Abstract
Chronic infection and inflammation are among the most important factors contributing to cancer development and growth. Toll-like receptors (TLRs) are important families of pattern recognition receptors, which recognize conserved components of microbes and trigger the immune response against invading microorganisms. TLR4 is the signaling receptor for lipopolysaccharide (LPS), the endotoxic component of Gram-negative bacteria. Recent studies demonstrate that TLRs are expressed in some tumor cells, and that the expression of TLRs in these cells is associated with tumorigenesis. Cervical intraepithelial neoplasia (CIN) is a key stage in the development of cervical cancer and human papillomavirus (HPV) infection is an essential factor in cervical carcinogenesis. As the cervix is in constant contact with bacteria, especially Gram-negative bacteria, we hypothesize that TLR4-mediated bacterial stimulation may be involved in the tumorigenesis of cervical cancer. In the present study, the expression and distribution of TLR4 in CIN and cervical squamous carcinoma were investigated by immunohistochemistry. To our surprise, we observed a decrease in the expression of TLR4 during the progression of cervical neoplasia and this down-regulation of TLR4 appeared to be associated with the expression of 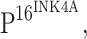 which is a crucial marker of HPV integration into host cells. These data offer further insight regarding the association of HPV infection and TLR signaling during the carcinogenesis of cervical cancer.
which is a crucial marker of HPV integration into host cells. These data offer further insight regarding the association of HPV infection and TLR signaling during the carcinogenesis of cervical cancer.
Keywords: Toll-like receptor 4, Cervical intraepithelial neoplasia, Cervical cancer, 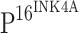
Introduction
Cervical carcinoma is the second most common form of cancer among women worldwide and human papillomavirus (HPV) infection is an essential factor in cervical carcinogenesis [33]. However, the role and potential effects of bacteria in the female reproduction tract have been largely unexplored. The healthy vagina contains numerous bacteria and microorganisms. Bacterial vaginosis is the most common type of vaginal infection in women prior to menopause, and at least one-third of all vaginal infections are due to bacterial vaginosis. In bacterial vaginosis, the Gram-negative bacteria, especially Gardnerella vaginalis, are most common type of bacteria associated with this condition. The cervix is therefore often exposed to the environment and various bacteria except for HPV. Previous evidence demonstrates that bacterial infections cause inflammation, local production of proinflammatory cytokines, and increases in the local endotoxin level [24]. However, the effect and significance of bacterial infection and the consequential inflammation in the development of cervical intraepithelial neoplasia (CIN) and cancer have not been thoroughly investigated.
Toll-like receptors (TLRs) function as molecular sensors to detect conserved components of microbes and play a crucial role in the host defense against invading pathogens. To date, 11 human TLRs have been identified, and each TLR recognizes distinct pathogen-associated molecular patterns (PAMPs) on various microorganisms [1]. For example, TLR4 is the signaling receptor for lipopolysaccharide (LPS), the endotoxic component of Gram-negative bacteria [14, 26]. TLR5 recognizes bacterial flagellin [10] and TLR9 recognizes unmethylated deoxycytidyl-phosphate-deoxyguanosine (CpG) motifs [13] in bacterial and viral genomes.
Interestingly, recent studies demonstrate that TLRs are expressed in some tumor cells, and the expression of TLRs in these cells has been associated with tumorigenesis [18, 28, 37]. CIN is a key stage in the development of cervical cancer. Expression of TLR5 and TLR9 is undetectable or weak in normal cervical squamous epithelial tissues, but gradually increases in accordance with the histopathologic grade ranging from low-grade CIN, high-grade CIN to invasive squamous cell carcinomas (ISCCs) [19, 22]. These findings suggest that TLR5 and TLR9 may play a significant role in the progression of cervical neoplasia and may represent a useful marker for malignant transformation of cervical squamous cells.
As the cervix cannot avoid coming into contact with bacteria and TLR4 recognizes the endotoxic component of Gram-negative bacteria, LPS, we hypothesize that TLR4-mediated signaling may be involved in the pathogenesis of CIN and carcinogenesis. In the present study, the expression and distribution of TLR4 in CIN and cervical squamous cancer were investigated by immunohistochemistry, and their relationship with the expression of 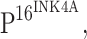 a crucial marker for the integration of HPV into host cells, was also examined.
a crucial marker for the integration of HPV into host cells, was also examined.
Materials and methods
Tissue samples
A total of 180 paraffin-embedded, formalin-fixed tissue specimens were used in this study. Specimens of 104 CINs and 26 ISCCs were selected from patients that were screened for CINs using liquid-based thin layer cytology at the Department of Pathology, the First Affiliated Hospital, Sun Yat-Sen University, and pathologically confirmed via cervical biopsy under colposcope. CINs are classified into three stages with one-, two- or three-thirds thickness being classified as dysplastic, and cases of CIN I, CIN II and CIN III being 49, 32 and 23, respectively. Invasive tumors were diagnosed when the epithelial basement membrane was breached. Normal control cervical tissues (n = 50) that were confirmed by histopathology reports to be either hysteromyoma (42 cases) or adenomyosis (8 cases) were obtained from surgically removed uteruses. In addition, 64 frozen biopsies that included 20 normal cervical epithelia, 21 CINs and 23 ISCCs were subjected to real-time reverse transcription-polymerase chain reaction (RT-PCR) for the detection of TLR4 and TLR9 mRNA expression.
