Abstract
Developing a cancer vaccine with a potent adjuvant, which is safe for human use, remains to be an unmet need. In this study, we developed a simple, safe, yet efficient, peptide-based therapeutic cancer vaccine, DOTAP/E7 complex, which comprises only two molecules: a DOTAP cationic lipid and a peptide antigen derived from E7 oncoprotein of human papillomavirus (HPV) type 16. The anti-cancer activity of DOTAP/E7 against existing HPV positive TC-1 tumor was compared to that of our previous LPD/E7 formulation, which contains bacterial DNA CpG motifs. Tumor-bearing mice showed significant tumor inhibition following a single vaccination of either formulation at the optimal lipid dose, suggesting that DOTAP liposome alone can provide a potent adjuvant activity without plasmid DNA. E7 peptide formulated with DOTAP induced migration of activated dendritic cells (DC) to the draining lymph node (DLN) and efficiently generated functional antigen-specific CD8+ T lymphocyte responses. Accumulation of CD8+ tumor infiltrating T cells and apoptosis at tumor sites were observed after treatment with DOTAP/E7 complexes, which was also associated with a decreased amount of CD25+Foxp3+ regulatory T cells in treated animals. Reactive oxygen species (ROS) induced by DOTAP cationic lipid in DLN revealed a plausible mechanism of the initial interaction between DC and DOTAP. An adequate amount of ROS generation was apparently required for the initiation of the vaccine mechanism; however, an overdose of DOTAP induced massive ROS production and apoptosis of DC in DLN, which led to diminished anti-cancer immunity. Overall, these results indicate that cationic lipid DOTAP alone serves as an efficient vaccine adjuvant for the induction of a therapeutic, antigen-specific anti-cancer activity.
Keywords: Cancer immunotherapy, Cationic liposomes, Cervical cancer, CTL, ROS, Vaccine adjuvant
Introduction
The development of safe and effective therapeutic cancer vaccines for human use remains an urgent and unmet medical need. In order to elicit appropriate protective immune responses, the use of immunologic adjuvant to enhance and direct the immune response is required in a rational vaccine design [1]. For vaccines composed of synthetic peptide or subunit antigen, which are often unable to induce adequate immune responses, an appropriate adjuvant is necessary to improve the immune response and process a weakly immunogenic antigen. An ideal adjuvant for a therapeutic cancer vaccine should: increase the biological or immunological half-life of the vaccine antigen; improve antigen delivery and/or processing in the antigen presenting cells (APC) [2]; reduce the number of immunizations or the amount of antigen required for an effective vaccine [3]; induce the production of immunomodulatory cytokines that favor the development of Th1 immune responses to the vaccine antigen, thus promoting cell-mediated immunity including CTL [4]; and finally, overcome immune tolerance to tumor by inhibiting immune suppressive factors [5].
Previous work in our lab has led to the development of a liposome-based, nanoparticle delivery system, called LPD [6]. LPD, originally designed as a gene delivery system, is self-assembled from cationic liposomes, polycations and plasmid DNA. LPD is also a novel vaccine adjuvant for stimulating both humoral and cellular immune responses [7]. Intravenous administration of LPD potentiates efficient anti-tumor activity in a murine tumor model 24 JK, even when the formulation contained an empty, non-coding plasmid [8]. It was further correlated with its ability to induce Th-1 cytokines such as TNF-α, IFN-β and IL-12 and to the development of tumor-specific CD8+ cytotoxic lymphocytes [9]. The observed anti-tumor activity originates from the innate immunostimulatory activity of the unmethylated CpG motif, abundantly present in the plasmid DNA [10]. Cationic liposomes are capable of protecting plasmid DNA from extracellular degradation and thus more DNA enters the endosomal compartment where the toll-like receptor 9 (TLR9) is selectively expressed [11], [12]. However, cationic liposomes alone are relatively inert in terms of activating immune responses and there have been no studies so far to support that the cationic liposome by itself can serve as an efficient adjuvant [13], [14].
Our previous studies have shown that a strong antigen-specific anti-tumor response was observed in mice, which received LPD particles loaded with an MHC class I-restricted peptide (RAHYNIVTF) epitope derived from the E7 protein of human papillomavirus (HPV) type 16 [15], [16]. It induced an E7-specific CTL response, which contributed both to protective and therapeutic effects against E7-expressing TC-1 tumors, a murine model for human cervical cancer [17]. Recently, we have also investigated structure-specific immunomodulatory effects of cationic lipids on dendritic cells [18]. Our data suggest that some of the cationic lipids alone can activate dendritic cells toward expression of surface markers, CD80 and CD86. Moreover, we found that cationic liposomes, such as DOTAP, could stimulate DC, resulting in MAP kinase ERK activation and chemokine induction, such as CCL-2 [19]. The fact that cationic lipids alone could stimulate the antigen presenting cells was of high importance, since the immunomodulatory adjuvant activities of LPD were initially thought to arise only by unmethylated CpG motifs of the plasmid DNA via the TLR9 [20], [21].
The results prompted us to initiate a series of studies to assess whether cationic DOTAP liposome by itself could function as an immune stimulator such that an effective vaccine could be formulated. In the present study, we examined for the first time the immunological mechanism and anti-cancer effect of an efficient therapeutic cancer vaccine formulation, DOTAP/E7, which contains only an antigen and a cationic lipid. We describe herein the anti-tumor activity of this simple vaccine.
Materials and methods
Lipids, reagents and murine tumor cell lines
DOTAP and other lipids were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). Protamine sulfate was purchased from Sigma-Aldrich (St. Louis, MO, USA) and plasmid DNA (pNGVL3) was obtained from the National Gene Vector Laboratory (Ann Arbor, MI, USA). CpG ODN1826 was requested from IDT, Inc. (Coralville, IA, USA) and the complete Freund’s adjuvant (CFA) was purchased from DIFCO Laboratories (Detroit, MI, USA). Murine TC-1 cells were kindly provided by Dr. T.C. Wu at Johns Hopkins University (Baltimore, MD, USA). TC-1 cells are C57BL/6 mouse lung epithelial cells transformed with HPV 16 E6 and E7 oncogenes and the activated H-ras. BL6 are melanoma cells derived from C57BL/6. Both murine tumor cell lines were maintained in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen).
Peptide synthesis
H-2Db restricted peptide RAHYNIVTF derived from HPV 16 E7 protein (amino acid 49–57) was synthesized and purified in Molecular Medicine Institute Peptide Synthesis Facility in the University of Pittsburgh.
Preparation and characterization of DOTAP/E7 and other liposomal complexes
Cell culture grade water (Cambrex, Walkersville, MD, USA) was used in all liposome preparation procedures. Briefly, lipid films were made in a glass vial by evaporating the chloroform solution under a steady stream of dry nitrogen gas. Traces of organic solvent were removed by keeping the films under vacuum with desiccators overnight. Lipid films were hydrated for 12 h by adding the required amount of water to make a final concentration of 10 mg/ml. The suspensions were then sonicated in a bath-type sonicator for 10 min followed by extrusion (Hamilton Co., Reno, NV, USA) through 400, 200 and 100 nm membrane filters and were stored at 4°C before use. For the preparation of DOTAP/E7, the lipid film was hydrated with an aqueous solution of E7 peptide instead of water. LPD formulation comprises DOTAP lipid, protamine and plasmid DNA at a ratio of 8.6:0.6:1 (w/w/w). LPD was made as described [16] and the complex was allowed to stay at room temperature for at least 20 min prior to the injection.
The particle size and the zeta (ζ) potential of the liposomal complexes were measured following the manufacturer’s instruction using a Coulter N4 Plus particle sizer (Beckman Coulter, San Francisco, CA, USA) and a ZetaPlus (Brookhaven Instruments, Corp., Holtsville, NY, USA). Peptide encapsulation was determined by the percentage of liposome-bound peptide. In brief, the unbound peptide from DOTAP/E7 and LPD/E7 was separated from the complex by a Microcon® centrifugal filtrate device (Millipore, Bedford, MA, USA) and the concentration of unbound peptide was measured by Micro BCATM protein assay kit (Pierce, Rockford, IL, USA). The efficiency of peptide encapsulation was determined as (1-% unbound peptide) and was reported as mean ± SD (n = 3).
Mice and immunizations
All work performed on animals was in accordance with and permitted by our institutional IACUC. C57BL/6 female mice, 6–7 weeks old were purchased from Charles River Laboratories (Wilmington, MA, USA) and were used in all animal studies. Subcutaneous tumors were established by injecting TC-1 cells (105 cells) into the hair-trimmed flank of the mouse on day 0. On day 6, mice (n = 6–12) were injected subcutaneously (sc) at the opposite side of the flank with 150 μl of selected formulations containing 10 μg of the E7 peptide. The size of the tumor was measured using a caliper two or three times a week. Tumor size was determined by multiplying the two largest dimensions of the solid tumor.
