2.60), n = 68, broken line
Table 4.
Univariate and multivariate analyses of the associations between prognostic variables and overall survival in 192 cases of ovarian cancer
| Overall survival hazard ratio (95% CIa), P value | ||
|---|---|---|
| Univariate analysis | Multivariate analysis | |
| CA125 positive | NSb | NS |
| NLR positive | 6.05 (1.77–20.71), 0.004 | 8.42 (1.09–64.84), 0.041 |
| Neutrophil | NS | NS |
| Lymphocyte | 0.99 (0.99–1.00), 0.008 | NS |
| Monocyte | NS | NS |
| Eosinophil | 0.99 (0.98–1.00), 0.040 | NS |
| Basophil | NS | NS |
| Age | 1.06 (1.02–1.10), 0.001 | 1.08 (1.03–1.13), 0.001 |
| Stage | ||
| Stage I/II | Reference | Reference |
| Stage III/IV | 4.60 (1.06–19.87), 0.041 | 6.09 (1.03–36.00), 0.046 |
| Grade | ||
| Grade 1 | Reference | Reference |
| Grade 2/3 | NS | NS |
| Cell type | ||
| Serous | Reference | Reference |
| Others | NS | NS |
aConfidence interval
bNot significant
Discussion
Ovarian cancer is often fatal simply because it is not discovered until it is in the late stages. Therefore, the incentive for early detection is enormous. Microarrays are ideally positioned to provide data on potential biomarkers for early detection, and indeed, a number of such genes have been identified. Since the ultimate aim is a non-invasive test, focusing on upregulated genes encoding transmembrane receptors and secreted proteins is crucial. Unfortunately, none of these potential biomarkers is observed in 100% of ovarian cancer patient samples tested, and all are frequently expressed to some degree in non-malignant conditions, compromising the prospect of any potential assay’s specificity and sensitivity. This is a major problem, given the need for an inexpensive assay as a routine screening procedure.
Chronic inflammation has become a recognized risk factor for epithelial-derived malignancies [11, 12]. Chronic inflammation can be caused by infection, autoimmune disease, malignant and benign tumors, or other pathologies and results in the infiltration of inflammatory cells at specific sites in the body. Inflammation is thought to contribute to the development and progression of various cancers, including lung [13], breast [14], gastrointestinal [11, 12, 15], ovarian [16], prostate [17], skin [18], and liver cancers [19].
It is only been in the past decade, however, that the complexities of the tumor inflammatory microenvironment and the host’s response to tumor induced inflammatory pathways have begun to be understood, resulting in an improved ability to prevent and treat malignancy. Elevated markers of inflammation, especially elevated C-reactive protein (CRP), have been used as prognostic tools in patients undergoing curative resection for primary colorectal tumors [20, 21]. In our study, analyses of leukocyte subtypes in ovarian cancer revealed significantly higher neutrophil and monocyte counts and significantly lower lymphocyte, eosinophil, and basophil counts as compared to the benign and control groups.
The explanation for the association between elevated neutrophil or monocyte counts and progression of tumors is not fully understood. The new paradigm in tumor immunology states that the tumor microenvironment can educate and control invading leukocytes to promote angiogenesis, viability, motility, and invasion [22–25]. Tumor-associated macrophages in particular, which arise from blood monocytes, seem to play a crucial role in this interaction [26]. Neutrophils represent 50–60% of total leukocytes and their cytoplasm is rich in granules with high toxic potential against various types of tumor cells. Additionally, neutophils express membrane receptors necessary for the recognition and elimination of microorganisms and tumor cells [27].
