Abstract
Radiolabeled monoclonal antibodies (mAb) have demonstrated measurable antitumor effects in hematologic malignancies. This outcome has been more difficult to achieve for solid tumors due, for the most part, to difficulties in delivering sufficient quantities of mAb to the tumor mass. Previous studies have shown that nonlytic levels of external beam radiation can render tumor cells more susceptible to T cell-mediated killing. The goal of these studies was to determine if the selective delivery of a radiolabeled mAb to tumors would modulate tumor cell phenotype so as to enhance vaccine-mediated T-cell killing. Here, mice transgenic for human carcinoembryonic antigen (CEA) were transplanted with a CEA expressing murine carcinoma cell line. Radioimmunotherapy consisted of yttrium-90 (Y-90)-labeled anti-CEA mAb, used either alone or in combination with vaccine therapy. A single dose of Y-90-labeled anti-CEA mAb, in combination with vaccine therapy, resulted in a statistically significant increase in survival in tumor-bearing mice over vaccine or mAb alone; this was shown to be mediated by engagement of the Fas/Fas ligand pathway. Mice receiving the combination therapy also showed a significant increase in the percentage of viable tumor-infiltrating CEA-specific CD8+ T cells compared to vaccine alone. Mice cured of tumors demonstrated an antigen cascade resulting in CD4+ and CD8+ T-cell responses not only for CEA, but for p53 and gp70. These results show that systemic radiotherapy in the form of radiolabeled mAb, in combination with vaccine, promotes effective antitumor response, which may have implications in the design of future clinical trials.
Keywords: mAb, Radiation, Y-90, Vaccine, Immunotherapy
Introduction
Cancer vaccines have proven effective in generating antigen-specific T-cell responses and antitumor effects in a wide range of preclinical models. Clinical studies have also demonstrated that many different types of cancer vaccines can induce T-cell responses to specific tumor-associated antigens (TAAs) in patients with a variety of carcinomas. Antitumor effects in patients, however, have been less frequently observed. One reason for this discrepancy between cancer vaccines’ ability to generate antigen-specific T-cell responses and to induce antitumor effects is the possibility that tumor cells express insufficient quantities of either TAAs or accessory molecules to render them susceptible to T-cell killing.
Recent studies have demonstrated that nonlytic doses of external beam radiation to tumor cells ex vivo can render them more susceptible to T-cell killing [4]. Subsequent studies have also shown that nonlytic doses of external beam radiation therapy (EBRT) directed against solid tumor masses alter the phenotype of tumor cells, rendering them more susceptible to vaccine-mediated T-cell killing [5, 11]. However, in patients with metastatic cancer, tumor cells may not be amenable to EBRT because they are either too diffuse or in inaccessible locations.
In studies involving the delivery of systemic radiation through radioimmunotherapy (RAIT) to patients with hemopoietic malignancies, monoclonal antibodies (mAb) conjugated to radionuclides such as yttrium-90 (Y-90) or iodine-131 have demonstrated substantial antitumor effects [12, 41–43]. Similar antitumor effects with radiolabeled mAb, however, have rarely been achieved in the therapy of solid tumor masses, which comprise the majority of human carcinomas. While radiolabeled mAb directed against TAAs of solid tumors have proven capable of selectively targeting tumor masses in a range of cancer types, one of the major limitations of this modality is that limited doses of the radionuclide are actually deposited at the tumor site.
We thus hypothesized that delivering nonlytic doses of a radiolabeled mAb to a tumor mass might sufficiently alter the phenotype of tumor cells to render them more susceptible to vaccine-mediated T-cell killing. The studies reported here utilized mice transgenic (Tg) for human carcinoembryonic antigen (CEA), where CEA is expressed in normal GI tissue and embryonic tissue in levels similar to those found in humans, and tumors expressing CEA as a self-antigen. We employed a vaccine and a radiolabeled mAb, both directed against CEA. While each regimen alone proved to be marginally effective in reducing tumor burden, utilizing both in combination demonstrated statistically significant increases in antitumor effects. We demonstrate here that the combined agents work synergistically: the radiolabeled mAb up-regulates the accessory molecule Fas on tumor cells, rendering the tumor cells more susceptible to vaccine-mediated T-cell killing.
Materials and methods
COL-1 mAb
COL-1 (a murine IgG2a) is a murine mAb specific for CEA, which is overexpressed in a wide variety of human tumor cells. COL-1’s range of relativities to CEA-related normal antigens, biochemical characteristics, and comparative binding capabilities have been previously described [26, 32].
Chelate synthesis and mAb conjugation
COL-1 (4.8 mg/ml) was reacted with the A” stereoisomer of 2-(p-isothiocyanatobenzyl)-cyclohexyl-diethylenetriamine pentaacetic acid (CHX-A”; Macrocyclics, Dallas, TX, USA) at a COL-1:CHX-A” molar ratio of 10 in 0.05 M sodium borate buffer at pH 9 for 24 h. The resulting COL-1/CHX-A” conjugate was purified with a size exclusion PD-10 column (Pharmacia Biotech AB, Uppsala, Sweden) eluted with saline.
Y-90 radiolabeling
The purified COL-1/CHX-A” conjugate (400 μg) was reacted with Y-90 (11.5 mCi) in 175 μL of aqueous medium containing 0.2 M sodium acetate and 0.028 M sodium ascorbate, pH 4.2. The labeling yield was 83%. To quench any nonincorporated free Y-90, diethylenetriamine pentaacetic acid (DTPA) at a final concentration of 0.2 mM was added and the labeled product was purified using a PD-10 column. The Y-90-labeled COL-1/CHX-A” at a specific activity of 23.8 μCi/μg was admixed with bovine serum albumin (2.5%) and used within 6 h. Immunoreactivity was determined by incubating COL-1/CHX-A”-Y-90 (1 μg) with CEA at 8 times molar excess in 43 μL PBS, pH 7.2 at room temperature for 30 min and analyzing the complex with size exclusion high-performance liquid chromatography equipped with a TSK 3000 SWXL column (TosoHaas, Philadelphia, PA, USA; 0.067 M sodium phosphate—0.1 M KCl, pH 6.8; 1 ml/min). Over 95% of COL-1/CHX-A”-Y-90 was bound to CEA, producing a higher molecular complex than either entity alone.
CEA-Tg mice
In vivo studies were conducted in 6- to 8-week-old female CEA-Tg mice that were housed and maintained in pathogen-free microisolator cages. The generation and characterization of the CEA-Tg mouse have been previously described [8].