Antibodies and flow cytometric analysis
All anti-mouse antibodies used for flow cytometric analysis were purchased from BD Pharmingen (San Diego, CA, USA) or eBioscience, Inc. (San Diego, CA, USA). Spleen cells or lymph node cells were harvested and dissociated by incubating with 1 mg/ml collagenase (Sigma-Aldrich) for 20 min and passing through a 70 μm cell strainer (BD Biosciences). After removal of red blood cells (RBC), the cell suspensions were incubated with anti-CD16/CD32 (24.G2) on ice for 15 min to block non-specific binding, followed by immunostaining with fluorescently conjugated antibodies to surface antigens for 30 min at 4°C. The following Abs were used: anti-CD3e (145-2c11), anti-CD4 (RM4–5), anti-CD8a (53–6.7), anti-CD11c (HL3), anti-CD25 (pc61.5), anti-CD80 (16-10A1), anti-CD86 (GL1) and anti-NK1.1 (pk136). Isotype control Abs were used to set the background for the surface Ab labels. Prior to staining intracellular molecules such as Foxp3 and IFN-γ, cells were fixed and permeabilized using the Cytofix/Cytoperm™ kit (BD Pharmingen) according to the manufacturer’s instruction. Cells were finally resuspended in 300 μl stain buffer and analyzed using a BD FACSCanto digital flow cytometer (San Diego, CA, USA).
Tracking uptake of fluorescent DOTAP/E7 complexes in vivo
The mice were sc injected with DOTAP/E7 containing 100 nmol total lipid with 0.05% (w/w) NBD-DOTAP. The draining lymph nodes (DLNs) were isolated at various time points after injection. Cell suspensions were stained with appropriate antibodies and analyzed by flow cytometry. Analysis gates were set on live cells based on the forward and side scatter characteristics.
Immunohistochemistry and TUNEL
Solid TC-1 tumors were established as described and the mice were given treatments on day 6. On day 14, tumor samples were dissected and embedded in Tissue-Tek® OCT compound (Sakura Finetek, Torrance, CA, USA) followed by cryosection preparation. The samples were cut into 8 μm thick sections with a cryostat (Hacker Instruments & Industries Inc., Winnsboro, SC, USA). The sections were stained with FITC-conjugated anti-CD8 or anti-CD4 antibodies (Miltenyi Biotec Inc. Auburn, CA, USA) to determine tumor-infiltrating T cells. The samples were mounted on a coverslip using Vectashield® mounting solution (Vector Laboratories, Inc., Burlingame, CA, USA) containing DAPI to counterstain nuclei. Images of the sections were taken using a Leica SP2 confocal microscope.
TUNEL assay was conducted using a TACSTM TdT Kit (R&D Systems, Minneapolis, MN, USA) and developed with DAB according to manufacturer’s instructions. Samples were imaged using a Nikon Microphot SA microscope.
Analysis of Ag specific CTL and immune response
Naïve C57BL/6 mice were immunized with 10 μg E7 peptide formulated in DOTAP liposomal formulations on day 0 and 7. For in vitro CTL assay, the mice were killed 7 days after the last immunization and spleen cells were isolated and dissociated. After RBC lysis, the total spleen cells (responder cells) were in vitro stimulated for 5 days with E7 peptide (10 μg/ml) in the presence of β-mercaptoethanol and 40 U/ml recombinant mIL-2 (R&D Systems) in complete RPMI-1640 medium. After 5-day of CTL expansion, responder cells were used as CTL effectors while TC-1 tumor cells were used as target cells in this assay. To discriminate between effectors and targets, TC-1 cells were labeled with PKH-67 (Sigma-Aldrich) according to manufacturer’s instructions. The mixture of effectors and labeled targets were plated into 96-well U-bottom plates at various effector:target (E:T) ratios and the cell lysis reactions were carried out for 4 h at 37°C. The cells were then harvested and stained with propidium iodide (Sigma Aldrich) prior to flow cytometric analysis. The percentage of E7-specific lysis was determined by the proportion of PI positive cells within the FL1 (PKH-67) positive region.
In vivo CTL activity of E7-specific cytotoxic T cells was enumerated according to the protocol of Byers et al. [22] with minor modifications. In brief, spleen cells from syngenic mice were RBC lysed followed by pulsing with 10 μM E7 peptide or without peptide in complete medium for 1 h at 37°C. Both spleen cell populations were stained with equal amount of 2 μM PKH-26 (Sigma-Aldrich) according to manufacturer’s instruction. The peptide pulsed and unpulsed populations were loaded with 4 and 0.4 μM CFSE (Molecular Probe), respectively, at 37°C for 15 min. The two cell populations were mixed together (1:1) before tail vein injection to the control or DOTAP/E7 immunized mice (107 cells per mouse injection). At 16 h after injection, spleen cells from the recipient mice were isolated and single cell suspension were prepared prior to flow cytometric analysis. The number of CFSEhigh and CFSElow population were determined and the in vivo E7 specific lysis percentage was enumerated according to a published equation [22].
For the measurement of IFN-γ producing CD8+ T cells, spleen cells were isolated from the control or immunized mice 7–10 days after the last immunization. For 6 h in the presence of 1 μl/ml of GolgiStopTM (BD Pharmingen), 2 × 106 spleen cells were incubated with 5 μg/ml E7 peptide or without peptide. After washing with FACS buffer, cells were stained with Abs to surface markers and intracellularly with IFN-γ mAb (XMG1.2) prior to analysis by flow cytometry.
Analysis of ROS cytotoxicity in the DLN
The mice were sc injected with DOTAP/E7 containing 0, 15, 100 or 600 nmol total lipid and the DLNs were isolated at a 2 h time point after injection for the analysis of ROS production. Single cell suspensions were prepared and incubated with 20 μM 2′, 7′-dichlorodihydrofluorescein diacetate (DCFH-DA) (Sigma-Aldrich) in serum free medium in the dark for 30 min at 37°C. The non-fluorescent DCFH-DA readily diffuses into the cells where it is hydrolyzed to become the polar derivative DCFH, which is oxidized in the presence of ROS to the highly fluorescent 2′, 7′-dichlorofluorescein (DCF) [23]. The cells were quickly washed with pulse spin and immediately analyzed by flow cytometry. For determining cytotoxicity induced by the high dose of DOTAP lipid, DLNs from the immunized mice were collected at 10 h after injection, followed by subsequent staining with Abs to the surface markers. Cell suspensions were added with propidium iodide prior to flow cytometric analysis. The percentage of cell death of DC in the DLN was determined by the proportion of PI+ cells within the CD11c+ region.
Statistical analysis
Data were analyzed statistically using a two-tailed Student’s t-test. Data were considered statistically significant when the P value was less than 0.05.
Results
Cationic DOTAP liposome as a potent cancer vaccine adjuvant, which provides a major contribution toward the anti-tumor activity of LPD vaccine formulation
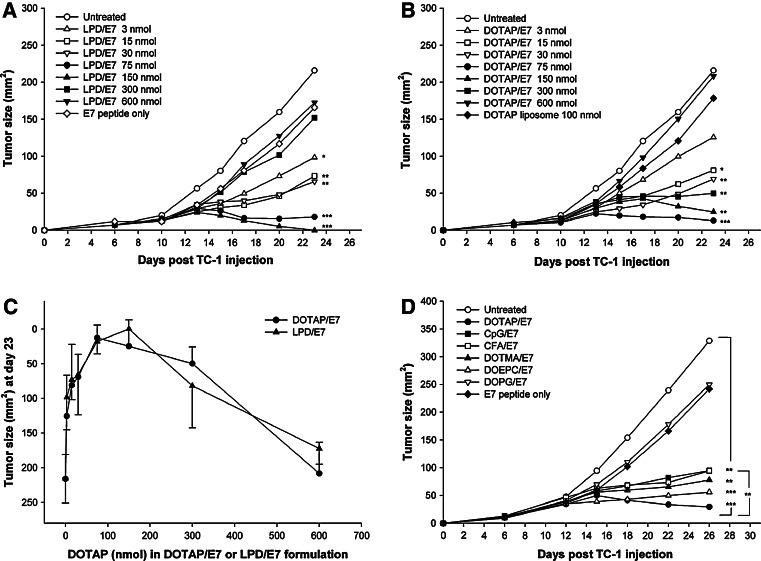
Previous investigations by our group showed that LPD encapsulated with an MHC class I peptide epitope derived from HPV (type 16) E7 protein can generate a preventive as well as a therapeutic effect against the HPV16 E7 positive tumor cell line, TC-1 [17], [16]. However, there are concerns regarding LPD/E7 vaccine formulation such as the possible toxicity, which can be induced by a high dose of lipid–DNA complex (lipoplex) [24]. To assess the adjuvant activity of lipoplex, we examined anti-TC-1 tumor activity of LPD/E7 formulation at various DOTAP lipid concentrations. TC-1 tumor bearing mice sc received 10 μg E7 peptide formulated in LPD on day 6 post tumor inoculation (Fig. 1a). DOTAP lipid concentrations in LPD varying from 3 to 600 nmol were investigated and the L:P:D weight ratio (8.6:0.6:1) was kept the same in all groups. The mice that received LPD/E7 containing the lowest amount of DOTAP (3 nmol) showed partial tumor inhibition (P < 0.05) compared to the untreated control group on day 23. LPD/E7 with DOTAP at 15 or 30 nmol exhibited an enhanced efficacy (P < 0.01), while LPD/E7 with 75 or 150 nmol lipid showed the most significant tumor regression (P < 0.001). Interestingly, mice given high doses of LPD (300 or 600 nmol) did not show significant tumor inhibition.
Fig. 1.
