Abstract
The mucin MUC1 molecule is overexpressed on a variety of adenocarcinomas and is thus, a potential target for immunotherapy. Of the MUC1 peptides that bind to HLA-A*0201(A2), M1.2 (LLLLTVLTV) from the signal sequence appears to be the most immunogenic in humans. Here we have shown that large numbers (109) of tetramer-binding M1.2-specific cytotoxic T lymphocytes (CTL) can be generated ex vivo from circulating precursors, derived from healthy adults. However, there was significant interpersonal variation in the level of co-stimulatory signal required. Tetramer-binding cells also required maturation in culture to become proficient killers of the HLA-A2+ MUC1+ MCF7 cell line, known to express a low number of endogenously processed M1.2. The functional avidity of M1.2-specific CTL, however, was low as compared to CTL specific for an HIV-1 epitope. Despite the low avidity, M1.2-specific CTL were polyfunctional, secreting multiple cytokines upon degranulation with antigen recognition. To identify potential agonist peptides that may be superior immunogens, an M1.2-specific CTL culture was used to scan a large nonameric combinatorial peptide library. Of 54 predicted peptides, 4 were “consensus” agonists because they were recognized by CTL from two other donors. Two agonists, p29 (LLPWTVLTV) and p15 (VLLWTVLTV), were equally stimulatory when loaded onto C1R target cells transfected with wild-type HLA-A2. Both agonists induced IL-2, TNF-α, IFN-γ, and degranulation with M1.2-specific CTL. In contrast, production of these cytokines, which are tightly regulated by specific activation through the T cell receptor, was restricted when the CTL were stimulated with peptides loaded onto C1R cells that were transfected with an HLA-A2 molecule bearing a mutation that abrogates binding to the CD8 co-receptor. Thus, activation by both M1.2 and its agonists was dependent upon CD8, showing that compensation by the co-receptor was necessary for the human T cell response to M1.2.
Keywords: Cancer, CTL epitope, Agonist peptide, Immunotherapy
Introduction
Active specific immunotherapy has a potential role in one or more of the common adenocarcinomas. For example, 25% of women with breast cancer die despite advances in detection and conventional (surgical and cytoreductive) treatments. When breast cancer is detected early it is theoretically curable, but once it has spread it is not. Nevertheless, many patients with metastatic disease remain viable despite having exhausted all forms of conventional hormonal or cytotoxic therapy. Specific immunotherapy with potent vaccines, with their intrinsic lack of toxicity, would be desirable in such patients, but especially in others with early, minimal residual disease.
The ubiquitous cancer mucin, MUC1, may be a good target for the development of specific immunotherapy, and eventually a preventive vaccine. MUC1 is overexpressed on 90% of breast carcinomas [1], in contrast to HER2/neu, for example, which is amplified in only 20–30% of breast cancers [2]. In addition, MUC1 is overexpressed on adenocarcinomas including those of the pancreas, ovary, and prostate, as well as on hematologic malignancies [3, 4]. This highly glycosylated and complex molecule is anchored within the cell surface by a transmembrane domain. The bulk of the molecule is an extensive extracellular domain with a variable number of 20 amino acid tandem repeats (TR) [5, 6]. Since aberrant glycosylation of the TR results in exposure of tumor-specific epitopes that are normally cryptic, much attention has been given to this region of MUC1 as a possible immunogen [1]. However, in clinical trials using TR domain conjugated to adjuvants such as BCG [7–9], the dominant response observed has been humoral with minimal induction of cytotoxic T cells [8, 10].
Two HLA-A2-binding peptides, M1.1 (STTPPVHNV) and M1.2 (LLLLTVLTV) were identified through computer analysis in the terminal TR and the signal sequence of the MUC1 protein, respectively [11, 12]. With dendritic cells (DC) loaded with these peptides as antigen-presenting cells (APC), cytotoxic T lymphocytes (CTL) were generated that lysed tumors endogenously expressing MUC1 in an antigen-specific and HLA-A2-restricted fashion, showing that T cell immune responses to MUC1 protein are not limited to the TR domain [13]. More importantly, MUC1-specific CTL were induced after vaccination of breast and ovarian cancer patients with DC pulsed with these peptides, with M1.2 eliciting a stronger CTL response than M1.1 [13]. A potential advantage of using an epitope such as M1.2 is its location in the signal sequence of MUC1. These peptides could be presented in malignant cells without functional TAP, utilizing an alternative pathway for the generation of MHC class I-restricted peptides [14]. Of several peptides that bind to HLA-A2 in the MUC1 molecule, only M1.2 was recognized by T cells in the bone marrow [15] or the peripheral blood in a proportion of patients with breast cancer [15, 16].
Selecting and expanding antigen-specific T cells ex vivo can provide new insights into T cell antigen recognition, co-stimulation, and repertoire. CD8+ T cells with specificities for cytomegalovirus or Epstein–Barr virus appear to grow sufficiently well in vitro to allow for adoptive T cell therapy in immunocompromised allogeneic bone marrow recipients [17]. We have also generated stable HIV-1 epitope-specific CD8+ T cell cultures from seronegative healthy donors which have provided unique insights into the corresponding T cell responses elicited by the natural infection [18, 19]. In contrast, it has been significantly more challenging to obtain CD8+ T cell cultures to non-viral tumor antigens from cancer patients, with rare exceptions being a few epitopes in melanoma (Melan-A and gp100 epitopes) [20] that appear to have unusual properties [21]. In general, reports on CTL with specificity to tumor-associated antigens, including MUC1 [13, 16, 22], have been restricted to short-lived cultures with modest reactivities that did not allow for detailed characterization. The poor in vitro T cell responses to non-viral tumor antigens suggest immunological tolerance in that clonotypes with high avidity have been deleted or made anergic by the autochthonous tumors.
Here we characterized M1.2-specific CTL cultures with high levels of tetramer-binding cells that were generated from circulating CD8+ precursor T cells of healthy normal donors, using protocols established for HIV-1 peptide-specific CTL [18]. To identify agonists that may be more effective than the native epitope, we used one of the M1.2-specific CTL cultures to probe a combinatorial peptide library consisting of 340 billion nonapeptides in a positional scanning format [19, 23]. “Consensus” peptides—those recognized by several donors—have been identified. These were used to further analyze the characteristics of human CTL against M1.2.
Materials and methods
Donors and HLA typing
Healthy male and female donors were prescreened by flow cytometry with the monoclonal antibody (mAb) BB7.2 specific for the HLA-A2 supertype [24] and subsequently confirmed by sequence-specific primer PCR by the Immunogenetics Laboratory at the National Cancer Institute. Only HLA-A*0201+ (abbreviated hereafter as HLA-A2+) individuals were studied. This study was approved by the Human Investigation Committee of Wayne State University School of Medicine and all subjects gave written informed consent.
Preparation of monocyte-derived dendritic cells
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll–Hypaque density gradients. Immature DC were generated from plastic-adherent monocytes by culturing in 500 U/ml of rIL4 and 1,000 U/ml of GM-CSF for 7 days. Maturation of DC was achieved by exposing day 6 immature DC to 1 μg/ml LPS (E. coli serotype 026:B6, Sigma, St Louis, MO, USA) for 16 h or to a cocktail of 1,000 U/ml IL-6, 10 ng/ml IL-1β, and 10 ng/ml TNF-α for 36 h [25]. Maturation was confirmed by enhanced expression of HLA-DR, CD40, and CD86 in lineage-negative cells (CD3, CD14, and CD19). Induction of CD83 was also used as an index for maturation.
In vitro immunization of human CD8+ T cells with M1.2 peptide
The procedures for ex vivo generation of peptide-specific CTL have been described previously [18]. In brief, CD8+ T cells were positively selected from PBMC with immunomagnetic beads (Dynal, Oslo, Norway). DC were irradiated at 4,000 cGy and pulsed with 20 μg/ml M1.2 peptide for 2 h at 37°C in serum-free HEPES-buffered RPMI 1640 supplemented with penicillin and streptomycin, sodium pyruvate, non-essential amino acids, and l-glutamine (complete medium). After removing the medium, DC were pulsed for an additional 1.5 h with 20 μg/ml peptide, 3 μg/ml β2-microglobulin, and 1% human serum albumin. Purified CD8+ T cells and irradiated, peptide-pulsed DC at a 5:1 (T:DC) ratio were cultured in complete medium supplemented with 10% autologous serum and 10 ng/ml rIL-7 (Genzyme, Cambridge, MA, USA). On days 1 and 4, IL-2 (Chiron, Harefield, Middlesex, UK) was added at 20 U/ml. CTL were re-stimulated weekly with M1.2-pulsed irradiated autologous monocytes [18]. The resulting CTL cultures were tested at week 4 and subsequent weeks for antigen-specificity. In a few experiments, M1.2-specific CTL (2 × 105) were expanded with the anti-CD3 mAb (OKT3, Ortho Biotech, Raritan, NJ, USA) at 30 ng/ml in the presence of IL-2 (20 U/ml added day 1 and every 3 day thereafter), irradiated PBMC (4,000 cGy, 25 × 106) were used as feeder in complete media supplemented with 10% autologous serum.
Cytotoxicity assay
Cytotoxicity was determined by the ability of CTL to lyse peptide-treated or known MUC1+ target cells. T2 cells (HLA-A2+, TAP deficient) or tumor cells were labeled with sodium [51Cr] chromate (NEN, Boston, MA, USA) and incubated for 1 h at 37°C. After washing to remove soluble 51Cr, 1 × 104 labeled peptide-pulsed T2 cells or tumor cells were mixed with T cells at different E:T ratios in 96-round bottom plates. Supernatants were harvested after 4 h and mixed with scintillation fluid (Optiphase SuperMix; PerkinElmer-Wallac) for counting in a MicroBeta counter (PerkinElmer-Wallac). T2 cells not pulsed with peptide were used as negative controls. Percent lysis was calculated as: 100 × (experimental release − spontaneous release)/(total release − spontaneous release).
