Abstract
In order to study the effect of glycosylation on its biological activities, and to develop TNFα with less deleterious effects, recombinant human TNFα was chemically coupled with N-acetylneuraminic acid (NeuAc). NeuAc with C9 spacer was coupled to TNFα by acyl azide method. Two glycosylated TNFαs, designated L NeuAc-TNFα and H NeuAc-TNFα, were purified by anion-exchange chromatography. NeuAc coupling to TNFα was confirmed by lectin blotting. Average number of carbohydrate molecules introduced per molecule of L NeuAc-TNFα and H NeuAc-TNFα were estimated to be 1.0 and 1.5, respectively. We examined a variety of TNFα activities in vitro, including antiproliferative or cytotoxic activities to tumor cells, proliferative effect on fibroblast cells, stimulatory effects on IL-6 production by melanoma cells and NF-κB activation in hepatoma cells. L NeuAc-TNFα and H NeuAc-TNFα exhibited reduced activities about 1/3 and 1/10 as compared to native TNFα in all the activities performed in vitro.
Keywords: Neoglycoprotein, Sialic acid, Tumor necrosis factor, Cytokine
Introduction
Most of the natural cytokines are glycosylated. Although the recombinant cytokines generated by E. coli exhibit comparable biological activities in vitro as natural glycosylated counterparts in several cytokines, carbohydrates appeared to play an important role in their stability and biological activities. Recombinant interferons (IFN) generated by E. coli are unstable [1] and removal of carbohydrates from erythropoietin results in inactivation [2]. Carbohydrates in cytokine receptors are also important. In cytokines and chemokines, carbohydrates in receptor molecule contribute to the ligand binding affinity [3, 4]. It is also known that many kinds of lectin molecules are expressed on the cell surface through which cell–cell or cell–glycoproteins interactions and recognitions are initiated [5]. Lectin-independent interactions between proteins and carbohydrates are also important in exerting their biological activities. The binding of IL-8 to heparan sulfate proteoglycans on the surface of endothelial cells is crucial for the recruitment of neutrophils to an inflammatory site [6]. Binding of TGFβ to cell surface proteoglycan (Type III receptor) facilitates the binding of TGFβ to TGFβ receptors I and II [7]. Therefore, it is possible that the mode of actions of recombinant cytokines in vivo is different from those of glycosylated natural counterparts. Also it is possible to manipulate the biological activity, tissue distribution, and stability of cytokines by coupling carbohydrates into recombinant cytokines.
Tumor necrosis factor α (TNFα) is a cytokine produced mainly by macrophages and monocytes. TNFα exhibits a variety of biological activities and plays an important role in immunologic and inflammatory reactions [8, 9]. TNFα has been expected as a useful therapeutic agent for certain tumors. However, because of its proinflammatory activity and toxicity its clinical application is limited.
In the previous studies, we synthesized d-Manα(1–6)Man [Man2α(1–6)] conjugated-human recombinant interleukin-1α (IL-1α), neoglyco IL-1α, to know the effect of glycosylation on its activities and to develop IL-1 with less deleterious effects [10–12]. Man2α(1–6)-IL-1α exhibited impairment in both biological activities in all the experiments in vitro [10]. However, Man2α(1–6)-IL-1α exhibited comparable activities as nonglycosylated IL-1α in down-regulation of serum level of glucose and recovery of peripheral white blood cells from myelosuppression in 5-fluorouracil-treated mice, irrespective of the decrease of all the other activities in vivo [11]. In addition, tissue distribution of Man2α(1–6)-IL-1α in mice differed from that of nonglycosylated IL-1α [12]. We also synthesized D-Gal conjugated IL-1α [13]. It exhibited the similar decrease in its biological activities in vitro [14]. However, in vivo the magnitude of its decrease was less than that in vitro [15].
Sialic acid is present at the non-reducing position of oligosaccharide in glycoproteins and glycolipids, and plays an important role in function, stability, and tissue distribution of glycoproteins [16]. Sialic acid is especially important in preventing the clearance of sialoglycoproteins from serum because asialoglycoproteins are rapidly cleared through Gal/GalNAc binding lectins present in the liver [17]. Furthermore, sialic acid is also important as a ligand for selectins [18] and Siglecs, members of the Ig superfamily. Siglecs are consist of sialoadhesion, CD22, myelin associated glycoprotein and CD33, present in macrophage subsets, B lymphocytes, oligodendrocytes/Schwann cells and myeloid cells, respectively [19]. A subset CD33-related Siglecs are expressed in immune cells and modulate immune function by down-regulating innate immune cell activation via cytosolic immunoreceptor tyrosine-based inhibitory motifs [20]. Therefore, it is possible that coupling of sialic acid enables its conjugate to bind to a variety of cell types, which may lead to the alteration of its activities in vivo.