The low lymphocyte count observed here may possibly be related to the existence of a primary immunodeficiency at the start of ovarian cancer. Reduction of total lymphocyte counts and lymphocyte function within the periphery seems to be associated with a poorer outcome in a variety of tumors including breast [28], renal [29], pancreatic [30], and ovarian cancers [31]. Both eosinophil and basophil counts were significantly lower in the ovarian cancer patient group as compared to the benign disease and control patient groups. It is not clear whether eosinophils are indicative of a better immunologic response against tumors. Several studies have suggested that eosinophils may play a major role in the complex anticancer activity of the immune system activated by interleukin-2 administration [32]. Our findings are in contrast to the increased eosinophil counts in patients with other malignancies [33], and might be a phenomenon associated with ovarian cancer, specifically. Several in vitro studies have demonstrated that the incidence of tumors and metastases was inversely correlated with the number of blood basophils. The same was found for histamine produced by the basophils [34]. A low basophil count therefore would have a detrimental effect in patients with cancer.
A further preoperative marker, an elevated NLR, has also been linked with clinical outcome in patients with primary colorectal carcinoma [35]. Our study demonstrates that preoperative NLR in combination with CA125 is a useful discriminative marker for epithelial ovarian cancer. The AUC for NLR (0.77 ± 0.04) was larger than those of other leukocyte subsets, and the mean NLR in ovarian cancer was significantly higher than those of the other study groups. In benign ovarian tumor and benign gynecologic disease patients, immune system surveillance might not be required and therefore the value of NLR is not elevated. It is important to consider that the majority of pelvic masses (80%) are benign with cystic, solid, or mixed characteristics and have a favorable prognosis, while only 20% are malignant tumors [36]. Considering that most benign tumors are found in women of fertile age, the preservation of the ovary is important. However, accurate diagnosis of the suspicious mass, whether malignant or benign, is often difficult. Our results showed that NLR is elevated in the sera from ovarian cancer patients but not that from benign ovarian tumor or benign gynecologic disease patients. In benign ovarian tumor patients, 97 (56.1%) of 173 patients were CA125 negative and NLR negative. CA125 was elevated in 35 (20.2%) out of 173 patients and of these 35 patients, 22 (62.9%) were NLR negative and 13 were NLR positive (cutoff value: 2.60) (data not shown). Low NLR levels in patients with pelvic masses indicate the likelihood of a benign mass, while elevation of either marker could suggest a malignancy and would warrant a more thorough diagnostic procedures and faster treatment of the patient.
As more biomarkers are discovered and validated, efforts will focus on combined use of multiple serum tumor markers. Although NLR cannot provide all of the necessary information for optimal ovarian cancer diagnosis, our results suggest that it could complement CA125, especially in early stage ovarian cancer. Moreover, NLR can be calculated from data that are already routinely available. It does not require any additional expenditure.
Several possible reasons exist to explain the association between elevated NLR and poor prognosis. The immune response of host to tumor is lymphocyte dependent. Patients with elevated NLR have a relative lymphocytopenia and, as a result, may exhibit a poorer lymphocyte-mediated immune response to malignancy, thereby worsening their prognosis and increasing the potential for the tumor to recur. Alternatively, circulating neutrophils have been shown to contain and secrete the vast majority of circulating VEGF, a pro-angiogenic factor that is thought to play an integral role in tumor development [37, 38].
Multivariate analysis in our study showed that increasing age, advanced FIGO-stage, and elevated NLR value at primary diagnosis are prognostic indicators of poor outcome in ovarian cancer. The survival of patients depends on tumor stage and age, but it is also affected by the NLR value, suggesting that the immune system plays an important role in ovarian cancer immunosurveillance. We therefore believe that the restoration of immunocompetence and nutritional status might be useful aids in ameliorating the prognosis of patients with ovarian cancer. The ability to successfully predict poor prognosis in ovarian cancer patients using NLR would be valuable in directing both pre-and postoperative therapies in order to improve prognosis.