Scanning of the nonamer library with M1.2-specific CTL
The combinatorial nonamer peptide library was scanned with an M1.2-specific CTL culture from donor CC, and cytolytic activity was determined by the 51Cr release assay. The CTL were incubated with 51Cr-labeled T2 cells in the presence of 100 μg/ml of each peptide library mixture. This nonamer library consists of 180 equimolar peptide mixtures in the OX8 format where O represents one each of the 20 natural l-amino acids in a defined position and X represents all of the natural amino acids, with the exception of cysteine (C) in each of the remaining positions. For example, the first mixture has alanine (A) in position 1 (A1X8), while mixture number 180 has tyrosine (Y) in position 9 (X8Y9). Each OX8 mixture consists of 1.9 × 109 (198) different nonamer peptides in approximately equimolar concentration, and the total X9 library consists of 3.8 × 1010 (20 × 198) different peptides. Assuming an average molecular weight of 1,080 for a nonamer peptide mixture and a concentration of 100 μg/ml (100 μM), the concentration of each individual library nonapeptide is 5.3 × 10−14 M.
Individual peptides were synthesized by the simultaneous multiple peptide synthesis method [26]. The identity and purity of each peptide were characterized using an electrospray mass spectrometer interfaced with a liquid chromatography system.
Intracellular cytokine staining and degranulation assays
To assess the contribution of the CD8 co-receptor to CTL functions, C1R stably transfected with wild-type HLA-A2 (C1R-A2wt) or an HLA-A2 variant encoding a mutation in the (3 domain that abrogate CD8 binding without affecting the integrity of TCR interaction (C1R-A2CD8null) [27] were used as target cells. CTL were stimulated with peptide-loaded (10 (g/ml) target cells at 37°C in the presence of anti-CD107a/b mAb. Monensin (GolgiStop, Pharmingen/BD, San Diego, CA) was added from the beginning to prevent cytokine secretion. After 4 h, cells were washed twice, fixed, permeabilized and stained with either PE-conjugated anti-isotype, anti-IL-2, anti-TNF-α, anti-IFN-γ, anti-MIP-1α, or FITC-conjugated anti-CD8 according to the manufacturer’s instructions (Pharmingen/BD, San Diego, CA). Analysis was performed by gating on CD8+ T cells and collecting 10,000 events.
T2 stabilization assay
For each peptide to be tested, 2 × 105 T2 cells in triplicates were washed and incubated overnight in 100 μl of complete medium supplemented with 1% FBS, 4 μg/ml β2-microglobulin, and 200 μM peptide (saturating level) at 37°C. Peptide-loaded cells were washed with cold PBS/0.05% bovine serum albumin/0.02% NaN3 and stained with the HLA-A*0201-specific BB7.2 mAb (Pharmingen/BD, San Diego, CA) followed with a FITC-labeled goat anti-mouse IgG secondary antibody at 4°C. Mean fluorescence intensity (MFI) was determined by analysis with a FACSCalibur cytofluorometer.
Results
Comparison of LPS-matured, TNF-α-matured, or immature DC for primary stimulation
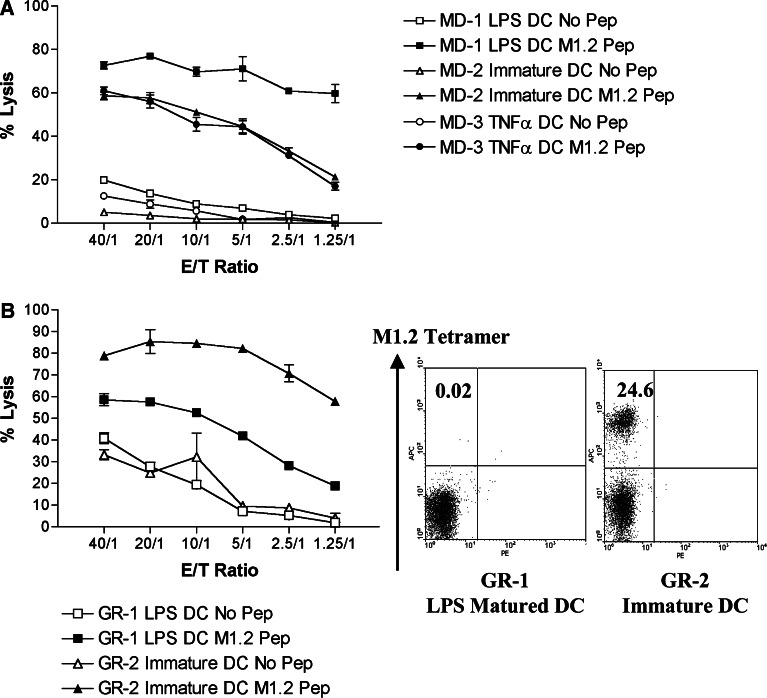
Since CTL cultures specific for the HIV-1 Gag p17 epitope SLYNTVATL (SL9) could be generated only by priming with immature DC [18], we first determined whether there was a similar maturation restriction for M1.2-specific CTL. As shown in Fig. 1a, M1.2-specific cytotoxicity was elicited in parallel cultures from donor MD by priming with autologous immature DC or with LPS-matured DC (LPS DC) or cytokine-matured DC (TNF-α DC). The level of cytotoxicity elicited with LPS-matured DC was significantly higher than that achieved by immature or cytokine-treated DC, particularly at the lower E/T ratio. Thus, all three types of DC could prime M1.2-specific CTL. However, we did not find tetramer-positive T cells in these cultures, suggesting that either the frequencies of antigen-specific cells were below the level of detection (<1/10,000) or, more likely, that their avidity was too low to bind stably with tetramers. From a different donor, GR, priming by immature DC produced more cytotoxic T cells than LPS-matured DC in parallel cultures (Fig. 1b, right panel). Moreover, as many as 25% of the CD8+ T cells in the former stained positively for tetramers (Fig. 1b, left panel). These results indicate person-to-person variability in the co-stimulatory requirements for the priming of CD8+ T cells specific for M1.2 peptide.
Fig. 1.
Cytotoxicity of parallel cultures of M1.2-specific CTL from donors MD (a) and GR (b) primed by immature and matured DC generated as described in Sect. ”Materials and methods.” Percent lysis was measured by a standard 4-h 51Cr release assay using as targets T2 cells pulsed with 1 μg/ml of M1.2 peptide at various E/T ratios. M1.2-tetramer-binding CD8+ T cells were detected only in cultures from GR (b, scatter plots)
Re-stimulation with adherence-purified monocytes versus DC
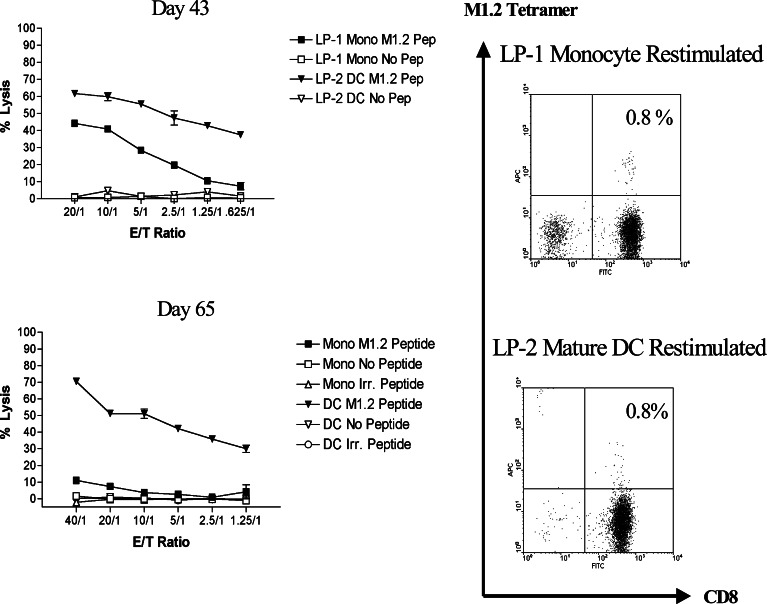
CD8+ T cells improve their responsiveness to antigen after successive re-stimulations with APC [28, 29]. We therefore compared re-stimulation by DC with that by monocytes in producing highly cytotoxic, tetramer-positive M1.2-specific CTL. Parallel CD8+ T cell cultures primed by LPS-matured DC from donor LD were re-stimulated with autologous M1.2-pulsed matured DC or monocytes. As shown in Fig. 2, both methods generated the same percentage of tetramer-binding CTL, but the CTL culture re-stimulated by DC displayed significantly greater cytotoxicity, which was also retained for a longer period of time. By day 65, LP-1 cells re-stimulated by monocytes had lost their ability to kill M1.2-pulsed T2 cells in the microcytotoxicity assay. In contrast, LP-2 remained functionally active when the cells were assessed 14 days later (data not shown). Thus, it appears that cytotoxicity, and perhaps other differentiated functions as well, of the M1.2-specific CTL may be improved through the modulation of co-stimulatory signals.
Fig. 2.