In the previous studies, we coupled N-acetylneuraminic acid (NeuAc), a major constituent of sialic acid, to rhIL-1α [21]. NeuAc coupled IL-1α exhibited selective activities in vivo as Man2α(1–6)-coupled IL-1α and enhanced tissue distribution [22, 23]. In this report we synthesized NeuAc-coupled TNFα and studied its in vitro biological activities.
Materials and methods
Reagents
RPMI 1640 and Dulbecco’s modified Eagle’s medium (D-MEM) were purchased from Sigma Chemical Co. (St.Louis, MO). Fetal bovine serum (FBS) was from JRH Biosciences (Lenexa, KS). Human recombinant TNFα (rhTNFα) was provided by Dainippon Pharmaceutical Co. (Osaka, Japan). The specific activity of rhTNFα was 6 × 107 U/mg based on the cytotoxic assay using L929 cells cultured with actinomycin D. Human recombinant IL-6 (rhIL-6) was provided by Ajinomoto Co. (Tokyo, Japan). The specific activity of rhIL-6 was 5 × 106 U/mg based on the proliferative assay using MH60·BSF2 cells. LFA (limax flavus) lectin was purchased from CALBIOCHEM (Darmstadt, Germany), and it was biotynated with ECL protein biotinylation module (Amersham Biosciences, Piscatway, NJ).
Coupling of NeuAc with C9 spacer to TNFα
NeuAc with C9 spacer, 8-(hydrazinocarbonyl)octyl 5-acetamido-3, 5-dideoxy-D-glycero-α-D-galacto-2-nonulo-pyranosidonic acid potassium salt was synthesized as described previously (21). The compound (6.73 mg, 12.8 μmol) was dissolved in water (325 μL) and the solution chilled on ice. To the chilled solution (288 μL), cold 4 M HCl (40 μL), and 2 M sodium nitrite (20 μL) were added. After the solution was kept at room temperature for 15 min, 2 M ammonium sulfamate (20 μL) was added and the mixture was kept at room temperature for 15 min in order to inactivate excess HNO2. This mixture (containing the acyl azide) was added to ice-cooled 0.4 M sodium borate buffer (pH 10.0, 100 μL) and PBS (400 μL) containing 1,984 mg of rhIL-1α. The pH was quickly adjusted to 9.0 with 4 N NaOH and kept for 60 min at room temperature. In this step it is essential to use micro stirring bar. The reaction mixture was then filtrated with 0.2 μm nylon membrane filter and desalted with 20 mM Tris–HCl buffer (pH 7.5) with Hi Trap Desalting column (Pharmacia). TNFα treated with the same manner without the compound was used as control (treated) TNFα.
Purification of NeuAc-coupled TNFα
Purification was carried out at room temperature employing the FPLC system (Pharmacia). The desalted sample was loaded onto an anion-exchange chromatography column (Mono Q, Pharmacia) equilibrated with 20 mM Tris–HCl buffer (pH 7.5) at flow rate of 1 ml/min, eluted with a 30 mL liner NaCl gradient (0–0.5 M) in the same buffer, and fractions (1 ml) were collected. The buffer of fractions containing NeuAc-coupled TNFα was exchanged to PBS using HiTrap Desalting column and concentrated in Centriplus-3 (Amicon, Mr 30,000 cut-off).
Electrophoresis
Analytical sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to the method of Laemmli [24] on 15% polyacrylamide gel in the presence of 0.1% SDS using a vertical slab minigel apparatus. Protein bands were visualized with silver staining. Molecular weight of NeuAc-coupled TNFα was estimated by comparision of the electrophoretic mobility with those of standard molecular weight markers (ovalbumin, Mr 43,000; carbonic anhydrase, Mr 30,000; soybean trypsin inhibitor, Mr 20,100; α-lactalbumin, Mr 14,300).
Confirmation of NeuAc coupling
Size fractionated proteins were transferred from gels to immobilon polyvinylidene difluoride membrane (Milipore Corporation, Bedford, MA) using a semidry apparatus (Marysol, Tokyo, Japan) for 1 h with a current of 100 mA at room temperature. The coupled NeuAc was detected by using biotinated LFA lectin and reacted with horseradish peroxidase (HRP)-conjugated avidin.
Time-of-mass spectrometry (TOF-MS) analysis
TOF-MS analysis was performed according to the procedure of the supplier using Voyage Elite (PE Biosystems, Foster, CA).
Determination of protein content
The amount of protein was determined using a BCA protein assay kit (Pierce, Rockford, IL) with bovine serum albumin as a standard.
Cell culture
Murine hybridoma MH60·BSF2 cells provided by Dr. T. Hirano (University of Osaka) were maintained in culture medium (RPMI1640 supplemented with 100 U/ml of penicillin, 100 μg/ml of streptomycin, 15 mM HEPES) supplemented with 10% FBS and 1 U/ml rhIL-6 [25]. Human melanoma A375-6 is an IL-1 sensitive subclone of human melanoma cell line A375 [26]. A375-6 cells and mouse transformed fibroblast L929 cells were maintained in the culture medium supplemented with 5% FBS. Human histiocytic cell line U937 and human embryonic fibroblast cell line TIG-1 were obtained from the Japanese Cancer Research Resources Bank (Tokyo, Japan). U937 and TIG-1 cells were maintained in the culture medium supplemented with 10% FBS.
