Abstract
Adoptive immunotherapy of cancer patients with cytolytic T lymphocytes (CTL) has been hampered by the inability of the CTL to home into tumors in vivo. Chemokines can attract T lymphocytes to the tumor site, as demonstrated in animal models, but the role of chemokines in T-lymphocyte trafficking toward human tumor cells is relatively unexplored. In the present study, the role of chemokines and their receptors in the migration of a colon carcinoma (CC) patient’s CTL toward autologous tumor cells has been studied in a novel three-dimensional organotypic CC culture. CTL migration was mediated by chemokine receptor CXCR3 expressed by the CTL and CXCL11 chemokine secreted by the tumor cells. Excess CXCL11 or antibodies to CXCL11 or CXCR3 inhibited migration of CTL to tumor cells. T cell and tumor cell analyses for CXCR3 and CXCL11 expression, respectively, in ten additional CC samples, may suggest their involvement in other CC patients. Our studies, together with previous studies indicating angiostatic activity of CXCL11, suggest that CXCL11 may be useful as an immunotherapeutic agent for cancer patients when transduced into tumor cells or fused to tumor antigen-specific Ab.
Keywords: CTL, Chemokines, Chemotaxis, Apoptosis, Tumor immunity
Introduction
Chemokines produced by tumor cells have been demonstrated to attract chemokine receptor-positive T lymphocytes into the tumor area, potentially leading to tumor growth inhibition in vitro and in vivo [13, 22, 48]. These studies have been largely confined to mouse tumor systems, whereas the role of human chemokines in human T-lymphocyte trafficking toward human tumor cells is relatively unexplored. In mouse systems, in vivo transduction of mouse tumor cells with chemokines led to T-cell infiltration into the tumor area and rejection of both transduced and non-transduced tumor cells [13, 22, 48]. CD4+ T-cell subsets have been implicated in tumor rejection induced by vaccination of mice with CCL19-transduced tumor cells [6], and CD8+ cytolytic T lymphocytes (CTL) were instrumental in tumor growth rejection in mice following intratumoral delivery of CCL20 via adenovirus vector [15] or injection of CCL16-expressing tumor cells [16]. Both CD4+ and CD8+ T cells were required for tumor growth inhibition to occur in mice injected intratumorally with CCL21 [41].
Adoptive immunotherapy of cancer patients with CTL has been hampered by the inability of the CTL to home to tumors in vivo [30]. Chemokines may be fused to tumor antigen (Ag)-specific Ab to attract adoptively transferred T cells to the tumor site.
To develop immunotherapeutic strategies for cancer patients based on chemokines and their receptors, similar to the approaches already successfully used in mice (see above), we must identify chemokines and their receptors involved in T-cell migration toward tumor cells. Such information is scarce in human tumor systems, including colon carcinoma (CC). In related studies Musha et al. demonstrated CCR5 and CXCR3 expressing infiltrating T cells in colorectal tumors, and the presence of CCL5 and CXCL10 in the same areas of tumor tissues [33]. In another study in colorectal cancer patients, the expression level of CX3CL1 in tumors correlated with the density of tumor infiltrating lymphocytes (TIL), and patients with strong CX3CL1 expression showed significantly better prognosis than those with weak expression [35].
Recently, we have shown that migration of CTL derived from melanoma patients toward autologous tumor cells is dependent on CXCR4 or CCR4 expressed by the CTL and CXCL12 or CCL2 produced by the melanoma cells, respectively [52, 53]. However, it was not known whether similar results would be obtained in patients with other malignancies.
In the present study, we have established a CTL line from the PBMC of a patient with CC and studied migration in a three-dimensional organotypic culture system (reconstruct) described previously [52, 53]. The CTL migrated toward and induced apoptosis of autologous CC cells. CTL migration was mediated by CXCR3 and CXCL11.
Materials and methods
Patients
Patient 020 (Caucasian female) underwent resection of a primary cecal adenocarcinoma, stage Dukes’ B. There was transmural tumor penetration through the bowel wall, but no evidence of lymph node or distant metastatic disease. This patient’s lymphocytes provided the basis for T-cell migration studies. Ten additional primary tumor tissues were obtained from CC patients of various disease stages (see Table 1). These patients’ lymphocytes were used in chemokine receptor analyses. Blood and tissue were obtained under informed consent approved by the Institutional Review Board of the Fox Chase Cancer Center, University of Pennsylvania, and The Wistar Institute.
Table 1.
CXCR3 expression by T-cell lines established from TIL of CC tissues in MLTC or reconstruct
| Patient | T cell | Expression of CXCR3a (% positive cells) | |||
|---|---|---|---|---|---|
| # | Disease stage (Dukes’) | Culture | Phenotype | Function | |
| 03123 | B | MLTC | CD4+ | CTL | 76.2 |
| 05193 | B | MLTC | CD4+ | CTL | 81.7 |
| 28712 | B | MLTC | CD4+ | CTL | 87.7 |
| 274405 | B | Reconstruct | CD8+ | NT | 42.4 |
| 280150 | C | MLTC | CD4+ | Th | 88.7 |
| 280291 | A | Reconstruct | CD4+ | Th | 43.7 |
| 280321 | A | MLTC | CD4+ | NT | 71.7 |
| 281412 | C | Reconstruct | CD4+ | NT | 85.0 |
| 281638 | B | Reconstruct | CD4+/CD8+ | NT | 30.4 |
| 1003485 | A | Reconstruct | CD4+/CD8+ | NT | 47.0 |
NT not tested
aCXCR3 expression was determined by FACS analysis
Cell lines
Colon carcinoma cell lines were established from tumor tissues as previously described [23]. CC cells were maintained in 75% MCDB 201-L15 medium (Sigma-Aldrich, St Louis, MO, USA) and 25% DMEM (GIBCO-Invitrogen, Carlsbad, CA, USA) supplemented with 2% FBS. HLA types of WC020 CC cells were A30, B13, DR13, 15, and DQ06. Fibroblast cell line FCFB/1 was established from fetal colon and maintained in DMEM (GIBCO-Invitrogen) supplemented with 10% FBS. NK cell target K562 (human erythroleukemia cell line) and LAK cell target Daudi (human lymphoblastoid cell line) were obtained from ATCC (Rockville, MD, USA). Both lymphoid cell lines were maintained in RPMI 1640 medium (GIBCO-Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS.
Reagents
The following monoclonal antibodies (mAbs) were used: HLA-class I-specific mAb W6/32 and HLA-class II-specific mAb B33.1 and D1.B6 (obtained from Dr B. Perussia, Thomas Jefferson University, and Dr G. Trinchieri, The Wistar Institute); mAb Nok-1 to Fas ligand (PharMingen, Los Angeles, CA, USA); mAb CH-11 to CD95 and anti-CD11a mAb (Immunotech, Westbrook, ME, USA); fluoresceinated or phycoerythrin-labeled anti-CD:4, 8, 25, 29, 40, 40L, 44, 49a, 49b, 80, and 54 (PharMingen, Los Angeles, CA, USA); anti-CXCL-11 mAb; anti-human CCR:1, 2, 3, 5, 6, 7, 9, and CXCR:1, 2, 3, 4, 5, and 6 mAbs (R&D Systems, Minneapolis, MN, USA); anti-human CCR:4, 8, and 10 mAbs (Imgenex, San Diego, CA, USA); anti-CCR11 and -CX3CR1 polyclonal Abs (Abcam, Cambridge, MA, USA); fluoresceinated goat anti-mouse IgG (Molecular Probes, Eugene, OR, USA). Recombinant human CXCL11 was purchased from R&D Systems (Minneapolis, MN, USA).
