Abstract
Many tumors down-regulate major histocompatibility complex (MHC) antigen expression to evade host immune surveillance. However, there are very few in vivo models to study MHC antigen expression during tumor spontaneous regression. In addition, the roles of transforming growth factor beta1 (TGF-β1), interferon gamma (IFN-γ), and interleukin (IL)-6 in modulating MHC antigen expression are ill understood. We previously reported that tumor infiltrating lymphocyte (TIL)-derived IL-6 inhibits TGF-β1 and restores natural killing (NK) activity. Using an in vivo canine-transmissible venereal tumor (CTVT) tumor model, we presently assessed IL-6 and TGF-β involvement associated with the MHC antigen expression that is commonly suppressed in cancers. IL-6, IFN-γ, and TGF-β1, closely interacted with each other and modulated MHC antigen expression. In the presence of tumor-derived TGF-β1, host IFN-γ from TIL was not active and, therefore, there was low expression of MHC antigen during tumor progression. TGF-β1-neutralizing antibody restored IFN-γ-activated MHC antigen expression on tumor cells. The addition of exogenous IL-6 that has potent anti-TGF-β1 activity restored IFN-γ activity and promoted MHC antigen expression. IFN-γ and IL-6 in combination acted synergistically to enhance the expression of MHC antigen. Thus, the three cytokines, IL-6, TGF-β1, and IFN-γ, closely interacted to modulate the MHC antigen expression. Furthermore, transcription factors, including STAT-1, STAT-3, IRF-1, NF-κB, and CREB, were significantly elevated after IL-6 and IFN-γ treatment. We conclude that the host IL-6 derived from TIL works in combination with host IFN-γ to enhance MHC molecule expression formerly inhibited by TGF-β1, driving the tumor toward regression. It is suggested that the treatment of cancer cells that constitutively secrete TGF-β1 should incorporate anti-TGF-β activity. The findings in this in vivo tumor regression model have potential applications in cancer immunotherapy.
Keywords: MHC, IFN-γ, IL-6, TGF-β1, Transcription factors
Introduction
Major histocompatibility complex (MHC) antigen expression is pivotal to the initiation of immune responses. MHC-dependent activities are important for dendritic cells to present self or foreign antigens including those of cancer cells, and for antigen-specific cytotoxicity of T cells. Tumor cells often suppress the expression of MHC antigen through a variety of mechanisms to evade immune surveillance [1], facilitating their progressive growth. Antagonism of MHC expression activates anti-tumor immune responses in vitro [2-4]. However, in vivo models are presently lacking, so it is unclear how host interaction with tumors regulates MHC antigen expression.
Canine-transmissible venereal tumor (CTVT) is a unique tumor caused by transfer of the cancer cell itself [5]. One of the characteristic features of the tumor is spontaneous regression following progressive growth with significantly elevated MHC antigen expression [6]. CTVT is the sole model to study the mechanisms of host-cancer cell interactions during tumor growth and spontaneous regression. That CTVT has an aneuploid karyotype and a long interspersed nuclear element insertion near c-myc is globally evident in tumors [7,8]. During CTVT progression, MHC class I and class II antigens are scarcely expressed [6], while transforming growth factor-beta1 (TGF-β1) produced by the tumor cells is elevated [9]. After progressive growth for 3–4 months, the tumor usually spontaneously regresses, with MHC antigen being expressed in up to 40% of the regressing cells [6].
Cytokines produced by tumor cells and tumor-infiltrating lymphocyte (TIL) play significant roles in regulating tumor cell growth and in the cytotoxic activity of TIL. Interleukin (IL)-6 produced by TIL antagonizes TGF-β and restores natural-killer (NK) activity [9]. TGF-β1 has been detected in tissue specimens from a variety of tumor types and is a pleiotropic growth factor with wide-ranging effects on proliferation, differentiation, migration, apoptosis, and extra-cellular matrix remodeling [10]. In addition, TGF-β1 exerts strong immunosuppressive effects by inhibiting the proliferation of B and T cells, allowing tumors to escape immune surveillance [11,12]. This cytokine directly targets CD8+ cytotoxic T lymphocytes (CTL) by inhibiting the expression of perforin, granzymes, and other gene products responsible for CTL-mediated tumor cytotoxicity during tumor immune evasion [12]. Finally, TGF-β1 has an inhibitory effect on interferon gamma (IFN-γ)-induced MHC class I and class II antigen expression [13,14] by attenuating IFN-γ-induced expression of MHC genes. IFN-γ is one of the major cytokines responsible for up-regulating MHC class I antigen expression and also for inducing MHC class II antigens on a variety of leukocytes and epithelial cells [15].
IL-6 is another pleiotropic cytokine produced by a variety of cells that act on a wide range of tissues. The functions of IL-6 depend on sources and/or types of tumors. IL-6 can enhance tumor growth or assist host immunity against tumor cells, and promote cervical cancer growth by vascular endothelial growth factor-mediated angiogenesis [16]. We have previously demonstrated that IL-6 produced by TIL in CTVT increases the host immune responses [9]. During CTVT regression, high concentrations of IL-6 secreted by TIL block TGF-β1 inhibition of LAK natural-killing activity [9]. In addition, IL-6 significantly antagonizes the immunosuppressive effects of TGF-β1 on T cell proliferation in the eyes with endotoxin-induced uveitis [17]. IL-6 also induces T-cell-mediated anti-tumor effects in animal models and directly activates human NK cells [18, 19]. Combined IL-2 and IL-6 gene therapy involving liposome-mediated intra-tumoral transfer of the genes to mice bearing B16F10 melanoma significantly enhances CTL and NK activities of splenocytes and TIL [20].
Using the CTVT model, we presently studied the host/cancer interactions in vivo, focusing on cytokine-mediated modulation of MHC antigen expression. We demonstrate that the anti-TGF-β1 effect of IL-6 restores the ability of IFN-γ to promote MHC class I and II antigen expressions. IFN-γ alone does little in promoting MHC expression during tumor progression. However, treatment with both IL-6 and IFN-γ (IL-6/IFN-γ) markedly increases the expression of MHC antigens on tumor cells. The transcription factors (TF) associated with these activities were also studied.
Materials and methods
In vivo tumor growth
Six beagles (three males, three females) were used for the transplantation of tumors at eight sites subcutaneously in the back. Tumor introduction and excision have been described previously [6]. The size of each tumor was measured weekly with calipers, and tumor volume (cm3) was calculated as π × length × width × thickness/4. Animals were maintained in the Veterinary Central Animal Facility of National Taiwan University, Taipei, Taiwan, in accordance with the guidelines of the Institutional Animal Care and Use Committee.
Purification of CTVT cells and TIL
One tumor mass was surgically removed from each experimental dog every 2–3 weeks post-implantation. Those collected after 6–8 weeks were used as the progression phase samples, and those collected 1–2 weeks after tumors decreased in size represented regression phase samples. Tumor cells and TIL were isolated as described previously [6]. Briefly, aseptic tumor tissue was minced in Hank’s buffered salt solution (HBSS: Invitrogen, Carlsbad, CA). Samples were mechanically crushed using a stainless steel mesh, and the resulting single cells were overlaid on a gradient of 42% Percoll (Amersham Pharmacia Biotech, Piscataway, NJ) and centrifuged (820g, 4°C, 25 min). CTVT cells and TIL located in the liquid–air interface and the tube bottom, respectively, were harvested and washed three times with Dulbecco’s Modified Eagle medium (DMEM; Invitrogen) supplemented with 10% fetal calf serum. The purities were determined by staining with Hemacolor (Merck, Whitehouse Station, NJ).
Antibodies and reagents
TGF-β and β-actin antibodies were purchased from Sigma–Aldrich (St. Louis, MO). Mouse anti-canine MHC class I H58A and MHC class II H42A monoclonal antibody (mAb) and appropriate isotype controls were purchased from VMRD (Pullman, WA). Goat anti-canine IFN-γ, anti-canine IL-6 mAb, recombinant canine IFN-γ (rcIFN-γ), and recombinant canine IL-6 (rcIL-6) were purchased from R&D systems (Minneapolis, MN). The phosphor-STAT1 (Try701) and phosphor-STAT3 (Try705) rabbit mAbs were from Cell Signaling Technology (Beverly, MA). STAT1 and STAT3 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell lysis and Western immunoblotting
Progression or regression phase CTVT cells and TILs were lysed in lysis buffer containing protease inhibitors. Equal amounts of protein were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidenedifluoride membrane. Each membrane was blocked, probed with primary antibody, and then with the appropriate horseradish peroxidase-conjugated secondary antibody. The results were visualized using the Supersignal West™ Pico detection system (Pierce Biotechnology, Rockford, IL), according to the manufacturer’s instructions. Intensities of immunoreactive bands (target gene normalized to actin) were quantified using Multi-Gauge Version 2.2 software (Fuji Film, Tokyo, Japan).
Immunohistochemistry staining
Super sensitive non-biotin horseradish peroxidase detection system (BioGenex Laboratories, San Ramon, CA) was used to detect TGF-β1 in the deparaffined tumor sections that were adhered to glass slides and stained following the procedures described previously [21]. Antigen unmasking was performed by the immersion of sections in TrilogyTM (CellMarque, Hot Springs, AR) and heating at 121°C for 15 min in a SA-252F autoclave (Sturdy Industrial, Taipei, Taiwan). Sections were immediately transferred to fresh hot TrilogyTM solution for 10 min at 80°C. Antibody against TGF-β1 (T 9429; Sigma–Aldrich) diluted 50× was applied for 24 h at 4°C followed by Super EnhancerTM (BioGenex, San Ramon, CA) treatment for another hour at room temperature. Tris-buffered saline (TBS; DakoCytomation, Carpinteria, CA) was used to wash the slides following every staining step. The slides were treated with the substrate, diaminobenzidine tetrahydrochloride (BioGenex), for 1–2 min and counterstained with hematoxylin for 1–2 min.