Cytotoxic assay for L929 cells
Cell suspension of 50 μl (1 × 106 cells/ml) in culture medium containing actinomycin D (6 μg/ml) was added to each well of a 96 well micro titer plate, to which 100 μl of medium containing TNFαs were added, and the plates were incubated for 18 h at 37°C in 5% CO2 in air. After culture, the plate were washed, and cell lysis was determined by staining the plates with crystal violet (0.5%) in methanol–water with 0.1 ml of 0.1% SDS, the dye uptake was calculated by measuring the absorbance at 595 nm using an ELISA auto reader (Bio-Rad Laboratories, Richmond, CA). The percentage of cytotoxicity was calculated as follows:
 |
Growth inhibition assay for A375-6 cells
A375-6 cells were detached from culture dish with 0.02% EDTA-PBS. The cells were washed with the culture medium and 100 μl of cell suspension (4 × 104 cells/ml) was added to each well of a 96 well micro titer plate. After 24 h culture at 37°C in 5% CO2 in air, 100 μl of medium containing TNFαs were added, and the plates were incubated for another 72 h under the same condition. The cell growth was determined by the crystal violet-staining method. After solubilization of the dye staining the absorbance at 595 nm was determined. The percentage of cell growth was calculated using the same formula as the cytotoxic assay.
Growth inhibition assay for U937 cells
About 50 μl of medium containing TNFαs were added to each well of a 96 well micro titer plate, to which 50 μl of cell suspension (4 × 105 cells/ml) was added, and the plates were incubated for 72 h at 37°C in 5% CO2 in air. Proliferation of the cells was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay [27]. The percentage of cell growth was calculated using the same formula as the cytotoxic assay.
Growth promoting assay for TIG-1 cells
About 200 μl medium containing 2 × 104 TIG-1cells were added to each well of a 96-well micro titer plate with varying concentrations of TNFαs, and the plates were incubated for 72 h at 37°C in 5% CO2 in air. After culture, cells were stained with crystal violet, and the percentage of cell growth was calculated using the same formula as the cytotoxic assay.
Apoptotic cell death assay
A375-R8 cells are very sensitive to the apoptotic effect of TNFα [28]. Four ml of medium containing 2 × 105 cells was added to 60 mm culture plate, and the cells were cultured with or without TNFα for 3 days at 37°C in 5% CO2 in air. After culture, cells were collected by centrifugation, and treated with 1% glutaraldehyde solution for 30 min at room temperature. After washing with PBS, the cells were treated with 0.2% Triton for 8 min at room temperature, washed again with PBS, and then stained with DAPI (0.2 μg/ml). Apoptotic cells exhibiting nuclear fragmentation were counted by using a florescence microscopy (OLYMPUS BX50). Percent of apoptotic cells was determined by counting at least 400 cells.
Measurement of NF-κB activity
NF-κB activity was determined by using reporter plasmid 4κBw-luc containing four tandem copies of the human immunodeficiency virus-κB sequence (from Dr. T. Okamoto, Nagoya City University Medical School, Japan) [29]. HepG2 cells were transiently transfected with lipofection with reporter plasmid and pCMV-β-gal in 12-well plate. One day later, the medium was replaced with a fresh one. After 24 h, cells were treated with TNFαs for 24 h. The cells were then washed with PBS and then lysed. Luciferase assay was performed with Luciferase Reporter Gene Assay Kit (Boehringer, Mannheim, Germany) according to the manufacturer’s instructions. The light emission was measured by Multi-label counter 1420 ARVO (Pharmacia, San Francisco, CA). Luciferase activity was determined by using β-galactosidase value as a basis for normalization.
Results
Coupling of NeuAc with rhIL-1α
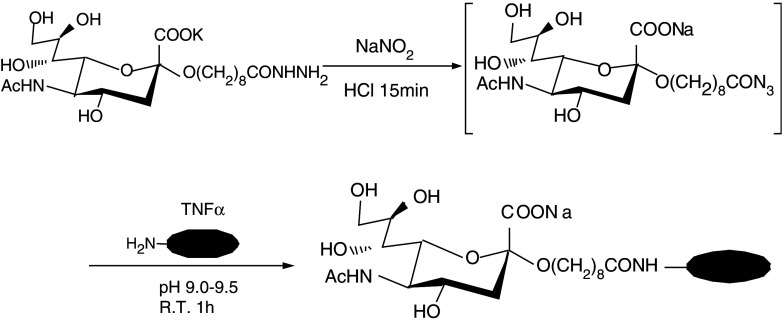
NeuAc with C9 spacer, 8-(hydrazinocarbonyl)octyl 5-acetamido-3, 5-dideoxy-D-glycero-α-D-galacto-2-nonulo-pyranosidonic acid potassium salt was transformed to an acyl azide derivative by reaction with hydrogen nitrite. The acyl azide derivative was coupled with rhTNFα to yield a glycosylated rhTNFα (Fig. 1). rhTNFα treated in the same manner without the acyl azide derivative was used as a control and designated treated TNFα.