Tumor cells
The murine colon adenocarcinoma cell line MC38 (H-2b) has been described previously [38]. MC38 cells expressing human CEA (MC38-CEA+) were generated by retroviral transduction with CEA cDNA. For the mechanism studies, MC38-CEA+ cells were transfected with an expression vector encoding dominant-negative (DN-1) Fas [28] (donated by Dr. Robert H. Wiltrout, NCI, NIH, Frederick, MD, USA), and designated MC38-CEA-DN-1.
Tumor targeting and biodistribution
Before undertaking RAIT studies, we performed a tumor-targeting experiment to analyze the selective localization and retention of Y-90-labeled COL-1 administered i.v. MC38 or MC38-CEA+ tumor cells (3 × 105) in 100 μL PBS were injected s.c. into the quadriceps area of the right hind limb of the mice described above. After 14 days, groups of mice were injected i.v. with 0, 25, 50, 100, or 150 μCi of Y-90-labeled COL-1. All dose-level groups received exactly the same amount of antibody (6.5 mg) per mouse (cold + labeled COL-1). The mice were sacrificed 96 h after i.v. injection of the Y-90-labeled COL-1. Blood, tumors, and various organs were removed, weighed, and analyzed for radioactivity by beta counter. Percentages of injected dose (ID)/g normalized to a 20-g mouse were determined and expressed as %ID/g ± SD.
Staining and immunofluorescence
Cell-surface staining was performed with primary fluorescein isothiocyanate (FITC)-labeled mAb COL-1 (anti-CEA), H-2Kb, and intercellular adhesion molecule (ICAM)-1 (CD54). Fas cell-surface staining was performed with primary PE-labeled mAb. For analysis of tumor-infiltrating cells, tumor cell suspensions were stained with primary labeled antibody specific for CD3, CD4, CD8, CD19, and natural killer cells. All of the mAb’s were purchased from Pharmingen (San Diego, CA, USA). Immunofluorescence was analyzed and compared with the appropriate isotype-matched controls (Pharmingen) with a FACScan cytometer using CellQuest™ software (Becton Dickinson, Mountain View, CA, USA). Nonviable cells were electronically excluded from analysis based on propidium iodide exclusion.
External beam radiation
For some control studies, mice were injected with tumor cells as described above. When tumors reached a mean volume of 75–100 mm3 as measured by digital caliper, they were irradiated as previously described [5].
Fas up-regulation
To determine if Fas up-regulation on tumor cells after systemic irradiation was similar to Fas up-regulation after EBRT, CEA-Tg mice were injected s.c. with MC38 or MC38-CEA+ tumor cells. After 14 days, groups of mice were injected i.v. with 0, 50, 100 or 150 μCi of Y-90-labeled COL-1. Tumors were excised after 96 h and cells were examined for Fas up-regulation by flow cytometric analysis. As a control, another group of MC38 and MC38-CEA+ tumor cells was subjected to 10 Gy EBRT.
Recombinant poxvirus vaccines
Recombinant vaccinia and fowlpox viruses containing genes for human CEA and the murine costimulatory molecules B7-1, ICAM-1, and leukocyte function-associated antigen (LFA)-3 genes (designated rV-CEA/TRICOM and rF-CEA/TRICOM, respectively) have been previously described [6, 17, 18]. The rF virus containing the gene for murine granulocyte-macrophage colony-stimulating factor (GM-CSF) has been previously described [23]. Dr. A. Gomez-Yafal, Dr. D. Panicali, Dr. G. Mazzara, and Dr. L. Gritz of Therion Biologics Corporation (Cambridge, MA, USA) provided the orthopox viruses as part of a collaborative research and development agreement between the NCI/NIH and Therion Biologics Corporation.
Vaccine regimen
To examine whether irradiation of growing tumors by radiolabeled antibody improves the efficacy of a recombinant anticancer vaccine regimen, we first treated CEA-Tg mice with a CEA/TRICOM vaccine in a diversified prime-and-boost regimen. The advantages of this regimen have been previously described [16, 21, 30]. MC38, MC38-CEA+, or MC38-CEA-DN-1 cells (3 × 105) were injected s.c. into the quadriceps area of the right hind limb of the mice described above. After 8 days, the mice were vaccinated s.c. with 1 × 108 plaque-forming units (PFUs) rV-CEA/TRICOM admixed with 1 × 107 PFUs rF-GM-CSF. On day 14 post-tumor transplant, mice were injected with 100 μCi of Y-90-labeled COL-1, a dose that does not have an impact on tumor growth (data not shown) but does change the phenotype of tumor cells. On days 15, 22, and 29, mice were boosted with 1 × 108 PFUs rF-CEA/TRICOM admixed with 1 × 107 PFUs rF-GM-CSF. Tumors were measured daily by digital caliper in two dimensions, and volumes were calculated as previously described [4]. Animals were sacrificed when tumors reached >20 mm in any dimension. To determine whether radiation-induced Fas up-regulation on MC38-CEA+ tumor cells was responsible for tumor regression, we conducted an experiment wherein CEA-Tg mice were transplanted with MC38-CEA-DN-1 tumors and treated with a combination of CEA/TRICOM vaccine in the regimen described above and Y-90-labeled COL-1 irradiation at the doses described above.
Immunologic assays
To evaluate CD4+ T-cell responses, spleens were removed as indicated, dispersed into single-cell suspensions, and pooled. CD4+ T cells were enriched using autoMACS® (Miltenyi Biotec, Auburn, CA, USA). CEA-specific CD4+ T-cell responses were analyzed as previously described [16]. p53-specific CD4+ T-cell responses were analyzed in a similar manner, using a major histocompatibility complex (MHC) class II p53108–122 peptide (LGFLQSGTAKSVMCT) (0.16–2.5 μg/ml) [46]. To evaluate CD8+ T-cell responses, spleens were removed as indicated, dispersed into single-cell suspensions, and pooled. CD8+ T cells were enriched using autoMACS®. Purified CD8+ T cells were coincubated with the CEA peptide (10 μg/ml), the H-2Db-restricted peptide p53232–240 (KYMCNSSCM) (2 μg/ml) [15, 27], or the H-2Kb-restricted peptide p15E604–611 (KSPWFTTL; gp70 peptide) (1 μg/ml) [45] for 7 days along with freshly irradiated naive splenocytes. Lymphocytes were recovered by centrifugation through a Ficoll-Hypaque gradient (Beckman Coulter, Fullerton, CA, USA). T cells were restimulated with freshly irradiated naive splenocytes and the corresponding peptide for 24 h. As controls, VSVN was used for H-2Db-restricted peptides, and OVA (ovalbumin257–264, SIINFEKL) for H-2Kb-restricted peptides [9]. Supernatant was collected and analyzed for murine IFN-γ by BD™ Cytometric Bead Array (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. To analyze the tumor-infiltrating T-cell population, tumors were harvested and mechanically dispersed into single-cell suspension. Antibodies used for analysis included FITC-conjugated anti-CD3e (hamster IgG1) and CyChrome-conjugated anti-CD8 mAb (rat IgG2a) (Pharmingen) and appropriate isotype control antibodies. To evaluate the generation of CEA-specific CTLs, cells were stained with phycoerythrin-conjugated CEA526–533/H-2Db-tetramer (CEA-tetramer, Beckman Coulter). Cell viability was tested by Annexin V-FITC staining.