The prognostic significance of preoperative serum CA125 level remains controversial in the literature. Although some publications have described an association between CA125 serum levels and the stage and histology of tumors [39, 40], only a few studies have demonstrated an association of CA125 with prognosis in epithelial ovarian cancer. Paramasivam et al. reported preoperative serum CA125 of more than 30 U/mL was significantly associated with impaired survival (Hazard Ratio = 2.40 [95% CI: 1.26–4.59], P = 0.028) in stage I epithelial ovarian cancer [41]. In our study, using Cox multivariate analysis, preoperative CA125 level had no independent prognostic significance. The different results of these several studies could be explained by different mixtures of tumor types and grades, and different residual tumor status.
In conclusion, we have documented changes in preoperative NLR in some women with ovarian cancer. Many patients with ovarian cancer have a significantly elevated NLR indicating that NLR measurement could become the part of the routine diagnosis of early stage ovarian cancer. In addition, the patients with an elevated NLR at diagnosis had significantly worse overall survival rates. Preoperative NLR measurement in such patients may provide a simple method of identifying patients with a poorer prognosis and aid in guiding treatment effectively.
Acknowledgments
This work was supported in part by National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (7-2006-0153), CMB-YUHAN research grant of Yonsei University College of Medicine for 2006 (6-2006-0030), and the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2006-311-e00339).
References
- 1.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1998. CA Cancer J Clin. 1998;48(1):6–29. doi: 10.3322/canjclin.48.1.6. [DOI] [PubMed] [Google Scholar]
- 2.Pauler DK, Menon U, McIntosh M, Symecko HL, Skates SJ, Jacobs IJ. Factors influencing serum CA125II levels in healthy postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2001;10(5):489–493. [PubMed] [Google Scholar]
- 3.Bast RC, Jr, Badgwell D, Lu Z, Marquez R, Rosen D, Liu J, Baggerly KA, Atkinson EN, Skates S, Zhang Z, Lokshin A, Menon U, Jacobs I, Lu K. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005;15(Suppl 3):274–281. doi: 10.1111/j.1525-1438.2005.00441.x. [DOI] [PubMed] [Google Scholar]
- 4.Bandera CA, Ye B, Mok SC. New technologies for the identification of markers for early detection of ovarian cancer. Curr Opin Obstet Gynecol. 2003;15(1):51–55. doi: 10.1097/00001703-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Rosen DG, Wang L, Atkinson JN, Yu Y, Lu KH, Diamandis EP, Hellstrom I, Mok SC, Liu J, Bast RC., Jr Potential markers that complement expression of CA125 in epithelial ovarian cancer. Gynecol Oncol. 2005;99(2):267–277. doi: 10.1016/j.ygyno.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 6.Terry KL, Sluss PM, Skates SJ, Mok SC, Ye B, Vitonis AF, Cramer DW. Blood and urine markers for ovarian cancer: a comprehensive review. Dis Markers. 2004;20(2):53–70. doi: 10.1155/2004/241982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bast RC, Jr, Xu FJ, Yu YH, Barnhill S, Zhang Z, Mills GB. CA 125: the past and the future. Int J Biol Markers. 1998;13(4):179–187. doi: 10.1177/172460089801300402. [DOI] [PubMed] [Google Scholar]