Cytotoxicity and percent tetramer-binding cells in parallel M1.2-specific CTL cultures generated from donor LP over time (day 43 and day 65) using either monocytes or DC for re-stimulation. Panels on the left show cytotoxicity, which was measured in a standard 51Cr release assay with T2 cells, pulsed either with 1 μg/ml of M1.2, no peptide, or an irrelevant HIV-1 peptide, TV9 (TLNAWVKVV) on day 43 and day 65. Panels on the right show the percent CD8+ T cells that stained with an APC-conjugated tetramer and FITC-conjugated anti-CD8 mAb on those days
Acquisition of cytotoxicity to HLA-A2+MUC+ breast cancer cell line with maturation by tetramer-positive, M1.2-specific CTL
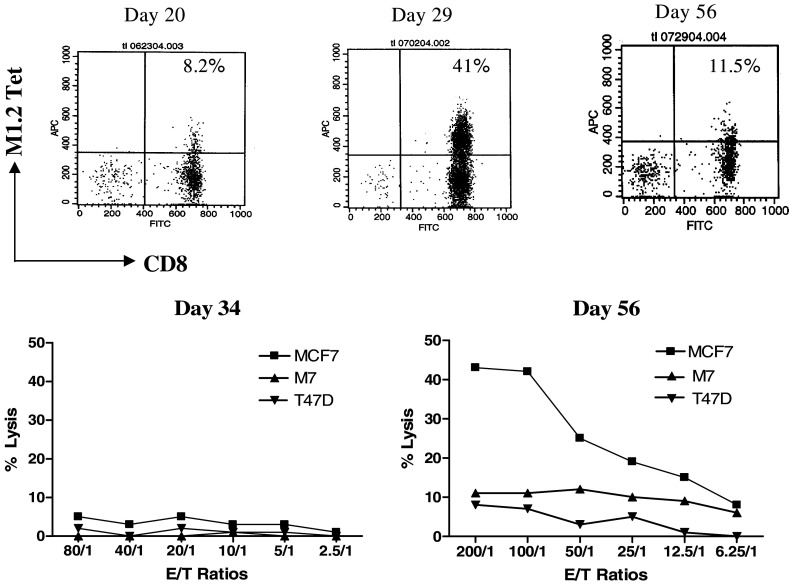
Tetramer-rich M1.2-specific CTL generated with immature DC from donor JF were cytotoxic not only to M1.2-pulsed T2 cells but also to the MUC1+/A2+ MCF-7 breast cancer cell line. In contrast, control melanoma MSM-M7 (MUC1-/A2+) and T47D cells (MUC1+/A2-) were not lysed. Interestingly, M1.2-tetramer-binding T cells showed an increase in antigen responsiveness of their cytotoxicity with maturation (day 56 versus day 29). MCF7 cells were not lysed by the day 34 cultures despite the presence of 40% tetramer-binding T cells. In contrast, on day 56 JF-1 CTL caused >40% lysis of MCF7 cells at an E/T ratio of 100/1 and 25% lysis at a 25/1 ratio despite a lower percentage of tetramer-binding cells (Fig. 3). These results suggest a dissociation between proliferation and functional maturation during the early stages of expansion by antigen-specific human CD8+ T cells, a phenomenon also observed for HIV-1-specific CTL [19].
Fig. 3.
Dissociation between proliferation of M1.2-tetramer-binding T cells and acquisition of functional maturation during the early stages of expansion in M1.2-specific CTL from donor JF. The number of tetramer-binding T cells over the course of this study was shown for days 20, 29, and 56 (top). Human breast cancer cell lines MCF-7 (MUC1+, HLA-A2+) and T47D (MUC1+, HLA-A2−), and melanoma MSM-M7 (MUC1−, HLA-A2+) were used as targets in a standard 4-h 51Cr release assay (bottom)
Nonamer library scan and screening of subsequently synthesized peptides as potential agonistic peptide analogs
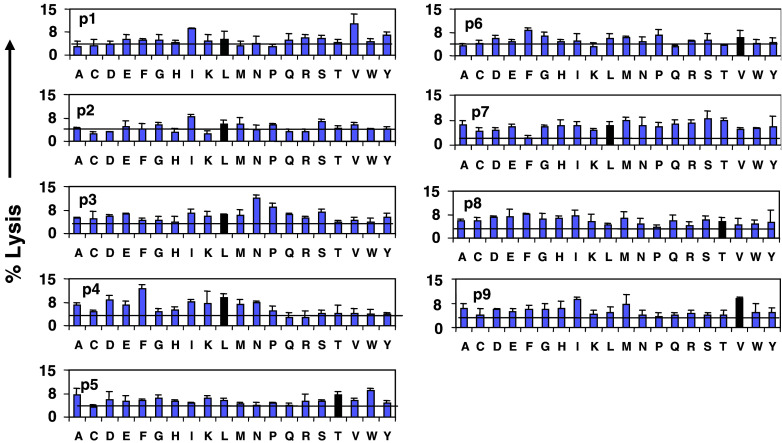
The peptide library was scanned with M1.2-specific CTL from donor CC. This culture was highly specific for M1.2 in the T2 microcytotoxicity assay (50% lysis at E/T ratio of 20:1). CTL were incubated with 51Cr-labeled T2 cells at this ratio as described in Sect. “Materials and methods.” Each assay was performed in triplicate; mean lysis and standard deviations are shown in Fig. 4. It was striking that a high level of cytotoxicity (as much as 12%) was observed with some peptide mixtures, each of which comprised 1.7 × 1010 (198) peptides, in which the concentration of a single peptide was only 5.3 × 10−14 M. The standard deviations were small even though the triplicate assays were set in three separate microtiter plates for each mixture. Three of the mixtures defined with residues corresponding to the native M1.2 sequence (dark bars) were among the most stimulatory mixtures. They were position 4-L, position 5-T, and position 9-V. Moreover, the mixtures defined at the anchor positions of this scan confirmed its predictive power and accuracy. L and M are the preferred hydrophobic residues for the position 2 anchor position of HLA-A2. Accordingly, the L and M mixtures at position 2 were equally stimulatory. Similarly, V is preferred for the position 9-anchor of HLA-A2, and the scan showed the V mixture to be most active. For positions 1, 3, 4, 7, and 8, at least two other mixtures were equal or more stimulatory than the mixture containing the amino acid of the native peptide. Thus, variability at these positions is well tolerated by the TCR, making them likely to be the most “degenerate.”
Fig. 4.
Scanning of the nonapeptide combinatorial peptide library. Cytotoxicity of the index M1.2-specific CTL culture (donor CC) to the 180 mixtures of the library is presented. Each graph, designated P1 to P9, represents a set of 20 mixtures having the defined amino acid listed on the X-axis at a given position. The horizontal line in each graph shows the average cytotoxicity for the 20 mixtures of that position. Percent lysis is plotted on the Y-axis. The dark bar in each graph is the lysis generated by the amino acid of the M1.2 peptide at that position
Based upon these data, a panel of 54 candidate peptides was designed and synthesized (Table 1) with single, double, or triple amino acid substitutions. There were no substitutions at positions 2, 5, 7, or 9. The peptides were screened with LP-2 and JF-1, which were 0.8 and 41% tetramer-positive, respectively (Figs. 2, 3). Nonetheless, both cultures were highly cytotoxic to T2 cells pulsed with M1.2 over a range of E/T ratios ranging from 6.25/1 to 20/1. Maximum % lysis was achieved at 10/1 (65 and 80%, respectively), with <3% background lysis of T2 cells alone or T2 pulsed with irrelevant HIV peptide. Table 2 summarizes the reactivities of these CTL against the predicted peptides at four different concentrations. Thirty percent (16 of 54) of the peptides predicted from the reactivity of M1.2-specific CTL from one donor were recognized by CTL from the two other donors. LP-2 recognized 6 of the 54 (11%), while JF-2 recognized an additional 10 peptides (19%).
Table 1.
Library predictions from the scans with CC-CTL
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| M1.2 | L | L | L | L | T | V | L | T | V |
| Library predictions | V | A | F | P | S | ||||
| P | T | N | E | ||||||
| W |
Panel to be tested: 9 single substituted, 39 double substituted, 6 triple substituted, 54 in total
Table 2.