Statistical analysis
Tests of significance are reported as P values, derived from Student’s t test using a 2-tailed distribution and calculated at 95% using StatView 4.1 software (Abacus Concepts, Berkeley, CA, USA).
Results
Tumor targeting and biodistribution
Before undertaking RAIT studies, we performed a tumor-targeting experiment to analyze the selective localization and retention of Y-90-labeled COL-1 administered i.v. MC38 or MC38-CEA+ tumor cells (3 × 105) in 100 ml PBS were injected s.c. into the quadriceps area of the right hind limb of CEA-Tg mice. After 14 days, groups of mice were injected i.v. with 0, 25, 50, 100, or 150 μCi of Y-90-labeled COL-1. At 96 h postinjection, there were no significant differences in accumulation of Y-90-labeled COL-1 in the blood, liver, kidney, stomach, lung, muscle, bone, or heart of mice bearing MC38-CEA+ tumors or MC38 tumors at any dose examined (Fig. 1a–d). There was no significant uptake of radioactivity in GI tissue, even though CEA expression in normal GI tissue is similar in mice and in humans. MC38-CEA+ tumors (Fig. 1a) showed a significantly higher accumulation of Y-90-labeled COL-1 than MC38 tumors (Fig. 1b), with the highest accumulation at a dose of 150 μCi Y-90-labeled COL-1 (35 ± 14%).
Fig. 1.
Biodistribution of Y-90-labeled COL-1. CEA-Tg mice were injected with 3 × 105 MC38-CEA+ (white), (a) or MC38 (gray), (b) tumor cells s.c. After 14 days, mice were given 25, 50, 100, or 150 μCi Y-90-labeled COL-1 i.v (a–d). At 96 h postinjection of radiolabeled mAb, organs and tissues (a–d) and tumors (e–f) were isolated and assessed for radioactivity. Percent of injected dose per gram (%ID/g) is depicted, along with SD. (Note, a–d, spleen not included due to tissue processing error)
Up-regulation of Fas postirradiation
As Fas has been shown to be up-regulated on tumor cells in vitro after EBRT [4], we sought to determine if Fas would be up-regulated in a similar manner by Y-90-labeled COL-1. CEA-Tg mice were injected s.c. with MC38 or MC38-CEA+ tumor cells. After 14 days, groups of mice were injected i.v. with 0, 50, 100 or 150 μCi of Y-90-labeled COL-1. Tumors were excised after 96 h and cells were examined for Fas up-regulation by flow cytometric analysis. As a control, another group of MC38 and MC38-CEA+ tumor cells was subjected to 10 Gy EBRT (Fig. 2). For these tests, the population of cells positive for isotype control never exceeded 7% and viable CEA+ cells were gated to confirm them as tumor cells. Cell-surface expression of Fas was greater in irradiated than in nonirradiated MC38-CEA+ tumor cells, which exhibited 30% of Fas expression (Fig. 2b). Fas expression peaked (58%) at 50 μCi Y-90-labeled COL-1 and remained the same at 100 μCi (Fig. 2b). Mean fluorescence intensity (MFI) of Fas-positive cells increased from 210 in nonirradiated cells to up to 950 after 150 μCi of Y-90-labeled COL-1 (Fig. 2d). As the difference in MFI at doses of 100 and 150 μCi was not significant, we used a dose level of 100 μCi in all subsequent studies. Interestingly, Y-90-labeled COL-1 did not seem to have an effect on surface expression of Fas in MC38 cells (Fig. 2f, h), indicating the importance of retention of radiolabeled antibody in terms of phenotypic changes in tumor cells. Cells subjected to EBRT efficiently up-regulated both surface expression and MFI of Fas (Fig. 2a, c, e, g).
Fig. 2.
Expression of up-regulated Fas on tumor cells after administration of radiolabeled mAb. CEA-Tg mice were injected with 3 × 105 MC38-CEA+ (a–d) or MC38 (e – h) tumor cells s.c. After 14 days, mice were given either 10 Gy tumor-directed EBRT, or 0, 25, 50, 100, or 150 μCi Y-90-labeled COL-1 i.v. After 96 h tumors were excised and examined for Fas up-regulation by flow cytometric analysis
Inhibition of tumor growth with combination of radiolabeled mAb and vaccine therapy
To examine whether irradiation of growing tumors by radiolabeled antibody improves the efficacy of a recombinant anticancer vaccine regimen, we first treated CEA-Tg mice with a CEA/TRICOM vaccine in a diversified prime-and-boost regimen (Table 1). The vaccine regimen consisted of priming mice with rV-CEA/TRICOM admixed with rF-GM-CSF followed by 3 weekly boosts with rF-CEA/TRICOM admixed with rF-GM-CSF. MC38-CEA+ tumor cells were injected s.c. on the right hind leg of CEA-Tg mice. Eight days following tumor transplant, groups of mice were divided into those that received (a) no treatment; (b) vaccine alone; (c) 100 μCi of Y-90-labeled COL-1 alone; or (d) the combination of vaccine followed by Y-90-labeled COL-1 irradiation (Table 1a). In mice that received no treatment, tumors grew progressively (average tumor volume on day 28 was 3,311 mm3). Moreover, 100% of the animals died by day 30 (Table 1). In mice treated with vaccine alone in the regimen described above, CEA/TRICOM did not significantly inhibit tumor growth (P = 0.051 compared to no treatment). Y-90-labeled COL-1 as a monotherapy significantly inhibited tumor growth (P = 0.001 compared to no treatment) (Table 1). However, treating tumors with a combination of vaccine and radiolabeled mAb resulted in a marked decrease in tumor growth rate and tumor volume (P = 0.001 compared to no treatment and vaccine alone, and 0.0064 compared with irradiation alone; Table 1). Moreover, mice treated with the combination therapy not only showed delayed tumor progression, but 20% experienced a resolution of tumor mass by endogenous host response and remained tumor-free for the duration of the experiment (77 days).