Kinetics of TC-1 tumor growth in mice treated with (a) LPD/E7 or (b) DOTAP/E7 formulation. a C57BL/6 female mice of 7 weeks old (n = 8–12) were injected sc with TC-1 tumor on day 0. On day 6, the mice received 10 μg E7 peptide formulated in LPD at various DOTAP lipid concentrations. The untreated mice were used as a negative control. b On day 6 post TC-1 inoculation, the mice received treatment of 10 μg E7 peptide formulated in DOTAP liposomes at various lipid concentrations. Tumor sizes were measured with calipers and determined by multiplying the two largest dimensions of the tumor. Tumor size of each group on day 23 was compared to the untreated control group and was analyzed statistically (*P < 0.05, **P < 0.01, ***P < 0.001). c The anti TC-1 tumor activity of the formulations DOTAP/E7 and LPD/E7 was contrasted on day 23 by comparing the tumor size at each corresponding DOTAP lipid concentration. No statistically significant differences in tumor size were found between the two groups. d Anti-cancer activity of DOTAP/E7 was compared to that of other cationic lipids (DOEPC and DOTMA), an anionic lipid (DOPG), CFA and CpG ODN1826. Tumor-bearing mice (n = 6–12) received a single treatment on day 6 after tumor inoculation. TC-1 tumor sizes were measured and analyzed statistically
Upon receiving cationic lipoplex, bacterial DNA containing immunostimulatory CpG motif activates host innate immune response, which includes the induction of proinflammatory cytokines and immune cell activation, especially in human [25]. To avoid the drawbacks of CpG effect and to assess whether cationic lipid alone could function as a cancer vaccine adjuvant, we have investigated a new formulation that only contains cationic DOTAP liposomes and the E7 peptide. The new formulation, DOTAP/E7, without the help of plasmid DNA and protamine sulfate, also exhibited anti TC-1 tumor effect (Fig. 1b). The tumor growth kinetics in mice treated with DOTAP/E7 was similar to that of the LPD/E7 formulation. Low dose of DOTAP (15 nmol) showed partial TC-1 tumor inhibition effect (P < 0.05) compared to the untreated control on day 23, while DOTAP at 30, 150 or 300 nmol exhibited an enhanced efficacy (P < 0.01). DOTAP at 75 nmol showed the most significant tumor regression effect (P < 0.001). Again, mice given a high dose of DOTAP (600 nmol) did not show anti-tumor activity, suggesting that DOTAP liposomes at a high dose might have induced a negative regulation to the immune response. In addition, DOTAP liposomes at optimal dose, but without E7 peptide, did not show significant inhibition, indicating that the anti-tumor effect was antigen specific. Anti TC-1 tumor activity of both DOTAP/E7 and LPD/E7 formulations was compared on day 23 by plotting the tumor size at each corresponding DOTAP lipid concentration (Fig. 1c). The data is pooled from three independent experiments and it is evident that these two dose–response curves are almost identical to each other and no significant difference was found in any paired tumor size comparison. This result suggests that the DOTAP liposome is the major active ingredient in the LPD formulation to provoke immune response. Plasmid DNA and protamine are not necessary for the potent anti-tumor activity of the vaccine. We have also shown that low or non-detectable TNF-α production (<10 pg/ml) in the sera of mice injected with DOTAP/E7, indicating low toxicity of our vaccine formulation. Also, according to the bell-shape curves in Fig. 1c, the optimal dose of DOTAP liposome is around 100 nmol and both low and high doses compromise its anti-tumor activity. This finding tempted us to investigate the role of DOTAP as a vaccine adjuvant in supporting the anti-cancer activity of DOTAP/E7.
To assess the efficacy of DOTAP/E7-induced immune response compared to other adjuvants, tumor-bearing mice were treated on day 6 with E7 peptide formulated in DOTAP, two additional cationic liposomes, DOEPC and DOTMA, an anionic lipid, DOPG, CFA and CpG ODN1826. In Fig. 1d, the mice that received the above formulations showed significantly smaller tumor sizes compared to the untreated group on day 26, except for those that received DOPG/E7, which did not show tumor regression. More importantly, the mice that received DOTAP/E7 formulation exhibited a better anti-cancer activity (P < 0.01) compared to those that received CpG/E7 or CFA/E7 formulations.
Physical properties of DOTAP/E7 complexes and the efficiency of E7 peptide entrapment in the liposome
DOTAP liposomes and DOTAP/E7 particles were prepared at 0.1 mM and their particle sizes and zeta (ζ) potentials were characterized. The particle size of DOTAP/E7 was not significantly changed compared to that of the plain DOTAP liposome, which was 100 ± 24 nm in diameter. Also, the zeta potential of DOTAP/E7 particle was similar to that of DOTAP liposomes (43.5 ± 7.8 mV), indicating that the particle remains positively charged after changing the composition of DOTAP lipid and peptide. E7 peptide entrapment efficiency in DOTAP/E7 particles, which was determined by the percentage of liposome-bound E7 peptide was around 32 ± 4% in both DOTAP/E7 containing 100 or 600 nmol total lipids, whereas DOTAP/E7 with 15 nmol lipid showed a lower entrapment efficiency (17 ± 4%).
DOTAP/E7 particles are mainly taken up by dendritic cells after sc injection, resulting in the migration of activated DC to the DLN
Previous studies from our lab describe the ability of cationic liposome to activate mouse bone marrow dendritic cells in vitro [18], and the production of IL-12 cytokine by BMDC increases in response to DOTAP concentration (data not shown). The findings prompted us to hypothesize that DC, the professional APC, would be the direct responder after sc injection of DOTAP/E7 vaccine in vivo. To address the effect of DOTAP/E7 on DC activation and migration to the DLN, naïve mice were sc injected with DOTAP/E7 containing 0.5% (w/w) NBD-DOTAP at the flank. DLNs were harvested and analyzed by flow cytometry at different time points, post injection. The numbers of CD11c+ cells among total lymph node cells were increased more than 2.5-fold compared to the untreated at 4 h after NBD-DOTAP/E7 injection (Fig. 2a, b). The expression of costimulatory molecule CD86 on the NBD+ cells was investigated. NBD+ cells demonstrated high levels of CD86 (Fig. 2c, d), indicating that sc injection of DOTAP prompted DC activation. NBD uptake by other cell types such as T lymphocytes was also investigated by co-staining with anti-CD3, CD4 and CD8 Abs. The dot plots in Fig. 2e, f were gated on the CD3+ population. Neither of the CD8+ or CD8− cells showed NBD uptake after NBD-DOTAP injection. The results clearly demonstrated that NBD-DOTAP is mainly taken up by DC (∼80%) soon after immunization and DOTAP induces migration of activated DC to the DLN, resulting in DC–T cell interactions and eliciting T cell responses.
Fig. 2.
Subcutaneous injection of DOTAP induces DC activation and migration to the DLN. Naïve mice (n = 4–6) were injected sc with PBS control (a, c, e) or DOTAP/E7 containing 100 nmol total lipid with 0.5% NBD-DOTAP (b, d, f). At 4 h after the injection, DLNs were prepared and stained with appropriate antibodies to surface markers. The co-expression of NBD and CD11c (a, b) or CD86 (c, d) was analyzed within total lymph node cells, whereas the co-expression of NBD and CD8 (e, f) were gated and analyzed within the CD3+ population. The numbers represented the percentages of cells in the quadrants
Tumor infiltrating T lymphocytes were found in mice treated with DOTAP/E7 at optimal lipid dose
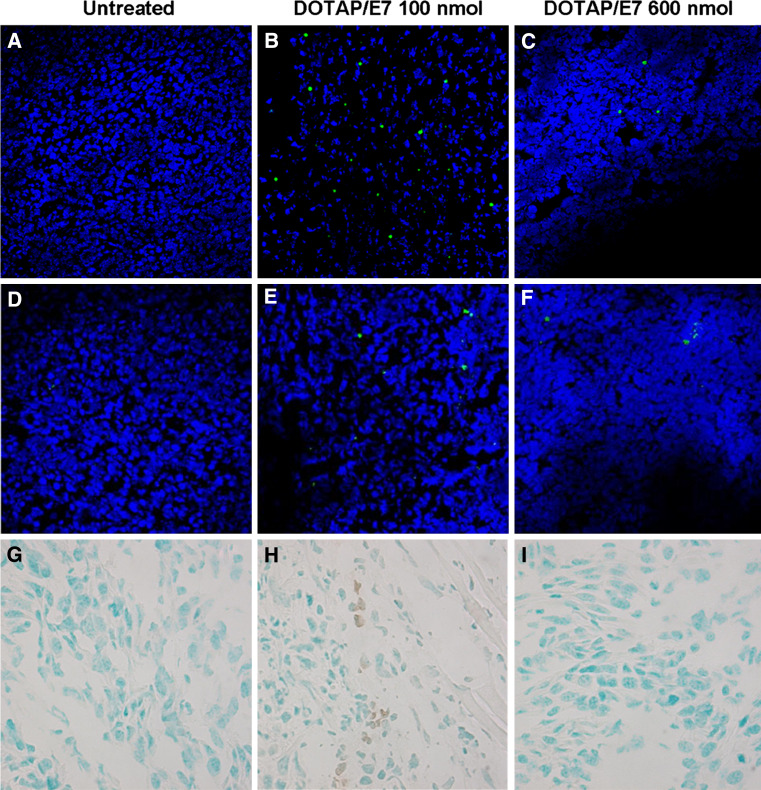
To understand whether the optimal DOTAP/E7 formulation induces migration of T lymphocytes to the tumor microenvironment, we performed immunohistochemistry staining on the frozen tumor tissues to examine tumor-infiltrating T lymphocytes (Fig. 3). TC-1 tumor-bearing mice were treated with PBS, DOTAP/E7 (containing 15, 100 or 600 nmol lipid) or LPD/E7 as described. On day 14 after TC-1 inoculation, solid tumors were harvested for preparation of cryosection and immunostaining. An increased amount of CD8+ T lymphocytes (∼5%) were found in mice that received LPD/E7 (data not shown) or DOTAP/E7 at the optimal lipid dose (100 nmol, Fig. 3b) compared to untreated (Fig. 3a) or those received DOTAP overdose (600 nmol, Fig. 3c). A similar result was found for CD4+ T cells; around 2% of tumor infiltrating CD4+ T lymphocytes were found in mice that received an optimal dose of DOTAP (Fig. 3e), but not in the untreated or overdosed group. TUNEL assay for determining apoptotic cells was also performed in tumor cryosections. A TUNEL-positive reaction was observed in the condensed and fragmented nuclei of the tumor cells after the mice were treated with LPD/E7 (data not shown) or DOTAP/E7 at an optimal dose (Fig. 3h) compared to the untreated (Fig. 3g) and overdosed group (Fig. 3i), which exhibited normal and vital tumor cells. Similar result, from Hematoxylin and Eosin staining (data not shown), showed that tumor necrosis was found only in the mice treated with E7 formulated with an optimal dose of DOTAP. The results indicate that the eliciting CD8+ T lymphocytes were attracted to the tumor microenvironment and tumor cells were undergoing apoptosis or necrosis upon DOTAP/E7 treatment.