- 8.Ueno H, Hawrylowicz CM, Banchereau J. Immunological intervention in human diseases. J Transl Med. 2007;5:59. doi: 10.1186/1479-5876-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jilma B, Blann A, Pernerstorfer T, Stohlawetz P, Eichler HG, Vondrovec B, Amiral J, Richter V, Wagner OF. Regulation of adhesion molecules during human endotoxemia. No acute effects of aspirin. Am J Respir Crit Care Med. 1999;159(3):857–863. doi: 10.1164/ajrccm.159.3.9805087. [DOI] [PubMed] [Google Scholar]
- 10.Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5–14. [PubMed] [Google Scholar]
- 11.Jaiswal M, LaRusso NF, Gores GJ. Nitric oxide in gastrointestinal epithelial cell carcinogenesis: linking inflammation to oncogenesis. Am J Physiol Gastrointest Liver Physiol. 2001;281(3):G626–G634. doi: 10.1152/ajpgi.2001.281.3.G626. [DOI] [PubMed] [Google Scholar]
- 12.Brower V. Feeding the flame: new research adds to role of inflammation in cancer development. J Natl Cancer Inst. 2005;97(4):251–253. doi: 10.1093/jnci/97.4.251. [DOI] [PubMed] [Google Scholar]
- 13.Ardies CM. Inflammation as cause for scar cancers of the lung. Integr Cancer Ther. 2003;2(3):238–246. doi: 10.1177/1534735403256332. [DOI] [PubMed] [Google Scholar]
- 14.Van der Auwera I, Van Laere SJ, Van den Eynden GG, Benoy I, van Dam P, Colpaert CG, Fox SB, Turley H, Harris AL, Van Marck EA, Vermeulen PB, Dirix LY. Increased angiogenesis and lymphangiogenesis in inflammatory versus noninflammatory breast cancer by real-time reverse transcriptase-PCR gene expression quantification. Clin Cancer Res. 2004;10(23):7965–7971. doi: 10.1158/1078-0432.CCR-04-0063. [DOI] [PubMed] [Google Scholar]
- 15.Biarc J, Nguyen IS, Pini A, Gosse F, Richert S, Thierse D, Van Dorsselaer A, Leize-Wagner E, Raul F, Klein JP, Scholler-Guinard M. Carcinogenic properties of proteins with pro-inflammatory activity from Streptococcus infantarius (formerly S.bovis) Carcinogenesis. 2004;25(8):1477–1484. doi: 10.1093/carcin/bgh091. [DOI] [PubMed] [Google Scholar]
- 16.Altinoz MA, Korkmaz R. NF-kappaB, macrophage migration inhibitory factor and cyclooxygenase-inhibitions as likely mechanisms behind the acetaminophen- and NSAID-prevention of the ovarian cancer. Neoplasma. 2004;51(4):239–247. [PubMed] [Google Scholar]
- 17.Wang W, Bergh A, Damber JE. Chronic inflammation in benign prostate hyperplasia is associated with focal upregulation of cyclooxygenase-2, Bcl-2, and cell proliferation in the glandular epithelium. Prostate. 2004;61(1):60–72. doi: 10.1002/pros.20061. [DOI] [PubMed] [Google Scholar]
- 18.Hussein MR, Ahmed RA. Analysis of the mononuclear inflammatory cell infiltrate in the non-tumorigenic, pre-tumorigenic and tumorigenic keratinocytic hyperproliferative lesions of the skin. Cancer Biol Ther. 2005;4(8):819–821. doi: 10.4161/cbt.4.8.1864. [DOI] [PubMed] [Google Scholar]
- 19.Bartsch H, Nair J. Oxidative stress and lipid peroxidation-derived DNA-lesions in inflammation driven carcinogenesis. Cancer Detect Prev. 2004;28(6):385–391. doi: 10.1016/j.cdp.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Gunter MJ, Stolzenberg-Solomon R, Cross AJ, Leitzmann MF, Weinstein S, Wood RJ, Virtamo J, Taylor PR, Albanes D, Sinha R. A prospective study of serum C-reactive protein and colorectal cancer risk in men. Cancer Res. 2006;66(4):2483–2487. doi: 10.1158/0008-5472.CAN-05-3631. [DOI] [PubMed] [Google Scholar]
- 21.Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60(1):184–190. [PubMed] [Google Scholar]
- 22.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 23.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 25.Lin EY, Pollard JW. Role of infiltrated leucocytes in tumour growth and spread. Br J Cancer. 2004;90(11):2053–2058. doi: 10.1038/sj.bjc.6601705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 27.Koga Y, Matsuzaki A, Suminoe A, Hattori H, Hara T. Neutrophil-derived TNF-related apoptosis-inducing ligand (TRAIL): a novel mechanism of antitumor effect by neutrophils. Cancer Res. 2004;64(3):1037–1043. doi: 10.1158/0008-5472.CAN-03-1808. [DOI] [PubMed] [Google Scholar]