Tumor-specific T cell cytotoxicity
CTVT cells at P and R phase (8 × 106 cells/ml) were incubated with mitomycin (15 μg/ml, Sigma–Aldrich) for 1 h. After three washes, CTVT cells (0.8 × 106 cells/ml) were incubated with peripheral blood monomuclear cells (3.2 × 106 cells/ml) for 6 days to prepare CTVT-specific CTLs (effector cells; E). Following washing, the freshly prepared target cells (T) were incubated at 37°C overnight in U-bottom microtiter plates (4 × 103 cells/well) with the effector cells at E:T ratios of 100:1, 50:1, 25:1, 12.5:1, or 6.25:1. After incubation, supernatants were collected and the CTL cytotoxic activity was measured by the CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI) following manufacturer’s instructions.
Effect of TGF-β, IFN-γ, and IL-6 interactions on MHC antigen expression
Purified progression phase CTVT cells (1 × 106) were treated with rcIFN-γ (250, 500, or 1,000 U/ml) or rcIL-6 (5, 10, 20, 40 ng/ml) for 24, 48, or 72 h. In another experiment, CTVT cells (1 × 106) were pre-incubated with anti-TGF-β antibody for 4 h and then treated with IFN-γ (1,000 U/ml), IL-6 (10 ng/ml), or IFN-γ/IL-6 (1,000 U/ml and 10 ng/ml, respectively) for another 72 h. Finally, the combined effects of IFN-γ and IL-6 were investigated by adding IFN-γ (1,000 U/ml) and 2.5, 5, 10, 20, 40, or 60 ng IL-6/ml, or 10 ng IL-6/ml and various amounts of IFN-γ (250, 500, or 1,000 U/ml) to progression phase CTVT cells and incubating for 72 h. MHC class I and class II antigen expressions were determined as described previously [9] by measuring the intensity of the positive surface immunofluorescence with a FACScaliber™ flow cytometer (BD Biosciences, Franklin Lakes, NJ).
Reverse transcription polymerase chain reaction
Total RNA from the P and R phase TIL were prepared using TRIzol™ (Invitrogen). ′The IFN-γ sense primer was 5′-CCAGATGTATCGGACGGTGG-3 and the anti-sense primer was 5′-TTATCGCCTTGCGCTGGACC-3′; the IL-6 sense primer was 5′-AACAAGTGTGAAGACAGCAAAGAGGCACTG-3′ and the anti-sense primer was 5′-CATTATCCGAACAGCCCTCA-3′. Amplification of β-actin cDNA served as an internal standard. Cycle conditions were 94°C for 30 s, 56°C for 30 s, and 72°C for 1 min, for a total of 34 cycles. The RT-PCR products were separated on 2% agarose gels.
Enzyme-linked immunosorbant assay
The concentration of TGF-β1 in the supernatant from the P and R phase tumor cells was measured with an ELISA using the TGF-β1 Emax ImmunoAssay system (Promega) according to the manufacturer’s instructions.
TranSignal™ protein/DNA array
A nuclear extraction kit (Panomics, Fremont, CA) and the procedures following the manufacture’s instructions were used to isolate nuclear extracts from progression phase CTVT cells that had been stimulated by IFN-γ (1,000 U/ml) or IL-6 (10 ng/ml), or IFN-γ (1,000 U/ml) plus IL-6 (10 ng/ml) for 72 h. TF levels were determined using TranSignalTM protein/DNA arrays (Panomics). DNA/protein hybridization was carried out according to the manufacturer’s instructions. The array membrane was spotted with biotinylated DNA in the right columns and bottom rows and was used as positive controls for the assay. We adjusted the exposure time such that the majority of the positive control spots displayed equal signal intensity at the same exposure time, ensuring that control spots had a similar intensity. Quantification of the data was done by spot-densitometry using NIH Image 1.61 software. Any spots with a twofold increase were considered significant.
Detection of transcription factors
The sequence of the single-stranded oligodeoxynucleotide (ODN) corresponding to the selected transcription factors was designed according to previous publications (underlined letters denote phosphorothioate-bonded bases) [22–24]. Double-stranded decoy ODN were prepared from complementary, single-stranded phosphorothioate-bonded ODN (MWG-Biotech, Ebersberg, Germany) by melting at 95°C for 5 min, followed by a 3-h cool-down at room temperature. The decoy ODN were: 5′-CATGTTATGCATATTCCTGTAAGTG-3′, signal transducer and activator of transcription-1 (STAT-1); 5′-AGTTGAGGGGACTTTCCCAGGC-3′, nuclear factor-κB (NF-κB); 5′-AGTTGAGGTGAGTTTCACAGGC-3′, NF-κB mutant control, NF-κB mut; 5′-GGAAGCGAAA ATGAAATTGAC-3′, interferon regulatory factor-1 (IRF-1) [22]; 5′-CCTGCATTCTGGGAACTGTAG-3′, signal transducer and activator of transcription-3 (STAT-3); 5′-TGACGTCATGACGTCATGACGTCA-3′, cyclic AMP response element (CRE); and 5′–CTAGCTAGCTAGCTAGCTAGCTA G-3′, nonsense sequence palindrome control ODN (CREC) [23,24]. CREC ODN does not bind to other TF DNA binding sites [23]. The effective concentrations for STAT-1, STAT-3, NF-κB, NF-κBmut, and IRF-1 decoy ODN were 7–10 μM. The effective concentrations for CRE and control CREC decoy ODN were 150–200 nM. The cationic lipid N-(2, 3-dioleoyloxy-1-propyl) trimethylammonium methyl sulfate (DOTAP) (Sigma–Aldrich) was used to increase the delivery of CRE-decoy ODN and control CREC-decoy ODN into cells [23]. However, the uptake of other decoy ODN was achieved without using cationic lipid. IFN-γ (1,000 U/ml) and IL-6 (10 ng/ml) were added to the P phase CTVT cells pretreated with decoy ODN.
Statistical analysis
Experiments were performed in triplicate and repeated at least six times. Results are expressed as mean ± SE. The statistical significance of differences between mean values was estimated using Student’s t-test (Microsoft Excel). Values of P < 0.05 were considered significant.
Results
MHC class I and II antigen expression during CTVT progression and regression
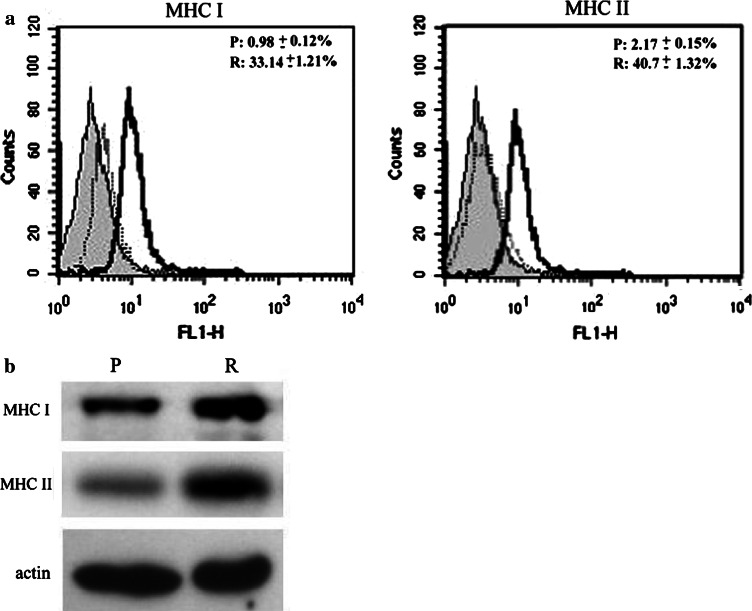
Flow cytometry determined MHC classe I and II antigen expressions on tumor cells during growth. These analyses confirmed the previous findings [6] that a much higher proportion of tumor cells (33.14 ± 1.21% for class I and 40.7 ± 1.32% for class II) expressed MHC antigen in the regression phase than those during the progression phase (0.98 ± 0.12% for class I and 2.17 ± 0.15% for class II) (P < 0.01) (Fig. 1a). Western blotting results analyzed by actin-normalized densitometry also revealed markedly higher levels of MHC class I (0.82 ± 0.1) and class II antigens (0.76 ± 0.1) in regression phase tumor cells compared to progression phase tumor cells (0.3 ± 0.08 for MHC I and 0.12 ± 0.05 for MHC II) (Fig. 1b).
Fig. 1.