Fig. 1.
Synthesis of NeuAc-coupled TNFα by acyl azide method
Purification of NeuAc-coupled TNFα
The NeuAc-coupled TNF (NeuAc-IL-1α) was purified employing FPLC system using an anion-exchange chromatography column. As shown in Fig. 2a and b, untreated TNFα and treated TNFα eluted in Fr. 9 and 10. In contrast, glycosylated TNFα eluted in Fr. 9–27 with major two peaks (Fig. 2c). As Fr. 9 and 10 may contain nonglycosylated TNFα, fractions 11–19 and 20–27 were pooled and designated L NeuAc-TNFα and H Neu-TNFα, respectively. The yields of L NeuAc-TNFα and H Neu-TNFα were 28.6 and 4.87%, respectively.
Fig. 2.

Purification of NeuAc-TNFα by anion exchange column chromatography. a Native TNFα (170 μg), b treated TNFα (425 μg) or c NeuAc-TNFα (1.71 mg) after reaction were applied to the anion-exchange chromatography column (Mono Q, Pharmacia) with FPLC system. The starting buffer was 20 mM Tris–HCl (pH 7.5), and the elution buffer was the starting buffer containing 0.5 M NaCl with a linear gradient. Fractions (1 ml) were collected at a flow rate of 1 ml/min
SDS-PAGE analysis of NeuAc-TNFα and confirmation of NeuAc coupling
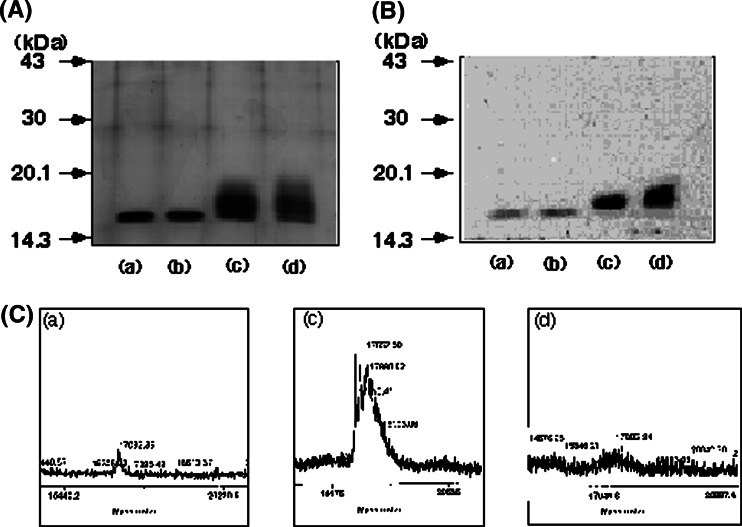
Native TNFα, treated TNFα, L NeuAc-TNFα and H Neu-TNFα were analyzed on SDS-PAGE. As shown in Fig. 3a, native and treated TNFα migrated at 17.0 kDa, while L NeuAc-TNFα and H Neu-TNFα migrated at 17.3–19.5 and 17.6–20.8 kDa, respectively. To confirm that NeuAc was coupled to TNFα, lectin-blotting analysis was performed using NeuAc-specific LFA lectin. As shown in Fig. 3b, L NeuAc-TNFα and H Neu-TNFα were markedly stained with the lectin. The faint staining of native and treated TNFα was nonspecific because nonglycosylated molecular weight markers were also stained in the same density (data not shown). The M.W. of NeuAc-TNFα was determined by TOF-MS analysis. Native TNFα, L NeuAc-TNFα and H Neu-TNFα exhibited a major peak at 17.08,17.55, and 17.80 kDa, respectively (Fig. 3c). Treated TNFα exhibited the same molecular weight as native TNFα (data not shown). Therefore, NueAc with C9 spacer introduced into L NeuAc-TNFα and H Neu-TNFα were averagely 1.00 and 1.51 mole per molar TNFα, respectively.
Fig. 3.