Screening of predicted peptides by cytotoxicity (% specific lysis) with M1.2-specific LP-2 and JF-1
| Peptide sequences | Number of substitutions | LP-2 | JF-1 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percent lysis at peptide concentrations (ng/ml) | ||||||||||||||||||
| 1,000 | 100 | 10 | 1 | 1,000 | 100 | 10 | 1 | |||||||||||
| Muc1.2 | L | L | L | L | T | V | L | T | V | 0 | 82 | 77 | 40 | 7 | 70 | 72 | 54 | 3 |
| 1 | V | – | – | – | – | – | – | – | 1 | 82 | 42 | 8 | 1 | 40 | 11 | 1 | −1 | |
| 2 | – | – | A | – | – | – | – | – | – | 78 | 38 | 7 | 4 | 73 | 66 | 53 | 0 | |
| 3 | – | – | P | – | – | – | – | – | – | 30 | 15 | 6 | 5 | 68 | 72 | 62 | 12 | |
| 4 | – | – | – | F | – | – | – | – | – | 4 | 0 | 3 | 3 | 0 | −1 | 1 | 0 | |
| 5 | – | – | – | T | – | – | – | – | – | 6 | 5 | 6 | −1 | 20 | −1 | 1 | −1 | |
| 6 | – | – | – | W | – | – | – | – | – | 5 | 2 | 2 | 2 | 12 | −1 | 0 | −1 | |
| 7 | – | – | – | – | – | P | – | – | – | 20 | 4 | 2 | 2 | 0 | 0 | −1 | −1 | |
| 8 | – | – | – | – | – | N | – | – | – | 40 | 22 | 4 | 2 | 0 | 0 | 0 | −1 | |
| 9 | – | – | – | – | – | – | – | S | – | 32 | 9 | 3 | 3 | 61 | 26 | −1 | −1 | |
| 10 | – | – | – | – | – | – | – | E | – | 20 | 12 | 3 | 0 | 63 | 38 | −1 | –2 | |
| 11 | V | – | A | – | – | – | – | – | – | 2 | 64 | 28 | 7 | 1 | 65 | 29 | −1 | –1 |
| 12 | V | – | P | – | – | – | – | – | – | 3 | 2 | 1 | 1 | 1 | −1 | –1 | –1 | |
| 13 | V | – | – | F | – | – | – | – | – | 65 | 24 | 5 | 1 | 24 | 2 | −1 | –1 | |
| 14 | V | – | – | T | – | – | – | – | – | 5 | 2 | −1 | 1 | 0 | −2 | –1 | –2 | |
| 15 | V | – | – | W | – | – | – | – | – | 83 | 63 | 28 | 3 | 47 | 24 | 0 | 1 | |
| 16 | V | – | – | – | – | P | – | – | – | 17 | −1 | –1 | 2 | −2 | –1 | 1 | −2 | |
| 17 | V | – | – | – | – | N | – | – | – | 10 | 2 | 0 | 1 | 34 | 4 | 1 | 0 | |
| 18 | V | – | – | – | – | – | – | S | – | 65 | 18 | 3 | 1 | 48 | 4 | 0 | −1 | |
| 19 | V | – | – | – | – | – | – | E | – | 36 | 14 | 2 | 1 | 24 | 1 | −1 | –2 | |
| 20 | – | – | A | F | – | – | – | – | – | 6 | 3 | 6 | 2 | −1 | –2 | –1 | –2 | |
| 21 | – | – | A | T | – | – | – | – | – | –1 | 0 | 6 | 2 | 53 | 31 | −1 | –1 | |
| 22 | – | – | A | W | – | – | – | – | – | 6 | 2 | 2 | 4 | 1 | −2 | –2 | –2 | |
| 23 | – | – | A | – | – | P | – | – | – | 1 | −1 | 3 | 2 | 2 | −1 | –1 | –2 | |
| 24 | – | – | A | – | – | N | – | – | – | 1 | −1 | 1 | 2 | 50 | 25 | 1 | −1 | |
| 25 | – | – | A | – | – | – | – | S | – | 29 | 8 | 4 | −2 | 52 | 15 | −1 | –3 | |
| 26 | – | – | A | – | – | – | – | E | – | 3 | 5 | 2 | −1 | 7 | 0 | −1 | −3 | |
| 27 | – | – | P | F | – | – | – | – | – | 2 | 5 | 4 | 4 | 0 | −1 | −1 | −2 | |
| 28 | – | – | P | T | – | – | – | – | – | 6 | 5 | 0 | 0 | 59 | 52 | 10 | −1 | |
| 29 | – | – | P | W | – | – | – | – | – | 84 | 78 | 55 | 9 | 64 | 59 | 15 | −1 | |
| 30 | – | – | P | – | – | N | – | – | – | 2 | 1 | 0 | 0 | 46 | 19 | −1 | −2 | |
| 31 | – | – | P | – | – | – | – | S | – | 3 | 1 | 1 | 1 | 58 | 43 | 4 | −2 | |
| 32 | – | – | P | – | – | – | E | – | 7 | 1 | 0 | −1 | 60 | 37 | 3 | −1 | ||
| 33 | – | – | – | F | – | N | – | – | – | 2 | 0 | 4 | 1 | 3 | 0 | 1 | −1 | |
| 34 | – | – | – | F | – | P | – | – | – | 1 | 4 | 5 | 1 | −1 | 1 | −2 | 0 | |
| 35 | – | – | – | F | – | – | – | S | – | 5 | 5 | 6 | 4 | 8 | 0 | 0 | −1 | |
| 36 | – | – | – | F | – | – | – | E | – | 5 | 3 | 3 | 0 | −1 | −1 | −1 | 0 | |
| 37 | – | – | – | T | – | N | – | – | – | 7 | 1 | 2 | 1 | −1 | 0 | −1 | −2 | |
| 38 | – | – | – | T | – | P | – | – | – | 0 | 2 | 2 | 1 | 2 | 0 | −1 | 1 | |
| 39 | – | – | – | T | – | – | – | S | – | 2 | 1 | 3 | 2 | 0 | −1 | −1 | −2 | |
| 40 | – | – | – | T | – | – | – | E | – | 3 | 2 | 2 | 1 | 0 | 1 | 0 | 0 | |
| 41 | – | – | – | W | – | N | – | – | – | 11 | 2 | 1 | 1 | 0 | −1 | −1 | −1 | |
| 42 | – | – | –– | W | – | P | – | – | – | 59 | 17 | 4 | 2 | 39 | 5 | 0 | −2 | |
| 43 | – | – | –– | W | – | – | –– | S | – | 2 | 2 | 3 | 1 | 0 | 0 | 0 | −2 | |
| 44 | – | – | – | W | – | – | – | E | – | 7 | 5 | 2 | 3 | 1 | −2 | 1 | −2 | |
| 45 | – | – | – | – | – | P | – | S | – | 8 | 5 | 3 | 3 | 0 | −1 | 0 | −1 | |
| 46 | – | – | – | – | – | P | – | E | – | 40 | 15 | 5 | 4 | −1 | −1 | 1 | −2 | |
| 47 | – | – | – | – | – | N | – | S | – | 5 | 3 | 2 | 1 | 1 | 0 | 0 | −2 | |
| 48 | – | – | – | – | – | N | – | E | – | 2 | 0 | −3 | 1 | 0 | 0 | 1 | −2 | |
| 49 | V | – | – | F | – | – | – | S | – | 3 | 1 | 1 | 1 | 0 | 0 | −1 | 0 | −2 |
| 50 | V | – | A | F | – | – | – | – | – | 3 | 4 | 2 | 2 | 0 | 0 | 0 | −1 | |
| 51 | V | – | A | – | – | – | – | S | – | 17 | 7 | 5 | 0 | 4 | −1 | 0 | −3 | |
| 52 | V | – | – | F | – | P | – | – | – | 1 | 3 | 2 | 3 | −1 | −1 | 0 | −1 | |
| 53 | V | – | – | – | – | P | – | S | – | 9 | 6 | 0 | 1 | 2 | −3 | 1 | −2 | |
| 54 | V | – | – | F | – | – | E | – | 0 | 2 | 4 | 3 | −2 | −2 | 1 | 1 | ||
Two thousand 51Cr-labeled T2 cells were co−cultured with T cells at the E/T ratio of 10/1 for 4 h at 37°C in the presence of 1,000, 100, 10, or 1 ng/ml of 1 of the 54 predicted peptides. The data are representative of two separate experiments. Background cytotoxicity was <5% at all E/T ratios and was subtracted from the corresponding value
From the screening results in Table 2, peptides were selected and grouped based upon their level of recognition and whether they were recognized by only one or both donors (Table 3). Peptides recognized by both donors were defined as “consensus peptides” in this study. Group I comprises consensus peptides, Group II, peptides recognized only by LP-2, and Group III, peptides recognized only by JF-1. Group I contained 4 of the 54 peptides (7%), and contained only single or double substitutions. Peptides with triple substitutions or more were not recognized. Only one of the ten monosubstituted peptides was a consensus epitope, which contained an L→A substitution at position 3 (p2, LLALTVLTV). Interestingly, antigenicity that was abrogated by an L→W substitution at position 4 (p6, LLLWTVLTV) was almost completely restored by a compensatory replacement at position 1 (p15, VLLWTVLTV) or position 3 (p29, LLPWTVLTV) (Table 2).
Table 3.
Grouping of peptides based upon recognition by LP−2 and JF−1 M1.2-specific CTL
| Group | Peptide sequences | Number of substitutions | LP−2 | JF-1 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percent lysis at peptide concentrations (ng/ml) | |||||||||||||||||||
| 1,000 | 100 | 10 | 1 | 1,000 | 100 | 10 | 1 | ||||||||||||
| Muc1.2 | L | L | L | L | T | V | L | T | V | 0 | 82 | 77 | 40 | 7 | 70 | 72 | 54 | 3 | |
| I | 2 | – | – | A | – | – | – | – | – | – | 1 | 78 | 38 | 7 | 4 | 73 | 66 | 53 | 0 |
| 11 | V | – | A | – | – | – | – | – | – | 2 | 64 | 28 | 7 | 1 | 65 | 29 | – | −1 | |
| 15 | V | – | – | W | – | – | – | – | – | 2 | 83 | 63 | 28 | 3 | 47 | 24 | 1 | 1 | |
| 29 | – | – | P | W | – | – | – | – | – | 2 | 84 | 78 | 55 | 9 | 64 | 59 | 15 | −1 | |
| II | 1 | V | – | – | – | – | – | – | – | – | 1 | 82 | 42 | 8 | 1 | 40 | 11 | 1 | −1 |
| 13 | V | – | – | F | – | – | – | – | – | 2 | 65 | 24 | 5 | 1 | 24 | 2 | – | −1 | |
| III | 3 | – | – | P | – | – | – | – | – | – | 1 | 30 | 15 | 6 | 5 | 68 | 72 | 62 | 12 |
| 9 | – | – | – | – | – | – | – | S | – | 1 | 32 | 9 | 3 | 3 | 61 | 26 | – | −1 | |
| 10 | – | – | – | – | – | – | – | E | – | 1 | 20 | 12 | 3 | 0 | 63 | 38 | −1 | −2 | |
| 21 | – | – | A | T | – | – | – | – | – | 2 | −1 | 0 | 6 | 2 | 53 | 3 | −1 | −1 | |
| 24 | – | – | A | – | – | N | – | – | – | 2 | 1 | −1 | 1 | 2 | 50 | 1 | 1 | −1 | |
| 28 | – | – | P | T | – | – | – | – | – | 2 | 6 | 5 | 0 | 0 | 59 | 5 | 10 | −1 | |
| 30 | – | – | P | – | – | N | – | – | – | 2 | 2 | 1 | 0 | 0 | 46 | 2 | −1 | −2 | |
| 31 | – | – | P | – | – | – | – | S | – | 2 | 3 | 1 | 1 | 1 | 58 | 9 | 4 | −2 | |
| 32 | – | – | P | – | – | – | E | – | 2 | 7 | 1 | 0 | −1 | 60 | 3 | 3 | −1 | ||
Group I represents consensus analog recognized by both donors. Groups II and III consist of peptides recognized only by donor LP or donor JF, respectively
The most promising peptide analogs appear to be the latter two: p29 and p15, each of which was recognized by both donors even at low peptide concentrations. The T2 stabilization assay was performed to measure binding to the HLA-A2 molecule. This assay affirmed that M1.2, p29, and p15 at 200 μg/ml all bound equally well and, surprisingly, as well as did our standard Flu virus peptide. MFI were: M1.2, 138.3 (1.73 S.D.), p29, 184.6 (3.11 S.D.), p15, 165 (0.70 S.D., and Flu, 184.6 (4.16 S.D.).