Fig. 3.
Tumor infiltrating T-lymphocytes were found in mice that received DOTAP/E7 at an optimal dose. TC-1 tumors were established as described and were left untreated or given treatment on day 6. Solid tumors were dissected on day 14 and examined for infiltrating lymphocytes. FITC conjugated anti-CD8 (a, b, c) and anti-CD4 (d, e, f) antibodies were used to determine tumor infiltrating T cells followed by counterstaining with DAPI. Representative tumor sections from groups of three mice were examined as described and imaged by confocal microscopy. TUNEL assay was conducted to detect cell apoptosis in the tumor sections (g, h, i)
Decreased amount of Treg population in DOTAP/E7 treated mice was correlated with the anti-tumor activity
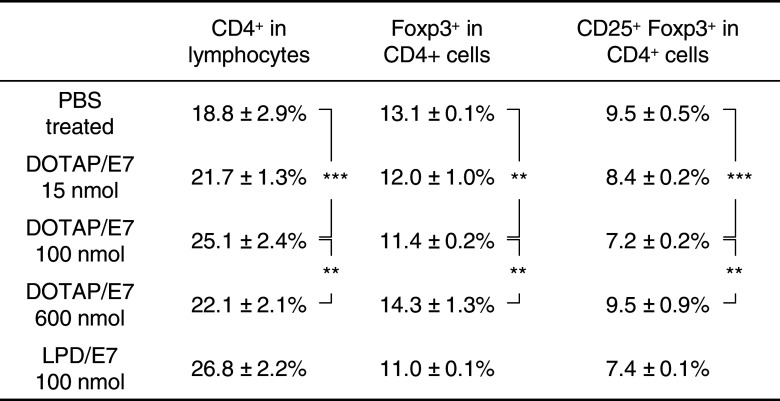
Despite intense recent interest, the suppressive mechanisms of regulatory CD4+CD25+ T cells (Tregs) remain poorly understood. Tregs are thought to dampen functional T cell immunity to tumor-associated antigens and to be the main obstacle, hampering successful immunotherapy and active vaccination [26]. It becomes necessary to monitor and characterize both effector and Treg responses in hosts that receive candidate tumor vaccine [5]. In Table 1, ten representative experiments are summarized on the phenotype analysis for the spleen cells obtained from tumor-bearing mice after vaccine treatment. The population of CD4+ T cells was significantly increased in spleen cells from mice that received DOTAP/E7 treatment with an optimal lipid dose. The percentage of CD4+ within the total lymphocyte population was shown. An increased amount of CD8+ T cells was also observed (data not shown). In addition, a significant decrease in the Treg population (CD4+Foxp3+ and CD4+CD25+Foxp3+) was found in this treatment group, which is correlated with the results of anti-tumor activity. Similar results were observed in the LPD/E7 treated group. On the other hand, spleen cells collected from mice that received overdosed DOTAP did not show significant difference in the Treg population compared to the untreated tumor bearing mice. This result indicates that there is evidence for preferential expansion of T effector cells relative to Tregs after the treatment of DOTAP/E7 vaccine at an optimal lipid dose.
Table 1.
Treg subpopulation profile in spleen cells obtained from tumor-bearing mice after treatment with DOTAP/E7 vaccine
** P < 0.01, *** P < 0.001 (n = 10 per group)
In vitro CTL-mediated and NK cell-mediated cytotoxicity
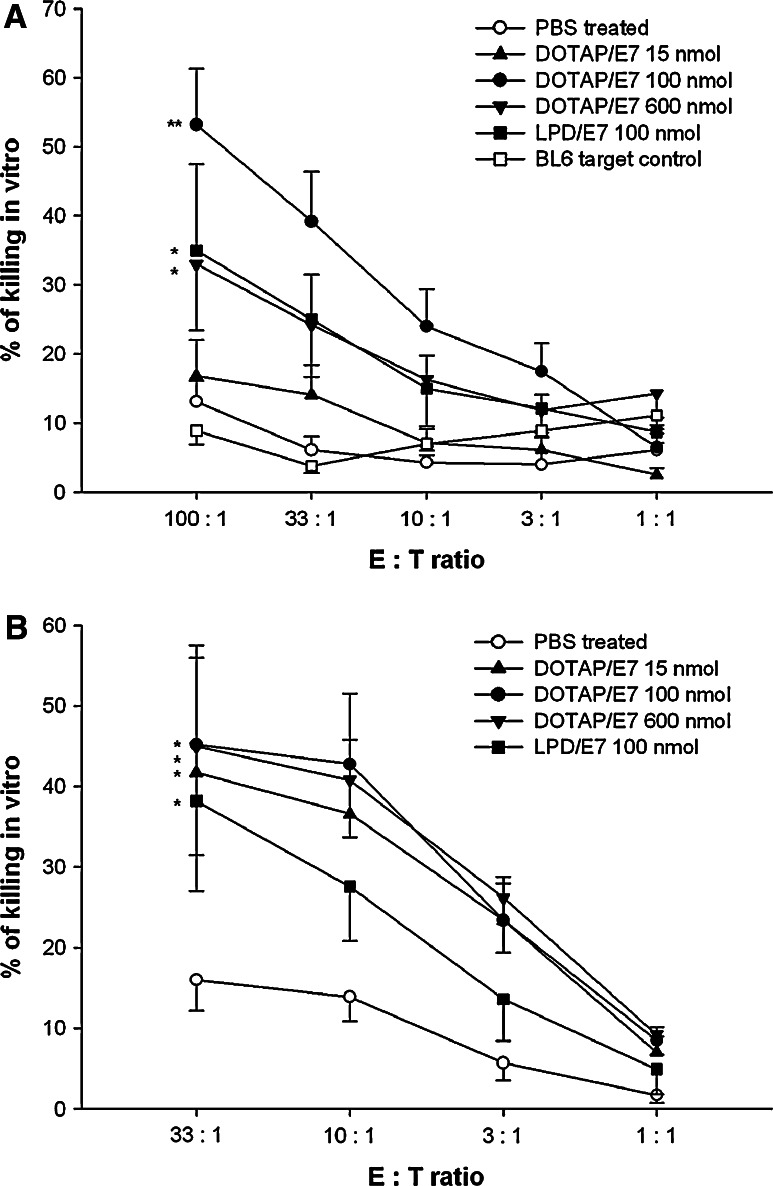
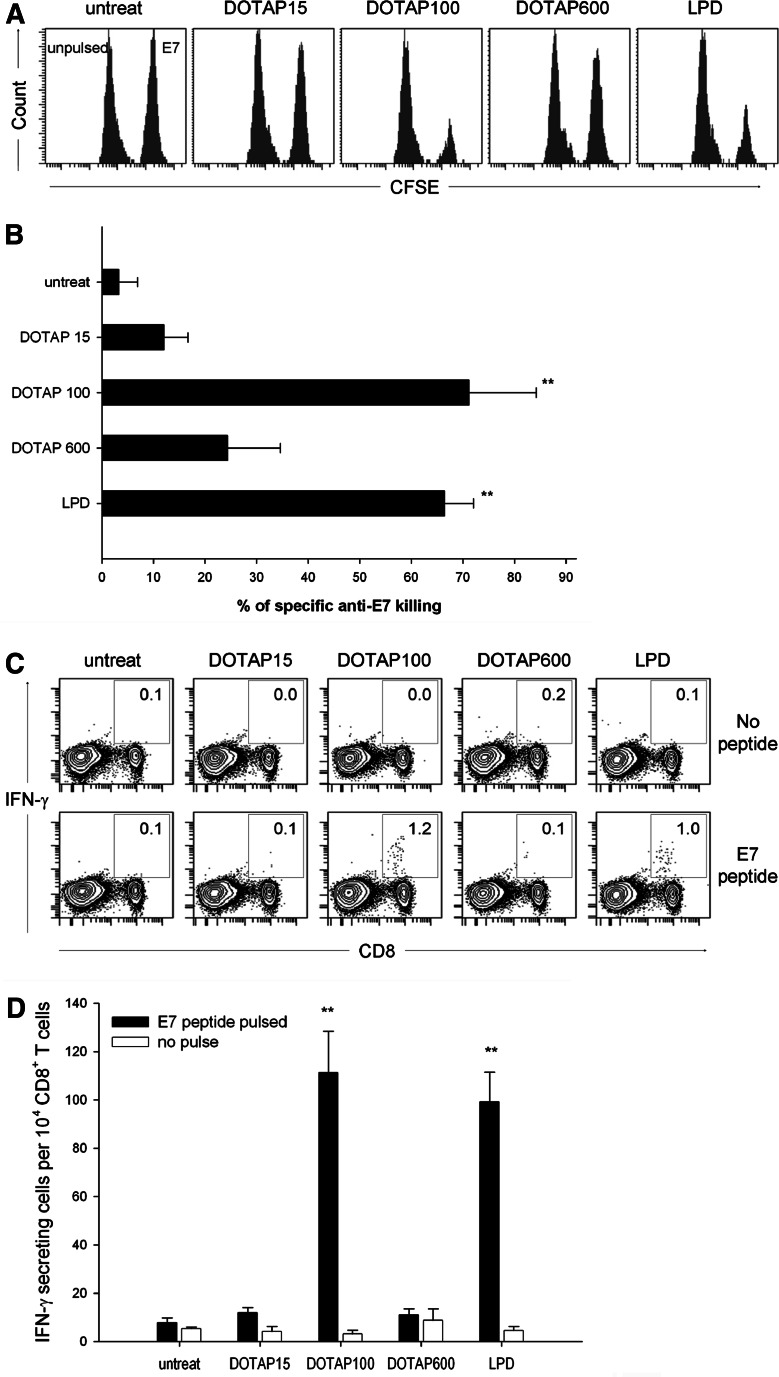
Since cytotoxic T lymphocytes (CTL) are most capable of killing tumor cells, vaccines that induce these immune responses are important for eradicating tumor growth or preventing cancer recurrences. To evaluate whether vaccination by DOTAP/E7 would be suitable for inducing a primary CTL response, we prepared effector cells by collecting spleen cells from immunized mice at 1 week after the last immunization, followed by in vitro culture with 5 μg/ml E7 peptide for 5 days. As depicted in Fig. 4a, a cytotoxicity assay was carried out by incubating effector cells with PKH-67 labeled TC-1 target cells for 4 h and the percentage of killing was revealed by adding propidium iodide to label the killed cells, followed by flow cytometric analysis. Spleen cells recovered from mice that received DOTAP/E7 at an optimal lipid dose exhibited significant CTL activity against TC-1 cells, while mice that received LPD/E7 or DOTAP overdose showed a moderate killing effect (Fig. 4a). We confirmed that the killing effect was E7-specific by incubating effector cells with E7-negative BL6 cells as targets, and negligible cytotoxicity was found. In addition to CTL-mediated killing, we also investigated natural killer (NK) cells-mediated cytotoxicity (Fig. 4b). NK cells are reported as being able to destroy tumor cells without deliberate immunization or activation and also play an important role in the innate immune response to interact with dendritic cells [27], [28]. In comparison with the PBS control, spleen cells directly harvested from mice that received liposomal peptide vaccines, without in vitro restimulation process, exhibited significant killing of YAC-1 cells, an NK susceptible target. The data indicated that DOTAP/E7 formulations were capable of stimulating NK cells, which were present in the effector cell population.
Fig. 4.