SDS-PAGE, lectin blotting with LFA and TOF-MS analysis. a)We analyzed 0.3 μg of TNFαs on SDS containing 15% polyacrylamide gel under reduced condition. TNFαs were visualized with Coomassie Brilliant Blue staining. b TNFαs 0.3 μg of were electrophoresed on SDS containing 15% polyacrylamide under reduced condition. After electrophoresis, TNFαs were transferred onto a PVDF membrane. The membrane was treated with a biotinyated Limax flavus (LFA) lectin and reacted with HRP-avidin. Molecular weight standard electrophoresed in parallel are indicated to the left in kilodaltons. a Native TNFα b treated TNFα c L NeuAc-TNFα (FPLC fractins, 11–19), d H NeuAc-TNFα (FPLC fractions, 20–27). Representative data of three independent experiments are shown (c). Native TNFα (a), L NeuAc-TNFα (c), H NeuAc-TNFα (d) were analyzed by TOF-MS
Antiproliferative or cytotoxic effect of TNFα on tumor cells
Biological activities of NeuAc-TNFα were compared to those of native TNFα and treated TNFα. TNFα exhibits cytotoxic or antiproliferative activities for many tumor cells. Cytotoxic effect of TNFαs for murine transformed fibroblast cells L929 was determined by culturing the cells with or without TNFαs for 24 h in the presence of actinomycin D. Antiproliferative effects of TNFα for human melanoma cells A375-6 and human histiocytic cells U937 were determined by culturing the cells with or without TNFαs for 3 days. As shown in Fig. 4, treated TNFα exhibited the comparative activities as native TNFα in both cytotoxic and antiproliferative activities. In contrast, L NeuAc-TNFα and H Neu-TNFα exhibited about 1/3 and 1/10 activities of native TNFα, respectively.
Fig. 4.

Antiproliferative or cytotoxic effects of TNFα on L929, A375-6 and U937 cells. a Mouse transformed fibroblast cell line L929 cells were cultured at 37°C for 18 h with or without varying doses of TNFαs in the presence of actinomycin D (2 μg.ml). After culture, cells were stained with crystal violet. b Human melanoma cell line A375-6 cells were cultured at 37°C for 3 days with or without varying doses of TNFαs. After culture, cells were stained with crystal violet. c Human myelomonocytic cell line U937 cells were cultured at 37°C for 3 days with or without varying doses of TNFαs. After culture, cell proliferation was determined by MTT method. Data shown are mean ± SD of four cultures. Representative data of three independent experiments are shown
Proliferative effect of TNFα on fibroblast cells
TNFα stimulates the proliferation of normal fibroblast cells. To determine the proliferative activity, human normal fibroblast cells TIG-1 were cultured with or without TNFαs for 3 days. As shown in Fig. 5, native TNFα and treated TNFα exhibited the comparable growth promoting activity. In contrast, H NeuAc-TNFα exhibited the reduced activity as compared to native TNFα. L NeuAc-TNFα also exhibited the reduced activity, but the decrease was less than that of H NeuAc-TNFα.
Fig. 5.
Proliferative effect of TNFα on TIG-1 cells. Human fibroblast cell line TIG-1 cells were cultured with or without varying doses of TNFαs. After 24 h culture, cells were stained with crystal violet. Data shown are mean ± SD of four cultures. Representative data of four independent experiments are shown
Apoptotic cell death induction for A375-R8 cells
To determine the apoptotic cell death inducing activity of TNFα, human melanoma A375-R8 cells, sensitive cells to the apoptotic effect of TNFα, were cultured with or without TNFα for 3 days. As shown in Fig. 6, native TNFα and treated TNFα exhibited the comparable activity. In contrast, H NeuAc-TNFα exhibited the reduced activity as compared to native TNFα. L NeuAc-TNFα also exhibited the reduced activity, but the decrease was less than that of H NeuAc-TNFα.
Fig. 6.
TNFα induction of apoptosis. Human melanoma cell line A375-R8 cells were cultured with or without TNFαs (50 ng/ml). After 3 days culture, apoptotic cells were counted using fluorescence microscope. Data shown are mean ± SD of three cultures. Representative data of two independent experiments are shown
TNFα induction of IL-6 by fibroblast cells
TNFα induces production of IL-6 from many cell types. To evaluate the IL-6 inducing activity of TNFα, TIG-1 was cultured with or without TNFαs for 24 h, and then the amount of IL-6 in the culture supernatants was determined. As shown in Fig. 7, treated TNFα exhibited the comparable IL-6 inducing activity as native TNFα. In contrast, L NeuAc-TNFα and H Neu-TNFα exhibited about 1/3 and 1/20 activities of native TNFα, respectively.
Fig. 7.