Table 1.
Effect of radiolabeled mAb and vaccine therapy on tumor volume
| Treatment | n | D28 tumor volume (mm3) | D75 tumor free | P vs. buffer | P vs. vaccine | P vs. Y-90 COL-1 |
|---|---|---|---|---|---|---|
| (A) | ||||||
| Buffer | 20 | 3,311 ± 321 | 0 | |||
| Vaccine | 20 | 2,653 ± 261 | 0 | 0.051 | <0.001 | |
| Y-90 COL-1 | 20 | 1,275 ± 167 | 1 | 0.001 | 0.0064 | |
| Vaccine+Y-90 COL-1 | 24 | 594 ± 111 | 5 | 0.001 | ||
| (B) Controls | ||||||
| Vaccine + COL-1 (cold) | 5 | 1,159 ± 130 | 0 | |||
| Vaccine+Y-90 COL-1 (DN-1 tumors) | 5 | 2,985 ± 565 | 0 | |||
| Vaccine+Y-90 COL-1 (CEA negative tumors) | 5 | 2,071 ± 50 | 0 | |||
CEA-Tg mice were injected with MC38-CEA+tumor cells s.c. A Mice either received no treatment, were vaccinated with rV-CEA/TRICOM on day 8 and boosted with rF-CEA/TRICOM on days 15, 22, and 29, were injected i.v. with 100 μCi Y-90-labeled COL-1 on day 14, or received rV-CEA/TRICOM vaccine on day 8 and 100 μCi Y-90-labeled COL-1 i.v. on day14, followed by boosts of rF-CEA/TRICOM on days 15, 22, and 29. All vaccines were given with rF-GM-CSF. Tumor volume was monitored for 56 days. B Role of Fas up-regulation and antigen specificity in tumor eradication by combination therapy. CEA-Tg mice were injected with either MC38-CEA + tumor cells, MC38-CEA-DN-1 tumor cells, or MC38-CEA-tumor cells s.c. Groups of mice were vaccinated with rV-CEA/TRICOM on day 8. Groups of mice were vaccinated with rV-CEA/TRICOM on day 8 and injected with 100 μCi Y-90-labeled COL-1 i.v. on day 14, followed by boosting with rF-CEA/TRICOM on days 15, 22, and 29. Groups of mice were vaccinated with rV-CEA/TRICOM on day 8 and injected with 6.5 μg unlabeled COL-1 i.v. on day 14, followed by boosting with rF-CEA/TRICOM on days 15, 22, and 29. Additionally, mice were vaccinated with rV-CEA/TRICOM on day 8 and injected with 100 μCi Y-90-labeled COL-1 i.v. on day 14, followed by boosting with rF-CEA/TRICOM on days 15, 22, and 29. All vaccines were given with rF-GM-CSF. Tumor volume was monitored for 56 days
Role of Fas up-regulation and antigen specificity in tumor eradication with combination of radiolabeled mAb and vaccine therapy
To determine directly whether Y-90-labeled COL-1-induced Fas up-regulation on MC38-CEA+ cells in vivo was responsible for tumor regression, groups of CEA-Tg mice were transplanted with MC38-CEA-DN1 tumors (Fas nonfunctional). In mice transplanted with MC38-CEA-DN-1 tumors, treatment with CEA/TRICOM vaccine and Y-90-labeled COL-1 failed to mediate tumor regression (Table 1b), defining the crucial role of the Fas signaling pathway in the murine model. Furthermore, treatment of MC38-CEA+ tumors with 6.5 μg unlabeled COL-1 mAb in combination with vaccine also failed to mediate tumor regression. To confirm the finding that a TAA-specific vaccine can work synergistically with a radiolabeled mAb to enhance antitumor activity, we employed the treatment regimen described above in a TAA-negative tumor model with parental MC38 (CEA−) tumor cells (Table 1b). Mice treated with CEA/TRICOM vaccine and Y-90-labeled COL-1 showed no significant delay in tumor growth compared to untreated mice, confirming the role of TAA-specific immune responses in our study involving MC38-CEA+ cells.
Increased survival with combination of radiolabeled mAb and vaccine therapy
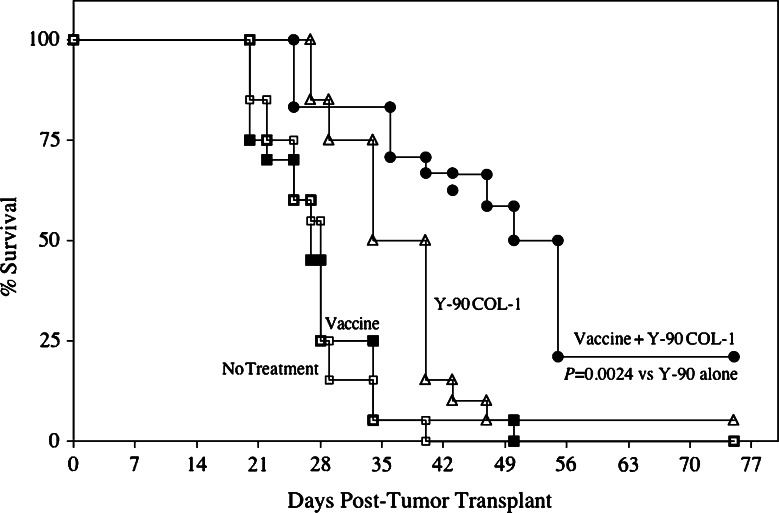
As shown in Fig. 3, 80–100% of untreated mice died by 35 days post-transplant. Antitumor effect was minimal in mice receiving vaccine only and moderate in mice receiving Y-90-labeled COL-1 only. Just 5% of mice receiving Y-90-labeled COL-1 only and none of the mice receiving vaccine only survived longer than 50 days post-treatment. However, the combination of vaccine and Y-90-labeled COL-1 resulted in a statistically significant increase in survival, with 50% of mice alive after 50 days of observation and 22% alive after 56 days of observation (P = 0.0024 compared to irradiation alone).
Fig. 3.