Immunohistochemistry
Sections (4 μm) were cut from formalin-fixed, paraffin-embedded blocks and were used to prepare routine histopathology and immunohistochemistry (IHC) slides. Sections were mounted on glass slides coated with poly-l-lysine, dried at 37°C overnight, deparaffinized in xylene, washed in ethanol, and finally washed in PBS (pH 7.4). To increase specificity and sensitivity, samples were pretreated with microwave for 20 min on high mode in Tris/EDTA buffer (pH 9.0, for 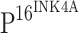 ) or in citrate buffer (pH 6.0, for TLR4). After cooling to room temperature and rinsing in distilled water, endogenous peroxidase activity was blocked with 3% H2O2 for 15 min. The tumor sections and sections of control mucosa were subjected to IHC with mouse anti-human
) or in citrate buffer (pH 6.0, for TLR4). After cooling to room temperature and rinsing in distilled water, endogenous peroxidase activity was blocked with 3% H2O2 for 15 min. The tumor sections and sections of control mucosa were subjected to IHC with mouse anti-human 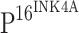 (Clone JC8) monoclonal antibody diluted at 1:50 (GeneTex, San Antonio, TX 78245, USA) or rabbit anti-human TLR4 (H-80) polyclonal antibody diluted at 1:50 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA 95060, USA) in accordance with the manufacturer’s instructions. Briefly, after incubation with primary antibody at 4°C overnight, the sections were washed with PBS three times followed by incubation for 20 min at room temperature with horseradish peroxidase (HRP)-labeled secondary antibody that was included in the MaxVision anti-mouse/rabbit and HRP conjugated Polymer IHC kit (MaxVision, Fuzhou, China). Color development was assessed following incubation at room temperature for 5–10 min with diaminobenzidine (DAB) (Dako Corp, Carpinteria, California, USA) for
(Clone JC8) monoclonal antibody diluted at 1:50 (GeneTex, San Antonio, TX 78245, USA) or rabbit anti-human TLR4 (H-80) polyclonal antibody diluted at 1:50 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA 95060, USA) in accordance with the manufacturer’s instructions. Briefly, after incubation with primary antibody at 4°C overnight, the sections were washed with PBS three times followed by incubation for 20 min at room temperature with horseradish peroxidase (HRP)-labeled secondary antibody that was included in the MaxVision anti-mouse/rabbit and HRP conjugated Polymer IHC kit (MaxVision, Fuzhou, China). Color development was assessed following incubation at room temperature for 5–10 min with diaminobenzidine (DAB) (Dako Corp, Carpinteria, California, USA) for 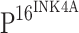 or with aminoethylcarbazole (AEC) (Dako Corp, Carpinteria, CA, USA) for TLR4. Slides were counterstained with hematoxylin, and then examined by light microscopy. Mouse or rabbit serum was used in place of the primary antibodies as a negative control.
or with aminoethylcarbazole (AEC) (Dako Corp, Carpinteria, CA, USA) for TLR4. Slides were counterstained with hematoxylin, and then examined by light microscopy. Mouse or rabbit serum was used in place of the primary antibodies as a negative control.
Two histopathologists blindly reviewed the slides and evaluated the immunohistochemical data. Red or purple red color in the cytoplasmic membrane and/or cytoplasm of cells was defined as TLR4 positive staining. Based on the staining intensity and the distribution and proportion of TLR4 positive cells per high-power field in squamous epithelium of CINs, TLR4 immunoreactivity was scored on a scale from − to 3+ as follows: −, no positive staining cells; 1+, less than 25% positive staining cells with weak intensity and focal distribution; 2+, 26–50% positive staining cells with moderate intensity and focal distribution; and 3+, more than 50% positive staining cells with strong intensity and diffuse distribution [19].
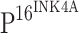 immunostaining appeared in brown or dark brown color in the nuclei and/or cytoplasm of cells. Based on the distribution of
immunostaining appeared in brown or dark brown color in the nuclei and/or cytoplasm of cells. Based on the distribution of 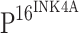 positive cells in squamous epithelium of CINs,
positive cells in squamous epithelium of CINs, 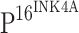 staining was also scored on a scale from − to 3+ as follows: −, no positive staining cells; 1+, focal or diffuse distribution (lower third); 2+, focal or diffuse distribution (lower third to two-thirds); and 3+, diffuse distribution (lower two-thirds to complete thickness of epithelium) [17].
staining was also scored on a scale from − to 3+ as follows: −, no positive staining cells; 1+, focal or diffuse distribution (lower third); 2+, focal or diffuse distribution (lower third to two-thirds); and 3+, diffuse distribution (lower two-thirds to complete thickness of epithelium) [17].
The double immunostaining of TLR4 and 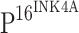 was performed with DouSP™ double staining kit (MaxVision, Fuzhou, China). The primary antibodies are the same as those used in the single staining experiments.
was performed with DouSP™ double staining kit (MaxVision, Fuzhou, China). The primary antibodies are the same as those used in the single staining experiments. 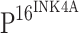 immunoreactivity was first examined and biotinylated second antibody (anti-mouse/rabbit)/streptavidin–alkaline phosphatase and BCIP-NBT were used for visualization. Biotinylated second antibody (anti-mouse/rabbit)/streptavidin–peroxidase and AEC were used for detection of TLR4 expression. The evaluation criteria are the same as that used in single antibody staining.
immunoreactivity was first examined and biotinylated second antibody (anti-mouse/rabbit)/streptavidin–alkaline phosphatase and BCIP-NBT were used for visualization. Biotinylated second antibody (anti-mouse/rabbit)/streptavidin–peroxidase and AEC were used for detection of TLR4 expression. The evaluation criteria are the same as that used in single antibody staining.