Expression of MHC classes I and II in purified progression (P) and regression (R) phase tumor cells. a Results of flow cytometry analysis. Surface expression of MHC antigens on isolated CTVT cells during R phase was higher (P < 0.01) than during P phase. Filled profile: isotype control. Open profile: P phase (light line) and R phase (bold line). b MHC class I and II protein expressions in P and R phase CTVT cells were detected by immunoblotting. Their expressions were higher in R phase CTVT cells than in P phase CTVT cells
TGF-β1 expression in CTVT progression and regression
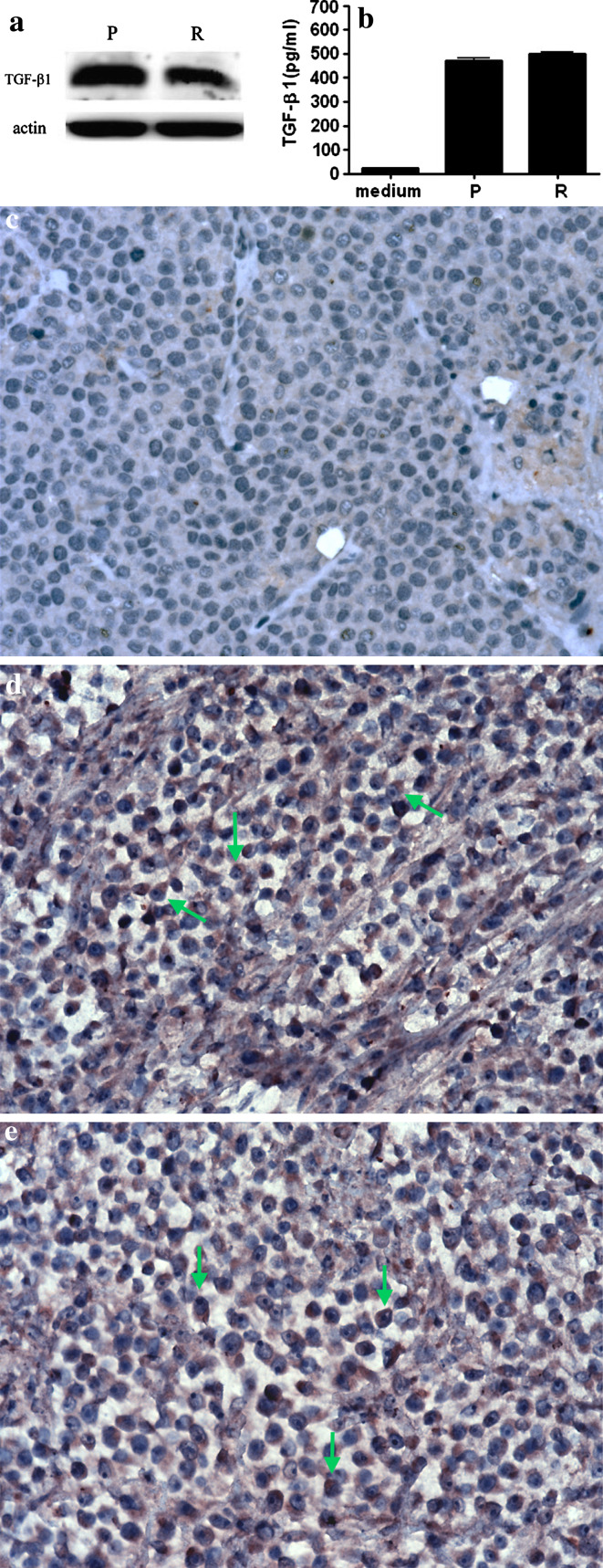
Similarly performed Western blotting established that the TGF-β1 protein was expressed at a high level both in the progression (0.93 ± 0.08) and regression phases (0.84 ± 0.11) (Fig. 2a). ELISA verified that the active form of TGF-β1 was highly expressed both in cultured progression phase cells (472.16 ± 17.85 pg/ml) and regression phase cells (499.96 ± 12.45 pg/ml). Expressions of active TGF-β1 were not significantly different (Fig. 2b). Immunohistochemistry was positive for TGF-β1 in the cytoplasm of most progression and regression phase tumor cells (Fig. 2c–e).
Fig. 2.
TGF-β1 expression in progression (P) and regression (R) phase CTVT cells. a Western blotting revealed high levels of the 12.5 kDa TGF-β1 protein in both P and the R phase cells. b Expression of the active form of TGF-β1 in P and R phase tumor cell supernatants was detected by ELISA. The amounts of active TGF-β1 in P and the R phase CTVT supernatants were not significantly different. Immunohistochemical analyses of deparaffinized formalin-fixed sections revealed that the majority of P phase (D) and R phase (E) tumor cells were positive for TGF-β1 (arrows). c Negative control without TGF-β1 antibody staining
Tumor-specific T cell cytotoxicity
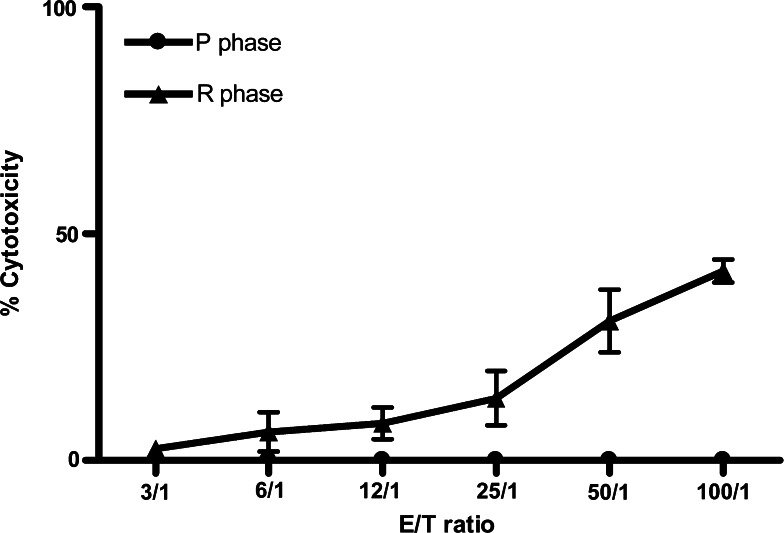
To further prove that the regression phase T cells were more potent in cytotoxicity against the tumor cells, regression and progression phase PBMC were collected to perform tumor-specific cytotoxicity assay. The cytotoxicity from regression phase PBMC had significant higher killing activity against CTVT than progression phase cells (Fig. 3).
Fig. 3.
Tumor specific T cell cytotoxicity. P phase and R phase CTVT cells (8 × 106 cells/ml) were incubated with mitomycin C and R phase peripheral blood mononuclear cells (PBMC; 3.2 × 106 cells/ml) for 6 days. Freshly prepared target (T) cells (CTVT; 4 × 103/well) were incubated at 37°C overnight with the effector (E) cells (R phase PBMC) at various E:T ratios (100:1, 50:1, 25:1, 12.5:1, 6.25:1). The supernatants were collected and the CTL cytotoxic activity was measured by the CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega, USA)
IFN-γ and IL-6 mRNA and proteins in progression and regression phase TIL
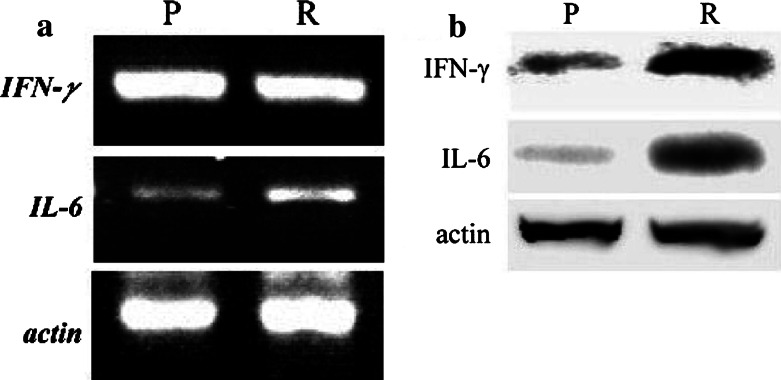
IFN-γ and IL-6 are important for MHC expression and TGF-β activities, respectively [9,15]. IL-6 mRNA (Fig. 4a) and protein (Fig. 4b) were significantly higher in the regression phase than the progression phase. Although progression and regression phase TILs both contained similarly high levels of IFN-γ, the level of IFN-γ protein in regression phase TIL was greater than in progression phase TIL (Fig. 4b). The IFN-γ and IL-6 sequences of the PCR products were confirmed by sequencing and analysis with BLAST software.
Fig. 4.
IFN-γ and IL-6 mRNA, and protein in TIL during the P and the R phases. a P and the R phase TIL expressed both IFN-γ and IL-6 mRNA. The levels of IFN-γ mRNA in the P and the R phase TIL were not significantly different. However, the levels of IL-6 mRNA were higher in R phase than in P phase. b A similar result for the IL-6 protein was obtained by Western blotting, but the level of IFN-γ protein was higher in R phase than in P phase
IFN-γ effect on MHC antigen expression
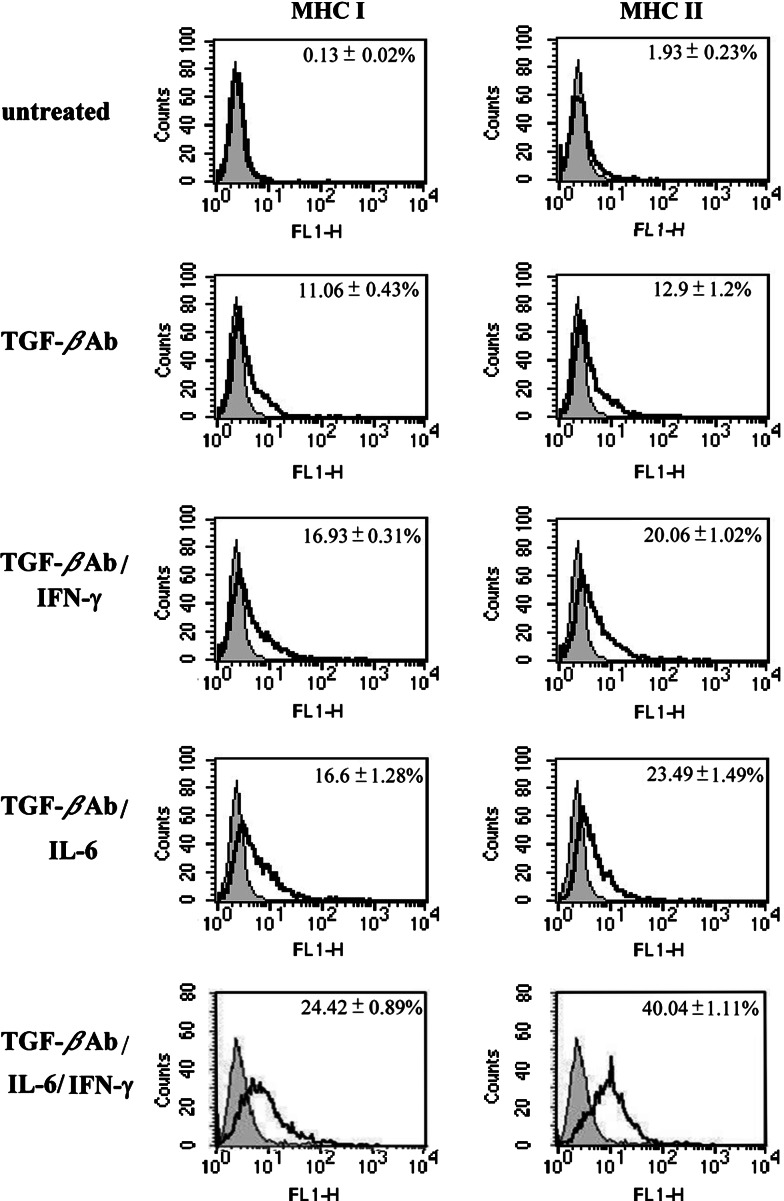
As IFN-γ was prominent in the progression phase (during which MHC antigen expression was very low), we assessed whether tumor IFN-γ was still able to promote MHC antigen expression. Accordingly, freshly purified progression phase tumor cells with low MHC class I and II antigens were treated with different concentrations of IFN-γ. Surprisingly, regardless of concentration or treatment length, IFN-γ did not induce further tumor cell expression of MHC class I or class II antigens (Fig. 5a, b). Because TGF-β hampers IFN-γ activity on MHC antigen expression [13], we tested if TGF-β was involved in the inhibition of the MHC antigen expression in this tumor system. After incubation with the anti-TGF-β specific antibody, expression of MHC class I and II antigens on the tumor cells isolated from six different dogs significantly increased (P < 0.01) from 0.31 ± 0.02 and 1.93 ± 0.23% in untreated cells to 11.06 ± 0.43 and 12.90 ± 1.2%, respectively (Fig. 6).