Combination of radiolabeled mAb and vaccine therapy increased survival in tumor-bearing mice. CEA-Tg mice (n = 20) were transplanted s.c. with MC38-CEA+ tumors on day 0. A control group (open squares) received HBSS buffer only. A second group (closed squares) received rV-CEA/TRICOM vaccine on day 8, boosted with rF-CEA/TRICOM on days 15, 22, and 29. A third group (open triangles) received 100 μCi Y-90-labeled COL-1 i.v. only on day 14. A fourth group (closed circles) received rV-CEA/TRICOM vaccine on day 8, boosted with rF-CEA/TRICOM on days 15, 22, and 29, as well as 100 μCi Y-90-labeled COL-1 i.v. on day 14. All vaccines were given with rF-GM-CSF. Mice were monitored weekly for tumor size and survival
Induction of antigenic cascade with combination of radiolabeled mAb and vaccine therapy
MC38-CEA+ tumor cells have been shown to express not only CEA, but also the gene products of murine retroviruses [20], including the endogenous retroviral env epitope gp70 [39, 45], which could act as targets of a CTL response. These cells also overexpress wild-type p53, another potential target antigen [15]. CEA-Tg mice that were cured of established tumor by treatment with the combination therapy described above were monitored for 2 months, along with a control group of age-matched CEA-Tg mice that received neither tumor transplant nor treatment. At the end of the 2-month observation period, control mice were tested for CD4+ and CD8+ T-cell responses specific for multiple TAAs. As seen in Table 2, there was no increase in proliferative T-cell responses to CEA protein at any of the concentrations used. In contrast, in mice cured by the combination therapy, there was a tenfold increase in proliferative T-cell responses at a 25 mg/ml concentration and a twofold increase at a 12.5 mg/ml concentration. CD4+ T-cell responses specific for an MHC class II-restricted p53 peptide were also analyzed. T cells of mice treated with the combination therapy showed a 5.3-fold increase in p53-specific proliferative response compared to the control group. T cells from both groups failed to react with a negative control protein (human serum albumin; data not shown).
Table 2.
Cellular immune responses to multiple turnor antigens after therapy with vaccine and radiolabeled antibody
| Treatmenta | Antigenb | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CEA (μg/ml) | p53 (μg/ml) | Peptidec | |||||||
| 25 | 12.5 | 6.25 | 5 | 2.5 | 1 | CEA | P53 | gp70 | |
| Vaccine + radiolabeled antibody | 10.4 | 3.3 | 1.7 | 3.2 | 3.0 | 1.6 | 6,280 | 3,560 | 36,410 |
| Control | 1.0 | 1.7 | 1.4 | 0.6 | 0.7 | 0.6 | 570 | 1,880 | 23,970 |
a3 CEA-Tg mice/group were given MC38-CEA+tumors s.c. Eight days later mice were vaccinated with rV-CEA/TRICOM admixed with rF-GM-CSF. On day 14, mice received 100 μCi Y-90-COL-1 (anti-CEA mAb). On days 15, 22, and 29 mice were boosted with rF-CEA/TRICOM admixed with rF-GM-CSF. Responses from pooled splenic T cells from cured mice were analyzed 2 months following tumor transplant for CEA protein and p53 MHC-II-peptide-specific proliferation and CEA, p53, and gp70 peptide-specific IFN-γ production. Control mice: normal age-matched CEA-Tg mice
bFor proliferation, each value represents the stimulation index of the mean CPM of triplicate samples versus media. SD never exceeded 10%
cFor peptide-specific IFN-γ production: concentrations of peptides CEA, p53, and gp70 were 10, 2, and 1 μg/ml, respectively. Each value represents IFN-γ (pg/ml/106 cells/24 h)
CD8+ T-cell responses against CEA peptide were then monitored by measuring IFN-gamma production (Table 2). CD8+ T cells from mice treated with the combination therapy showed much higher CEA-specific IFN-gamma production compared to the control group. To extend these findings to other potential TAAs, CD8+ T-cell responses were measured against an MHC class I-restricted p53 or gp70 peptide for both p53 and gp70. Greater production of IFN-gamma was again observed against the p53 and gp70 peptides from CD8+ T cells in mice receiving the combination therapy. T cells from the control group demonstrated no reactivity to CEA and p53 and only slight reactivity to gp70 peptide.
Characterization of infiltrating cells in the tumor microenvironment post-treatment
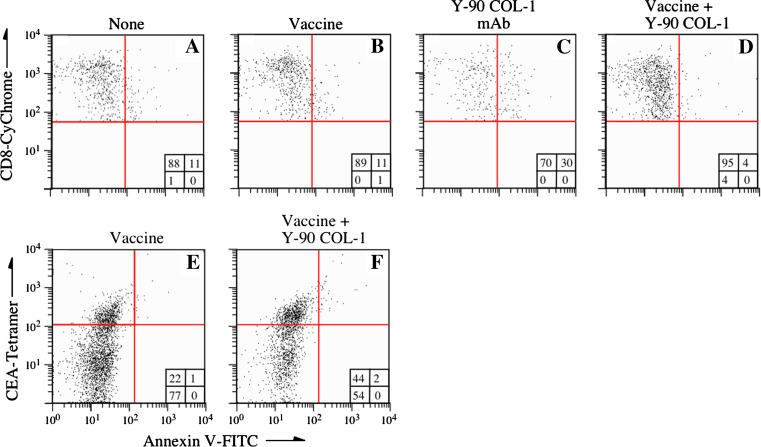
To evaluate the role of immune cells in the inhibition of tumor growth after combination therapy, we looked for infiltrating cells in the tumor microenvironment. CEA-Tg mice were transplanted with tumor cells as described above. Eight days later, groups of mice received either (a) no treatment; (b) CEA/TRICOM vaccine alone; (c) Y-90-labeled COL-1 irradiation alone; or (d) vaccine followed by Y-90-labeled COL-1 irradiation (Fig. 4). Tumors were excised 7 days postirradiation and analyzed for infiltrating CD8+ T cells by three-color flow cytometry (CD3-PE/CD8-CyChrome/Annexin V-FITC). In mice that received no treatment and mice that received vaccine only, 11% of tumor-infiltrating CD8+ T cells were Annexin-positive (Fig. 4a, b). In contrast, mice that received Y-90-labeled COL-1 alone had 30% more Annexin-positive cells than the other groups (Fig. 4c). Surprisingly, mice treated with the combination therapy had only 4% Annexin-positive tumor-infiltrating CD8+ T cells (Fig. 4d). We also analyzed the antigen specificity of CD8+ T cells by tetramer staining. The percentage of CEA tetramer-positive tumor-infiltrating cells doubled in the combination therapy group (Fig. 4e, f). Interestingly, these tetramer-positive cells did not show any increased positivity to Annexin V-FITC compared to mice treated with vaccine alone, indicating that they were less sensitive to radiation-induced damage.
Fig. 4.