RNA extraction and quantitative real-time RT-PCR
Biopsies were subjected to rapid cryo-sectioning, and microscopy was used to locate the areas of normal squamous epithelium, CIN and ISCC. The corresponding tissues were then isolated for RNA extraction. Total RNA was extracted using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) in accordance with the manufacturer’s instructions and treated with TURBO DNA-free™ DNase (Ambion, Austin, TX, USA) to remove the genomic DNA. RNA was reverse-transcribed with random hexamer primers using ReverTra Ace-α-(Toyobo Co., Osaka, Japan). Real-time PCR for TLR4 or TLR9 was performed in a 10 μl reaction volume using the Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen Life Technologies, Carlsbad, CA, USA) and the LightCycler 480 system (Roche Diagnostics, Penzberg, Germany). The primer sequences used for the RT-PCR were AATCTAGAGCACTTGGACCTTTCC (TLR4F), (GGGTTCAGGGACAGGTCTAAAGA) (TLR4R) [4], TGAAGACTTCAGGCCCAACTG (TLR9F), and TGCACGGTCACCAGGTTGT (TLR9R) [9]. The thermal cycling conditions included an initial denaturation step at 95°C for 1 min, followed by 40 cycles of amplification at 95°C for 10 s, 58°C for 30 s, and 72°C for 10 s. Reactions were performed in triplicate and the threshold cycles and relative fold differences were calculated.
Statistical analysis
SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis of the data. The Kruskal–Wallis test was used to compare the differences of cumulative TLR4 and 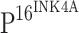 expression with CIN progression and ISCCs among five groups and the Bonferroni test was used for multiple comparisons between each two groups, and the significant level was adjusted to 0.005. The correlation of TLR4 immunoreactivity and
expression with CIN progression and ISCCs among five groups and the Bonferroni test was used for multiple comparisons between each two groups, and the significant level was adjusted to 0.005. The correlation of TLR4 immunoreactivity and 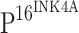 expression was examined by Spearman correlation test. Except for the multiple comparison test, the significance level of the other tests was established at 0.05. Student’s t test was used to compare the differences of TLR4 or TLR9 mRNA expression in normal cervical epithelia, CINs and ISCCs.
expression was examined by Spearman correlation test. Except for the multiple comparison test, the significance level of the other tests was established at 0.05. Student’s t test was used to compare the differences of TLR4 or TLR9 mRNA expression in normal cervical epithelia, CINs and ISCCs.
Results
TLR4 expression is decreased with progression of CIN
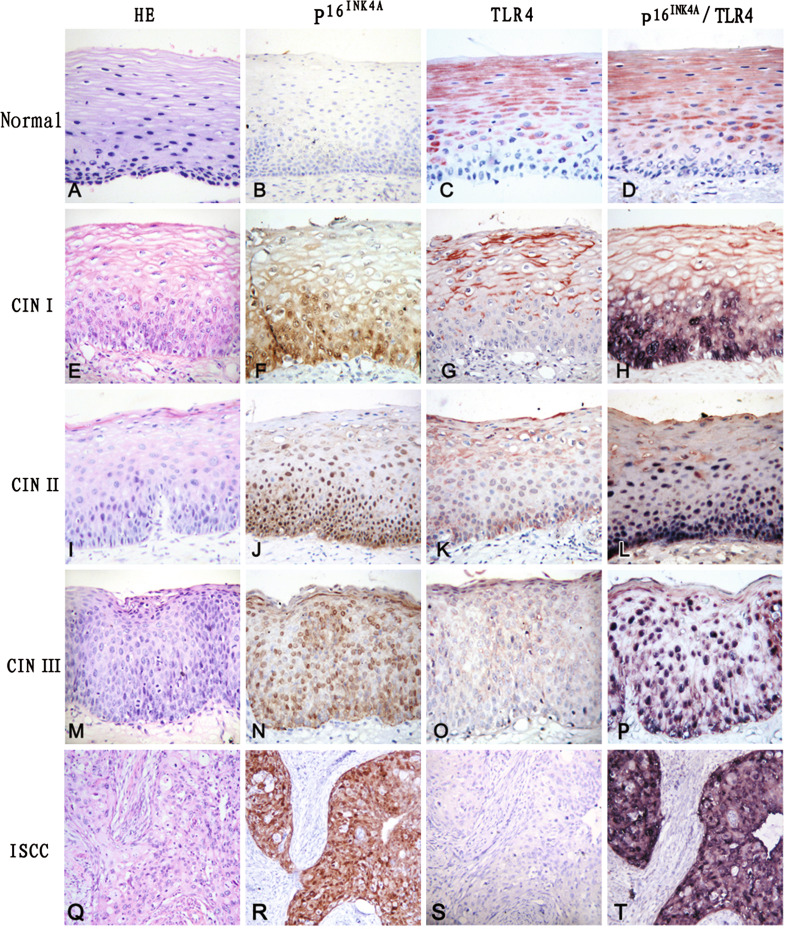
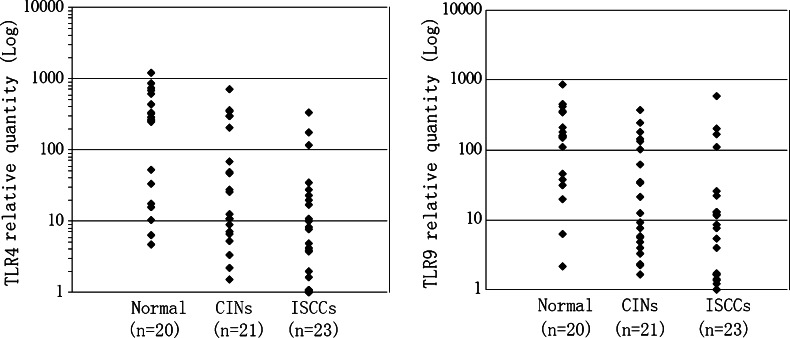
Surface and/or intracellular TLR4 expression was observed in normal cervical squamous epithelial cells. TLR4 positive cells were distributed mainly above the basal layer of squamous epithelium (Fig. 1c). The majority of normal cervical specimens (42/50, 84.0%) showed positive staining for TLR4 (Table 1). Staining with TLR4 antibody was not detected in cervical columnar epithelial cells except for the goblet cells (data not shown). The intensity of TLR4 immunostaining was gradually decreased with increasing histopathologic grade of CINs. TLR4 immunoreactivity in high-grade CINs (CIN II/III) was markedly weaker than the reactivity in normal cervical epithelium and CIN I, and positive TLR4 staining was usually not observed within dysplastic proliferating cells of CINs (Fig. 1g, k, o). The overall positive staining levels for TLR4 in CIN I, CIN II and CIN III were 69.4, 34.4, and 13.0%, respectively (Table 1). The positive detection levels of TLR4 between high-grade CINs (CIN II/III) and normal controls and CIN I were statistically different (P < 0.005). Detection of TLR4 immunostaining in cells within the cancer cell nests of ISCC specimens was difficult (Fig. 1s). To confirm the immunostaining results, TLR4 mRNA expression in cervical biopsies was assessed by real-time PCR. As observed with immunohistochemistry, TLR4 mRNA expression was decreased in CINs and ISCCs (Fig. 2). Although sample size was limited, the differences of TLR4 mRNA expression levels between normal cervical epithelia and CINs (P = 0.014), or expression between normal cervical epithelia and ISCCs (P < 0.001) were statistically significant. As previous studies have demonstrated that TLR9 expression is suppressed in cervical cancers [9], TLR9 mRNA expression in cervical biopsies was also measured. The results indicate that the TLR9 mRNA expression patterns in normal cervical tissues, CINs, and ISCCs were similar to what we observed for TLR4 (Fig. 2).