Fig. 5.
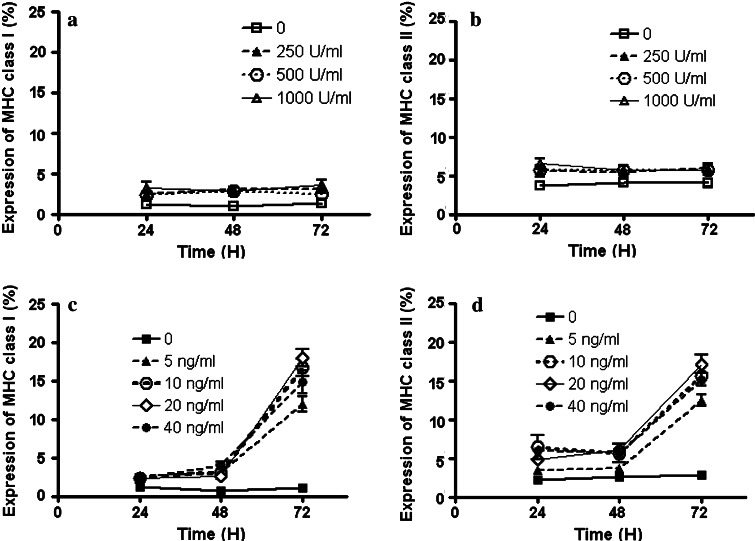
MHC class I and II antigen expressions on the P phase tumor cells stimulated by IFN-γ or IL-6. Isolated tumor cells from six tumor isolates were incubated with different concentrations of IFN-γ (250, 500, 1,000 U/ml) or IL-6 (5, 10, 20, 40 ng/ml) for 24, 48, or 72 h, and expression of MHC class I (a and c) and class II (b and d) antigens was determined by flow cytometry. None of the IFN-γ treatments increased MHC expression significantly. Different concentrations of IL-6 increased MHC class I and II antigen expressions, but only on tumor cells incubated with IL-6 for 72 h. Each experiment was done in triplicate
Fig. 6.
Effect of IFN-γ, IL-6, or IFN-γ/IL-6 on MHC class I and II antigen expressions in tumor cells pretreated with anti-TGF-β Ab. Progression phase CTVT cells were incubated with anti-TGF-β Ab for 4 h and then treated with IFN-γ (1,000 U/ml) or IL-6 (10 ng/ml) or IFN-γ/IL-6 for 24 h. Expression of MHC molecules was measured by flow cytometry. Data for each experimental group is shown with a bold line; data for the isotype control is shown with a light line. Numbers (top right) represent the percentage of cells showing significant induction compared to the control. Data are representative of least three separate experiments
IL-6 effect on MHC antigen expression
IL-6 inhibits TGF-β and restores NK-killing activities [9]. Appropriately, we investigated whether IL-6 also played a role in MHC antigen expression. All four tested concentrations of IL-6 induced moderate MHC class I and II antigen expressions on progression phase tumor cells from six tumor samples, while these cells produced high levels of TGF-β1. However, this activation of MHC antigen expression was evident only after 72 h (Fig. 5c, d).
Combinatorial effects of IL-6 and IFN-γ in MHC expression
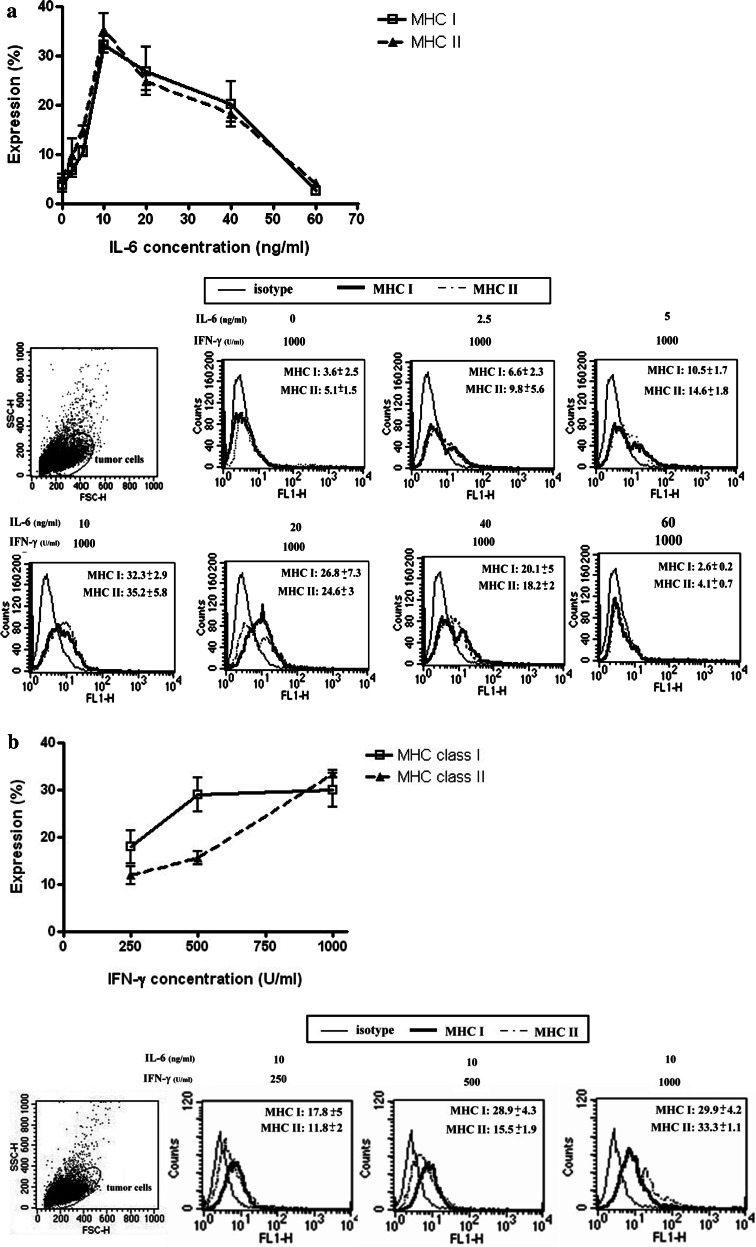
Since IFN-γ and IL-6 were both present in regression phase tumors, we further explored the effects of TGF-β1 on MHC antigen expression when IFN-γ and IL-6 were both added to the culture. After the effect of TGF-β1 was blocked by the antibody, the expression of MHC class I and II antigens were both increased from 0.31 ± 0.02 to 11.06 ± 0.43% and 1.93 ± 0.23 to 12.90 ± 1.2%, respectively (P < 0.01). The addition of IFN-γ or IL-6 further increased the expression to 16.93 ± 0.31% (class I) and 20.06 ± 1.02% (class II) for IFN-γ (P < 0.01), and 16.6 ± 1.28% (class I) to 23.49 ± 1.47% (class II) for IL-6 (P < 0.01). In addition, IFN-γ/IL-6 was the more potent inducer for both MHC antigen (24.42 ± 0.89% for class I and 40.04 ± 1.11% for class II) (P < 0.01) (Fig. 6). These synergistic effects of IFN-γ/IL-6 were further investigated using different cytokine dosages, alone or in combination. When progressive phase tumor cells were co-cultured with a fixed amount of IFN-γ (1,000 U/ml) and various amounts of IL-6, IFN-γ/IL-6 significantly increased the expression of MHC class I and II antigens. Peak expression was achieved with 1,000 U/ml IFN-γ and 10 ng/ml IL-6 (P < 0.05) (Fig. 7a). Nevertheless, IL-6 concentration > 10 ng/ml resulted in lower levels of MHC antigen expression. When the IL-6 concentration was fixed (10 ng/ml), MHC class I and II antigen expression in the progressive phase tumor cells from six tumor tissues increased significantly (P < 0.01) as the concentration of IFN-γ increased from 250 to 1,000 U/ (Fig. 7b).
Fig. 7.