Characterization of infiltrating cells in tumors treated with a combination of vaccine and radiolabeled mAb. CEA-Tg mice were transplanted s.c. with MC38-CEA+ tumors on day 0. a Mice received no treatment. b, e Mice received rV-CEA/TRICOM vaccine on day 8, followed by boosts of rF-CEA/TRICOM on days 15, 22, and 29. c Mice received 100 μCi Y-90-labeled COL-1 only on day 14. d, f Mice received rV-CEA/TRICOM vaccine on day 8, followed by boosts of rF-CEA/TRICOM on days 15, 22, and 29, along with 100 μCi Y-90-labeled COL-1 on day 14. All vaccines were given with rF-GM-CSF. Tumors were excised on day 28 and analyzed for infiltrating CD8+ and Annexin V-FITC-positive cells by flow cytometry (a–d). Tumor-infiltrating cells were analyzed by CEA-specific tetramer and Annexin V-FITC staining (e, f). Insets depict percent of positive cells in each quadrant
Discussion
Increased understanding of the development of antitumor immunity and the effects of radiation on tissues has spurred interest in the potential therapeutic benefit of combining radiation and immunotherapy [7, 37]. The targeted systemic radiation delivery known as RAIT has several potential advantages over EBRT, such as the ability to specifically target multiple tumor sites, minimize toxicity to normal tissue, and cause the death of adjacent tumor cells due to its large radiation path length. In addition, RAIT has been shown to increase expression of TAAs, thus enhancing the effectiveness of targeted immunotherapy [31]. RAIT has demonstrated effectiveness in treating hematologic cancers [19, 22, 36, 37, 44] and is more appropriate than EBRT for treatment of metastatic disease. However, to be a successful treatment for most solid tumors, RAIT will likely need to be employed with other therapeutic modalities. The study reported here explored the synergistic effect of RAIT combined with a vaccine.
One of the TAAs whose expression is enhanced by RAIT is Fas. Engagement of Fas by its cognate ligand (FasL) triggers recruitment of adaptor proteins, followed by activation of the caspase-signaling pathway, culminating in cell death. Ours is the first study to demonstrate the up-regulation of Fas expression on MC38-CEA+ tumor cells by radiolabeled mAb (Fig. 2b, d). Interestingly, Y-90-labeled COL-1 had no modulatory effect on Fas expression in MC38 cells (Fig. 2f, h). While the particular mechanism needs further study, it is possible that up-regulation of cell-surface Fas depends on tumor cells’ ability to retain the radiolabeled mAb.
Antigens from peripheral tumor cells can enter the MHC class I pathway and be presented to CD8+ T cells by antigen-presenting cells via cross-priming [34]. The antigen cascade reported here supports and extends previously reported results [5, 25, 29, 35]. For example, Kudo-Saito et al. [25] recently reported that in CEA+ tumor-bearing mice vaccinated with CEA/TRICOM, T-cell responses were detected not only to the CEA encoded in vaccine vectors, but also to other antigens expressed on the tumor itself, such as wild-type p53 and the endogenous retroviral epitope gp70. In fact, the magnitude of CD8+ T-cell responses to gp70 was far greater than that induced to CEA or p53, suggesting that immunization with dominant TAA vaccines, followed by a general enhancement of CD4+ T-cell activity to multiple TAAs via cross-priming, may confer long-term tumor protection in this particular mouse model. Similar antigenic cascades have also been reported in clinical studies [2, 3]. In our study, Y-90-labeled COL-1, in addition to up-regulating Fas (Fig. 2), may have induced apoptosis in a few of the tumor cells. This may have provided a boost to CEA-specific T cells and induced T-cell responses to additional tumor antigens such as p53 and gp70 through cross-priming (Table 2).
We have shown that destruction of MC38-CEA+ tumor cells in response to the combination of Y-90-labeled COL-1 and CEA/TRICOM vaccine was associated with increased infiltration of CD8+ T cells (Fig. 4). In mice receiving the combination therapy, CD8+ T cells were less apoptotic than those in other treatment groups, as determined by Annexin V-FITC staining (Fig. 4d, f). This intriguing finding may help to elucidate the mechanism of tumor destruction after combination therapy. In tumors overexpressing Fas, the mechanism of increased T-cell infiltration is still unclear. Radiation has complex effects on the tumor microenvironment and vessels. These effects facilitate homing of antigen-presenting effector cells to tumor [10, 33], possibly by radiation-induced inflammatory signals, changes in extracellular matrix proteins [1], or changes in expression of adhesion molecules by endothelial cells [14, 40]. We extended our study to analyze the induction of tumor-infiltrating antigen-specific CTLs. We examined the radiation sensitivity of antigen-specific CD8+ T cells by Annexin V-FITC staining. In mice receiving the combination therapy, apoptosis of T cells was the same as in mice receiving vaccine alone, indicating that the combination of RAIT and vaccine conferred a measure of resistance to radiation-induced destruction of T cells (Fig. 4). However, this finding is consistent with a preclinical study by Grayson et al. [13] which found that murine memory T cells were more resistant to apoptosis than naïve T cells after whole-body irradiation.
Radioimmunotherapy’s ability to target hematological malignancies more efficiently than solid tumors is due to a variety of factors, including a solid tumor’s limited vascular supply, heterogeneous uptake of antibody, and raised interstitial pressure. Several strategies have been employed to overcome these obstacles, including genetically engineered antibodies and pretargeting strategies [24]. The difficulty of finding a dose level that spares toxicity to normal tissues, especially the bone marrow, is another major obstacle to the therapeutic efficacy of RAIT. The dose of radiolabeled mAb (100 μCi) used in our study, employed in combination with vaccine therapy, eradicated established tumors in treated mice while causing no toxicity to normal tissue or abnormalities in serum chemistry. Further investigation of strategies to improve tumor targeting and expedite clearance are needed to further increase the efficacy of combination therapy.
Overall, we have shown that targeted tumor irradiation in combination with vaccine promotes a more effective antitumor response than either modality alone (Table 1). These findings may have implications for the design of future clinical trials.
Acknowledgments
The authors acknowledge the technical assistance of Marion Taylor and the editorial assistance of Bonnie L. Casey in the preparation of this manuscript.
Footnotes
This research was supported by the Intramural Research Program of the Center for Cancer Research, NCI, NIH.