Analysis for CTL-mediated (a) and NK cell-mediated (b) cytotoxicity by flow cytometry. a E7-specific CTL clones were prepared and expanded as described in “Materials and Methods”. After 5 days in vitro restimulation with E7 peptide, effector cells were incubated with PKH-67 labeled TC-1 target cells at the indicated effector:target ratio for 4 h at 37°C. BL-6 was used as a non-specific target cell control. Percentage of E7-specific killing was determined by the proportion of PI positive cells within the gating of PKH-67 positive target cells. Statistical analysis was calculated by comparing treatment group and control group at 100:1 E:T ratio (*P < 0.05, **P < 0.01, n = 5). b On day 7 after the last immunization, splenocytes from each group were harvested and incubated with PKH-67 labeled YAC-1 cells at the indicated E:T ratio for 4 h at 37°C. Percentage of killing was determined as described. Statistical analysis was done by comparing the treatment group and control group at 33:1 E:T ratio (*P < 0.05, n = 3)
CD8+ T cells elicited by DOTAP/E7 vaccination are functionally active in vivo
In vitro CTL was measured after expansion of the splenocytes with antigen in vitro; however, there is a possibility of over- or underestimating the CTL function due to the restimulation process. Thus, we evaluated whether the observed in vitro CTL was also relevant in vivo (Fig. 5a). A mixture containing equal amounts of E7-pulsed CFSEhigh and unpulsed CFSElow spleen cells from a syngenic donor was injected iv into the mice, 1 week after the last DOTAP/E7 immunization. The specific lysis of target cells was analyzed by flow cytometry at 16 h after adoptive transfer. The percentage of E7-specific killing from mice that received an optimal dose of DOTAP/E7 or LPD/E7 was more than 65%, while in the groups that received DOTAP at low dose and overdose showed only ∼15 and 25% specific killing, respectively (Fig 5b).
Fig. 5.
Immunization with E7 peptide formulated in DOTAP adjuvant elicits functional CD8+ T cells. a C57BL/6 mice were immunized sc with 10 μg E7 peptide formulated in DOTAP at low, optimal, overdose or in LPD on day 0 and day 10. Seven days after the last immunization, representative mice were injected iv with an equivalent amount of E7-pulsed (labeled with 4 μM CFSE) and non-pulsed (labeled with 0.4 μM CFSE) spleen cells from a syngenic donor. The spleen cells were harvested from the recipient mice 16 h later and the proportion of the CFSEhigh and CFSElow cells were analyzed by flow cytometry. b Percentage of specific anti-E7 killing was compared to that of the untreated control (n = 4) and was statistically analyzed by t-test (P < 0.01). c IFN-γ production by CD8+ cells after in vitro stimulation with E7 peptide. Seven days after the last immunization, the mice were killed and spleen cells were isolated. The total spleen cells were stimulated with 5 μg/ml E7 peptide for 6 h, followed by subsequent staining with surface CD8 marker and intracellular IFN-γ prior to FACS analysis. The numbers shown on contour plots represent the percentage of CD8+IFN-γ+ T cells gated on total CD8+ cells. Representative figures in the four experiments performed. d The numbers of CD8+IFN-γ+ cells per 104 CD8+ T cells were shown as mean ± SD and were compared to that of the untreated control (n = 4, **P < 0.01)
IFN-γ secreted by activated T cells or NK cells play an important role in Th1 type immune response as well as inducing CTL response [29]. To assess whether the functional CD8+ T lymphocytes induced by DOTAP/E7 vaccination would be able to produce the essential cytokine, spleen cells from control or immunized mice were isolated at 1 week after the final immunization and incubated with 5 μg/ml E7 peptide (Fig. 5c, lower panel) or without peptide (Fig. 5c, upper panel) for 6 h followed by intracellular staining of IFN-γ. The numbers represent the percentage of CD8 and IFN-γ double positive cells within the CD8+ population. As depicted in Fig. 5d, the numbers of IFN-γ producing CD8+ cells were significantly higher in mice that received E7 formulated in an optimal lipid dose of DOTAP and LPD than the control mice. The IFN-γ production by the CD8+ cells was in an E7-specific manner. These results show that DOTAP at an optimal dose is a potent vaccine adjuvant for the induction of CTLs as well as generation of IFN-γ producing CD8+ T lymphocytes in the systemic lymphoid organ.