Expression of MHC class I and II antigens on tumor cells stimulated by different concentrations of IFN-γ/IL-6 when anti-TGF-β Ab is not present. Effect of 72 h exposures a IFN-γ (1,000 U/ml) and IL-6 (2.5, 5, 10, 20, 40, or 60 ng/ml), and b IFN-γ (250, 500, or 1,000 U/ml) and IL-6 (10 ng/ml) on the expression of MHC class I and II antigens in six tumor isolates were determined by flow cytometry. The upper portions of a, b displayed the expression curves of MHC I and II antigens stimulated by different concentrations of IL-6 or IFN-γ, respectively. Peak expression was achieved with 1,000 U/ml IFN-γ and 10 ng/ml IL-6 (P < 0.05). Nevertheless, IL-6 concentration > 10 ng/ml resulted in lower levels of MHC antigen expression. The lower portions of a and b displayed the histograms corresponding to a and b
Transcription factors associated with IFN-γ/IL-6-inducible MHC antigen expression
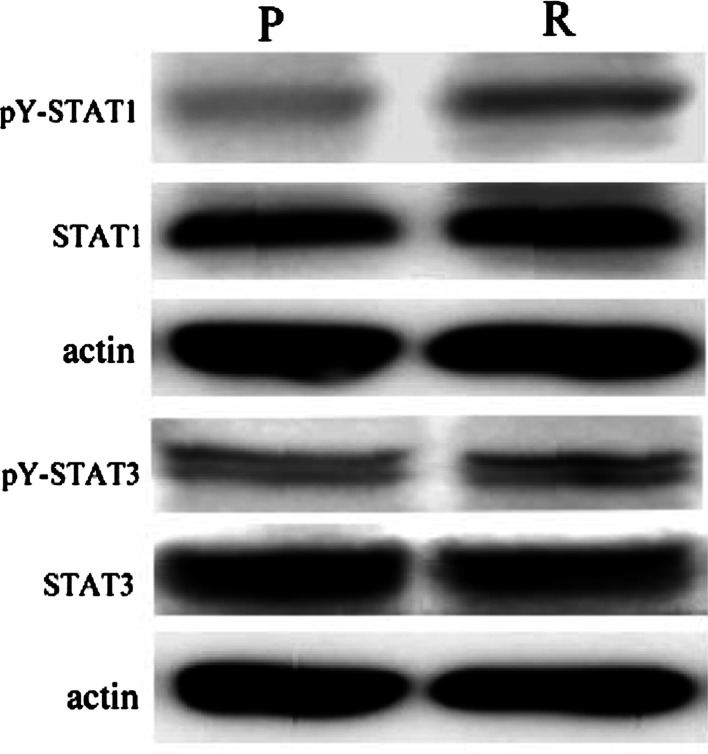
During tumor regression, because high levels of IFN-γ and IL-6 were secreted by TIL (Fig. 4b) and cellular responses to IFN-γ and IL-6 are known to be mediated largely by STAT1 and STAT3 activation, respectively [25,26], we first sought to determine the phosphorylation status of these two TFs in our cancer system. As expected, actin-normalized densitometry immunoblotting patterns revealed that STAT1 and STAT3 phosphorylation in tumor cells was higher in the regression phase (0.27 ± 0.01 for pY-STAT1 and 0.4 ± 0.09 for pY-STAT3) than in the progression phase (0.16 ± 0.01 for pY-STAT1 and 0.23 ± 0.04 for pY-STAT3) (Fig. 8).
Fig. 8.
Phosphorylation of STAT1 and STAT3 in tumor cells. Western blotting results showed that STAT1 and STAT3 were activated by phosphorylation at Try 701 and Try 705, respectively. Their phosphorylated levels were higher in R phase than in P phase. pY-STAT1 and pY-STAT3 were antibodies against the STAT1 and STAT3 when phosphorylated accordingly at tyrosine 701 and tyrosine 705
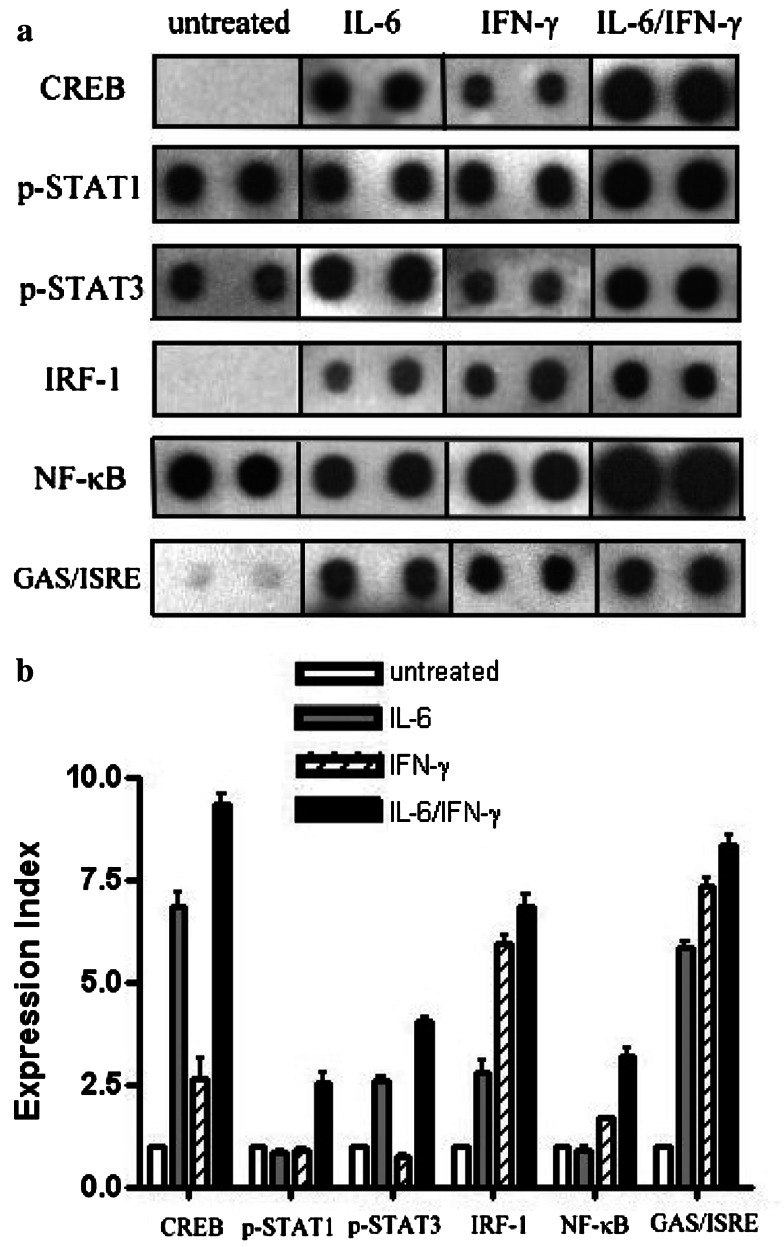
To further study the mechanism of IFN-γ and/or IL-6-inducible MHC antigen expression, the expression levels of 54 different kinds of TF in the tumor cells before and after IFN-γ/IL-6 treatments were determined using TranSignalTM protein/DNA arrays. The expression index is the average of the tested DNA hybridization signal divided by the average of the positive control DNA hybridization signal, and the value of untreated cells is set as 1. CREB, STAT-1, STAT-3, NF-κB, and IRF-1 increased significantly (more than twofold increase) after IFN-γ, IL-6, or IFN-γ/IL-6 treatment, except that IFN-γ did not stimulate STAT-1 (Fig. 9). The expression index of CREB in tumor cells induced by IFN-γ/IL-6 was almost tenfold greater than in untreated tumor cells (Fig. 9b). In tumor cells treated with IFN-γ or IL-6 alone, the CREB expression index was greater than in untreated cells, but it was significantly lower than in tumor cells with the IFN-γ/IL-6 treatment. The expression index of STAT-3 in tumor cells induced by IL-6 or IFN-γ/IL-6 was higher than in untreated cells. IRF-1 was higher in IFN-γ and IFN-γ/IL-6 treatments, but NF-κB increased only in IFN-γ/IL-6 treated cells (Fig. 9b). In addition, the protein/DNA arrays also revealed that the expression indexes of GAS/ISRE elements, which are STAT-binding sites in the nucleus [27], increased significantly in IFN-γ/IL-6 treatments. CRE, STAT-1, IRF-1, NF-κB, and STAT-3 decoy ODNs all effectively suppressed MHC class I and class II molecule expressions in IFN-γ/IL-6 treatments. The CREC and NF-κBmut decoy ODN controls did not have any effect (Fig. 10).
Fig. 9.
Transcription factor expression levels in tumor cells exposed to IFN-γ, IL-6, or IFN-γ/IL-6. a Transcription factor expression levels in nuclear extracts from P phase CTVT cells, which had been stimulated for 72 h by IFN-γ (1,000 U/ml), IL-6 (10 ng/ml), or IFN-γ (1,000U/ml) and IL-6 (10 ng/ml), were determined using TranSignalTM protein/DNA. On each array, genes were spotted in duplicate. Expressions of CREB, STAT-1, STAT-3, IRF-1, NF-κB, and GAS/ISRE were increased significantly in nuclei by IFN-γ/IL-6 treatment. b All spots were quantified by scanning densitometry. The expression index was the average of the tested DNA hybridization signal divided by the average of the positive control DNA hybridization signal and the value of untreated cells was set as 1. Data are representative of two experiments. Any spots with a twofold increase were considered significant
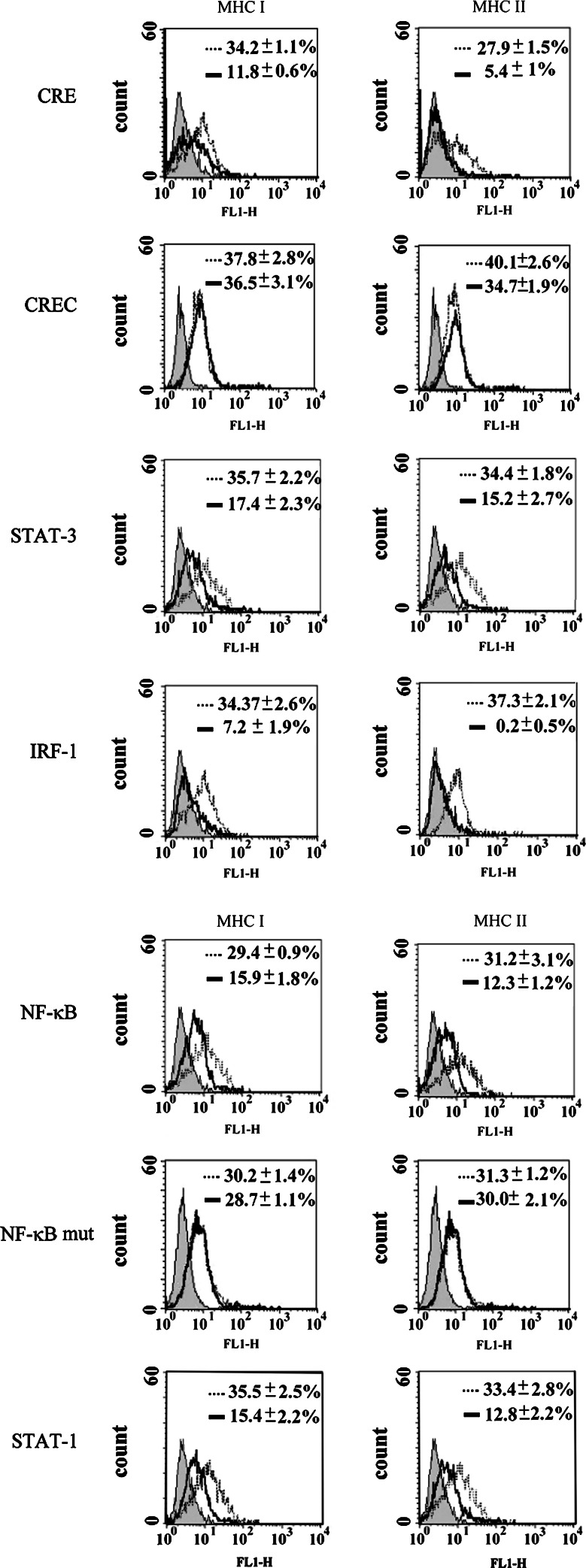
Fig. 10.