Table 4 shows a comparison of the structure of the two analogs with that of the native M1.2 molecule.
Table 4.
Comparison of agonists p29 and p15 with native M1.2
| M1.2 | L-L-L-L-T-V-L-T-V |
|---|---|
| Agonist peptides | |
| p29 | L-L-P-W-T-V-L-T-V |
| p15 | V-L-L-W-T-V-L-T-V |
Boldface indicates the differences from native M1.2 shown above
Avidity of CTL elicited by M1.2 against the peptide analogs
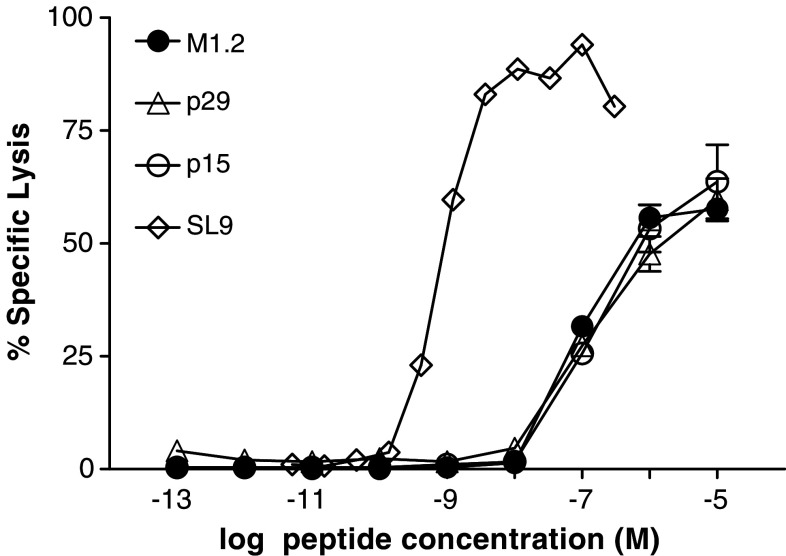
The avidity of antigen-specific CTL is a critical determinant for clearing viral infection and eliminating tumor. Here, the functional avidities of M1.2-specific MD-1 CTL were determined operationally as the negative logarithm of the peptide concentration that resulted in 50% maximal cytotoxicity with T2 cells (EC50), to approximate the capacity and efficiency of a CTL to recognize and lyse target cells in an antigen-specific fashion [30, 31]. The EC50 values of MD-1 for p15 and p29 were essentially identical to that of M1.2, indicating that these three peptides are equally antigenic. A comparison of the functional avidity of MD-1 CTL with that of CTL against SL9 [18] showed, not surprisingly, that the MD-1 CTL (EC50 8.8 × 10−8 M) were 100-fold less avid than CTL to SL9 (EC50 7.8 × 10−10 M) (Fig. 5). This re-affirms the concept that the T cell repertoire for MUC1, a self-antigen, does not contain high avidity clonotypes.
Fig. 5.
Functional avidities of M1.2-specific MD-1 CTL assessed against M1.2 and the agonists, p29 and p15. For comparison, the functional avidity of an HIV-1-specific CTL culture targeting the SL9 epitope is also shown. The EC50 values for M1.2 cells are: M1.2, 8.8 × 10−8 M, p29 and p15, 1.6 × 10−7 M, and for the SL9-CTL, SL9, 7.8 × 10−10 M
Role of the CD8 co-receptor in cytotoxicity effected by CTL against MUC1 epitopes
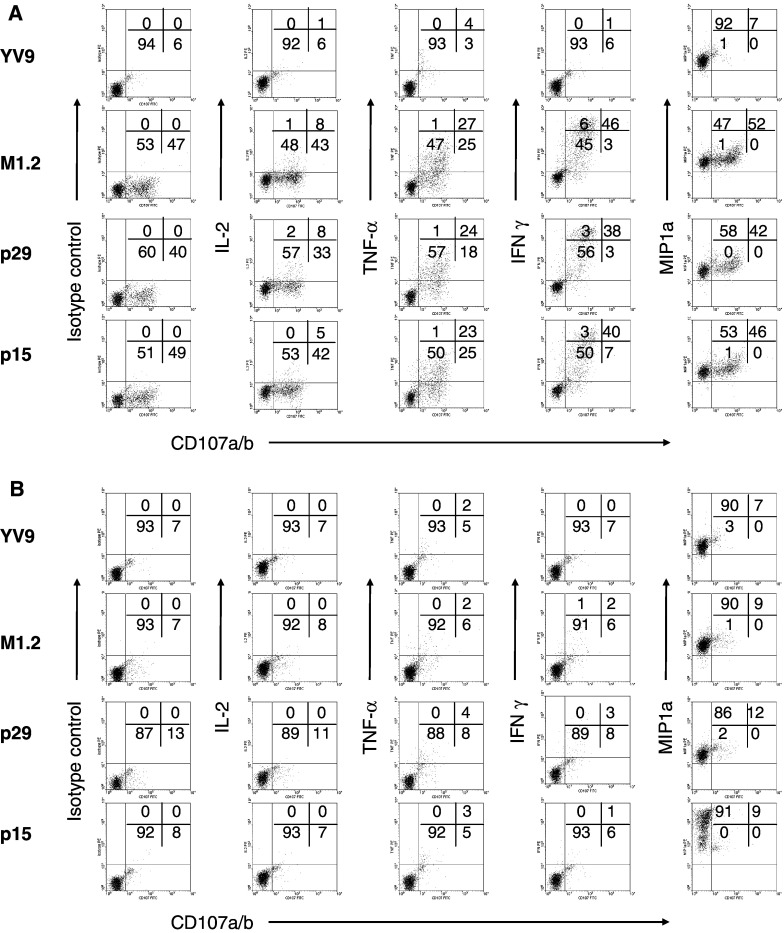
CD8 acts as a co-receptor for the peptide-MHC (pMHC) molecule in concert with the antigen-specific TCR during the process of T cell recognition and activation. Recent publications suggest that CD8 contributes significantly to the maintenance of clonal diversity [32] and responsiveness [33]. To examine whether CD8 is important for effector functions of M1.2-specific CTL, MD-1 cells were stimulated with peptide-pulsed C1R target cells stably transfected with either the wild-type HLA-A2 or one bearing a mutation that abrogates CD8 binding [34]. Figure 6a shows scatter plots correlating CD107a/b mobilization (as a measurement of degranulation) with the production of IL-2, TNF-α, IFN-γ, and MIP-1α in MD-1 cells after stimulation with C1R-A2wt cells pulsed with an irrelevant peptide (YV9), M1.2, p29, or p15. The percentages of the cells that were cytotoxic, as defined by CD107a/b mobilization, were just under 50%, showing a large fraction of peptide-specific cells. Moreover, degranulation was relatively consistent for the M1.2 native peptide or its agonists, p29 and p15 (first column, second to fourth row), showing that M1.2-specific cells recognized the peptides equally. Nearly all MD-1 cells (99%) produced MIP-1α after co-culture with C1R-A2wt cells pulsed with the irrelevant peptide (fourth column, first row). Thus, MIP-1α production is constitutive, that is, not regulated by TCR activation [35]. In contrast, TNF-α, IL-2, or IFN-γ were induced only after specific activation with M1.2, p29, or p15. After stimulation with these peptides, IL-2 was detected in 5–8% of CTL, while significantly higher proportions of MD-1 cells produced TNF-α (23–27%) and IFN-γ (38–46%). Since only three-color flow analysis was performed, we were unable to ascertain the number of cells that produced two or more cytokines upon degranulation. Nonetheless, these data show that MD-1 comprised several populations of functionally distinct M1.2-specific CTL.
Fig. 6.
Analysis of the functionality of individual CD8+ T cells of the MD-1 culture after specific stimulation with C1R-A2wt (a) or C1R-A2CD8null (b), loaded with M1.2, p29, or p15. CD8+ T cells were activated for 4 h and stained with a panel of mAb to examine degranulation (CD107a/b), cytokine production (IL-2, TNF-α, IFN-γ), and chemokine production (MIP-1α). The frequency of CD8+ T cells displaying production of each cytokine or chemokine is shown on the Y-axis. The frequency of CD8+ T cells that have undergone degranulation is shown on the X-axis
Figure 6b shows scatter plots of MD-1 cells after stimulation with the C1R-A2CD8null cells. In the absence of binding by the CD8 co-receptor to pMHC, MD-1 cells were neither cytotoxic nor able to secrete IL-2, TNF-α, and IFN-γ after specific activation (columns 1–4, rows 2–4). Thus, the CD8 enables low avidity M1.2-specific T cells to produce these inducible cytokines, which are tightly regulated by TCR activation [35]. We do not understand why some CD8+ T cells are more dependent on CD8 than others. However, those that are dependent are likely to have lower avidity, which had been our experience with HIV-1-specific CTL (unpublished data). Notably, all of the T cells produced MIP-1α constitutively when co-cultured with C1R-A2CD8null cells loaded with the various peptides, further confirming that MIP-1α secretion is independent of TCR activation (Fig. 6a).