Fig. 1.
Expression of TLR4 and 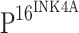 detected by immunohistochemisty. Hematoxylin and eosin (HE) staining showing that atypical hyperplasia is restricted within lower third (e), lower two-thirds (i), or exceeded over lower two-thirds (m) of squamous epithelium in CIN I, CIN II and CIN III, respectively, compared to normal cervical squamous epithelium (a). Cancer nests invaded into muscle tissue in ISCC (q). No
detected by immunohistochemisty. Hematoxylin and eosin (HE) staining showing that atypical hyperplasia is restricted within lower third (e), lower two-thirds (i), or exceeded over lower two-thirds (m) of squamous epithelium in CIN I, CIN II and CIN III, respectively, compared to normal cervical squamous epithelium (a). Cancer nests invaded into muscle tissue in ISCC (q). No 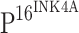 staining was detected in the control (b). Immunoreactivity of
staining was detected in the control (b). Immunoreactivity of 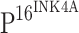 gradually increased from CIN I (f), CIN II (j) to CIN III (n), and
gradually increased from CIN I (f), CIN II (j) to CIN III (n), and 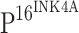 positive cells were diffusely distributed within the atypical hyperplasia tissue. Strong
positive cells were diffusely distributed within the atypical hyperplasia tissue. Strong 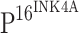 staining was observed in tumor epithelial cells of cancer nests in ISCC (r). In contrast to
staining was observed in tumor epithelial cells of cancer nests in ISCC (r). In contrast to 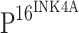 staining, TLR4 showed the strongest immunoreactivity in normal cervical tissue (c), and the staining intensity of TLR4 gradually decreased from CIN I (g), CIN II (k) to CIN III (o). No TLR4 immunostaining was observed in cells within cancer cell nests (s). Only TLR4 staining was observed in normal cervical tissue (d) with double immunostaining of TLR4 and
staining, TLR4 showed the strongest immunoreactivity in normal cervical tissue (c), and the staining intensity of TLR4 gradually decreased from CIN I (g), CIN II (k) to CIN III (o). No TLR4 immunostaining was observed in cells within cancer cell nests (s). Only TLR4 staining was observed in normal cervical tissue (d) with double immunostaining of TLR4 and 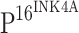 , and TLR4 staining was not observed within dysplastic proliferating cells of CINs (h, l, p), where
, and TLR4 staining was not observed within dysplastic proliferating cells of CINs (h, l, p), where 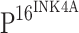 was positive. Strong
was positive. Strong 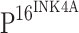 staining, but no TLR4 staining, was detected in the tumor cells of cancer nests (t)
staining, but no TLR4 staining, was detected in the tumor cells of cancer nests (t)
Table 1.
Expression of TLR4 in CINs and ISCCs
| Tissues | Degree of immunoreactivity (%) | |||
|---|---|---|---|---|
| – | 1+ | 2+ | 3+ | |
| Normala | 8/50 (16.0) | 17/50 (34.0) | 13/50 (26.0) | 12/50 (24.0) |
| CIN Ib | 15/49 (30.6) | 18/49 (36.7) | 10/49 (20.4) | 6/49 (12.2) |
| CIN IIc | 21/32 (65.6) | 9/32 (28.1) | 2/32 (6.3) | 0/32 (0.0) |
| CIN IIId | 20/23 (87.0) | 3/23 (13.0) | 0/23(0.0) | 0/23 (0.0) |
| ISCCse | 23/26 (88.5) | 3/26 (11.5) | 0/26 (0.0) | 0/26 (0.0) |
CIN cervical intraepithelial neoplasia, ISCCs invasive squamous cell carcinomas
a–b P > 0.005, a–c P < 0.005, a–d P < 0.005, a–e P < 0.005, b–c P < 0.005, b–d P < 0.005, b–e P < 0.005, c–d P > 0.005, c–e P > 0.005, and d–e P > 0.005
Fig. 2.