Generation of anti-CC CTL lines
CTL020 cells were obtained from co-culture of PBMC (obtained 7 months after surgery; 105 cells/well of 96-well round-bottom microtiter plates) of CC patient 020 with irradiated (30,000 rads, Cs source) autologous CC cells WC020 (105 cells/well) in T-cell medium containing RPMI 1640 medium, 10% human AB serum (Gemini, Calabasas, CA, USA), 10 mM HEPES (Sigma-Aldrich, St Louis, MO, USA), l-arginine (116 mg/l), l-asparagine (36 mg/l), l-glutamine (216 mg/l; all from GIBCO-Invitrogen, Carlsbad, CA, USA) and 2-mercaptoethanol (5 × 10−5 M; Sigma, Aldrict, St Louis, MO, USA), in a humidified 5% CO2 incubator. Cultures were stimulated with irradiated autologous tumor cells every 2 weeks in T-cell medium containing partially purified IL-2 (20 U/ml; ABI, Columbia, MA, USA), and characterized after 9–12 weeks in culture. Additional T-cell lines were established from TIL of fresh CC tissues. Tumor was minced and cultured in MLTC or in organotypic cultures (reconstructs). MLTC was initiated by culturing minced tumor in 24-well plates using 2 ml/well T-cell medium. Reconstructs were initiated by mixing minced tumor with collagen matrix [1.65 ml of 10 × Eagle’s MEM, 150 μl of 200 mM l-glutamine, 1.8 ml of heat inactivated human AB serum, 510 μl of 7.5% NaHCO3 and 13.8 ml of bovine collagen type I (1.17 mg/ml; Organogenesis, Canton, MA, USA)], and plating 1 ml of the mixture and 1 ml T-cell medium into wells of a 24-well plate. Natural IL-2 was added to MLTC and reconstruct cultures weekly, starting on day 7. After 7–8 weeks, cultured cells were isolated from MLTC or reconstruct and stimulated every 2 weeks with cultured, irradiated (10,000 rads, Cs source) tumor cells or uncultured tumor cells derived from cryopreserved tissues. T-cell function and CXCR3 expression was tested after 8–12 weeks in culture.
Chemokine determination by RT-PCR or ELISA
mRNA was extracted from CC cells (5 × 106) using Fast Track 2.0 mRNA isolation kit (Invitrogen, Carlsbad, CA, USA). The primers used were 5′-TGT AGG GCG ACG GTT TTA-3′ and 5′-TCC ACC ACA ACA TGC AG-3′ for CCL25; 5′-ATG GCC CTG CTA CTG GCC CTC AGC CTG-3′ and 5′-TTA ACT GCT GCG GCG CTT CAT CTT GGC-3′ for CCL19; 5′-CAT GCT GGT GAG CCA AGC AGT TTG AA-3′ and 5′-CAC TTC TGT GGG GTG TTG GGG ACA AG-3′ for CXCL9; 5′-CGA TGC CTA AAT CCC AAA TCG AAG CA-3′ and 5′-AAT TGC TGG ACT CCT TTG GGC AGT GG-3′ for CXCL11; 5′-ATG AAC GCC AAG GTC GTG GTC-3′ and 5′-TGG CTG TTG TGC TTA CTT GTT T-3′ for CXCL12; 5′-TCT CTC CAG GCC ACG GTA TTC-3′ and 5′ ACC ATT TGG CAC GAG GAT TCA C-3′ for CXCL13. PCR reactions were performed for 35 cycles (94°C, 45 s; 60°C for CCL19, CXCL9, and CXCL11; 56°C for CXCL12, 55°C for CCL25, 45 s; 72°C, 45 s) using the SuperScript One-Step RT-PCR kit (Invitrogen, Carlsbad, CA, USA). CCL21 primers were purchased from Biosource (Camarillo, CA, USA). PCR for CCL21 involved a 1-min 30-s denaturation step at 94°C, followed by 35 cycles of 30 s each at 94°C, 45 s at 60°C, and 45 s at 72°C, with a final 7-min extension at 72°C. CXCL10 primers were purchased from R&D Systems (Minneapolis, MN, USA), and PCR involved a 45-s denaturation step at 94°C, followed by 35 cycles of 45 s each at 94°C, 45 s at 55°C, and 45 s at 72°C, with a final 7-min extension at 72°C. All PCR products were analyzed on 10% novex-TBE gel (Invitrogen, Carlsbad, CA, USA). Supernatants obtained from CC cells on day 6 of culture were tested for the presence of CXCL9, CXCL11, CXCL12, CCL21, and CCL25 using DuoSet ELISA kits (R&D, Minneapolis, MN, USA).
Cytokine measurements
For cytokine production, cultured CTL were washed twice with serum-containing T-cell medium (see Sect. “Generation of anti-CC CTL lines”), incubated in serum-containing medium (without stimulants and IL-2) for 8 h at 37°C in a humidified 5% CO2 incubator and washed once. CTL (104 cells/well of 96-well round-bottom microtiter plates) were stimulated with different numbers (2.5 × 103, 5 × 103, or 104 cells/well) of irradiated autologous tumor cells and 20 U/ml recombinant human IL-2 (gift from Biological Resources Branch, NCI-Frederick Cancer Research and Development Center, Frederick, MD, USA) in 96-well microtiter plates. Supernatants obtained from cultured CTL after 2 days were tested for the presence of GM-CSF, TNF-α, and IFN-γ, and supernatants obtained from cultured CTL after 4 days were tested for the presence of IL-4. All cytokine determinations were performed using ELISA kits (Endogen, Rockford, IL, USA).
Cytotoxicity assay
Labeled targets [5 μCi of 51Cr, as Na2CrO4 (Perkin Elmer, Boston, MA, USA), per 5 × 103 cells] were mixed in 96-well round-bottom microtiter plates with effector cells at various E:T and incubated at 37°C for 10 h. Supernatants were harvested and tested for 51Cr release (experimental release). For maximal release, target cells were treated with 10 N HCl. Spontaneous release of radioactivity by target cells was determined in the absence of effector cells. The percentage of cytotoxicity was determined by the following formula:
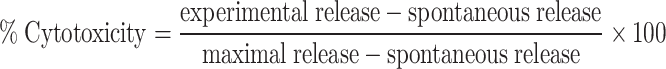 |
1 |
To determine HLA restriction of the CTL, tumor targets were incubated with saturating concentrations of anti-HLA class I mAb W6/32 (IgG2a), or anti-HLA class II mAb B33.1 (IgG2a) at a final concentration of 0.45 mg/ml. Isotype-matched control mAb was used at similar concentrations. All incubations were performed for 1 h at room temperature. Excess blocking mAb was removed and cytotoxicity assay was performed as described above. The percentage of CTL lysis inhibition was determined using the following formula:
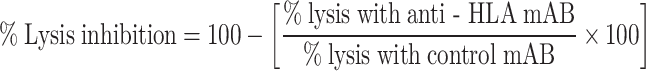 |
2 |
Phenotyping of tumor cells and T cells
Cultured T cells were incubated with saturating concentrations (5 μg/ml) of fluoresceinated or phycoerythrin-labeled mAbs detecting human lymphocyte and tumor markers (Tables 2, 3) in RPMI 1640 medium supplemented with 5% human AB serum for 1 h at 4°C. Binding of the mAbs was analyzed in the cytofluorograph. All values given in Sect. ”Results” are corrected for irrelevant, isotype-matched control Ab binding.
T-cell migration in organotypic CC culture (reconstruct)
Cultures were initiated by mixing 4.5 × 104 FCFB/1 cells with collagen matrix (see at Sect. ”Generation of anti-CC CTL lines”), and plating 450 μl of the mixture into wells of a 24-well plate. After 24 h, WC020 CC cells (1 × 105) were seeded on top of the collagen matrix. After a further 24 h, a separating layer of fibroblasts in collagen gel (100 μl, 500 μm) was added on top of the CC cells. Fibroblast–collagen overlay containing CTL was prepared by mixing 2.5 × 104 fibroblasts FCFB/1 and 3 × 105 CTL with 250 μl collagen matrix per well. In some cultures CC cells were stained with CellTracker Blue CMAC (Invitrogen, Carlsbad, CA, USA) and CTL were pre-stained with CFDA-Green (Invitrogen, Carlsbad, CA, USA). For control reconstruct, PHA blasts prepared from the PBMC of the same patient by incubating them with 5 μg/ml of PHA were used. Reconstructs were incubated in medium (50% DMEM, 50% CC medium supplemented with 2% human AB serum). Three or 4 days after the addition of T cells, reconstructs were fixed in 10% buffered formalin for 4 h at room temperature, and processed for histological evaluation. The percentage of apoptotic tumor cells was determined by counting apoptotic nuclei (based on nuclear morphology) and intact tumor cells in sections stained with H&E.