Table 5 summarizes the flow cytometric data for MD-1 in Fig. 6a, b as well as those for two other CTL cultures, JF-2 and GR-1. All three CTL cultures exhibited modest non-TCR-mediated (spontaneous) degranulation (6–10% CD107a/b+ cells) when co-cultured with either C1R target cells loaded with the irrelevant peptide YV9. Significantly higher numbers of CTL stained positively for CD107a/b after specific stimulation with M1.2-pulsed C1R-A2wt (47, 31, and 43% for MD-1, JF-2, and GR-1, respectively), showing that all cultures contained substantial proportions of antigen-specific cells. Incidentally, the percent of tetramer-binding cells at the time of this assay was 4, 1, and 6% for MD-1, JF-2, and GR-1, respectively (data not shown). Two (GR-1 and MD-1) of the three CTL recognized the agonist p29 and p15 almost as efficiently as M1.2. In contrast, JF-2 contained fewer CD107a/b+ cells after stimulation with p29 and p15 (37 and 18%, respectively) than with the native M1.2 (43%), showing that JF-2 CTL were less cross-reactive to the agonists. Antigen-specific degranulation for all three CTL was dependent upon compensation by CD8 co-stimulation, but to varying degrees, as evidenced by reductions in the percentages of CD107a/b+ cells after stimulation with cognate peptide-loaded C1R-A2CD8null target cells, as compared with C1R-A2wt. By this criterion, JF-2 and GR-1 would be considered more avid than MD-1, which showed 7, 13, and 8% CD107a/b+ cells after stimulation with C1R-A2CD8null target cells pulsed with M1.2, p29, and p15, respectively, essentially identical to spontaneous degranulation with YV9 (8%).
Table 5.
Production of cytokines after stimulation with an irrelevant peptide (YV9), M1.2, p29, or p15 loaded onto C1R-A2wt or C1R-A2CD8null cells in three M1.2-specific CTL cultures
| CTL | Peptide for re-stimulation | Target cell | % T cells expressing | ||||
|---|---|---|---|---|---|---|---|
| CD107a/b | CD107a/b IL−2 | CD107a/b TNF-α | CD107a/b IFN-γ | CD107a/b MIP-1α | |||
| MD-1 | YV9 (irrelevant peptide) | C1R-A2wt | 6 | 1 | 4 | 1 | 7 |
| M1.2 | 47 | 8 | 27 | 46 | 52 | ||
| P29 | 40 | 8 | 24 | 38 | 42 | ||
| P15 | 49 | 5 | 23 | 40 | 46 | ||
| YV9 | C1R-A2CD8null | 8 | 0 | 2 | 0 | 7 | |
| M1.2 | 7 | 0 | 2 | 2 | 9 | ||
| P29 | 13 | 0 | 4 | 3 | 12 | ||
| p15 | 8 | 0 | 3 | 1 | 9 | ||
| GR-1 | YV9 | C1R-A2wt | 13 | 3 | 4 | 2 | 13 |
| M1.2 | 31 | 18 | 22 | 24 | 33 | ||
| p29 | 30 | 12 | 22 | 24 | 34 | ||
| p15 | 31 | 14 | 22 | 26 | 32 | ||
| YV9 | C1R-A2CD8null | 11 | 3 | 4 | 3 | 15 | |
| M1.2 | 17 | 4 | 8 | 7 | N.D. | ||
| p29 | 13 | 2 | 5 | 2 | N.D. | ||
| p15 | 20 | 2 | 9 | 7 | N.D. | ||
| JF-2 | YV9 | C1R-A2wt | 10 | 0 | 2 | 0 | 13 |
| M1.2 | 43 | 3 | 3 | 33 | 41 | ||
| p29 | 37 | 0 | 3 | 24 | 34 | ||
| p15 | 18 | 1 | 2 | 5 | 19 | ||
| YV9 | C1R-A2CD8null | 13 | 0 | 1 | 0 | 15 | |
| M1.2 | 25 | 0 | 0 | 10 | 22 | ||
| p29 | 13 | 0 | 1 | 0 | 13 | ||
| p15 | 12 | 0 | 1 | 1 | 11 | ||
In addition, Table 5 compares the ability of the three CTL cultures to produce the inducible cytokines IL-2, TNF-α, and IFN-γ upon specific activation. CD107a/b+ MD-1 and GR-1 cells also stained positively for all three cytokines, although the percentage of cells making each cytokine was different. JF-2 cells produced only IFN-γ after specific stimulation. IL-2 was produced by the smallest proportion of cytotoxic cells in all three CTL cultures. It is difficult to conclude whether this reflects little synthesis, a shorter half-life, or poor sensitivity of the intracellular staining technique for this cytokine. The pattern of constitutive secretion of MIP-1α was essentially identical for all three CTL and has been discussed in the context of MD-1. Since polyfunctional human CTL may be more protective [36], the T cell repertoires of donors GR and MD may be more capable of responding to M1.2 than that of JF.
To show more clearly how degranulation was affected by the CD8 co-receptor, the data in Table 5 were re-analyzed to show only the percentages of cells that had been specifically activated to express CD107a/b (Table 6). In other words, background degranulation with YV9 was subtracted from these values. Table 6 shows all three M1.2-specific cultures to depend to a large extent on CD8 for degranulation. However, only MD-1 was completely dependent, suggesting that these cultures had different TCR affinities for M1.2. The agonist peptides, however, were more poorly recognized and thus, activation was more dependent on CD8 than with the native epitope.
Table 6.
Absolute percentage increase in CD107a/b+ CD8+ T cells over non-specific activation: M1.2-specific CTL stimulated by M1.2, p29, or p15 loaded onto C1R-A2wt or C1R-A2CD8null cells
| CTL | Target cell | % CD107a/b+ T cells above non-specific (YV9)-stimulated controls | ||
|---|---|---|---|---|
| M1.2 | p29 | p15 | ||
| MD-1 | C1R-A2wt | 41 | 34 | 43 |
| C1R-A2CD8null | −1 | 5 | 0 | |
| JF-2 | C1R-A2wt | 33 | 27 | 8 |
| C1R-A2CD8null | 12 | 0 | −1 | |
| GR-1 | C1R-A2wt | 18 | 17 | 18 |
| C1R-A2CD8null | 6 | 2 | 9 | |
Discussion
In murine TCR transgenic models, it has been shown that clonal expansion and acquisition of differentiation functions are imprinted upon naïve high affinity CD8+ T cells after a single optimized encounter with APC [28, 37, 38]. Important variables in this interaction include the level of co-stimulation afforded by DC, the cytokine milieu, and the intrinsic immunogenicity of the epitope [39, 40]. Less is known about the priming of human T cells. Thus, ex vivo priming affords an opportunity to compare signal strengths for priming and activation of human CTL to different epitopes [18, 41]. In this study, we studied in greater detail CTL directed to a self-antigen, the M1.2 epitope, that is capable of eliciting immunological responses from patients with advanced malignancies [13] as well as from healthy donors [12]. In particular, the M1.2-specific CTL cultures that we have expanded from several donors contained percentages of tetramer-binding cells (as much as 41%) that were significantly higher than that previously reported for this specificity [22]. This suggests that the CD8+ repertoires of healthy people contain fairly high frequencies of M1.2-reactive cells, and from this perspective, M1.2 can be considered moderately immunogenic. However, in contrast to our experience with HIV-1 epitopes, there was great donor-to-donor variability in terms of the requirement by M1.2 CTL precursors for co-stimulation afforded by DC used as APC in these cultures. This may explain why these CTL cultures are generally difficult to maintain, which may have implications in terms of using M1.2 as a target of active specific immunotherapy.
The strength of the signals delivered to T cells modulate their capacity to develop into fully functional effector and memory cells [42]. M1.2-specific cells proliferated less robustly and were more short-lived than those specific for HIV-1 under identical conditions of co-stimulation. M1.2-specific CTL also lost their ability to kill after several weeks of continuous culture, which was not observed for HIV-1-specific CTL [18, 19]. The loss of this differentiated function can be deferred by amplifying TCR signaling through compensatory co-stimulation with matured DC. Alternatively, the cytolytic function of tetramer-binding cells was also extended by stimulating tetramer-sorted cells through their TCR with an anti-CD3 mAb and expanding them in the presence of IL-2 and irradiated feeder cells. In general, poor in vitro proliferation and loss of differentiated functions of M1.2-specific CTL, even for cultures with high percentages of tetramer-stained cells, suggest that the TCR signal delivered by the pMHC molecule may be suboptimal. This is consistent with the premise that humans are tolerized to this self-antigen. Nonetheless, as many as 109 peptide-specific T cells were expanded from precursors isolated from 100 ml of heparinized blood from most of our donors. These CTL were also amenable to cryopreservation under standard conditions and could be further expanded with re-stimulation after thawing, albeit with a fairly precipitous loss of specificity.
M1.2-specific CTL were highly cytotoxic to peptide-pulsed T2 cells and were also able to lyse the HLA-A2+, MUC1+ MCF7 breast carcinoma cells, consistent with a previous report [12]. Flow analysis revealed significant discordance at the single cell level between the proportions of specific T cells determined by tetramer staining (less than 6%) versus functional assessment (∼50% CD107a/b+), which other investigators have also observed [43, 44]. A possible explanation for this finding was suggested by the measured functional avidities, which showed M1.2-specific CTL to be 100-fold less avid than SL9-specific CTL. Interestingly, the functional avidities of our ex vivo primed SL9-specific CTL were consistent with those of CTL isolated from patients with HIV-1 infection [18].