Quantitative comparison of the TLR4 or TLR9 mRNA expression detected by real-time RT-PCR in normal cervical epithelia (Normal), cervical intraepithelial neoplasias (CINs) and invasive squamous cell carcinomas (ISCCs). Statistical differences of TLR4 mRNA expression were significant between normal cervical epithelia and CINs (P = 0.014), or between normal cervical epithelia and ISCCs (P < 0.001), but significant differences were not found between CINs and ISCCs (P = 0.066). The differences of TLR9 mRNA expression between normal cervical epithelia and CINs (P = 0.008), or between normal cervical epithelia and ISCCs (P = 0.006) were also statistically significant. However, there was no significant difference between CINs and ISCCs (P = 0.681)
TLR4 immunostaining is inversely associated with 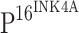 expression
expression
The down-regulation of TLR4 expression was unexpected, and it is believed to be associated with HPV infection. Thus, we investigated expression of 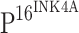 , a marker for the integration of high-risk HPV into host cells, in various cervical tissues. Positive immunostaining was not observed in 50 normal controls (Fig. 1b). The positive staining rates of
, a marker for the integration of high-risk HPV into host cells, in various cervical tissues. Positive immunostaining was not observed in 50 normal controls (Fig. 1b). The positive staining rates of 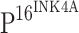 in CIN I, CIN II, CIN III and ISCCs were 81.6, 93.8, 95.7 and 96.2%, respectively (Table 2). The increase in the staining intensity of
in CIN I, CIN II, CIN III and ISCCs were 81.6, 93.8, 95.7 and 96.2%, respectively (Table 2). The increase in the staining intensity of 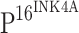 was associated with increased histopathologic grade of CINs (Fig. 1f, j, n). The differences of positive staining levels for
was associated with increased histopathologic grade of CINs (Fig. 1f, j, n). The differences of positive staining levels for 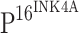 between normal controls and CINs, and between normal controls and ISCCs were statistically significant (P < 0.005).
between normal controls and CINs, and between normal controls and ISCCs were statistically significant (P < 0.005).
Table 2.
Expression of 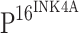 in CINs and ISCCs
in CINs and ISCCs
| Tissues | Degree of immunoreactivity (%) | |||
|---|---|---|---|---|
| – | 1+ | 2+ | 3+ | |
| Normala | 50/50 (100.0) | 0/50 (0.0) | 0/50 (0.0) | 0/50 (0.0) |
| CIN Ib | 9/49 (18.4) | 32/49 (65.3) | 5/49 (10.2) | 3/49 (6.1) |
| CIN IIc | 2/32 (6.3) | 6/32 (18.8) | 20/32 (62.5) | 4/32 (12.5) |
| CIN IIId | 1/23 (4.3) | 2/23 (8.7) | 3/23 (13.0) | 17/23 (73.9) |
| ISCCe | 1/26 (3.8) | 5/26 (19.2) | 2/26 (7.7) | 18/26 (69.2) |
CIN cervical intraepithelial neoplasia, ISCCs invasive squamous cell carcinomas
a–b P < 0.005, a–c P < 0.005, a–d P < 0.005, a–e P < 0.005, b–c P < 0.005, b–d P < 0.005, b–e P < 0.005, c–d P > 0.005, c–e P > 0.005 and d–e P > 0.005
When the slides stained with only TLR4 or 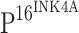 antibody were compared, it was observed that TLR4 was typically not stained in the cells that stained for
antibody were compared, it was observed that TLR4 was typically not stained in the cells that stained for 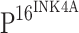 . To further explore the effect of
. To further explore the effect of 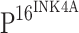 on TLR4 expression, we performed double immunostaining for both TLR4 and
on TLR4 expression, we performed double immunostaining for both TLR4 and 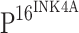 . Similar to what was observed in the single staining,
. Similar to what was observed in the single staining, 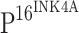 staining gradually increased with the histopathologic grade of CINs (Fig. 1h, l, p). However, TLR4 expression was not detected in
staining gradually increased with the histopathologic grade of CINs (Fig. 1h, l, p). However, TLR4 expression was not detected in 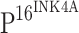 positive staining cells (Fig. 1h, l, p). This same staining phenomenon was observed in ISCCs. Expression of TLR4 is therefore inversely correlated to the expression of
positive staining cells (Fig. 1h, l, p). This same staining phenomenon was observed in ISCCs. Expression of TLR4 is therefore inversely correlated to the expression of 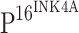 (P < 0.001; Table 3).
(P < 0.001; Table 3).
Table 3.