ROS production induced by DOTAP liposome
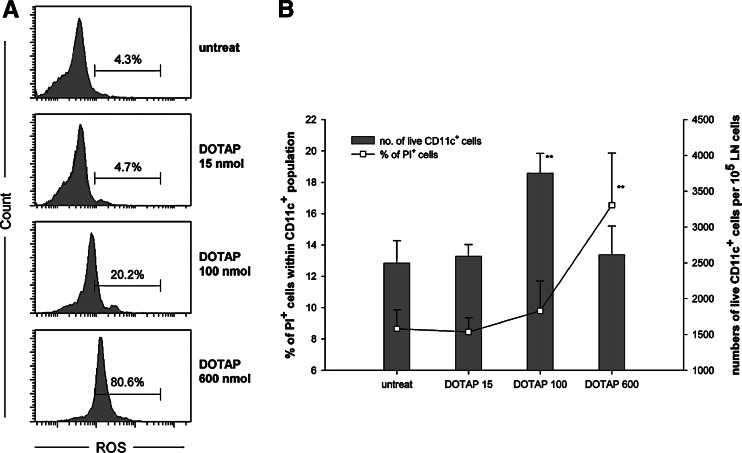
ROS, which has been regarded as a harmful signal, plays an important role in DC activation as well as antigen presentation to the T cells [30]. Our results demonstrated that DOTAP/E7 complexes were mainly taken up by DC in the DLN, and high dose DOTAP dramatically decreased the antigen-specific T cell responses. Since high levels of ROS generated in cells lead to cell death [31], we hypothesized that a high dose of DOTAP lipids may lead to massive ROS production in DC in the DLN, resulting in cell death of DC. To test out the hypothesis, we measured ROS levels induced by DOTAP/E7 in the DLNs at 2 h after sc injection. For characterization of DC in the DLN, large granular cells were gated and analyzed by flow cytometry. In Fig. 6a, cells from mice that received DOTAP 15 nmol exhibited a basal level of ROS production (<5%), whereas cells from mice injected with DOTAP at an optimal dose produced relatively higher levels of ROS (∼20%). Strikingly, a majority of large granular cells (∼80%) from the DOTAP 600 nmol group showed positive ROS signal. The subsequent question was whether the raised ROS induced by a high dose of DOTAP lipid triggered DC toward cell death. At 10 h post sc injection, DLNs were harvested and analyzed for cell death by flow cytometry (Fig. 6b). The percentage of cell death (propidium iodide positive) within the CD11c+ population showed positive correlation with the lipid dose as well as with the level of ROS production shown in Fig. 6a. The percentage of cell death in the DC population was twice as high for the group injected with DOTAP overdose compared to the group of untreated control. Also shown in Fig. 6b are the numbers of live CD11c+ cells per 105 lymph node cells as a function of the dose of DOTAP lipid. The mice that received DOTAP/E7 with lipid 100 nmol exhibited the highest amount (P < 0.01) of live DC among all treatment groups. Indeed, the DLN weighed larger at 2 days after the mice received the optimal formulation than other groups. Collectively, the results indicate that ROS toxicity induced by high dose DOTAP may cause cell death in DC.
Fig. 6.
High dose of cationic DOTAP liposome induces ROS production, resulting in cell death in DC. a DLNs from mice (n = 4) that received injection sc of DOTAP/E7 containing 0, 15, 100 or 600 nmol lipid were isolated 2 h after the injection. The total lymph node cells were incubated with DCFH-DA at 37°C for 30 min prior to the flow cytometric analysis. Large granular cells were gated and analyzed for the expression of ROS, where the fluorescent product DCF was generated in the presence of ROS. The relative percentages of cells with positive ROS signals are listed. b Cytotoxicity in the DLN was measured by collecting cells at 10 h after DOTAP/E7 injection by flow cytometry. The relative percentages of dead cells (PI positive) in DC (open square) and the numbers of live DC per 105 LN cells (bars) were shown and compared with that of the untreated control by paired Student’s t test (**P < 0.01)
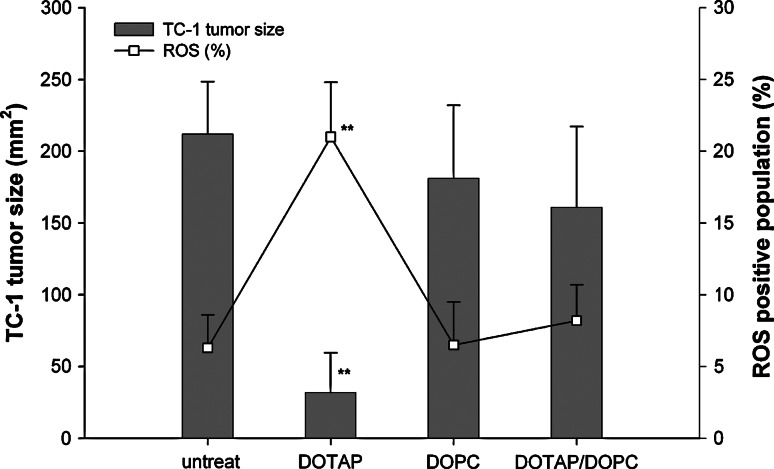
Since DOTAP-induced ROS signal initiates the vaccine mechanism owing to the interaction of DC and the lipid, we found that the anti-cancer activity can be diminished by co-formulation of an inert lipid, DOPC with DOTAP/E7. As shown in Fig. 7, DOTAP at the optimal dose could induce the maximal vaccine activity and the desirable ROS content. However, both activities were significantly reduced when the same amount of DOTAP was diluted with an inert neutral lipid, DOPC (1:5 dilution). In other words, the charge density of the cationic lipid could be an important parameter to ROS generation and the anti-cancer activity of the vaccine could be abolished by addition of an inert lipid.
Fig. 7.
ROS production in DLN was diminished by co-formulation of an inert neutral lipid, DOPC with DOTAP/E7 (mole ratio of DOPC/DOTAP = 5), resulting in decreased anti-tumor activity of the vaccine. ROS production in DLN was measured 2 h after receiving sc DOTAP/E7, DOPC/E7 or DOTAP/DOPC/E7 formulation as described. Large granular cells were gated and the relative percentage of ROS positive population are shown (open square). TC-1 tumor bearing mice were treated with the corresponding vaccine formulation on day 6 and tumor sizes (bars) on day 23 were measured and compared between each group. **P < 0.01 was found for the comparison between DOTAP/E7 and DOTAP/DOPC group by paired Student’s t test (n = 4–6)
Discussion
As shown in the data of this report, we have demonstrated that the majority of the adjuvant activity of LPD comes from the DOTAP liposome and, for the first time, we have shown that cationic lipid alone is sufficient for a cancer vaccine adjuvant. Indeed, upon receiving the optimal lipid dose of DOTAP/E7, the particles were mainly taken up by DC, the major professional antigen presenting cells. The initiation of DC activation and migration to the DLN facilitates immune responses against antigen-specific TC-1 tumor. Functional CD8+ T lymphocytes were generated upon receiving DOTAP/E7 vaccine. The size of the tumor decreased and the tumor exhibited enhanced apoptosis, likely owing to the increasing number of infiltrating T cells in the tumor microenvironment. According to the bell-shaped dose–response curve in Fig. 1c, the rising arc is very steep, indicating that DOTAP as a vaccine adjuvant is so potent that EC50 is as low as about 15 nmol per injection. Herein, we have demonstrated that DOTAP/E7 formulation is truly a simple, safe, yet very efficient, therapeutic vaccine against pre-existing HPV positive tumor.
The E7 peptide RAHYNIVTF (amino acid 49–57) is positively charged and somewhat hydrophobic. The association of the peptide with the cationic liposomes must be due to hydrophobic interaction, because the percent encapsulation far exceeded what could be expected from the internal volume of the liposomes [32]. The suboptimal anti-cancer effect observed when low dose of lipids were used (i.e., 15 nmol DOTAP per dose) might be due to the insufficient number of vehicles to carry the required antigen to the lymphoid organs.
Merck’s HPV vaccine, GARDASIL™, which is a preventive vaccine against HPV infection, is already available in the market. It contains HPV virus-like particles (VLPs), which mimic infectious virions in structure and morphology [33], [34] and potently induce high titered neutralizing antisera, even in the absence of any adjuvant [35], [36]. However, VLP vaccine is not likely to be active in treating established cervical cancer because the HPV coat proteins, which are major components of VLP, are not expressed in the cervical cancer cells. Our vaccine contains an epitope peptide of E7, which is expressed in the transformed cervical cells and can thus induce therapeutic immunity.
A great variety of experimental adjuvants are available for use in animals and some of them have been tested in clinical trials. They include several water-in-oil emulsions, liposomes and other chemical adjuvants [37]. However, only influenza virosomes [38] and Chiron’s MF59 [39] have already been launched into the market, in addition to aluminum salts. Similar to cationic liposome, the submicron emulsion-based adjuvant, MF59, is internalized by dendritic cells [40]. It stimulates a variety of immune activities that lead to high antibody and T-cell reactions against co-delivered antigens. However, according to the clinical trial report on HSV and influenza vaccines [41], [42], evidence from animal models suggests that the MF59 adjuvant enhances neutralizing antibodies rather than T-cell responses [43]. Therefore, DOTAP liposome as a vaccine adjuvant is different from MF59 in that it generates a strong cell-mediated immune response, as shown by our data. We have compared the adjuvant activity of DOTAP with those of two other well-known adjuvants, i.e., CpG ODN and CFA. As shown in Fig. 1d, DOTAP compares favorably with them.
Some recent developing adjuvants such as MPL [44] and CpG ODN [12] were meant to initiate innate immunity by triggering toll-like receptors (TLR) on dendritic cells and macrophages, thus inducing the production of NF-κB and inflammatory responses. We have previously demonstrated that DOTAP cationic lipid was unable to enhance the expression of NF-κB, suggesting that stimulation of dendritic cells by cationic lipids is signaled through an NF-κB independent mechanism [16]. Thus, cationic liposomes could belong to a unique class of adjuvants with an improved safety profile.