This method has been superior to other methods used for staining of apoptotic cells, such as caspase-3, TUNEL, and cytokeratin staining [11].
Blocking of T-cell migration in reconstruct
The bottom layer of reconstruct contained 4.5 × 104 fibroblasts in 450 μl type I collagen gel. After 24 h, WC020 CC cells (1 × 105) were seeded on top of the collagen matrix. After 48 h, a separating layer of fibroblasts in collagen gel (100 μl, 500 μm) was added on top of CC cells. After gel formation, anti-chemokine or chemokine receptor Abs, anti-CXCL11, anti-CXCR3, anti-CXCR4, anti-CCR7, and anti-CCR9 Ab (10 μg/ml each Ab), or isotype-matched control Ab were added, followed by the top layer containing CTL (3 × 105) mixed with 2.5 × 104 fibroblasts and type I collagen gel. To evaluate whether excess CXCL11 can block migration of T cells, the chemokine (50 ng/ml) was added into the medium on top of the T-cell layer. The percentage of apoptotic tumor cells in the presence and absence of inhibitor was determined and the percentage of inhibition of apoptosis by Abs or chemokines was calculated.
T helper assay
Helper activity of T cells was determined by measuring proliferative response of allogeneic PBMC in the presence of T cells. T cells (1 × 105/ml) were added to the top chamber of Transwell plates (Costar No. 3413; 0.4 μm pore size), and allogeneic PBMC (1 × 105/ml) to the bottom chamber. As negative control, medium or an EBV-transformed B cell line (EBVB013) was added to the top chamber. After 4 days of culture the top chamber was removed, and proliferative responses of the allogeneic PBMC in the bottom chamber were determined by 3H-thymidine (Amersham, Buckinghamshire, UK) incorporation assay. All determinations were performed in triplicate.
Statistical analyses
Differences between experimental and control values were analyzed for significance by two-sample Student’s t-test.
Results
Functional characteristics of CTL020 cells in MLTC
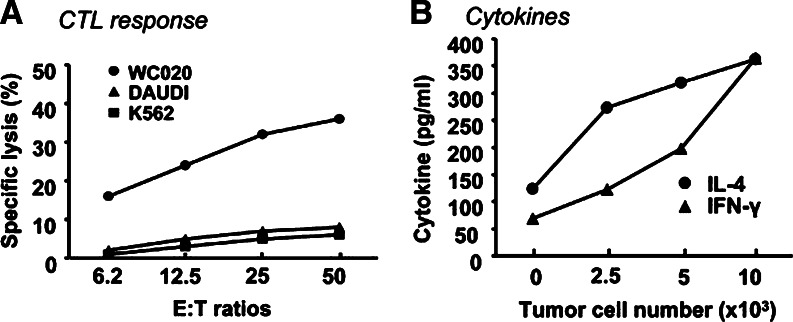
The phenotype of the CTL020 cell line changed from 66.8% CD4+ and 49% CD8+ at week 7, to 88.7% CD4+ and 5.8% CD8+ at week 12, relative to the start of PBMC and CC tumor cell co-culture (data not shown). CTL020 cells lysed autologous WC020 CC cells in a 10-h 51Cr-release assay and lysis was dependent on the E:T cell ratio. NK target cells K562 and LAK target cells Daudi were not significantly lysed in the same assay (Fig. 1a). Lysis of CC cells by the CTL was significantly inhibited by mAbs to HLA class I as well as HLA class II, while isotype-matched control Ab had no effect on cytotoxicity (Table 2). The demonstration of dual HLA restriction of the CTL is not surprising since uncloned CTL020 cells presumably comprise cells with different specificities. Thus, the uncloned CTL020 cell population most likely contains CD8+ HLA class I-restricted, and CD4+, HLA class II-restricted CTL. IFN-γ, and IL-4 production by CTL020 cells is dependent on the presence of tumor cells (Fig. 1b). CTL020 cells do not secrete measurable amounts of GM-CSF, or TNF-α. The T cells did not display helper activity in an assay which measures proliferation of allogeneic PBMC in the presence of the T cells (not shown).
Fig. 1.
Functions of CTL020 cells in MLTC. CTL020 was generated in MLTC by stimulating PBMC of CC patient 020 with irradiated autologous WC020 CC cells. Cultures were restimulated every 2 weeks with irradiated autologous CC cells and 20 U/ml of natural IL-2. a CTL responses. CTL020 cells were stimulated for 4 days with irradiated autologous tumor cells and IL-2. CTL lysis of 51Cr-labeled autologous CC cells and control target cells [allogeneic Daudi (LAK target) and K562 (NK target)] was determined in 10 h 51Cr-release assay at various E:T ratios. b Cytokine production. CTL020 cells were incubated with various numbers of irradiated WC020 CC cells or without tumor cells in the presence of recombinant IL-2. IFN-γ and IL-4 in supernatants obtained from cultured CTL after 2 and 4 days, respectively, were measured by ELISA. All values obtained from cultures stimulated with tumor cells are significantly (P < 0.01) different from controls (CTL020 cells incubated without tumor cells)
Table 2.
HLA restriction of CTL020
| Antibody treatment | % Specific lysisa | % Inhibition | Significance (P value) |
|---|---|---|---|
| Isotype-matched control | 12.3 | – | |
| Anti-HLA class I | 3.7 | 69.7 | 0.003 |
| Anti-HLA class II | 6.9 | 44 | 0.028 |
aE:T = 12.5
Functional characteristics of CTL020 cells in reconstruct
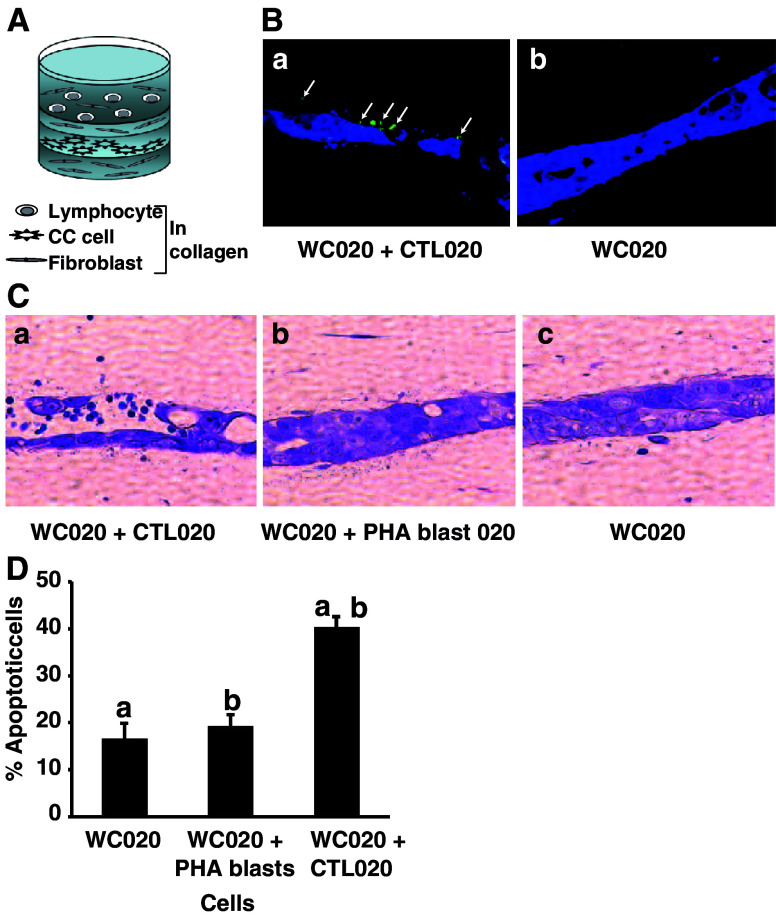
CTL020 cells labeled with CFDA-Green migrated from the top layer of collagen and fibroblasts through a separating layer of collagen and fibroblasts toward WC020 tumor cells labeled with CMAC-Blue (Fig. 2A, Ba), whereas no T cells were found in cultures with tumor cells only (Fig. 2Bb). Tumor cell apoptosis was determined microscopically in H&E-stained cultures (Fig. 2C) and by enumerating apoptotic tumor cells (Fig. 2D). The CTL-induced significant apoptosis in the autologous CC cells, as compared to reconstructs with tumor cells alone or tumor cells plus PHA blasts (P < 0.0001, Fig. 2D). Although a small proportion of PHA blasts migrated toward tumor cells (not shown), the PHA blasts did not induce apoptosis in the tumor cells (Fig. 2C, D). CTL020 cells did not mediate significant apoptosis of allogeneic HLA-mismatched 05193 or 281638 CC cell lines (Table 3).