A cross tabulation of TLR4 and 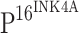 expression
expression
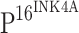 expression level (cases) expression level (cases) |
TLR4 expression level (cases) | Total | |||
|---|---|---|---|---|---|
| – | 1+ | 2+ | 3+ | ||
| – | 10 | 24 | 15 | 14 | 63 |
| 1+ | 21 | 12 | 9 | 3 | 45 |
| 2+ | 17 | 11 | 1 | 1 | 30 |
| 3+ | 39 | 3 | 0 | 0 | 42 |
| Total | 87 | 50 | 25 | 18 | 180 |
180 specimens that include 104 CINs, 26 ISCCs and 50 controls
Correlation of TLR4 and 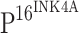 expression: rs = −0.599, P < 0.001
expression: rs = −0.599, P < 0.001
Discussion
Vaginal infection is commonly due to Gram-negative bacteria, and as the cervix is frequently exposed to a bacterial environment, it is important to explore the expression of TLR4 in cervical tissues and determine its effects on preneoplasia CINs and cervical cancer. In the present study, we demonstrate that TLR4 expression is down-regulated during the progression of cervical neoplasia and this TLR4 down-regulation appears to be associated with the expression of 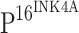 .
.
The mammalian TLR4 is a key component in the innate immune response to Gram-negative bacterial infection and plays a crucial role in the defense against bacterial invasion. For example, TLR4 signaling is important for an effective innate immune response to respiratory tract infections [27, 35] and to bacterial invasion of bladder epithelial cells [29, 30]. In addition, studies in mice have shown that TLR4 recognizes not only bacterial motifs, but also viral motifs. TLR4-deficient mice infected with respiratory syncytial virus (RSV) resulted in impaired pulmonary cellular responses and delayed viral clearance [11, 21], and it was further shown that TLR4 polymorphisms mediated the impaired responses to RSV and LPS [32]. Although adenovirus proteins were reported to inhibit LPS signaling [5], Hantaan virus induced TLR4 expression which led to enhanced production of beta interferon, interleukin-6 and tumor necrosis factor-alpha [16].
In addition to its expression in immune cells, TLR expression is also observed in many epithelial cells. Constitutive expression and differential distribution of TLRs 1–6 have been observed in the female reproductive tract [6, 25]. TLR4 was found to be localized mainly in the upper parts of the female reproductive tract, and not expressed in the vagina and ectocervix areas [25]. The functional TLR3 and TLR9 in cervical mucosal epithelial cells were reported to respond to microbial pathogens [2]. Interestingly, the expression of TLRs is also detected in some tumor cell lines and tissues [37]. TLR4 was expressed in several tumor cells, such as human lung cancer cells [12], epithelial ovarian cancer cells [18] and melanoma cells [23]. The TLR4 expression intensity was also correlated with the tumor grade in human head and neck squamous cell carcinomas [31]. The engagement of TLR4 promoted tumor immune escape by inducing immunosuppressive cytokines and apoptosis resistance [12], induced the production of proinflammatory cytokines directly from the tumor, and promoted tumor growth and paclitaxel chemoresistance [18]. Triggering of TLR4 expressed on certain tumors promoted tumor development and protected the tumor from immune attack [7, 31]. Blockade of the TLR4 pathway delayed tumor growth and prolonged the survival of tumor-bearing mice [15]. The wild-type mice exhibited a more severe cachexia than the TLR4-deficient mice [3]. The impaired ability to secrete proinflammatory cytokines such as interleukin 1beta in the TLR4-deficient mice may protect these animals from developing severe cancer cachexia [3].
The regulation of TLR expression in tumors has not been fully elucidated, and the current evidence is controversial. Increased expression of TLR5 and TLR9 was associated with neoplastic progression in the uterine cervix [19, 22]. But in another report, it was demonstrated that TLR9 expression was suppressed in primary cervical cancers [9]. This pattern of expression is similar for TLR4. As previously mentioned, TLR4 was expressed or up-regulated in some tumor cells [12, 18, 23, 31]. However, current evidence indicates that TLR4 gene expression is decreased in leukemic leukocyte populations [34], although the relevance of this decreased TLR4 expression to the pathogenesis of leukemia requires further investigation. We initially expected that TLR4 would also be up-regulated in CINs and that the TLR4-mediated inflammation might be a critical cofactor for cervical lesion. But to our surprise, we observed a down-regulation of TLR4 expression. Our observations are based on the finding that TLR9 expression was suppressed in HPV16-positive cancer-derived cell lines and primary cervical cancers [9]. The suppressive effect was mainly attributed to HPV16 E6 and E7 proteins because infection of human primary keratinocytes with HPV16 E6 and E7 recombinant retroviruses inhibits TLR9 transcription [9]. Cervical cancer development is linked to the persistent infection of high-risk HPVs. The E6 and E7, which are major oncoproteins of HPV, play a key role in the tumorigenesis of cervical epithelium. The above-mentioned study suggests that a novel mechanism is used by HPV to suppress the host immune response by deregulating the TLR9 transcript, and also indicates that abolishing innate responses may be a crucial step in the carcinogenic events mediated by HPVs [9]. Consistent with this finding, we also observed a down-regulation of TLR9 mRNA expression in CINs and ISCCs as detected by RT-PCR. Furthermore, it has been reported that hepatitis B virus (HBV) is able to suppress the TLR-induced antiviral activity of liver cells [36].