DOTAP/E7, the therapeutic cancer vaccine described in this report, contains only two molecules, i.e., an antigen and a carrier. In addition to the delivery of the E7 peptide to the cytoplasm of the APC such DC, DOTAP must also lead DC to activation and maturation. To elucidate the mechanism of DOTAP as vaccine adjuvant, we have demonstrated that the ability of cationic lipids to activate DC depends on the structure of the cationic lipid. Cationic lipids with a quaternary ammonium group are more potent than those with a tertiary ammonium group [18]. More recently, we have reported that DOTAP and other active cationic lipids can activate MAP kinase ERK and p38 pathway [19] in DC, resulting in chemokines and cytokines production, especially CCL2. All the preceding results support our hypothesis that DC plays an important role in response to DOTAP stimulation.
The importance of ROS has been implicated in innate immune response and T cell activation [30], [45], and excess of ROS production leads to cell death [46], [47]. We have shown in this report that ROS was induced by DOTAP in cells in the DLN and a high dose of DOTAP lipid led to killing of DC. Indeed, Iwaoka et al. have shown that cationic liposomes can induce ROS in macrophages in vitro [31]. Data shown in Fig. 6a clearly demonstrate that DOTAP was capable of generating ROS signal in vivo in the DLN after sc injection of DOTAP/E7 complexes. The same data also suggest that excess ROS generated by a high dose of DOTAP leads to enhanced death of DC. There might be other possible reasons related to the loss of anti-cancer activity by overdose of DOTAP/E7; however, the decreased amount of activated APC in the DLN should definitely play an important role in the observed decrease in the lymphocyte infiltration (Fig. 3), antigen-specific CTL activities and IFN-γ production (Figs. 4 and 5) and, most importantly, the anti-tumor activity (Fig. 1) at the high vaccine dose. On the other hand, a desirable level of ROS production is required since DOTAP-induced ROS is probably the initial signal to mediate the subsequent signal transduction, such as the ERK and p38 MAP kinases, necessary for the vaccine activity [19]. We have demonstrated the importance of the density of the cationic lipid to the anti-cancer activity in Fig. 7 where we showed that both ROS generation and anticancer activity could be diminished by co-formulation of an inert, neutral lipid, DOPC with DOTAP/E7. The result indicates that the simple cationic DOTAP/E7 formulation is not just a sufficient, but also a necessary, vaccine formulation for the treatment of cancer.
A high dose of DOTAP seems capable of stimulating CTL, in Fig. 4a, using a traditional assay method, which requires in vitro stimulation of responder T cells with the antigen for 5 days. We cannot rule out the possibility that the true functionality of CD8+ cells was overestimated after in vitro expansion of the subset cells. An in vivo CTL result (Fig. 5a) gave us a better estimation of the functionality of CD8+ T cells elicited in the mice and the data showed that a high dose of DOTAP does not generate sufficient CTL in vivo. Although DOTAP at high dose could also generate a good NK response toward non-specific killing (Fig. 4b), the effect contributed little to the anti-tumor activity. It is not clear, at present, the role that NK cells play in the overall anti-tumor activity induced by DOTAP/E7. We have previously shown that the IFN-γ activity in the splenocytes of mice treated with LPD/E7 was independent of NK, since mice injected with NK1.1 antibody to eliminate the NK activity showed unchanged IFN-γ activity (Dileo et al., unpublished data). Mice injected with DOTAP alone without E7 did not show significant anti-tumor activity (Fig. 1b), suggesting that the antigen independent component was minor.
T regulatory cells (Tregs) were initially described by Gershon et al. [48, 49] in the 1970s and were called suppressive T cells. Recent studies have explored the role of CD4+CD25+ Tregs in the suppression of tumor immunity in several murine models as well as in cancer patients [50]. The frequency of Treg population increases in the peripheral blood of cancer patients [51]. They are also enriched among the tumor infiltrating lymphocytes and in the DLNs [52]. Also, accumulation of Treg in tumor-associated tissue predicts poor prognosis or survival [5]. Although the detailed mechanism of how Treg dampens normal T-cell immunity is not well understood, it has been reported that the anti-tumor activity is enhanced by using anti-CD25 antibody to block Treg cells [53]. Indeed, it has become apparent that it will be necessary to monitor and characterize both the effector and Treg responses in patients that receive candidate human tumor vaccines [5]. In the present studies, we found a correlation of the anti-tumor activity of the DOTAP/E7 (Fig. 1b) with the reduction of Treg cells (Table 1). It was evident that the reduced anti-tumor activity of DOTAP/E7 at a high dose was associated with a higher level of Tregs, probably owing to the excess ROS production and cell death in DC. However, we do not have direct evidence to show that a high dose of DOTAP increases Tregs subpopulation.
In summary, the promising features of the DOTAP/E7 formulation, which contains only two molecules, include the safety and stability of the complexes and the abilities to elicit DC activation, generate antigen-specific CTL, as well as to inhibit an established tumor after a single dose of immunization. It also lends itself to the mechanistic studies of how DC can be activated by cationic lipids and how liposomes deliver the peptide antigen to DC. Although the dose of cationic DOTAP lipid as a vaccine adjuvant should be carefully studied before going to the clinical trial, it possesses great potentials that deserve further studies.
Acknowledgments
This work was supported by Mary Kay Ash Charitable Foundation for women cancers. We thank Shyh-Dar Li, Histopathology Facility and Michael Hooker Microscopy Facility in the University of North Carolina at Chapel Hill for the frozen section and microscopy assistance.
Disclosures
LH has financial interest in PDS Biotechnology, Inc., which has licensed the basic intellectual properties described here.
Abbreviations
- LPD
Liposome-polycations-DNA
- DLN
Draining lymph node
- Treg
Regulatory T cell
- DOTAP
1,2-Dioleoyl-3-trimethylammonium propane
- NBD-DOTAP
1-Oleoyl-2-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl]-3-trimethylammonium propane
- DOEPC
1,2-Dioleoyl-sn-glycero-3-ethylphosphocholine
- DOTMA
N-[1-(2,3-Dioleyloxy)propyl] -N,N,N-trimethylammonium chloride
- DOPG
1,2-Dioleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)]
- DOPC
1,2-Dioleoyl-sn-glycero-3-phosphocholine
References
- 1.Gregoriadis G. Immunological adjuvants: a role for liposomes. Immunol Today. 1990;11:89–97. doi: 10.1016/0167-5699(90)90034-7. [DOI] [PubMed] [Google Scholar]
- 2.Wang RF, Wang HY. Enhancement of antitumor immunity by prolonging antigen presentation on dendritic cells. Nat Biotechnol. 2002;20:149–154. doi: 10.1038/nbt0202-149. [DOI] [PubMed] [Google Scholar]
- 3.Vogel FR. Improving vaccine performance with adjuvants. Clin Infect Dis. 2000;30(Suppl 3):S266–270. doi: 10.1086/313883. [DOI] [PubMed] [Google Scholar]
- 4.Vogel FR. Role of adjuvants in HIV vaccine design. Antibiot Chemother. 1996;48:84–91. doi: 10.1159/000425161. [DOI] [PubMed] [Google Scholar]
- 5.Baecher-Allan C, Anderson DE. Immune regulation in tumor-bearing hosts. Curr Opin Immunol. 2006;18:214–219. doi: 10.1016/j.coi.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Li S, Huang L. In vivo gene transfer via intravenous administration of cationic lipid-protamine-DNA (LPD) complexes. Gene Ther. 1997;4:891–900. doi: 10.1038/sj.gt.3300482. [DOI] [PubMed] [Google Scholar]
- 7.Chen WC, Huang L. Non-viral vector as vaccine carrier. Adv Genet. 2005;54:315–337. doi: 10.1016/S0065-2660(05)54013-6. [DOI] [PubMed] [Google Scholar]
- 8.Whitmore M, Li S, Huang L. LPD lipopolyplex initiates a potent cytokine response and inhibits tumor growth. Gene Ther. 1999;6:1867–1875. doi: 10.1038/sj.gt.3301026. [DOI] [PubMed] [Google Scholar]
- 9.Whitmore MM, Li S, Falo L, Jr, Huang L. Systemic administration of LPD prepared with CpG oligonucleotides inhibits the growth of established pulmonary metastases by stimulating innate and acquired antitumor immune responses. Cancer Immunol Immunother. 2001;50:503–514. doi: 10.1007/s002620100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishii KJ, Kawakami K, Gursel I, Conover J, Joshi BH, Klinman DM, Puri RK. Antitumor therapy with bacterial DNA and toxin: complete regression of established tumor induced by liposomal CpG oligodeoxynucleotides plus interleukin-13 cytotoxin. Clin Cancer Res. 2003;9:6516–6522. [PubMed] [Google Scholar]