Fig. 2.
CTL020 cell migration toward autologous WC020 CC cells in reconstruct. A Schema of reconstruct. B CTL020 cell migration. The bottom layer of reconstructs contained 4.5 × 104 fibroblasts in 450 μl type I collagen gel. After 24 h, CC cells (1 × 105) were seeded on top of the bottom layer. After 24 h, CC cells were stained with CellTracker Blue CMAC. A separating layer of fibroblasts in collagen gel (500 μm) was then placed on top of the CC cell layer, followed by addition of a top layer containing 3 × 105 pre-stained (CFDA-Green, Invitrogen) CTL020, mixed with 2.5 × 104 fibroblasts and type I collagen gel (a); control cultures were prepared without lymphocytes (b). Reconstructs were harvested on day 6 (3 days after adding T cells), fixed in buffered formalin, and embedded in paraffin. Sections were photographed in the Nikon fluorescence microscope using appropriate filters. Magnification ×400 . Note the presence of CFDA-Green-labeled CTL in the tumor cell layer (a). C Induction of apoptosis in WC020 cells by CTL020—qualitative analysis. The reconstructs were harvested on day 7, fixed in buffered formalin, embedded in paraffin, and stained with H&E. Magnification ×400 . Note the presence of apoptotic tumor cells in cultures with WC020 plus CTL020 cells (a), but not in control cultures with WC020 plus PHA blasts (b), or WC020 only (c). D Induction of apoptosis in WC020 cells by CTL020—quantitative analysis. The percentage of apoptotic tumor cells was determined by counting apoptotic nuclei, based on nuclear morphology, and intact tumor cells in 20 fields of sections stained with H&E. Data represent means + SD of 20 fields. Values with the same symbol are significantly different from each other (P < 0.0001)
Table 3.
Apotosis induction by CTL020 cells in reconstructs with allogeneic CRC cells
| Tumor cells | Apoptotic tumor cells (%) | ||
|---|---|---|---|
| Code | Production of chemokines binding to CXCR3 | CTL020 | No CTL |
| 281638 | CXCL11 | 10.1 ± 2.7 | 10.0 ± 5 |
| 05193 | CXCL9 | 6.09 ± 3 | 5.6 ± 3 |
| WC020 | CXCL11 | 25.2 ± 5a | 10.5 ± 2.1a |
aSignificant difference (P < 0.001)
Phenotypic characteristics of CTL020 and WC020 CC cells
CTL020 and WC020 CC cells were phenotyped with special emphasis on molecules that might be involved in the interactions of these cells with each other and components of the reconstruct (Table 4). CTL020 cell line is predominantly of CD4 phenotype (88.7% of cells positive). The cells also express CD95 (FAS) as well as adhesion and co-stimulatory molecules (Table 4). Expression of α2 (CD49b) and β1 (CD29) integrins by the T cells (Table 4) is important for T-cell interaction with collagen in the reconstruct, which results in T-cell activation [14, 37]. LFA-1a (CD11a), ICAM-1 (CD54), and CD44 expressed by the CTL (Table 4) facilitate interaction of the lymphocytes with fibroblasts in the reconstruct [1, 32]. This interaction results in the activation of both lymphocytes and fibroblasts through secretion of growth and survival factors, cytokines, and fibronectin [1, 10, 28, 32].
Table 4.
Phenotypic and functional markers of anti-CC CTL020 cells and autologous WC020 tumor cell line
| Parameter investigateda | Cell lines (% cells positive) | |
|---|---|---|
| CC WC020 | CTL020 | |
| HLA class I | 99.6 | 99.5 |
| HLA class II | 51.7 | 92.5 |
| CD4 | NA | 88.7 |
| CD8 | NA | 5.8 |
| CD25 | 1.1 | 33.6 |
| CD40 | 0.3 | 0.3 |
| CD40L | 3.2 | 6.9 |
| CD44 | 94.9 | 66.7 |
| CD80 (B7-1) | 1.2 | 52.8 |
| CD49a?? (α1 ?integrin) | 57.8 | 22.9 |
| CD49b?? (α2 ?integrin) | 93.1 | 70.7 |
| CD29?? (β1 ?integrin) | 83.8 | 96.6 |
| CD95 (FAS) | 78.0 | 90.2 |
| CD95L (FASL) | 1.4 | 1.6 |
| CD54 (ICAM-1) | 20.0 | 71.5 |
| CD11a (LFA-1a) | 1.5 | 91.8 |
NA not applicable
aAll markers were determined by FACS analysis
WC020 CC cells expressed both HLA class I and II molecules, FAS, ICAM-1, and various integrins. Expression of α2 and β1 integrins by the CC cells indicates that these cells potentially can bind to collagen [27]. The cells did not express FAS ligand and B7-1 (Table 2). ICAM-1 on the CC cells potentially interacts with LFA-1a on the CTL, which may result in T-cell stimulation [3].
Thus, several phenotypic markers are expressed by CTL020 and WC020 CC cells, which may facilitate interactions between these cells and between the lymphocytes or tumor cells and collagen or fibroblasts in the reconstruct, leading to activation of T cells as well as T-cell migration toward tumor cells.
Chemokine and chemokine receptor involved in CTL020 cell migration toward WC020 cells
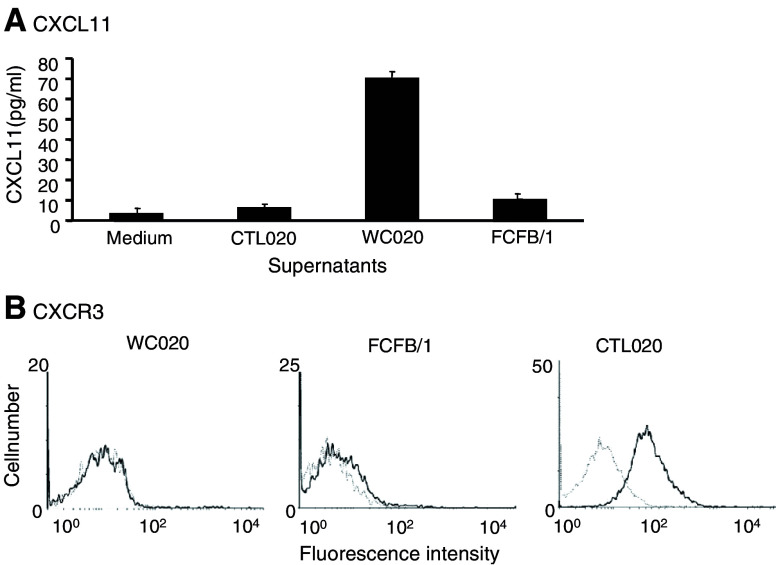
CTL020 cells expressed the chemokine receptors CCR7, CCR9, CXCR3, and CXCR4 (Table 3). For each chemokine receptor, with the exception of CXCR4, the corresponding chemokine(s) was expressed by WC020 CC cells, as determined by RT-PCR (Table 5). However, chemokine protein expression by the tumor cells could only be shown for CXCL11 (Fig. 3a). No significant amounts of CXCL11 were produced by FCFB/1 fibroblasts used in the reconstruct or CTL020 cells (Fig. 3a). CXCL11 produced by WC020 CC cells most likely binds to CXCR3 on CTL020 cells [9]. CXCR3, while significantly expressed by CTL020 cells (48.7% of cells positive), showed no significant expression by WC020 CC cells or FCFB/1 fibroblasts (Fig. 3b).
Table 5.