- 28.Blake-Mortimer JS, Sephton SE, Carlson RW, Stites D, Spiegel D. Cytotoxic T lymphocyte count and survival time in women with metastatic breast cancer. Breast J. 2004;10(3):195–199. doi: 10.1111/j.1075-122X.2004.21290.x. [DOI] [PubMed] [Google Scholar]
- 29.Fumagalli LA, Vinke J, Hoff W, Ypma E, Brivio F, Nespoli A. Lymphocyte counts independently predict overall survival in advanced cancer patients: a biomarker for IL-2 immunotherapy. J Immunother. 2003;26(5):394–402. doi: 10.1097/00002371-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Fogar P, Sperti C, Basso D, Sanzari MC, Greco E, Davoli C, Navaglia F, Zambon CF, Pasquali C, Venza E, Pedrazzoli S, Plebani M. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. 2006;32(1):22–28. doi: 10.1097/01.mpa.0000188305.90290.50. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 32.Porta C, Moroni M, De Amici M. Eosinophils and serum eosinophilic cationic proteins in interleukin-2-based immunotherapy for cancer. Br J Haematol. 1998;100(3):607–609. doi: 10.1046/j.1365-2141.1998.0636d.x. [DOI] [PubMed] [Google Scholar]
- 33.Stefanini M, Claustro JC, Motos RA, Bendigo LL. Blood and bone marrow eosinophilia in malignant tumors. Role and nature of blood and tissue eosinophil colony-stimulating factor(s) in two patients. Cancer. 1991;68(3):543–548. doi: 10.1002/1097-0142(19910801)68:3<543::AID-CNCR2820680317>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Burtin C, Ponvert C, Fray A, Scheinmann P, Lespinats G, Loridon B, Canu P, Paupe J. Inverse correlation between tumor incidence and tissue histamine levels in W/WV, WV/+, and +/+ mice. J Natl Cancer Inst. 1985;74(3):671–674. [PubMed] [Google Scholar]
- 35.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91(3):181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 36.Pelusi G, Taroni B, Flamigni C. Benign ovarian tumors. Front Biosci. 1997;2:g5–7. [PubMed] [Google Scholar]
- 37.Fondevila C, Metges JP, Fuster J, Grau JJ, Palacin A, Castells A, Volant A, Pera M. p53 and VEGF expression are independent predictors of tumour recurrence and survival following curative resection of gastric cancer. Br J Cancer. 2004;90(1):206–215. doi: 10.1038/sj.bjc.6601455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6(4):283–287. doi: 10.1023/B:AGEN.0000029415.62384.ba. [DOI] [PubMed] [Google Scholar]
- 39.Hwang GI, Yoo CH, Sohn BH, Shin JH, Park YL, Kim HD, Kim YS, Han WK, Pae WK. Predictive value of preoperative serum CEA, CA19-9 and CA125 levels for peritoneal metastasis in patients with gastric carcinoma. Cancer Res Treat. 2004;36(3):178–181. doi: 10.4143/crt.2004.36.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuxen MK, Soletormos G, Dombernowsky P. Tumor markers in the management of patients with ovarian cancer. Cancer Treat Rev. 1995;21(3):215–245. doi: 10.1016/0305-7372(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 41.Paramasivam S, Tripcony L, Crandon A, Quinn M, Hammond I, Marsden D, Proietto A, Davy M, Carter J, Nicklin J, Perrin L, Obermair A. Prognostic importance of preoperative CA–125 in International Federation of Gynecology and Obstetrics stage I epithelial ovarian cancer: an Australian multicenter study. J Clin Oncol. 2005;23(25):5938–5942. doi: 10.1200/JCO.2005.08.151. [DOI] [PubMed] [Google Scholar]