Effect of decoy ODN on cytokine-mediated MHC class I and II antigen expressions. Double-stranded, decoy ODN were prepared from complementary single-stranded, phosphorothioate-bonded bases. Decoy ODN were added 1 day after seeding) to the wells for 24 h. IFN-γ (1,000U/ml) and IL-6 (10 ng/ml) were added to the CTVT cells pretreated with decoy ODN. After incubation at 37°C for 72 h, MHC class I and II antigen expressions were d ODN (CRE, STAT-1, STAT-3, IRF-1, NF-κB) significantly decreased the expression of MHC class I and II molecules on the surface of tumor cells exposed to IFNγ/IL-6 for 72 h. Filled profile: isotype control. Open profile: with ODN (bold line) and without ODN (light line). Data are representative of three experiments
Discussion
MHC antigens play a critical role in immune responses to neoplastically transformed cells. Tumor cells commonly reduce MHC antigen expression to escape host immune responses [1]. The host/cancer interactions among TGF-β1, IFN-γ, and IL-6 in modulating MHC antigen expression are not fully understood. Here, we used a spontaneous regression canine tumor model to demonstrate that during progression phase, tumor-derived TGF-β1 inhibits the host IFN-γ and down-regulates tumor MHC antigen expression. However, during the regression phase, TIL produce high level of IL-6 that antagonizes TGF-β activities and restores IFN-γ-mediated MHC antigen expression.
We and other investigators [5,6] have found that this spontaneously transmissible tumor expresses extremely low levels of MHC class I and class II antigens. At the same time, a large amount of activated TGF-β1 is secreted during the growth of the tumor [9]. This was presently reconfirmed by RT-PCR, Western blotting, and immunohistochemical staining. Although Treg cells might be one possible source, CD4 positive cells comprised only around 10% of total the TIL; considering Foxp-3 reduces the percentage further, such that the numbers of Treg cells is non-proportional to tumor cells. Thus, the total contribution of TGF-β originating from cells other than the tumor cells could be minimal.
TGF-β inhibits MHC antigen expression through the inhibition of type III and type IV-CIITA mRNA in microglial cells [28] and can down-regulate MHC I antigen expression in ocular melanoma cells [29], the high concentrations of TGF-β secreted by the tumor cells could be one of the factors causing low MHC antigen expression of this tumor. Thus, when the activity of TGF-β is neutralized by anti-TGF-β polyclonal antibody, tumor cell expression of MHC class I and II antigens increases significantly. In addition, IFN-γ is one of the most potent cytokines modulating the expression of MHC antigens [15]. The high level of IFN-γ expression in TIL during the tumor growth was a reason to suspect that TIL-secreted IFN-γ might lose its capability to induce MHC antigen expression during tumor progression. This was confirmed by the failure to promote MHC antigen expression on the tumor cells by addition of up to 1000 U/ml of exogenous IFN-γ to the culture. Unresponsiveness of MHC antigen expression to IFN-γ in tumors that secrete TGF-β1 is not common. However, in TGF-β−/− mice, IFN-γ mRNA and circulating levels of IFN-γ are increased [30] as are IRF-1 and STAT1a [31], and expression of MHC class I and class II antigens are also increased in TGF-β deficient mice [32]. Presently, the observation that addition of IFN-γ in the anti-TGF-β polyclonal Ab-treated tumor cells further promoted the MHC antigen expression provides additional supportive evidence. Although tumor-derived TGF-β efficiently inhibited the function of IFN-γ secreted by TIL during the tumor’s progression phase, we are not able to rule out the possibility that other unknown factors might also be involved in the suppression of MHC antigen expression.
The observation that exogenous IL-6 alone can induce the expression of MHC antigens on tumor cells supports the view that in the presence of TGF-β1, TIL-derived IL-6 could also promote MHC antigen expression of the tumor cells through its anti-TGF-β1 activity. Further support comes from our observation that STAT3, a major TF associated with IL-6 activity [26], was significantly activated only when IL-6 was used. In addition, as described previously when the tumor cells were pre-treated with anti-TGF-β antibody, exogenous IL-6 further enhanced the expression of MHC class I and II antigens. Thus, IL-6 secreted by TIL in the regression phase of the tumor might be a key element in promoting tumor MHC antigen expression.
IL-6 is multivalent cytokine. In some cases, IL-6 enhances tumor growth [33], whereas in others, it assists host immune activity against tumor cells [33,34], and IL-6 gene has also been used to treat cancers such as melanoma [34] and Lewis mouse lung cancer [35]. In addition, IL-6 function may depend, in part, on the type of cells that produces it. IL-6 secreted by a tumor cell may protect the tumor from host immune attack [36]. In other cases, as shown previously [9] and presently, IL-6 produced by TIL stimulates host immune responses. Thus, IL-6 activities rely on the situations that it encounters.
Furthermore, co-application of IFN-γ and IL-6 promoted the highest MHC antigen expression among all of the tested groups. Therefore, TIL-derived IL-6 may play a dual role in regression phase tumor cells. First, IL-6 overcomes TGF-β-mediated suppression, which likely constitutes the most important activity in promoting or initiating the MHC antigen expression, abrogating IFN-γ inhibition. Second, because when the tumor cells were pre-treated with anti-TGF-β antibody, exogenous IFN-γ were then able to enhance the expression of MHC class I and II antigens and IL-6 might act in combination with the functionally restored IFN-γ in increasing MHC antigen expression. On the other hand, TGF-β1 is the major obstacle for IFN-γ to overcome to promote MHC antigen expression. Thus, IL-6, IFN-γ, and TGF-β1 work alone and/or together to modulate MHC antigen expression on the tumor cells. These three cytokines should be important in controlling the tumor MHC antigen expression. As far as we know, this is the first experimental in vivo system that has demonstrated the combinatorial effect of host IFN-γ/IL-6 in the promotion of MHC antigen expression in TGF-β-producing tumor during tumor regression. This appears to be an important mechanism used by the host to inhibit tumor-progressive growth and eventually to cause its regression. Knowing that as long as the TGF-β1 activities are present IFN-γ will not work efficiently, the blocking of TGF-β1 effect may allow for the treatment of cancer cells that constitutionally produce this cytokine and suppress immune responses.
Regulatory T cell is an important cell type in regulating immune homeostasis [37]. Treg cells increase in numbers in the tumor microenvironment and induce tumor-specific immune tolerance [38]. TGF-β1 actively promotes the expansion and differentiation of Treg cells [39]. TGF-β1 converts CD4+CD8− cells into CD4+CD8+ Treg cells by inducing transcription factor Foxp3 [40]. Treg cells were not presently examined. While IL-6 is a potent anti-TGF-β1 cytokine, the high concentration of IL-6 secreted by TIL during regression phase might block the expansion and differentiation of Treg cells, which could possibly explain the tumor regression.
During regression phase, up to 60% of the tumor cells still lack MHC antigen expression. However, in this phase, cytotoxic T cells increased in numbers with enhanced tumor-specific T cell cytotoxicity, and the inhibited NK cytotoxicity by TGF-β1 was also restored [9]. Therefore, the remaining 60% of regression phase CTVT cells that still lacked sufficient MHC antigen expression were presumably targeted by the reactivated NK cells during the regression phase.
The proper regulation of the cytokine-activated JAK/STAT pathway is critical because abnormal JAK/STAT signaling is closely associated with some cancers [41] and immune disorders [42]. The cytokine-activated JAK tyrosine kinases recruit STATs that are latent TFs in the cytoplasm, facilitating phosphorylation of the STATs. Tyrosine phosphorylation of STATs is required for their dimerization, nuclear translocation, and DNA binding [25]. The significant increase of phosphorylation of STAT1 at Try701 and STAT3 at Try705 in the regression phase tumor cells suggests the involvement of STAT1- and STAT3-associated activities. Cellular responses to IFN-γ are largely mediated by STAT1 activation and are rarely associated with STAT3 [25,43]. In contrast, IL-6 strongly activates STAT3 and only weakly activates STAT1 [26]. Similar relationships between these cytokines and STATs were found ex vivo in this tumor and the combinatorial effect of IFN-γ/IL-6 that activated both STAT1 and STAT3 in the tumor cells also highlights these activities. The coincident low expression of STAT1 in the presence of IFN-γ and inability of IFN-γ to stimulate MHC expression suggest that TGF-β1 inhibits IFN-γ-activated MHC antigen expression during the progression phase of a tumor at the transcription level via STAT1. Also noteworthy is our finding of the decreased protein level during this phase compared to regression phase TIL, but that the mRNA level of IFN-γ was similarly high during both phases. Thus, in addition to the inhibitory effect to the IFN-γ activities through the STAT1 activation, this tumor might also inhibit IFN-γ protein expression. STATs cooperate with the histone acetyltransferase CREB-binding protein (CBP)/p300 for gene activation [44] and activate numerous genes including the MHC class II and the CIITA by directly binding to the promoters [45, 46]. We found that both IL-6 alone and IFN-γ/IL-6 treatment groups, strongly activated CREB in the tumor cells, suggesting that CREB activity is also associated with the MHC antigen expression in this tumor under the influence of the cytokines. This might explain the present results in cells exposed solely to IFN-γ, where there was no promotion in MHC antigen expression and much less of an effect on CREB.