Chemokine receptors expressed by CTL020 cells, and chemokines produced by WC020 CC cells
| Chemokine receptors expressed by CTL020a | Chemokines | |
|---|---|---|
| Known to bind to receptor | Expressed by WC020b | |
| CCR7 | CCL21 | +/− |
| CCL19 | − | |
| CCR9 | CCL25 | +/− |
| CXCR3 | CXCL9 | +/− |
| CXCL10 | − | |
| CXCL11 | + | |
| CXCL13 | − | |
| CXCR4 | CXCL12 | − |
aThe following receptors were not expressed by CTL020: CCR1, CCR2, CCR3, CCR4, CCR5, CCR6, CCR8, CCR10, CCR11, CXCR1, CXCR2, CXCR5, CXCR6, CX3CR1
bChemokine expression was detected by RT-PCR. CXCL11 protein was detected in WC020 supernatant by ELISA (see Fig. 3a). CCL21, CCL25, and CXCL9 mRNA were detected in WC020 cells by RT-PCR, but the protein was not expressed as determined by ELISA
Fig. 3.
Autologous CC cells WC020 produce CXCL11, and CTL020 cells express the corresponding receptor CXCR3. a Production of CXCL11. CTL020 cells were cultured with irradiated WC020 tumor cells in T-cell medium for 4 days. WC020 CC cells were cultured in CC medium; after 2 days of culture the medium was replaced and supernatants were collected 4 days later. FCFB/1 fibroblast cells were cultured in DMEM for 4 days. CXCL11 production in supernatants obtained from various cells was measured in ELISA. b Expression of CXCR3. Cultured cells of CTL020, FCFB/1, and WC020 were incubated with saturating concentration (5 μg/ml) of anti-CXCR3 MAB (solid line) or mouse IgG control (dotted line) in RPMI-1640 with 5% FBS for 1 h at 4°C. After washing, fluoresceinated goat anti-mouse IgG Ab was added. The expression of CXCR3 was detected by flow cytometry
We evaluated a possible role of CXCL11 produced by WC020 cells and CXCR3 present on CTL020 cells in the migration of the T cells toward CC cells in the reconstruct. T-cell migration was measured as a function of tumor cell apoptosis and not absolute numbers of T cells at the tumor cell layer, since T cells may themselves apoptose after inducing tumor cell apoptosis and one T cell may induce apoptosis in several tumor cells. Only apoptotic tumor cells, and not T cells, were counted. Apoptotic tumor cells could be easily distinguished from apoptotic T cells based on size difference. However, evaluation of apoptotic tumor cells does not allow us to distinguish between T cells with high migratory and low lytic activity and T cells with low migratory and high lytic activity. Blocking of CXCR3, but not CCR7, CCR9, or CXCR4 on CTL020 cells with antibodies significantly inhibited tumor cell apoptosis (Table 6). We therefore tested whether the ligand of CXCR3 expressed by WC020 tumor cells (CXCL11) was involved in T-cell migration and tumor cell apoptosis. Excess recombinant CXCL11 (50 ng/ml), when added on top of the T-cell layer, significantly (P < 0.01) inhibited CC cell apoptosis induction by CTL020 cells (Table 6). Anti-CXCL11 Ab when added on top of the separating layer, significantly (P < 0.01) inhibited tumor cell apoptosis (Table 6). In all migration blocking experiments shown in Table 6, blocking of tumor cell apoptosis was incomplete, suggesting that factors other than CXCL11 might attract CTL to the tumor site. Alternatively, Abs used for CTL migration blocking may have low affinity. CTL020 cells did not induce apoptosis of allogeneic HLA-mismatched 05193 or 281638 CC cells producing ligands of CXCR3 (CXCL9 or CXCL11, respectively) (Table 3).
Table 6.
Blocking of CTL020 cell migration in reconstruct
| Treatmenta | Total number of tumor cells mean ± SD/field (10 fields) | Number of apoptotic tumor cells mean ± SD/field (10 fields) | Percentage of apoptotic tumor cells mean ± SD/field (10 fields) | Percentage of tumor cell apoptosis inhibition |
|---|---|---|---|---|
| WC020 | 69.9 ± 15.8 | 11.1 ± 3.4 | 15.8 ± 2.5 | NA |
| WC020 + CTL020 | 58.9 ± 7.5 | 23.9 ± 4.2 | 40.8 ± 6.9b | 0.0 |
| WC020 + CTL020 + control IgG | 49.3 ± 9.1 | 18.5 ± 4.0 | 38.9 ± 6.9c,d | 4.7 |
| WC020 + CTL020 + α-CXCR3 Ab | 72.7 ± 7.0 | 18.0 ± 4.3 | 24.9 ± 6.2c | 39.0 |
| WC020 + CTL020 + α-CCR7 Ab | 59.7 ± 8.2 | 24.0 ± 5.2 | 39.8 ± 4.6 | 2.5 |
| WC020 + CTL020 + α-CCR9 Ab | 59.9 ± 11.1 | 21.7 ± 4.8 | 36.4 ± 6 | 10.8 |
| WC020 + CTL020 + α-CXCR4 Ab | 60.4 ± 5.1 | 23.1 ± 5.2 | 38.7 ± 8.7 | 5.2 |
| WC020 + CTL020 + CXCL11 | 69.4 ± 6.8 | 20.1 ± 2.5 | 29.1 ± 4.8b | 28.7 |
| WC020 + CTL020 + α-CXCL11 Ab | 74.8 ± 6.0 | 17.4 ± 5.7 | 23.4 ± 8.3d | 42.7 |
Ab antibody, NA not applicable
aReconstructs consisted of a bottom layer of collagen and fibroblasts, followed by a tumor cell layer and a separating layer of collagen and fibroblasts. Anti-chemokine or anti-chemokine receptor or control Abs were added at 10 μg/ml, followed by a top layer containing CTL mixed with fibroblasts and collagen (E:T = 3:1). Separate cultures received CXCL11 or CXCL9 (50 ng/ml) on top of the T-cell layer. Percentage of apoptotic tumor cells in 6-day cultures was determined
b–dValues with same letter differ significantly from each other (P < 0.01, Student’s two-sample t-test)
FCFB/1 fibroblasts used in the reconstruct did not produce CXCL11 (Fig. 3) as detected by ELISA, and therefore they are not expected to contribute to T-cell migration. However, CXCL12 (the ligand for CXCR4 which is expressed by CTL020), was produced by the fibroblasts (1,991 pg/ml in culture supernatants). Fibroblasts are evenly distributed over all layers in the reconstruct, and thus they are not expected to provide a chemokine gradient and contribute to T-cell migration. Furthermore, anti-CXCR4 Ab did not inhibit tumor cell apoptosis (Table 6). These data suggest that CXCL12 produced by fibroblasts had no effect on T-cell migration.
CXCR3 and CXCL11 expression by T cells and tumor cell lines, respectively, derived from additional CC patients
Since CXCR3 and CXCL11 mediated CTL020 cell migration toward tumor cells followed by tumor cell apoptosis (see above), we investigated the distribution of CXCR3 and CXCL11 in T cells and tumor cell lines, respectively, recently established from specimens of additional CC patients. Expression of the receptor and its ligand by the cells of additional CC patients would suggest that involvement of the receptor/ligand in T-cell migration toward tumor cells may not be a unique observation made in a single CC patient, but may be found in a larger population of these patients. Tumor-reactive T-cell lines derived from TIL of ten additional CC patients all expressed CXCR3 (Table 1). Furthermore, two of the three established CC cell lines produced CXCL11 (not shown).
Discussion
We have described here a CTL line (CTL020) that lysed autologous WC020 CC cells in culture. Only a few examples of CC-specific CTL have been described in cancer patients [17, 23, 42].
We have shown here that CTL020 cells migrate through a 500-μm collagen/fibroblast separating layer toward tumor cells, resulting in tumor cell apoptosis. We have also shown that migration is dependent on CXCL11 produced by the tumor cells and CXCR3 expressed by the T cells. To our knowledge, this is the first demonstration of chemokine dependency of human CTL migration toward CC cells. In addition to CXCL11 (this study), CC cells can produce CXCL8 [7], CXCL1, CXCL3, CCL2, CCL5 [51], CXCL9, and CXCL10 [20].