- 11.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 12.Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2003;2:305–315. doi: 10.1586/14760584.2.2.305. [DOI] [PubMed] [Google Scholar]
- 13.Marciani DJ. Vaccine adjuvants: role and mechanisms of action in vaccine immunogenicity. Drug Discov Today. 2003;8:934–943. doi: 10.1016/S1359-6446(03)02864-2. [DOI] [PubMed] [Google Scholar]
- 14.Jiang ZH, Koganty RR. Synthetic vaccines: the role of adjuvants in immune targeting. Curr Med Chem. 2003;10:1423–1439. doi: 10.2174/0929867033457340. [DOI] [PubMed] [Google Scholar]
- 15.Cui Z, Huang L. Liposome-polycation-DNA (LPD) particle as a carrier and adjuvant for protein-based vaccines: therapeutic effect against cervical cancer. Cancer Immunol Immunother. 2005;54:1180–1190. doi: 10.1007/s00262-005-0685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui Z, Han SJ, Vangasseri DP, Huang L. Immunostimulation mechanism of LPD nanoparticle as a vaccine carrier. Mol Pharm. 2005;2:22–28. doi: 10.1021/mp049907k. [DOI] [PubMed] [Google Scholar]
- 17.Dileo J, Banerjee R, Whitmore M, Nayak JV, Falo LD, Jr, Huang L. Lipid-protamine-DNA-mediated antigen delivery to antigen-presenting cells results in enhanced anti-tumor immune responses. Mol Ther. 2003;7:640–648. doi: 10.1016/S1525-0016(03)00064-9. [DOI] [PubMed] [Google Scholar]
- 18.Vangasseri DP, Cui Z, Chen W, Hokey DA, Falo LD, Huang L. Immunostimulation of dendritic cells by cationic liposomes. Mol Membr Biol. 2006;23:385–395. doi: 10.1080/09687860600790537. [DOI] [PubMed] [Google Scholar]
- 19.Yan W, Chen W, Huang L. Mechanism of adjuvant activity of cationic liposome: phosphorylation of a MAP kinase, ERK and induction of chemokines. Mol Immunol. 2007;44:3672–3681. doi: 10.1016/j.molimm.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Gursel I, Gursel M, Ishii KJ, Klinman DM. Sterically stabilized cationic liposomes improve the uptake and immunostimulatory activity of CpG oligonucleotides. J Immunol. 2001;167:3324–3328. doi: 10.4049/jimmunol.167.6.3324. [DOI] [PubMed] [Google Scholar]
- 21.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, Bosio C, Dow S. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176:7335–7345. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 22.Byers AM, Kemball CC, Moser JM, Lukacher AE. Cutting edge: rapid in vivo CTL activity by polyoma virus-specific effector and memory CD8+ T cells. J Immunol. 2003;171:17–21. doi: 10.4049/jimmunol.171.1.17. [DOI] [PubMed] [Google Scholar]
- 23.Hempel SL, Buettner GR, O’Malley YQ, Wessels DA, Flaherty DM. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2′,7′-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic Biol Med. 1999;27:146–159. doi: 10.1016/S0891-5849(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 24.Tan Y, Liu F, Li Z, Li S, Huang L. Sequential injection of cationic liposome and plasmid DNA effectively transfects the lung with minimal inflammatory toxicity. Mol Ther. 2001;3:673–682. doi: 10.1006/mthe.2001.0311. [DOI] [PubMed] [Google Scholar]
- 25.Yew NS, Scheule RK. Toxicity of cationic lipid–DNA complexes. Adv Genet. 2005;53:189–214. doi: 10.1016/S0065-2660(05)53007-4. [DOI] [PubMed] [Google Scholar]
- 26.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 27.Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003;90:127–156. doi: 10.1016/s0065-230x(03)90004-2. [DOI] [PubMed] [Google Scholar]
- 28.Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18:391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 30.Matsue H, Edelbaum D, Shalhevet D, Mizumoto N, Yang C, Mummert ME, Oeda J, Masayasu H, Takashima A. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J Immunol. 2003;171:3010–3018. doi: 10.4049/jimmunol.171.6.3010. [DOI] [PubMed] [Google Scholar]
- 31.Iwaoka S, Nakamura T, Takano S, Tsuchiya S, Aramaki Y. Cationic liposomes induce apoptosis through p38 MAP kinase-caspase-8-Bid pathway in macrophage-like RAW264.7 cells. J Leukoc Biol. 2006;79:184–191. doi: 10.1189/jlb.0405181. [DOI] [PubMed] [Google Scholar]
- 32.Enoch HG, Strittmatter P. Formation and properties of 1000-A-diameter, single-bilayer phospholipid vesicles. Proc Natl Acad Sci USA. 1979;76:145–149. doi: 10.1073/pnas.76.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagensee ME, Olson NH, Baker TS, Galloway DA. Three-dimensional structure of vaccinia virus-produced human papillomavirus type 1 capsids. J Virol. 1994;68:4503–4505. doi: 10.1128/jvi.68.7.4503-4505.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen XS, Garcea RL, Goldberg I, Casini G, Harrison SC. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol Cell. 2000;5:557–567. doi: 10.1016/S1097-2765(00)80449-9. [DOI] [PubMed] [Google Scholar]
- 35.Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christensen ND, Hopfl R, DiAngelo SL, Cladel NM, Patrick SD, Welsh PA, Budgeon LR, Reed CA, Kreider JW. Assembled baculovirus-expressed human papillomavirus type 11 L1 capsid protein virus-like particles are recognized by neutralizing monoclonal antibodies and induce high titres of neutralizing antibodies. J Gen Virol. 1994;75(Pt 9):2271–2276. doi: 10.1099/0022-1317-75-9-2271. [DOI] [PubMed] [Google Scholar]
- 37.Vogel FR, Powell MF. A compendium of vaccine adjuvants and excipients. Pharm Biotechnol. 1995;6:141–228. doi: 10.1007/978-1-4615-1823-5_7. [DOI] [PubMed] [Google Scholar]
- 38.Gluck R, Walti E. Biophysical validation of Epaxal Berna, a hepatitis A vaccine adjuvanted with immunopotentiating reconstituted influenza virosomes (IRIV) Dev Biol (Basel) 2000;103:189–197. [PubMed] [Google Scholar]
- 39.Kahn JO, Sinangil F, Baenziger J, Murcar N, Wynne D, Coleman RL, Steimer KS, Dekker CL, Chernoff D. Clinical and immunologic responses to human immunodeficiency virus (HIV) type 1SF2 gp120 subunit vaccine combined with MF59 adjuvant with or without muramyl tripeptide dipalmitoyl phosphatidylethanolamine in non-HIV-infected human volunteers. J Infect Dis. 1994;170:1288–1291. doi: 10.1093/infdis/170.5.1288. [DOI] [PubMed] [Google Scholar]
- 40.Dupuis M, Murphy TJ, Higgins D, Ugozzoli M, van Nest G, Ott G, McDonald DM. Dendritic cells internalize vaccine adjuvant after intramuscular injection. Cell Immunol. 1998;186:18–27. doi: 10.1006/cimm.1998.1283. [DOI] [PubMed] [Google Scholar]
- 41.Jones CA, Cunningham AL. Vaccination strategies to prevent genital herpes and neonatal herpes simplex virus (HSV) disease. Herpes. 2004;11:12–17. [PubMed] [Google Scholar]
- 42.Minutello M, Senatore F, Cecchinelli G, Bianchi M, Andreani T, Podda A, Crovari P. Safety and immunogenicity of an inactivated subunit influenza virus vaccine combined with MF59 adjuvant emulsion in elderly subjects, immunized for three consecutive influenza seasons. Vaccine. 1999;17:99–104. doi: 10.1016/S0264-410X(98)00185-6. [DOI] [PubMed] [Google Scholar]
- 43.Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11:S63–68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 44.Evans JT, Cluff CW, Johnson DA, Lacy MJ, Persing DH, Baldridge JR. Enhancement of antigen-specific immunity via the TLR4 ligands MPL adjuvant and Ribi.529. Expert Rev Vaccines. 2003;2:219–229. doi: 10.1586/14760584.2.2.219. [DOI] [PubMed] [Google Scholar]
- 45.Kantengwa S, Jornot L, Devenoges C, Nicod LP. Superoxide anions induce the maturation of human dendritic cells. Am J Respir Crit Care Med. 2003;167:431–437. doi: 10.1164/rccm.200205-425OC. [DOI] [PubMed] [Google Scholar]
- 46.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aramaki Y, Takano S, Arima H, Tsuchiya S. Induction of apoptosis in WEHI 231 cells by cationic liposomes. Pharm Res. 2000;17:515–520. doi: 10.1023/A:1007552529280. [DOI] [PubMed] [Google Scholar]
- 48.Eardley DD, Hugenberger J, McVay-Boudreau L, Shen FW, Gershon RK, Cantor H. Immunoregulatory circuits among T-cell sets. I. T-helper cells induce other T-cell sets to exert feedback inhibition. J Exp Med. 1978;147:1106–1115. doi: 10.1084/jem.147.4.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cantor H, McVay-Boudreau L, Hugenberger J, Naidorf K, Shen FW, Gershon RK. Immunoregulatory circuits among T-cell sets. II. Physiologic role of feedback inhibition in vivo absence in NZB mice. J Exp Med. 1978;147:1116–1125. doi: 10.1084/jem.147.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Comes A, Rosso O, Orengo AM, Di Carlo E, Sorrentino C, Meazza R, Piazza T, Valzasina B, Nanni P, Colombo MP, Ferrini S. CD25+ regulatory T cell depletion augments immunotherapy of micrometastases by an IL-21-secreting cellular vaccine. J Immunol. 2006;176:1750–1758. doi: 10.4049/jimmunol.176.3.1750. [DOI] [PubMed] [Google Scholar]
- 51.Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98:1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 52.Baecher-Allan C, Hafler DA. Suppressor T cells in human diseases. J Exp Med. 2004;200:273–276. doi: 10.1084/jem.20040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28:582–592. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]