Although TLR4 expression is inversely related to the expression of 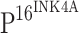 , it is difficult to believe that it is
, it is difficult to believe that it is 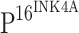 that directly regulates TLR4 expression. We conclude that the down-regulation of TLR4 observed in our study is likely the consequence of HPV persistent infection.
that directly regulates TLR4 expression. We conclude that the down-regulation of TLR4 observed in our study is likely the consequence of HPV persistent infection. 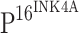 is a cell cycle inhibitor, and the down-regulation of
is a cell cycle inhibitor, and the down-regulation of 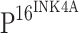 has been associated with carcinogenesis in a variety of organ systems. However, integration of HPV DNA into the host genome results in overexpression of viral proteins and induces the production of
has been associated with carcinogenesis in a variety of organ systems. However, integration of HPV DNA into the host genome results in overexpression of viral proteins and induces the production of 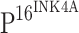 by a negative feedback loop [20].
by a negative feedback loop [20]. 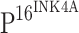 is typically used as a surrogate marker of high-risk HPV infection and high-grade CIN [8]. We hypothesize that the transcriptional inactivation of TLRs by HPV proteins may be an important mechanism utilized by HPV to suppress the host immune response. The down-regulation of TLR4 expression and the subsequent TLR4-mediated cytokine production would likely be beneficial to HPV persistent infection and enhance the transformation of cervical epithelial cells. Although further studies are required to elucidate the mechanism and significance of TLR4 down-regulation in the pathogenesis of cervical neoplasia, the current study provides a basis for more detailed investigations of TLR-mediated signaling in the carcinogenesis of cervical cancers.
is typically used as a surrogate marker of high-risk HPV infection and high-grade CIN [8]. We hypothesize that the transcriptional inactivation of TLRs by HPV proteins may be an important mechanism utilized by HPV to suppress the host immune response. The down-regulation of TLR4 expression and the subsequent TLR4-mediated cytokine production would likely be beneficial to HPV persistent infection and enhance the transformation of cervical epithelial cells. Although further studies are required to elucidate the mechanism and significance of TLR4 down-regulation in the pathogenesis of cervical neoplasia, the current study provides a basis for more detailed investigations of TLR-mediated signaling in the carcinogenesis of cervical cancers.
Acknowledgments
We are most grateful to Dr. Jacob Couturier, Department of internal medicine, University of Texas-Health Science Center at Houston (Houston, TX, USA), for language review. This study was funded by the Ph.D. Program Foundation of Ministry of Education of China (20090171110029) and grants from Yuexiu Science and Technology Bureau of Guangzhou, China (2005-WS-001, 2008-WS-003).
Contributor Information
Li Yu, Email: liyuuk@yahoo.co.uk.
Shangwu Chen, Email: lsschshw@mail.sysu.edu.cn.
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Andersen JM, Al-Khairy D, Ingalls RR. Innate immunity at the mucosal surface: role of toll-like receptor 3 and toll-like receptor 9 in cervical epithelial cell responses to microbial pathogens. Biol Reprod. 2006;74:824–831. doi: 10.1095/biolreprod.105.048629. [DOI] [PubMed] [Google Scholar]
- 3.Cannon TY, Guttridge D, Dahlman J, George JR, Lai V, Shores C, Buzková P, Couch ME. The effect of altered Toll-like receptor 4 signaling on cancer cachexia. Arch Otolaryngol Head Neck Surg. 2007;133:1263–1269. doi: 10.1001/archotol.133.12.1263. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Cheng Y, Xu Y, Liao J, Zhang X, Hu Y, Zhang Q, Wang J, Zhang Z, Shen F, Yuan Z. Expression profiles and function of Toll-like receptors 2 and 4 in peripheral blood mononuclear cells of chronic hepatitis B patients. Clin Immunol. 2008;128:400–408. doi: 10.1016/j.clim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Delgado-Lopez F, Horwitz MS. Adenovirus RIDalphabeta complex inhibits lipopolysaccharide signaling without altering TLR4 cell surface expression. J Virol. 2006;80:6378–6386. doi: 10.1128/JVI.02350-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fazeli A, Bruce C, Anumba DO. Characterization of Toll-like receptors in the female reproductive tract in humans. Hum Reprod. 2005;20:1372–1378. doi: 10.1093/humrep/deh775. [DOI] [PubMed] [Google Scholar]
- 7.Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ, Subbaramaiah K, Cooper HS, Itzkowitz SH, Abreu MT. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halloush RA, Akpolat I, Jim Zhai Q, Schwartz MR, Mody DR. Comparison of ProEx C with p16 and Ki-67 immunohistochemical staining of cell blocks prepared from residual liquid-based cervicovaginal material: a pilot study. Cancer. 2008;114:474–480. doi: 10.1002/cncr.23951. [DOI] [PubMed] [Google Scholar]