We have developed a novel three-dimensional culture system in which the migration of leukocytes toward tumor cells and the factors that influence leukocyte migration can be studied under physiological conditions [52]. In the reconstruct, human CC is recapitulated in vitro using a mixture of collagen and fibroblasts (lattices or matrices). Ag-elicited T cells are stimulated by collagen, most likely through the interaction of α2 (CD49b) and β1 (CD29) integrins on T cells with collagen [14, 37]. Since activated fibroblasts play an important role in the activation of T lymphocytes, they were included in the reconstruct. T lymphocytes bind to fibroblasts via LFA-1a (CD11a), ICAM-1 (CD54), and CD44. The adhesive interaction stimulates fibroblasts to secrete inflammatory cytokines such as IL-1, IL-6 and IL-7 [1, 32], and fibronectin [28]. The main biological role of IL-1 is the stimulation of T cells to express IL-2 receptor and secrete IL-2 [44]. IL-6 and IL-7 are T-cell survival factors [38], and fibronectin stimulates predominantly resting lymphocytes [28].
Other investigators have used collagen matrices to study interaction of leukocytes with tumor cells, but they have not demonstrated CTL migration resulting in tumor cell apoptosis in a culture system similar to the reconstruct shown here. Thus, Wei et al. [49] have demonstrated inhibition of mouse tumor cell growth by peptide-specific CTL in a three-dimensional collagen matrix. However, in contrast to our studies, T cells were not shown to migrate in that culture system. In an organotypic culture of human papilloma virus-transformed keratinocytes, allogeneic lymphocytes induced apoptosis in the tumor cells [24]. This effect most likely was induced by NK, but not T cells. In another study, a two-layer collagen matrix culture model that consisted of a collagen gel containing human DCs as the lower layer and a collagen gel containing necrotic human tumor cells and T cells as the upper layer was used to demonstrate DC migration toward tumor and T cells and tumor Ag-presentation to T cells by the DCs [46].
In the present study, CXCL11 produced by CC cells attracted CTL through binding to CXCR3 on the T cells. This was demonstrated by blocking CTL migration toward tumor cells with high concentrations of CXCL11, applied on top of the T-cell layer, or antibodies to CXCR3 or CXCL11, each applied on top of the separating collagen/fibroblast layer. Each of the compounds inhibited CTL migration, most likely by blocking CXCR3 on the CTL.
CXCL11 binds to the receptor CXCR3 as does CXCL9, CXCL10, and CXCL13 [9, 25, 26]. Of the four CXCR3 ligands, three (CXCL9, CXCL10, and CXCL11) show close similarities in structure and function [34], are induced primarily by IFN-γ, and are produced by macrophages and some other cell types [9]. However, CXCL11 shows higher affinity for CXCR3 than either CXCL9 or CXCL10, and consequently is a more potent activator of the receptor [8].
CXCL11 mRNA is constitutively expressed by astrocytes, monocytes, SV-A3 microglial cell line, and in the pancreas, lung, and liver. Stimulation of these cells with IFN-γ results in increased CXCL11 production [9]. CXCL11 expression (along with CXCL9 and CXCL10) was demonstrated at inflammatory sites in human bronchoalveolar lavage fluid of patients with peribronchial inflammation after lung transplantation; keratinocytes in a variety of skin disorders, including allergic contact dermatitis and mycosis fungoides; and human atheroma-associated endothelial cells and macrophages [2, 12, 29, 47]. CXCL11 efficiently attracted activated T cells, NK cells, and a subset of B lymphocytes in in vitro chemotaxis assays [9, 19, 39]. CXCL11 is displayed on the surface of human endothelial cells, and was a potent inducer of transendothelial migration of activated T cells in an in vitro Transwell plate assay [40]. CXCL11 also induced migration of T lymphoblasts and spleen T cells in an in vivo rat model and had anti-tumor activity in a mouse model [21, 43]. In addition to its chemotactic effects, this chemokine stimulated proliferation of mouse CD4 T lymphocytes in a mixed leukocyte reaction assay [50].
In agreement with the above studies, high levels of the cognate CXCR3 receptor expression were found on T cells activated by anti-CD3 Ab and IL-2; a proportion of circulating blood T cells, B cells, and NK cells from healthy individuals also expressed the receptor [26, 36]. Virtually all T cells within inflamed tissues expressed CXCR3, whereas far fewer T cells within normal lymph nodes were positive for the receptor [36]. CXCR3 expression by melanoma patients’ lymphocytes was associated with enhanced patient survival, suggesting that CXCR3 is of central importance in maintaining effective anti-tumor immunity [31]. CXCR3 is also expressed by a small percentage of endothelial cells in human normal and pathological tissues, and CXCL11 blocks endothelial cell proliferation in vitro [45]. Thus, CXCL11 may inhibit tumor growth by attenuation of tumor angiogenesis.
In the present study, a new role for CXCL11 in human CTL migration toward tumor cells, which is followed by tumor cell apoptosis, has been established. The two functions of CXCL11, inhibition of angiogenesis [45] and attraction of tumor-specific CTL (this study) may act synergistically and lead to tumor regression.
Although a role of CXCL11 and CXCR3 in T-cell migration toward CC cells has been established in a single patient, this chemokine and its receptor may be involved in the migration of other patients’ T cells. CC-reactive T cells from two additional CC patients expressed CXCR3 and the autologous tumor cells produced CXCL11. In addition, our studies suggest the potential usefulness of CXCL11 in immunotherapy of patients with CXCR3-expressing T lymphocytes, since T-cell lines of ten out of ten CC patients expressed CXCR3.
CXCL11 may be useful for immunotherapy of cancer patients. Thus, patients may be vaccinated with CXCL11-transduced tumor cells (analogous to CCL3-transduced tumor cells used as effective vaccines in mice [54]) or tumor-associated Ags fused to chemokines [5]; alternatively, patients may be treated with anti-tumor Ab/chemokine fusion protein, which will attract lymphocytes to the tumor area [4, 5, 22].
In addition to therapeutic implications, the results of our study have prognostic potential. Infiltration of CC with T lymphocytes is correlated with a favorable prognosis [18], and CXCR3 expression by T lymphocytes as well as CXCL11 expression by tumor cells should be explored for their possible association with prognosis. Thus, the expression of CXCR3 on CD8+ T cells correlated with statistically significant survival advantage in melanoma patients with stage III disease [31], suggesting that expression of this receptor may serve as a biomarker of potential clinical responses to immunotherapy.
Acknowledgments
We thank James Hayden for his advice in microscopy imaging and Jeffrey S. Faust for assistance in flow cytometry analyses. We also thank Elsa Aglow for providing assistance in histotechnology. This work is supported by National Institute of Health grants CA74294, and CA10815, by a grant from Corixa Corporation, by Intramural National Cancer Institute Funds, and by the Commonwealth Universal Research Enhancement program, Pennsylvania Department of Health.