The biological functions of STAT3 are diversified. STAT3 is constitutively expressed in a number of primary human tumors [47] and is critical in maintaining cancer cell proliferation [48] and immunosuppression [49]. STAT3 is inducible by many cytokines and growth factors including TGF-alpha, EGF, hepatocyte growth factor, vascular endothelial growth factor, and IL-6, and oncogenic kinases as well [47,50]. Therefore, the activities also depend on substance(s) that initiate STAT3 activation or induction. IL-6 production increases significantly by TILs isolated from regression rather than progression phase, and functions as an anti-TGF beta agent [9]. Signaling of IL-6 follows the STAT3 pathway [51]. Thus, it is reasonable to suggest that the increased pSTAT3 presently demonstrated was a result of increased concentration of IL-6, which preludes anti-TGF beta activity. Our evidence also indicates that IL-6 acts indirectly in promoting MHC expression and the decoy ODN of STAT3 inhibits the expression of MHC expression. Therefore, IL-6 is activated during regression phase, by a yet unknown mechanism, to transduce a STAT3-mediated signal, antagonize the TGF-beta activities, and promote MHC expression. In addition, IL-6 displayed a concentration threshold phenomenon. When IL-6 concentration exceeded 10 ng/ml, MHC expression was suppressed. Thus, it is also possible that in CTVT, IL-6 concentration and pSTAT3 are at relatively low levels that favor the activation of MHC expression of CTVT, while, in other cancers, IL-6 and pSTAT3 are relatively more abundant and mediate cancer cell proliferation.
IRF-1 is expressed at low levels in unstimulated cells and can be activated by many cytokines including type I and II IFN, TNF-α, IL-1, and IL-6 [52,53]. IRF-1 is mediated through STAT1 and NF-κB, whose binding sites are in the IRF-1 promoter [54,55]. The interaction of NF-κB with IRF-1 is critical for promoter activation [55]. Presently, both STAT1 and NF-κB were activated in the IFN-γ/IL-6 treatment. Gamma-activated sites (GAS) are associated with the induction of transcription by IFN-γ [56] and as a binding site for STATs [57]. IFN-stimulated response element (ISRE) is also important in MHC I gene promoters. Upon treatment of IFN-γ, IRF-1 serves as an adapter protein for the binding of STATs to the ISRE or GAS, which in turn induces MHC I transactivation [56,58]. Presently, IFN-γ/IL-6 treatment activated GAS/ISRE most effectively. Thus, IFN-γ/IL-6 treatment activates STAT1, which binds to GAS/ISRE to activate the promoter for IRF-1, ultimately promoting MHC I antigen expression. In the present IRF-1 decoy ODN study, MHC expression was almost totally suppressed. Thus, IRF-1 should play a more important role in IFN-γ- and IL-6-stimulated MHC antigen expression. However, exogenous IFN-γ alone significantly activated the interferon-inducible genes GAS/ISRE, but did not activate STATs. IRF dimers that do not form a complex with STATs can also regulate interferon-stimulating genes by directly binding the ISRE and bypassing STATs [56]. The activation of the GAS/ISRE observed in the presence of only IFN-γ likely occurred via this STATs-independent pathway. In addition, IFN-γ alone can activate IRF-1 even in the presence of TGF-β effect, but does not promote MHC antigen expression on the progression phase tumor cells, even when a high concentration of exogenous IFN-γ is added to the culture. This dichotomy can be at least partially explained by the findings that TGF-β inhibits IFN-γ-induced MHC antigen expression, but is incapable of acting globally to inhibit IFN-γ-induced gene expression including IRF-1[13]. Taken together, the presence of high level of IRF-1 does not necessarily relate to STATs or promote the MHC expression.
In conclusion, CTVT, a TGF-β-producing transmissible tumor, can markedly suppress the expression levels of MHC. In the presence of TGF-β, IFN-γ is not active. However, the host TIL produces high concentrations of IL-6, which show powerful anti-TGF-β activity and activate MHC antigen expression. Furthermore, IFN-γ and IL-6 act synergistically in combination to enhance the expression of MHC antigens through mechanisms associated with TFs such as STAT-1, STAT-3, CREB, NF-κB, and IRF-1. Relationships between the TFs are closely interactive and complicated. It is suggested that the regimen for treating cancer cells that constitutively secrete TGF-β should consider incorporating a strategy of anti-TGF-β activity. Finally, the significance of this research is that the results and the mechanisms depicted were obtained from an in vivo system of the tumor presented with spontaneous regression. This finding should prove useful for designing effective immunotherapies for cancer patients.
Acknowledgments
This project was supported by National Science Council of Taiwan (NSC95-2313-B-002-375) and Council of Agriculture of Taiwan (96AS-1.2.1-AD.U1(20)).
References
- 1.Garrido F, Ruiz-Cabello F, Cabrera T, Perez-Villar JJ, Lopez-Botet M, Duggan-Keen M, Stern PL. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today. 1997;18:89–95. doi: 10.1016/S0167-5699(96)10075-X. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann TK, Meidenbauer N, Muller-Berghaus J, Storkus WJ, Whiteside TL. Proinflammatory cytokines and CD40 ligand enhance cross-presentation and cross-priming capability of human dendritic cells internalizing apoptotic cancer cells. J Immunother. 2001;24:162–171. doi: 10.1097/00002371-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Overwijk WW. Breaking tolerance in cancer immunotherapy: time to ACT. Curr Opin Immunol. 2005;17:187–194. doi: 10.1016/j.coi.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Yu Z, Restifo NP. Cancer vaccines: progress reveals new complexities. J Clin Invest. 2002;110:289–294. doi: 10.1172/JCI16216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murgia C, Pritchard JK, Kim SY, Fassati A, Weiss RA. Clonal origin and evolution of a transmissible cancer. Cell. 2006;126:477–487. doi: 10.1016/j.cell.2006.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsiao YW, Liao KW, Hung SW, Chu RM. Effect of tumor infiltrating lymphocytes on the expression of MHC molecules in canine transmissible venereal tumor cells. Vet Immunol Immunopathol. 2002;87:19–27. doi: 10.1016/S0165-2427(02)00026-0. [DOI] [PubMed] [Google Scholar]
- 7.Katzir N, Arman E, Cohen D, Givol D, Rechavi G. Common origin of transmissible venereal tumors (TVT) in dogs. Oncogene. 1987;1:445–448. [PubMed] [Google Scholar]
- 8.Liao KW, Lin ZY, Pao HN, Kam SY, Wang FI, Chu RM. Identification of canine transmissible venereal tumor cells using in situ polymerase chain reaction and the stable sequence of the long interspersed nuclear element. J Vet Diagn Invest. 2003;15:399–406. doi: 10.1177/104063870301500501. [DOI] [PubMed] [Google Scholar]
- 9.Hsiao YW, Liao KW, Hung SW, Chu RM. Tumor-infiltrating lymphocyte secretion of IL-6 antagonizes tumor-derived TGF-beta 1 and restores the lymphokine-activated killing activity. J Immunol. 2004;172:1508–1514. doi: 10.4049/jimmunol.172.3.1508. [DOI] [PubMed] [Google Scholar]
- 10.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 11.Cross D, Cambier JC. Transforming growth factor beta 1 has differential effects on B cell proliferation and activation antigen expression. J Immunol. 1990;144:432–439. [PubMed] [Google Scholar]
- 12.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Panek RB, Lee YJ, Benveniste EN. TGF-beta suppression of IFN-gamma-induced class II MHC gene expression does not involve inhibition of phosphorylation of JAK1, JAK2, or signal transducers and activators of transcription, or modification of IFN-gamma enhanced factor X expression. J Immunol. 1995;154:610–619. [PubMed] [Google Scholar]
- 14.Ljunggren G, Anderson DJ. Cytokine induced modulation of MHC class I and class II molecules on human cervical epithelial cells. J Reprod Immunol. 1998;38:123–138. doi: 10.1016/S0165-0378(98)00009-6. [DOI] [PubMed] [Google Scholar]
- 15.Ting JP, Baldwin AS. Regulation of MHC gene expression. Curr Opin Immunol. 1993;5:8–16. doi: 10.1016/0952-7915(93)90074-3. [DOI] [PubMed] [Google Scholar]
- 16.Wei L-H, Kuo M-L, Chen C-A, Chou C-H, Lai K-B, Lee C-N, Hsieh C-Y. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- 17.Ohta K, Yamagami S, Taylor AW, Streilein JW. IL-6 antagonizes TGF-beta and abolishes immune privilege in eyes with endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2000;41:2591–2599. [PubMed] [Google Scholar]
- 18.Rabinowich H, Sedlmayr P, Herberman RB, Whiteside TL. Response of human NK cells to IL-6 alterations of the cell surface phenotype, adhesion to fibronectin and laminin, and tumor necrosis factor-alpha/beta secretion. J Immunol. 1993;150:4844–4855. [PubMed] [Google Scholar]