TNFα induction of IL-6 by TIG-1 cells. TIG-1 cells were cultured with or without varying doses of TNFαs. After 24 h culture, IL-6 activity in the supernatants was determined by proliferation of IL-6 dependent MH60-BSF2 cells. Data shown is the mean of triplicate cultures. Representative data of three independent experiments are shown
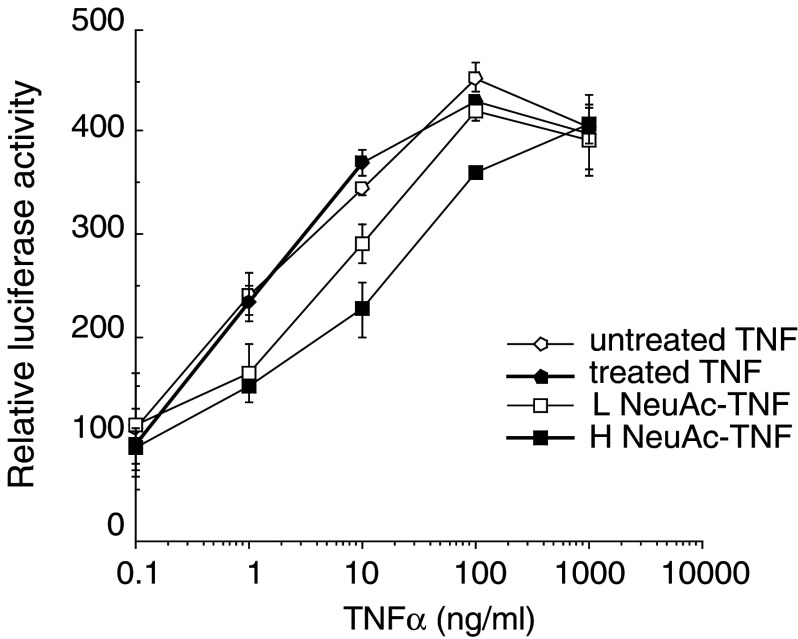
TNFα activation of NF-κB in human hepatoma cells
NF-κB is activated by TNFα, and plays a key role in mediating TNFα functions through inducing cytokines, chemokines, growth factors, cell adhesion molecules and a variety of enzymes. To evaluate the activitiy of TNFα for NF-κB activation, the reporter plasmid 4κBw-luc, in which four tandem copies of the human immunodeficiency virus κB sequence are fused to the luciferase reporter gene, was transfected into HepG2 cells. After treatment with TNFαs, NF-κB activity was determined by measuring luciferase activity in the cell lysate. As shown in Fig. 8, treated TNFα exhibited the comparable activity as native TNFα, and L NeuAc-TNFα and H Neu-TNFα exhibited about 1/3 and 1/10 activities of native TNFα, respectively.
Fig. 8.
TNFα activation of NF-κB. Human hepatoma cell line HepG2 cells were transiently transfected with 4κBw-luc and pCMV-β-gal. After 1 day culture, cells were treated for 24 h with or without varying doses of TNFαs. After treatment, cell lysates were prepared and the luciferase activity was determined. The relative promoter activity indicates the ratio between the luciferase activity in untreatment and in others after normalization with β-galactosidase activity. Data shown are mean of triplicate cultures. Representative data of three independent experiments are shown
Discussion
This is the first report demonstrating the synthesis of NeuAc-coupled TNFα. NeuAc-coupled TNFα exhibited reduction in all the biological activities examined in vitro. However, as control (treated) TNFα exhibited comparable activities as native TNFα, the coupling condition did not affect TNFα activity. This was the same in cases of Man dimmer (Man2), Gal or NeuAc introduction into IL-1α [11, 14, 22]. The retarded elution profile of glycosylated TNFα in anion exchange column chromatography suggests that amino residues of TNFα reacted with NeuAc-C9 and subsequently NeuAc-derived negative charge affected the elution profile. We obtained two major preparations of NeuAc-coupled TNFα, L NeuAc-TNFα and H NeuAc-TNFα. Coupling of NeuAc into TNFα was confirmed by lectin blotting with NeuAc-specific LFA, and by the increase of molecular weight (m.w.) on SDS-PAGE and by the analysis with TOF-MS. The average number of NueAc with C9 spacer coupled with L NeuAc-TNFα and H Neu-TNFα were 1.00 and 1.51 mole per molar TNFα, respectively.
Human TNFα contains 15 amino residues, 6 Lys, 8 Arg and 1N-terminal amino acid [30]. Based on the X-ray crystallographic analysis, all these residues are potentially able to react with NeuAc [31]. In case of human IL-1α, Man2(α1,4), Man2(α1,6), Gal and NeuAc were coupled to IL-1α, 5.5, 4.7, 9.1 and 2.7 molar per molecule of IL-1, respectively [10, 13, 21]. Based on these findings, the number of carbohydrate coupled to cytokine seems to be affected by both the size and charge of the carbohydrate. NeuAc appeared to be most difficult to couple with cytokines by acyl azide method probably because NeuAc is relative large in size and has negative charge in neutral condition. It is also possible that TNFα exists as a trimmer in solution [33], so that only limited amino residues are exposed on the surface of the complex. The high isoelectric point of TNFα (pI 5.9) relative to human IL-1α (pI 5.0) may also contribute to the less number of NeuAc coupled to TNFα.
When compared to native TNFα and treated TNFα, NeuAc-coupled TNFα exhibited reduced activities in all the assays performed in vitro, including antiproliferative, cytotoxic and apoptotic effects on tumor cells, proliferative effect on normal fibroblast cells, IL-6 induction by fibroblast cells and NF-κB activation in hepatoma cells. The reduction of the activities was greater in H NeuAc-TNFα than L NeuAc-NFα. The activities of L NeuAc-TNFα were 1/3–1/6, and those of H NeuAc-TNFα were 1/10–1/20 of native TNFα.