References
- 1.Brooks PC, Roth JM, Lymberis SC, DeWyngaert K, Broek DFormenti SC. Ionizing radiation modulates the exposure of the HUIV26 cryptic epitope within collagen type IV during angiogenesis. Int J Radiat Oncol Biol Phys. 2002;54(4):1194–1201. doi: 10.1016/S0360-3016(02)03748-3. [DOI] [PubMed] [Google Scholar]
- 2.Butterfield LH, Ribas A, Dissette VB, Amarnani SN, Vu HT, Oseguera D, Wang HJ, Elashoff RM, McBride WH, Mukherji B, Cochran AJ, Glaspy JAEconomou JS. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin Cancer Res. 2003;9(3):998–1008. [PubMed] [Google Scholar]
- 3.Cavacini LA, Duval M, Eder JPPosner MR. Evidence of determinant spreading in the antibody responses to prostate cell surface antigens in patients immunized with prostate-specific antigen. Clin Cancer Res. 2002;8(2):368–373. [PubMed] [Google Scholar]
- 4.Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CNHodge JW. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170(12):6338–6347. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 5.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom JHodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64(12):4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain RS, Carroll MW, Bronte V, Hwu P, Warren S, Yang JC, Nishimura M, Moss B, Rosenberg SARestifo NP. Costimulation enhances the active immunotherapy effect of recombinant anticancer vaccines. Cancer Res. 1996;56(12):2832–2836. [PMC free article] [PubMed] [Google Scholar]
- 7.Demaria S, Bhardwaj N, McBride WHFormenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys. 2005;63(3):655–666. doi: 10.1016/j.ijrobp.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eades-Perner AM, van der Putten H, Hirth A, Thompson J, Neumaier M, von Kleist SZimmermann W. Mice transgenic for the human carcinoembryonic antigen gene maintain its spatiotemporal expression pattern. Cancer Res. 1994;54(15):4169–4176. [PubMed] [Google Scholar]
- 9.el-Shami K, Tirosh B, Bar-Haim E, Carmon L, Vadai E, Fridkin M, Feldman MEisenbach L. MHC class I-restricted epitope spreading in the context of tumor rejection following vaccination with a single immunodominant CTL epitope. Eur J Immunol. 1999;29(10):3295–3301. doi: 10.1002/(SICI)1521-4141(199910)29:10<3295::AID-IMMU3295>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 10.Ganss R, Ryschich E, Klar E, Arnold BHammerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62(5):1462–1470. [PubMed] [Google Scholar]
- 11.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom JHodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64(21):7985–7994. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 12.Goldenberg DM. Targeted therapy of cancer with radiolabeled antibodies. J Nucl Med. 2002;43(5):693–713. [PubMed] [Google Scholar]
- 13.Grayson JM, Harrington LE, Lanier JG, Wherry EJAhmed R. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J Immunol. 2002;169(7):3760–3770. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- 14.Hallahan D, Kuchibhotla JWyble C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res. 1996;56(22):5150–5155. [PubMed] [Google Scholar]
- 15.Hilburger Ryan Abrams M SI. Characterization of CD8+ cytotoxic T lymphocyte/tumor cell interactions reflecting recognition of an endogenously expressed murine wild-type p53 determinant. Cancer Immunol Immunother. 2001;49(11):603–612. doi: 10.1007/s002620000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodge JW, McLaughlin JP, Kantor JASchlom J. Diversified prime and boost protocols using recombinant vaccinia virus and recombinant non-replicating avian pox virus to enhance T-cell immunity and antitumor responses. Vaccine. 1997;15(6–7):759–768. doi: 10.1016/S0264-410X(96)00238-1. [DOI] [PubMed] [Google Scholar]
- 17.Hodge JW, Rad AN, Grosenbach DW, Sabzevari H, Yafal AG, Gritz LSchlom J. Enhanced activation of T cells by dendritic cells engineered to hyperexpress a triad of costimulatory molecules. J Natl Cancer Inst. 2000;92(15):1228–1239. doi: 10.1093/jnci/92.15.1228. [DOI] [PubMed] [Google Scholar]
- 18.Hodge JW, Sabzevari H, Yafal AG, Gritz L, Lorenz MGSchlom J. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59(22):5800–5807. [PubMed] [Google Scholar]
- 19.Horning SJ. Future directions in radioimmunotherapy for B-cell lymphoma. Semin Oncol. 2003;30(6 Suppl 17):29–34. doi: 10.1053/j.seminoncol.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Huang AY, Gulden PH, Woods AS, Thomas MC, Tong CD, Wang W, Engelhard VH, Pasternack G, Cotter R, Hunt D, Pardoll DMJaffee EM. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc Natl Acad Sci USA. 1996;93(18):9730–9735. doi: 10.1073/pnas.93.18.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irvine KR, Chamberlain RS, Shulman EP, Surman DR, Rosenberg SARestifo NP. Enhancing efficacy of recombinant anticancer vaccines with prime/boost regimens that use two different vectors. J Natl Cancer Inst. 1997;89(21):1595–1601. doi: 10.1093/jnci/89.21.1595. [DOI] [PubMed] [Google Scholar]
- 22.Kaminski MS, Estes J, Zasadny KR, Francis IR, Ross CW, Tuck M, Regan D, Fisher S, Gutierrez J, Kroll S, Stagg R, Tidmarsh GWahl RL. Radioimmunotherapy with iodine (131)I tositumomab for relapsed or refractory B-cell non-Hodgkin lymphoma: updated results and long-term follow-up of the University of Michigan experience. Blood. 2000;96(4):1259–1266. [PubMed] [Google Scholar]
- 23.Kass E, Panicali DL, Mazzara G, Schlom JGreiner JW. Granulocyte/macrophage-colony stimulating factor produced by recombinant avian poxviruses enriches the regional lymph nodes with antigen-presenting cells and acts as an immunoadjuvant. Cancer Res. 2001;61(1):206–214. [PubMed] [Google Scholar]
- 24.Koppe MJ, Bleichrodt RP, Oyen WJ, Boerman OC. Radioimmunotherapy and colorectal cancer. Br J Surg. 2005;92(3):264–276. doi: 10.1002/bjs.4936. [DOI] [PubMed] [Google Scholar]