Abbreviations
- Ab
Antibody
- Ag
Antigen
- CC
Colon carcinoma
- CTL
Cytotoxic T lymphocyte
- DC
Dendritic cell
- EBV
Epstein–Barr virus
- E:T
Effector-to-target ratio
- FCFB/1
Fetal colon fibroblast
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- IFN
Interferon
- IL
Interleukin
- LAK
Lymphokine-activated killer
- mAb
Monoclonal antibody
- MLTC
Mixed lymphocyte tumor cell culture
- PBMC
Peripheral blood mononuclear cells
- PCR
Polymerase chain reaction
- TIL
Tumor infiltrating lymphocytes
- TNF
Tumor necrosis factor
References
- 1.Aiba S, Nakagawa S, Hara M, Tomioka Y, Deguchi M, Tagami H. Cultured murine dermal cells can function like thymic nurse cells. J Invest Dermatol. 1994;103:162–167. doi: 10.1111/1523-1747.ep12392632. [DOI] [PubMed] [Google Scholar]
- 2.Belperio JA, Keane MP, Burdick MD, Lynch JP, 3rd, Xue YY, Li K, Ross DJ, Strieter RM. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol. 2002;169:1037–1049. doi: 10.4049/jimmunol.169.2.1037. [DOI] [PubMed] [Google Scholar]
- 3.Binnerts ME, van Kooyk Y, Simmons DL, Figdor CG. Distinct binding of T lymphocytes to ICAM-1, -2 or -3 upon activation of LFA-1. Eur J Immunol. 1994;24:2155–2160. doi: 10.1002/eji.1830240933. [DOI] [PubMed] [Google Scholar]
- 4.Biragyn A, Kwak LW. Designer cancer vaccines are still in fashion. Nat Med. 2000;6:966–968. doi: 10.1038/79649. [DOI] [PubMed] [Google Scholar]
- 5.Biragyn A, Tani K, Grimm MC, Weeks S, Kwak LW. Genetic fusion of chemokines to a self tumor antigen induces protective, T-cell dependent antitumor immunity. Nat Biotechnol. 1999;17:253–258. doi: 10.1038/6995. [DOI] [PubMed] [Google Scholar]
- 6.Braun SE, Chen K, Foster RG, Kim CH, Hromas R, Kaplan MH, Broxmeyer HE, Cornetta K. The CC chemokine CK beta-11/MIP-3 beta/ELC/Exodus 3 mediates tumor rejection of murine breast cancer cells through NK cells. J Immunol. 2000;164:4025–4031. doi: 10.4049/jimmunol.164.8.4025. [DOI] [PubMed] [Google Scholar]
- 7.Brew R, Erikson JS, West DC, Kinsella AR, Slavin J, Christmas SE. Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine. 2000;12:78–85. doi: 10.1006/cyto.1999.0518. [DOI] [PubMed] [Google Scholar]
- 8.Clark-Lewis I, Mattioli I, Gong JH, Loetscher P. Structure–function relationship between the human chemokine receptor CXCR3 and its ligands. J Biol Chem. 2003;278:289–295. doi: 10.1074/jbc.M209470200. [DOI] [PubMed] [Google Scholar]
- 9.Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, Sahagan BG, Neote K. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187:2009–2021. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowston JG, Salmon M, Khaw PT, Akbar AN. T-lymphocyte–fibroblast interactions. Biochem Soc Trans. 1997;25:529–531. doi: 10.1042/bst0250529. [DOI] [PubMed] [Google Scholar]
- 11.Duan WR, Garner DS, Williams SD, Funckes-Shippy CL, Spath IS, Blomme EA. Comparison of immunohistochemistry for activated caspase-3 and cleaved cytokeratin 18 with the TUNEL method for quantification of apoptosis in histological sections of PC-3 subcutaneous xenografts. J Pathol. 2003;199:221–228. doi: 10.1002/path.1289. [DOI] [PubMed] [Google Scholar]
- 12.Flier J, Boorsma DM, Bruynzeel DP, Van Beek PJ, Stoof TJ, Scheper RJ, Willemze R, Tensen CP. The CXCR3 activating chemokines IP-10, Mig, and IP-9 are expressed in allergic but not in irritant patch test reactions. J Invest Dermatol. 1999;113:574–578. doi: 10.1046/j.1523-1747.1999.00730.x. [DOI] [PubMed] [Google Scholar]
- 13.Frederick MJ, Clayman GL. Chemokines in cancer. Expert Rev Mol Med. 2001;2001:1–18. doi: 10.1017/S1462399401003301. [DOI] [PubMed] [Google Scholar]
- 14.Friedl P, Entschladen F, Conrad C, Niggemann B, Zänker KS. CD4+ T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize β1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur J Immunol. 1998;28:2331–2343. doi: 10.1002/(SICI)1521-4141(199808)28:08<2331::AID-IMMU2331>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 15.Fushimi T, Kojima A, Moore MA, Crystal RG. Macrophage inflammatory protein 3alpha transgene attracts dendritic cells to established murine tumors and suppresses tumor growth. J Clin Invest. 2000;105:1383–1393. doi: 10.1172/JCI7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giovarelli M, Cappello P, Forni G, Salcedo T, Moore PA, LeFleur DW, Nardelli B, Carlo ED, Lollini PL, Ruben S, Ullrich S, Garotta G, Musiani P. Tumor rejection and immune memory elicited by locally released LEC chemokine are associated with an impressive recruitment of APCs, lymphocytes, and granulocytes. J Immunol. 2000;164:3200–3206. doi: 10.4049/jimmunol.164.6.3200. [DOI] [PubMed] [Google Scholar]
- 17.Gohara R, Nakao M, Ogata Y, Isomoto H, Oizumi K, Itoh K. Histocompatibility leukocyte antigen-A2402-restricted cytotoxic T lymphocytes recognizing adenocarcinoma in tumor-infiltrating lymphocytes of patients with colon cancer. Jpn J Cancer Res. 1997;88:198–204. doi: 10.1111/j.1349-7006.1997.tb00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidoboni M, Gafa R, Viel A, Doglioni C, Russo A, Santini A, Del Tin L, Macri E, Lanza G, Boiocchi M, Dolcetti R. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol. 2001;159:297–304. doi: 10.1016/S0002-9440(10)61695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauser AE, Debes GF, Arce S, Cassese G, Hamann A, Radbruch A, Manz RA. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J Immunol. 2002;169:1277–1282. doi: 10.4049/jimmunol.169.3.1277. [DOI] [PubMed] [Google Scholar]
- 20.Hellmuth M, Paulukat J, Ninic R, Pfeilschifter J, Muhl H. Nitric oxide differentially regulates pro- and anti-angiogenic markers in DLD-1 colon carcinoma cells. FEBS Lett. 2004;563:98–102. doi: 10.1016/S0014-5793(04)00275-3. [DOI] [PubMed] [Google Scholar]
- 21.Hensbergen PJ, Wijnands PG, Schreurs MW, Scheper RJ, Willemze R, Tensen CP. The CXCR3 targeting chemokine CXCL11 has potent antitumor activity in vivo involving attraction of CD8+ T lymphocytes but not inhibition of angiogenesis. J Immunother. 2005;28:343–351. doi: 10.1097/01.cji.0000165355.26795.27. [DOI] [PubMed] [Google Scholar]
- 22.Homey G, Müller A, Zlotnik A. Chemokines: agents for the immunotherapy of cancer? Nat Rev Immunol. 2002;2:175–184. doi: 10.1038/nri748. [DOI] [PubMed] [Google Scholar]
- 23.Jacob L, Somasundaram R, Smith W, Monos D, Basak S, Marincola F, Pereira S, Herlyn D. Cytotoxic T-cell clone against rectal carcinoma induced by stimulation of a patient’s peripheral blood mononuclear cells with autologous cultured tumor cells. Int J Cancer. 1997;71:325–332. doi: 10.1002/(SICI)1097-0215(19970502)71:3<325::AID-IJC3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs N, Moutschen MP, Franzen-Detrooz E, Boniver V, Boniver J, Delvenne P. Organotypic culture of HPV-transformed keratinocytes: a model for testing lymphocyte infiltration of (pre)neoplastic lesions of the uterine cervix. Virchows Arch. 1998;432:323–330. doi: 10.1007/s004280050173. [DOI] [PubMed] [Google Scholar]
- 25.Jenh CH, Cox MA, Hipkin W, Lu T, Pugliese-Sivo C, Gonsiorek W, Chou CC, Narula SK, Zavodny PJ. Human B cell-attracting chemokine 1 (BCA-1; CXCL13) is an agonist for the human CXCR3 receptor. Cytokine. 2001;15:113–121. doi: 10.1006/cyto.2001.0923. [DOI] [PubMed] [Google Scholar]