TNFα activities are mediated through its specific receptor on cell surface. There are two types of receptors for TNFα. Type I receptor (TNFRI) with molecular weight (m.w.) of 55 kDa is expressed in many cell types, and type II receptor (TNFRII) with m.w. of 75 kDa is expressed in lymphoid cells [32]. TNFRI transduces signals for most of the TNFα activities, including antiproliferative or cytotoxic effects on tumor cells, proliferative effect on fibroblast cells, induction of IL-6, NF-κB activation and antiviral activity. In contrast, TFRII transduces signals for proliferatative activities for thymocytes and NK cells and induction of GM-CSF [33]. The decreased activities of NeuAc-coupled TNFα may be ascribable to its decreased binding affinity for TNFR. Although in this study we were unable to determine the affinity of TNFα for TNFR because of experimental difficulties, in our previous studies all the carbohydrate coupled IL-1αs exhibited reduced binding affinity to IL-1R. NeuAc may cause conformational changes that lead to the decrease in binding affinity, or NeuAc interferes TNFα binding to TNFR.
There has been no attempt to couple carbohydrates with TNFα. In this study we succeeded in the preparation of NeuAc-coupled TNFα. Although its biological activities in vitro are all reduced, in the accompanying paper we show that this is not the case in vivo.
Acknowledgments
This work was supported in part by grant-in aid for grant-in-aids for scientific research on priority areas (C) from The Ministry of Education, Science, Sports and Culture.
References
- 1.Tanaka T, Naruto M, Kawano G. Production of recombinant mouse beta interferon. J Interferon Res. 1986;6:429–435. doi: 10.1089/jir.1986.6.429. [DOI] [PubMed] [Google Scholar]
- 2.Tsuda E, Kawanishi G, Ueda M, Masuda S, Sasaki R. The role of carbohydrate in recombinant human erythropoietin. Eur J Biochem. 1990;188:405–411. doi: 10.1111/j.1432-1033.1990.tb15417.x. [DOI] [PubMed] [Google Scholar]
- 3.Fischer T, Thoma B, Scheurich P, Pfizenmaier K. Glycosylation of the human interferon-gamma receptor. N-linked carbohydrates contribute to structural heterogeneity and are required for ligand binding. J Biol Chem. 1990;265:1710–1717. [PubMed] [Google Scholar]
- 4.Mancilla J, Ikejima T, Dinarello CA. Glycosylation of the interleukin-1 receptor type I is required for optimal binding of interleukin-1. Lymphokine Cytokine Res. 1992;11:197–205. [PubMed] [Google Scholar]
- 5.Ashwell G, Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- 6.Webb LM, Ehrengruber MU, Clark-Lewis I, Baggiolini M, Rot A. Binding to heparan sulfate or heparin enhances neutrophil responses to interleukin 8. Proc Natl Acad Sci U S A. 1993;90:7158–7162. doi: 10.1073/pnas.90.15.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Casillas F, Cheifetz S, Doody J, Andres JL, Lane WS, Massague J. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell. 1991;67:785–795. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- 8.Old J. Tumor necrosis factor (TNF) Science. 1985;230:6630–6632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- 9.Beutler B, Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Ann Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 10.Takei Y, Wada K, Chiba T, Hayashi H, Ishihara H, Onozaki K. Development of glycosylated human interleukin-1 alpha, neoglyco IL-1 alpha, coupled with D-mannose dimer: synthesis and biological activities in vitro. Lymphokine Cytokine Res. 1994;13:265–270. [PubMed] [Google Scholar]
- 11.Takei Y, Wada K, Chiba T, Hayashi H, Yamada M, Kuwashima J, Onozaki K. Glycosylated human recombinant interleukin-1 alpha, neo interleukin-1 alpha, with D-mannose dimmer exhibits selective activities in vivo. Lymphokine Cytokine Res. 1995;15:713–719. doi: 10.1089/jir.1995.15.713. [DOI] [PubMed] [Google Scholar]
- 12.Takei Y, Yang D, Chiba T, Nabeshima S, Naruoka M, Wada K, Onozaki K. D-mannose dimmer introduced human recombinant interleukin-1 alpha, neo IL-1 alpha exhibits altered tissue distribution in mice. J Interferon Cytokine Res. 1996;16:333–336. doi: 10.1089/jir.1996.16.333. [DOI] [PubMed] [Google Scholar]
- 13.Chiba T, Nabeshima S, Takei Y, Onozaki K. Development of glycosylated human interleukin-1alpha, neoglyco IL-1alpha, by coupling with D-galactose monosaccharide: synthesis and purification. Glycoconjugate J. 1998;15:63–67. doi: 10.1023/A:1006991416735. [DOI] [PubMed] [Google Scholar]