- 25.Kudo-Saito C, Schlom JHodge JW. Induction of an antigen cascade by diversified subcutaneous/intratumoral vaccination is associated with antitumor responses. Clin Cancer Res. 2005;11(6):2416–2426. doi: 10.1158/1078-0432.CCR-04-1380. [DOI] [PubMed] [Google Scholar]
- 26.Kuroki M, Greiner JW, Simpson JF, Primus FJ, Guadagni FSchlom J. Serologic mapping and biochemical characterization of the carcinoembryonic antigen epitopes using fourteen distinct monoclonal antibodies. Int J Cancer. 1989;44(2):208–218. doi: 10.1002/ijc.2910440204. [DOI] [PubMed] [Google Scholar]
- 27.Lacabanne V, Viguier M, Guillet JGChoppin J. A wild-type p53 cytotoxic T cell epitope is presented by mouse hepatocarcinoma cells. Eur J Immunol. 1996;26(11):2635–2639. doi: 10.1002/eji.1830261114. [DOI] [PubMed] [Google Scholar]
- 28.Lee JK, Sayers TJ, Brooks AD, Back TC, Young HA, Komschlies KL, Wigginton JMWiltrout RH. IFN-gamma-dependent delay of in vivo tumor progression by Fas overexpression on murine renal cancer cells. J Immunol. 2000;164(1):231–239. doi: 10.4049/jimmunol.164.1.231. [DOI] [PubMed] [Google Scholar]
- 29.Markiewicz MA, Fallarino F, Ashikari AGajewski TF. Epitope spreading upon P815 tumor rejection triggered by vaccination with the single class I MHC-restricted peptide P1A. Int Immunol. 2001;13(5):625–632. doi: 10.1093/intimm/13.5.625. [DOI] [PubMed] [Google Scholar]
- 30.Marshall JL, Hoyer RJ, Toomey MA, Faraguna K, Chang P, Richmond E, Pedicano JE, Gehan E, Peck RA, Arlen P, Tsang KYSchlom J. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18(23):3964–3973. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- 31.Modrak DE, Gold DV, Goldenberg DMBlumenthal RD. Colonic tumor CEA, CSAp and MUC-1 expression following radioimmunotherapy or chemotherapy. Tumour Biol. 2003;24(1):32–39. doi: 10.1159/000070658. [DOI] [PubMed] [Google Scholar]
- 32.Muraro R, Wunderlich D, Thor A, Lundy J, Noguchi P, Cunningham RSchlom J. Definition by monoclonal antibodies of a repertoire of epitopes on carcinoembryonic antigen differentially expressed in human colon carcinomas versus normal adult tissues. Cancer Res. 1985;45(11 Pt 2):5769–5780. [PubMed] [Google Scholar]
- 33.Nikitina Gabrilovich EY DI. Combination of gamma-irradiation and dendritic cell administration induces a potent antitumor response in tumor-bearing mice: approach to treatment of advanced stage cancer. Int J Cancer. 2001;94(6):825–833. doi: 10.1002/1097-0215(20011215)94:6<825::AID-IJC1545>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 34.Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, Frelinger JARobinson BW. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170(10):4905–4913. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 35.Pilon SA, Kelly CWei WZ. Broadening of epitope recognition during immune rejection of ErbB-2-positive tumor prevents growth of ErbB-2-negative tumor. J Immunol. 2003;170(3):1202–1208. doi: 10.4049/jimmunol.170.3.1202. [DOI] [PubMed] [Google Scholar]
- 36.Press OW. Radioimmunotherapy for non-Hodgkin’s lymphomas: a historical perspective. Semin Oncol. 2003;30(2 Suppl 4):10–21. doi: 10.1053/sonc.2003.23798. [DOI] [PubMed] [Google Scholar]
- 37.Press OW, Unger JM, Braziel RM, Maloney DG, Miller TP, LeBlanc M, Gaynor ER, Rivkin SEFisher RI. A phase 2 trial of CHOP chemotherapy followed by tositumomab/iodine I 131 tositumomab for previously untreated follicular non-Hodgkin lymphoma: Southwest Oncology Group Protocol S9911. Blood. 2003;102(5):1606–1612. doi: 10.1182/blood-2003-01-0287. [DOI] [PubMed] [Google Scholar]
- 38.Robbins PF, Kantor JA, Salgaller M, Hand PH, Fernsten PDSchlom J. Transduction and expression of the human carcinoembryonic antigen gene in a murine colon carcinoma cell line. Cancer Res. 1991;51(14):3657–3662. [PubMed] [Google Scholar]
- 39.Rosato A, Santa SD, Zoso A, Giacomelli S, Milan G, Macino B, Tosello V, Dellabona P, Lollini PL, De Giovanni CZanovello P. The cytotoxic T-lymphocyte response against a poorly immunogenic mammary adenocarcinoma is focused on a single immunodominant class I epitope derived from the gp70 Env product of an endogenous retrovirus. Cancer Res. 2003;63(9):2158–2163. [PubMed] [Google Scholar]
- 40.Ryschich E, Harms W, Loeffler T, Eble M, Klar ESchmidt J. Radiation-induced leukocyte adhesion to endothelium in normal pancreas and in pancreatic carcinoma of the rat. Int J Cancer. 2003;105(4):506–511. doi: 10.1002/ijc.11073. [DOI] [PubMed] [Google Scholar]
- 41.Sharkey Goldenberg RM DM. Perspectives on cancer therapy with radiolabeled monoclonal antibodies. J Nucl Med. 2005;46(Suppl 1):115S–127S. [PubMed] [Google Scholar]
- 42.Tempero M, Leichner P, Baranowska-Kortylewicz J, Harrison K, Augustine S, Schlom J, Anderson J, Wisecarver JColcher D. High-dose therapy with 90Yttrium-labeled monoclonal antibody CC49: a phase I trial. Clin Cancer Res. 2000;6(8):3095–3102. [PubMed] [Google Scholar]
- 43.Tempero M, Leichner P, Dalrymple G, Harrison K, Augustine S, Schlam J, Anderson J, Wisecarver JColcher D. High-dose therapy with iodine-131-labeled monoclonal antibody CC49 in patients with gastrointestinal cancers: a phase I trial. J Clin Oncol. 1997;15(4):1518–1528. doi: 10.1200/JCO.1997.15.4.1518. [DOI] [PubMed] [Google Scholar]
- 44.Witzig TE, Gordon LI, Cabanillas F, Czuczman MS, Emmanouilides C, Joyce R, Pohlman BL, Bartlett NL, Wiseman GA, Padre N, Grillo-Lopez AJ, Multani PWhite CA. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20(10):2453–2463. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 45.Yang Perry-Lalley JC D. The envelope protein of an endogenous murine retrovirus is a tumor-associated T-cell antigen for multiple murine tumors. J Immunother. 2000;23(2):177–183. doi: 10.1097/00002371-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Zwaveling S, Vierboom MP, Ferreira Mota SC, Hendriks JA, Ooms ME, Sutmuller RP, Franken KL, Nijman HW, Ossendorp F, Van Der Burg SH, Offringa RMelief CJ. Antitumor efficacy of wild-type p53-specific CD4(+) T-helper cells. Cancer Res. 2002;62(21):6187–6193. [PubMed] [Google Scholar]