- 26.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maaser K, Wolf K, Klein CE, Niggemann B, Zänker KS, Bröker E-B, Friedl P. Functional hierarchy of simultaneously expressed adhesion receptors: integrin alpha2beta1 but not CD44 mediated MV3 melanoma cell migration and matrix reorganization within three-dimensional hyaluronan-containing matrices. Mol Biol Cell. 1999;10:3067–3079. doi: 10.1091/mbc.10.10.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maas-Szabowski N, Stark HJ, Fusenig NE. Keratinocyte growth regulation in defined organotypic cultures through IL-1-induced keratinocyte growth factor expression in resting fibroblasts. J Invest Dermatol. 2000;114:1075–1084. doi: 10.1046/j.1523-1747.2000.00987.x. [DOI] [PubMed] [Google Scholar]
- 29.Mach F, Sauty A, Iarossi AS, Sukhova GK, Neote K, Libby P, Luster AD. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J Clin Invest. 1999;104:1041–1050. doi: 10.1172/JCI6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell MS, Darrah D, Yeung D, Halpern S, Wallace A, Voland J, Jones V, Kan-Mitchell J. Phase I trial of adoptive immunotherapy with cytolytic T lymphocytes immunized against a tyrosinase epitope. J Clin Oncol. 2002;20:1075–1086. doi: 10.1200/JCO.20.4.1075. [DOI] [PubMed] [Google Scholar]
- 31.Mullins IM, Slingluff CL, Lee JK, Garbee CF, Shu J, Anderson SG, Mayer ME, Knaus WA, Mullins DW. CXC chemokine receptor 3 expression by activated CD8+ T cells is associated with survival in melanoma patients with stage III disease. Cancer Res. 2004;64:7697–7701. doi: 10.1158/0008-5472.CAN-04-2059. [DOI] [PubMed] [Google Scholar]
- 32.Murakami S, Okada H. Lymphocyte–fibroblast interactions. Crit Rev Oral Biol Med. 1997;8:40–50. doi: 10.1177/10454411970080010201. [DOI] [PubMed] [Google Scholar]
- 33.Musha H, Ohtani H, Mizoi T, Kinouchi M, Nakayama T, Shiiba K, Miyagawa K, Nagura H, Yoshie O, Sasaki I. Selective infiltration of CCR5(+)CXCR3(+) T lymphocytes in human colorectal carcinoma. Int J Cancer. 2005;116:949–956. doi: 10.1002/ijc.21135. [DOI] [PubMed] [Google Scholar]
- 34.O’Donovan N, Galvin M, Morgan JG. Physical mapping of the CXC chemokine locus on human chromosome 4. Cytogenet Cell Genet. 1999;84:39–42. doi: 10.1159/000015209. [DOI] [PubMed] [Google Scholar]
- 35.Ohta M, Tanaka F, Yamaguchi H, Sadanaga N, Inoue H, Mori M. The high expression of Fractalkine results in a better prognosis for colorectal cancer patients. Int J Oncol. 2005;26:41–47. [PubMed] [Google Scholar]
- 36.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao WH, Hales JM, Camp RDR. Potent costimulation of effector T lymphocytes by human collagen type I. J Immunol. 2000;165:4935–4940. doi: 10.4049/jimmunol.165.9.4935. [DOI] [PubMed] [Google Scholar]
- 38.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- 39.Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71:173–183. [PubMed] [Google Scholar]
- 40.Sauty A, Colvin RA, Wagner L, Rochat S, Spertini F, Luster AD. CXCR3 internalization following T cell–endothelial cell contact: preferential role of IFN-inducible T cell alpha chemoattractant (CXCL11) J Immunol. 2001;167:7084–7093. doi: 10.4049/jimmunol.167.12.7084. [DOI] [PubMed] [Google Scholar]
- 41.Sharma S, Stolina M, Luo J, Strieter RM, Burdick M, Zhu LX, Batra RK, Dubinett SM. Secondary lymphoid tissue chemokine mediates T cell-dependent antitumor responses in vivo. J Immunol. 2000;164:4558–4563. doi: 10.4049/jimmunol.164.9.4558. [DOI] [PubMed] [Google Scholar]
- 42.Somasundaram R, Jacob L, Swoboda R, Caputo L, Song H, Basak S, Monos D, Peritt D, Marincola F, Cai D, Birebent B, Bloome E, Kim J, Berencsi K, Mastrangelo M, Herlyn D. Inhibition of cytolytic T lymphocyte proliferation by autologous CD4+/CD25+ regulatory T cells in a colorectal carcinoma patient is mediated by transforming growth factor-beta. Cancer Res. 2002;62:5267–5272. [PubMed] [Google Scholar]
- 43.Stanford MM, Issekutz TB. The relative activity of CXCR3 and CCR5 ligands in T lymphocyte migration: concordant and disparate activities in vitro and in vivo. J Leukoc Biol. 2003;74:791–799. doi: 10.1189/jlb.1102547. [DOI] [PubMed] [Google Scholar]
- 44.Starnes HF., Jr Biological effects and possible clinical applications of interleukin 1. Semin Hematol. 1991;28:34–41. [PubMed] [Google Scholar]
- 45.Strieter RM, Belperio JA, Phillips RJ, Keane MP. CXC chemokines in angiogenesis of cancer. Semin Cancer Biol. 2004;14:195–200. doi: 10.1016/j.semcancer.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 46.Tasaki A, Yamanaka N, Kubo M, Matsumoto K, Kuroki H, Nakamura K, Nakahara C, Onishi H, Kuga H, Baba E, Tanaka M, Morisaki T, Katano M. Three-dimensional two-layer collagen matrix gel culture model for evaluating complex biological functions of monocyte-derived dendritic cells. J Immunol Methods. 2004;287:79–90. doi: 10.1016/j.jim.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 47.Tensen CP, Flier J, Van Der Raaij-Helmer EM, Sampat-Sardjoepersad S, Van Der Schors RC, Leurs R, Scheper RJ, Boorsma DM, Willemze R. Human IP-9: a keratinocyte-derived high affinity CXC-chemokine ligand for the IP-10/Mig receptor (CXCR3) J Invest Dermatol. 1999;112:716–722. doi: 10.1046/j.1523-1747.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- 48.Vicari AP, Caux C. Chemokines in cancer. Cytokine Growth Factor Rev. 2002;13:143–154. doi: 10.1016/S1359-6101(01)00033-8. [DOI] [PubMed] [Google Scholar]
- 49.Wei WZ, Miller B, Gutierrez RM. Inhibition of tumor growth by peptide specific cytotoxic T lymphocytes in a three-dimensional collagen matrix. J Immunol Methods. 1997;200:47–54. doi: 10.1016/S0022-1759(96)00196-2. [DOI] [PubMed] [Google Scholar]
- 50.Whiting D, Hsieh G, Yun JJ, Banerji A, Yao W, Fishbein MC, Belperio J, Strieter RM, Bonavida B, Ardehali A. Chemokine monokine induced by IFN-gamma/CXC chemokine ligand 9 stimulates T lymphocyte proliferation and effector cytokine production. J Immunol. 2004;172:7417–7424. doi: 10.4049/jimmunol.172.12.7417. [DOI] [PubMed] [Google Scholar]
- 51.Yang SK, Eckmann L, Panja A, Kagnoff MF. Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells. Gastroenterology. 1997;113:1214–1223. doi: 10.1053/gast.1997.v113.pm9322516. [DOI] [PubMed] [Google Scholar]
- 52.Zhang T, Somasundaram R, Berencsi K, Caputo L, Rani P, Guerry D, Furth E, Rollins BJ, Putt M, Gimotty P, Swoboda R, Herlyn M, Herlyn D. CXC chemokine ligand 12 (stromal cell-derived factor 1{alpha}) and CXCR4-dependent migration of CTLs toward melanoma cells in organotypic culture. J Immunol. 2005;174:5856–5863. doi: 10.4049/jimmunol.174.9.5856. [DOI] [PubMed] [Google Scholar]
- 53.Zhang T, Somasundaram R, Berencsi K, Caputo L, Gimotty P, Rani P, Guerry D, Swoboda R, Herlyn D. Migration of cytotoxic T lymphocytes toward melanoma cells in three-dimensional organotypic culture is dependent on CCL2 and CCR4. Eur J Immunol. 2006;36:457–467. doi: 10.1002/eji.200526208. [DOI] [PubMed] [Google Scholar]
- 54.Zibert A, Balzer S, Souquet M, Quang TH, Paris-Scholz C, Roskrow M, Dilloo D. CCL3/MIP-1alpha is a potent immunostimulator when coexpressed with interleukin-2 or granulocyte–macrophage colony-stimulating factor in a leukemia/lymphoma vaccine. Hum Gene Ther. 2004;15:21–34. doi: 10.1089/10430340460732436. [DOI] [PubMed] [Google Scholar]