Comparative analysis of the binding of 16 different soluble recombinant TCRs to their corresponding ligands by surface plasmon resonance has revealed that anti-pathogen TCRs bind with a significantly stronger affinity than those raised against tumor epitopes (Cole, A.K. Sewell et al., unpublished data, 2006). Anti-tumor CTL clones were also found to exhibit greater dependence on the pMHCI/CD8 interaction than anti-viral CTL clones. This finding is consistent with the notion that the off-rate, and hence half-life, of TCR/pMHC interactions is the critical parameter for TCR triggering and with the observation that CD8 acts to extend this half-life [53].
Because we do not know which ex vivo CTL function defines a clinically relevant T cell response, the ability of M1.2 CTL to secrete many different factors upon degranulation after antigen recognition is encouraging. Cytokine secretion may be equally important as target lysis in terms of clinical efficacy help to amplify or prolong the cytotoxic response. Polyfunctional T cells may be the most protective, extrapolating from studies of T cell immune responses to viral infections [36].
The combinatorial peptide library screening with an M1.2-specific CTL culture predicted acceptable substitutions at positions 1, 3, 4, 6, and 8 that were then verified by M1.2-specific CTL from other donors. This approach does not make any assumptions regarding TCR or MHC contact positions and has identified even highly substituted peptide analogs with little homology to the native peptide [45, 46]. Recognition of the peptide analogs by M1.2-specific CTL appeared to be limited to the least substituted peptides (1–2 positions), since peptides with three substitutions were not recognized. It appears that the TCR of M1.2-specific T cells may be more discerning that those we have previously studied with this approach [19, 47, 48]. This may also imply that the human T cell repertoire to M1.2 peptide has very limited diversity [49], consistent with the likelihood that high affinity clonotypes to the MUC1 self-antigen may have been deleted during thymic development, leaving only those recognizing low affinity complexes [50]. Thus the formidable challenge in developing cancer vaccines is to create a qualitatively and quantitatively superior CTL response to weak protein antigens, while avoiding autoimmunity.
The importance of the CD8 molecule in CTL function has been known for some time. The binding of CD8 to the alpha 3 chain of the MHC1 molecule per se augments TCR binding to the same molecule, and the effect of CD8 on CTL function does not require signal transduction [51]. Blocking CD8 with antibody during the interaction of CD8+ T cells with target cells can inhibit lysis [52, 53]. The heterogeneous peptide-specific memory CTL repertoire in vivo can accommodate a range of TCR affinities and T cell avidities [52, 53], through variable compensation by the CD8 co-receptor [27, 32, 34, 52–55]. Here we showed that the three M1.2-specific CTL cultures required participation by CD8 for activation of degranulation and inducible cytokine production, albeit to different degrees (Table 6). Both CD8-dependent and -independent T cell clonotypes were identified in JF-2 and GR-1 cultures. Thus, clones primed ex vivo with lower “intrinsic avidity” may compete with those of higher avidity through the elicitation of CD8 compensation [32]. These results showed indirectly that the affinity of M1.2-specific CTL for the p15-MHC and p29-MHC molecules may be less than that for the cognate pMHC complex, since specific activation by the agonists was more sensitive to the loss of CD8 participation.
We have attempted to identify agonists of MUC1 epitope M1.2 that are recognized better than the native epitope. However, the pattern of recognition of p15 and p29 and the avidity (EC50 measured by cytotoxicity) were very similar to the native epitope, which suggest a similar TCR affinity for the agonist peptides that we have focused on. Our study shows that the human CD8+ repertoire may contain significant numbers of potentially polyfunctional M1.2-specific T cells. Given the limited avidity, it may be worthwhile to continue seeking “superagonists” for the M1.2 epitope using this or other approaches. In addition, our results suggest that enhancing co-stimulation perhaps through the use of matured DC may be helpful in generating robust CTL responses to this epitope. Our study attests to the difficulty of generating potent CTL responses to non-viral tumor antigens.
Acknowledgments
Supported by grants to M.S.M. from the Susan G. Komen Breast Cancer Foundation, Expedition Inspiration, and the Department of Defense; to D.B.W. from the Alzheimer’s and Aging Research Center, the Osteoporosis and Breast Cancer Research Center, Mixture Sciences Inc., and the National Institutes of Health (CA78040); and to J.K.M. from the Michigan Life Sciences Corridor Program 1659 and National Institutes of Health (R21-AI44372, R01-AI064069).
Abbreviation
- M1.2
MUC1 epitope LLLLTVLTV
References
- 1.Taylor-Papadimitriou J, Burchell JM, Plunkett T, Graham R, Correa I, Miles D, Smith M. MUC1 and the immunobiology of cancer. J Mammary Gland Biol Neoplasia. 2002;7:209–221. doi: 10.1023/A:1020360121451. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 3.Gendler S, Taylor-Papadimitriou J, Duhig T, Rothbard J, Burchell J. A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J Biol Chem. 1988;263:12820–12823. [PubMed] [Google Scholar]
- 4.Brossart P, Schneider A, Dill P, Schammann T, Grunebach F, Wirths S, Kanz L, Buhring HJ, Brugger W. The epithelial tumor antigen MUC1 is expressed in hematological malignancies and is recognized by MUC1-specific cytotoxic T-lymphocytes. Cancer Res. 2001;61:6846–6850. [PubMed] [Google Scholar]
- 5.Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J, Pemberton L, Lalani EN, Wilson D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990;265:15286–15293. [PubMed] [Google Scholar]
- 6.Ligtenberg MJ, Vos HL, Gennissen AM, Hilkens J. Episialin, a carcinoma-associated mucin, is generated by a polymorphic gene encoding splice variants with alternative amino termini. J Biol Chem. 1990;265:5573–5578. [PubMed] [Google Scholar]
- 7.Karanikas V, Hwang LA, Pearson J, Ong CS, Apostolopoulos V, Vaughan H, Xing PX, Jamieson G, Pietersz G, Tait B, Broadbent R, Thynne G, McKenzie IF. Antibody and T cell responses of patients with adenocarcinoma immunized with mannan-MUC1 fusion protein. J Clin Invest. 1997;100:2783–2792. doi: 10.1172/JCI119825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musselli C, Ragupathi G, Gilewski T, Panageas KS, Spinat Y, Livingston PO. Reevaluation of the cellular immune response in breast cancer patients vaccinated with MUC1. Int J Cancer. 2002;97:660–667. doi: 10.1002/ijc.10081. [DOI] [PubMed] [Google Scholar]
- 9.Schmielau J, Nalesnik MA, Finn OJ. Suppressed T-cell receptor zeta chain expression and cytokine production in pancreatic cancer patients. Clin Cancer Res. 2001;7:933s–939s. [PubMed] [Google Scholar]
- 10.Musselli C, Livingston PO, Ragupathi G. Keyhole limpet hemocyanin conjugate vaccines against cancer: the memorial sloan kettering experience. J Cancer Res Clin Oncol. 2001;127(Suppl 2):R20–R26. doi: 10.1007/BF01470995. [DOI] [PubMed] [Google Scholar]
- 11.Apostolopoulos V, Karanikas V, Haurum JS, McKenzie IF. Induction of HLA-A2-restricted CTLs to the mucin 1 human breast cancer antigen. J Immunol. 1997;159:5211–5218. [PubMed] [Google Scholar]
- 12.Brossart P, Heinrich KS, Stuhler G, Behnke L, Reichardt VL, Stevanovic S, Muhm A, Rammensee HG, Kanz L, Brugger W. Identification of HLA-A2-restricted T-cell epitopes derived from the MUC1 tumor antigen for broadly applicable vaccine therapies. Blood. 1999;93:4309–4317. [PubMed] [Google Scholar]
- 13.Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96:3102–3108. [PubMed] [Google Scholar]
- 14.Henderson RA, Michel H, Sakaguchi K, Shabanowitz J, Appella E, Hunt DF, Engelhard VH. HLA-A2.1-associated peptides from a mutant cell line: a second pathway of antigen presentation. Science. 1992;255:1264–1266. doi: 10.1126/science.1546329. [DOI] [PubMed] [Google Scholar]
- 15.Feuerer M, Beckhove P, Bai L, Solomayer EF, Bastert G, Diel IJ, Pedain C, Oberniedermayr M, Schirrmacher V, Umansky V. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat Med. 2001;7:452–458. doi: 10.1038/86523. [DOI] [PubMed] [Google Scholar]
- 16.Correa I, Plunkett T, Coleman J, Galani E, Windmill E, Burchell JM, Taylor-Papdimitriou J. Responses of human T cells to peptides flanking the tandem repeat and overlapping the signal sequence of MUC1. Int J Cancer. 2005;115:760–768. doi: 10.1002/ijc.20949. [DOI] [PubMed] [Google Scholar]
- 17.Riddell SR. Finding a place for tumor-specific T cells in targeted cancer therapy. J Exp Med. 2004;200:1533–1537. doi: 10.1084/jem.20042004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kan-Mitchell J, Bisikirska B, Wong-Staal F, Schaubert KL, Bajcz M, Bereta M. The HIV-1 HLA-A2-SLYNTVATL is a help-independent CTL epitope. J Immunol. 2004;172:5249–5261. doi: 10.4049/jimmunol.172.9.5249. [DOI] [PubMed] [Google Scholar]