- 9.Hasan UA, Bates E, Takeshita F, Biliato A, Accardi R, Bouvard V, Mansour M, Vincent I, Gissmann L, Iftner T, Sideri M, Stubenrauch F, Tommasino M. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J Immunol. 2007;178:3186–3197. doi: 10.4049/jimmunol.178.5.3186. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 11.Haynes LM, Moore DD, Kurt-Jones EA, Finberg RW, Anderson LJ, Tripp RA. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75:10730–10737. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He W, Liu Q, Wang L, Chen W, Li N, Cao X. TLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Mol Immunol. 2007;44:2850–2859. doi: 10.1016/j.molimm.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 14.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 15.Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, Mayer L, Unkeless JC, Xiong H. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 16.Jiang H, Wang PZ, Zhang Y, Xu Z, Sun L, Wang LM, Huang CX, Lian JQ, Jia ZS, Li ZD, Bai XF. Hantaan virus induces toll-like receptor 4 expression, leading to enhanced production of beta interferon, interleukin-6 and tumor necrosis factor-alpha. Virology. 2008;380:52–59. doi: 10.1016/j.virol.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Kalof AN, Evans MF, Simmons-Arnold L, Beatty BG, Cooper K. P16INK4A immunoexpression and HPV in situ hybridization signal patterns: potential markers of high-grade cervical intraepithelial neoplasia. Am J Surg Pathol. 2005;29:674–679. doi: 10.1097/01.pas.0000155164.78785.c2. [DOI] [PubMed] [Google Scholar]
- 18.Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, Visintin I, Rutherford T, Mor G. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006;66:3859–3868. doi: 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- 19.Kim WY, Lee JW, Choi JJ, Choi CH, Kim TJ, Kim BG, Song SY, Bae DS. Increased expression of Toll-like receptor 5 during progression of cervical neoplasia. Int J Gynecol Cancer. 2008;18:300–305. doi: 10.1111/j.1525-1438.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- 20.Klaes R, Benner A, Friedrich T, Ridder R, Herrington S, Jenkins D, Kurman RJ, Schmidt D, Stoler M, von Knebel Doeberitz M. p16 immunohistochemistry improves interobserver agreement in the diagnosis of cervical intraepithelial neoplasia. Am J Surg Pathol. 2002;26:1389–1399. doi: 10.1097/00000478-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 22.Lee JW, Choi JJ, Seo ES, Kim MJ, Kim WY, Choi CH, Kim TJ, Kim BG, Song SY, Bae DS. Increased toll-like receptor 9 expression in cervical neoplasia. Mol Carcinog. 2007;46:941–947. doi: 10.1002/mc.20325. [DOI] [PubMed] [Google Scholar]
- 23.Molteni M, Marabella D, Orlandi C, Rossetti C. Melanoma cell lines are responsive in vitro to lipopolysaccharide and express TLR-4. Cancer Lett. 2006;235:75–83. doi: 10.1016/j.canlet.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Nikolaitchouk N, Andersch B, Falsen E, Strömbeck L, Mattsby-Baltzer I. The lower genital tract microbiota in relation to cytokine-, SLPI- and endotoxin levels: application of checkerboard DNA–DNA hybridization (CDH) APMIS. 2008;116:263–277. doi: 10.1111/j.1600-0463.2008.00808.x. [DOI] [PubMed] [Google Scholar]
- 25.Pioli PA, Amiel E, Schaefer TM, Connolly JE, Wira CR, Guyre PM. Differential expression of toll-like receptors 2 and 4 in tissues of the human female reproductive tract. Infect Immun. 2007;72:5799–5806. doi: 10.1128/IAI.72.10.5799-5806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice:mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 27.Ramphal R, Balloy V, Jyot J, Verma A, Si-Tahar M, Chignard M. Control of Pseudomonas aeruginosa in the lung requires the recognition of either lipopolysaccharide or flagellin. J Immunol. 2008;181:586–592. doi: 10.4049/jimmunol.181.1.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmausser B, Andrulis M, Endrich S, Muller-Hermelink HK, Eck M. Toll-like receptors TLR4, TLR5 and TLR9 on gastric carcinoma cells: an implication for interaction with Helicobacter pylori . Int J Med Microbiol. 2005;295:179–185. doi: 10.1016/j.ijmm.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Song J, Bishop BL, Li G, Duncan MJ, Abraham SN. TLR4-initiated and cAMP-mediated abrogation of bacterial invasion of the bladder. Cell Host Microbe. 2007;1:287–298. doi: 10.1016/j.chom.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song J, Duncan MJ, Li G, Chan C, Grady R, Stapleton A, Abraham SN. A novel TLR4-mediated signaling pathway leading to IL-6 responses in human bladder epithelial cells. PLoS Pathog. 2007;3:e60. doi: 10.1371/journal.ppat.0030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szczepanski MJ, Czystowska M, Szajnik M, Harasymczuk M, Boyiadzis M, Kruk-Zagajewska A, Szyfter W, Zeromski J, Whiteside TL. Triggering of toll-like receptor 4 expressed on human head and neck squamous cell carcinoma promotes tumor development and protects the tumor from immune attack. Cancer Res. 2009;69:3105–3113. doi: 10.1158/0008-5472.CAN-08-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tulic MK, Hurrelbrink RJ, Prêle CM, Laing IA, Upham JW, Le Souef P, Sly PD, Holt PG. TLR4 polymorphisms mediate impaired responses to respiratory syncytial virus and lipopolysaccharide. J Immunol. 2007;179:132–140. doi: 10.4049/jimmunol.179.1.132. [DOI] [PubMed] [Google Scholar]
- 33.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 34.Webb RN, Cruse JM, Lewis RE. Decreased TLR4 gene expression in leukemic leukocyte populations. Exp Mol Pathol. 2009;87:117–126. doi: 10.1016/j.yexmp.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Wieland CW, Florquin S, Maris NA, Hoebe K, Beutler B, Takeda K, Akira S, van der Poll T. The MyD88-dependent, but not the MyD88-independent, pathway of TLR4 signaling is important in clearing nontypeable haemophilus influenzae from the mouse lung. J Immunol. 2005;175:6042–6049. doi: 10.4049/jimmunol.175.9.6042. [DOI] [PubMed] [Google Scholar]
- 36.Wu J, Meng Z, Jiang M, Pei R, Trippler M, Broering R, Bucchi A, Sowa JP, Dittmer U, Yang D, Roggendorf M, Gerken G, Lu M, Schlaak JF. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology. 2009;49:1132–1140. doi: 10.1002/hep.22751. [DOI] [PubMed] [Google Scholar]
- 37.Yu L, Chen S. Toll-like receptors expressed in tumor cells: targets for therapy. Cancer Immunol Immunother. 2008;57:1271–1278. doi: 10.1007/s00262-008-0459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]