- 19.Scheid C, Young R, McDermott R, Fitzsimmons L, Scarffe JH, Stern PL. Immune function of patients receiving recombinant human interleukin-6 (IL-6) in a phase I clinical study: induction of C-reactive protein and IgE and inhibition of natural killer and lymphokine-activated killer cell activity. Cancer Immunol Immunother. 1994;38:119–126. doi: 10.1007/BF01526207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao X, Wang Q, Ju DW, Tao Q, Wang J. Efficient inducation of local and systemic antitumor immune response by liposome-mediated intratumoral co-transfer of interleukin-2 gene and interleukin-6 gene. J Exp Clin Cancer Res. 1999;18:191–200. [PubMed] [Google Scholar]
- 21.Liang CT, Chueh LL, Pang VF, Zhuo YX, Liang SC, Yu CK, Chiang H, Lee CC, Liu CH. A non-biotin polymerized horseradish-peroxidase method for the immunohistochemical diagnosis of canine distemper. J Comp Pathol. 2007;136:57–64. doi: 10.1016/j.jcpa.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Wagner AH, Gebauer M, Pollok-Kopp B, Hecker M. Cytokine-inducible CD40 expression in human endothelial cells is mediated by interferon regulatory factor-1. Blood. 2002;99:520–525. doi: 10.1182/blood.V99.2.520. [DOI] [PubMed] [Google Scholar]
- 23.Park YG, Nesterova M, Agrawal S, Cho-Chung YS. Dual blockade of cyclic AMP response element- (CRE) and AP-1-directed transcription by CRE-transcription factor decoy oligonucleotide. gene-specific inhibition of tumor growth. J Biol Chem. 1999;274:1573–1580. doi: 10.1074/jbc.274.3.1573. [DOI] [PubMed] [Google Scholar]
- 24.Krzesz R, Wagner AH, Cattaruzza M, Hecker M. Cytokine-inducible CD40 gene expression in vascular smooth muscle cells is mediated by nuclear factor kappaB and signal transducer and activation of transcription-1. FEBS Lett. 1999;453:191–196. doi: 10.1016/S0014-5793(99)00683-3. [DOI] [PubMed] [Google Scholar]
- 25.Darnell JE, Kerr I, Stark G. Jak-STAT pathways and transcriptional activation in response to interferons and other extracellular signaling proteins. Science. 1994;264:1415–1420. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 26.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334(Pt 2):297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehret GB, Reichenbach P, Schindler U, Horvath CM, Fritz S, Nabholz M, Bucher P. DNA binding specificity of different STAT proteins. Comparison of in vitro specificity with natural target sites. J Biol Chem. 2001;276:6675–6688. doi: 10.1074/jbc.M001748200. [DOI] [PubMed] [Google Scholar]
- 28.Pazmany T, Tomasi TB. The major histocompatibility complex class II transactivator is differentially regulated by interferon-gamma and transforming growth factor-beta in microglial cells. J Neuroimmunol. 2006;172:18–26. doi: 10.1016/j.jneuroim.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Ma D, Niederkorn JY. Transforming growth factor-beta down-regulates major histocompatibility complex class I antigen expression and increases the susceptibility of uveal melanoma cells to natural killer cell-mediated cytolysis. Immunology. 1995;86:263–269. [PMC free article] [PubMed] [Google Scholar]
- 30.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCartney-Francis NL, Wahl SM. Dysregulation of IFN-gamma signaling pathways in the absence of TGF-beta 1. J Immunol. 2002;169:5941–5947. doi: 10.4049/jimmunol.169.10.5941. [DOI] [PubMed] [Google Scholar]
- 32.Geiser AG, Letterio JJ, Kulkarni AB, Karlsson S, Roberts AB, Sporn MB. Transforming growth factor beta 1 (TGF-beta 1) controls expression of major histocompatibility genes in the postnatal mouse: aberrant histocompatibility antigen expression in the pathogenesis of the TGF-beta 1 null mouse phenotype. Proc Natl Acad Sci USA. 1993;90:9944–9948. doi: 10.1073/pnas.90.21.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shouda T, Hiraoka K, Komiya S, Hamada T, Zenmyo M, Iwasaki H, Isayama T, Fukushima N, Nagata K, Yoshimura A. Suppression of IL-6 production and proliferation by blocking STAT3 activation in malignant soft tissue tumor cells. Cancer Lett. 2006;231:176–184. doi: 10.1016/j.canlet.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 34.Mule JJ, Custer MC, Travis WD, Rosenberg SA. Cellular mechanisms of the antitumor activity of recombinant IL-6 in mice. J Immunol. 1992;148:2622–2629. [PubMed] [Google Scholar]
- 35.Usuda J, Okunaka T, Furukawa K, Tsuchida T, Kuroiwa Y, Ohe Y, Saijo N, Nishio K, Konaka C, Kato H. Increased cytotoxic effects of photodynamic therapy in IL-6 gene transfected cells via enhanced apoptosis. Int J Cancer. 2001;93:475–480. doi: 10.1002/ijc.1374. [DOI] [PubMed] [Google Scholar]
- 36.Code S, Simard C, Lemieux R. Regulation of growth-related genes by interleukin-6 in muring myeloma cells. Cytokine. 2002;20:113–120. doi: 10.1006/cyto.2002.1988. [DOI] [PubMed] [Google Scholar]
- 37.Sakaguichi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 38.Wang HY, Wang RF. Regulatory T cells and cancer. Curr Opin Immunol. 2007;19:217–223. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Yi H, Xia XP, Zhao Y. Transforming growth factor-beta: an important role in CD4+CD25+ regulatory T cells and immune tolerance. Autoimmunity. 2006;39:269–276. doi: 10.1080/08916930600753903. [DOI] [PubMed] [Google Scholar]
- 40.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 42.Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev. 2003;3:900–911. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- 43.Balhoff JP, Stephens JM. Highly specific and quantitative activation of STATs in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1998;247:894–900. doi: 10.1006/bbrc.1998.8890. [DOI] [PubMed] [Google Scholar]
- 44.Christian M, Marangos P, Mak I, McVey J, Barker F, White J, Brosens JJ. Interferon-gamma modulates prolactin and tissue factor expression in differentiating human endometrial stromal cells. Endocrinology. 2001;142:3142–3151. doi: 10.1210/en.142.7.3142. [DOI] [PubMed] [Google Scholar]
- 45.Moreno CS, Beresford GW, Louis-Plence P, Morris AC, Boss JM. CREB regulates MHC class II expression in a CIITA-dependent manner. Immunity. 1999;10:143–151. doi: 10.1016/S1074-7613(00)80015-1. [DOI] [PubMed] [Google Scholar]
- 46.van der Stoep N, Quinten E, van den Elsen PJ. Transcriptional regulation of the MHC class II trans-activator (CIITA) promoter III: identification of a novel regulatory region in the 5’-untranslated region and an important role for cAMP-responsive element binding protein 1 and activating transcription factor-1 in CIITA-promoter III transcriptional activation in B lymphocytes. J Immunol. 2002;169:5061–5071. doi: 10.4049/jimmunol.169.9.5061. [DOI] [PubMed] [Google Scholar]
- 47.Huang S. Regulation of metastases by signal transducer and activator of transcription 3 signaling pathway: clinical implications. Clin Cancer Res. 2007;13:1362–1366. doi: 10.1158/1078-0432.CCR-06-2313. [DOI] [PubMed] [Google Scholar]
- 48.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 49.Kortylewski M, Kujawski M, Wang TH, Wei S, Zhang SM, Guilian STP, Kay NHK, William JM, Richard GK, Pardoll JW, Yu H. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 50.Aggarwal B, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci. 2006;1091:151–169. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- 51.Wagenka UM, Buschmann L, Lutticken C, Heinrich PC, Horn F. Acute-phase response factor, a muclear factor binding to acute-phase response elements, is rapidly activated by interleukin-6 at the posttranslational level. Mol Cell Biol. 1993;13:2762–2788. doi: 10.1128/mcb.13.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Revel M, Katz A, Eisenbach L, Feldman M, Haran-Ghera N, Harroch S, Chebath J. Interleukin-6: effects on tumor models in mice and on the cellular regulation of transcription factor IRF-1. Ann N Y Acad Sci. 1995;762:342–355. doi: 10.1111/j.1749-6632.1995.tb32338.x. [DOI] [PubMed] [Google Scholar]
- 53.Agresti C, Bernardo A, Del Russo N, Marziali G, Battistini A, Aloisi F, Levi G, Coccia EM. Synergistic stimulation of MHC class I and IRF-1 gene expression by IFN-gamma and TNF-alpha in oligodendrocytes. Eur J Neurosci. 1998;10:2975–2983. doi: 10.1111/j.1460-9568.1998.00313.x. [DOI] [PubMed] [Google Scholar]
- 54.Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, Kaplan DH, Riley JK, Greenlund AC, Campbell D, Carver-Moore K, DuBois RN, Clark R, Aguet M, Schreiber RD. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/S0092-8674(00)81288-X. [DOI] [PubMed] [Google Scholar]
- 55.Chelbi-alix MK, Bobe P, Benoit G, Canova A, Pine R. Arsenic enhances the activation of Stat1 by interferon gamma leading to synergistic expression of IRF-1. Oncogene. 2003;22:9121–9130. doi: 10.1038/sj.onc.1207090. [DOI] [PubMed] [Google Scholar]
- 56.Wesoly J, Szweykowska-Kulinska Z, Bluyssen HA. STAT activation and differential complex formation dictate selectivity of interferon responses. Acta Biochim Pol. 2007;54:27–38. [PubMed] [Google Scholar]
- 57.Kim OS, Park EJ, Joe EH, Jou I. JAK-STAT signaling mediates gangliosides-induced inflammatory responses in brain microglial cells. J Biol Chem. 2002;277:40594–40601. doi: 10.1074/jbc.M203885200. [DOI] [PubMed] [Google Scholar]
- 58.Gobin SJ, van Zutphen M, Woltman AM, van den Elsen PJ. Transactivation of classical and nonclassical HLA class I genes through the IFN-stimulated response element. J Immunol. 1999;163:1428–1434. [PubMed] [Google Scholar]