- 19.Kan-Mitchell J, Bajcz M, Schaubert KL, Price DA, Brenchley JM, Asher TE, Douek DC, Ng HL, Yang OO, Rinaldo CR, Benito JM, Bisikirska B, Hegde R, Marincola FM, Boggiano C, Wilson D, Abrams J, Blondelle SE, Wilson DB. Degeneracy and repertoire of the human HIV-1 Gag p17(77−85) CTL response. J Immunol. 2006;176:6690–6701. doi: 10.4049/jimmunol.176.11.6690. [DOI] [PubMed] [Google Scholar]
- 20.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pittet MJ, Zippelius A, Valmori D, Speiser DE, Cerottini JC, Romero P. Melan-A/MART-1-specific CD8 T cells: from thymus to tumor. Trends Immunol. 2002;23:325–328. doi: 10.1016/S1471-4906(02)02244-5. [DOI] [PubMed] [Google Scholar]
- 22.Bohnenkamp HR, Coleman J, Burchell JM, Taylor-Papadimitriou J, Noll T. Breast carcinoma cell lysate-pulsed dendritic cells cross-prime MUC1-specific CD8+ T cells identified by peptide-MHC-class-I tetramers. Cell Immunol. 2004;231:112–125. doi: 10.1016/j.cellimm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Pinilla C, Rubio-Godoy V, Dutoit V, Guillaume P, Simon R, Zhao Y, Houghten RA, Cerottini JC, Romero P, Valmori D. Combinatorial peptide libraries as an alternative approach to the identification of ligands for tumor-reactive cytolytic T lymphocytes. Cancer Res. 2001;61:5153–5160. [PubMed] [Google Scholar]
- 24.Parham P, Brodsky FM. Partial purification and some properties of BB7.2. A cytotoxic monoclonal antibody with specificity for HLA-A2 and a variant of HLA-A28. Hum Immunol. 1981;3:277–299. doi: 10.1016/0198-8859(81)90065-3. [DOI] [PubMed] [Google Scholar]
- 25.Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 26.Houghten RA. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen–antibody interaction at the level of individual amino acids. Proc Natl Acad Sci USA. 1985;82:5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purbhoo MA, Boulter JM, Price DA, Vuidepot AL, Hourigan CS, Dunbar PR, Olson K, Dawson SJ, Phillips RE, Jakobsen BK, Bell JI, Sewell AK. The human CD8 coreceptor effects cytotoxic T cell activation and antigen sensitivity primarily by mediating complete phosphorylation of the T cell receptor zeta chain. J Biol Chem. 2001;276:32786–32792. doi: 10.1074/jbc.M102498200. [DOI] [PubMed] [Google Scholar]
- 28.Kersh EN, Kaech SM, Onami TM, Moran M, Wherry EJ, Miceli MC, Ahmed R. TCR signal transduction in antigen-specific memory CD8 T cells. J Immunol. 2003;170:5455–5463. doi: 10.4049/jimmunol.170.11.5455. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan S, Farber DL, Tsokos GC. T cell rewiring in differentiation and disease. J Immunol. 2003;171:3325–3331. doi: 10.4049/jimmunol.171.7.3325. [DOI] [PubMed] [Google Scholar]
- 30.Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, Lee PP. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–1382. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 31.Snyder JT, Alexander-Miller MA, Berzofskyl JA, Belyakov IM. Molecular mechanisms and biological significance of CTL avidity. Curr HIV Res. 2003;1:287–294. doi: 10.2174/1570162033485230. [DOI] [PubMed] [Google Scholar]
- 32.Price DA, Brenchley JM, Ruff LE, Betts MR, Hill BJ, Roederer M, Koup RA, Migueles SA, Gostick E, Wooldridge L, Sewell AK, Connors M, Douek DC. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J Exp Med. 2005;202:1349–1361. doi: 10.1084/jem.20051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maile R, Siler CA, Kerry SE, Midkiff KE, Collins EJ, Frelinger JA. Peripheral “CD8 tuning” dynamically modulates the size and responsiveness of an antigen-specific T cell pool in vivo. J Immunol. 2005;174:619–627. doi: 10.4049/jimmunol.174.2.619. [DOI] [PubMed] [Google Scholar]
- 34.Hutchinson SL, Wooldridge L, Tafuro S, Laugel B, Glick M, Boulter JM, Jakobsen BK, Price DA, Sewell AK. The CD8 T cell coreceptor exhibits disproportionate biological activity at extremely low binding affinities. J Biol Chem. 2003;278:24285–24293. doi: 10.1074/jbc.M300633200. [DOI] [PubMed] [Google Scholar]
- 35.Slifka MK, Whitton JL. Antigen-specific regulation of T cell-mediated cytokine production. Immunity. 2000;12:451–457. doi: 10.1016/S1074-7613(00)80197-1. [DOI] [PubMed] [Google Scholar]
- 36.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T-cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 38.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 40.Langenkamp A, Casorati G, Garavaglia C, Dellabona P, Lanzavecchia A, Sallusto F. T cell priming by dendritic cells: thresholds for proliferation, differentiation and death and intraclonal functional diversification. Eur J Immunol. 2002;32:2046–2054. doi: 10.1002/1521-4141(200207)32:7<2046::AID-IMMU2046>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 41.Ho WY, Nguyen HN, Wolfl M, Kuball J, Greenberg PD. In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naive repertoire. J Immunol Methods. 2006;310:40–52. doi: 10.1016/j.jim.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 42.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 43.Whiteside TL, Zhao Y, Tsukishiro T, Elder EM, Gooding W, Baar J. Enzyme-linked immunospot, cytokine flow cytometry, and tetramers in the detection of T-cell responses to a dendritic cell-based multipeptide vaccine in patients with melanoma. Clin Cancer Res. 2003;9:641–649. [PubMed] [Google Scholar]
- 44.Comin-Anduix B, Gualberto A, Glaspy JA, Seja E, Ontiveros M, Reardon DL, Renteria R, Englahner B, Economou JS, Gomez-Navarro J, Ribas A. Definition of an immunologic response using the major histocompatibility complex tetramer and enzyme-linked immunospot assays. Clin Cancer Res. 2006;12:107–116. doi: 10.1158/1078-0432.CCR-05-0136. [DOI] [PubMed] [Google Scholar]
- 45.Wilson DB, Pinilla C, Wilson DH, Schroder K, Boggiano C, Judkowski V, Kaye J, Hemmer B, Martin R, Houghten RA. Immunogenicity. I. Use of peptide libraries to identify epitopes that activate clonotypic CD4+ T cells and induce T cell responses to native peptide ligands. J Immunol. 1999;163:6424–6434. [PubMed] [Google Scholar]
- 46.Hemmer B, Vergelli M, Gran B, Ling N, Conlon P, Pinilla C, Houghten R, McFarland HF, Martin R. Predictable TCR antigen recognition based on peptide scans leads to the identification of agonist ligands with no sequence homology. J Immunol. 1998;160:3631–3636. [PubMed] [Google Scholar]
- 47.Nino-Vasquez JJ, Allicotti G, Borras E, Wilson DB, Valmori D, Simon R, Martin R, Pinilla C. A powerful combination: the use of positional scanning libraries and biometrical analysis to identify cross-reactive T cell epitopes. Mol Immunol. 2004;40:1063–1074. doi: 10.1016/j.molimm.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Hernandez J, Schoeder K, Blondelle SE, Pons FG, Lone YC, Simora A, Langlade-Demoyen P, Wilson DB, Zanetti M. Antigenicity and immunogenicity of peptide analogues of a low affinity peptide of the human telomerase reverse transcriptase tumor antigen. Eur J Immunol. 2004;34:2331–2341. doi: 10.1002/eji.200425134. [DOI] [PubMed] [Google Scholar]
- 49.Jones EY. Favorite flavors of surfaces. Nat Immunol. 2005;6:365–366. doi: 10.1038/ni0405-365. [DOI] [PubMed] [Google Scholar]
- 50.Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NR. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 51.Tanabe M, Karaki S, Takiguchi M, Nakauchi H. Antigen recognition by the T cell receptor is enhanced by CD8 alpha-chain binding to the alpha 3 domain of MHC class I molecules, not by signaling via the cytoplasmic domain of CD8 alpha. Int Immunol. 1992;4:147–152. doi: 10.1093/intimm/4.2.147. [DOI] [PubMed] [Google Scholar]
- 52.Couedel C, Bodinier M, Peyrat MA, Bonneville M, Davodeau F, Lang F. Selection and long-term persistence of reactive CTL clones during an EBV chronic response are determined by avidity, CD8 variable contribution compensating for differences in TCR affinities. J Immunol. 1999;162:6351–6358. [PubMed] [Google Scholar]
- 53.Levitsky V, de Campos-Lima PO, Frisan T, Masucci MG. The clonal composition of a peptide-specific oligoclonal CTL repertoire selected in response to persistent EBV infection is stable over time. J Immunol. 1998;161:594–601. [PubMed] [Google Scholar]
- 54.Wooldridge L, Hutchinson SL, Choi EM, Lissina A, Jones E, Mirza F, Dunbar PR, Price DA, Cerundolo V, Sewell AK. Anti-CD8 antibodies can inhibit or enhance peptide-MHC class I (pMHCI) multimer binding: this is paralleled by their effects on CTL activation and occurs in the absence of an interaction between pMHCI and CD8 on the cell surface. J Immunol. 2003;171:6650–6660. doi: 10.4049/jimmunol.171.12.6650. [DOI] [PubMed] [Google Scholar]
- 55.Wooldridge L, van den Berg HA, Glick M, Gostick E, Laugel B, Hutchinson SL, Milicic A, Brenchley JM, Douek DC, Price DA, Sewell AK. Interaction between the CD8 coreceptor and major histocompatibility complex class I stabilizes T cell receptor–antigen complexes at the cell surface. J Biol Chem. 2005;280:27491–27501. doi: 10.1074/jbc.M500555200. [DOI] [PMC free article] [PubMed] [Google Scholar]