- 14.Nabeshima S, Chiba T, Takei Y, Watanabe S, Okuyama H, Onozaki K. Development of glycosylated human interleukin-1alpha, neoglyco IL-1alpha, by coupling with D-galactose monosaccharide: biological activities in vitro. Glycoconjugate J. 1998;15:69–74. doi: 10.1023/A:1006943500806. [DOI] [PubMed] [Google Scholar]
- 15.Nabeshima S, Chiba T, Takei Y, Ono A, Moriya K, Onozaki K. Development of glycosylated human interleukin-1alpha, neoglyco IL-1alpha, coupled with D-galactose monosaccharide: biological activities in vivo. Glycoconjugate J. 1998;15:491–498. doi: 10.1023/A:1006987020372. [DOI] [PubMed] [Google Scholar]
- 16.Schauer R. Sialic acids: fascinating sugars in higher animals and man. Zoology (Jena) 2004;107:49–64. doi: 10.1016/j.zool.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Ashwell G, Morell AG. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41:99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- 18.van Rijk, Heinsius HL, van den Hamer CJ. Preparation of 131I-asialo-alpha1-acid glycoprotein. Vox Sang. 1976;30:412–419. doi: 10.1111/j.1423-0410.1976.tb02846.x. [DOI] [PubMed] [Google Scholar]
- 19.Crocker PR, Varki A. Siglecs, sialic acids and innate immunity. Trends Immunol. 2001;22:337–42. doi: 10.1016/S1471-4906(01)01930-5. [DOI] [PubMed] [Google Scholar]
- 20.Crocker PR. Siglecs in innate immunity. Curr Opin Pharmacol. 2005;5:431–437. doi: 10.1016/j.coph.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Chiba T, Moriya K, Nabeshima S, Hayashi H, Kobayashi Y, Sasayama S, Onozaki K. Synthesis of glycosylated human interleukin-1alpha, neoglyco IL-1alpha, coupled with N-acetylneuraminic acid. Glycoconj J. 1999;16:499–505. doi: 10.1023/A:1007017920392. [DOI] [PubMed] [Google Scholar]
- 22.Moriya K, Chiba T, Nabeshima S, Hayashi H, Onozaki K. In vitro biological activities of glycosylated human interleukin-1alpha, neoglyco IL-1alpha, coupled with N-acetylneuraminic acid. Glycoconj J. 1999;16:563–568. doi: 10.1023/A:1007082207188. [DOI] [PubMed] [Google Scholar]
- 23.Sasayama S, Moriya K, Chiba T, Matsumura T, Hayashi H, Hayashi A, Onozaki K. Glycosylated human interleukin-1alpha, neoglyco IL-1alpha, coupled with N-acetylneuraminic acid exhibits selective activities in vivo and altered tissue distribution. Glycoconj J. 2000;17:353–359. doi: 10.1023/A:1007181929405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda T, Hirano T, Kishimoto T. Establishment of an interleukin 6 (IL 6)/B cell stimulatory factor 2-dependent cell line and preparation of anti-IL 6 monoclonal antibodies. Eur J Immunol. 1988;18:951–956. doi: 10.1002/eji.1830180618. [DOI] [PubMed] [Google Scholar]
- 26.Onozaki K, Matsushima K, Aggarwal BB, Oppenheim JJ. Human interleukin 1 is a cytocidal factor for several tumor cell lines. J Immunol. 1985;135:3962–3968. [PubMed] [Google Scholar]
- 27.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 28.Hattori T, Hayashi H, Chiba T, Onozaki K. Activation of two distinct anti-proliferative pathways, apoptosis and p38 MAP kinase-dependent cell cycle arrest, by tumor necrosis factor in human melanoma cell line A375. Eur Cytokine Netw. 2001;12:244–252. [PubMed] [Google Scholar]
- 29.Sato T., Asamitsu K., Yang JP, Takahashi N, Tetsuka T, Yoneyama A, Kanagawa A., Okamoto T. Inhibition of human immunodeficiency virus type 1 replication by a bioavailable serine/threonine kinase inhibitor, fasudil hydrochloride. AIDS Res Hum Retroviruses. 1998;14:293–298. doi: 10.1089/aid.1998.14.293. [DOI] [PubMed] [Google Scholar]
- 30.Pennica D, Nedwin GE, Hayflick JS, Seeburg PF, Derynck R, Palladino MA, Kohr WJ, Aggarwal BB, Goeddel DV. Nature. 1984;312:724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- 31.Eck MJ, Sprang SR. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. J Biol Chem. 1989;264:17595–17605. doi: 10.2210/pdb1tnf/pdb. [DOI] [PubMed] [Google Scholar]
- 32.Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA, Jr, Goeddel DV. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci USA. 1991;88:9292–9296. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis M, Tartaglia LA, Lee A, Bennett GL, Rice GC, Wong GH, Chen EV, Goeddel DV. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc Natl Acad Sci USA. 1991;88:2830–2834. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]